Abstract
Background
Whether to cover cardiovascular disease costs is an increasingly pressing question for low- and middle-income countries. We sought to identify the impact of expanding national insurance to cover primary prevention, secondary prevention, and tertiary treatment for cardiovascular disease in India.
Methods and Results
We incorporated data from coverage experiments into a validated microsimulation model of myocardial infarction and stroke in India to evaluate the cost-effectiveness of alternate coverage strategies. Coverage of primary prevention alone saved 3.6 million disability-adjusted life-years (DALY) per annum at an incremental cost-effectiveness ratio of $469 per DALY averted when compared with the status quo of no coverage. Coverage of primary and secondary preventions was dominated by a strategy of covering primary prevention and tertiary treatment, which prevented 6.6 million DALYs at an incremental cost-effectiveness ratio of $2241 per DALY averted, when compared with that of primary prevention alone. The combination of all 3 categories yielded the greatest impact at an incremental cost per DALY averted of $5588 when compared with coverage of primary prevention plus tertiary treatment. When compared with the status quo of no coverage, coverage of all 3 categories of prevention/treatment yielded an incremental cost-effectiveness ratio of $1331 per DALY averted. In sensitivity analyses, coverage of primary preventive treatments remained cost-effective even if adherence and access to therapy were low, but tertiary coverage would require avoiding unnecessary procedures to remain cost-effective.
Conclusions
Coverage of all 3 major types of cardiovascular treatment would be expected to have high impact and reasonable cost-effectiveness in India across a broad spectrum of access and adherence levels.
Keywords: cost-effectiveness, developing countries, health policy, healthcare economics and organizations, insurance, myocardial infarction, stroke
The treatment of cardiovascular disease (CVD) risk factors has improved dramatically in the past few decades with highly efficacious therapeutic drugs and procedures. Although this has resulted in a decline in CVD burden among Western countries, low- and middle-income countries (LMICs) are now experiencing a growing burden of CVD such that 80% of CVD deaths now occur in LMICs.1 Furthermore, outcomes of CVD events are worse in LMICs than in high-income countries, and a higher proportion of out-of-hospital deaths attributable to cardiovascular events occur in LMICs than in high-income countries.1 Also, contrary to popular belief, CVD is frequently more common among the poorest members of these countries rather than among the affluent.1
To combat this epidemic of CVD among LMICs, major policy changes may be required. The objective of the current study is to estimate the cost-effectiveness of expanding government healthcare insurance coverage in India—a prototypical LMIC facing a surge of CVD—to incorporate treatment for cardiovascular risk factors and CVD events (ie, myocardial infarctions and strokes). Historically, national insurance programs in LMICs have covered maternal and child healthcare, or episodic acute primary healthcare services that excluded chronic noncommunicable diseases.2 However, some question whether government insurance programs should cover treatment for cardiovascular risk factors and events—and, if so, what components of treatment.3
Previous projections suggested that whether access and adherence are high, primary prevention of CVD events through the treatment of risk factors (statins for hypercholesterolemia and blood pressure medications for hypertension) is likely be highly cost-effective in LMICs.4–7 Additional studies suggested that secondary prevention of cardiovascular events through the prescribing of aspirin, blood pressure medications, and a statin for persons with a history of previous myocardial infarction or stroke would also be cost-effective in LMICs under similar assumptions of high access and adherence.4–6 However, real-world effectiveness of providing coverage for primary and secondary preventions might be more limited than suggested by these previous assessments, as studies of observed access to therapy suggest that only a minority of adults in LMICs are receiving cardiovascular treatment even when covered by insurance programs8; similarly, adherence to pharmacotherapy in long-term observational cohorts averages ≈50%.9–14 We are unaware of any assessments of the cost-effectiveness of tertiary treatment for CVD events that include coverage for hospitalizations and procedures including percutaneous coronary interventions and coronary artery bypass grafting (CABG). A recent community-based evaluation observed significant reductions in CVD mortality among patients receiving government coverage for tertiary care in a region of India than those against control regions not receiving such coverage.15 Given the high costs of tertiary care, several current government programs in middle-income countries such as India are offering tertiary treatment coverage as a means to avert catastrophic expenditure for families; however, it is unclear whether this is an optimal strategy for improving population health.15,16
We used simulation models that synthesize the best available clinical and epidemiological data to estimate the health and economic implications of alternative strategies for providing national coverage for primary preventive, secondary preventive, and tertiary treatments of CVD risk factors and events among Indian adults. Building on previous modeling work,5,6 we incorporate not only pharmaceutical treatments but also procedural and surgical treatments for cardiovascular events (ie, tertiary treatments such as CABG) and incorporate empirical estimates of increased utilization and access to treatment after coverage, as well as the impact of varying levels of adherence and quality of care on both myocardial infarction and stroke outcomes.
Methods
Analytic Overview
We used an empirically validated microsimulation model to estimate the costs and benefits of expanding government healthcare insurance coverage for primary prevention, secondary prevention, and tertiary treatment of CVD among adults in India. In the status quo scenario, current access to therapy was simulated using estimates from a nationally representative survey in India.17 This survey revealed 17% (95% confidence interval [CI], 16%–18%) access to primary prevention therapies for cardiovascular risk factors (ie, statins and blood pressure treatments), and 54% (95% CI, 50%–58%) access to secondary prevention therapies and tertiary treatment among persons without any government insurance coverage (Table 1).
Table 1.
Values for Cardiovascular Disease Variables in the Model for India
| Variable | Values | Reference |
|---|---|---|
| Myocardial infarction | ||
| Incidence(n/1000) | 4.7–87.7 | World Health Organization18 |
| Mortality(n/1000) | 0.0–15.8 | World Health Organization18 |
| Disabilityweight | 0.04–0.56 (across all types; disaggregated estimates are available in the Data Supplement) | Global Burden of Disease Study19 |
| Stroke | ||
| Incidence(n/1000) | 0.0–10.9 | World Health Organization18 |
| Mortality(n/1000) | 0.0–0.5 | World Health Organization18 |
| Disabilityweight | 0.01–0.74 (across all types; disaggregated estimates are available in the Data Supplement) | Global Burden of Disease Study19 |
| Treatments | ||
| Cost of thiazide ($ per y) | 2–3 | International Drug Price Indicator Guide20 |
| Cost of ACEI, enalapril ($ per y) | 4–5 | International Drug Price Indicator Guide20 |
| Cost of calcium channel blocker, amlodipine ($ per y) | 6–7 | International Drug Price Indicator Guide20 |
| Cost of aspirin ($ per y) | 2–3 | International Drug Price Indicator Guide20 |
| Cost of statin, atorvastatin ($ per y) | 11–12 | International Drug Price Indicator Guide20 |
| Cost of β-blocker, metoprolol ($ per y) | 7–8 | International Drug Price Indicator Guide20 |
| Cost of service for primary and secondary treatment, including screening, monitoring, and patient costs ($ per y) | 5–10 | World Health Organization21 |
| Cost of tertiary care services for myocardial infarction or stroke per event ($ per event) | 160–2080 | Community-based trial15 |
| Cost of postmyocardial infarction care ($ per y) | 54–64 | Prior cost tabulation22 |
| Cost of poststroke care ($ per y) | 408–775 | Previous cost tabulation22 |
| Relative risk for myocardial infarction from primary prevention with ACEI and calcium channel blocker | 0.60–0.71 | Meta-analyses22–26 |
| Relative risk for stroke from primary prevention with ACEI and calcium channel blocker | 0.45–0.58 | Meta-analyses22–26 |
| Relative risk for myocardial infarction from primary prevention with statin | 0.55–0.74 | Meta-analyses22,27,28 |
| Relative risk for stroke from primary prevention with statin | 0.78–1.00 | Meta-analyses22,27,28 |
| Relative risk for myocardial infarction from secondary treatment with aspirin | 0.60–0.72 | Meta-analyses22,29,30 |
| Relative risk for stroke from secondary treatment with aspirin | 0.72–0.84 | Meta-analyses22,29,30 |
| Relative risk for myocardial infarction from secondary treatment with β-blocker | 0.73–0.87 | Meta-analyses22–26 |
| Relative risk for stroke from secondary treatment with β-blocker | 0.68–0.74 | Meta-analyses22–26 |
| Relative risk for myocardial infarction from secondary treatment with ACEI | 0.70–0.90 | Meta-analyses22–26 |
| Relative risk for stroke from secondary treatment with ACEI | 0.56–0.84 | Meta-analyses22–26 |
| Relative risk for myocardial infarction from secondary treatment with statin | 0.62–0.82 | Meta-analyses22,27,28 |
| Relative risk for stroke from secondary treatment with statin | 0.66–1.00 | Meta-analyses22,27,28 |
| Relative risk for death with secondary treatment with aspirin | 0.81–0.89 | Meta-analyses22,29,30 |
| Relative risk for death with secondary treatment with β-blocker | 0.68–0.85 | Meta-analyses22–26 |
| Relative risk for death with secondary treatment with ACEI | 0.75–0.95 | Meta-analyses22–26 |
| Relative risk for death with secondary treatment with statin | 0.69–0.87 | Meta-analyses22,27,28 |
| Relative risk of death from myocardial infarction with tertiary treatment (acute period of 28 d, then death rate subject to secondary therapy) | 0.17–0.28 | Community-based trial15 |
| Relative risk of death from stroke with tertiary treatment (acute period of 28 d, then death rate subject to secondary therapy) | 0.42–0.80 | Pooled randomized trials31–33 |
| Access and utilization to primary prevention without coverage | 0.16–0.18 | World Health Organization17 |
| Access and utilization to secondary prevention or tertiary treatment without coverage | 0.50–0.58 | World Health Organization17 |
| Access and utilization to primary prevention with coverage | 0.31–0.43 | World Health Organization17 |
| Access and utilization to secondary prevention or tertiary treatment with coverage | 0.61–0.88 | World Health Organization17 |
| Adherence to pharmacological therapy (primary or secondary, %) | 20–80 | Observational cohorts9–14 |
| Covariates to incidence or mortality Non-CVD mortality rates (n/1000) | 2.5–78.7 (across all age/sex/location groups; disaggregated estimates are available in Table X in the Data Supplement) | World Health Organization34 |
| Systolic blood pressure, mm Hg | 82–177 (across all age/sex/location groups; disaggregated estimates are available in Table I in the Data Supplement) | World Health Organization17 |
| Total cholesterol, mmol/L | 3.7–8.1 (across all age/sex/location groups; disaggregated estimates are available in Table II in the Data Supplement) | Global Burden of Disease Study35 |
| Diabetes mellitus prevalence, % | 0.0–6.8 (across all age/sex/location groups; disaggregated estimates are available in Table IV in the Data Supplement) | Global Burden of Metabolic Risk Factors Study36 |
| Tobacco smoking, % | 1.8–32.6 (across all age/sex/location groups; disaggregated estimates are available in Table III in the Data Supplement) | World Health Organization37 |
| History of previous myocardial infarction, % | 0.7–30.4 (across all age/sex/location groups; disaggregated estimates are available in Table V in the Data Supplement) | World Health Organization18 |
| History of previous stroke, % | 0.0–0.95 (across all age/sex/location groups; disaggregated estimates are available in Table VI in the Data Supplement) | World Health Organization18 |
Ranges represent 95% CIs across age categories, as fully disaggregated in the Data Supplement. Rates are annual rates unless otherwise noted. The cost per case is expressed in 2014 US dollars and represents the average lifetime discounted costs of disease, including all treatments (ie, the costs of procedures, hospitalizations, and medications). As in previous assessments,22statins were modeled as conferring diminishing relative risk of myocardial infarction from 0.89 at 1 year, 0.76 at 2 years, 0.67 at 3–5 years, and 0.64 in subsequent years, and β-blocker effects included a decreasing benefit over time with the relative risk in first 3 years of 0.77 for death and 0.73 for myocardial infarction, 0.93 at 4–6 years for either outcome, and 0.99 in subsequent years for either outcome. Based on differences in utilization of services among those without and with coverage of primary, secondary or tertiary treatments for cardiovascular disease in the World Health Organization Study on Global Aging and Adult Health,17and controlling for age, sex, urban/rural residence and income, access to primary prevention would be expected to increase from 17% (95% CI, 16%–18%) without coverage to 37% (95% CI, 31%–43%) with coverage, whereas access to secondary prevention or tertiary treatment would be expected to improve from 54% without coverage (95% CI, 50%–58%) to 75% with coverage (95% CI, 61%–88%). ACEI indicates angiotensin-converting enzyme inhibitor; CI, confidence interval; and CVD, cardiovascular disease.
To examine the benefits of expanding government insurance coverage, we assessed the expanded use of therapies after locally implemented insurance treatment coverage programs (eg, local primary preventive, secondary preventive, or tertiary treatment access schemes), which vary across populations and provinces of India.16 After government insurance coverage for primary prevention, access increased to 37% (95% CI, 31%–43%) on average, whereas coverage for secondary prevention or tertiary treatment increased to 75% (95% CI, 61%–88%; estimation details are available in the Data Supplement).
In our base case analysis, we evaluated the incremental health benefits and cost-effectiveness of expansion in access to care, studying primary preventive, secondary preventive, and tertiary treatment coverage in isolation and in plausible combinations. Our model outcomes included incidence and mortality from myocardial infarctions and strokes per 1000 population. We adopted a societal perspective, discounted costs and benefits by 3% annually, and expressed benefits in disability-adjusted life-years (DALYs) averted (more details on the derivation of DALYs can be found in the Data Supplement). Incremental cost-effectiveness ratios (ICER) were calculated as the additional cost in 2014 US dollars divided by the additional health benefit in DALYs associated with 1 strategy when compared with the next-less-costly cost-effective strategy. For reference, interventions are considered cost-effective if they cost less than gross domestic product (GDP) per capita per DALY averted, and cost-effective between 1× and 3× GDP per capita, where 2014 GDP per capita for India was $1523 in 2014 US dollars.21
Model
Our model simulates Indians with risk factors for ischemic heart disease and cerebrovascular disease and probabilities of experiencing myocardial infarction and stroke, receiving treatment before and after such events, and dying from such events or other causes. The model has been previously validated against independent data.38,39 The population is characterized by age, sex, and residential location (urban or rural). Individuals are assigned these demographic characteristics to match demographic estimates of population size and age distribution in India from the United Nations for the year 2015; individuals then age and experience fertility and mortality, such that population size and age distribution estimates over the course of the baseline simulation match the projections from the United Nations for the period 2015 to 2035.40 Mortality rates in each demographic group include deaths from myocardial infarctions or strokes based on individual risk factors and from all other causes. Risk factors include tobacco smoking, systolic blood pressure, total cholesterol, diabetes mellitus, and a previous history of myocardial infarction or stroke. These risk factors were chosen for their predictive power, their wide-spread availability from epidemiological studies in India, and their common usage in Indian medical practices (as discussed in the Data Supplement). The risk factor distributions are detailed across all demographic cohorts along with a full description of the simulation methods in Tables I to IX in the Data Supplement.
Individuals in each year of the simulation may experience myocardial infarction or stroke, based on their individual risk factors (Figure I in the Data Supplement). We adopted an alternative approach to classical (eg, Framingham) risk calculation that has been validated among diverse populations including Indians.5 The approach uses observed incidence rates relative to risk factor prevalence (Table X in the Data Supplement), rather than an absolute risk score, to calculate the relationships between incidence and risk, allowing us to calibrate the model to any given population rather than assuming universal validity of the Framingham risk equations, which are thought to misestimate risk among South Asians.41 In each year of the simulation, individuals also experienced a probability of mortality from other causes specific to their demographic cohort, based on World Health Organization (WHO) mortality estimates by cause of death.34
The model included secular trends in all variables to account for dynamic changes in risk and disease rates (Table XI in the Data Supplement) and was validated by ensuring that model estimates of myocardial infarction and stroke DALY losses and mortality were within the margin of error from independent estimates among demographic groups (Figure II in the Data Supplement).
Coverage Scenarios
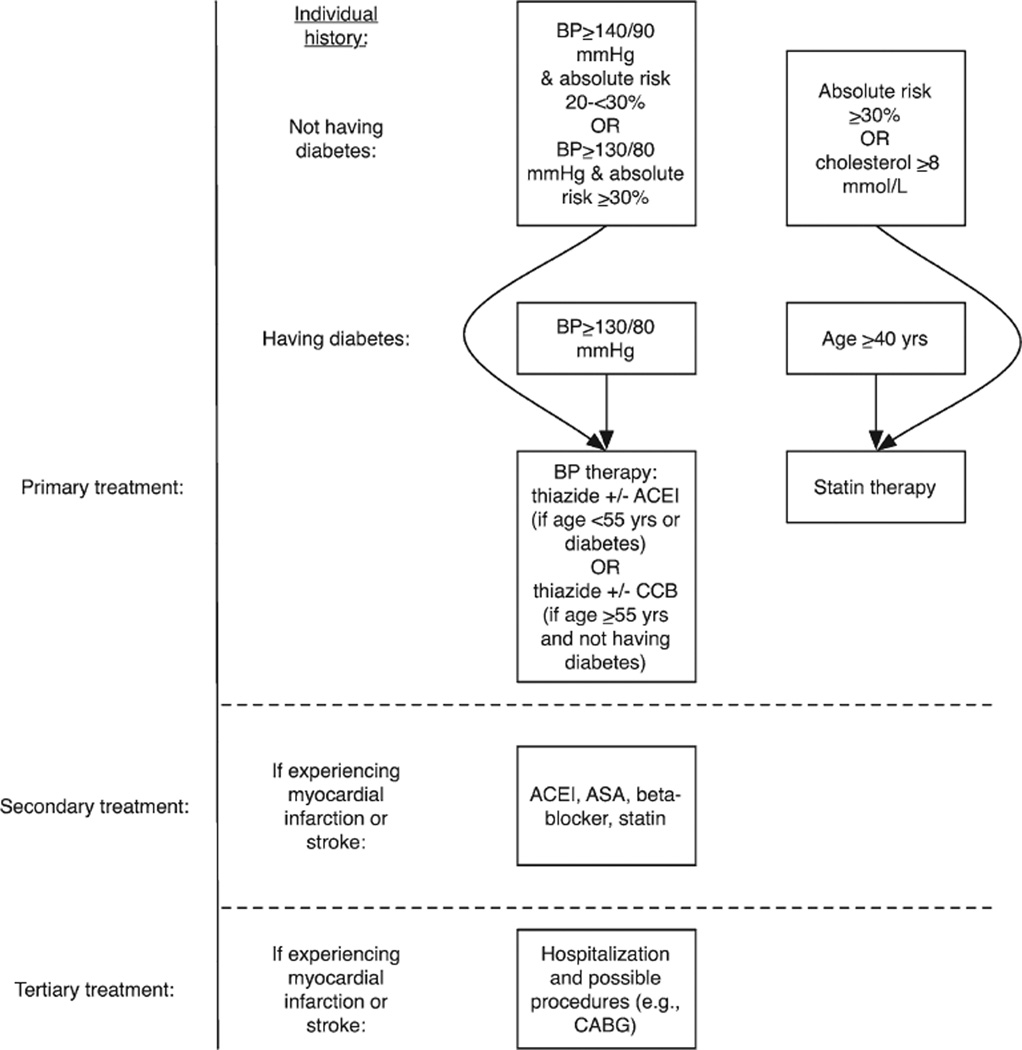
The simulated coverage strategies included primary prevention through pharmacological therapy recommended by the WHO for LMICs (Figure 1).42 As shown in Figure 1, we specifically applied the relative risk reductions associated with antihypertensive therapy to those individuals who have access to therapy (as detailed above) and would be recommended therapy per the WHO guidelines, which recommend therapy to individuals with a blood pressure >160/90 mm Hg, those with a calculated 10-year cardiovascular event risk of 20 to <30% with a pressure >140/90 mm Hg, or patients with risk >30% or having diabetes mellitus or known CVD with a pressure >130/80 mm Hg. The treatment includes thiazide or angiotensin-converting enzyme inhibitors for patients aged <55 years or having diabetes mellitus, and thiazide or calcium channel blockers for others. Similarly, the guidelines recommend statin therapy to all individuals with 10-year risk >30%, patients aged >40 years with type 2 diabetes mellitus, and individuals with total cholesterol of at least 8 mmol/L (individuals likely with familial hypercholesterolemia).42 Secondary prevention was modeled per the WHO,43 which prescribes an angiotensin-converting enzyme inhibitor, aspirin, β-blocker, and statin for individuals with a history of previous myocardial infarction or stroke. Individuals surviving first myocardial infarctions or strokes during the simulation period were switched to the secondary prevention regimen. Relative risk reductions from these measures were estimated from systematic reviews and meta-analyses including Indian subjects (Table 1). Using standard approaches,5 we multiplied the independent relative risks of each drug by the baseline hazard of myocardial infarction to estimate overall effect on myocardial infarction and stroke incidence and mortality. We incorporated directly observed mortality risk reduction estimates from the multidrug regimens22–28 to account for the fact that some of the mortality benefits may not be through risk factor modification. We estimated the benefits of tertiary treatment as the reduction in case fatality rate among patients with myocardial infarctions and strokes in a tertiary insurance experiment in Karnataka, India (Table 1).15 We also included sensitivity analyses in which case fatality rates reduced further by 10% among patients presenting to tertiary care, testing sensitivity of the model to future quality improvement. Additional sensitivity analyses simulated poor quality care under expanded coverage, to determine what rate of inappropriate primary, secondary, and tertiary care (eg, conducting inappropriate procedures such as unnecessary CABG) would cause coverage to no longer be cost-effective. Note that we did not include expanded type 2 diabetes mellitus treatment in our model because glycemic control is not thought to significantly alter the primary macrovascular outcomes in our model (myocardial infarction and stroke).44
Figure 1.
Ischemic heart disease treatment strategies in India.42,43 Absolute risk is calculated over a 10-y horizon using the Framingham risk score, per current World Health Organization guidelines.42,43 Note that the inclusion of statin criteria of treating people with total cholesterol >8 mmol/L is meant to capture high-risk subgroups (ie, familial hypercholesterolemia). ACEI indicates angiotensin-converting enzyme inhibitor; ASA, aspirin; BP, blood pressure; and CABG, coronary artery bypass grafting.
Table XII in the Data Supplement provides details on the scenarios considered, which include primary prevention, secondary prevention, and tertiary treatment coverage in isolation and combination. Access to treatment before and after coverage was assessed from the nationally representative Indian cohort in the WHO Study on Global Aging and Adult Health (n=11 230),17 which captured changes in utilization before and after provision of insurance coverage (Table 1). Access to and utilization of primary prevention was defined as diagnosis and treatment of hypertension, hyperlipidemia, and diabetes mellitus. Access to and utilization of secondary prevention was defined as having received pharmacological treatment after survival from myocardial infarction or stroke. Access to and utilization of tertiary treatment was defined as having been able to obtain hospital-level care for acute myocardial infarction or stroke. We included scenarios in which all individuals receiving tertiary treatment were covered for secondary prevention, and scenarios in which procedural expenditures alone were covered (Table XII in the Data Supplement). We also assessed the implications of improved access, up to a range of 80% for primary and secondary preventions and tertiary treatment.5 Adherence to therapy was varied from a baseline value of 50% across a range from 20% to 80% as observed in previous studies of CVD pharmacotherapy.9–14 In sensitivity analyses, we calculated the minimum threshold level of adherence that would be necessary to ensure cost-effectiveness. Given the potentially diminished long-term role of β-blockers after myocardial infarction and angiotensin-converting enzyme inhibitors among patients with normal left ventricular systolic function, we also conducted a sensitivity analysis in which only aspirin and a statin are given to patients as secondary prevention after myocardial infarction.
Costs (in 2014 US dollars; Table 1) included the direct medical costs associated with screening, diagnosis, and treatment.20,21 Tertiary hospitalization and care costs included the frequency and type of intervention, including procedures and surgeries such as percutaneous coronary intervention and CABG.15 Direct nonmedical costs such as patients’ out-of-pocket expenditures, time and transportation were included for all strategies.21 We followed up the data sources from the WHO’s CHOICE database, which are widely used,45 and consistent with recent subnational surveys from India.46 To estimate CIs, all modeled scenarios were repeated 10 000× while sampling from the ranges of input variable values, listed in Table 1. Modeling was performed in MATLAB (version R2015a; The MathWorks, Cambridge, MA).
Cost-Effectiveness Analysis
To estimate the long-term outcomes associated with coverage of CVD treatments, we projected the health and economic consequences for all cohorts of adult men and women in the first 20 years of the program, from 2015 to 2035. The ICER in terms of incremental costs per DALY averted was used as the index of cost-effectiveness.
We first compare all singular strategies (coverage of primary prevention only, secondary prevention only, or tertiary treatment only) to the status quo of no coverage. The most cost-effective singular strategy is then used as the comparator in the evaluation of cost-effectiveness of combinations of strategies, beginning with dual combinations. The series of nondominated strategies, with increasing levels of costs and effectiveness represent the cost-effectiveness frontier. We use the term absolute dominance to refer to a strategy that is both more costly and less effective (with respect to DALYs averted) than the comparator strategy (including the strategy of maintaining the status quo), and extended dominance to refer to a strategy that is not absolutely dominated but for which the ratio of incremental costs:incremental DALYs averted is higher than that of a combination of other strategies (potentially including the status quo strategy in the combination).18
Ethics Approval
Ethics committee approval for the study was obtained from the Stanford University Institutional Review Board (reference number eP-28811).
Results
Morbidity and Mortality Impact of Coverage
At existing levels of treatment in India, myocardial infarctions generated a loss of 37.9 million DALYs and strokes generated a loss of 24.6 million DALYs each year on average over the period 2015 to 2035. The DALY losses included 835 000 deaths per year from myocardial infarctions and 489 000 deaths from strokes. These model estimates reflect a 8.1 million DALY increase from the 33.1 million DALYs lost to myocardial infarctions and 21.3 million DALYs lost to strokes in the year 2012 (the most recent year estimated by the WHO), which included the effects of 734 000 deaths from myocardial infarctions and 426 000 deaths from strokes.34
Coverage of primary prevention only averted 2544 DALYs per year per million population (Table 2) or 3.6 million DALYs overall (DALYs; 95% CI, 3.1–4.1 million) relative to the status quo of no coverage; these estimates are similar to previous independent estimates of 2984 DALYs averted per million population by primary prevention in the overall South and Southeast Asian region.4
Table 2.
Cost-Effectiveness of National Coverage for Cardiovascular Disease Prevention and Treatment in India
| Comparison to Status Quo | Incremental Analysis | ||||||
|---|---|---|---|---|---|---|---|
| Strategy | Annual DALYs Averted (Per Million Pop) |
Annual Cost ($US Per Capita) |
Incremental DALYs Averted (Per Million Pop) |
Incremental Cost ($US Per Capita) |
ICER in $/DALY Averted Relative to Reference Category |
Reference Category |
Rational Decision |
| Status quo Primary prevention only | 2544.47 (2190.84 to 2898.1) |
$1.19 ($1.03 to $1.36) |
2544.47 (2190.84 to 2898.1) |
$1.19 ($1.03 to $1.36) |
$469 (355 to 620); INR30100 |
Status quo | … First point on cost-effectiveness frontier |
| Secondary prevention only | 147.87 (138.57 to 157.16) |
$0.36 ($0.33 to $0.38) |
147.87 (138.57 to 157.16) |
$0.36 ($0.33 to $0.38) |
$2404 (2120 to 2727); INR154400 |
Status quo | Extended dominance by status quo plus primary prevention |
| Tertiary treatment only | 2076.77 (1738.19 to 2415.35) |
$4.68 ($3.92 to $5.44) |
2076.77 (2415.35 to 1738.19) |
$4.68 (3.92 to 5.44) |
$2253 (3131 to 1621); INR144700 |
Status quo | Extended dominance by primary prevention plus tertiary treatment |
| Primary plus secondary | 2689.47 (2315.12 to 3063.81) |
$1.55 ($1.33 to $1.76) |
145 (124.28 to 165.71) |
$0.35 (0.3 to 0.4) |
$2431 (2425 to 2440); INR156100 |
Primary prevention only | Extended dominance by primary prevention plus tertiary treatment |
| Primary plus tertiary | 4629.02 (3979.91 to 5278.12) |
$5.86 ($5.04 to $6.69) |
2084.55 (1789.07 to 2380.02) |
$4.67 (4.01 to 5.33) |
$2241 (2238 to 2244); INR143900 |
Primary prevention only | Second point on cost-effectiveness frontier |
| Secondary plus tertiary | 2185.03 (1890.18 to 2479.88) |
$5.05 ($4.37 to $5.73) |
−359.44 (−418.22 to −300.66) |
$3.86 (3.34 to 4.37) |
$−10728 (−11110 to −10452); INR−688800 |
Primary prevention only | Absolute dominance by primary prevention |
| All 3 | 4699.02 (4102.6 to 5295.44) |
$6.26 ($5.46 to $7.05) |
70 (17.32 to 122.69) |
$0.39 (0.36 to 0.42) |
$5588 (3419 to 7756); INR358800 |
Primary prevention and tertiary treatment only | Third point on cost-effectiveness frontier |
The values represent average annual DALYs averted, costs conferred, and ICERs (ie, the additional cost divided by the additional health benefit compared with the next-best strategy, defined as the strategy to the left of the data point on the cost-effectiveness frontier illustrated in Figure 2) expressed as cost per DALY for the period 2015–2035. All costs are expressed in 2014 US dollars. 95% confidence intervals are listed in parentheses, estimated from 10 000 repeated iterations of the model while sampling from the uncertainty ranges of each input variable. DALY indicates disability-adjusted life-years; ICER, incremental cost-effectiveness ratios; and INR, Indian Rupees.
Coverage of primary and secondary preventions averted 2689 DALYs per year per million population or 3.8 million DALYs in the overall population (95% CI, 3.3–4.3 million), whereas coverage of primary prevention and tertiary treatment averted 4629 DALYs per year per million population or a total of 6.6 million DALYs (95% CI, 5.7–7.5 million). Coverage of primary, secondary, and tertiary treatments averted 4699 DALYs per year per million population or a total of 6.7 million DALYs (95% CI, 5.8–7.5 million; Table 2).
Cost-Effectiveness of Coverage
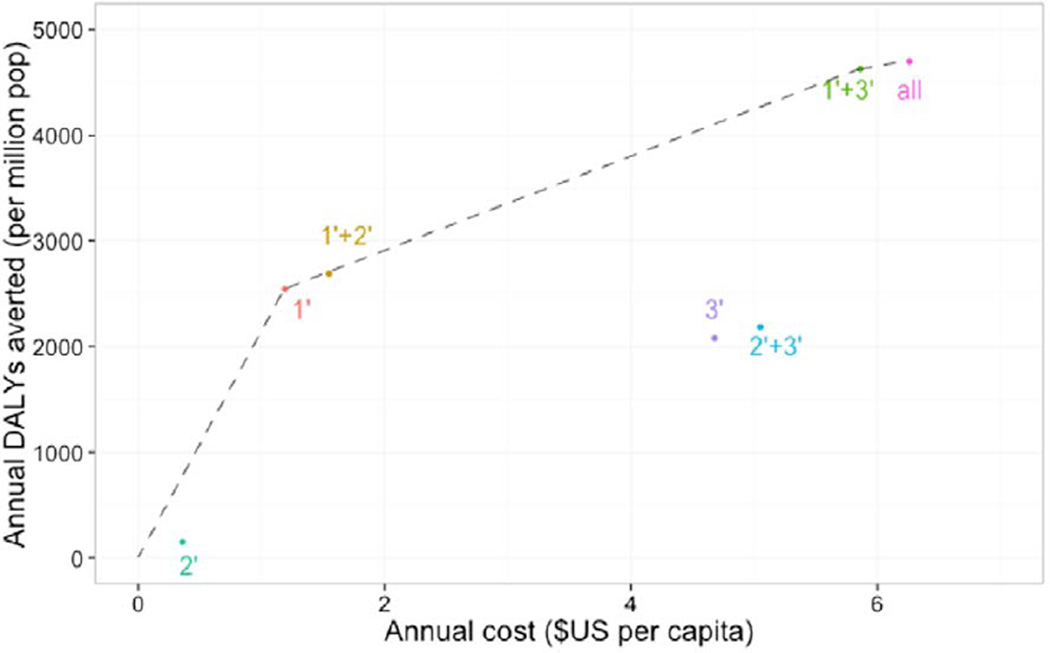
Table 2 and Figure 2 present the results of the cost-effectiveness analysis. As shown in Table 2, primary prevention alone for CVD costs $1.19 per capita and averted 2544 DALYs per million population each year for an ICER of $469 per DALY averted (95% CI, $355–$620) when compared with the status quo. Secondary prevention alone and tertiary treatment alone also averted DALYS at increased cost; however, each of these strategies yielded higher ICER estimates and were, therefore, extendedly dominated by primary prevention alone, which is therefore the first point on the cost-effectiveness frontier (Figure 2). Adding coverage for either secondary prevention or tertiary treatment to primary prevention coverage yielded gains with respect to DALYs averted, at increased costs and ICERs that were close in magnitude. However, the combination of primary prevention and tertiary treatment coverage had an incremental cost of $5.86 per capita and gains in DALYs averted of 4629, yielding an ICER of $2241 (95% CI, $2440–$2425), which was most favorable strategy, becoming the second point on the cost-effectiveness frontier. The strategy that covered all 3 types of care—primary and secondary preventions and tertiary treatment—compared with primary prevention plus tertiary treatment was associated with an ICER of $5588 per DALY averted and becomes the third point on the cost-effectiveness frontier (Figure 2). When compared with the status quo of no coverage, the strategy of coverage for all 3 was associated with an ICER of $1331 per DALY averted.
Figure 2.
Efficiency frontier of alternative coverage strategies for cardiovascular disease in India. The points reveal that all 3 major types of treatment—primary, secondary, and tertiary—for cardiovascular disease would be expected to be cost-effective in India, but strategies excluding primary preventive interventions would be less efficient (lower and to the right, meaning less effective but more costly) than strategies including primary prevention. 1′ indicates primary prevention coverage; 2′, secondary treatment coverage; and 3′, tertiary treatment coverage; all, coverage of primary, secondary, and tertiary care.
Sensitivity Analysis
Impact of Increased Access to Treatment
If postcoverage access to primary therapies improved from 37% in the base case analysis after insurance coverage to 50%, cost-effectiveness of primary prevention coverage would not significantly change ($472 per DALY averted), and the ICER for the combination of all 3 categories of coverage would be reduced to $2295 per DALY averted when compared with coverage for primary prevention and tertiary treatment, and $1097 per DALY averted compared with the status quo of no coverage (Table XIII in the Data Supplement). The cost-effectiveness of secondary and tertiary coverage alone or in combination was largely unaffected.
Impact of Increased Adherence to Therapy
cost-effectiveness improved more substantially as adherence increased from the base case of 50% to 80%. With 80% adherence, cost-effectiveness of coverage for primary prevention alone improved to $288 per DALY averted (95% CI, $213–$389), whereas the ICER for the combination of all 3 categories of coverage decreased to $1796 per DALY averted relative to coverage of primary prevention and tertiary treatment (95% CI, $1481–$2110, a $3792 per DALY savings) and to $960 per DALY averted compared with no coverage (95% CI, $756–$1219, a $371 per DALY savings; Table XIV in the Data Supplement). We estimated that 5% adherence to primary prevention therapies would be necessary for coverage of primary prevention to remain cost-effective, and a minimum of 35% adherence to secondary prevention therapies would be necessary for secondary prevention to remain cost-effective at the cost-effectiveness threshold of 3× GDP per DALY averted.
Impact of Suboptimal Care
The cost-effectiveness of primary and secondary preventions was relatively insensitive to rates of inappropriate therapy, whereas tertiary treatments were more sensitive to inappropriate therapy. Even if 90% of people with new access to primary therapy received medical visits but did not receive the indicated medications for their condition (ie, providers charging for visits but not following guidelines to provide indicated medication prescriptions, as observed throughout South Asia8), primary prevention coverage would remain below the cost-effectiveness threshold because of the profound benefits of treatment at relatively low costs to the remaining 10%; by contrast, the maximum level of suboptimal treatment for secondary prevention coverage was 52%. Tertiary treatment coverage was more sensitive to inappropriate care; if >11% of patients receiving treatment were in fact receiving inappropriate therapy (ie, unnecessary procedures), then the treatment would no longer be cost-effective given the high DALY and financial costs of tertiary care.
Impact of Mortality Reduction With Tertiary Treatment
The estimated mortality benefits of tertiary treatment in the primary data set from India were ≈6% lower than those observed in high-income settings.15,47 There were significant improvements in cost-effectiveness with higher quality of tertiary care. With a 10% improvement in the relative risk reduction of death conferred by tertiary care (Table 1), the cost-effectiveness of tertiary care coverage alone was improved from $2253 to $1961 per DALY averted (95% CI, $1443–$2664; Figure III in the Data Supplement); the incremental cost per DALY of coverage for all 3 treatments also improved from $5588 to $2432 per DALY averted (95% CI, $523–$4341) over the cost of primary prevention and tertiary treatment alone and to $1232 per DALY averted (95% CI, $1092–$1527) over no coverage.
Impact of More Limited Secondary Prevention
If only aspirin and a statin are given to patients as secondary prevention after myocardial infarction, secondary prevention would shift from averting 148 DALYs per year per million population to averting 94.7 DALYs per year per million (95% CI, 88.7–100.6) at a cost of $0.28 per capita (95% CI, $0.25–$0.29; rather than $0.36 per capita in the baseline simulation) for a higher ICER of $2957 per DALY averted ($2485–$3269, from $2404 in the baseline simulation) relative to the status quo of no coverage. Overall this improvement did not significantly change our baseline estimates of the relative cost-effectiveness of secondary prevention alone versus the combination of primary and secondary preventions or the overall cost-effectiveness of all 3 types of therapy combined.
Discussion
Our estimates imply that universal coverage of therapies for primary prevention, secondary prevention and tertiary cardiovascular treatments would avert 6.8 million DALYs per year at the population level at a cost of ≈$1300 per DALY averted versus the status quo of no coverage. The results show that universal coverage of all 3 categories of treatment remains cost-effective under plausible scenarios about the effectiveness of coverage in improving access to care, as well as under existing estimates of adherence to therapy. Of note, currently, several Indian government-based coverage strategies are providing only tertiary coverage alone under the premise that such coverage would avert the most catastrophic expenditures; yet our analysis finds that such coverage should be expanded to include primary and secondary prevention coverage. In addition, partial coverage alone may not be capable of averting impoverishment and distress financing. Our findings also suggested that it would be particularly important to avoid the expansion of inappropriate therapies (eg, unnecessary CABG procedures) if offering tertiary treatment coverage for the coverage to remain cost-effective.
The results also show that coverage of primary prevention should be the cornerstone of any policy for universal healthcare coverage of CVD and associated risk factor control. We found that all strategies that were incrementally cost-effective above less-costly strategies involved coverage of primary prevention. Coverage of primary prevention remained cost-effective even if adherence to therapy was low and even if coverage did not result in significant improvement in access to care. Coverage of primary prevention alone would save 3.6 million DALYs per year at the population level even if adherence was only 50% and access did not improve above currently observed levels; however, this outcome is significantly lower than the population level benefit of covering all treatments. Thus, coverage of primary prevention alone is only sensible in environments where coverage of all 3 treatments is simply unaffordable. We note that a fully universal plan of coverage for all 3 categories of treatment, while cost-effective, would generate a total societal cost of $13.6 billion per year (Rs. 873 billion or 87 300 crore; $2.6 billion for primary prevention, $0.8 billion for secondary prevention, and $10.2 billion for tertiary treatment), most of which might have to be borne by the government to finance national coverage, which is far larger than the current $4 billion government healthcare budget; India remains among the countries with the lowest spending as a proportion of GDP (4%), despite its growing economy.48
Our results of covering primary and secondary preventions are consistent with those reported in the literature, but further enhance our understanding of actual utilization and costs, including the role of tertiary treatment coverage. Our estimates of DALYs lost to CVD are similar to independent assessments assuming continuation of the status quo in treatment access and adherence,49 as are our estimates of DALYs potentially averted from primary preventions4 and the relative impact of secondary versus primary preventive treatment.7 Our findings are the first, however, to be based on actual observed changes in access and utilization after localized coverage expansions in India, as well as directly observed risk factor values rather than imputed estimates. In addition, our study directly estimates risk reductions from tertiary care from a controlled study in India that included both the public and the private sectors,15 providing real-world estimates of care quality. Furthermore, the bulk purchasing effect of population-wide insurance programs allows us to take into account the price-lowering effect of such insurance programs on treatment costs.
Our analysis has important limitations. Data on CVD were limited to large surveys of myocardial infarctions and strokes, to the omission of less common but nevertheless prevalent forms of CVD in India such as rheumatic heart disease. Furthermore, as risk equations are updated from the traditional Framingham equations used in current WHO guidelines to versions that may compensate for the observed bias in risk calculations among South Asians, it is possible that treatment strategies will change and improve the targeting and efficacy of primary and secondary preventions among Indians. We intentionally omitted the controversial use of aspirin as a primary preventive strategy because it is explicitly excluded from recent WHO guidelines42; whereas it is widely agreed that patients at low risk of cardiovascular events should not take aspirin for primary prevention at even a low dose, 3 trials are still ongoing to determine whether patients at higher than average risk should take low-dose aspirin.50 If those trials prove successful despite negative results in the past, then our cost-effectiveness estimates of primary preventive therapy coverage may be viewed as conservative. Our estimates of tertiary care impact are also based on a province that is considered to have less care infrastructure than some others15; hence our cost-effectiveness estimates of tertiary treatment coverage may also be conservative for the overall country. By contrast, our estimates of the cost-effectiveness of tertiary treatment assume that there is sufficient capacity to provide it, and the cost of capacity building will be a considerable added expense in some locations that is not factored into our analysis.
Overall, coverage of primary prevention, secondary prevention, and tertiary treatment for CVD would be expected to have the largest population impact on morbidity and mortality and be cost-effective relative to the status quo of non-coverage. This critically informs ongoing efforts in India and other LMICs that are seeking to expand government-based national insurance coverage strategies, but have traditionally not included coverage for noncommunicable chronic diseases that are now leading causes of catastrophic expenditure and premature morbidity and mortality.
Supplementary Material
WHAT IS KNOWN
Most cardiovascular disease deaths occur in low- and middle-income countries.
National insurance programs in these countries have historically excluded coverage of cardiovascular disease therapies because the cost-effectiveness of providing such coverage has been unclear.
WHAT THE STUDY ADDS
This study incorporates data on observed enrollment and treatment access rates after subnational expansion of insurance programs in India into a microsimulation model to assess the cost-effectiveness of public insurance coverage of cardiovascular disease therapies.
The study reveals that coverage of all 3 major types of treatment–primary prevention, secondary prevention, and tertiary treatment—would be expected to have high impact and reasonable cost-effectiveness in India across a broad spectrum of access and adherence levels.
Covering only secondary prevention or only tertiary treatment was not as cost-effective as strategies that incorporated primary prevention coverage.
Acknowledgments
We thank Zachary Wagner for providing demographic data used in the model.
Sources of Funding
This study was supported by The World Bank, Rosenkranz Prize for Healthcare Research.
Footnotes
The Data Supplement is available at http://circoutcomes.ahajournals.org/lookup/suppl/doi:10.1161/CIRCOUTCOMES.115.001994/-/DC1.
Disclosures
None.
References
- 1.Alwan A. World Health Organization; 2011. [Accessed July 31, 2015]. Global status report on noncommunicable diseases 2010. [Internet] [cited 2015 Jan 28]. http://www.cabdirect.org/abstracts/20113168808.html. [Google Scholar]
- 2.McKee M, Balabanova D, Basu S, Ricciardi W, Stuckler D. Universal health coverage: a quest for all countries but under threat in some. Value Health. 2013;16(1 suppl):S39–S45. doi: 10.1016/j.jval.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Evans DB, Etienne C. Health systems financing and the path to universal coverage. Bull World Health Organ. 2010;88:402. doi: 10.2471/BLT.10.078741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ortegón M, Lim S, Chisholm D, Mendis S. Cost effectiveness of strategies to combat cardiovascular disease, diabetes, and tobacco use in sub-Saharan Africa and South East Asia: mathematical modelling study. BMJ. 2012;344:e607. doi: 10.1136/bmj.e607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lim SS, Gaziano TA, Gakidou E, Reddy KS, Farzadfar F, Lozano R, Rodgers A. Prevention of cardiovascular disease in high-risk individuals in low-income and middle-income countries: health effects and costs. Lancet. 2007;370:2054–2062. doi: 10.1016/S0140-6736(07)61699-7. [DOI] [PubMed] [Google Scholar]
- 6.Megiddo I, Chatterjee S, Nandi A, Laxminarayan R. Cost-effectiveness of treatment and secondary prevention of acute myocardial infarction in India: a modeling study. Glob Heart. 2014;9:391–398. e3. doi: 10.1016/j.gheart.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 7.Gaziano T, Srinath Reddy K, Paccaud F, Horton S, Chaturvedi V. Chapter 33: Cardiovascular disease [Internet]. Disease control priorities in developing countries. [Accessed July 31, 2015];World Bank Publications. 2006 [cited 2015 May 11]. https://books.google.com/books?hl=en&lr=&id=Ds93H98Z6D0C&oi=fnd&pg=PR7&dq=gaziano+cost+effectiveness+disease+control+priorities&ots=rhz-WQYCi6&sig=qQQTKsD4TF5kA3TWZHKPZJ0ylj0. [Google Scholar]
- 8.Basu S, Millett C. Social epidemiology of hypertension in middle-income countries: determinants of prevalence, diagnosis, treatment, and control in the WHO SAGE study. Hypertension. 2013;62:18–26. doi: 10.1161/HYPERTENSIONAHA.113.01374. [DOI] [PubMed] [Google Scholar]
- 9.Jackevicius CA, Mamdani M, Tu JV. Adherence with statin therapy in elderly patients with and without acute coronary syndromes. JAMA. 2002;288:462–467. doi: 10.1001/jama.288.4.462. [DOI] [PubMed] [Google Scholar]
- 10.Kopjar B, Sales AE, Piñeros SL, Sun H, Li YF, Hedeen AN. Adherence with statin therapy in secondary prevention of coronary heart disease in veterans administration male population. Am J Cardiol. 2003;92:1106–1108. doi: 10.1016/j.amjcard.2003.07.008. [DOI] [PubMed] [Google Scholar]
- 11.Benner JS, Glynn RJ, Mogun H, Neumann PJ, Weinstein MC, Avorn J. Long-term persistence in use of statin therapy in elderly patients. JAMA. 2002;288:455–461. doi: 10.1001/jama.288.4.455. [DOI] [PubMed] [Google Scholar]
- 12.Newby LK, LaPointe NM, Chen AY, Kramer JM, Hammill BG, DeLong ER, Muhlbaier LH, Califf RM. Long-term adherence to evidence-based secondary prevention therapies in coronary artery disease. Circulation. 2006;113:203–212. doi: 10.1161/CIRCULATIONAHA.105.505636. [DOI] [PubMed] [Google Scholar]
- 13.Bovet P, Burnier M, Madeleine G, Waeber B, Paccaud F. Monitoring one-year compliance to antihypertension medication in the Seychelles. Bull World Health Organ. 2002;80:33–39. [PMC free article] [PubMed] [Google Scholar]
- 14.Yusuf S, Islam S, Chow CK, Rangarajan S, Dagenais G, Diaz R, Gupta R, Kelishadi R, Iqbal R, Avezum A, Kruger A, Kutty R, Lanas F, Lisheng L, Wei L, Lopez-Jaramillo P, Oguz A, Rahman O, Swidan H, Yusoff K, Zatonski W, Rosengren A, Teo KK. Prospective Urban Rural Epidemiology (PURE) Study Investigators. Use of secondary prevention drugs for cardiovascular disease in the community in high-income, middle-income, and low-income countries (the PURE Study): a prospective epidemiological survey. Lancet. 2011;378:1231–1243. doi: 10.1016/S0140-6736(11)61215-4. [DOI] [PubMed] [Google Scholar]
- 15.Sood N, Bendavid E, Mukherji A, Wagner Z, Nagpal S, Mullen P. Government health insurance for people below poverty line in India: quasi-experimental evaluation of insurance and health outcomes. BMJ. 2014;349:g5114. doi: 10.1136/bmj.g5114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reddy S, Mary I. Rajiv Aarogyasri Community Health Insurance Scheme in Andhra Pradesh, India: a comprehensive analytic view of private public partnership model. Indian J Public Health. 2013;57:254–259. doi: 10.4103/0019-557X.123264. [DOI] [PubMed] [Google Scholar]
- 17.Kowal P, Chatterji S, Naidoo N, Biritwum R, Fan W, Lopez Ridaura R, Maximova T, Arokiasamy P, Phaswana-Mafuya N, Williams S, Snodgrass JJ, Minicuci N, D’Este C, Peltzer K, Boerma JT SAGE Collaborators. Data resource profile: the World Health Organization Study on global AGEing and adult health (SAGE) Int J Epidemiol. 2012;41:1639–1649. doi: 10.1093/ije/dys210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shah B, Kumar N, Menon G, Khurana S, Kumar H. Assessment of Burden of Non-Communicable Diseases. Delhi: WHO India; 2010. [Google Scholar]
- 19.Salomon JA, Vos T, Hogan DR, Gagnon M, Naghavi M, Mokdad A, Begum N, Shah R, Karyana M, Kosen S, Farje MR, Moncada G, Dutta A, Sazawal S, Dyer A, Seiler J, Aboyans V, Baker L, Baxter A, Benjamin EJ, Bhalla K, Bin Abdulhak A, Blyth F, Bourne R, Braithwaite T, Brooks P, Brugha TS, Bryan-Hancock C, Buchbinder R, Burney P, Calabria B, Chen H, Chugh SS, Cooley R, Criqui MH, Cross M, Dabhadkar KC, Dahodwala N, Davis A, Degenhardt L, Díaz-Torné C, Dorsey ER, Driscoll T, Edmond K, Elbaz A, Ezzati M, Feigin V, Ferri CP, Flaxman AD, Flood L, Fransen M, Fuse K, Gabbe BJ, Gillum RF, Haagsma J, Harrison JE, Havmoeller R, Hay RJ, Hel-Baqui A, Hoek HW, Hoffman H, Hogeland E, Hoy D, Jarvis D, Karthikeyan G, Knowlton LM, Lathlean T, Leasher JL, Lim SS, Lipshultz SE, Lopez AD, Lozano R, Lyons R, Malekzadeh R, Marcenes W, March L, Margolis DJ, McGill N, McGrath J, Mensah GA, Meyer AC, Michaud C, Moran A, Mori R, Murdoch ME, Naldi L, Newton CR, Norman R, Omer SB, Osborne R, Pearce N, Perez-Ruiz F, Perico N, Pesudovs K, Phillips D, Pourmalek F, Prince M, Rehm JT, Remuzzi G, Richardson K, Room R, Saha S, Sampson U, Sanchez-Riera L, Segui-Gomez M, Shahraz S, Shibuya K, Singh D, Sliwa K, Smith E, Soerjomataram I, Steiner T, Stolk WA, Stovner LJ, Sudfeld C, Taylor HR, Tleyjeh IM, van der Werf MJ, Watson WL, Weatherall DJ, Weintraub R, Weisskopf MG, Whiteford H, Wilkinson JD, Woolf AD, Zheng ZJ, Murray CJ, Jonas JB. Common values in assessing health outcomes from disease and injury: disability weights measurement study for the Global Burden of Disease Study 2010. Lancet. 2012;380:2129–2143. doi: 10.1016/S0140-6736(12)61680-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Management Sciences for Health. Cambridge, MA: Management Sciences for Health; 2014. [Accessed July 31, 2015]. International drug price indicator guide [Internet] [cited 2015 Jan 28]. http://www.msh.org/sites/msh.org/files/international-drug-price-indicator-guide.pdf. [Google Scholar]
- 21.WHO. Choosing interventions that are cost effective (WHO-CHOICE) [Internet] [Accessed July 31, 2015];2014 http://www.who.int/choice/cost-effectiveness/en/
- 22.Gaziano TA, Opie LH, Weinstein MC. Cardiovascular disease prevention with a multidrug regimen in the developing world: a cost-effectiveness analysis. Lancet. 2006;368:679–686. doi: 10.1016/S0140-6736(06)69252-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Law MR, Wald NJ, Morris JK, Jordan RE. Value of low dose combination treatment with blood pressure lowering drugs: analysis of 354 randomised trials. BMJ. 2003;326:1427. doi: 10.1136/bmj.326.7404.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Collins R, Peto R, MacMahon S, Hebert P, Fiebach NH, Eberlein KA, Godwin J, Qizilbash N, Taylor JO, Hennekens CH. Blood pressure, stroke, and coronary heart disease. Part 2, Short-term reductions in blood pressure: overview of randomised drug trials in their epidemiological context. Lancet. 1990;335:827–838. doi: 10.1016/0140-6736(90)90944-z. [DOI] [PubMed] [Google Scholar]
- 25.MacMahon S, Peto R, Cutler J, Collins R, Sorlie P, Neaton J, Abbott R, Godwin J, Dyer A, Stamler J. Blood pressure, stroke, and coronary heart disease. Part 1, Prolonged differences in blood pressure: prospective observational studies corrected for the regression dilution bias. Lancet. 1990;335:765–774. doi: 10.1016/0140-6736(90)90878-9. [DOI] [PubMed] [Google Scholar]
- 26.The Blood Pressure Lowering Treatment Trialists’ Collaboration. Blood pressure-lowering treatment based on cardiovascular risk: a meta-analysis of individual patient data. Lancet. 2014;384:591–598. doi: 10.1016/S0140-6736(14)61212-5. [DOI] [PubMed] [Google Scholar]
- 27.Law MR, Wald NJ, Rudnicka AR. Quantifying effect of statins on low density lipoprotein cholesterol, ischaemic heart disease, and stroke: systematic review and meta-analysis. BMJ. 2003;326:1423. doi: 10.1136/bmj.326.7404.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cholesterol Treatment Trialists’ (CTT) Collaborators. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet. 2012;380:581–590. doi: 10.1016/S0140-6736(12)60367-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Antithrombotic Trialists’ (ATT) Collaboration. Baigent C, Blackwell L, Collins R, Emberson J, Godwin J, Peto R, Buring J, Hennekens C, Kearney P, Meade T, Patrono C, Roncaglioni MC, Zanchetti A. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373:1849–1860. doi: 10.1016/S0140-6736(09)60503-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Antithrombotic Trialists’ Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324:71–86. doi: 10.1136/bmj.324.7329.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.The ATLANTIS, ECASS, and NINDS rt-PA Study Group Investigators. Association of outcome with early stroke treatment. Lancet. 2004;363:768–774. doi: 10.1016/S0140-6736(04)15692-4. [DOI] [PubMed] [Google Scholar]
- 32.Multicentre Acute Stroke Trial—Italy (MAST-I) Group. Randomised controlled trial of streptokinase, aspirin, and combination of both in treatment of acute ischaemic stroke. Lancet. 1995;346:1509–1514. [PubMed] [Google Scholar]
- 33.Albers GW, Amarenco P, Easton JD, Sacco RL, Teal P. Antithrombotic and thrombolytic therapy for ischemic stroke. Chest. 2001;119(1 suppl):300S–320S. doi: 10.1378/chest.119.1_suppl.300s. [DOI] [PubMed] [Google Scholar]
- 34.World Health Organization. WHO Global InfoBase. Geneva: WHO; 2014. [Google Scholar]
- 35.Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, Amann M, Anderson HR, Andrews KG, Aryee M, Atkinson C, Bacchus LJ, Bahalim AN, Balakrishnan K, Balmes J, Barker-Collo S, Baxter A, Bell ML, Blore JD, Blyth F, Bonner C, Borges G, Bourne R, Boussinesq M, Brauer M, Brooks P, Bruce NG, Brunekreef B, Bryan-Hancock C, Bucello C, Buchbinder R, Bull F, Burnett RT, Byers TE, Calabria B, Carapetis J, Carnahan E, Chafe Z, Charlson F, Chen H, Chen JS, Cheng AT, Child JC, Cohen A, Colson KE, Cowie BC, Darby S, Darling S, Davis A, Degenhardt L, Dentener F, Des Jarlais DC, Devries K, Dherani M, Ding EL, Dorsey ER, Driscoll T, Edmond K, Ali SE, Engell RE, Erwin PJ, Fahimi S, Falder G, Farzadfar F, Ferrari A, Finucane MM, Flaxman S, Fowkes FG, Freedman G, Freeman MK, Gakidou E, Ghosh S, Giovannucci E, Gmel G, Graham K, Grainger R, Grant B, Gunnell D, Gutierrez HR, Hall W, Hoek HW, Hogan A, Hosgood HD, III, Hoy D, Hu H, Hubbell BJ, Hutchings SJ, Ibeanusi SE, Jacklyn GL, Jasrasaria R, Jonas JB, Kan H, Kanis JA, Kassebaum N, Kawakami N, Khang YH, Khatibzadeh S, Khoo JP, Kok C, Laden F, Lalloo R, Lan Q, Lathlean T, Leasher JL, Leigh J, Li Y, Lin JK, Lipshultz SE, London S, Lozano R, Lu Y, Mak J, Malekzadeh R, Mallinger L, Marcenes W, March L, Marks R, Martin R, McGale P, McGrath J, Mehta S, Mensah GA, Merriman TR, Micha R, Michaud C, Mishra V, Mohd Hanafiah K, Mokdad AA, Morawska L, Mozaffarian D, Murphy T, Naghavi M, Neal B, Nelson PK, Nolla JM, Norman R, Olives C, Omer SB, Orchard J, Osborne R, Ostro B, Page A, Pandey KD, Parry CD, Passmore E, Patra J, Pearce N, Pelizzari PM, Petzold M, Phillips MR, Pope D, Pope CA, III, Powles J, Rao M, Razavi H, Rehfuess EA, Rehm JT, Ritz B, Rivara FP, Roberts T, Robinson C, Rodriguez-Portales JA, Romieu I, Room R, Rosenfeld LC, Roy A, Rushton L, Salomon JA, Sampson U, Sanchez-Riera L, Sanman E, Sapkota A, Seedat S, Shi P, Shield K, Shivakoti R, Singh GM, Sleet DA, Smith E, Smith KR, Stapelberg NJ, Steenland K, Stöckl H, Stovner LJ, Straif K, Straney L, Thurston GD, Tran JH, Van Dingenen R, van Donkelaar A, Veerman JL, Vijayakumar L, Weintraub R, Weissman MM, White RA, Whiteford H, Wiersma ST, Wilkinson JD, Williams HC, Williams W, Wilson N, Woolf AD, Yip P, Zielinski JM, Lopez AD, Murray CJ, Ezzati M, AlMazroa MA, Memish ZA. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2224–2260. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Danaei G, Finucane MM, Lu Y, Singh GM, Cowan MJ, Paciorek CJ, Lin JK, Farzadfar F, Khang YH, Stevens GA, Rao M, Ali MK, Riley LM, Robinson CA, Ezzati M Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating Group (Blood Glucose) National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2·7 million participants. Lancet. 2011;378:31–40. doi: 10.1016/S0140-6736(11)60679-X. [DOI] [PubMed] [Google Scholar]
- 37.Tobacco Free Initiative. Global Adult Tobacco Survey (GATS) India Report 2009–2010. Geneva: WHO; 2011. [Google Scholar]
- 38.Basu S, Babiarz KS, Ebrahim S, Vellakkal S, Stuckler D, Goldhaber-Fiebert JD. Palm oil taxes and cardiovascular disease mortality in India: economic-epidemiologic model. BMJ. 2013;347:f6048. doi: 10.1136/bmj.f6048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Basu S, Glantz S, Bitton A, Millett C. The effect of tobacco control measures during a period of rising cardiovascular disease risk in India: a mathematical model of myocardial infarction and stroke. PLoS Med. 2013;10:e1001480. doi: 10.1371/journal.pmed.1001480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.United Nations Population Division. World Population Prospects: The 2012 Revision. United Nations: United Nations Population Division; 2013. [Google Scholar]
- 41.Bhopal R, Fischbacher C, Vartiainen E, Unwin N, White M, Alberti G. Predicted and observed cardiovascular disease in South Asians: application of FINRISK, Framingham and SCORE models to Newcastle Heart Project data. J Public Health (Oxf) 2005;27:93–100. doi: 10.1093/pubmed/fdh202. [DOI] [PubMed] [Google Scholar]
- 42.World Health Organization. Package of Essential Noncommunicable (PEN) Disease Interventions for Primary Health Care in Low-Resource Settings. Geneva: WHO; 2013. [Google Scholar]
- 43.World Health Organization. Prevention of Recurrent Heart Attacks and Strokes in Low and Middle Income Populations: Evidence Based Recommendations for Policy-Makers and Health Professionals. Geneva: WHO; 2013. [Google Scholar]
- 44.Mannucci E, Dicembrini I, Lauria A, Pozzilli P. Is glucose control important for prevention of cardiovascular disease in diabetes? Diabetes Care. 2013;36(suppl 2):S259–S263. doi: 10.2337/dcS13-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Salomon JA, Carvalho N, Gutiérrez-Delgado C, Orozco R, Mancuso A, Hogan DR, Lee D, Murakami Y, Sridharan L, Medina-Mora ME, González-Pier E. Intervention strategies to reduce the burden of non-communicable diseases in Mexico: cost effectiveness analysis. BMJ. 2012;344:e355. doi: 10.1136/bmj.e355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Srivastava A, Mohanty SK. Age and sex pattern of cardiovascular mortality, hospitalisation and associated cost in India. PLoS One. 2013;8:e62134. doi: 10.1371/journal.pone.0062134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Krumholz HM, Wang Y, Chen J, Drye EE, Spertus JA, Ross JS, Curtis JP, Nallamothu BK, Lichtman JH, Havranek EP, Masoudi FA, Radford MJ, Han LF, Rapp MT, Straube BM, Normand SL. Reduction in acute myocardial infarction mortality in the United States: risk-standardized mortality rates from 1995–2006. JAMA. 2009;302:767–773. doi: 10.1001/jama.2009.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.World Bank. World Development Indicators. Washington, DC: IBRD; 2014. [Google Scholar]
- 49.Moran AE, Tzong KY, Forouzanfar MH, Rothy GA, Mensah GA, Ezzati M, Murray CJ, Naghavi M. Variations in ischemic heart disease burden by age, country, and income: the Global Burden of Diseases, Injuries, and Risk Factors 2010 study. Glob Heart. 2014;9:91–99. doi: 10.1016/j.gheart.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gaziano JM, Greenland P. When should aspirin be used for prevention of cardiovascular events? JAMA. 2014;312:2503–2504. doi: 10.1001/jama.2014.16047. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.