Significance
Eukaryotic genomes have distinct heterochromatic regions that silence gene transcription and other DNA transactions. In yeast, these regions are controlled by activities of the silent information regulator (SIR) complex, which are influenced by NAD+ metabolism. We report results, starting from an in silico screen, that proteins functioning in a diverse range of amino acid metabolic activities influence chromatin silencing, thus expanding knowledge of connections between metabolism and epigenetic processes. In a case study, we found that glutamate dehydrogenase 1 (Gdh1) has two chromatin-based functions: controlling recruitment of the SIR complex and negatively regulating proteolysis of a conserved core histone to modulate gene expression. Mechanistically, Gdh1 contributes to silencing through α-ketoglutarate consumption, and high levels of α-ketoglutarate are shown to be detrimental to telomeric silencing.
Keywords: yeast chromatin, metabolism, SIR complex, epigenetic silencing, glutamate dehydrogenase
Abstract
Growing evidence demonstrates that metabolism and chromatin dynamics are not separate processes but that they functionally intersect in many ways. For example, the lysine biosynthetic enzyme homocitrate synthase was recently shown to have unexpected functions in DNA damage repair, raising the question of whether other amino acid metabolic enzymes participate in chromatin regulation. Using an in silico screen combined with reporter assays, we discovered that a diverse range of metabolic enzymes function in heterochromatin regulation. Extended analysis of the glutamate dehydrogenase 1 (Gdh1) revealed that it regulates silent information regulator complex recruitment to telomeres and ribosomal DNA. Enhanced N-terminal histone H3 proteolysis is observed in GDH1 mutants, consistent with telomeric silencing defects. A conserved catalytic Asp residue is required for Gdh1’s functions in telomeric silencing and H3 clipping. Genetic modulation of α-ketoglutarate levels demonstrates a key regulatory role for this metabolite in telomeric silencing. The metabolic activity of glutamate dehydrogenase thus has important and previously unsuspected roles in regulating chromatin-related processes.
In eukaryotic nuclei, DNA is wrapped around histones to form nucleosomes, the basic subunits of chromatin (1). The physical and chemical properties of chromatin are regulated by at least two types of enzymatic activities: chromatin remodeling and posttranslational modifications of histones and chromatin-associated proteins (2, 3). These enzymatic activities directly determine the accessibility of DNA for transcription, replication, and repair.
In Saccharomyces cerevisiae, three genomic regions are known to contain loci repressed by chromatin-related activities. These include the HM silent mating-type loci (HMR and HML), regions within the ribosomal DNA (rDNA), and some telomeres. Transcriptional silencing at these loci is established and maintained by a number of factors, including the Sirtuin class III deacetylase activity of silent information protein 2 (Sir2). Notably, Sir2 uses NAD+ as a cofactor to deacetylate histones H3 and H4 in newly deposited nucleosomes. Other than Sir2, the composition of the silencing complexes and the mechanisms of silencing are different for the three silenced regions, with the telomeres and the HM loci more similar to each other and the rDNA locus having distinct features (reviewed in ref. 4). Silencing at telomeres and the HM loci was long considered to follow a sequential model: initial deacetylation by Sir2 creates binding sites for Sir3 and Sir4, which in turn regulate the spreading of Sir2 across these regions (5). More recent high-resolution studies report a less uniform landscape for silenced chromatin (6), although the central importance of the Sir proteins remains clear. They, together with transcription factors including Rap1 and Abf1, form a multisubunit silencing complex known as the silent information regulator (SIR) complex. Silencing at the rDNA locus does not involve the SIR complex but rather requires the regulator of nucleolar silencing and telophase exit (RENT) complex, which consists of Sir2, Net1, and Cdc14 (4).
A growing field in the study of chromatin is the intersection of epigenetic and chromatin dynamics with cellular metabolic processes (7–9). Although most metabolic proteins localize to the cytoplasm, a number of metabolic proteins are found in the nucleus, where they can regulate chromatin dynamics through at least two distinct mechanisms. In one, the metabolic activities of these proteins modulate the levels of substrates or cofactors available to chromatin-modifying enzymes. For instance, the acetyl-CoA synthesizing machinery in both yeast and mammalian cells exists in multiple cellular compartments, and its metabolic activity in the nucleus directly determines the amount of acetyl-CoA available as a cofactor available for lysine acetyltransferases (10, 11).
In an alternative mechanism, metabolic proteins have evolved distinct nuclear functions. For example, the mammalian pyruvate kinase PKM2 is translocated to the nucleus when the EGF receptor is activated, where it phosphorylates histone H3 instead of its usual metabolic substrate (12). A second example is the yeast homocitrate synthase, encoded by the genes LYS20 and LYS21. This protein is constitutively localized to the nucleus (13). It acts as a dosage suppressor of the DNA damage sensitivity of esa1-414, a hypomorphic allele of the essential lysine acetyltransferase encoded by ESA1 (14). The nuclear function of Lys20 is independent of its lysine biosynthetic activity but requires a separate domain that facilitates the recruitment of the INO80 complex to DNA damage sites (14, 15). Lys20 is thus defined as a moonlighting protein (16, 17) because of its two distinct functions.
The findings with Lys20 prompted us to ask if other nuclear amino acid metabolic proteins function in chromatin regulation. This was an attractive possibility for two reasons. First, many amino acid metabolic reactions consume or produce metabolites, such as NAD+ and acetyl-CoA, that influence the activities of chromatin-modifying enzymes by modulating their cofactor pools. Second, amino acid metabolic enzymes are ancient proteins with a rich repertoire of biochemical activities, thereby making them ideal candidates to evolve multiple functions, which is indeed a common feature observed for well-established multifunctional proteins (18). By screening information curated in the Saccharomyces Genome Database (19), combined with results from transcriptional silencing reporter assays, we identified a diverse range of candidate proteins with potential functions in chromatin regulation. Among these, we focused on the role of glutamate dehydrogenase 1 (Gdh1) in the regulation of telomeric silencing. We report that α-ketoglutarate is an important regulatory metabolite for telomeric silencing and that Gdh1 regulates histone H3 clipping and binding of the SIR complex to the telomeres.
Results
A Screen Revealed Diverse Amino Acid Metabolic Enzymes with Chromatin Functions.
To discover additional amino acid metabolic proteins with chromatin functions, we designed an in silico screen as outlined in Fig. 1A. The screen began with a search of the yeast proteome for proteins annotated to participate in amino acid metabolism. Next, we identified proteins with reported nuclear pools (Tables S1 and S2). Transcription factors were excluded because their nuclear functions have been established. Finally, to increase the likelihood of finding candidates with chromatin regulatory functions, we selected candidates based on three additional criteria: First, they must have interactions with chromatin regulators as reported in the Saccharomyces Genome Database (19). Second, their metabolic function must directly involve a metabolite needed for chromatin-modifying enzymes. The metabolites considered included acetyl-CoA, S-adenosylmethionine, α-ketoglutarate, and NAD(P)+, which are cofactors for histone acetyltransferases, histone methyltransferases, JMJC-domain–containing histone demethylases, and class III histone deacetylase, respectively (20). Alternatively, metabolic enzymes with similar catalytic activities as those established for chromatin-modifying enzymes were selected, because they might act on chromatin substrates through catalytic promiscuity (21).
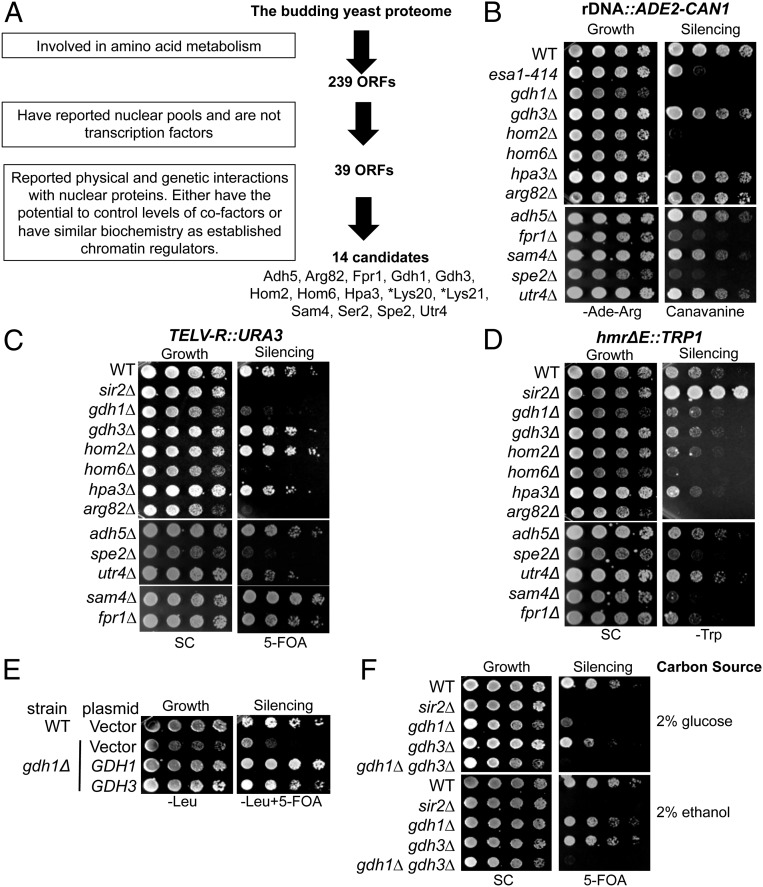
Fig. 1.
A number of amino acid metabolic proteins regulate chromatin silencing. (A) The in silico screen identified 14 proteins that potentially function in chromatin regulation. The search was performed using the Advanced Search Engine in the Saccharomyces Genome Database: yeastmine.yeastgenome.org/yeastmine/begin.do. Search criteria for each step were boxed at Left. (B–D) Strains carrying triple silencing reporters (Table S3) were plated on the indicated media to assess chromatin silencing. The esa1-414 and sir2Δ strains served as controls with established silencing defects. (B) Deletion of FPR1, GDH1, HOM2, HOM6, and SPE2 caused defects in rDNA reporter silencing. Decreased growth on canavanine indicates defective rDNA silencing. (C) Deletion of ARG82, GDH1, HOM6, and SPE2 caused defective telomeric silencing, whereas deletion of SAM4 improved silencing. Reduced growth on 5-FOA indicates defective telomeric silencing. (D) Deletion of ARG82, HOM6, SAM4, and SPE2 enhanced HMR silencing. Reduced growth on SC-Trp indicates enhanced HMR silencing. (E) Increased dosage of GDH3 suppressed gdh1Δ’s silencing defect. WT and gdh1Δ strains were transformed with 2-μ plasmids: vector (pRS425), GDH1 (pLP2764), or GDH3 (pLP2662). (F) GDH1 and GDH3 single and double mutants showed different telomeric silencing phenotypes on glucose and ethanol-containing media. WT (LPY4916), sir2∆ (LPY4979), gdh1∆ (LPY16033), gdh3∆ (LPY16785), and gdh1∆ gdh3∆ (LPY17916) strains were plated on 5-FOA with the indicated carbon sources. *Lys20 and Lys21 were identified in the screen but are not analyzed further here because of their previous characterization (14).
Table S1.
Summary of metabolic function, rationale for selection, and nuclear interactors of candidate proteins (www.yeastgenome.org; 19)
| Candidate | Metabolic function | Rationale | Examples of nuclear interactors |
| Adh5 | Alcohol dehydrogenase | NAD+ homeostasis | Nab2, Npl3 (nuclear RNA binding) |
| Arg82 | Transcriptional activation; inositol polyphosphate kinase | Kinase activity | Hir1,Hir2, Hir3 (HIR complex) |
| Rad53 (DNA damage response) | |||
| Yaf9 (shared subunit of NuA4 and Swr1 complexes) | |||
| Fpr1 | Prolyl-isomerase | Prolyl-isomerase activity | Cdc73 (Paf1 complex) |
| Gcn5 (shared subunit of ADA and SAGA complex) | |||
| Hmo1 (HMG family) | |||
| H2A.Z | |||
| Gdh1 | Glutamate dehydrogenase | NAD(P)+ and α-ketoglutarate homeostasis | Ceg1, Cet1 (nuclear RNA binding proteins) |
| Ess1 (nuclear prolyl-isomerase) | |||
| Gdh3 | |||
| Gdh3 | Glutamate dehydrogenase | NAD(P)+ and α-ketoglutarate homeostasis | Gdh1 |
| Htl1(Rsc complex) | |||
| YEL057C (unknown protein in telomere maintenance) | |||
| Hom2 | Methionine–threonine biosynthesis | NAD(P)+ homeostasis | Cdc13 (telomere binding protein) |
| Seh1 and Sec13 (Nup84 complex) | |||
| Hom6 | Methionine–threonine biosynthesis | NAD(P)+ homeostasis | Eaf1, Esa1 (NuA4 complex) |
| Nat1 Histone H3, H2A.Z | |||
| Ard1 (NatA complex) | |||
| Rad50, Mre11 (MRX complex) | |||
| Rad51, Rad52, Rad54, Rad55, and Rad57 (homologous end repair) | |||
| Hpa3 | d-amino acid N-acetyltransferase | Acetyl-CoA | Asa1 (ASTRA chromatin remodeling complex) |
| Nab2, Npl3 (nuclear RNA binding) | |||
| Sam4 | SAM-homocysteine methyltransferase | SAM homeostasis | Sif2 (Set3C complex) |
| Slx5 (STUbL) | |||
| Ser2 | Phosphoserine phosphatase for the phosphoglycerate pathway | Phosphatase activity | Ard1, Nat1 (NatA complex) |
| Cse2, Med1, Srb8 (mediator complex) | |||
| Eaf1 (NuA4 complex) | |||
| Spe2 | SAM decarboxylase | SAM homeostasis | Cdc13 (telomere binding) |
| Histone H2A and H2B | |||
| Rad4, Rtt109 (DNA damage repair) | |||
| Rpd3 | |||
| Utr4 | Enolase phosphatase | Phosphatase activity | Asf1 (nucleosome assembly) |
| Nab2 (nuclear RNA binding) | |||
| Swc4 (Swr1 complex) |
Table S2.
Noncandidate amino acid metabolic proteins with reported nuclear pools
| ORF | Gene | Enzymatic activity of the encoding protein |
| YMR009W | ADI1 | Acireductone dioxygenease involved in the methionine salvage pathway |
| YDR035W | ARO3 | 3-deoxy-d-arabino-heptulosonate-7-phosphate (DAHP) synthase |
| YBR249C | ARO4 | DAHP synthase |
| YPR060C | ARO7 | Chorismate mutase |
| YHR137W | ARO9 | Aromatic aminotransferase II |
| YLR155C | ASP3-1 | Cell-wall l-asparaginase II involved in asparagine catabolism |
| YJR148W | BAT2 | Cytosolic branched-chain amino acid aminotransferase |
| YPL111W | CAR1 | Arginase |
| YLR438W | CAR2 | l-ornithine transaminase (OTAse) |
| YLL018C | DPS1 | Aspartyl-tRNA synthetase |
| YBR281C | DUG2 | Probable di- and tripeptidase |
| YML004C | GLO1 | Monomeric glyoxalase I |
| YJR070C | LIA1 | Deoxyhypusine hydroxylase |
| YPR118W | MRI1 | 5′-methylthioribose-1-phosphate isomerase |
| YIL145C | PAN6 | Pantothenate synthase |
| YLR044C | PDC1 | Major of three pyruvate decarboxylase isozymes |
| YLR134W | PDC5 | Minor isoform of pyruvate decarboxylase |
| YOR323C | PRO2 | Gamma-glutamyl phosphate reductase |
| YPR069C | SPE3 | Spermidine synthase |
| YJR130C | STR2 | Cystathionine gamma-synthase |
| YDL080C | THI3 | Probable alpha-ketoisocaproate decarboxylase |
| YCR053W | THR4 | Threonine synthase |
| YDR354W | TRP4 | Anthranilate phosphoribosyl transferase of the tryptophan biosynthetic pathway |
| YGL026C | TRP5 | Tryptophan synthase |
| YGR185C | TYS1 | Cytoplasmic tyrosyl-tRNA synthetase |
The screen identified 14 candidates with potential functions in chromatin regulation (Fig. 1A). Notably, the screen recovered not only Lys20 and its homolog Lys21 but also Arg82 (also known as Ipk2), another established moonlighting protein (22, 23). Therefore, the screen served as a promising approach for discovering candidate metabolic proteins with potential chromatin regulatory functions. For the new candidate proteins, the metabolic function, the rationale of the selection, and examples of nuclear proteins that they interact with are briefly summarized in Table S1.
To assess the candidates’ roles in chromatin function, we took advantage of a yeast strain in which reporter genes were integrated at three silenced regions (24). Of note, we grew the null strains in synthetic complete (SC) medium throughout this study, because SC is made with defined nitrogen sources (Materials and Methods) and is likely to introduce fewer experimental variables than rich medium for analyzing strains deleted for amino acid metabolic genes. We did not include Lys20 and Lys21 in this analysis because their roles in silencing have been examined previously (25), and we were unable to assess the silencing function of Ser2 because of the poor growth of the null strain in our SC medium.
We first assessed rDNA silencing in strains deleted for each of the remaining 11 candidate genes. The rDNA reporter is an ADE2–CAN1 cassette inserted at the 25S transcription unit. CAN1 encodes a plasma membrane permease that imports arginine. Natural silencing within the rDNA locus represses CAN1 expression, thus blocking the import of the toxic arginine analog canavanine (Fig. 1B). Mutants with rDNA silencing defects, for example esa1-414 (26), are sensitive to canavanine, because elevated import and incorporation of canavanine compromise protein functions. Strains deleted for FPR1, GDH1, HOM2, HOM6, and SPE2 strains were sensitive to canavanine, suggesting that these genes may normally promote rDNA silencing (Fig. 1B).
The reporter strain also has the URA3 gene inserted on the right arm of telomere V (TELV-R). Cells expressing URA3 are sensitive to 5-fluoroorotic acid (5-FOA). Natural silencing at TELV-R represses URA3 transcription, allowing cells to grow on 5-FOA. Defective silencing, such as that caused by SIR2 deletion, results in cell death on 5-FOA (27). Deletion of ARG82, GDH1, HOM6, and SPE2 resulted in varying degrees of 5-FOA sensitivity (Fig. 1C), suggesting that their gene products normally promote telomeric silencing. In contrast, deletion of SAM4 improved growth on 5-FOA, suggesting an inhibitory role of Sam4 in telomeric silencing. Notably, unlike the SIR2 deletion mutant that showed complete death on 5-FOA, deleting GDH1 and SPE2 led to small colony size and delayed growth on 5-FOA (Fig. 1C). This phenotype is similar to that observed upon deletion of CAC1, which encodes a component of the CAF1 chromatin assembly complex (28–30).
For monitoring silent mating-type control, the reporter strain carries the TRP1 gene in the HMR silent mating-type locus. Natural silencing of HMR represses TRP1 transcription, causing poor growth on medium lacking tryptophan. Loss of silencing in the SIR2 deletion strain improved growth on SC-Trp (27). In contrast, deletion of ARG82, HOM6, SAM4, and SPE2 worsened growth on SC-Trp (Fig. 1D), suggesting that HMR silencing is enhanced in these mutants. The phenotype for the ARG82 mutant was unexpected because it was reported to have a mating defect (31), whereas enhanced HM silencing usually results in a higher mating efficiency. Nonetheless, it is known that deletion of PLC1, which acts upstream of ARG82, similarly enhances HMR silencing when deleted (32). Therefore, Arg82 is likely to have a complex role in the regulation of mating-type control.
The divergent results for silencing for GDH1 and GDH3 upon first consideration might be surprising, as the two are paralogs (33), with the two proteins more than 90% similar in amino acid sequence. However, the genes are differentially regulated by carbon sources: Gdh1 is expressed and active in medium containing either glucose or ethanol, whereas Gdh3 is only detectable and active with ethanol as a carbon source (34). We speculate that no defect in its telomeric silencing was observed upon deletion of GDH3 because of its low level of expression in glucose. In fact, increased gene dosage of GDH3 suppressed the telomeric silencing defect of the gdh1Δ mutant (Fig. 1E). Also, deletion of either GDH gene only caused a mild loss of silencing when grown in ethanol (Fig. 1F), suggesting that the two paralogs may have overlapping functions in telomeric silencing when they are both expressed. Moreover, the gdh1Δ gdh3Δ double mutant showed defective telomeric silencing in both glucose- and ethanol-containing media (Fig. 1F). Therefore, both proteins contribute to telomeric silencing, with Gdh1 as the primary player when glucose is the carbon source.
Overall, our screen revealed that the deletion of ARG82, FPR1, GDH1, GDH3, HOM2, HOM6, SAM4, and SPE2 altered silencing of integrated reporter genes, whereas the deletion of ADH5, HPA3, and UTR4 had no apparent effect. We focused on GDH1 because it encodes the broadly conserved glutamate dehydrogenase enzyme that lies at the nexus between the citric acid cycle and nitrogen metabolism. Dysregulation of this enzyme has been directly associated with congenital hyperinsulinism (35) and is indirectly implicated in cancer through the glutamine production pathway (36). Therefore, it is of great importance to determine whether GDH has a role in chromatin regulation in addition to or independent of its role in metabolism.
Gdh1 Regulates Recruitment of SIR Proteins to Silent Chromatin and Is Bound to Telomeric Chromatin.
Because the silencing reporters assayed in Fig. 1 are metabolic in nature and Gdh1 is a metabolic protein, we further evaluated Gdh1’s role in silencing through independent assays. Recent studies showed that 5-FOA–based telomeric silencing assays could give false-positive results for mutants that elevate ribonucleotide reductase (RNR) activities (37, 38). One way to eliminate the confounding effect is to inhibit RNR activity with 10 mM hydroxyurea (HU) (37). Adding HU did not rescue the 5-FOA sensitivity of gdh1∆ TELV-R::URA3, suggesting that the 5-FOA readout is likely a true reflection of gdh1∆’s silencing defect (Fig. 2A).
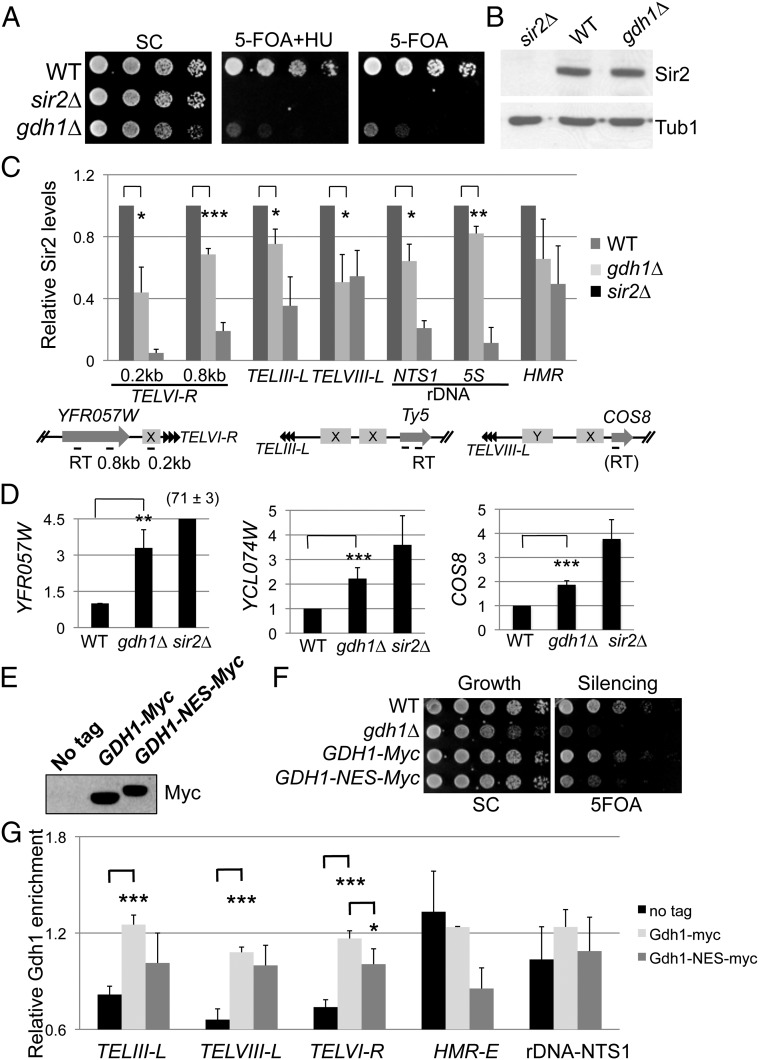
Fig. 2.
Independent assays support GDH1’s function in telomeric silencing. (A) The gdh1Δ silencing phenotype on 5-FOA was not a result of elevated RNR activity. WT (LPY4916), sir2∆ (LPY4979), and gdh1∆ (LPY16033) strains were assayed on 5-FOA with 10 mM HU, a RNR inhibitor. (B) Sir2 protein levels were unaffected in the gdh1Δ mutant. Whole-cell extracts of WT (LPY5), gdh1∆ (LPY16026), and sir2∆ (LPY11) were immunoblotted with antiserum for Tub1 (loading control) or Sir2. (C) Sir2 binding was significantly reduced in the gdh1Δ mutant. Sheared chromatin was prepared from strains in B. Approximate positions of the primers used for ChIP analysis are indicated. Subtelomeric structures are indicated, including designations of the X and Y elements (boxed) and the TG1–3n repeats (arrowheads). Sir2 enrichment at each locus was normalized to an established ChrV control locus (76) with WT set to 1 for each experiment. For all experiments of this study, error bars represent SEs and P values represent results of one-tailed Student’s t test. *P < 0.05, **P < 0.01, ***P < 0.005. (D) Expression of telomere-proximal transcripts was increased in the gdh1∆ mutant. The mRNA from strains in B was analyzed by quantitative RT-PCR, using primers indicated as “RT” in C. Expression levels were normalized to ACT1 with the WT value set to 1. (E) Gdh1-Myc and Gdh1-NES-Myc were stably expressed. Whole-cell extracts from no tag (LPY5), GDH1-Myc (LPY16784), and GDH1-NES-Myc (LPY17738) strains were immunoblotted for Myc. (F) Ectopic integration of an NES at the C terminus of Gdh1 caused a moderate telomeric silencing defect. WT, gdh1Δ, GDH1-Myc (LPY16782), and GDH1-NES-Myc (LPY17736) strains were assayed on 5-FOA for telomeric silencing. (G) Gdh1 is enriched at the telomeres, but the artificial addition of NES did not efficiently or uniformly deplete Gdh1 from the binding sites. No tag (LPY5), GDH1-myc (LPY16784), and GDH1-NES-myc (LPY17738) strains were analyzed by ChIP. The signal at each locus was normalized to that at the ChrV control locus.
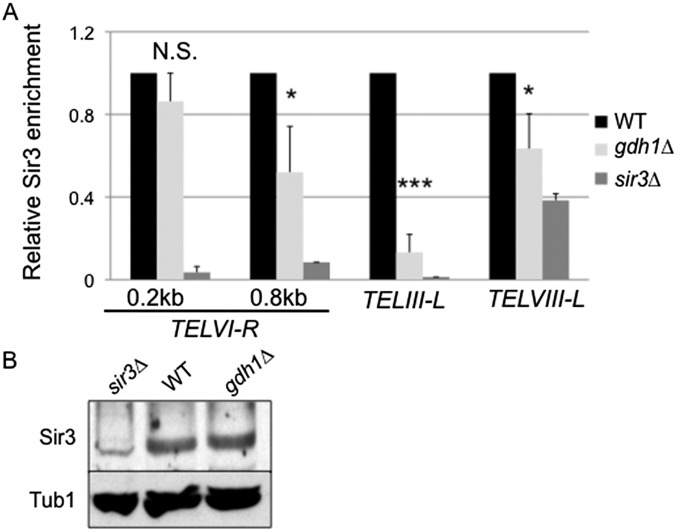
Next, we assessed Gdh1’s impact on the recruitment of the SIR proteins at natural telomeres. Deletion of GDH1 did not change Sir2 protein levels (Fig. 2B), however it did reduce Sir2 binding at all natural telomeric loci studied (Fig. 2C). In contrast, deletion of GDH1 did not significantly change Sir2 binding at the HMR locus (Fig. 2C), consistent with the result of the hmr∆E::TRP1 reporter assay. Sir3 binding was also reduced at telomeres (Fig. S1A).
Fig. S1.
Sir3 binding is reduced at three telomeric loci in the gdh1Δ mutant. (A) Telomeric Sir3 binding was significantly reduced in the gdh1Δ mutant. WT (LPY5), gdh1Δ (LPY16026), and sir3Δ (LPY10) strains were analyzed by ChIP for the indicated loci and the ChrV intergenic control locus. The WT values were set to 1. Data represent the averages from three independent experiments. (B) Sir3 protein levels were unchanged in the gdh1Δ mutant. Whole-cell extracts from the strains used in A were immunoblotted for Sir3 or Tub1 (loading control).
To directly assess the effect of the loss of the SIR complex in the absence of Gdh1, we measured the transcript levels of three genes close to their native telomeres (Fig. 2D). We found increased gene expression in the GDH1 deletion mutant (Fig. 2D). Although less severe than the effect of deleting SIR2 (Fig. 2D), these results parallel those of the silencing reporter assay in which the remaining silencing activity is likely to be mediated by the residual presence of the SIR complex at the telomeres. These independent assays collectively support a role for Gdh1 in telomeric silencing through modulating the recruitment of the SIR complex.
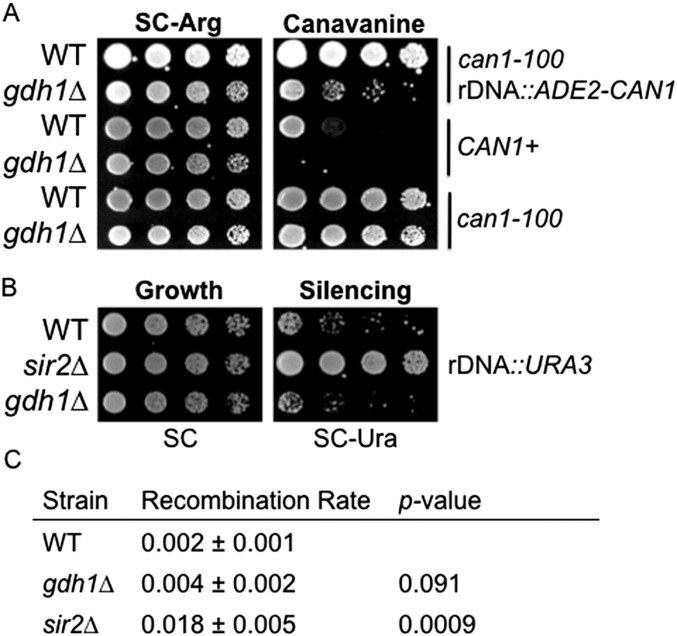
In contrast to the silencing effects for telomeres, we found that the rDNA reporter assay was confounded by a silencing-independent effect of Gdh1 on CAN1. A GDH1 deletion mutant with CAN1 at its endogenous locus was hypersensitive to canavanine (Fig. S2A), suggesting that Gdh1 influences canavanine sensitivity outside the context of rDNA. We did observe decreased Sir2 binding at the rDNA NTS1 spacer and 5S loci (Fig. 2C). However, we did not observe loss of rDNA silencing using alternative silencing assays: The gdh1∆ mutant did not show changes in silencing a URA3 reporter integrated at the NTS1 spacer region (39) (Fig. S2B), neither did it increase the rDNA recombination rate significantly (40) (Fig. S2C).
Fig. S2.
GDH1 has a minimal role in rDNA silencing. (A) The gdh1Δ strain showed canavanine sensitivity in a silencing-independent manner. The following strains were plated on SC-Arg (control) and SC-Arg + canavanine (0.75 μg/mL): WT rDNA::ADE2-CAN1 (LPY4909), gdh1Δ rDNA::ADE2-CAN1 (LPY16019), WT CAN1 (LPY17954), gdh1Δ CAN1 (LPY17951), WT can1-100 (LPY5), and gdh1Δ can1-100 (LPY16026). Note that this was a lower concentration of canavanine than used in the silencing assay. Decreased growth indicates increased sensitivity to canavanine. (B) rDNA silencing at NTS1 was unaffected in the gdh1Δ mutant. WT (LPY2446), sir2Δ (LPY2445), and gdh1Δ (LPY16009) strains carried the rDNA Ty mURA3 insertion, and silencing was assessed on SC-Ura. Increased growth indicates defective silencing. (C) Mitotic recombination within the rDNA array was mildly elevated in the gdh1Δ mutant. WT (LPY4909), gdh1Δ (LPY16019), and sir2Δ (LPY5013) strains were assessed as described in SI Materials and Methods. Data represent the averages from three to four independent experiments.
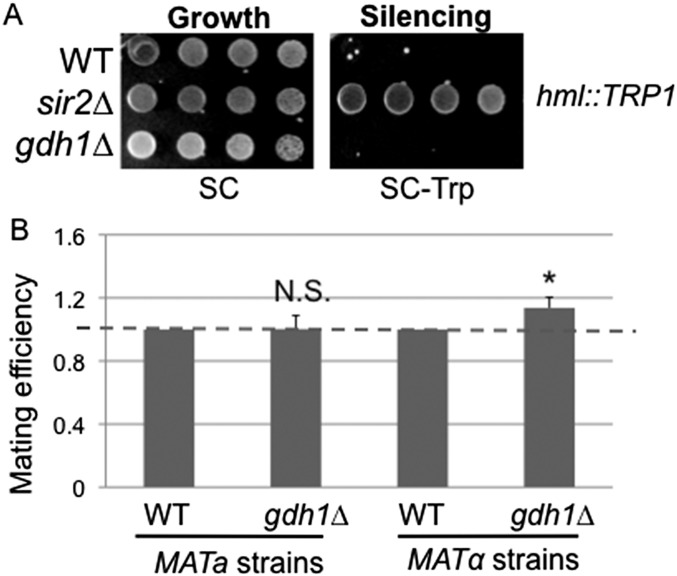
We also assessed the effect of deletion of GDH1 on the HML silent mating-type locus. The gdh1∆ strain silenced HML as efficiently as wild-type cells (Fig. S3A). Quantitative mating analysis showed that deletion of GDH1 moderately improved mating in the MATα strain (Fig. S3B), suggesting that Gdh1 contributes to the regulation of silent mating type.
Fig. S3.
Gdh1 has a modest role in HM silencing. (A) Silencing at the HML locus was unaffected in the gdh1Δ mutant. WT (LPY309), sir2Δ (LPY1401), and gdh1Δ (LPY18979) strains were plated on SC-Trp, where increased growth indicates defective silencing. (B) MATα mating efficiency was moderately increased in the gdh1Δ mutant. MATa WT (LPY5), MATα WT (LPY79), MATa gdh1Δ (LPY16026), and MATα gdh1Δ (LPY16560) strains were assessed as described in SI Materials and Methods. Data represent the averages from five independent experiments.
Because deletion of GDH1 resulted in phenotypes consistent with contributions to chromatin-based functions and because the protein has both nuclear and cytoplasmic pools (41, 42), it was important to establish if nuclear localization was relevant for its silencing functions. In ChIP experiments, we found that Myc-tagged Gdh1 (Fig. 2E) is enriched at all three native telomeres tested (Fig. 2G). Because Gdh1 does not have a canonical Nuclear Localization Signal, we added a well-established heterologous Nuclear Export Sequence (NES) (43) to investigate the effect of depleting the nuclear pool of Gdh1 on silencing. This construct is stably expressed (Fig. 2E) and, when provided as the only source of Gdh1 in the telomeric reporter strain, caused reduced telomeric silencing (Fig. 2F). The silencing defect of the Gdh1–NES mutant was not as strong as that of the gdh1∆ mutant. This may be because the endogenous localization mechanism is intact, so the nuclear pool is likely to be diminished but not eliminated. This is consistent with the observation that the artificial addition of NES did not effectively or uniformly deplete Gdh1 from the telomeric sites (Fig. 2G).
Gdh1 Is a Negative Regulator of H3 N-Terminal Clipping in Vivo.
Transcriptional silencing and activation are influenced by both the structure and modification of the nucleosomal histones. For example, it is known that the N-terminal tail of histone H3 itself, along with its modifications, can be critical for gene regulation. In classic experiments, deletion of the H3 N terminus was found to aberrantly activate transcription of a number of genes (44). This interesting biological result correlates with the long-standing observation that H3 exists in two pools in vivo, one full-length and the other as a proteolytically cleaved population (45). Histone H3 “clipping” is the term used to denote the process in which the H3 N-terminal tail is cleaved. Over the years, a number of studies have been performed to characterize clipping activity and to identify the enzyme(s) that may be responsible. Clipping was originally attributed to an unidentified serine protease, and the cleavage site was proposed to be at residue H3-S22 in budding yeast (46).
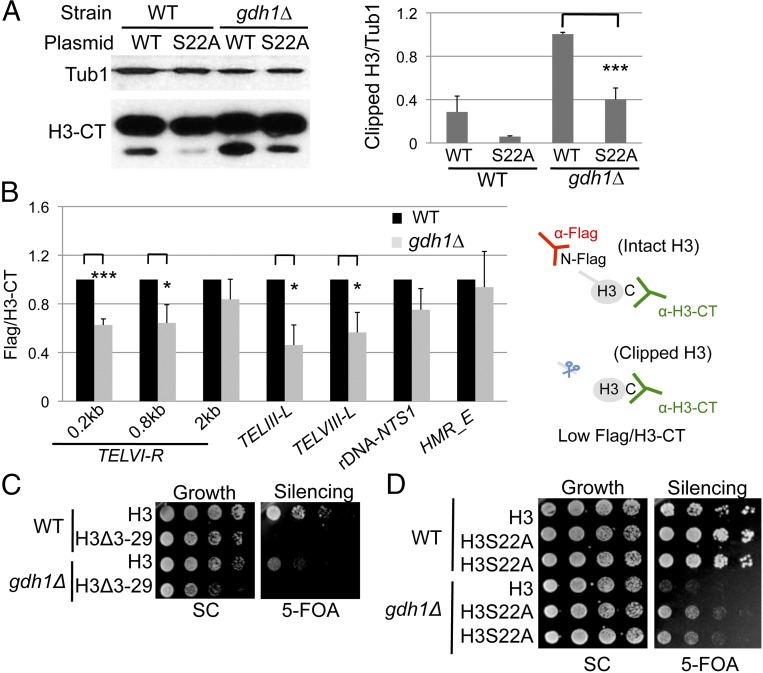
In more recent studies, glutamate dehydrogenase purified from vertebrate microsomes appeared to catalyze H3 clipping in vitro (47), suggesting a potential moonlighting role for GDH. By contrast, yeast studies showed that Gdh1 was present in a fraction with clipping activity, but whole-cell extracts from GDH1 mutants did not affect clipping in vitro (48). Because we showed that Gdh1 had a role in regulating chromatin silencing, we asked whether it also functioned in H3 clipping in vivo through both biochemical and genetic approaches.
Nuclear extracts were prepared from log-phase cells followed by immunoblotting to evaluate both intact and clipped H3. The gdh1Δ mutant showed increased global H3 clipping (Fig. 3A), suggesting an inhibitory role of Gdh1 on H3 clipping under the conditions tested. The protease inhibited is likely to be a serine protease, because the H3-S22A mutation reduced the amount of clipped H3 in both the WT and the gdh1∆ cells (Fig. 3A). Moreover, a ChIP experiment measuring H3 N- and C-terminal signals at each locus showed that Gdh1 also inhibited H3 clipping locally at specific genomic loci, such as regions close to the telomeres but not the rDNA or the HMR loci (Fig. 3B).
Fig. 3.
Gdh1 is a negative regulator of histone H3 N-terminal clipping in budding yeast in vivo. (A) The H3S22A mutation interferes with H3 clipping. Nuclear extracts of strains were prepared from WT and gdh1Δ histone shuffle strains (LPY20470, -16155, -20623, and -16162) and blotted anti–H3-CT or anti-Tub1 (loading control). Blots from two sets of independent extracts were quantified. (B) H3 N-terminal clipping was increased at telomeres in the gdh1∆ mutant. WT (LPY19789) and gdh1∆ (LPY19794) histone shuffle strains carried H3 with an N-terminal Flag tag (pLP2129). Anti-Flag antibody precipitated unclipped H3, whereas anti–H3-CT antibody precipitated both clipped and unclipped H3. Flag and H3-CT signals at each locus were normalized to the respective signals for the control locus ACT1. The Flag/H3-CT ratio for the WT strain was set to 1 for each experiment. (C) The H3Δ3–29 mutation was epistatic to the gdh1Δ mutant with respect to telomeric silencing. WT and gdh1Δ histone shuffle strains (LPY20665, -20695, -20021, and -20023) were assayed on 5-FOA. (D) The H3S22A mutation improved silencing in both the WT and the gdh1Δ background. The strains used in A were assayed on 5-FOA.
To evaluate the functional significance of H3 clipping in GDH1-mediated silencing, we first evaluated the N-terminal truncation (44), with a histone-shuffle experiment, in which plasmid-borne mutant or wild-type H3 is the only source of that protein in the cell (49). We found that the genetically clipped version of H3 led to loss of telomeric silencing in both WT and the GDH1 deletion mutant and also had a modest inhibition of growth in the GDH1 mutant (Fig. 3C). In an independent histone shuffle, in which the H3-S22A mutant that interfered with clipping was the only source of H3, we observed improved silencing in both WT and the GDH1 mutant cells (Fig. 3D). These results support the idea that clipped H3 contributes to loss of silencing in the promotion of gene activation, consistent with the defective silencing observed in the GDH1 deletion mutant.
Gdh1’s Chromatin Functions Require Its Metabolic Activity.
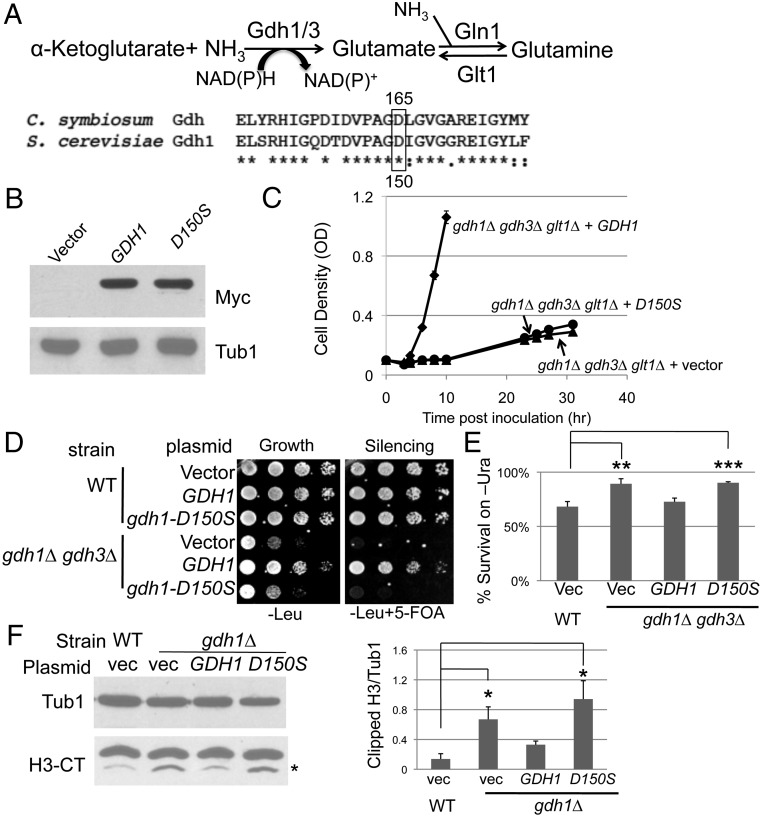
To determine whether Gdh1 had a distinct moonlighting activity or whether the silencing defects observed for the GDH mutants were intrinsic to the established catalytic activity, we asked if the chromatin function of Gdh1 was dependent on its metabolic activity. The well-defined functions of Gdh1 and Gdh3 in S. cerevisiae are in nitrogen assimilation: The enzymes catalyze the synthesis of glutamate from α-ketoglutarate and ammonium. Of note, budding yeast has an alternative pathway to synthesize glutamate, using the glutamate synthase encoded by GLT1 (Fig. 4A).
Fig. 4.
The metabolic activity of the GDH homologs is important for its chromatin functions. (A) The catalytic Asp residue is conserved in S. cerevisiae Gdh1. (Top) The enzymatic reaction catalyzed by the GDH enzymes. (Bottom) Alignment of the C. symbiosum GDH with S. cerevisiae Gdh1. Boxed is the conserved catalytic Asp residue at position 165 in the Clostridium protein. (B) gdh1-D150S-13Myc was stably expressed. gdh1Δ (LPY16026) cells were transformed with vector (pRS316), GDH1-13Myc (pLP2833), or gdh1-D150S-13Myc (pLP2834). Whole-cell extracts were immunoblotted for Myc or Tub1 (loading control). (C) The gdh1-D150S mutant had diminished catalytic activity required to assimilate ammonium. The gdh1Δ gdh3Δ glt1Δ strain (LPY17131) was transformed with vector (pRS315), GDH1 (pLP2631), or gdh1-D150S (pLP2638) and assayed for growth. (D) The conserved Asp residue contributes to Gdh1’s function in telomeric silencing. WT and gdh1Δ gdh3Δ cells were transformed with vector (pRS425), GDH1 (pLP2764), or gdh1-D150S (pLP2698), and telomeric silencing was assessed. (E) Colony counting assay on SC-Ura confirmed the lack of silencing activity of the gdh1-D150S mutant. The assay was described in Materials and Methods. (F) Gdh1’s inhibitory function in H3 clipping is dependent on the conserved Asp residue. Nuclear extracts were prepared from WT (LPY4916) and gdh1∆ (LPY16033) strains transformed with vector (pRS314), GDH1 (pLP3082), or gdh1-D150S (pLP3083) and immunoblotted for H3-CT and Tub1 (loading control). N-terminally clipped H3 was highlighted by the asterisk. Blots from two sets of independent extracts were quantified.
Studies on GDH from Clostridium symbiosum reported that the Asp165 residue is required for catalysis because a D165S mutant lost its catalytic activity in vitro without affecting substrate binding (50). Sequence alignment revealed that this residue is conserved in S. cerevisiae (Fig. 4A).
We constructed a plasmid-borne gdh1-D150S mutant, Myc-tagged and expressed from its endogenous promoter. The mutant protein had stable expression compared with wild type (Fig. 4B), demonstrating that the mutation did not perturb protein stability. The catalytic activity of the gdh1-D150S mutant was assessed in vivo by a growth assay. The assay is based on the principle that the gdh1Δ gdh3Δ glt1Δ triple mutant strain cannot catalyze the anabolic reactions to synthesize glutamate from ammonium sulfate (Fig. 4A) and thus grows poorly in ammonium sulfate-based minimal medium. Transformation of the triple mutant with wild-type GDH1 rescued the growth defect, whereas the D150S mutant was unable to do so within the same time course (Fig. 4C). Therefore, the D150S mutant had lost much of its catalytic activity in vivo. Because Gdh1 and Gdh3 were reported to form hetero-hexamers (34), we assessed the silencing function of the gdh1-D150S mutant in the gdh1Δ gdh3Δ background to avoid potential dominant effects. The gdh1-D150S mutant failed to suppress the telomeric silencing defect of the gdh1Δ gdh3Δ strain, as demonstrated by dilution assay (Fig. 4D) and independently counting colonies grown on medium lacking uracil (Fig. 4E). Some silencing activity was observed upon prolonged incubation, likely due to low or residual metabolic activity of the D150S mutant. Also, the conserved Asp residue is required for Gdh1’s inhibitory effect on H3 clipping (Fig. 4F). Hence, we conclude that the metabolic activity of Gdh1 is required for its chromatin functions.
Elevated α-Ketoglutarate Levels Result in Telomeric Silencing Defects.
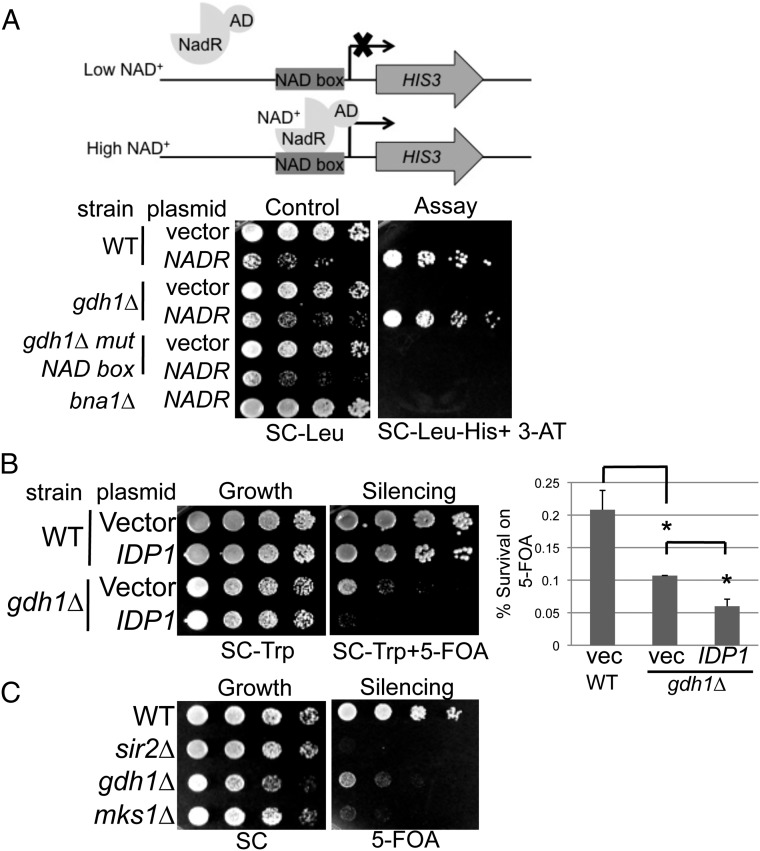
Gdh1 was selected by our in silico screen because its metabolic activity involves NAD(P)+ and α-ketoglutarate, which serve as cofactors for class III HDACs and JMJC-domain–containing demethylases, respectively. Because we observed defective telomeric silencing in the gdh1Δ mutant and Sir2’s HDAC activity depends on NAD+ (51), we asked if Gdh1’s metabolic activity influences the nuclear pool of NAD+.
In budding yeast, nuclear NAD+ levels can be measured in strains with a cis-acting NadR binding-site box at the promoter of the HIS3 gene that is transformed with a plasmid-borne NadR-AD transcriptional activator (Fig. 5A). The levels of free NAD+ in the nucleus influence the binding affinity of NadR-AD for the NAD box, thus modulating the ability of the strain to grow on medium lacking histidine (52). In this assay, the gdh1Δ mutant showed similar growth as the wild-type strain (Fig. 5A); thus, it is unlikely that GDH1 influences telomeric silencing through nuclear NAD+ levels.
Fig. 5.
Gdh1 likely regulates telomeric silencing through modulating α-ketoglutarate levels. (A) The gdh1Δ cells have normal levels of nuclear NAD+. WT (LPY20466) and gdh1Δ (LPY20477) reporter strains were transformed with vector (pLP3227) or NADR-AD (pLP3228). Nuclear NAD+ levels were measured by growth on SC-His-Trp + 10 mM 3-AT. 3-AT prevents leaky transcription of HIS3. The bna1Δ strain (LPY20468) was a positive control for reduced NAD+ levels, and the gdh1Δ strain with mutant NAD boxes (LPY20480) was a negative control to test for NadR-AD–specific effects. (B) Increased dosage of IDP1 worsened telomeric silencing in gdh1Δ. WT, and gdh1Δ cells transformed with vector (pRS314) or IDP1 (pLP3238) were assayed on SC-Trp + 5-FOA. Survival rate on 5-FOA was quantified as described in Materials and Methods. (C) Deletion of MKS1 caused defective telomeric silencing. WT, sir2Δ, gdh1Δ, and mks1Δ (LPY16796) strains were assayed on 5-FOA.
We next considered the possibility that Gdh1 regulates chromatin functions through modulating α-ketoglutarate levels. This metabolite serves as a cofactor for many enzymes, including the JMJC-domain–containing histone demethylases (53). The gdh1Δ mutant has reduced ability to use α-ketoglutarate as a substrate to assimilate ammonium (Fig. 4A) and caused an ∼33% increase in α-ketoglutarate levels when grown in ammonium sulfate-based SC medium (34). It is worth noting that no experimental tool has yet been developed to evaluate nuclear levels of α-ketoglutarate, however our finding that an NES-tagged Gdh1 construct caused a moderate loss of telomeric silencing (Fig. 2F) suggested that the nuclear pool of α-ketoglutarate may be altered when Gdh1 is depleted from the nucleus. Also, we observed physical association of Gdh1 at telomeric loci (Fig. 2G), and thus it is possible that Gdh1 influences α-ketoglutarate levels locally at specific genomic regions.
To assess whether elevated α-ketoglutarate levels could contribute to the silencing defect of the gdh1∆ mutant, we took two separate approaches. First, we transformed gdh1∆ with plasmid-borne IDP1, which encodes a mitochondrial NADP-specific isocitrate dehydrogenase (IDH) that is known to contribute to the synthesis of α-ketoglutarate (54, 55). Increased dosage of IDP1 further worsened telomeric silencing in gdh1Δ cells by ∼50% (Fig. 5B), consistent with the concept that these two genetic manipulations had additive effects on increasing α-ketoglutarate levels. In an independent approach, we deleted the gene MKS1, because a previous study showed that this mutant caused a 600% increase in α-ketoglutarate levels (56). The mks1Δ mutant showed an even stronger telomeric silencing defect than the gdh1Δ mutant (Fig. 5C). These findings suggest that α-ketoglutarate levels are important regulators of telomeric silencing.
GDH1 and JHD2 Exhibit Complex Genetic Interactions.
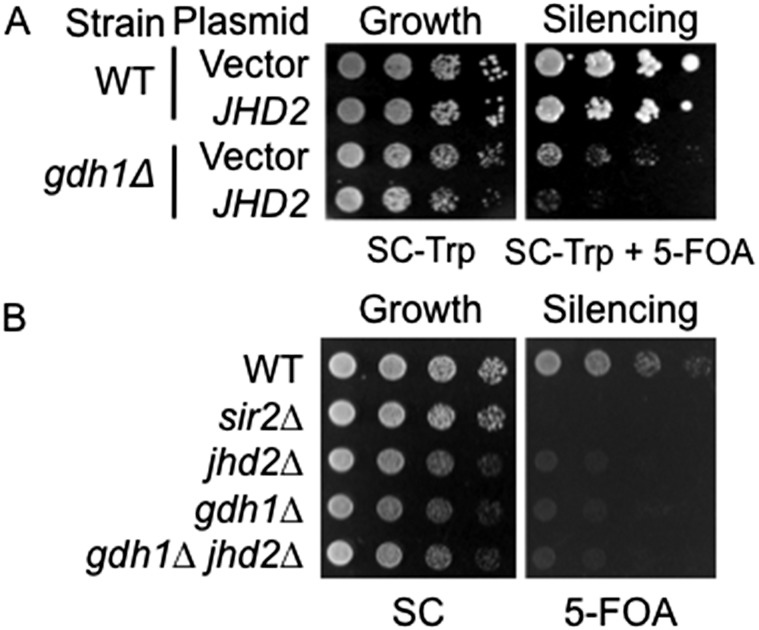
Because we observed that high levels of α-ketoglutarate caused a silencing defect, we sought to identify the molecular mechanism that links Gdh1 directly to telomeric silencing. The best established group of α-ketoglutarate–regulated nuclear proteins is the JMJC-domain–containing demethylases. Budding yeast has five known enzymes of this family, of which only the histone H3 Lys4 demethylase Jhd2 specifically removes methyl groups from trimethylated histone H3 Lys4 (57). Because H3 Lys4 methylation marks are known to be important for telomeric silencing (58, 59), we tested the hypothesis that the increased α-ketoglutarate levels in the gdh1Δ mutant hyperactivated Jhd2 to alter telomeric silencing. To test the hypothesis, we first took a gene overexpression approach. We found that increased dosage of JHD2 worsened telomeric silencing in the gdh1Δ mutant (Fig. S4A), suggesting that the deletion of GDH1 creates a condition under which Jhd2 has a greater impact on telomeric silencing. Furthermore, deletion of JHD2 did not rescue the silencing defect of the gdh1Δ mutant (Fig. S4B). Rather, the jhd2Δ gdh1Δ double mutant showed a silencing defect similar to each single mutant (Fig. S4B). Within the limits of these experiments, we concluded that although Gdh1 and Jhd2 may regulate telomeric silencing through a common pathway, the simple hypothesis of Jhd2 as a target of Gdh1 is unlikely to be correct. Thus, the mechanism through which Jhd2 and Gdh1 may regulate telomeric silencing is likely to be complex.
Fig. S4.
GDH1 has complex genetic interactions with JHD2. (A) Increased dosage of JHD2 worsens the telomeric silencing phenotype of the gdh1∆ mutant. WT (LPY4916) and gdh1∆ (LPY16033) strains were transformed with vector (pRS314) and JHD2 (pLP2928) and plated on SC-Trp + 5-FOA to assess telomeric silencing. (B) Deletion of JHD2 does not rescue the silencing defect of the gdh1∆ mutant. WT (LPY4916), sir2∆ (LPY4979), jhd2∆ (LPY18880), gdh1∆ (LPY16033), and gdh1∆ jhd2∆ (LPY18414) strains were plated on 5-FOA to assess telomeric silencing.
Discussion
Increasing evidence suggests that multiple pathways connect metabolism to chromatin regulation and epigenetic processes (8, 9, 20). In this study, our in silico screen revealed that multiple proteins with established roles in amino acid metabolism also have potential functions in chromatin. Our focus on glutamate dehydrogenase revealed that both Gdh1 and Gdh3 regulated telomeric silencing. Gdh1 acts as the primary regulator when glucose is the carbon source, whereby it regulated recruitment of the SIR complex to telomeres. Multiple genetic analyses demonstrated that increased α-ketoglutarate levels are detrimental to telomeric silencing and that Gdh1 has complex genetic interactions with the Jhd2 histone demethylase.
Deletion of Multiple Candidate Genes Encoding Metabolic Proteins Altered Chromatin Silencing.
We found that deletion of ARG82, FPR1, GDH1, GDH3, HOM2, HOM6, SAM4, and SPE2 could cause silencing defects. The gene products of these candidates represent five distinct metabolic pathways: arginine biosynthesis, proline isomerization, assimilation of ammonium, methionine and threonine biosynthesis, and SAM metabolism. Future studies will establish if the other candidate proteins contribute to chromatin-mediated silencing through metabolism-dependent or -independent mechanisms.
It is noteworthy that our in silico search revealed that 39 amino acid metabolic proteins have reported nuclear pools, 25 of which were eliminated from immediate consideration as chromatin regulators by one or more of our search criteria (Table S2). We note, however, that some of these proteins may regulate chromatin functions in diverse ways. For example, they may use the same catalytic site for entirely different biochemical reactions (21). Further, the candidate genes whose deletion did not show silencing phenotypes may contribute to other aspects of chromatin function, such as DNA damage repair, replication, or other elements of transcriptional regulation.
Gdh1 Is a Negative Regulator of H3 N-Terminal Clipping in Vivo.
The N terminus of histone H3 contributes significantly to gene regulation. In a recent report, GDH isolated from vertebrate cell microsomes was reported to clip free and chromatin-bound H3 in vitro (47). In marked contrast to this report, we found instead that deletion of GDH1 increased H3 clipping globally and locally during log phase and that the increase was dependent on the conserved catalytic residue controlling its metabolic activity (Fig. 4F). Thus, yeast Gdh1 can inhibit rather than catalyze H3 N-terminal clipping in vivo with functional consequences for gene regulation (Fig. 3).
Current knowledge on histone H3 clipping is rather limited. Recent work suggested that a vacuolar protease encoded by PRB1 has clipping activity in budding yeast during the early stationary phase (48). Nonetheless, it remains unclear whether, when, and how Prb1 might enter the nucleus from the vacuole to access chromatin. Also, Prb1 cleaved H3 at lysine residues in vitro, but independent studies confirmed that the in vivo clipping activity is sensitive to serine protease inhibitors (46, 48) and mutation of H3 to H3-S22A diminishes H3 clipping (Fig. 3A). Future work is required to establish the physiological conditions under which Prb1 is active, to identify any additional H3 proteases, and to determine the protease regulated by Gdh1.
α-Ketoglutarate Is an Important Metabolic Regulator of Telomeric Silencing.
We showed that Gdh1’s roles in both telomeric silencing and H3 clipping are dependent on its metabolic activity and that high α-ketoglutarate levels are detrimental to telomeric silencing. Recent studies revealed that oncogenic mutations in human IDH result in the synthesis of 2-hydroyglutarate (2-HG) instead of the normal α-ketoglutarate product. 2-HG competitively inhibits JMJC-domain histone demethylases, resulting in increased H3K9 methylation (60, 61). Further, levels of α-ketoglutarate are key for transcriptional and epigenetic processes in stem cell maintenance (62). Our work demonstrates that elevated α-ketoglutarate levels caused telomeric silencing defects (Fig. 5 B and C). These findings collectively suggest that it is crucial to maintain the homeostasis of α-ketoglutarate, as both low and high levels have detrimental effects on chromatin functions. This point will be particularly important when developing therapies against the increasingly recognized IDH mutant-bearing tumors (63, 64), because strategies to balance or compensate for decreased α-ketoglutarate may cause negative secondary effects.
In sum, we identified glutamate dehydrogenase homologs Gdh1 and Gdh3 as positive regulators of telomeric silencing. Gdh1’s silencing function requires its catalytic activity, and high α-ketoglutarate levels are detrimental to silencing. Gdh1 represses H3 N-terminal clipping and regulates the recruitment of the SIR complex to the telomeres. These results, and the other candidates identified in our screen (Fig. 1), thus emphasize the emerging concept that epigenetic processes are tightly regulated by cellular metabolic status and that mutations in metabolic genes may cause diseases through changes both in the cytoplasm and in the nucleus.
Materials and Methods
Yeast Strains and Plasmids.
Strains are listed in Table S3. All mutants are null alleles except esa1-414 (26). Gene deletions were constructed by amplifying kanMX from the Saccharomyces Genome Deletion Project strains (65, 66) (oligonucleotides are listed in Table S4) and transforming it into the silencing reporter strain (24). Strains for the GDH1 study were backcrossed before use. Double mutants were constructed through standard crosses. Histone shuffle strains were chromosomally deleted for both HHT-HHF loci and were originally covered with a plasmid carrying the wild-type copy of HHT2-HHF2 (67). Histone mutant strains were made by transforming the shuffle strains with plasmids carrying histone mutants and counterselecting the wild-type plasmid.
Table S3.
Strains used in this study
| Strain, alias | Genotype | Reference/source |
| LPY5 | MATa ade2-1 can1-100 his3-11 leu2-3,112 trp1-1 ura3-1 (W303-1a) | R. Rothstein (Columbia University, New York) |
| LPY10 | MATa sir3∆::TRP1 | |
| LPY11 | MATa sir2∆::HIS3 | |
| LPY78* | MATα his4 | |
| LPY79 | MATα W303 | |
| LPY142* | MATa his4 | |
| LPY309 | MATa hml::TRP1 | |
| LPY1401 | MATa sir2∆::HIS3 hml::TRP1 | |
| LPY2445* | MATα his3∆200 leu2∆1 ura3-52 sir2∆::HIS3 with rDNA Ty mURA3 insertion | (39) |
| LPY2446* | MATα his3∆200 leu2∆1 ura3-52 with rDNA Ty mURA3 insertion | (39) |
| LPY4654 | MATα hmr∆E::TRP1 rDNA::ADE2-CAN1 TELV-R::URA3 | (24) |
| LPY4909 | MATα rDNA::ADE2-CAN1 | (26) |
| LPY4916 | MATa TELV-R::URA3 | (26) |
| LPY4977 | MATα sir2∆::HIS3 hmr∆E::TRP1 rDNA::ADE2-CAN1 TELV-R::URA3 | (26) |
| LPY4979 | MATα sir2∆::HIS3 TELV-R::URA3 | (26) |
| LPY5013 | MATα sir2∆::TRP1 rDNA::ADE2-CAN1 | |
| LPY11113 | MATa esa1-414 hmr∆E::TRP1 rDNA::ADE2-CAN1 TELV-R::URA3 | |
| LPY15962 | MATα hom2∆::kanMX hmr∆E::TRP1 rDNA::ADE2-CAN1 TELV-R::URA3 | |
| LPY15964 | MATα utr4∆::kanMX hmr∆E::TRP1 rDNA::ADE2-CAN1 TELV-R::URA3 | |
| LPY15966 | MATα hpa3∆::kanMX hmr∆E::TRP1 rDNA::ADE2-CAN1 TELV-R::URA3 | |
| LPY15968 | MATα arg82∆::kanMX hmr∆E::TRP1 rDNA::ADE2-CAN1 TELV-R::URA3 | |
| LPY15970 | MATα gdh1∆::kanMX hmr∆E::TRP1 rDNA::ADE2-CAN1 TELV-R::URA3 | |
| LPY15972 | MATα gdh3∆::kanMX hmr∆E::TRP1 rDNA::ADE2-CAN1 TELV-R::URA3 | |
| LPY16009* | MATα his3∆200 leu2∆1 ura3-52 gdh1∆::kanMX with rDNA Ty mURA3 insertion | |
| LPY16019 | MATα gdh1∆::kanMX rDNA::ADE2-CAN1 | |
| LPY16026 | MATa gdh1∆::kanMX | |
| LPY16033 | MATα gdh1∆::kanMX TELV-R::URA3 | |
| LPY16155 | MATα hht1-hhf1∆::kanMX hht2-hhf2∆::kanMX hta2-htb2∆::HPH TELV-R::URA3+pLP2438 | |
| LPY16161 | MATα hht1-hhf1∆::kanMX hht2-hhf2∆::kanMX hta2-htb2∆::HPH TELV-R::URA3+pLP2438 | |
| LPY16162 | MATα gdh1∆::kanMX hht1-hhf1∆::kanMX hht2-hhf2∆::kanMX hta2-htb2∆::HPH TELV-R::URA3+pLP2438 | |
| LPY16163 | MATα gdh1∆::kanMX hht1-hhf1∆::kanMX hht2-hhf2∆::kanMX hta2-htb2∆::HPH TELV-R::URA3+pLP2438 | |
| LPY16560 | MATα gdh1∆::kanMX | |
| LPY16774 | MATα fpr1∆::kanMX rDNA::ADE2-CAN1 | |
| LPY16775 | MATa fpr1∆::kanMX hmr∆E::TRP1 | |
| LPY16776 | MATa fpr1∆::kanMX TELVR::URA3 | |
| LPY16782 | MATa GDH1-13Myc::kanMX TELV-R::URA3 | |
| LPY16784 | MATa GDH1-13Myc::kanMX | |
| LPY16785 | MATa gdh3∆::kanMX TELV-R::URA3 | |
| LPY16796 | MATα mks1∆::kanMX TELV-R::URA3 | |
| LPY17061 | MATa sam4∆::kanMX rDNA::ADE2-CAN1 | |
| LPY17062 | MATα sam4∆::kanMX hmr∆::TRP1 | |
| LPY17064 | MATα sam4∆::kanMX TELV-R::URA3 | |
| LPY17131* | MATα his3∆1 leu2∆0 met15∆0 ura3∆0 gdh1∆::kanMX gdh3∆::kanMX glt1∆::kanMX | |
| LPY17406 | MATα hom6∆::kanMX hmr∆E::TRP1 rDNA::ADE2-CAN1 TELV-R::URA3 | |
| LPY17736 | MATa GDH1-13Myc-NES::kanMX TELV-R::URA3 | |
| LPY17738 | MATα GDH1-13Myc-NES::kanMX | |
| LPY17916 | MATα gdh1∆::kanMX gdh3∆::kanMX TELV-R::URA3 | |
| LPY17951 | MATa ade2-1 CAN1 his3-11,15 leu2-3,112 trp1-1 ura3-1 gdh1∆::kanMX | |
| LPY17954 | MATa ade2-1 CAN1 his3-11,15 leu2-3,112 trp1-1 ura3-1 | |
| LPY18411 | MATa jhd2∆::kanMX mks∆::kanMX TELV-R::URA3 | |
| LPY18415 | MATα jhd2∆::kanMX gdh1∆::kanMX TELV-R::URA3 | |
| LPY18669 | MATα jhd2∆::kanMX TELV-R::URA3 | |
| LPY18979 | MATa gdh1∆::kanMX hml::TRP1 | |
| LPY19789 | MATα hht1-hhf1∆::kanMX hht2-hhf2∆::kanMX hta2-htb2∆::HPH +pLP2129 | |
| LPY19794 | MATα gdh1∆::kanMX hht1-hhf1∆::kanMX hht2-hhf2∆::kanMX hta2-htb2∆::HPH+pLP2129 | |
| LPY19922 | MATα adh5∆::kanMX hmr∆E::TRP1 rDNA::ADE2-CAN1 TELV-R::URA3 | |
| LPY19973 | MATα spe2∆::kanMX hmr∆E::TRP1 rDNA::ADE2-CAN1 TELV-R::URA3 | |
| LPY20021 | MATa gdh1Δ::kanMX hht1-hhf1Δ::kanMX hht2-hhf2Δ::kanMX hta2-htb2Δ::HPH TELV-R::URA3+pLP1490 | |
| LPY20023 | MATa gdh1Δ::kanMX hht1-hhf1Δ::kanMX hht2-hhf2Δ::kanMX hta2-htb2Δ::HPH TELV-R::URA3+pLP1491 | |
| LPY20665 | MATα hht1-hhf1Δ::kanMX hht2-hhf2Δ::kanMX hta2-htb2Δ::HPH TELV-R::URA3 +pLP1490 | |
| LPY20695 | MATα hht1-hhf1Δ::kanMX hht2-hhf2Δ::kanMX hta2-htb2Δ::HPH TELV-R::URA3 +pLP1491 | |
| LPY20466* | leu2 his3 (4×NAD boxes)-HIS3 (YSH896) | (78) |
| LPY20468* | bna1∆ leu2 his3 (4×NAD boxes)-HIS3+ BSH484 (YSH898) | (78) |
| LPY20470 | MATα hht1-hhf1∆::kanMX hht2-hhf2∆::kanMX hta2-htb2∆::HPH TELV-R::URA3+pLP1775 | |
| LPY20623 | MATα gdh1∆::kanMX hht1-hhf1∆::kanMX hht2-hhf2∆::kanMX hta2-htb2∆::HPH TELV-R::URA3+pLP1775 | |
| LPY20477* | gdh1∆::kanMX leu2 his3 (4×NAD boxes)-HIS3 | |
| LPY20480* | gdh1∆::kanMX leu2 his3 (4×mutated NAD boxes)-HIS3 |
Unless otherwise noted, strains were constructed during this study or are part of the standard laboratory collection. All strains are in the W303 background, except where indicated (*).
Table S4.
Oligonucleotides used in this study
| Oligo no. | Name | Sequence, 5′–3′ | Source/reference |
| 764 | TELVI-R 0.75KB F | ATATATGCACTAGTTGCACTAGGCG | |
| 765 | TELVI-R 0.75KB R | CTTCCAGTAAATTTCTCTTTGAGTGG | |
| 776 | rDNA.5S.f | CATGGAGCAGTTTTTTCCGC | (84) |
| 777 | rDNA.5S.r | TACAAGCACTCATGTTTGCCG | |
| 778 | TELVI-R0.200.f | AAATGGCAAGGGTAAAAACCAG | |
| 779 | TELVI-R0.200.r | TCGGATCACTACACACGGAAAT | |
| 798 | ACT1_F1 | GGTGGTTCTATCTTGGCTTC | (71) |
| 799 | ACT1_R1 | ATGGACCACTTTCGTCGTAT | |
| 852 | rDNA NTS1-1f | AGGGCTTTCACAAAGCTTCC | (85) |
| 853 | rDNA NTS1-1r | TCCCCACTGTTCACTGTTCA | |
| 871 | ChrV_sense | GTGTTTGACCCGAGGGTATG | (76) |
| 872 | ChrV_antisense | TAAGGTCCACACCGTCATCA | |
| 1516 | HOM2_5_KO | CGACGGAGAAGAAGGAGAC | |
| 1517 | HOM2_3_KO | CTTGGGTCAGCGAGAGAATTAC | |
| 1520 | GDH1_5_KO | CACGTCCAATCAGCAGAGAG | |
| 1521 | GDH1_3_KO | CAATAAGCCTGGTGTCCAATC | |
| 1522 | GDH3_5_KO | CCGTTCAGTTTGCTTGATTG | |
| 1523 | GDH3_3_KO | CACTATCCCCCTTCAAATTG | |
| 1524 | UTR4_5_KO | CCTTGCGGCCACTTATAG | |
| 1525 | UTR4_3_KO | CTATTTCGCGCCTCTGTG | |
| 1526 | SAM4_5_KO | GGGTGTTCCGTGGAGAATC | |
| 1527 | SAM4_3_KO | GGATCTCGACAGGTTCGTG | |
| 1528 | ARG82_5_KO | GACAGGCTTGTTGTGTGTG | |
| 1529 | ARG82_3_KO | CATAGCAGCCGGTTTTTC | |
| 1530 | HPA3_5_KO | CCCGACATTCAGACGTACAC | |
| 1531 | HPA3_3_KO | GACGGTGTCCATTGCTTATATAG | |
| 1556 | gdh1-D150S F | GACGTGCCAGCTGGTTCTATCGGTGTTGGTGGTC | |
| 1557 | gdh1-D150S R | GACCACCAACACCGATAGAACCAGCTGGCACGTC | |
| 1598 | GDH1 XmaI F | CCAAGGTGATGTATTTCCCGGGGTCTAAAAGAAAGAAAAGAGG | |
| 1599 | GDH1 XmaI R | CCTCTTTTCTTTCTTTTAGACCCCGGGAAATACATCACCTTGG | |
| 1629 | GDH1_CMyc F | CAAGTTTCATCAAGGTCTCTGATGCTATGTTTGACCAAGGTGATGTATTTCGGATCCCCGGGTTAATTAA | |
| 1630 | GDH1_CMyc R | AAAAGAAAGAACTTTTTATGAACTTTCCTCTTTTCTTTCTTTTAGACTATGAATTCGAGCTCGTTTAAAC | |
| 1667 | FPR1_5_KO | CCATTTGGCACGTTTACTTTG | |
| 1668 | FPR1_3_KO | GAAACTCTTGTGGCAGGAGG | |
| 1753 | XmaI_pFA6a_R | TCCCCCCGGGGGACGAGGCAAGCTAAACAG | |
| 1769 | HOM6_new5KO | CAATAACGCACATGGTGG | |
| 1770 | HOM6_new3KO | GCCCCATGACATGGATGAG | |
| 1815 | YFR057W_F | CTAGTGTCTATAGTAAGTGCTCGG | |
| 1816 | YFR057W_R | CTCTAACATAACTTTGATCCTTACTCG | |
| 1819 | YFR074W_F | ACGGCGGTATGTATGGACTC | |
| 1820 | YFR074W_R | GGGACCATCAGATGTGGAAC | |
| 1941 | HMRE_F | GGAGTCTTAATTTCCCTGATTTTAGTTTAG | (86) |
| 1942 | HMRE_R | CCCGTCCAAGTTATGAGCTTAATCT | |
| 2023 | YCL075W_F(TELIII-L) | GTTCCAGCTACCAAATCCTCAGA | M. Oki (University of Fukui, Fukui, Japan) |
| 2024 | YCL075W_R (TELIII-L) | CGTGGCACATGTGGGAAGTA | |
| 2134 | COS8_For (TELVIII-L) | CCGTTCTACCTCAAGATGTTTTCCG | (87) |
| 2135 | COS8_Rev (TELVIII-L) | CCAGGAACAGGACAAGAAGTGAAAC | |
| ADH5_5_KO | GGCGGCATTGTAAAAAGTTC | ||
| ADH5_3_KO | CCTCCTGGTTTCAGTTTCCA | ||
| SPE2_5_KO | CTGCATCCAATTTCAGCGTA | ||
| SPE2_3_KO | ACATCGGGCATAAACCTTTG |
Unless otherwise noted, oligos were designed during this study.
Plasmids are listed in Table S5. Each gene was subcloned from the Yeast Genomic Tiling Library (68), including endogenous 5′ and 3′ sequences. The D150S mutation was introduced by site-directed mutagenesis using primers listed in Table S4. Plasmid-borne Myc-tagged Gdh1 was constructed by replacing the natural stop codon of GDH1 with an XmaI site using primers oLP1598 and oLP1599 and ligating the digested plasmid with an XmaI-digested DNA fragment containing the 13Myc epitope and a terminator sequence made by amplification of pLP1651 with primers oLP1629 and oLP1753.
Table S5.
Plasmids used in this study
| Plasmid, alias | Description | Reference/source |
| pRS314 | vector TRP1 CEN | (79) |
| pRS315 | vector LEU2 CEN | |
| pRS316 | vector URA3 CEN | |
| pRS424 | vector TRP1 2μ | (80) |
| pRS425 | vector LEU2 2μ | |
| pLP1490 | pRS414-HHT2 HHF2 | (81) |
| pLP1491 | pRS414-hht2Δ3–29 HHF2 | |
| pLP1651 | pFA6a-13Myc-kanMX | (69) |
| pLP1775 | pRS314-HHT2 HHF2 | |
| pLP2129 | pRS314-FLAG-HHT2 HHF2 | (82) |
| pLP2438 | pRS314-hht2-S22A HHF2 | (83) |
| pLP2637 | pRS315-GDH1 | |
| pLP2638 | pRS315-gdh1-D150S | |
| pLP2662 | pRS425-GDH3 | |
| pLP2698 | pRS425-gdh1-D150S | |
| pLP2764 | pRS425-GDH1 | |
| pLP2928 | pRS314-JHD2 | |
| pLP2829 | pFA6a-13Myc-Gly-Gly-NES-kanMX | |
| pLP2833 | pRS316-GDH1-13Myc | |
| pLP2834 | pRS316-gdh1-D150S-13Myc | |
| pLP3082 | pRS314-GDH1 | |
| pLP3083 | pRS314-gdh1-D150S | |
| pLP3227 | pACT2 (V172) | (78) |
| pLP3228 | pACT2-NadR-AD (BSH 484) | |
| pLP3238 | pRS314-IDP1 |
Unless otherwise noted, plasmids were constructed during this study or are part of the standard laboratory collection.
The chromosomal GDH1 locus was tagged by amplifying the 13Myc-kanMX cassette from pLP1651 (69) with oligonucleotides OLP1629 and OLP1630 and transforming it into the WT W303 strain. The NES used was a variant of the strong NES from PKI with the sequence ELALKLAGLDINLI (43). A 13Myc-Gly-Gly-NES-kanMX fragment was integrated to tag the C terminus of Gdh1 by amplifying this cassette from pLP2829 with OLP1629 and OLP1630 and transforming it into the WT W303 strain.
Growth Assays and Silencing Reporter Assays.
SC medium was made with a 0.67% ammonium sulfate-based yeast nitrogen base without amino acids (Difco) supplemented with amino acids (standard laboratory recipe). For all experiments in this study, freshly thawed/transformed cells were inoculated in 3 mL SC medium for 2 d before plating/diluting for log-phase growth. For dilution assays, A600 of 1 OD of culture (∼6 × 106 cells) was pelleted, resuspended in 1 mL H2O, plated in fivefold serial dilutions, and incubated at 30 °C. For rDNA silencing assays, strains were plated on SC-Ade-Arg (growth control) and SC-Ade-Arg + 8 μg/mL l-canavanine (Sigma). For HM silencing assays, strains were plated on SC (growth control) and SC-Trp (silencing). For telomeric silencing assays, strains were plated on SC (growth control) and SC + 1 g/L 5-FOA (US Biological). For the nuclear NAD+ assays, strains were plated on SC-Leu (growth control) and SC-Leu-His + 10 mM 3-amino-1,2,4-triazole (3-AT) (Sigma). Images were captured after 1.5–3 d. To assess the catalytic activity of gdh1-D150S, starter cultures of transformants were diluted to A600 of 0.1 in minimal medium [0.67% ammonium sulfate-based yeast nitrogen base supplemented with required amino acids and 2% (wt/vol) glucose]. A600 was measured at indicated times. Quantification of growth on media lacking uracil was done by plating 200–500 log-phase cells on SC-Trp (control) or SC-Trp-Ura (silencing) media. Quantification of survival rate on 5-FOA was done by plating 500–1,000 log-phase cells on SC-Trp (control) and SC-Trp + 5-FOA (silencing). For both quantitative assays, cells were counted 48 h after plating.
mRNA Quantification.
Starter cultures were diluted in 50 mL SC medium and harvested at A600 of 0.8–1.0 (∼5 × 106–6.5 × 106 cells per mL). RNA was extracted using the hot acid phenol method (70), except that harvested cells were resuspended in sodium acetate buffer (50 mM sodium acetate pH 5.3, 10 mM EDTA). RNA was reverse transcribed with random hexamers using TaqMan Reverse Transcription Reagents (Life Technologies). cDNA was diluted five- or 10-fold and analyzed by real-time PCR on DNA Engine Opticon 2 (MJ Research) with primers listed in Table S4.
ChIP.
Strains were grown as for mRNA quantification and fixed in 1% formaldehyde for 15 min (25 min for anti-Myc ChIP). ChIP was performed as described (71). Briefly, sheared chromatin was incubated overnight at 4 °C with anti-Sir2 (1:1,000) (72), anti-Myc 9E10 (1:100, Sigma), anti-Flag (1:1,000, Sigma), or anti-H3-CT (1:500, Millipore). Input DNA and immunoprecipitated (IP) samples were diluted 10-fold and analyzed by real-time PCR (primers listed in Table S4). Anti-Flag specificity was verified with an untagged strain.
Preparation of Yeast Nuclei and Whole-Cell Extract.
Strains were grown as for mRNA quantification. Nuclei were prepared as described (73). Whole-cell extract was prepared by resuspending each A600 1.0 of cells in 25 μL PBS with protease inhibitors and vortexing it with glass beads. Protein samples were denatured by boiling in sample loading buffer.
Protein Immunoblotting.
Proteins were separated on SDS-polyacrylamide gels (18% acrylamide for the separation of histones and 8% for the other proteins) and transferred to 0.2 μm nitrocellulose. Primary antisera used were anti–H3-CT (1:10,000, Millipore), anti-Myc (1:10,000) (74), anti-Sir2 (1:10,000) (72), and anti–β-tubulin (1:10,000) (75). Secondary antisera used were goat anti-mouse for anti-Myc or goat anti-rabbit (conjugated to horseradish peroxidase, 1:10,000, Promega). Signals were detected with Pierce ECL substrate (Thermo Scientific) on Hyblot CL films (Denville Scientific). Quantification was done using ImageJ.
SI Materials and Methods
Silencing and rDNA Recombination Assays.
For the rDNA::mURA3 silencing assay, strains were plated on SC (growth control) and SC-Ura (silencing). For the canavanine sensitivity test, strains were plated on SC-Arg (growth control) and SC-Arg + 0.75 μg/mL l-canavanine (Sigma).
To measure the rDNA recombination rate, strains were grown in SC-Ade-Arg to an A600 of 0.8–1.0. We plated 400–500 cells on yeast peptone dextrose with adenine (YPAD), incubated them at 30 °C for 2 d, and then moved them to 4 °C to promote color development. We assessed 1,500–2,500 colonies for each strain. Half-sectored colonies originated from cells that had lost the ADE2 reporter at the first mitotic division. The recombination rate was calculated as the number of half-sectored colonies divided by the total number of colonies.
Quantitative Mating Assay.
Query strains were grown in SC, and mating testers were grown in YPAD. Approximately 500 log-phase cells were either plated in triplicate on YPAD or mixed with an equal number of mating tester cells (LPY78 or LPY142). After 3 h of incubation at 30 °C, the mating mixtures were plated on minimal medium in triplicate. Raw mating efficiency was calculated as the number of diploids (i.e., colonies growing on the minimal plate) divided by the number of haploids (i.e., colonies growing on the YPAD plate). Mating efficiency for the WT strain was set to 1.
Immunoblotting and ChIP.
Experimental procedures were described in Materials and Methods. Anti-Sir3 (77) was diluted 1:2,000 for ChIP and 1:5,000 for immunoblotting.
Acknowledgments
We thank A. L. Torres-Machorro for critical discussion throughout the course of the work. We also thank M. Oki, S. Holmes, K. Runge, J. Kadonaga, and A. Marston for sharing reagents and equipment. We thank N. Yi for help constructing the sam4∆ strains, and the L.P. laboratory members and D. J. Forbes for comments on the manuscript. This work was initiated with funding from the NIH and completed with support from the UC Cancer Research Coordinating Committee and the University of California, San Diego Academic Senate Committee on Research.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1518707113/-/DCSupplemental.
References
- 1.Kornberg RD, Lorch Y. Twenty-five years of the nucleosome, fundamental particle of the eukaryote chromosome. Cell. 1999;98(3):285–294. doi: 10.1016/s0092-8674(00)81958-3. [DOI] [PubMed] [Google Scholar]
- 2.Kouzarides T. Chromatin modifications and their function. Cell. 2007;128(4):693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 3.Rando OJ, Winston F. Chromatin and transcription in yeast. Genetics. 2012;190(2):351–387. doi: 10.1534/genetics.111.132266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kueng S, Oppikofer M, Gasser SM. SIR proteins and the assembly of silent chromatin in budding yeast. Annu Rev Genet. 2013;47:275–306. doi: 10.1146/annurev-genet-021313-173730. [DOI] [PubMed] [Google Scholar]
- 5.Rusché LN, Kirchmaier AL, Rine J. Ordered nucleation and spreading of silenced chromatin in Saccharomyces cerevisiae. Mol Biol Cell. 2002;13(7):2207–2222. doi: 10.1091/mbc.E02-03-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ellahi A, Thurtle DM, Rine J. The chromatin and transcriptional landscape of native Saccharomyces cerevisiae telomeres and subtelomeric domains. Genetics. 2015;200(2):505–521. doi: 10.1534/genetics.115.175711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wellen KE, Thompson CB. A two-way street: Reciprocal regulation of metabolism and signalling. Nat Rev Mol Cell Biol. 2012;13(4):270–276. doi: 10.1038/nrm3305. [DOI] [PubMed] [Google Scholar]
- 8.Kaelin WG, Jr, McKnight SL. Influence of metabolism on epigenetics and disease. Cell. 2013;153(1):56–69. doi: 10.1016/j.cell.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Janke R, Dodson AE, Rine J. Metabolism and epigenetics. Annu Rev Cell Dev Biol. 2015;31:473–496. doi: 10.1146/annurev-cellbio-100814-125544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takahashi H, McCaffery JM, Irizarry RA, Boeke JD. Nucleocytosolic acetyl-coenzyme a synthetase is required for histone acetylation and global transcription. Mol Cell. 2006;23(2):207–217. doi: 10.1016/j.molcel.2006.05.040. [DOI] [PubMed] [Google Scholar]
- 11.Wellen KE, et al. ATP-citrate lyase links cellular metabolism to histone acetylation. Science. 2009;324(5930):1076–1080. doi: 10.1126/science.1164097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang W, et al. ERK1/2-dependent phosphorylation and nuclear translocation of PKM2 promotes the Warburg effect. Nat Cell Biol. 2012;14(12):1295–1304. doi: 10.1038/ncb2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen S, Brockenbrough JS, Dove JE, Aris JP. Homocitrate synthase is located in the nucleus in the yeast Saccharomyces cerevisiae. J Biol Chem. 1997;272(16):10839–10846. doi: 10.1074/jbc.272.16.10839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scott EM, Pillus L. Homocitrate synthase connects amino acid metabolism to chromatin functions through Esa1 and DNA damage. Genes Dev. 2010;24(17):1903–1913. doi: 10.1101/gad.1935910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Torres-Machorro AL, Aris JP, Pillus L. A moonlighting metabolic protein influences repair at DNA double-stranded breaks. Nucleic Acids Res. 2015;43(3):1646–1658. doi: 10.1093/nar/gku1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Copley SD. Moonlighting is mainstream: Paradigm adjustment required. BioEssays. 2012;34(7):578–588. doi: 10.1002/bies.201100191. [DOI] [PubMed] [Google Scholar]
- 17.Jeffery CJ. Moonlighting proteins--An update. Mol Biosyst. 2009;5(4):345–350. doi: 10.1039/b900658n. [DOI] [PubMed] [Google Scholar]
- 18.Gancedo C, Flores CL. Moonlighting proteins in yeasts. Microbiol Mol Biol Rev. 2008;72(1):197–210. doi: 10.1128/MMBR.00036-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cherry JM, et al. Saccharomyces Genome Database: The genomics resource of budding yeast. Nucleic Acids Res. 2012;40(Database issue):D700–D705. doi: 10.1093/nar/gkr1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gut P, Verdin E. The nexus of chromatin regulation and intermediary metabolism. Nature. 2013;502(7472):489–498. doi: 10.1038/nature12752. [DOI] [PubMed] [Google Scholar]
- 21.Khersonsky O, Tawfik DS. Enzyme promiscuity: A mechanistic and evolutionary perspective. Annu Rev Biochem. 2010;79:471–505. doi: 10.1146/annurev-biochem-030409-143718. [DOI] [PubMed] [Google Scholar]
- 22.Dubois E, Dewaste V, Erneux C, Messenguy F. Inositol polyphosphate kinase activity of Arg82/ArgRIII is not required for the regulation of the arginine metabolism in yeast. FEBS Lett. 2000;486(3):300–304. doi: 10.1016/s0014-5793(00)02318-8. [DOI] [PubMed] [Google Scholar]
- 23.Odom AR, Stahlberg A, Wente SR, York JD. A role for nuclear inositol 1,4,5-trisphosphate kinase in transcriptional control. Science. 2000;287(5460):2026–2029. doi: 10.1126/science.287.5460.2026. [DOI] [PubMed] [Google Scholar]
- 24.Roy N, Runge KW. Two paralogs involved in transcriptional silencing that antagonistically control yeast life span. Curr Biol. 2000;10(2):111–114. doi: 10.1016/s0960-9822(00)00298-0. [DOI] [PubMed] [Google Scholar]
- 25.Scott EM. 2010. Characterization of the esa1 Suppressor LYS20 and Discovery of Its Novel Nuclear Functions. PhD thesis (University of California, San Diego, La Jolla, CA)
- 26.Clarke AS, Samal E, Pillus L. Distinct roles for the essential MYST family HAT Esa1p in transcriptional silencing. Mol Biol Cell. 2006;17(4):1744–1757. doi: 10.1091/mbc.E05-07-0613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aparicio OM, Billington BL, Gottschling DE. Modifiers of position effect are shared between telomeric and silent mating-type loci in S. cerevisiae. Cell. 1991;66(6):1279–1287. doi: 10.1016/0092-8674(91)90049-5. [DOI] [PubMed] [Google Scholar]
- 28.Enomoto S, McCune-Zierath PD, Gerami-Nejad M, Sanders MA, Berman J. RLF2, a subunit of yeast chromatin assembly factor-I, is required for telomeric chromatin function in vivo. Genes Dev. 1997;11(3):358–370. doi: 10.1101/gad.11.3.358. [DOI] [PubMed] [Google Scholar]
- 29.Kaufman PD, Kobayashi R, Stillman B. Ultraviolet radiation sensitivity and reduction of telomeric silencing in Saccharomyces cerevisiae cells lacking chromatin assembly factor-I. Genes Dev. 1997;11(3):345–357. doi: 10.1101/gad.11.3.345. [DOI] [PubMed] [Google Scholar]
- 30.Monson EK, de Bruin D, Zakian VA. The yeast Cac1 protein is required for the stable inheritance of transcriptionally repressed chromatin at telomeres. Proc Natl Acad Sci USA. 1997;94(24):13081–13086. doi: 10.1073/pnas.94.24.13081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dubois E, Messenguy F. Pleiotropic function of ArgRIIIp (Arg82p), one of the regulators of arginine metabolism in Saccharomyces cerevisiae. Role in expression of cell-type-specific genes. Mol Gen Genet. 1994;243(3):315–324. doi: 10.1007/BF00301067. [DOI] [PubMed] [Google Scholar]
- 32.Galdieri L, Chang J, Mehrotra S, Vancura A. Yeast phospholipase C is required for normal acetyl-CoA homeostasis and global histone acetylation. J Biol Chem. 2013;288(39):27986–27998. doi: 10.1074/jbc.M113.492348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Avendaño A, Deluna A, Olivera H, Valenzuela L, Gonzalez A. GDH3 encodes a glutamate dehydrogenase isozyme, a previously unrecognized route for glutamate biosynthesis in Saccharomyces cerevisiae. J Bacteriol. 1997;179(17):5594–5597. doi: 10.1128/jb.179.17.5594-5597.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DeLuna A, Avendano A, Riego L, Gonzalez A. NADP-glutamate dehydrogenase isoenzymes of Saccharomyces cerevisiae. Purification, kinetic properties, and physiological roles. J Biol Chem. 2001;276(47):43775–43783. doi: 10.1074/jbc.M107986200. [DOI] [PubMed] [Google Scholar]
- 35.James C, Kapoor RR, Ismail D, Hussain K. The genetic basis of congenital hyperinsulinism. J Med Genet. 2009;46(5):289–299. doi: 10.1136/jmg.2008.064337. [DOI] [PubMed] [Google Scholar]
- 36.Burgess DJ. Metabolism: Glutamine connections. Nat Rev Cancer. 2013;13(5):293. doi: 10.1038/nrc3515. [DOI] [PubMed] [Google Scholar]
- 37.Rossmann MP, Luo W, Tsaponina O, Chabes A, Stillman B. A common telomeric gene silencing assay is affected by nucleotide metabolism. Mol Cell. 2011;42(1):127–136. doi: 10.1016/j.molcel.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takahashi YH, et al. Dot1 and histone H3K79 methylation in natural telomeric and HM silencing. Mol Cell. 2011;42(1):118–126. doi: 10.1016/j.molcel.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith JS, Boeke JD. An unusual form of transcriptional silencing in yeast ribosomal DNA. Genes Dev. 1997;11(2):241–254. doi: 10.1101/gad.11.2.241. [DOI] [PubMed] [Google Scholar]
- 40.Gottlieb S, Esposito RE. A new role for a yeast transcriptional silencer gene, SIR2, in regulation of recombination in ribosomal DNA. Cell. 1989;56(5):771–776. doi: 10.1016/0092-8674(89)90681-8. [DOI] [PubMed] [Google Scholar]
- 41.Camardella L, Di Prisco G, Garofano F, Guerrini AM. Purification and properties of NADP-dependent glutamate dehydrogenase from yeast nuclear fractions. Biochim Biophys Acta. 1976;429(2):324–330. doi: 10.1016/0005-2744(76)90280-1. [DOI] [PubMed] [Google Scholar]
- 42.Huh WK, et al. Global analysis of protein localization in budding yeast. Nature. 2003;425(6959):686–691. doi: 10.1038/nature02026. [DOI] [PubMed] [Google Scholar]
- 43.Gadal O, et al. Nuclear export of 60s ribosomal subunits depends on Xpo1p and requires a nuclear export sequence-containing factor, Nmd3p, that associates with the large subunit protein Rpl10p. Mol Cell Biol. 2001;21(10):3405–3415. doi: 10.1128/MCB.21.10.3405-3415.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mann RK, Grunstein M. Histone H3 N-terminal mutations allow hyperactivation of the yeast GAL1 gene in vivo. EMBO J. 1992;11(9):3297–3306. doi: 10.1002/j.1460-2075.1992.tb05408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Allis CD, Glover CV, Bowen JK, Gorovsky MA. Histone variants specific to the transcriptionally active, amitotically dividing macronucleus of the unicellular eucaryote, Tetrahymena thermophila. Cell. 1980;20(3):609–617. doi: 10.1016/0092-8674(80)90307-4. [DOI] [PubMed] [Google Scholar]
- 46.Santos-Rosa H, et al. Histone H3 tail clipping regulates gene expression. Nat Struct Mol Biol. 2009;16(1):17–22. doi: 10.1038/nsmb.1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mandal P, Verma N, Chauhan S, Tomar RS. Unexpected histone H3 tail-clipping activity of glutamate dehydrogenase. J Biol Chem. 2013;288(26):18743–18757. doi: 10.1074/jbc.M113.462531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xue Y, Vashisht AA, Tan Y, Su T, Wohlschlegel JA. PRB1 is required for clipping of the histone H3 N terminal tail in Saccharomyces cerevisiae. PLoS One. 2014;9(2):e90496. doi: 10.1371/journal.pone.0090496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Megee PC, Morgan BA, Mittman BA, Smith MM. Genetic analysis of histone H4: Essential role of lysines subject to reversible acetylation. Science. 1990;247(4944):841–845. doi: 10.1126/science.2106160. [DOI] [PubMed] [Google Scholar]
- 50.Dean JL, et al. The catalytic role of aspartate in the active site of glutamate dehydrogenase. Biochem J. 1994;301(Pt 1):13–16. doi: 10.1042/bj3010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Imai S, Armstrong CM, Kaeberlein M, Guarente L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature. 2000;403(6771):795–800. doi: 10.1038/35001622. [DOI] [PubMed] [Google Scholar]
- 52.Anderson RM, et al. Yeast life-span extension by calorie restriction is independent of NAD fluctuation. Science. 2003;302(5653):2124–2126. doi: 10.1126/science.1088697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schneider J, Shilatifard A. Histone demethylation by hydroxylation: Chemistry in action. ACS Chem Biol. 2006;1(2):75–81. doi: 10.1021/cb600030b. [DOI] [PubMed] [Google Scholar]
- 54.Haselbeck RJ, McAlister-Henn L. Isolation, nucleotide sequence, and disruption of the Saccharomyces cerevisiae gene encoding mitochondrial NADP(H)-specific isocitrate dehydrogenase. J Biol Chem. 1991;266(4):2339–2345. [PubMed] [Google Scholar]
- 55.Haselbeck RJ, McAlister-Henn L. Function and expression of yeast mitochondrial NAD- and NADP-specific isocitrate dehydrogenases. J Biol Chem. 1993;268(16):12116–12122. [PubMed] [Google Scholar]
- 56.Feller A, Ramos F, Piérard A, Dubois E. Lys80p of Saccharomyces cerevisiae, previously proposed as a specific repressor of LYS genes, is a pleiotropic regulatory factor identical to Mks1p. Yeast. 1997;13(14):1337–1346. doi: 10.1002/(SICI)1097-0061(199711)13:14<1337::AID-YEA186>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 57.Liang G, Klose RJ, Gardner KE, Zhang Y. Yeast Jhd2p is a histone H3 Lys4 trimethyl demethylase. Nat Struct Mol Biol. 2007;14(3):243–245. doi: 10.1038/nsmb1204. [DOI] [PubMed] [Google Scholar]
- 58.Krogan NJ, et al. COMPASS, a histone H3 (Lysine 4) methyltransferase required for telomeric silencing of gene expression. J Biol Chem. 2002;277(13):10753–10755. doi: 10.1074/jbc.C200023200. [DOI] [PubMed] [Google Scholar]
- 59.Nislow C, Ray E, Pillus L. SET1, a yeast member of the trithorax family, functions in transcriptional silencing and diverse cellular processes. Mol Biol Cell. 1997;8(12):2421–2436. doi: 10.1091/mbc.8.12.2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Turcan S, et al. IDH1 mutation is sufficient to establish the glioma hypermethylator phenotype. Nature. 2012;483(7390):479–483. doi: 10.1038/nature10866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lu C, et al. IDH mutation impairs histone demethylation and results in a block to cell differentiation. Nature. 2012;483(7390):474–478. doi: 10.1038/nature10860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Carey BW, Finley LW, Cross JR, Allis CD, Thompson CB. Intracellular α-ketoglutarate maintains the pluripotency of embryonic stem cells. Nature. 2015;518(7539):413–416. doi: 10.1038/nature13981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Krell D, et al. IDH mutations in tumorigenesis and their potential role as novel therapeutic targets. Future Oncol. 2013;9(12):1923–1935. doi: 10.2217/fon.13.143. [DOI] [PubMed] [Google Scholar]
- 64.Cairns RA, Mak TW. Oncogenic isocitrate dehydrogenase mutations: Mechanisms, models, and clinical opportunities. Cancer Discov. 2013;3(7):730–741. doi: 10.1158/2159-8290.CD-13-0083. [DOI] [PubMed] [Google Scholar]
- 65.Winzeler EA, et al. Functional characterization of the S. cerevisiae genome by gene deletion and parallel analysis. Science. 1999;285(5429):901–906. doi: 10.1126/science.285.5429.901. [DOI] [PubMed] [Google Scholar]
- 66.Giaever G, et al. Functional profiling of the Saccharomyces cerevisiae genome. Nature. 2002;418(6896):387–391. doi: 10.1038/nature00935. [DOI] [PubMed] [Google Scholar]
- 67.Ahn SH, et al. Sterile 20 kinase phosphorylates histone H2B at serine 10 during hydrogen peroxide-induced apoptosis in S. cerevisiae. Cell. 2005;120(1):25–36. doi: 10.1016/j.cell.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 68.Jones GM, et al. A systematic library for comprehensive overexpression screens in Saccharomyces cerevisiae. Nat Methods. 2008;5(3):239–241. doi: 10.1038/nmeth.1181. [DOI] [PubMed] [Google Scholar]
- 69.Longtine MS, et al. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast. 1998;14(10):953–961. doi: 10.1002/(SICI)1097-0061(199807)14:10<953::AID-YEA293>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 70.Collart MA, Oliviero S. Preparation of yeast RNA. Curr Protoc Mol Biol. 2001;Chapter 13:Unit13.12. doi: 10.1002/0471142727.mb1312s23. [DOI] [PubMed] [Google Scholar]
- 71.Darst RP, Garcia SN, Koch MR, Pillus L. Slx5 promotes transcriptional silencing and is required for robust growth in the absence of Sir2. Mol Cell Biol. 2008;28(4):1361–1372. doi: 10.1128/MCB.01291-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Garcia SN, Pillus L. A unique class of conditional sir2 mutants displays distinct silencing defects in Saccharomyces cerevisiae. Genetics. 2002;162(2):721–736. doi: 10.1093/genetics/162.2.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kizer KO, Xiao T, Strahl BD. Accelerated nuclei preparation and methods for analysis of histone modifications in yeast. Methods. 2006;40(4):296–302. doi: 10.1016/j.ymeth.2006.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Evan GI, Lewis GK, Ramsay G, Bishop JM. Isolation of monoclonal antibodies specific for human c-myc proto-oncogene product. Mol Cell Biol. 1985;5(12):3610–3616. doi: 10.1128/mcb.5.12.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bond JF, Fridovich-Keil JL, Pillus L, Mulligan RC, Solomon F. A chicken-yeast chimeric beta-tubulin protein is incorporated into mouse microtubules in vivo. Cell. 1986;44(3):461–468. doi: 10.1016/0092-8674(86)90467-8. [DOI] [PubMed] [Google Scholar]
- 76.Hess D, Liu B, Roan NR, Sternglanz R, Winston F. Spt10-dependent transcriptional activation in Saccharomyces cerevisiae requires both the Spt10 acetyltransferase domain and Spt21. Mol Cell Biol. 2004;24(1):135–143. doi: 10.1128/MCB.24.1.135-143.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Palladino F, et al. SIR3 and SIR4 proteins are required for the positioning and integrity of yeast telomeres. Cell. 1993;75(3):543–555. doi: 10.1016/0092-8674(93)90388-7. [DOI] [PubMed] [Google Scholar]
- 78.Ringel AE, et al. Yeast Tdh3 (glyceraldehyde 3-phosphate dehydrogenase) is a Sir2-interacting factor that regulates transcriptional silencing and rDNA recombination. PLoS Genet. 2013;9(10):e1003871. doi: 10.1371/journal.pgen.1003871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sikorski RS, Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122(1):19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Christianson TW, Sikorski RS, Dante M, Shero JH, Hieter P. Multifunctional yeast high-copy-number shuttle vectors. Gene. 1992;110(1):119–122. doi: 10.1016/0378-1119(92)90454-w. [DOI] [PubMed] [Google Scholar]
- 81.Zhang W, Bone JR, Edmondson DG, Turner BM, Roth SY. Essential and redundant functions of histone acetylation revealed by mutation of target lysines and loss of the Gcn5p acetyltransferase. EMBO J. 1998;17(11):3155–3167. doi: 10.1093/emboj/17.11.3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nathan D, et al. Histone sumoylation is a negative regulator in Saccharomyces cerevisiae and shows dynamic interplay with positive-acting histone modifications. Genes Dev. 2006;20(8):966–976. doi: 10.1101/gad.1404206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nakanishi S, et al. A comprehensive library of histone mutants identifies nucleosomal residues required for H3K4 methylation. Nat Struct Mol Biol. 2008;15(8):881–888. doi: 10.1038/nsmb.1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Emre NC, et al. Maintenance of low histone ubiquitylation by Ubp10 correlates with telomere-proximal Sir2 association and gene silencing. Mol Cell. 2005;17(4):585–594. doi: 10.1016/j.molcel.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 85.Huang J, Moazed D. Association of the RENT complex with nontranscribed and coding regions of rDNA and a regional requirement for the replication fork block protein Fob1 in rDNA silencing. Genes Dev. 2003;17(17):2162–2176. doi: 10.1101/gad.1108403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yang B, Britton J, Kirchmaier AL. Insights into the impact of histone acetylation and methylation on Sir protein recruitment, spreading, and silencing in Saccharomyces cerevisiae. J Mol Biol. 2008;381(4):826–844. doi: 10.1016/j.jmb.2008.06.059. [DOI] [PubMed] [Google Scholar]
- 87.Ehrentraut S, Weber JM, Dybowski JN, Hoffmann D, Ehrenhofer-Murray AE. Rpd3-dependent boundary formation at telomeres by removal of Sir2 substrate. Proc Natl Acad Sci USA. 2010;107(12):5522–5527. doi: 10.1073/pnas.0909169107. [DOI] [PMC free article] [PubMed] [Google Scholar]