Abstract
Data from epidemiological studies suggest that individual differences in cigarettes per day (CPD) and duration of smoking account for only a small portion of the variance in Diagnostic and Statistical Manual of Mental Disorders (4th ed.) (DSM-IV) nicotine dependence. However, DSM-IV may be an insensitive measure of nicotine dependence; other measures might better reflect the true nature of the relationship between use and dependence. This paper describes the relationship between cigarettes per day (CPD) and years smoking and the severity of nicotine dependence as measured by the Nicotine Dependence Syndrome Scale (NDSS). Furthermore, we assessed the validity of individual differences in nicotine dependence by determining whether they related to cue-evoked craving during abstinence. Data were pooled from five laboratory studies of 489 regular (i.e., 15+ CPD) smokers. In contrast to previously reported data demonstrating a relatively strong relationship between CPD and dependence in chippers (Shiffman & Sayette, 2005), CPD and years smoking accounted for a statistically significant, but small (<6%), portion of the variance in nicotine dependence in daily smokers. Individual differences in both CPD and years smoking had little or no relationship with craving. However, the magnitude of craving was significantly related to the degree of nicotine dependence even after controlling for use variables and excluding craving-related items on the NDSS. These data suggest that among moderate to heavy daily smokers, meaningful individual differences in nicotine dependence are observed independent of differences in current daily cigarette consumption and duration of smoking. Further research into the sources of this variance is critical to understanding the process of and risk for nicotine dependence.
Introduction
Nicotine dependence is hypothesized to be a central process underlying why people continue to smoke and experience great difficulty when attempting to stop. Individual variation in vulnerability to nicotine dependence has important implications for theoretical models of dependence and for research into both prevention and treatment of nicotine dependence. Most theoretical models of dependence emphasize increased tobacco and nicotine use as the primary pathway to greater dependence. Increasing use is thought to lead to tolerance and withdrawal, core processes in the development of dependence (Eissenberg, 2004) that likely reflect more extensive neuroadaptation (Balfour, 1994), as well as increased opportunities for dependence-linked learning processes to occur. However, recent data suggest that marked individual differences in dependence arise independent of differences in major indices of tobacco use such as cigarettes per day (CPD) and duration of smoking. For example, a sizable minority of moderate to heavy (10+ CPD) daily smokers (~38%) fail to meet DSM-IV dependence criteria (Donny & Dierker, 2007), whereas a substantial proportion of less-than-daily smokers meet DSM-IV dependence (Dierker et al., 2007). Current models offer relatively little explanation of why two individuals with similar tobacco use should have divergent levels of dependence. One possibility is that although nicotine use is necessary for dependence to develop, other factors moderate the strength of this relationship and may even be necessary for dependence to evolve in some individuals.
An important question is whether individual differences in dependence that cannot be explained by nicotine use are “real” or represent measurement error. One source of error may be in the measurement of dependence. DSM-IV measures of nicotine dependence may be relatively insensitive, misclassifying a large portion of smokers as nondependent. Some loss of sensitivity may arise from the use of a dichotomous classification, requiring smokers to be classified as either dependent or nondependent, without recognizing degrees of dependence. Other assessments of nicotine dependence that provide a quantitative and multidimensional description of nicotine dependence may more accurately characterize the relationship between use and dependence and thereby reduce the variance in dependence that cannot be explained by use.
Previous research relating tobacco use to other measures of dependence has generally been conducted to establish the construct validity of the measure of dependence based on the assumption that dependence should be closely related to the duration (e.g., years smoking), frequency (e.g., smoking days per week), and quantity (e.g., CPD) of tobacco use. This assumption is logical given the hypothesized bidirectional relationship between use and dependence; greater use is thought to increase risk for dependence and greater dependence is thought to drive further use. Indeed, the widely used Fagerström Tolerance Questionnaire (FTQ) and Fagerström Test for Nicotine Dependence (FTND) rely heavily on use items as an index of dependence; two items in particular, time to first cigarette and CPD, account for most of the variance in FTQ scores (Heatherton, Kozlowski, Frecker, Rickert, & Robinson, 1989; Lichtenstein & Mermelstein, 1986). When CPD is removed, the remaining items are modestly correlated with smoking days per month (r=.25) and CPD (r=.41) (Wellman, et al., 2006).
Other scales that do not directly measure use as an index of dependence show varying degrees of association between the two constructs. The Hooked on Nicotine Checklist (HONC) (DiFranza et al., 2002) is only weakly correlated with smoking days per month (r=.22) and CPD (r=.22) in adult smokers (Wellman, et al., 2006). The Wisconsin Inventory on Smoking Dependence Motives (WISDM-68) (Piper et al., 2004), a 68-item scale that assesses 13 types of smoking motives, demonstrates a wide range of correlations with CPD for individual subscales (.23–.76); when all 13 subscales were entered simultaneously into a multiple regression, they explained 58% of the variance in smoking rate in a sample of daily and non-daily smokers. The Nicotine Dependence Syndrome Scale (NDSS) demonstrates a high degree of accuracy in discriminating between chippers and regular smokers (Shiffman & Sayette, 2005) but a relatively modest correlation between CPD and total dependence scores in treatment-seeking, regular smokers (r=.37–.48) (Shiffman, Waters, & Hickcox, 2004).
Furthermore, little attention is given to the degree to which the relationship between cigarette use and nicotine dependence changes as a function of use history. If a certain amount of nicotine exposure is necessary for dependence to develop but other factors partially determine the degree of dependence, one might expect use and dependence to be more closely related at low to moderate levels of use but more weakly related once use becomes extensive (i.e., above a hypothetical threshold). From this perspective, the unexplained variance in dependence in individuals with moderate to heavy use presents the greatest challenge for theories of nicotine dependence (Donny & Dierker, 2007).
Another way to address concerns that the variability in nicotine dependence is meaningful and not simply the result of measurement error is to demonstrate that individual differences in nicotine dependence are related to another theoretically-related construct. Craving, which can be elicited by both abstinence and exposure to smoking-related cues, has been proposed as a symptom of nicotine dependence in several contemporary measures of dependence (DiFranza et al., 2002; Piper et al., 2004; Shiffman et al., 2004). An association between nicotine dependence and craving that persists independent of cigarette use supports the hypothesis that individual differences in nicotine dependence amongst moderate to heavy smokers represent variance in the latent construct of dependence.
Although abstinent smokers exposed to lit cigarettes consistently report robust cravings (Wertz & Sayette, 2001b), relatively little is known about individual differences in the magnitude of this craving response. Individual differences in cue-evoked craving are related to nicotine dependence as measured by the FTQ (Payne, Smith, Sturges, & Holleran, 1996), but whether dependence continues to predict craving after accounting for tobacco use has received little attention. Indeed, several prominent theories of drug craving (Rohsenow, Niaura, Childress, Abrams, & Monti, 1990) would seem to predict that differences in tobacco use should account for a substantial proportion of the variance in craving because the source of this variance is either differential drug exposure, e.g., craving as a withdrawal state (Baker, Piper, McCarthy, Majeskie, & Fiore, 2004) or differential behavioral histories, e.g., automaticity (Tiffany, 1990). An alternative hypothesis is that individual differences in craving result from other characteristics (for example, propensity for stimulus-response learning) that also may convey risk for dependence.
The primary purpose of the present study was to determine the relationship between common measures of cigarette use (i.e., CPD, years smoking) and dependence, as measured by the NDSS, in smokers recruited to participate in human laboratory research. In addition, the study also assessed whether the variability in nicotine dependence predicted individual differences in craving before and after exposure to an in vivo smoking cue in abstinent smokers.
Method
Overview
Data from five laboratory studies (described in detail below) from the Sayette laboratory at the University of Pittsburgh were collapsed to provide a large sample size with adequate power for detecting individual differences. Cigarette use and nicotine dependence assessments were administered to all participants (489 daily smokers). Additionally, 347 daily smokers were required to abstain from smoking for at least 7 hrs and exposed to in vivo cigarette cues while asked to report their urge to smoke.
Participants
The combined sample included 489 daily, non-treatment-seeking smokers (47% female). Among the sample, 85% were White, 11% were Black, and 4% were Hispanic or Asian American. Selection criteria were applied at screening. Participants were excluded if they currently were trying to quit smoking, if they reported a medical condition that contraindicated nicotine, or if they were illiterate. Participants ranged in age from 18 to 40 years (M=25.3, SD=5.8), and they had to report smoking at least 15 CPD (M=21.8, SD=5.2) for at least 24 continuous months (years smoking: M=9.81, SD=5.9).
Cigarette use and nicotine dependence data for 123 tobacco chippers (Shiffman, 1989) are included for comparison purposes (Sayette et al., 2003; Shiffman & Sayette, 2005); data on craving were available only on a small subset of chippers and therefore are not included here. Chippers (59% female) had to report smoking at least 2 days per week but not more than five cigarettes on the days they smoked during initial screening. On average, chippers were 24.0 years old (SD=3.9) and smoked 4.0 CPD (SD=1.7) on 4.7 days per week (SD=0.12) for 6.4 years (SD=4.4).
Baseline assessment measures
Nicotine Dependence Syndrome Scale
This multidimensional measure of nicotine dependence yields a single summary score (NDSS-Total) and five factor-analytically derived dimensions of dependence (Shiffman et al., 2004). Drive measures craving, withdrawal avoidance, and felt compulsion to smoke. Priority measures the degree to which smoking becomes prioritized over other reinforcers. Tolerance represents a self-reported decreased sensitivity to nicotine or the escalation of smoking to overcome such decreases. Stereotypy and continuity indicate the development of a rigid and consistent smoking pattern that is not much influenced by other stimuli.
Smoking history and demographics
Participants were asked to complete a questionnaire assessing smoking history (Shiffman et al., 2004) in a nonabstinent state and prior to the cue exposure protocol. Daily smokers reported the current average number of cigarettes they smoke per day (CPD), as well as the total number of years they had smoked (years smoking). Tobacco chippers were asked to report the number of smoking days per week, the average number of cigarettes smoked on smoking days (CPD), and the total number of years they had smoked (years smoking). These data were taken from an assessment battery and not from the screening data used to determine eligibility. A composite measure of cigarette use also was computed for each group of smokers by converting CPD and years smoking into z scores and calculating the average z score.
Procedures
All participants were recruited through advertisements in local and campus newspapers to participate in a laboratory experiment. The individual studies are described in more detail below, but the cue-exposure protocol is nearly identical across all five experiments.
Cigarette cue exposure
A research assistant placed a tray containing a plastic cover on the desk in front of each participant. The research assistant then left the room and instructed the participant over an intercom system to pick up the cover, which revealed their pack of cigarettes with a lighter and an ashtray. Participants were instructed to remove a cigarette and light it without placing it in their mouths. They were then asked to put down the lighter and to hold the cigarette comfortably. Participants rated their urge to smoke immediately prior to lifting the cover from the tray (precue) and 31 seconds after lighting the cigarette (postcue).
Reported urge to smoke
Participants reported their urge to smoke on a rating scale ranging from 0 (“absolutely no urge to smoke at all”) to 100 (“strongest urge to smoke I’ve ever experienced”) (Sayette, Martin, Wertz, Shiffman, & Perrot, 2001).
Datasets
The first dataset included 67 daily smokers who participated in an experiment that examined the performance of a broad range of craving response measures (Sayette et al., 2001). Half of the participants were randomly assigned to a 7-hr nicotine-deprived condition; the other half could smoke freely prior to entering the laboratory. All participants were exposed to control cues (a small roll of electrical tape) and smoking cues (participants’ own lit cigarette). The present analyses focused on all 67 participants’ NDSS and cigarette use data; craving response was examined only in participants who were nicotine deprived during the cigarette cue exposure (n=34).
The second dataset included 77 daily smokers who participated in a study that examined the effects of craving on temporal cognition (Sayette, Loewenstein, Kirchner, & Travis, 2005). Following baseline assessment, participants were randomly assigned to either abstain from smoking for at least 12 hr (high-crave condition) or smoke normally (low-crave condition) before a 2-hr laboratory session. The present analyses focused on all participants’ (n=77) NDSS and cigarette use data; craving responses were assessed only in participants in the high-crave condition (n=40).
The third dataset included 72 heavy smokers who participated in a study that examined the effects of alcohol consumption on cigarette craving (Sayette, Martin, Wertz, Perrott, & Peters, 2005). Although participants participated in cue exposure, they were given either a moderate dose of alcohol or placebo beforehand. Therefore, current analyses focused only on the NDSS and cigarette use data.
The fourth dataset included 172 daily smokers (unpublished data) and examined how accurately smokers can anticipate the strength of their own future cigarette cravings. Participants were randomly assigned to (a) abstain from smoking for 12 hrs before two sessions, (b) smoke regularly before the first session and abstain before the second, or (c) abstain from smoking before a single session. Current analyses focused on the NDSS and cigarette use data from all participants. Craving response was examined only during nicotine deprivation; for participants who participated in two deprivation sessions, only data from the first session were used.
The fifth dataset included 101 daily smokers participating in a study designed to test an attentional coping mechanism during cigarette craving and the accuracy of smokers’ craving recollections (unpublished data). Smokers were required to attend either three or four experimental sessions in either a nicotine-deprived or a nondeprived state. Current analyses focused on all NDSS and cigarette use data; craving response was examined from the first deprivation session in participants who did not participate in the coping exercise during cigarette cue exposure.
Data analyses
Regression analyses including both linear and quadratic estimates were used to assess the association between use and dependence. The quadratic parameter was not significant in any of the analyses and was therefore dropped from the model. Percent variance accounted for is presented as adjusted r-squared. The strength of the linear parameter (i.e., correlation) was compared in chippers and daily smokers using Fisher’s z test. Linear regression was used to determine the relationship between use/dependence and craving. Use measures were entered as covariates in the model relating dependence to craving to determine if dependence measures provided incremental predictive validity. Regression parameters are reported in the original measurement units (i.e., B) rather than standardized units (i.e., β).
Results
Relating use to dependence
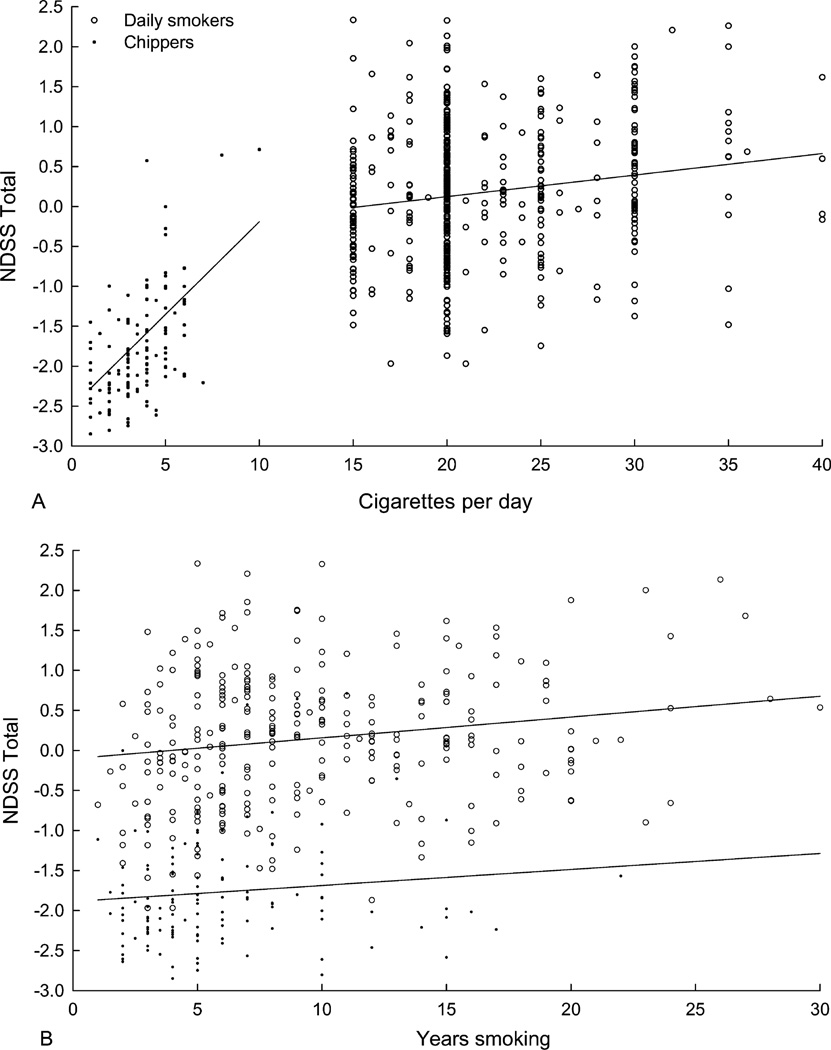
The relationship between cigarette use (CPD and years smoking) and nicotine dependence (NDSS-Total) is illustrated in Figure 1 and summarized in Table 1. Previously reported data relating use and dependence in 123 chippers are included in Figure 1 for comparison (Shiffman & Sayette, 2005). In daily smokers, NDSS-Total scores increased with increasing CPD, but CPD accounted for little variance in NDSS-Total. Similarly, analyses of the NDSS subscales revealed a significant positive linear relationship between CPD and the Priority and Tolerance subscales, but CPD accounted for less than 5% of the variance in these subscales. Other subscales were not significantly related to CPD. These findings are in contrast to the stronger relationship between use and dependence observed in chippers. These data, previously reported by Shiffman and Sayette (2005), indicated that CPD accounted for approximately 36% of the variance in total NDSS scores, 20% of the variance in Drive, and 3% of the variance in Stereotypy in chippers. Similar results were found when running these analyses using the number of cigarettes smoked per week: NDSS-Total (B=0.031, p<.0001, adjusted r2=.31); Drive (B=0.034, p<.0001, adjusted r2=.18); Stereotypy (B=0.015, p<.02, adjusted r2=.04); data not previously reported. The strength of the association between CPD and NDSS-Total was significantly lower in daily smokers (r=.18) than in chippers (r=.61; z=5.07, p<.0001).
Figure 1.
NDSS-Total scores as a function of cigarettes per day (upper panel) and years smoking (lower panel) in both chippers (n for CPD 121; n for years smoking =120) and heavy smokers (n for CPD=474, n for years smoking =299). Sample sizes differ from total sample as a result of missing data and/or years smoking not being assessed in some datasets.
Table 1.
Relating cigarette use to nicotine dependence in daily smokers.
| Cigarettes per day | Years smoking | Composite measure | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Scale | B | Adjusted r2 | p value | B | Adjusted r2 | p value | B | Adjusted r2 | p value |
| NDSS-Total | 0.03 | .03 | <.0001 | 0.03 | .04 | <.0001 | 0.28 | .06 | <.0001 |
| Drive | 0.01 | .00 | .306 | 0.03 | .02 | <.005 | 0.17 | .01 | <.01 |
| Priority | 0.04 | .04 | <.0001 | 0.02 | .01 | <.05 | 0.29 | .05 | <.0001 |
| Tolerance | 0.02 | .01 | <.05 | −0.01 | .00 | .103 | 0.01 | .00 | .903 |
| Stereotypy | 0.01 | .00 | .233 | 0.02 | .01 | <.05 | 0.16 | .01 | <.05 |
| Continuity | 0.02 | .00 | .068 | 0.02 | .01 | .054 | 0.19 | .02 | <.005 |
The relationship between years smoking and dependence tended to be comparable (daily smokers) or weaker (chippers) than what was observed for CPD. In daily smokers, years smoking was related to NDSS-Total, the Drive subscale, the Priority subscale, and the Stereotypy subscale but accounted for less than 5% of the variance in each. In chippers, years smoking was only related to NDSS-Total (B=0.039, p<.02), accounting for approximately 5% of the variance (not previously reported). None of the subscales were related to years smoking in tobacco chippers. Furthermore, combining CPD and years smoking into a composite variable did not substantially increase the strength of the association between use and dependence compared to CPD alone in daily smokers. Similarly, a composite measure of cigarette use did not increase the strength of the relationship in tobacco chippers: NDSS-T (B=0.46, p<.0001, adjusted r2=.31); Drive (B=0.54, p<.0001, adjusted r2=.20); Stereotypy (B=.23, p<.02, adjusted r2=.04); other subscales: ns.
Relating use and dependence to craving in daily smokers
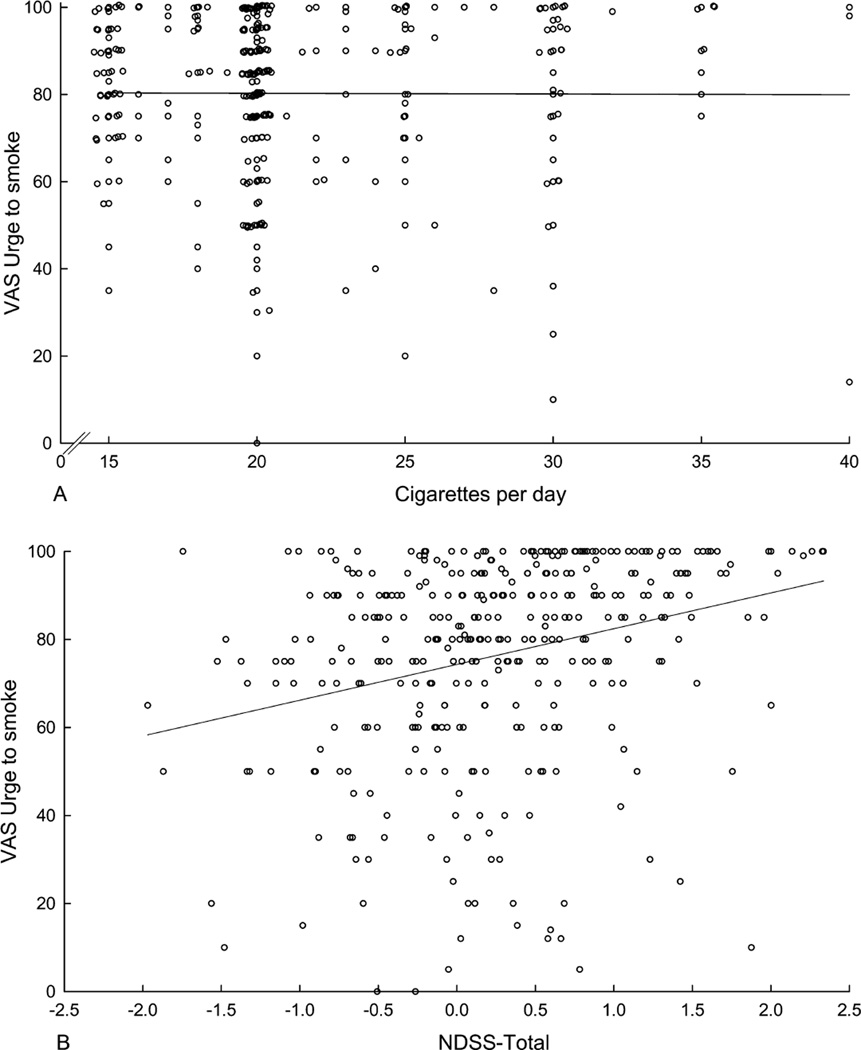
Figure 2 illustrates the relationship between postcue craving and both CPD (upper panel) and NDSS-Total (lower panel). Table 2 summarizes the results. CPD failed to predict the magnitude of the craving response after cigarette cue exposure or during the precue craving assessment. The change in craving from pre- to postcue exposure also was not related to CPD. Years smoking failed to predict the magnitude of the craving response after cigarette cue exposure. Years smoking was related to precue craving and showed a significant inverse relationship to the change in craving from pre- to postcue exposure, although the magnitude of effects was extremely small. Combining years smoking and CPD did not improve the predictions.
Figure 2.
Postcue ratings of urge as a function of cigarettes per day (upper panel; n=347) and NDSS-Total scores (lower panel; n=338) in daily smokers. Sample sizes differ from total sample as a result of missing data. Overlapping data points were randomly displaced slightly (<0.5) on both the abscissa and the ordinate in the upper panel to make all points visible; the regression line was fit to the raw data.
Table 2.
Relating cigarette use and nicotine dependence to cravings in daily smokers.
| Precue | Postcue | Pre–post | |||||||
|---|---|---|---|---|---|---|---|---|---|
| B | Adjusted r2 | p value | B | Adjusted r2 | p value | B | Adjusted r2 | p value | |
| Cigarettes/day | 0.11 | .00 | .624 | −0.02 | .00 | .934 | −0.13 | .00 | .406 |
| Years smoking | 0.46 | .01 | <.05 | 0.13 | .00 | .453 | −0.33 | .01 | <.05 |
| Composite | 0.00 | .00 | .14 | 0.00 | .00 | .85 | −0.01 | .01 | .06 |
| NDSS-Total | 9.42 | .11 | <.0001 | 7.63 | .10 | <.0001 | −1.79 | .01 | .08 |
| Drive | 4.66 | .04 | <.0001 | 4.59 | .06 | <.0001 | −0.07 | .00 | .94 |
| Priority | 5.02 | .04 | <.0001 | 4.50 | .05 | <.0001 | −0.51 | .00 | .56 |
| Tolerance | 0.96 | .00 | .446 | 0.90 | .00 | .40 | −0.06 | .00 | .94 |
| Stereotypy | 2.71 | .01 | <.05 | 1.19 | .00 | .30 | −1.52 | .01 | .09 |
| Continuity | 3.04 | .01 | <.05 | 4.07 | .04 | <.001 | 1.03 | .00 | .25 |
In contrast, NDSS-Total was a significant predictor of both pre- and postcue craving, as were the Drive, Priority, Continuity, and Stereotypy subscales. Neither NDSS-Total nor any of the NDSS subscales was significantly related to the change in craving from pre- to postcue exposure.
Direct comparison of the strength of the association between use and craving versus NDSS-Total and craving revealed that both precue craving (z=4.27, p<.0001) and postcue craving (z=4.31, p<.0001) was significantly more closely related to NDSS-Total than to CPD. This was also true when years smoking was entered into the analyses instead of CPD (precue: z=3.03, p<.01; postcue: z=3.79, p<.001). Furthermore, the relationship between NDSS-Total and both precue craving (B=9.68, p<.0001, adjusted r2=.11) and postcue craving (B=8.04, p<.0001, adjusted r2=.10) remained in an expanded model that included CPD and years smoking as covariates. Finally, similar findings were observed in this expanded model even after removing craving-related items on the NDSS (items 1–4 in Shiffman et al., 2004). This modified NDSS-Total predicted both precue craving (B=14.88, p<.0001, adjusted r2=.11) and postcue craving (B=11.86, p<.0001, adjusted r2=.09), suggesting that the relationship cannot be accounted for by the inclusion of craving as part of the measurement of dependence.
Discussion
Epidemiological evidence suggests that DSM-IV nicotine dependence and smoking history are not isomorphic; the association between nicotine dependence and the quantity, frequency, and duration of use is relatively weak (Dierker et al., 2007; Donny & Dierker, 2007). The modest correlation between cigarette use measures and the NDSS in the present sample of daily-smoking laboratory volunteers is consistent with these epidemiological findings. There is marked variance in dependence that cannot be easily explained by individual differences in CPD or years smoking. The source of this variance is largely unknown.
The relationship between use and dependence was steeper and stronger in chippers than in daily smokers, suggesting that a curvilinear association may exist between use and dependence. A similar curvilinear relationship between cigarettes smoked per week and DSM-IV nicotine dependence has been reported in a sample of college smokers (Dierker et al., 2007). Risk for dependence may increase substantially from light to moderate cigarette use, but slow as cigarette use becomes frequent. Such a relationship could be due to use being the predominant determinant of individual differences in dependence when dependence is low, but other factors determining individual differences in dependence or use as use becomes more frequent and extensive. The relationship between daily consumption and use may flatten out at higher levels of dependence because once individuals become at least minimally dependent, they settle into an individually determined “setpoint” for preferred daily smoking. It also could be argued that environmental factors not relevant to dependence (e.g., opportunities to smoke, environmental restrictions constraints) may control daily cigarette use among dependent smokers (Chandra, Shiffman, Scharf, Dang, & Shadel, 2007). This would be consistent with the boundary model of nicotine intake (Kozlowski & Herman, 1984), which suggests that minimal and maximal tobacco use are determined by the insufficient intake and the rate-limiting effects (i.e., toxic), respectively, but that in between these limits, use is less biologically determined and more dependent on psychosocial factors. Larger samples that assess nicotine dependence across the entire range of smoking quantity and frequency are needed to confirm this hypothesized curvilinear relationship and to explore the nondependence factors that influence daily cigarette use as well as the nonuse factors that determine the magnitude of dependence.
Dependence was a better predictor of craving than either CPD or years smoking. Indeed, use measures were of little to no value in predicting individual differences in craving, either pre- or postcue exposure. This observation was somewhat surprising given the widely held assumptions that both deprivation- induced and cue-induced craving are related to history of cigarette use; more extensive use would be expected to result in greater physical dependence and a more extensive history of classically conditioned associations between cues and nicotine delivery. In contrast, both pre- and postcue craving were related to nicotine dependence independent of cigarette use even when item content related to craving was removed from the NDSS. This observation suggests that the variance in dependence among people with comparable CPD and years smoking is not the result of error in the measurement of dependence, but instead represents meaningful individual differences in dependence (i.e., construct validity). Furthermore, these differences may relate to, and possibly mediate, the mechanisms underlying individual differences in craving. Which factors, besides cigarette use, drive these differences deserves further attention.
Although dependence predicted the base level of craving that participants reported, it did not predict the change in craving associated with exposure to the in vivo smoking cue. Several possible explanations for this result need to be considered. First, dependence may predict abstinence-induced or background craving, but not cue-induced craving, though this conclusion assumes that our approach of subtracting pre-cue urge from post-cue urge provides a true index of cue-induced craving. This position requires that the entire urge rating prior to cue exposure be attributed to abstinence effects (e.g., withdrawal) and thus be entirely uncued. It is likely, however, that simply asking abstinent smokers to rate their urge to smoke—while they are in a smoking laboratory and have signed a consent form that mentions smoking—cues them into their urge state, much like asking people who have not eaten all day if they are hungry reminds them of their appetite. As we have noted elsewhere, merely providing information to abstinent smokers about the opportunity to smoke affected responses to smoking-related words on a color-naming emotional Stroop task (Wertz & Sayette, 2001a). In other words, when smokers are deprived of nicotine, it may not take a particularly strong or explicit smoking cue to cue cravings. Accordingly, the assumption that the entire urge reported prior to cue exposure by abstinent smokers reflects an uncued urge that needs to be subtracted from the urge reported during smoking cue exposure can be challenged (Sayette et al., 2000).
In addition to the concern that our index of cue-induced urge underestimated the impact of the smoking cues administered in the laboratory, ceiling effects may have further clouded interpretation of cue-induced urge. Prior to smoking cue exposure, our heavy-smoking participants already were reporting urges substantially above the midpoint of the scale (M=69.7, SD=22.34). Previously, we reported that these types of abstinent smokers, when using a magnitude estimation measure, reported that their urges nearly tripled following smoking cue exposure (Sayette et al., 2001). Clearly an increase of such magnitude cannot be detected on the visual analog scale when presmoking levels already exceed the midpoint. Indeed, smokers who reported the strongest pre-cue urges, and were more dependent, were the ones who were most likely to confront a ceiling on their smoking cue urges. Taken together, these findings indicate that it is exceedingly difficult to identify a true index of cue-induced craving using the traditional change score from pre- to postcue exposure, which makes it difficult to interpret its lack of association with dependence.
Several limitations are worth noting. First, the sample of smokers reported here was one of convenience and may not represent the general population. Second, the range of CPD was restricted by the inclusion criteria of 15+ CPD; a wider range of current cigarette consumption may have revealed a different pattern of results. Third, self-reported CPD and years smoking are likely to be relatively poor proxies for both current nicotine consumption and history of exposure. In addition to the potential limitation of self-report, these measures also may fail to capture critical aspects of tobacco use or nicotine exposure (for instance, smoking topography, change in smoking patterns over time, nicotine metabolism). Fourth, a single-item measure of craving was used. In our hand, single-item measures of urge and craving demonstrate high reliability (e.g., Cronbach’s alpha >.96; S. Shiffman, unpublished observations), suggesting that reliability was not a problem with our measure (see Figure 1 of Sayette et al., 2000).
Relatively little is known about the sources of individual variability in nicotine dependence beyond those associated with nicotine use. Indeed, the list of possible sources of this variance is long and includes many different levels of analysis and their interactions, such as genetic, neurobiological, pharmacokinetic, pharmacodynamic, psychiatric, and contextual. Analysis of how these factors interact with use to produce risk for nicotine dependence will be essential for progress on both a theoretical and a prevention front.
Acknowledgments
The studies described in this report were funded by National Institute on Drug Abuse grant DA10605 to Michael Sayette. Eric Donny’s and Saul Shiffman’s participation was funded by National Institute on Drug Abuse grants R21DA019626 (ECD), R21DA023459 (ECD).
References
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: An affective processing model of negative reinforcement. Psychological Review. 2004;111:33–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- Balfour DJ. Neural mechanisms underlying nicotine dependence. Addiction. 1994;89:1419–1423. doi: 10.1111/j.1360-0443.1994.tb03738.x. [DOI] [PubMed] [Google Scholar]
- Chandra S, Shiffman S, Scharf DM, Dang Q, Shadel WG. Daily smoking patterns, their determinants, and implications for quitting. Experimental and Clinical Psychopharmacology. 2007;15:67–80. doi: 10.1037/1064-1297.15.1.67. [DOI] [PubMed] [Google Scholar]
- Dierker LC, Donny E, Tiffany S, Colby SM, Perrine N, Clayton RR. The association between cigarette smoking and DSM-IV nicotine dependence among first year college students. Drug and Alcohol Dependence. 2007;86:106–114. doi: 10.1016/j.drugalcdep.2006.05.025. [DOI] [PubMed] [Google Scholar]
- DiFranza JR, Savageau JA, Fletcher K, Ockene JK, Rigotti NA, McNeill AD, Coleman M, Wood C. Measuring the loss of autonomy over nicotine use in adolescents: The DANDY (Development and Assessment of Nicotine Dependence in Youths) study. Archives of Pediatric and Adolescent Medicine. 2002;156:397–403. doi: 10.1001/archpedi.156.4.397. [DOI] [PubMed] [Google Scholar]
- Donny EC, Dierker LC. The absence of DSM-IV nicotine dependence in moderate-to-heavy daily smokers. Drug and Alcohol Dependence. 2007;89:93–96. doi: 10.1016/j.drugalcdep.2006.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eissenberg T. Measuring the emergence of tobacco dependence: The contribution of negative reinforcement models. Addiction. 2004;99(Suppl. 1):5–29. doi: 10.1111/j.1360-0443.2004.00735.x. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Rickert W, Robinson J. Measuring the heaviness of smoking: Using self-reported time to the first cigarette of the day and number of cigarettes smoked per day. British Journal of Addiction. 1989;84:791–799. doi: 10.1111/j.1360-0443.1989.tb03059.x. [DOI] [PubMed] [Google Scholar]
- Kozlowski LT, Herman CP. The interaction of psychosocial and biological determinants of tobacco use: More on the boundary model. Journal of Applied Social Psychology. 1984;14:244–256. [Google Scholar]
- Lichtenstein E, Mermelstein RJ. Some methodological cautions in the use of the Tolerance Questionnaire. Addictive Behaviors. 1986;11:439–442. doi: 10.1016/0306-4603(86)90024-9. [DOI] [PubMed] [Google Scholar]
- Payne TJ, Smith PO, Sturges LV, Holleran SA. Reactivity to smoking cues: Mediating roles of nicotine dependence and duration of deprivation. Addictive Behaviors. 1996;21:139–154. doi: 10.1016/0306-4603(95)00043-7. [DOI] [PubMed] [Google Scholar]
- Piper ME, Piasecki TM, Federman EB, Bolt DM, Smith SS, Fiore MC, Baker TB. A multiple motives approach to tobacco dependence: The Wisconsin Inventory of Smoking Dependence Motives (WISDM-68) Journal of Consulting and Clinical Psychology. 2004;72:139–154. doi: 10.1037/0022-006X.72.2.139. [DOI] [PubMed] [Google Scholar]
- Rohsenow DJ, Niaura RS, Childress AR, Abrams DB, Monti PM. Cue reactivity in addictive behaviors: Theoretical and treatment implications. Internal Journal of Addiction. 1990;25:957–993. doi: 10.3109/10826089109071030. [DOI] [PubMed] [Google Scholar]
- Sayette MA, Loewenstein G, Kirchner TR, Travis T. Effects of smoking urge on temporal cognition. Psychology of Addictive Behaviors. 2005;19:88–93. doi: 10.1037/0893-164X.19.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayette MA, Martin CS, Wertz JM, Perrott MA, Peters AR. The effects of alcohol on cigarette craving in heavy smokers and tobacco chippers. Psychology of Addictive Behaviors. 2005;19:263–270. doi: 10.1037/0893-164X.19.3.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayette MA, Martin CS, Wertz JM, Shiffman S, Perrott MA. A multi-dimensional analysis of cue-elicited craving in heavy smokers and tobacco chippers. Addiction. 2001;96:1419–1432. doi: 10.1080/09652140120075152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayette MA, Shiffman S, Tiffany ST, Niaura RS, Martin CS, Shadel WG. The measurement of drug craving. Addiction. 2000;95(Suppl. 2):S189–S210. doi: 10.1080/09652140050111762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayette MA, Wertz JM, Martin CS, Cohn JF, Perrott MA, Hobel J. Effects of smoking opportunity on cue-elicited urge: A facial coding analysis. Experimental and Clinical Psychopharmacology. 2003;11:218–227. doi: 10.1037/1064-1297.11.3.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S. Tobacco “chippers”—Individual differences in tobacco dependence. Psychopharmacology. 1989;97:539–547. doi: 10.1007/BF00439561. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Sayette MA. Validation of the Nicotine Dependence Syndrome Scale (NDSS): A criterion-group design contrasting chippers and regular smokers. Drug and Alcohol Dependence. 2005;79:45–52. doi: 10.1016/j.drugalcdep.2004.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Waters A, Hickcox M. The Nicotine Dependence Syndrome Scale: A multidimensional measure of nicotine dependence. Nicotine & Tobacco Research. 2004;6:327–348. doi: 10.1080/1462220042000202481. [DOI] [PubMed] [Google Scholar]
- Tiffany ST. A cognitive model of drug urges and drug-use behavior: Role of automatic and nonautomatic processes. Psychological Review. 1990;97:147–168. doi: 10.1037/0033-295x.97.2.147. [DOI] [PubMed] [Google Scholar]
- Wellman RJ, DiFranza JR, Pbert L, Fletcher KE, Flint A, Young MH, Druker S. A comparison of the psychometric properties of the Hooked on Nicotine Checklist and the modified Fagerström Tolerance Questionnaire. Addictive Behaviors. 2006;31:486–495. doi: 10.1016/j.addbeh.2005.05.031. [DOI] [PubMed] [Google Scholar]
- Wellman RJ, Savageau JA, Godiwala S, Savageau N, Friedman K, Hazelton J, DiFranza J. A comparison of the Hooked on Nicotine Checklist and the Fagerström Test for Nicotine Dependence in adult smokers. Nicotine & Tobacco Research. 2006;8:575–580. doi: 10.1080/14622200600789965. [DOI] [PubMed] [Google Scholar]
- Wertz JM, Sayette MA. Effects of smoking opportunity on attentional bias in smokers. Psychology of Addictive Behaviors. 2001a;15:268–271. [PMC free article] [PubMed] [Google Scholar]
- Wertz JM, Sayette MA. A review of the effects of perceived drug use opportunity of self-reported urge. Experimental and Clinical Psychopharmacology. 2001b;9:3–13. doi: 10.1037/1064-1297.9.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]