Abstract
Purpose
The muscarinic M4 receptor antagonist MT3 (muscarinic toxin 3) is effective at inhibiting the development of myopia in response to form deprivation, and prevents the deprivation-induced choroidal thinning. We asked if it was equally effective in eyes wearing negative lenses.
Methods
Chicks wore monocular diffusers or –15 D lenses for 7 days. Intravitreal injections of MT3 (90 nmoles) were given on days 2, 4 and 6 (diffusers: n = 13; lenses: n = 12); saline was used as injection controls (diffusers: n = 11; lenses: n = 13). Ocular dimensions were measured with A-scan ultrasound on days 1 and 7. Refractions were measured using a Hartinger's refractometer. A third group of “normal” chicks received monocular injections of drug (n = 7) or saline (n = 7), and eyes were measured 3 and 72 h later.
Results
MT3 inhibited the myopia in response to form deprivation, but did not affect the compensation to negative lenses (drug versus saline: FD: –3.2 versus –7.4 D; p < 0.001; Lenses: –4.5 versus –4.9 D). The myopia inhibition in deprived eyes was due to inhibition of axial growth (610 μm versus 827 μm; p < 0.005); lens-wearing eyes grew similar to saline controls (747 μm versus 743 μm). There was no effect of the drug on the choroidal thinning in either condition. Unexpectedly, MT3 produced choroidal thinning in normal eyes (drug versus saline: –45 versus 16 μm/3 h; p < 0.05), but had no effect on refractions or ocular growth.
Conclusions
MT3 does not inhibit the development of myopia in response to hyperopic defocus. It also causes choroidal thinning, an anomalous effect for a muscarinic receptor antagonist. These results support the existence of different muscarinic mechanisms in the excessive eye growth resulting from the open-loop condition of form deprivation, versus that of hyperopic defocus, a closed-loop condition.
Keywords: Chick, choroids, form deprivation, myopia, muscarinic
INTRODUCTION
Animal models have definitively shown that the growing eye uses visual feedback to guide it to emmetropia, in which the image plane of distant objects lies on the retina.1 When this feedback system fails, eyes become too long for the front optics, and myopic. Despite decades of research, why this failure of emmetopization occurs in some people but not others is unknown, and the signal molecules that mediate ocular growth rate changes remain elusive, impeding the development of drugs that could slow down the progression of myopia in children. One drug that has long been the focus of attention, and the only one currently known to inhibit myopia progession in children, is the non-selective acetylcholine muscarinic antagonist atropine,2 however, atropine has the deleterious side effects of cycloplegia and photophobia. Pirenzepine, a more specific receptor antagonist (M2/M4) and one with milder side effects, was in clinical trials for several years, before the study was abruptly terminated.3–5 Hence the search for relatively specific antagonists that have fewer side effects than atropine continues in animal models.
Recently, McBrien et al. reported that the highly specific M4 antagonist MT3 effectively inhibited the development of form deprivation myopia in chickens, and prevented the choroidal thinning that is concomitant with myopia.6 However, an abstract by another group reported only a partial protection against lens-induced (–7 D) myopia,7 complicating interpretation. Historically, form deprivation and negative lens-wear have been more or less used interchangeably in the study of the visual regulation of eye growth, but despite their similar outcomes of excessive eye elongation and myopia, the visual stimuli differ: form deprivation provides no visual feedback and so constitutes an open-loop system, while negative lenses provide a focal plane, and so is closed-loop. Increasing evidence suggests that the underlying mechanisms may differ between the two,8–12 a non-trivial issue when considering the implications of translating results from both paradigms to the etiology of human myopia, and the search for potential pharmacological interventions. With this in mind, we studied the effects of MT3 in parallel experiments on chicks wearing negative lenses or diffusers, using the lower of the two effective concentrations in the McBrien study.6 We also asked if the choroidal response differed in the two paradigms, because a pilot study injecting MT3 into normal eyes showed choroidal thinning,13 the opposite of what would be predicted based on other muscarinic antagonists.14 Parts of this manuscript have been presented in abstract form.13
METHODS
Subjects
White Leghorn chicks (Gallus gallus domesticus) were hatched in an incubator and raised from day one in temperature – controlled brooders. The light cycle was 12 L/12D (8:30 am to 8:30 pm). Food and water were supplied ad libitum. Care and use of the animals conformed to the ARVO Resolution for the Care and Use of Animals in Research.
Injections and Measurements
At 12–14 days of age, translucent diffusers or –15 D lenses were affixed to the right eye using matching Velcro rings; the left eye was untreated. The diffusers and lenses were worn for 7 days. Starting on day 2, experimental eyes were injected with 10 μM 2.5 μm MT3 (~90 nmoles, the lower of the two effective doses used by McBrien6) (diffusers: n = 13; lenses: n = 12) or 10 μl 0.75% saline (diffusers: n = 11; lenses: n = 13); subsequent injections were given on days 4 and 6. Eyes were measured using high frequency A-scan ultrasonography (30 MHz transducer (Panametrics), sampled at 100 MHz with a Sonix 8100 A/D board in a computer; details in Nickla et al.15) on day 1 prior to the injections, and on day 7 (6 full days). Refractive errors were measured using a Hartinger's coincidence refractometer on day 7. A third group of “untreated” birds did not wear lenses or diffusers, and received a single injection of MT3 (n = 7) or saline (n = 7). Eyes were measured prior to the injection, and at 3 and 72 hours after. Chicks were under isoflurane inhalation anesthesia (1% in oxygen) for all optometric measurements. Axial length is defined as the distance from the anterior cornea to the anterior sclera. Vitreous chamber depth is the distance from the posterior lens to the anterior retina. For both parameters, the data analyzed and shown in the graphs are the changes in length over the 6-day period of the experiment. Choroidal thickness was also determined, and the changes over the time courses were analyzed.
Injections into the vitreous were made using a Hamilton syringe with a 30 G needle under isoflurane inhalation anesthesia. The needle punctured the sclera at approximately a 45° angle from the temporal side of the eye after the removal of feathers and cleaning the skin with alcohol. The needle remained in place for approximately 30 s while the skin around the needle was held together using a small forceps to minimize leakage. For the multiple injections, we attempted to use the same injection site. Recovery from anesthesia occurred within a few minutes.
Statistics
Means and standard deviations for treated eyes and their fellow eyes for both paradigms are in Table 1. Comparisons between experimental (injected) groups and their contralateral fellow eyes were done by one-sample t-tests using interocular difference (IOD) data. Two-way ANOVAs were conducted to test for interactions between diffuser versus lens (“device”) differences and the drug effect using IOD data (i.e. whether device type alters the drug effect on the parameters of interest). Comparisons between drug and saline groups within each device type were done via two-sample t-tests.
TABLE 1.
Means and standard deviations (parentheses) for all groups and their fellow eyes.
| Form deprivation: Mean (SD) |
Lenses: Mean (SD) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Drug | Fellow drg | Saline | Fellow sal | Drug | Fellow drg | Saline | Fellow sal | |
| R.E. | –3.2 (2.0) | 1.3 (0.9) | –7.4 (2.4) | 1.2 (0.6) | –4.5 (2.0) | 0.9 (1.6) | –4.9 (3.1) | 0.5 (1.1) |
| AXIAL | 610 (175) | 568 (100) | 827 (195) | 533 (111) | 747 (235) | 604 (106) | 743 (177) | 568 (95) |
| VITREOUS | 466 (153) | 155 (73) | 646 (118) | 240 (82) | 524 (175) | 349 (55) | 531 (125) | 291 (91) |
| ANT. CH. | 227 (91) | 336 (71) | 246 (103) | 442 (48) | 191 (97) | 332 (52) | 200 (73) | 292 (58) |
| CHOROID | –52 (82) | 0.1 (0.0) | –68 (48) | –0.1 (0.1) | –66 (40) | 0 (0.0) | –38 (57) | 0.1 (0.0) |
R.E.: refractive error; ant. ch.: anterior chamber depth.
RESULTS
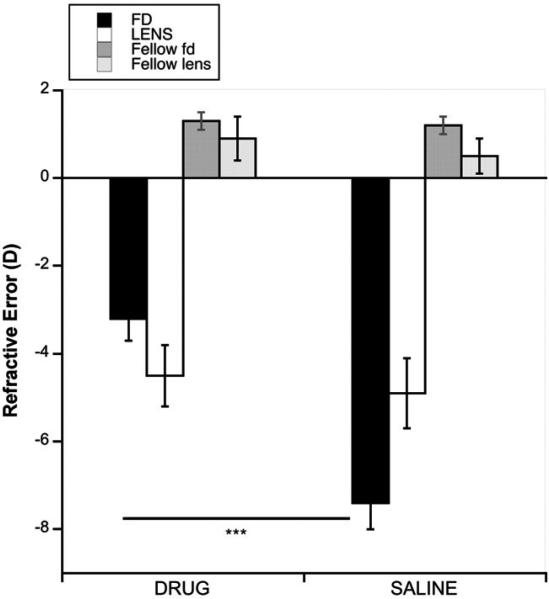
As expected,6 MT3 inhibited the development of myopia in response to form deprivation (Figure 1: black bars, drug versus saline: –3.2 versus –7.4 D; p = 0.0001), however, it had no effect on the myopia in eyes wearing –15 D lenses (white bars, drug versus saline: –4.5 versus –4.9 D; p = 0.3378). This difference was also shown in the IOD data (interaction between device type and drug with IOD data, p = 0.007, 2-way ANOVA). All 4 groups became significantly more myopic than their fellow eyes (IOD: FD, drug and saline: –4.5 D and –7.8 D, p < 0.0005 for both; Lens, drug and saline: –4.6 D and –4.2 D, p < 0.005 for both).
FIGURE 1.
Refractive errors at the end of the experiment in form-deprived (black bars) and negative lens-wearing (white bars) eyes, with their respective fellow eyes (dark and light grey bars), for both drug- and saline-injected eyes. Error bars are standard errors of the mean in all graphs. ***p = 0.0001.
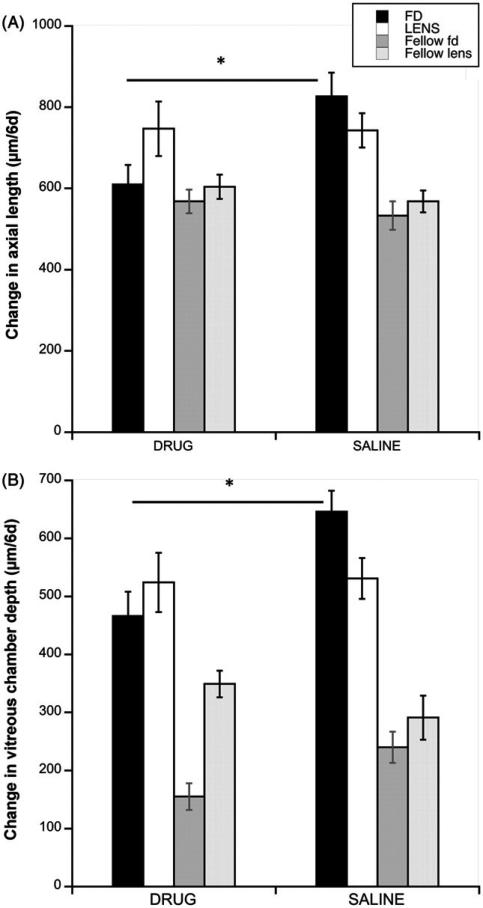
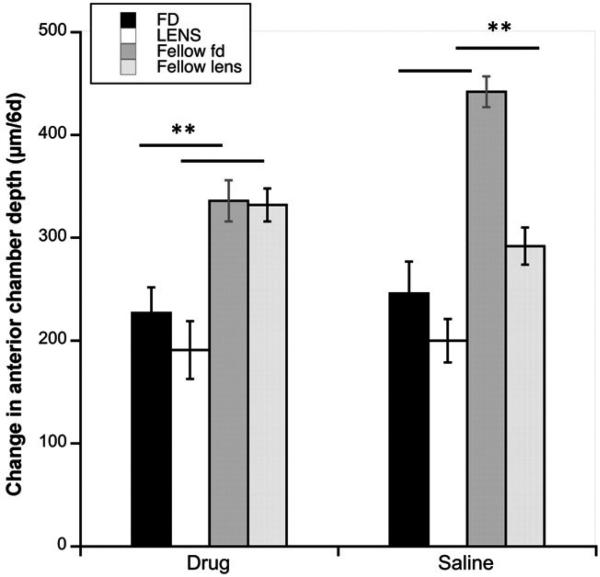
In form-deprived eyes, the inhibition of myopia was the result of inhibition of ocular elongation compared to saline controls (Figure 2A, black bars: 610 μm versus 827 μm/6d; p = 0.0028); elongation of the drug-injected eyes did not differ from that of fellow controls (610 versus 568 μm/6d; p = 0.42, Figure 2A, Table 1), while the saline-injected deprived eyes grew significantly faster than fellow eyes (827 versus 533 μm/6d; p = 0.0002, Table 1). By contrast, axial elongation of lens-wearing eyes injected with MT3 did not differ from that of saline controls (white bars: 747 μm versus 743 μm/6d; p = 0.956). In this group, both drug- and saline-injected eyes grew faster than fellow controls (respectively, 747 versus 604 μm/6d, p = 0.005; 743 versus 568 μm/6d; p = 0.027; Table 1). That axial elongation was inhibited by MT3 in eyes wearing diffusers but not in lens-wearing eyes is also seen in the IOD data (interaction between device and drug, p = 0.064, 2-way ANOVA). In both deprivation and lens groups, the pattern of the effects on vitreous chamber elongation was similar to that for axial length, in that the drug inhibited growth in form-deprived eyes but not in lens-wearing eyes (Figure 2B: black bars, FD: drug versus saline: 466 μm versus 646 μm/6d; p = 0.0045; white bars, Lens: 524 μm versus 531 μm/6d; p = 0.9073). Vitreous chambers in both drug and saline-injected eyes in both paradigms grew faster than their fellow controls (FD: drug: 466 versus 155 μm/6d, p < 0.0001; saline: 646 versus 240 μm/6d, p < 0.0001; LENS: drug: 524 versus 349 μm/6d, p = 0.0319; saline: 531 versus 291 μm/6d, p = 0.0003; Table 1). However, the interaction between device and drug in the IOD data was not significant (p = 0.39). This difference between experimental and fellow eyes here was larger than that for axial length due to the injection-induced inhibitory effect on anterior chamber growth (Figure 3; Table 1), which reduces the difference in axial length change when compared to non-injected fellow eyes.6 All differences in anterior chamber depth between experimental (injected) and fellow control eyes were statistically significant (p < 0.01 for all comparisons).
FIGURE 2.
(A) Change in axial length over 6 days in form-deprived (black bars) and negative lens-wearing (white bars) eyes, with their respective fellow eyes (dark and light grey bars), for both drug- and saline-injected eyes. (B) Change in vitreous chamber depth over 6 days in the same groups as in A. *p ≤ 0.005.
FIGURE 3.
Change in anterior chamber depth over 6 days in form-deprived (black bars) and negative lens-wearing eyes (white bars), with their respective fellow eyes (dark and light grey bars), for both drug- and saline-injected eyes. **p < 0.01 for all.
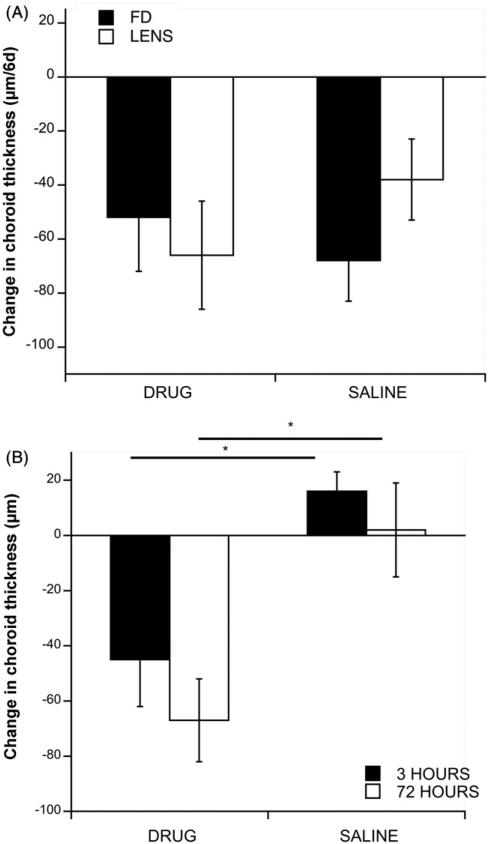
MT3 had no effect on the choroidal thinning in response to either form deprivation or negative lenses (Figure 4A, exp. versus saline controls: FD: –52 μm versus –68 μm/6d; p = 0.6075; Lens: –66 μm versus –38 μm/6d; p = 0.2844). However, MT3 did result in rapid thinning of choroids in normal (non-device-wearing) eyes (Figure 4B, black bars: –45 versus 16 μm/3 h; p = 0.012); choroids remained thinner than normal at 72 h (white bars: –67 μm versus 2 μm/72 h; p = 0.013). There was no effect on axial elongation (250 μm versus 280 μm/3d) or on refractive error (–0.4 D versus 1.1 D) at the end of 3 days (data not shown).
FIGURE 4.
(A) Change in choroid thickness over 6 days in form-deprived (black bars) and negative lens-wearing eyes (white bars) for drug- and saline-injected eyes. There are no significant differences between groups in either paradigm. (B) Change in choroid thickness in normal (non-device-wearing) eyes at 3 h (black bars) and 72 h (white bars). *p < 0.05.
DISCUSSION
Our results that MT3 inhibits the development of form deprivation myopia corroborate those of McBrien et al.,6 however, we find that MT3 is not effective at inhibiting the development of negative lens-induced myopia: refractive compensation did not differ between the drug and saline groups. Our results for lens-wearing eyes differ slightly from those of a prior study,7 which reported a partial inhibitory effect on refractive compensation in eyes wearing –7 D lenses. For our study we chose to use a higher-power lens (–15 D), and a longer duration of lens-wear (7 versus 4 days) in an attempt to maximize the effect of the hyperopic defocus, which may explain the discrepancy between our results. Our study also corroborates the injection-induced inhibition of anterior chamber growth in both saline- and drug-injected eyes.6
A contradictory and somewhat surprising finding is that in our hands, MT3 had no inhibitory effect on the choroidal thinning that normally occurs in response to either form deprivation or negative lenses, despite its ability to inhibit the deprivation-induced myopia. This absence of a choroidal effect differs from what was reported by McBrien et al. in which MT3 prevented the deprivation-induced choroidal thinning. Our lack of effect was surprising because atropine and pirenzepine, two muscarinic cholinergic antagonists that inhibit lens- and deprivation-induced myopia, also inhibit the choroidal thinning response in these eyes;16 because MT3 had similar inhibitory effects on myopia development in deprived eyes, we considered it likely to have the same effect on the choroid as the other two antagonists. One possible explanation for this discrepancy is the different strains of chicks used in the two studies (Cornell-K white leghorn versus White leghorn × Australorp), but this is unlikely because there has been no evidence to date for strain-related differences in drug studies. A more feasible explanation is that the prior study used daily intravitreal injections over 5 days while we used only 3 injections over 7 days: perhaps either the higher cumulative amount of drug injected, or the daily injection regimen, was the crucial factor in preventing the choroidal thinning.
In normal (non-device-wearing) eyes, MT3 resulted in rapid and long-lasting choroidal thinning. Again, this effect was unexpected, because the antagonist atropine causes choroidal thickening in normal eyes17, and muscarinic agonists cause choroidal thinning.14 Together these results suggest that the mechanism of action of MT3 on the choroid is via a non-muscarinic mechanism (see Stell's counterpoint in McBrien et al.).18 Furthermore, the fact that MT3 inhibited the deprivation-induced ocular growth stimulation, and that this occurs concomitant with the deprivation-induced choroidal thinning, is the first pharmacological evidence that choroidal thickening is not an essential aspect of the signal cascade mediating ocular growth inhibition.19 Finally, our findings support the existence of different muscarinic mechanisms underlying form deprivation- versus negative-lens induced myopia at the level of ocular (scleral) growth.
ACKNOWLEDGMENTS
The authors are grateful for the advice and statistical expertise of Dr. Li Deng (The New England College of Optometry).
Footnotes
DECLARATION OF INTEREST
The authors report no conflicts of interest, and are alone responsible for the content and writing of the paper. This work was supported by NIH-NEI-013636.
REFERENCES
- 1.Wallman J, Winawer J. Homeostasis of eye growth and the question of myopia. Neuron. 2004;43:447–468. doi: 10.1016/j.neuron.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 2.Bedrossian RH. The effect of atropine on myopia. Ann Ophthalmol. 1971;3:891–897. [PubMed] [Google Scholar]
- 3.Tan DTH, Lam DS, Chua WH, Shu-Ping DF, Crockett RS. One-year multicenter, double-masked, placebo-controlled, parallel saftey and efficacy study of 2% pirenzepine ophthalmic gel in children with myopia. Ophthalmology. 2005;112:84–91. doi: 10.1016/j.ophtha.2004.06.038. [DOI] [PubMed] [Google Scholar]
- 4.Siatkowski RM, Cotter S, Miller JM, Scher CA, Crockett RS, Novack GD. Safety and efficacy of 2% pirenzepine ophthalmic gel in children with myopia. Arch Ophthamol. 2004;122:1667–1674. doi: 10.1001/archopht.122.11.1667. [DOI] [PubMed] [Google Scholar]
- 5.Siatkowski R, Cotter S, Crockett R, Miller J, Novack G, Zadnik K. Two-year multicenter, randomized, double-masked, placebo-controlled, parallel safety and efficacy study of 2% pirenzepine ophthalmic gel in children with myopia. J AAPOS. 2008;12:332–339. doi: 10.1016/j.jaapos.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 6.McBrien N, Arumugam B, Gentle A, Chow A, Sahebjada S. The M4 muscarinic antagonist MT-3 inhbits myopia in chick: evidence for site of action. Ophthalmic Physiol Opt. 2011;31:529–539. doi: 10.1111/j.1475-1313.2011.00841.x. [DOI] [PubMed] [Google Scholar]
- 7.Diether S, Schaeffel F, Fritsch C, Trendelenburg A, Payor R, Lambrou GN. Role of M-1 and m-4 receptors in devolopment of myopia: study with muscarinic receptor antagonists and muscarinic toxins. Invest Ophthalmol Vis Sci. 2005;46:S3342. [Google Scholar]
- 8.Morgan IG, Ashby RS, Nickla DL. Form deprivation and lens-induced myopia: are they different? Ophthalmic Physiol Optics. 2013;33:355–361. doi: 10.1111/opo.12059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nickla DL, Totonelly K. Dopamine antagonists and brief vision distinguish lens-induced- and form-deprivation-induced myopia. Exp Eye Res. 2011;93:782–785. doi: 10.1016/j.exer.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmid KL, Wildsoet C. Inhibitory effects of apomorphine and atropine and their combination on myopia in chicks. Optometry Vis Sci. 2004;81:137–147. doi: 10.1097/00006324-200402000-00012. [DOI] [PubMed] [Google Scholar]
- 11.Nickla DL, Schroedl F. Parasympathetic influences on emmetropization in chicks: evidence for different mechanisms in form deprivation versus negative lens-induced myopia. Exp Eye Res. 2012;102:93–103. doi: 10.1016/j.exer.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schaeffel F, Hagel G, Bartmann M, Kohler K. 6-hydoxy dopamine does not affect lens-induced refractive errors but suppresses deprivation myopia. Vision Res. 1994;34:143–149. doi: 10.1016/0042-6989(94)90327-1. [DOI] [PubMed] [Google Scholar]
- 13.Totonelly K, Yusupova Y, Nickla DL. Muscarinic agents and emmetropization in chicks: evidence for separate pathways for form deprivation and lens-induced myopia. 2011 ARVO E-abstract #6319. [Google Scholar]
- 14.Nickla DL, Zhu X, Wallman J. Effects of muscarinic agents on chick choroids in intact eyes and eye-cups: evidence for a muscarinic mechanism in choroidal thinning. Ophthalmic Physiol Optics. 2013;33:245–256. doi: 10.1111/opo.12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nickla DL, Wildsoet C, Wallman J. Visual influences on diurnal rhythms in ocular length and choroidal thickness in chick eyes. Exp Eye Res. 1998;66:163–181. doi: 10.1006/exer.1997.0420. [DOI] [PubMed] [Google Scholar]
- 16.Nickla DL, Cheng AT. Atropine and apomorphine in emmetropization: evidence for inhibitory interactions on a common growth regulatory pathway. Invest Ophthalmol Vis Sci. 2009 ARVO E-abstract #3848. [Google Scholar]
- 17.Nickla D. The middle of the signal cascade in emmetropization: the choroid and the effects of nitric oxide synthase (NOS) inhibitors, muscarinic antagonists and dopaminergic agonists.. 12th International Myopia Conference; Queensland, Australia. 2008.p. 24. [Google Scholar]
- 18.McBrien N, Stell W, Carr B. How does atropine exert its anti-myopia effects? Ophthal Physiol Opt. 2013;33:373–377. doi: 10.1111/opo.12052. [DOI] [PubMed] [Google Scholar]
- 19.Nickla DL, Wallman J. The multifunctional choroid. Prog Retin Eye Res. 2010;29:144–168. doi: 10.1016/j.preteyeres.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]