Significance
Whether insect sesquiterpenes are synthesized de novo, derived from plant precursors, or produced by symbionts is often unknown. We identified an evolutionarily novel terpene synthase gene family in the striped flea beetle, a notorious pest of Brassica crops in North America and Asia, and one of these genes was shown to be directly involved in the biosynthesis of the male-specific sesquiterpene aggregation pheromone. Phylogenetic and gene structure analyses indicate that an expansion of the trans-isoprenyl diphosphate synthase gene family in the ancestor of the subfamily Galerucinae enabled functional diversification toward this terpene synthase gene family. These insights into how flea beetles synthesize their aggregation pheromones may lead to new approaches for pest management.
Keywords: isoprenyl diphosphate synthase; sesquiterpene synthase; (6R,7S)-himachala-9,11-diene; aggregation pheromone; flea beetle
Abstract
Sesquiterpenes play important roles in insect communication, for example as pheromones. However, no sesquiterpene synthases, the enzymes involved in construction of the basic carbon skeleton, have been identified in insects to date. We investigated the biosynthesis of the sesquiterpene (6R,7S)-himachala-9,11-diene in the crucifer flea beetle Phyllotreta striolata, a compound previously identified as a male-produced aggregation pheromone in several Phyllotreta species. A (6R,7S)-himachala-9,11-diene–producing sesquiterpene synthase activity was detected in crude beetle protein extracts, but only when (Z,E)-farnesyl diphosphate [(Z,E)-FPP] was offered as a substrate. No sequences resembling sesquiterpene synthases from plants, fungi, or bacteria were found in the P. striolata transcriptome, but we identified nine divergent putative trans-isoprenyl diphosphate synthase (trans-IDS) transcripts. Four of these putative trans-IDSs exhibited terpene synthase (TPS) activity when heterologously expressed. Recombinant PsTPS1 converted (Z,E)-FPP to (6R,7S)-himachala-9,11-diene and other sesquiterpenes observed in beetle extracts. RNAi-mediated knockdown of PsTPS1 mRNA in P. striolata males led to reduced emission of aggregation pheromone, confirming a significant role of PsTPS1 in pheromone biosynthesis. Two expressed enzymes showed genuine IDS activity, with PsIDS1 synthesizing (E,E)-FPP, whereas PsIDS3 produced neryl diphosphate, (Z,Z)-FPP, and (Z,E)-FPP. In a phylogenetic analysis, the PsTPS enzymes and PsIDS3 were clearly separated from a clade of known coleopteran trans-IDS enzymes including PsIDS1 and PsIDS2. However, the exon–intron structures of IDS and TPS genes in P. striolata are conserved, suggesting that this TPS gene family evolved from trans-IDS ancestors.
Terpenes play important roles in insect communication and defense, especially the C15 sesquiterpenes, which often act as sex, alarm, or aggregation pheromones or protection against enemies (1–3). To understand more about the biological function and evolution of sesquiterpenes in insects, it would be helpful to have more knowledge of their biosynthetic origins. Sesquiterpenes are biosynthesized from three C5 isopentenoid units supplied by the mevalonate pathway, which are then joined sequentially via the action of enzymes known as trans-isoprenyl diphosphate synthases (trans-IDS) to produce the linear C15 intermediate (E,E)-farnesyl diphosphate (FPP). Trans-IDS enzymes have been identified and characterized in a number of insect species (4–10). The huge diversity of sesquiterpene carbon skeletons are formed from FPP in the next step by the catalysis of terpene synthases (TPSs). Numerous TPSs have been identified in plants, fungi, and bacteria based on sequence similarity (11–13). However, no homologs of known terpene synthases have been reported from available insect genomic and transcriptomic sequences (3, 7). A unique bifunctional enzyme producing the C10 intermediate geranyl diphosphate (GPP) as well as the linear monoterpene myrcene in the bark beetle Ips pini represents the only insect terpene synthase known to date (14).
The reactions catalyzed by TPSs involve the generation of a highly reactive carbocation intermediate, which can undergo a wide array of different cyclizations, hydride shifts, and other rearrangements. The reaction cascade is either initiated by a metal ion-dependent ionization of the diphosphate moiety or a protonation of the substrate, and can be terminated by proton abstraction or water addition (11, 13, 15). Because the reaction cascade may be branched and termination may occur at multiple levels, many TPSs are multiproduct enzymes forming complex mixtures of compounds (16–19). Moreover, some TPSs also accept multiple substrates to produce monoterpenes, sesquiterpenes, and diterpenes (11).
Among sesquiterpene-producing insects are several genera in the leaf beetle subfamily Galerucinae in which males emit species-specific volatile sesquiterpene blends comprised mainly of himachalene-type compounds (20–23). In the flea beetles Phyllotreta cruciferae, Phyllotreta striolata, and Phyllotreta vittula, (6R,7S)-himachala-9,11-diene is a major male-produced sesquiterpene, and this compound was shown to be a key aggregation pheromone component (23–25).
We investigated the biosynthesis of the sesquiterpene aggregation pheromone in P. striolata, an important pest of crucifer crops in North America and Southeast Asia (26). Here, we report the identification of an evolutionarily novel family of terpene synthases in this flea beetle. No transcripts with similarity to plant or microbial terpene synthases were evident in a transcriptome of this species. However, a remarkably high number of genes were present that were predicted to encode enzymes similar to insect trans-IDSs, which produce geranyl diphosphate [(E)-GPP] and/or farnesyl diphosphate [(E,E)-FPP] from the C5 precursors isopentenyl diphosphate (IPP) and dimethylallyl diphosphate (DMAPP) (5, 9, 10). Functional characterization of the recombinant IDS-like enzymes led to the discovery of four TPS enzymes which formed a separate clade in a phylogenetic analysis of coleopteran trans-IDS and trans–IDS-like enzymes. We investigated the role of these TPS in the aggregation pheromone biosynthesis of P. striolata as well as their putative evolutionary origin.
Results
TPS Activity Is Present in P. striolata Crude Protein Extracts.
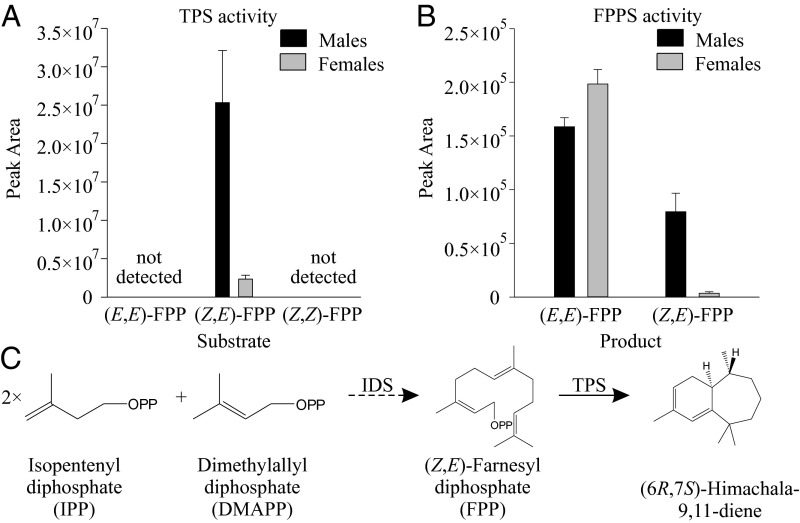
To search for TPS activity in P. striolata, we incubated crude protein extracts prepared from male or female adults with (E,E)-FPP, the canonical substrate for sesquiterpene synthases; however, no activity was observed. Because some plant sesquiterpene synthases use other FPP stereoisomers, we conducted further incubations and found to our surprise that both sexes converted (Z,E)-FPP to the aggregation pheromone compound (6R,7S)-himachala-9,11-diene and other known flea beetle sesquiterpenes (Fig. 1A), whereas no sesquiterpene products were detected when (Z,Z)-FPP was used as a substrate (Fig. 1A and Fig. S1A). A search for FPP synthase (FPPS) activity in the same crude protein extracts using DMAPP and IPP as substrates revealed (E,E)-FPPS as well as (Z,E)-FPPS activity (Fig. 1B and Fig. S1B). Both TPS and (Z,E)-FPPS activity were much higher in crude extracts from male P. striolata compared with females (11- and 23-fold, respectively; n = 3) as would be expected if these activities were associated with the formation of the male-specific aggregation pheromone (6R,7S)-himachala-9,11-diene (Fig. 1C).
Fig. 1.
Sesquiterpene synthase (TPS) activity (A) and farnesyl diphosphate synthase (FPPS) activity (B) in crude protein extracts of male and female Phyllotreta striolata adults. Crude protein extracts were incubated with the substrates (Z,E)-FPP, (E,E)-FPP, and (Z,Z)-FPP (A), or with IPP and DMAPP (B), respectively. TPS enzyme products were collected using a solid-phase microextraction (SPME) fiber and analyzed with GC-MS. The peak areas for the four products (6R,7S)-himachala-9,11-diene, trans-α-himachalene, (6R,7S)-2,2,6-trimethyl-10-methylenebicyclo[5.4.0]undec-1(11)-ene, and γ-cadinene were summed up to calculate the relative TPS activity. FPPS enzyme products were analyzed using LC-MS/MS. Means and SEs are shown (n = 3). A proposed pathway for the formation of sesquiterpenes from IPP and DMAPP in P. striolata is shown in C.
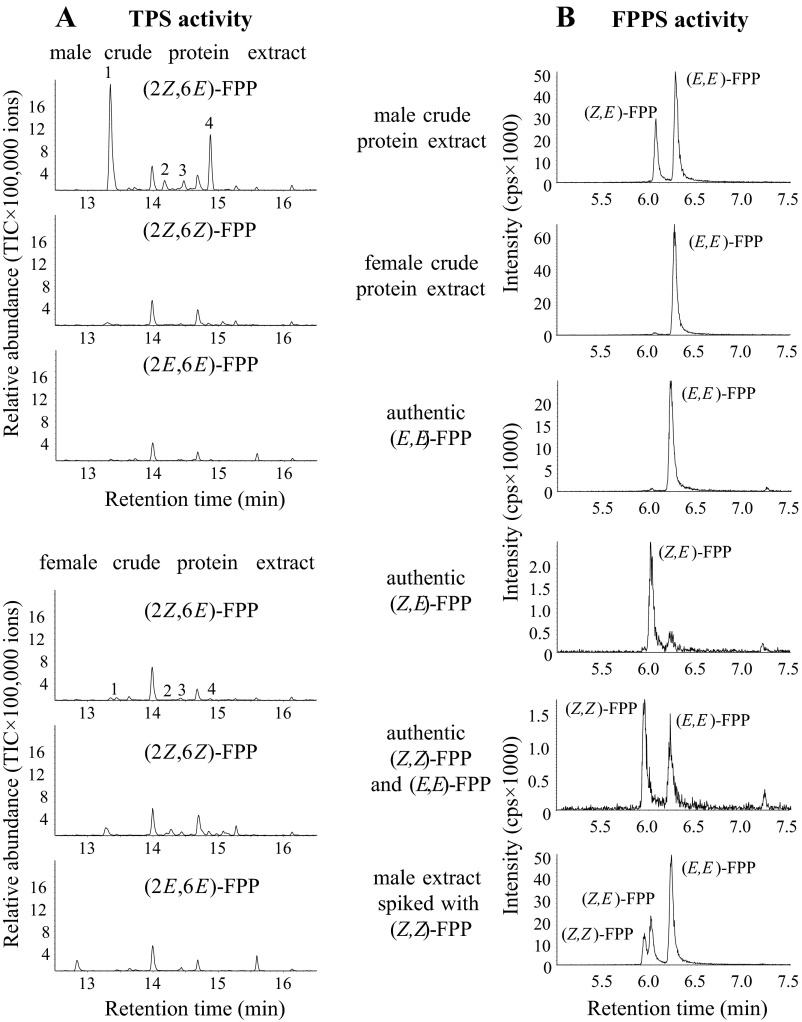
Fig. S1.
Sesquiterpene synthase (TPS) activity (A) and farnesyl diphosphate synthase (FPPS) activity (B) in crude protein extracts of male and female Phyllotreta striolata adults. Crude protein extracts were incubated with the substrates (2Z,6E)-FPP, (2Z,6Z)-FPP, and (2E,6E)-FPP (A), or IPP and DMAPP (B), respectively. (A) TPS enzyme products were collected using a SPME fiber and analyzed with GC-MS. 1, (6R,7S)-himachala-9,11-diene; 2, trans-α-himachalene; 3, (6R,7S)-2,2,6-trimethyl-10-methylenebicyclo[5.4.0]undec-1(11)-ene; 4, γ-cadinene. (B) FPPS enzyme products were analyzed using LC-MS/MS. Authentic standards were used to identify the different FPP isomers.
Identification and Functional Characterization of IDS-like Genes from P. striolata.
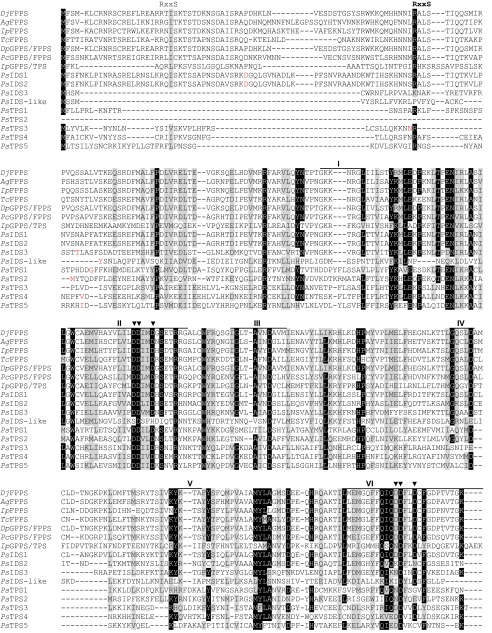
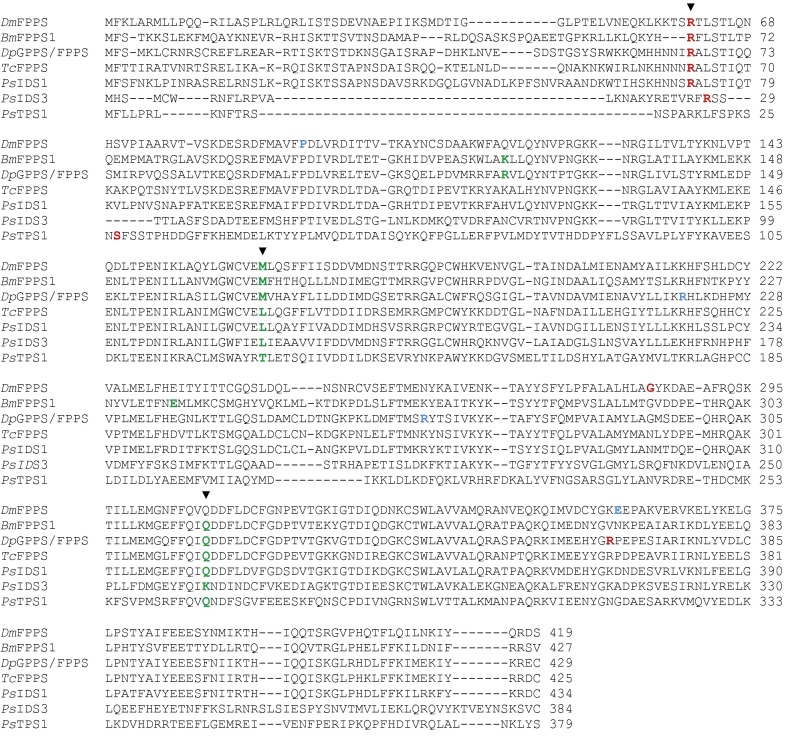
We searched for candidate genes encoding TPS enzymes in a P. striolata transcriptome database (27) based on amino acid sequence similarity, but no sequences homologous to known plant, fungal, or bacterial TPS were found. However, nine transcripts were predicted to encode trans-IDS enzymes (i.e., GPPS or FPPS). Full-length ORFs of these genes were obtained using rapid amplification of cDNA ends–PCR (RACE-PCR) (Dataset S1). An alignment of the corresponding amino acid sequences (Fig. S2) revealed considerable sequence divergence as amino acid identities ranged from 13.5% to 72.8%. The active site of trans-IDS enzymes generally contains two aspartate-rich motifs (DDxxD), which are critical for catalytic activity (28), but only three out of the nine proteins possessed both motifs. In five proteins, the second aspartate-rich motif was altered, and in one protein both motifs were modified (Fig. S2).
Fig. S2.
(Continued)
Fig. S2.
Amino acid sequence alignment of coleopteran trans-IDS, the GPPS/TPS from Ips pini, and IDS-like as well as TPS enzymes from P. striolata. The RxxS motif represents the putative mitochondrial cleavage site; I–VII indicate the conserved regions identified in trans-IDS. Two aspartate-rich motifs (DDxxD) in conserved regions II and VI are involved in catalysis and substrate binding. The first amino acid of the truncated heterologously expressed protein is shown in red. Ag, Anthonomus grandis; Dj, Dendroctonus jeffreyi; Dp, Dendroctonus ponderosae; Ip, Ips pini; Pc, Phaedon cochleariae; Ps, Phyllotreta striolata; Tc, Tribolium castaneum. The threshold for similarity/identity shading was set to 70%.
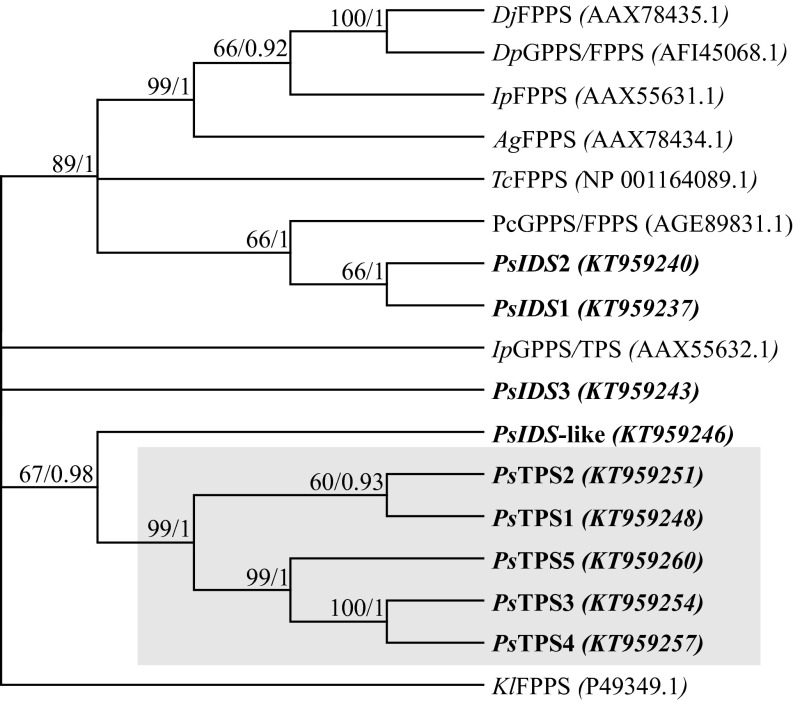
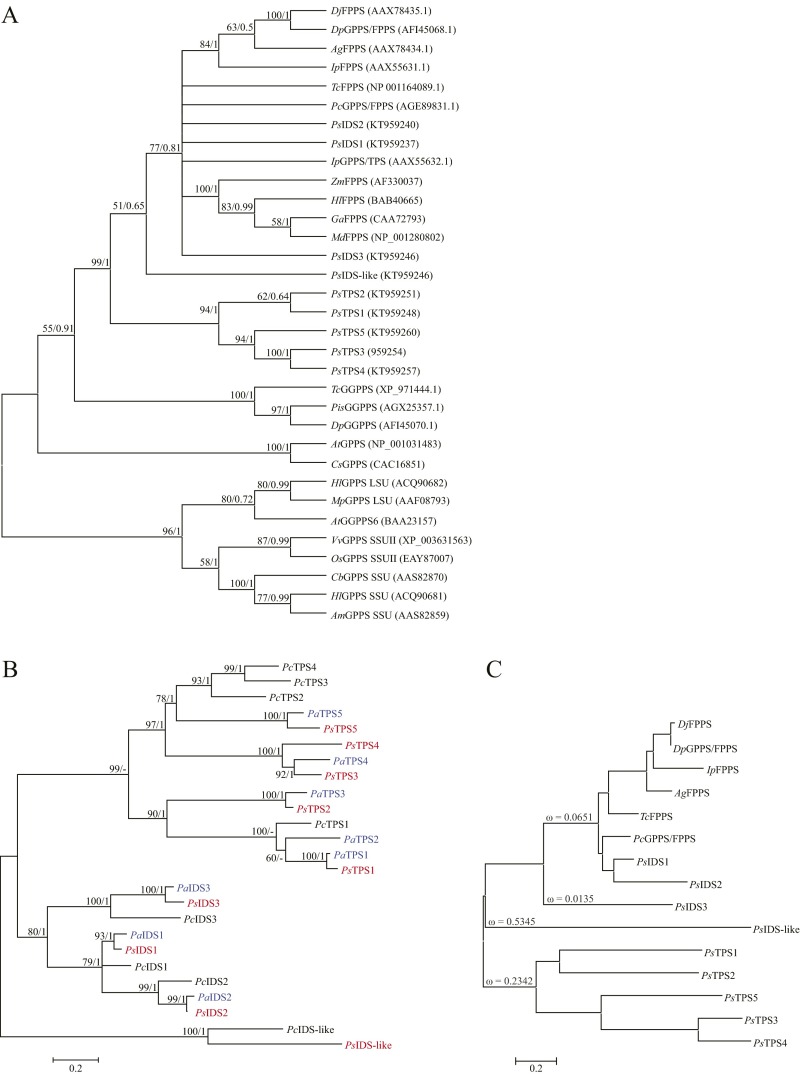
To determine the enzymatic activities of the P. striolata IDS-like gene products, the ORFs lacking the putative signal peptides (Fig. S2) were heterologously expressed as N-terminal His-tag fusions in Escherichia coli. Eight out of nine candidate genes were successfully expressed with this approach. Gene names were assigned according to the functional characterization of the corresponding recombinant proteins and the results of a phylogenetic analysis of P. striolata IDS-like enzymes with known trans-IDS from Coleoptera (Fig. 2).
Fig. 2.
Majority-rule cladogram inferred from maximum-likelihood analysis of IDS and TPS enzymes from P. striolata (shown in bold) along with other coleopteran trans-IDSs, and GPPS/TPS from Ips pini. The shaded box highlights the clade of evolutionarily novel TPS enzymes. The tree was rooted using a fungal FPPS from Kluyveromyces lactis (Kl). Bootstrap values (1,000 replicates) and posterior probability values from a Bayesian analysis using the same data set are shown next to each node. Sequences included in the analysis are as follows: Coleoptera: Ag, Anthonomus grandis; Dj, Dendroctonus jeffreyi; Dp, Dendroctonus ponderosae; Ip, Ips pini; Pc, Phaedon cochleariae; Ps, Phyllotreta striolata; Tc, Tribolium castaneum.
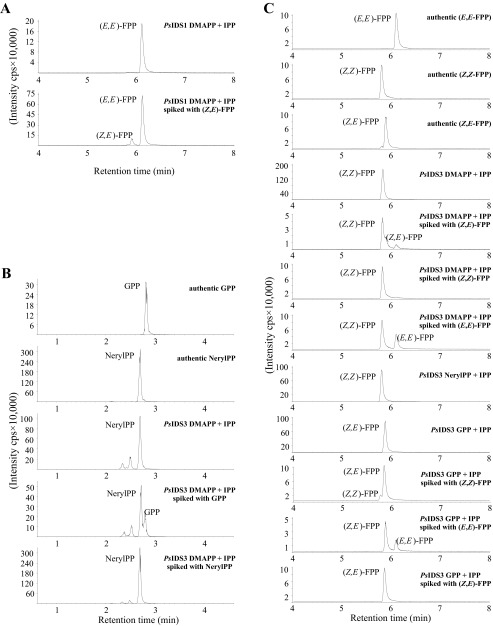
Interestingly, only two recombinant enzymes, PsIDS1 and PsIDS3, showed IDS activity in assays with DMAPP and IPP as substrates. PsIDS1 produced (E,E)-FPP (Fig. S3A), but PsIDS3, unprecedented for a trans-IDS, generated cis double bonds synthesizing neryl diphosphate [NerylPP = (Z)-GPP; Fig. S3B] as well as (Z,Z)-FPP (Fig. S4C). When (E)-GPP and IPP were provided as substrates, PsIDS3 synthesized (Z,E)-FPP (Fig. S3C).
Fig. S3.
Biochemical characterization of PsIDS1 (A) and PsIDS3 (B and C). The genes were overexpressed in Escherichia coli and purified recombinant proteins were incubated with potential isoprenyl diphosphate substrates in the presence of the cofactor magnesium. IDS enzyme products were analyzed using LC-MS/MS.
Fig. S4.
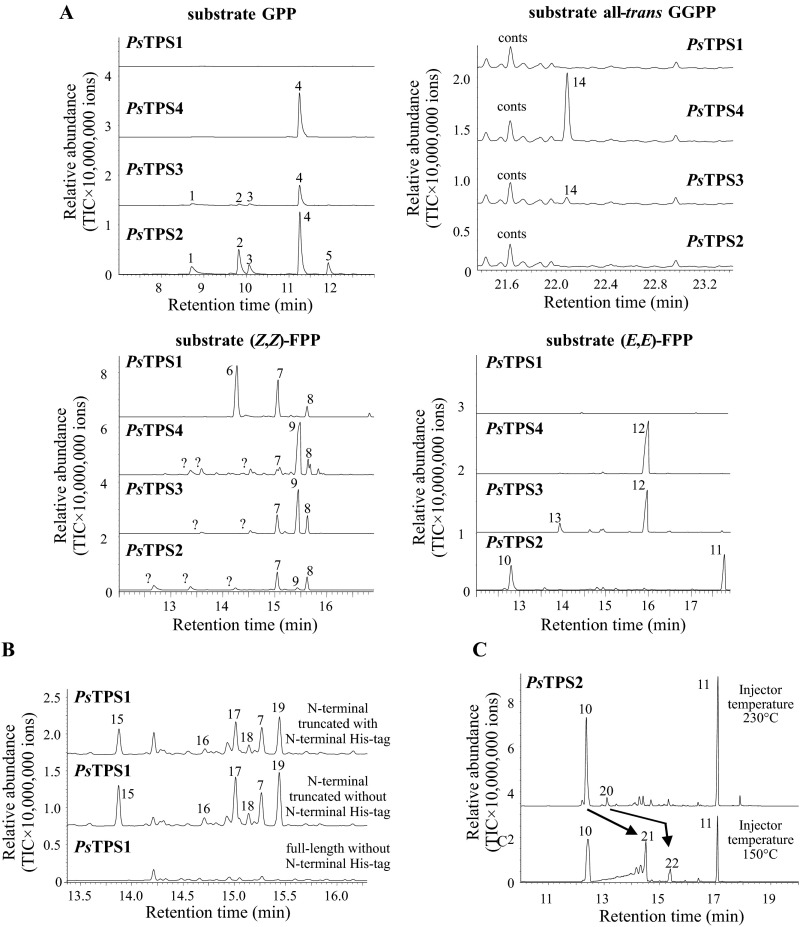
Biochemical characterization of PsTPSs. (A) Substrate and product specificity of PsTPS1-4. Purified recombinant proteins were incubated with the potential substrates (E)-GPP, (E,E)-FPP, (Z,Z)-FPP, or all-trans GGPP. Monoterpene and sesquiterpene products were collected using SPME and diterpene products were extracted with hexane. TPS products were analyzed with GC-MS. (B) The influence of the His-tag on enzyme activity of PsTPS1. Crude protein extracts of E. coli expressing either the N-terminal truncated PsTPS1 with and without an N-terminal His-tag or the full-length protein without His-tag were incubated with the substrate (Z,E)-FPP. Enzyme products were collected using SPME and analyzed with GC-MS. (C) Thermal rearrangement of PsTPS2 enzyme products during hot GC injection. Purified recombinant protein was incubated with the substrate (E,E)-FPP. Enzyme products were collected by SPME and analyzed with GC-MS using different injector temperatures. High-temperature injection resulted in the thermal rearrangement of germacrene A and germacrene B into β-elemene and γ-elemene, respectively. 1, myrcene; 2, (Z)-β-ocimene; 3, (E)-β-ocimene; 4, linalool; 5, unidentified monoterpene hydrocarbon; 6, unidentified sesquiterpene hydrocarbon; 7, β-bisabolene; 8, (E)-α-bisabolene; 9, (Z)-nerolidol; 10, β-elemene; 11, unidentified sesquiterpene alcohol; 12, (E)-nerolidol; 13, (E)-β-farnesene; 14, geranyllinalool; 15, (6R,7S)-himachala-9,11-diene; 16, trans-α-himachalene; 17, (6R,7S)-2,2,6-trimethyl-10-methylenebicyclo[5.4.0]undec-1(11)-ene; 18, (Z)-α-bisabolene; 19, γ-cadinene; 20, γ-elemene; 21, germacrene A; 22, germacrene B; conts, contaminations; ?, unidentified.
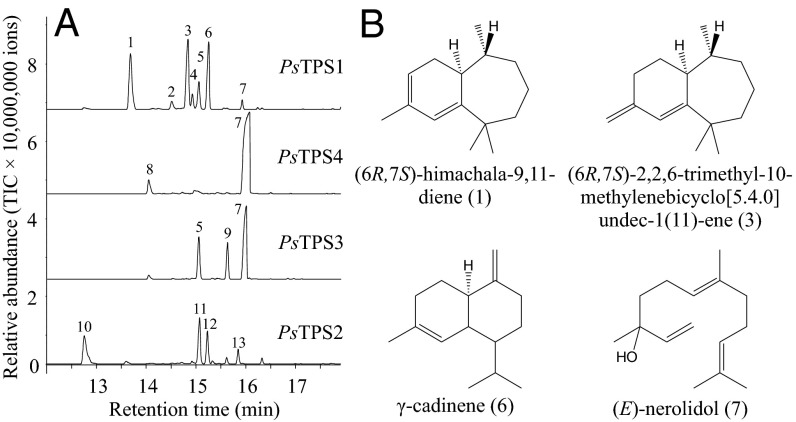
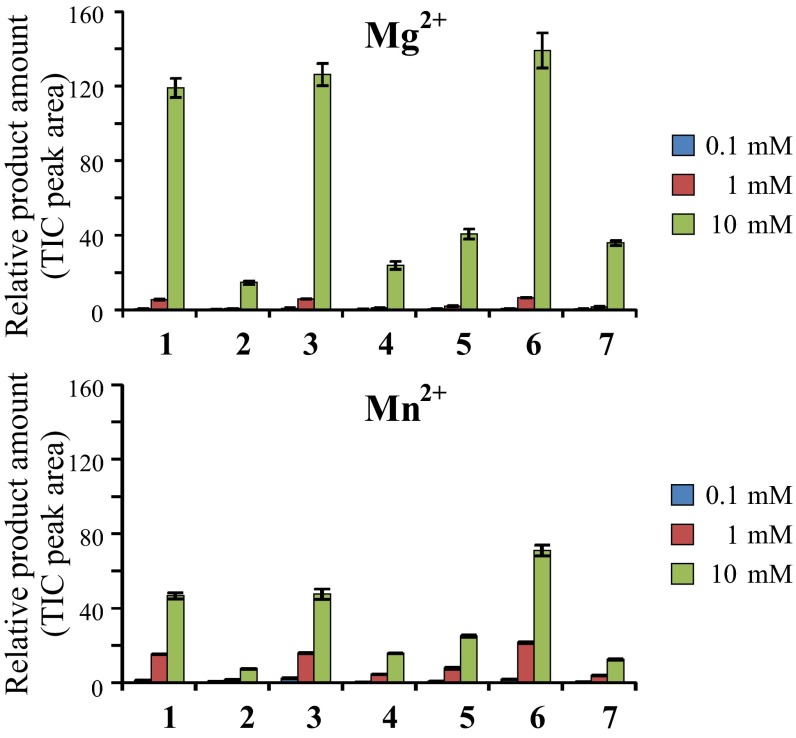
To analyze potential TPS activities of the P. striolata IDS-like proteins, assays were performed with the substrates (E)-GPP, (E,E)-FPP, (Z,E)-FPP, (Z,Z)-FPP, and (E,E,E)-GGPP. Although PsIDS1, PsIDS2, PsIDS3, and PsIDS-like showed no detectable activity with the tested potential substrates, recombinant PsTPS1, PsTPS2, PsTPS3, and PsTPS4 all demonstrated TPS activity. PsTPS1 converted (Z,E)-FPP into a mixture of sesquiterpenes with (6R,7S)-himachala-9,11-diene, trans-α-himachalene, (6R,7S)-2,2,6-trimethyl-10-methylenebicyclo[5.4.0]undec-1(11)-ene, and γ-cadinene being the predominant products (Fig. 3). The enzyme was also able to accept (Z,Z)-FPP as substrate, converting it into (R)-β-bisabolene and an unidentified sesquiterpene hydrocarbon (Fig. S4A). In contrast, enzyme assays with (E,E)-FPP, (E)-GPP, and (E,E,E)-GGPP revealed no detectable product formation for PsTPS1 (Fig. S4A). The N-terminal His-tag had no influence on PsTPS1 product specificity, but expression of the full-length PsTPS1 with its putative N-terminal signal peptide was not successful (Fig. S4B). Because divalent metal cofactors often influence the activity and product specificity of terpene synthases (29, 30), we tested PsTPS1 in the presence of different concentrations of magnesium and manganese. Although enzyme activity increased with higher magnesium and manganese ion concentrations, the product specificity of PsTPS1 was not influenced by these metal cofactors (Fig. S5).
Fig. 3.
Biochemical characterization of TPS enzymes from Phyllotreta striolata. (A) Genes were overexpressed in Escherichia coli, and purified recombinant proteins were incubated with (Z,E)-FPP. Enzyme products were collected using SPME and analyzed with GC-MS. 1, (6R,7S)-himachala-9,11-diene; 2, trans-α-himachalene; 3, (6R,7S)-2,2,6-trimethyl-10-methylenebicyclo[5.4.0]undec-1(11)-ene; 4, (Z)-α-bisabolene; 5, β-bisabolene; 6, γ-cadinene; 7, (E)-nerolidol; 8, (E)-β-farnesene; 9, (E)-α-bisabolene; 10, unidentified sesquiterpene hydrocarbon; 11–13, unidentified sesquiterpene hydrocarbons. (B) Structures of major PsTPS products.
Fig. S5.
Cofactor dependency of PsTPS1. Purified recombinant protein was incubated with the substrate (Z,E)-FPP in the presence of different divalent metal ions. Enzyme assays were overlaid with hexane. After incubation, enzyme products were extracted and analyzed using GC-MS. 1, (6R,7S)-himachala-9,11-diene; 2, trans-α-himachalene; 3, (6R,7S)-2,2,6-trimethyl-10-methylenebicyclo[5.4.0]undec-1(11)-ene; 4, (Z)-α-bisabolene; 5, β-bisabolene; 6, γ-cadinene; 7, unidentified sesquiterpene alcohol.
Like PsTPS1, PsTPS2 also showed sesquiterpene synthase activity. (E,E)-FPP was converted into β-elemene, a thermal rearrangement product formed from germacrene A upon injection in the heated inlet of the GC, and an unidentified sesquiterpene alcohol (Fig. S4C). Catalysis of (Z,E)-FPP and (Z,Z)-FPP resulted in complex sesquiterpene mixtures consisting mainly of unidentified compounds (Fig. 3 and Fig. S4). In contrast to PsTPS1, PsTPS2 also accepted (E)-GPP as substrate and produced several monoterpenes with linalool being the predominant one (Fig. S4A). Diterpene formation from (E,E,E)-GGPP could not be observed for PsTPS2.
PsTPS3 and PsTPS4 accepted all tested prenyl diphosphates as substrates and produced mainly acyclic alcohols resulting from the initial ionization of the substrate and a subsequent capture of the carbocation formed by addition of a water molecule. (E)-GPP was converted into linalool, (E,E)-FPP and (Z,E)-FPP were converted into (E)-nerolidol, (Z,Z)-FPP was converted into (Z)-nerolidol, and (E,E,E)-GGPP was converted into geranyllinalool (Fig. 3 and Fig. S4A).
Two recombinant proteins, PsIDS2 and PsIDS-like, showed neither IDS, nor TPS activity under our assay conditions. However, the aspartate-rich motifs required for catalysis were modified in both proteins (Fig. S2), which might explain the lack of enzyme activity with these substrates.
Enzyme activity of the putative terpene synthase PsTPS5 was not tested because this protein could be expressed neither in E. coli nor in insect cells (SI Materials and Methods).
PsTPS1 Is Involved in Aggregation Pheromone Biosynthesis.
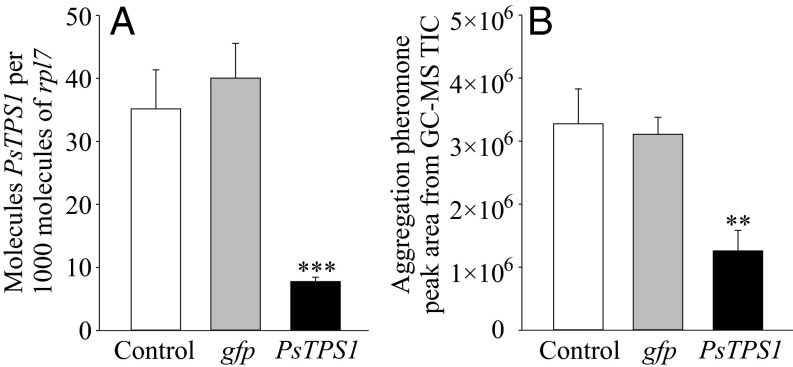
Because recombinant PsTPS1 converted (Z,E)-FPP to the major component of the male-produced aggregation pheromone, (6R,7S)-himachala-9,11-diene, as observed in P. striolata crude protein extracts, we used RNAi to examine the role of PsTPS1 in aggregation pheromone biosynthesis in vivo. Males that were injected with PsTPS1-derived dsRNA showed significantly reduced PsTPS1 transcript abundance after 11 d compared with gfp-injected or noninjected adults (by >77%; P < 0.001; Fig. 4A). Correspondingly, PsTPS1-injected males also emitted significantly fewer male-specific sesquiterpenes compared with controls (by >56%; P < 0.01; Fig. 4B), confirming a significant role of PsTPS1 in aggregation pheromone biosynthesis.
Fig. 4.
Knockdown of PsTPS1 in male P. striolata adults by RNAi. (A) PsTPS1 mRNA expression levels in P. striolata males 11 d after dsRNA injection of PsTPS1 or gfp and in uninjected adults (control). Copy number estimates are given per 1,000 copies of mRNA for the reference gene rpl7 (n = 6, +SEM). (B) Aggregation pheromone emission of control, gfp-, and PsTPS1 dsRNA-injected male P. striolata. The GC-MS peak areas of four male-specific sesquiterpenes, (6R,7S)-himachala-9,11-diene, trans-α-himachalene, γ-cadinene, and ar-himachalene were summed up (n = 6, +SEM). **P < 0.01; ***P < 0.001.
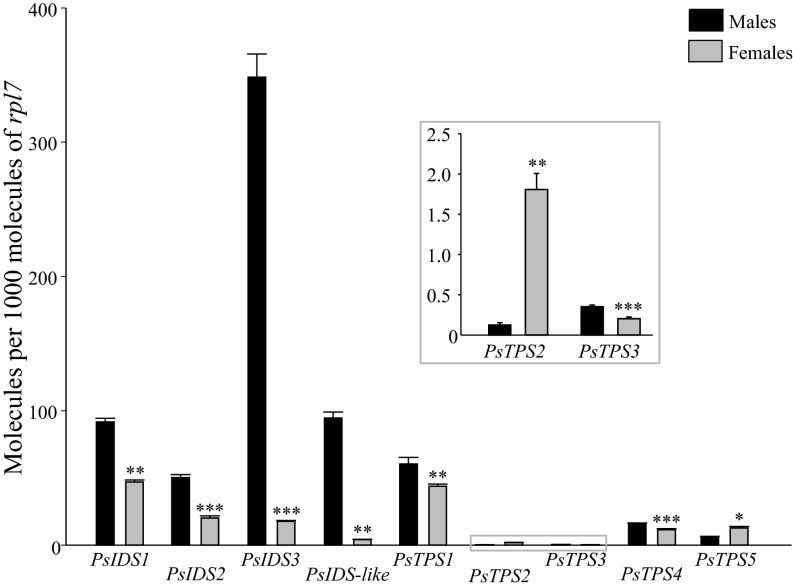
Transcript Levels of PsIDS, PsIDS-like, and PsTPS Genes in Male and Female P. striolata.
Expression levels of PsIDS, PsIDS-like, and PsTPS genes in male and female P. striolata were compared by quantitative RT-PCR (qRT-PCR). Most genes, including PsIDS1, PsIDS3, and PsTPS1, were significantly more expressed in males compared with females (Fig. 5). However, with PsTPS1 transcript abundance in females corresponding to 72.3% of that in males, expression levels were similar in both sexes. Expression of PsIDS3, on the other hand, was about 20 times higher in males than in females.
Fig. 5.
mRNA expression levels of IDS, IDS-like, and TPS genes in male and female P. striolata as determined by RT-qPCR. Copy number estimates are given per 1,000 copies of mRNA for reference gene rpl7 (n = 5, +SEM). *P < 0.05; **P < 0.01; ***P < 0.001.
Evolution of P. striolata TPS and cis-IDS Genes.
The evolutionary relationship between the nine P. striolata trans–IDS-like enzymes and trans-IDS from Coleoptera as well as the GPPS/TPS enzyme from I. pini was inferred in a maximum-likelihood analysis. PsIDS1 and PsIDS2 clustered together with a GPPS/FPPS from the leaf beetle Phaedon cochleariae in a clade with all known coleopteran trans-IDS enzymes included in the dataset (Fig. 2). The four TPS enzymes formed a separate clade including PsTPS5 (not expressed), which was supported by high bootstrap and posterior probability values (99/1). PsIDS3 as well as the I. pini GPPS/TPS were separated from both clades (Fig. 2). The evolutionary origin of the P. striolata cis-IDS and TPS enzymes from trans-IDS was confirmed in a broader phylogenetic analysis including coleopteran GGPPS as well as trans-IDS enzymes from plants. All P. striolata enzymes clustered with insect trans-IDS and plant FPPS, and were separated from plant GPPS and plant and insect GGPPS (Fig. S6A).
Fig. S6.
Phylogenetic tree of (A) plant and insect GPPS, FPPS, and GGPPS, and P. striolata IDS-like and TPS enzymes, and (B) IDS, IDS-like, and TPS enzymes from P. striolata (Ps), P. armoraciae (Pa), and P. chrysocephala (Pc), inferred by maximum-likelihood analysis. Bootstrap values (1,000 replicates) and posterior probability values from a Bayesian analysis using the same datasets are shown next to each node (−, not supported in the Bayesian phylogeny). (C) The result of a selection analysis is shown on a maximum-likelihood analysis of coleopteran trans-IDS and P. striolata IDS-like and TPS sequences. All clades and genes are under purifying selection. Insects: Ag, Anthonomus grandis; Dj, Dendroctonus jeffreyi; Dp, Dendroctonus ponderosae; Ip, Ips pini; Pc, Phaedon cochleariae; Pis, Pissodes strobi; Tc, Tribolium castaneum. Plants: Am, Antirrhinum majus; At, Arabidopsis thaliana; Cb, Clarkia breweri; Cs, Citrus sinensis; Ga, Gossypium arboretum; Hl, Humulus lupulus; Md, Malus domestica; Mp, Mentha x piperita; Os, Oryza sativa; Vv, Vitis vinifera; Zm, Zea mays. LSU, large subunit; SSU, small subunit.
To investigate the evolution of the IDS/TPS gene family in the leaf beetle subfamily Galerucinae, we identified putative members in the transcriptomes of two related species, the horseradish flea beetle, Phyllotreta armoraciae, and the cabbage stem flea beetle, Psylliodes chrysocephala. From both beetle species, eight transcripts were obtained as full-length sequences using RACE-PCR, except for one partial sequence from P. chrysocephala (PcTPS3). The corresponding amino acid sequences were aligned with PsIDS, PsIDS-like, PsTPS sequences to conduct a phylogenetic analysis (Fig. S6B). The resulting phylogenetic tree revealed three putative IDS enzymes in P. armoraciae as well as in P. chrysocephala clustering with PsIDS1, PsIDS2, and PsIDS3 according to the species phylogeny, suggesting that they are orthologs. On the other hand, we found species-specific sequence diversification in the TPS clade. For example, two putative TPS from P. armoraciae, PaTPS1 and PaTPS2, clustered together with PsTPS1 and PcTPS1 (Fig. S6B).
We analyzed whether different selection pressures act on the ancestral coleopteran trans-IDS (GPPS/FPPS) clade compared with the PsTPS clade, PsIDS3, and PsIDS-like, respectively. The result indicates different strengths of purifying selection acting on the different branches/genes (Fig. S6C). The trans-IDS clade representing the ancestral function and the cis-IDS PsIDS3 are under strong purifying selection (ω ≤ 0.065), whereas the PsTPS clade, and PsIDS-like, which showed neither IDS nor TPS activity in our experiments, appear to be under more relaxed constraints (ω = 0.234 and ω = 0.5345, respectively; Fig. S6C).
To further test the hypothesis that PsTPS and PsIDS3 genes evolved from insect trans-IDS genes, we analyzed the exon–intron structures of PsIDS1, PsIDS3, and PsTPS1, and compared them to the structures of coleopteran (Tribolium castaneum and Dendroctonus ponderosae), dipteran (Drosophila melanogaster), and lepidopteran (Bombyx mori) trans-IDS genes. The number of introns per gene ranged from three introns present in genes of P. striolata and T. castaneum to seven introns in the GPPS/FPPS gene of D. ponderosae (8) (Fig. S7). The positions and corresponding intron phases of the three introns found in P. striolata genes were conserved in all analyzed insect IDS genes, except for the third conserved intron, which was not present in the putative FPPS gene from D. melanogaster but is otherwise conserved. We assume that the poor alignment of the first intron position in PsIDS3 and PsTPS1 is caused by an inaccurate sequence correspondence in the N-terminal region due to extensive sequence diversification.
Fig. S7.
Amino acid sequence alignment of insect trans-IDS enzymes and PsTPS1 showing intron positions in the corresponding genes. The intron positions in three representatives from the P. striolata IDS/TPS gene family are compared with intron positions in trans-IDS genes from Drosophila melanogaster (Dm), Bombyx mori (Bm), Dendroctonus ponderosae (Dp), and Tribolium castaneum (Tc). Intron phases are highlighted in blue (phase 0), red (phase 1), and green (phase 2), respectively. Black arrowheads above the alignment indicate three conserved introns present in Coleoptera.
SI Materials and Methods
Plants and Insects.
Seeds of Brassica juncea cv. “Bau Sin” or Brassica rapa cv. “Yo Tsai Ching” were purchased from Known-You Seed Company. Field-collected Phyllotreta striolata adults were provided from the Entomology Unit of AVRDC–The World Vegetable Center in Shanhua, Taiwan. The import authorization for Brassica seeds and P. striolata adults to Germany was obtained under Directive 2008/61/EC. Beetles were reared on potted 3- to 5-wk-old B. juncea or B. rapa plants in a controlled environment chamber (24 °C, 65% relative humidity, 14-h/10-h light/dark period).
In September and October 2012, Phyllotreta armoraciae and Psylliodes chrysocephala adults were collected from host plants (Armoraciae rusticana and Brassica rapa subsp. rapa plants, respectively) in Laasdorf, Thuringia, Germany. Laboratory rearings of P. armoraciae and P. chrysocephala were established on potted B. juncea and B. rapa plants, respectively, in a controlled environment chamber (conditions as described above).
Foliage of Abies nordmanniana for volatile collections was provided by the botanical garden in Jena, Germany.
RNASeq and de Novo Transcriptome Assembly.
The preparation of the P. striolata transcriptome was described previously (27). RNASeq was performed with adult P. armoraciae and Psylliodes chrysocephala flea beetles. Total RNA was extracted from whole adults as well as from adults dissected into gut tissue and remaining body tissues using the InnuPREP RNA Mini Kit (Analytik Jena). An additional sample was prepared from P. chrysocephala beetles treated with a mixture of Gram-positive and Gram-negative bacteria and fungi for 48 h to induce immune responses. RNA integrity was verified using an Agilent 2100 Bioanalyzer with the RNA 6000 Nano Kit (Agilent Technologies), and RNA quantity was determined using a Nanodrop ND-1000 spectrophotometer. RNASeq was performed on the HiSeq 2500 Sequencing System from Illumina (www.illumina.com/) using paired end (2 × 100 bp) read technology with RNA fragmented to an average of 150 nt. Library construction and sequencing was performed by the Max Planck Genome Center (Cologne, Germany) (mpgc.mpipz.mpg.de/home/). Illumina sequencing resulted in a total of ∼25 million reads for each of the three P. armoraciae and four P. chrysocephala tissue and treatment samples, respectively. Quality control including filtering of high-quality reads based on the score value given in “fastq” files, and trimming of read length was done using the CLC Genomics Workbench software, version 7.0.1 (www.clcbio.com). After these initial filtering steps, 70 million total sequence reads were used for each species for a de novo transcriptome assembly using the CLC Genomics Workbench software by comparing an assembly with standard settings and two additional CLC-based assemblies as described in more detail previously (27). Scaffolding was selected and conflicts among individual bases were resolved in all assemblies by voting for the base with the highest frequency. Contigs shorter than 250 bp were removed from the final analysis.
Annotation.
Annotation of the consensus transcriptome used BLAST searches conducted on a local server using the National Center for Biotechnology Information blastall program. Homology searches (BLASTx and BLASTn) of unique sequences and functional annotation by gene ontology terms (GO) terms (geneontology.org/), InterPro terms (InterProScan, EBI), enzyme classification codes (EC), and metabolic pathways (KEGG, Kyoto Encyclopedia of Genes and Genomes) were determined using the BLAST2GO PRO software suite v2.6.1 (https://www.blast2go.com/) as described in ref. 27.
Protein Extraction from P. striolata.
To establish the presence of farnesyl diphosphate synthase (FPPS) and sesquiterpene synthase activity in P. striolata, crude protein extracts were prepared separately from male and female adults with three biological replicates for each sex. Beetles were cold anesthetized by placing them for 2 min at −20 °C. For each sample, 40 adults were pooled (30–40 mg fresh weight) and homogenized in 350 µL of extraction buffer [25 mM 3-(N-morpholino)-2-hydroxypropanesulfonic acid (MOPSO) (pH 7.2), 10% (vol/vol) glycerol] supplemented with protease inhibitors (cOmplete EDTA-free; Roche) using a 2-mL potter tissue grinder. After an initial centrifugation step at 4 °C for 10 min at 16,000 × g, the supernatant was ultracentrifuged at 4 °C for 45 min at 100,000 × g. The protein concentration in each sample was determined using the Bradford protein assay (Bio-Rad) according to the manufacturer’s instructions.
Identification of Putative trans-IDS Genes from Flea Beetles.
Several putative trans-IDS genes were identified in transcriptomes of P. striolata (nine transcripts), P. armoraciae (eight transcripts), and P. chrysocephala (eight transcripts) based on homology to known insect trans-IDS enzymes. Total RNA isolation and cDNA synthesis was carried out as described by Beran et al. (27). Because most transcripts in the database were truncated, we performed rapid amplification of cDNA ends–PCR (RACE-PCR) using the Advantage 2 Polymerase mix according to the manufacturer’s protocols (Clontech) to obtain the complete ORFs (primer sequences are listed in Dataset S1). RACE-PCR products were cloned into the TOPO TA 2.1 vector (Invitrogen) and sequenced. The manually curated sequences have been deposited in the GenBank database under accession numbers KT959237–KT959261.
Cloning and Expression of Putative trans-IDS Genes from P. striolata.
The putative trans-IDSs from P. striolata were amplified from cDNAs by PCR (Phusion High-Fidelity DNA Polymerase; Finzymes), using gene-specific primers (primer sequences listed in Dataset S1). The primers were designed to amplify a 5′-truncated sequence (Fig. S2) including the native stop codon, except for PsTPS2, which was amplified full-length. Eukaryotic signal peptides may affect protein expression in E. coli and the activity of the recombinant enzymes as previously shown for IDSs (10). The PCR products were ligated into the expression vector pET100/D-TOPO or pET200/D-TOPO (Invitrogen). Both vectors encode an N-terminal His-tag and only differ in the selection marker (ampicillin and kanamycin, respectively). Full-length and 5′-truncated PsTPS1 including the native stop codon were additionally cloned into the pET101/D-TOPO expression vector for heterologous expression without His-tag. Constructs were checked by sequencing and transformed in Escherichia coli strain BL21(DE3)pLysS (Life Technologies) for heterologous gene expression. A 20-mL preculture was inoculated from a single colony and incubated at 18 °C and 220 rpm using a Certomat IS orbital shaker (Sartorius) overnight. The Overnight Express Autoinduction System 1 (Novagen) was used for the 200-mL main culture. To start the protein production, the main culture was inoculated with the preculture to an OD600 of 0.05 and then cultivated 3 d at 18 °C and 220 rpm on a Certomat IS orbital shaker (Sartorius). Afterward, the cells were harvested by centrifugation at 4 °C for 10 min at 4,000 × g. The pellets were resuspended in 5 mL of extraction buffer [50 mM Tris·HCl, 100 mM NaCl, 10% (vol/vol) glycerol, 10 mM MgCl2, 2 mM DTT, 20 mM imidazol, 10% (vol/vol) glycerol (pH 7.4)] supplemented with 0.3 mg/mL lysozyme (AppliChem), 2.5 U/mL benzonase (Novagen), and proteinase inhibitors (Protease Inhibitor Mix HP; SERVA) and incubated at 4 °C for 30 min on a laboratory shaker. Afterward, cells were disrupted by sonication using a sonotrode (40% amplitude, 5-s pulse, 10-s pause, 100-s overall pulse duration). The lysate was centrifuged at 4 °C for 30 min at 15,000 × g to obtain the soluble fraction. The heterologously expressed protein was purified via the His-tag using 1 mL of HisPur Ni-NTA Resin (Life Technologies) according to the manufacturer’s instructions. After washing of the column with extraction buffer, protein was eluted with 1 mL of elution buffer [50 mM Tris·HCl, 100 mM NaCl, 10% (vol/vol) glycerol, 10 mM MgCl2, 2 mM DTT, 400 mM imidazol (pH 7.5)]. For enzymatic assays, the buffer was exchanged to 25 mM MOPSO, 10% (vol/vol) glycerol, 1 mM DTT, 10 mM MgCl2 (pH 7.2) using PD-10 Desalting Columns (GE Healthcare Life Sciences).
PsTPS5 could not be expressed successfully in E. coli, neither when it was cloned into the high-copy expression vector pET100/D-TOPO nor cloned into the low-copy expression vector pASK-IBA37plus (IBA GmbH). Attempts to express this gene in insect cells (Sf9 and High Five cell lines) after cloning into the pIB/V5-His TOPO TA vector (Invitrogen) failed as well.
Western Blots.
Heterologously expressed proteins were detected in Western blots using a rabbit anti–6-His antibody (1:10,000; Bethyl), followed by horseradish peroxidase-linked anti-rabbit IgG (1:10,000; GE Healthcare Life Sciences) as a secondary antibody.
IDS Activity Assay and Analysis.
Enzyme assays with crude protein extracts of males and females were carried out using 160 µg of total protein (50 µL) in 200 µL of assay buffer [25 mM MOPSO buffer (pH 7.2), 10% (vol/vol) glycerol, 10 mM MgCl2] and 50 µM isopentenyl diphosphate (IPP) (Sigma) and 50 µM dimethylallyl diphosphate (DMAPP) (Sigma) at 30 °C for 2 h. Heterologously expressed proteins (50 µL) were incubated with 50 µM IPP or 50 µM neryl diphosphate (NerylPP) together with 50 µM DMAPP as described above to determine IDS activity. Quantification was done using an Agilent 1260 HPLC system (Agilent Technologies) coupled to an API 5000 triple-quadrupole mass spectrometer (AB Sciex Instruments). For separation, a ZORBAX Extended C-18 column (1.8 μm, 50 mm × 4.6 mm; Agilent Technologies) was used. The mobile phase consisted of 5 mM ammonium bicarbonate in water as solvent A and acetonitrile as solvent B, with the flow rate set at 1.2 mL/min and the column temperature kept at 20 °C. Separation was achieved by using a gradient starting at 5% (vol/vol) B, increasing to 47% (vol/vol) B in 9 min and 100% (vol/vol) B in 0.1 min (0.9-min hold), followed by a change to 5% (vol/vol) B in 0.1 min (2.9-min hold) before the next injection. The injection volume for samples and standards was 2 μL; autosampler temperature was 4 °C. The mass spectrometer was used in the negative electrospray ionization mode. Optimal settings were determined using standards. Levels of ion source gases 1 and 2 were set at 60 and 70 psi, respectively, with a temperature of 700 °C. Curtain gas was set at 30 psi and collision gas was set at 7 psi, with all gases being nitrogen. Ion spray voltage was maintained at −4,200 V. Multiple-reaction monitoring was used to monitor analyte parent ion-to-product ion formation: m/z 312.9/79 for (E)-GPP/NPP, m/z 380.9/79 for all FPP isomers, and m/z 449/79 for GGPP. Data analysis was performed using Analyst Software 1.6 Build 3773 (AB Sciex).
Terpene Synthase Activity Assays.
Sesquiterpene synthase activity in crude protein extracts was determined with enzyme assays consisting of 50 µL of protein extract and 50 µL of assay buffer [10 mM Tris·HCl, 10% (vol/vol) glycerol, 1 mM DTT (pH 7) with 10 mM MgCl2] and 10 µM (E,E)-FPP (Sigma), (Z,Z)-FPP (Echelon), or (Z,E)-FPP as substrate. (Z,E)-FPP was synthesized according to method of Cane et al. (44) and was kindly provided by Nathalie Gatto and Wilhelm Boland, Max Planck Institute for Chemical Ecology, Jena, Germany. Heterologously expressed TPS enzymes (50 µL) were incubated with geranyl diphosphate [(E)-GPP] (Sigma), all available FPP substrates, as well as geranylgeranyl diphosphate [(E,E,E)-GGPP] (Sigma) as described above. Assays were performed in 1-mL glass vials, and enzyme products were collected using solid-phase microextraction (SPME). After penetrating the PTFE-lined silicone septum of the cap with a SPME fiber holder, a SPME fiber coated with 100-µm polydimethylsiloxane (Supelco) was exposed to the headspace in the vial for 2 h at 30 °C. Afterward, the SPME fiber was directly inserted into the inlet of a gas chromatograph. For collection and analysis of (E,E,E)-GGPP–derived diterpene products, assays were overlaid with 100 µL of hexane and incubated for 2 h at 30 °C. After mixing for 60 s, 1 µL of the organic phase was removed and directly injected into a gas chromatograph.
For analyzing the influence of different divalent metal cofactors on PsTPS1 activity and product specificity, enzyme assays with (Z,E)-FPP as substrate and three different concentrations (0.1, 1, and 10 mM) of each MgCl2 and MnCl2 were performed as described above. Assays were overlaid with 100 µL of hexane and incubated for 2.5 h at 30 °C. After mixing for 60 s, 1 µL of the organic phase was removed and directly injected into a gas chromatograph.
Gas Chromatography–Mass Spectrometry.
Assay products were analyzed using an Agilent 6890 N gas chromatograph (GC) equipped with a ZB-5MSi capillary column (30 m × 0.25 mm i.d., 0.25-μm film thickness; Phenomenex) coupled to a quadrupole mass spectrometer (Agilent 5973). The carrier gas was helium at a constant flow rate (1 mL/min). The inlet temperature was set to 240 °C for simultaneous conditioning of the SPME fiber. The oven program started at 60 °C for 3 min, increased at 7 °C/min to 240 °C, and then with 100 °C/min to 300 °C held for 1 min. MS conditions were electron impact mode (70 eV), and scan mode of 41–360 atomic mass units. Assay products were identified based on comparison of mass spectra and retention times to the National Institute of Standards mass spectral library and authentic reference compounds. The configuration of himachala-9,11-diene, trans-α-himachalene, (6R,7S)-2,2,6-trimethyl-10-methylenebicyclo[5.4.0]undec-1(11)-ene, and bisabolene were ascertained via GC with a chiral stationary phase (Cyclosil-B column; 30 m × 0.25 mm i.d. with 0.25-μm film thickness; Agilent) and comparison of mass spectra and retention times to samples of known chemical composition. The oven program started at 60 °C for 2 min, increased at 10 °C/min to 100 °C held for 2 min, then with 2 °C/min to 160 °C, and finally with 20 °C to 230° held for 5 min. The carrier gas was helium at a constant flow rate (1 mL/min). MS conditions were electron impact mode (70 eV), and scan mode of 33–250 atomic mass units.
Himachala-9,11-diene produced by recombinant PsTPS1 was compared with himachala-9,11-diene present in volatile collection of P. striolata and A. nordmanniana (23). The PsTPS1 products α-himachalene and 2,2,6-trimethyl-10-methylenebicyclo[5.4.0]undec-1(11)-ene had the same retention time as trans-α-himachalene and (6R,7S)-2,2,6-trimethyl-10-methylenebicyclo[5.4.0]undec-1(11)-ene isolated and purified from the leaf beetle Aphthona flava (provided by Dr. Allard Cossé, US Department of Agriculture–Agricultural Research Service, Peoria, IL) when separated by chiral stationary-phase GC (21, 42). The stereochemistry of β-bisabolene produced by PsTPS1 was identified by comparison with a racemic mixture of (S)- and (R)-β-bisabolene, which was prepared by dehydration of racemic α-bisabolol at 140 °C with acidic aluminum oxide as catalyst. The β-bisabolene enantiomers were identified by comparison with the bergamot (Citrus bergamia) essential oil, which contains only the (S)-enantiomer. According to the result of the chiral stationary-phase GC (isotherm at 110 °C), PsTPS1 produced (R)-β-bisabolene.
Phylogenetic Analyses.
Protein sequences from insect IDS were retrieved from publicly available databases, and aligned with the protein sequences of P. striolata IDS, IDS-like, and TPS enyzmes using MAFFT with default settings (version 7; mafft.cbrc.jp/alignment/server/; L-INS-I algorithm). Poorly aligned regions were removed from the alignment using GBlocks (version 0.91b) with parameters set as follows: minimum length of a block after cleaning: 5; minimum number of sequences for a conserved position and for a flank position: 14; maximum number of contiguous nonconserved positions: 8; allowed gap positions: one-half. The maximum-likelihood tree was reconstructed in MEGA 6.06 using the WAG+G model with pairwise deletion of gaps, and tested with 1,000 bootstrap replicates. A Bayesian phylogenetic analysis consisting of four Markov chains with 10,000,000 generations that were sampled every 100 generations was carried out in MrBayes, version 3.2.1. The first 25% of saved trees were discarded.
We performed a second broader phylogenetic analysis by complementing the above-mentioned dataset with insect GGPPS as well as plant GPPS, FPPS, and GGPPS. The protein sequences were aligned using MAFFT with default settings (version 7; mafft.cbrc.jp/alignment/server/; L-INS-I algorithm). The alignment was curated manually and then used for a maximum-likelihood analysis in MEGA 6.06 using the WAG+G model with pairwise deletion of gaps, and tested with 1,000 bootstrap replicates. A Bayesian phylogenetic analysis was performed with the same dataset as described above.
In addition, we analyzed the phylogenetic relationship of members of the IDS/IDS-like/TPS gene family within the subfamily Galerucinae. The deduced protein sequences of putative IDS and TPS from P. armoraciae (eight full-length sequences) and P. chrysocephala (seven full-length sequences and one partial sequence) were aligned with the corresponding sequences from P. striolata using MAFFT with default settings (version 7, mafft.cbrc.jp/alignment/server/; L-INS-I algorithm). Poorly aligned regions were eliminated from the alignment using GBlocks (version 0.91b) with parameters set as follows: minimum length of a block after cleaning: 5; minimum number of sequences for a conserved position and for a flank position: 13; maximum number of contiguous nonconserved positions: 8; allowed gap positions: one-half. The maximum-likelihood tree was reconstructed in MEGA 6.06 using the JTT+G model with pairwise deletion of gaps, and tested with 1,000 bootstrap replicates. A Bayesian phylogenetic analysis of the same dataset was carried out as described above.
Exon–Intron Structure of Insect trans-IDS and P. striolata IDS and TPS Genes.
Genomic DNA was extracted from P. striolata adults using hexadecyltrimethylammonium bromide (CTAB) (protocol modified from ref. 45). PsIDS1, PsIDS3, and PsTPS1 were amplified from genomic DNA using TaKaRa LA Taq polymerase (Clontech) according to the manufacturer’s protocol and cloned into the TOPO TA 2.1 vector. Intron positions in PsIDS1 (genomic DNA sequence, about 10 kb) were determined by using primers flanking the introns for sequencing. PsIDS3 and PsTPS1 were sequenced completely (1,460, 1,947 bp, respectively). Sequences of primers used for sequencing are listed in Dataset S1. For comparison, genomic and mRNA sequences of trans-IDS genes from Drosophila melanogaster (genomic DNA scaffold accession no. AE013599.5; mRNA accession no. NM_058032.5), Bombyx mori (FPPS1; NW_004582035.1; NM_001043424.1), Tribolium castaneum (NC_007422.4; NM_001170618.1), and Dendroctonus ponderosae (KB741247.1; JQ855705.1) were retrieved from publicly available databases. Genomic and mRNA sequences were aligned using Spidey (www.ncbi.nlm.nih.gov/spidey/) to determine intron positions.
Selection Analysis.
A selection analysis was performed with a dataset containing all members of the PsIDS/TPS gene family and all coleopteran trans-IDS (except for the insect GGPPS and the IpGPPS/TPS) included in the phylogenetic analyses. The coding sequences were translated into protein sequences, aligned using the MUSCLE algorithm implemented in MEGA 6.06 and reverse translated into the corresponding nucleotide alignment. Poorly aligned regions were removed using the software GBlocks (version 0.91b) with parameters set as follows: minimum length of a block after cleaning: 5; minimum number of sequences for a conserved position and for a flank position: 8; maximum number of contiguous nonconserved positions: 8; allowed gap positions: one-half. A maximum-likelihood tree was reconstructed in MEGA 6.06 using the JTT+G+I model with pairwise deletion of gaps, and tested with 1,000 bootstrap replicates. We used the codeml software implemented in the PAML package (46) to analyze the selection pressure on the P. striolata genes that evolved a novel function. Analyses were conducted under two assumptions: the one-ratio model which allows one ω value [ratio of nonsynonymous substitutions (dN) and synonymous substitutions (dS)] for all branches (ω = 0.0926) and the four-ratio model, which allows different selection pressures acting on the common insect IDS clade including PsIDS1 and PsIDS2, the PsTPS clade, PsIDS3, and PsIDS-like, respectively (Fig. S6C). Likelihood ratio tests (47) revealed that the four-ratio model (ln L −12,647.29) was significantly better than the one ratio model (ln L −12,672.48) (χ2 = 50.39, P < 0.001).
Analysis of Predicted Amino Acid Sequences.
The molecular weight and isoelectric point (pI) of predicted PsIDS, PsIDS-like, and PsTPS proteins were calculated using the ProtParam tool (48). Prediction of subcellular protein localization was done with targetP (49) and iPSORT (50).
RNAi.
Double-stranded RNA of PsTPS1 was prepared from a 266-bp fragment using the MEGAscript RNAi Kit (Life Technologies) according to the manufacturer’s instructions. The sequence was amplified from the corresponding expression vector (pET200 PsTPS1) using primers with overhangs containing the minimum T7 polymerase promotor sequence needed for transcription (Dataset S1). Off-target effects were predicted by searches of all possible 21-mers of both RNA strands against the local P. striolata transcriptome database allowing for two mismatches. Male P. striolata adults were immobilized on sticky tape and injected with 100 nL containing 90 ng of dsRNA of PsTPS1 or 66 ng of dsRNA of gfp using a Nano2010 injector (World Precision Instruments) oriented with a manual micromanipulator (MM 33; Narishige). Injected and uninjected males were kept in groups of 30–40 individuals in clear plastic tubes (160-mL volume) containing a moistened tissue and a cut B. juncea leaf as food under ambient conditions in the laboratory. After 10 d, headspace collections were performed to compare sesquiterpene emissions of males subjected to different treatments. Afterward, i.e., 11 d after injection, RNA was extracted from adults for analysis of PsTPS1 gene expression.
Volatile Collection.
The headspace of groups of 17 male P. striolata that were injected with dsRNA of PsTPS1 or gfp (injection negative control) and of uninjected beetles feeding on a cut B. juncea leaf was collected on SuperQ filters (25 mg; ARS, Inc.) using humidified and active-charcoal cleaned pressurized air (flow, <100 mL/min) for 24 h. Volatile collections from Abies nordmanniana foliage [producing (6S,7R)-himachala-9,11-diene] were performed analogously. Filters were eluted with 100 μL of hexane containing 10 ng/μL bromodecane (Sigma-Aldrich) as an internal standard. One microliter of each sample was analyzed by GC-MS as described above.
Analysis of PsIDS, PsIDS-like, and PsTPS Gene Expression.
To compare gene expression in males and females, as well as in male P. striolata injected with dsRNA of PsTPS1 or gfp, we performed RT-qPCR. Total RNA was extracted using the InnuPREP RNA Mini Kit (Analytik Jena), treated with TURBO DNase (Ambion, Life Technologies), and purified using the RNeasy MinElute Cleanup Kit (Qiagen). RNA integrity was verified using an Agilent 2100 Bioanalyzer with the RNA 6000 Nano Kit (Agilent Technologies), and RNA quantity was determined using a Nanodrop ND-1000 spectrophotometer. First-strand cDNA was synthesized using the Verso cDNA Synthesis Kit (Thermo Fisher Scientific) from 500 ng of total RNA and a blend of random hexamer and anchored oligo-dT 3:1 (vol/vol) primers according to the instructions of the manufacturer. Quantitative PCR was performed in optical 96-well plates (Bio-Rad) on a Bio-Rad CFX Connect Real-Time System using the ABsolute Blue qPCR SYBR Green Kit (Thermo Fisher Scientific). The PCR program was as follows: 95 °C for 15 min, then 40 cycles at 95 °C for 15 s, 57 °C for 30 s, and 72 °C for 30 s, and afterward a melt cycle from 55 to 95 °C in 0.5-s increments. All primers were designed using Primer3Plus (www.bioinformatics.nl/cgi-bin/primer3plus/primer3plus.cgi/) (Dataset S1). Melting-curve analyses and sequencing of PCR products cloned into the TOPO TA 2.1 vector (Invitrogen) were used to verify amplification specificity. Primer efficiencies were calculated from a cDNA template dilution series. Eukaryotic initiation factor 4A (eIF4A) and ribosomal protein L7 (rpl7) were used as reference genes. The expression of both eIF4A as well as rpl7 was comparable between treatments (beetle sex and injection) and replicates [cycle threshold (Ct) values difference, <1]. We analyzed five biological replicates from males and females, each with two technical replicates. PsTPS1 expression in dsRNA-injected and noninjected adults was analyzed using six biological replicates again each with two technical replicates. For each biological replicate, 15–17 P. striolata adults were pooled. For data analysis, only rpl7 expression was used as reference because the corresponding Ct values were less variable. The relative gene expression in males and females was compared using Student’s t test or Mann–Whitney U test if data were not normally distributed. Analyses were done in GraphPadInStat, version 3.06.
Discussion
P. striolata Possesses an Evolutionarily Novel Class of TPS That Likely Originated from Insect trans-IDSs.
Although TPSs are well known in plants, fungi, and bacteria, sesquiterpene synthases have not been described in insects so far. In our attempt to study the molecular basis of sesquiterpene pheromone production in P. striolata, we identified and characterized a small gene family encoding enzymes with TPS activity. We found that P. striolata contains at least four different TPS enzymes, and biochemical characterization of the recombinant enzymes revealed broad substrate and product specificity (Fig. 3 and Fig. S4A). The identified P. striolata enzymes shared no amino acid sequence similarity with other TPSs from plants, fungi, and bacteria, but were similar to insect trans-IDS enzymes (Figs. S2 and S6A). In contrast to P. striolata TPSs, which showed no IDS activity in our enzyme assays, the bark beetle I. pini possesses a bifunctional enzyme that synthesizes (E)-GPP as well as the monoterpene myrcene (14).
Although insects usually contain up to three trans-IDS enzymes producing (E,E)-FPP or both (E)-GPP and (E,E)-FPP (7, 9, 10, 31, 32), we identified at least eight genes similar to insect trans-IDS in the transcriptomes of each of the three galerucine flea beetle species studied. Several predicted enzymes from P. armoraciae and P. chrysocephala also cluster in the TPS clade; however, whether these species also produce sesquiterpenes is unknown.
A comparison of the exon–intron structures of P. striolata TPS and trans-IDS genes with known insect trans-IDS genes revealed three conserved intron positions to be present in all analyzed coleopteran trans-IDS genes (Fig. S7). Altogether, this indicates that P. striolata TPSs evolved from an insect trans-IDS ancestor, thus representing an evolutionarily novel class of TPS enzymes.
PsTPS1 Is Responsible for Sesquiterpene Pheromone Production in P. striolata Using the Unusual (Z,E)-FPP Isomer as Its Substrate.
The majority of characterized terpene synthases from plants, fungi, and bacteria accept exclusively all-trans-prenyl diphosphates as substrates in vivo. However, several plant sesquiterpene synthases from the Solanaceae were recently reported to convert (Z,Z)-FPP into sesquiterpenes in planta (33, 34), and one report indicates that (Z,E)-FPP might act as a TPS substrate in plants (35). Here, we demonstrated that one of the characterized terpene synthase enzymes from P. striolata enzymes, PsTPS1, converted (Z,E)-FPP into a mixture of sesquiterpene olefins matching most of the major sesquiterpenes emitted by adult male P. striolata (23) and detected in enzyme activity assays using crude protein extracts from male P. striolata (Fig. 3 and Fig. S1B). Although the recombinant enzyme was also able to accept (Z,Z)-FPP as a substrate (Fig. S4A), the product derived from this compound was not detected in the volatile blend of the beetles. Moreover, crude protein extracts from adult male beetles showed PsTPS1 activity only when provided with (Z,E)-FPP (Fig. 1A), suggesting that the enzyme accepts this uncommon (Z,E)-isomer as its native substrate to produce the aggregation pheromone. Indeed, silencing of PsTPS1 using RNAi in male beetles resulted in a significantly reduced pheromone emission (Fig. 4B), which indicates a crucial role of PsTPS1 in pheromone production in P. striolata. Because sesquiterpene emission is restricted to males (23), we were surprised to find similar expression levels of PsTPS1 in males and females (Fig. 5). However, sesquiterpene synthase activity was ∼11-fold higher in crude protein extracts prepared from males compared with females when (Z,E)-FPP was used as substrate, suggesting that sesquiterpene pheromone production might be regulated at a posttranscriptional level.
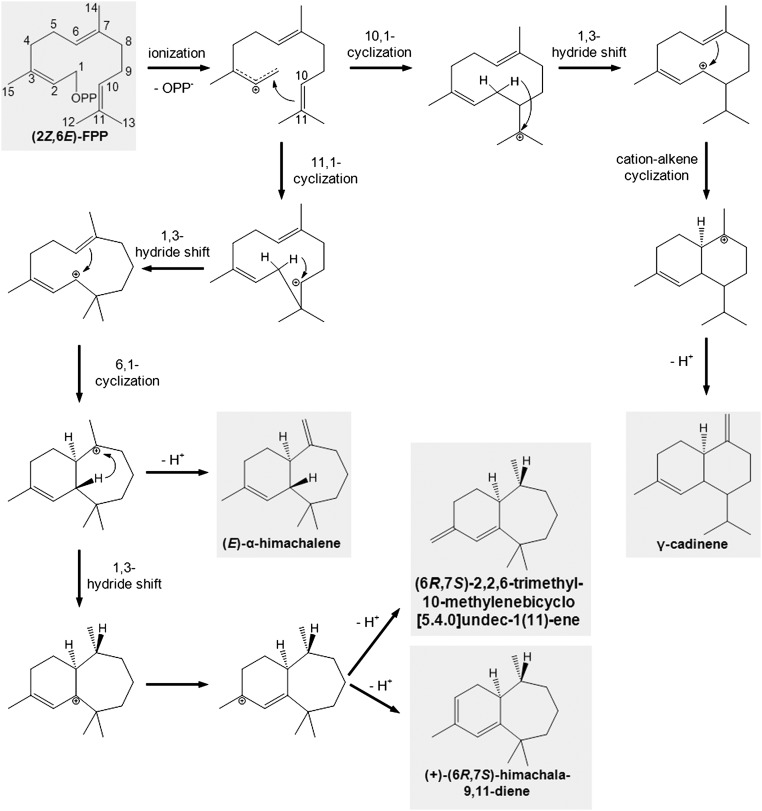
A proposed reaction mechanism for the formation of the (Z,E)-FPP–derived enzyme products is provided in Fig. S8. The cofactor-dependent initial ionization of (Z,E)-FPP results in the formation of the cisoid farnesyl carbocation, which then might undergo sequential 11,1-cyclization, a 1,3-hydride shift, and a 6,1-cyclization leading to the himachalane skeleton, followed by another 1,3-hydride shift, and further rearrangements and proton abstraction to give the end products (6R,7S)-himachala-9,11-diene, (6R,7S)-2,2,6-trimethyl-10-methylenebicyclo[5.4.0]undec-1(11)-ene, and trans-α-himachalene. γ-Cadinene is likely formed by an alternative 10,1-cyclization of the initial farnesyl carbocation followed by a 1,3-hydride shift and a 6,1-cyclization. Notably, PsTPS1 was not able to accept (E,E)-FPP as a substrate, suggesting that either the binding of this FPP isomer is impaired by steric constraints in the active site or that the isomerization of the ionization-derived transoid farnesyl carbocation into the cisoid form, which is required for subsequent 6,1-cyclization, cannot be catalyzed by this enzyme.
Fig. S8.
Proposed reaction mechanism for the formation of sesquiterpene products of PsTPS1. The substrate (2Z,6E)-FPP and the enzyme products (6R,7S)-himachala-9,11-diene, (E)-α-himachalene, (6R,7S)-2,2,6-trimethyl-10-methylenebicyclo[5.4.0]undec-1(11)-ene, and γ-cadinene are shown in boxes.
Although several PsTPS enzymes also showed monoterpene, sesquiterpene, and diterpene synthase activity in vitro (Fig. 3 and Fig. S4A), the respective enzyme products were not observed in the P. striolata volatile blend. Furthermore, in the sesquiterpene synthase activity assays conducted with crude protein extracts and the three FPP isomers, none of the other TPS products was detectable (Fig. S1). These findings might be due to the overall low expression levels of PsTPS2, PsTPS3, and PsTPS4 in P. striolata adults (Fig. 5). Moreover, it is conceivable that PsTPS2, PsTPS3, and PsTPS4 are expressed only in specific cells of adults with limited substrate availability, or in other developmental stages of the beetle. Further studies are needed to elucidate the biological role of the other TPS enzymes in P. striolata.
Both trans- and cis-IDSs Are Present in P. striolata.
To determine the origin of the unusual TPS substrate, (Z,E)-FPP, in P. striolata, we analyzed IDS activity in the beetle. It was not possible to detect prenyl diphosphates by liquid chromatography–tandem mass spectrometry (LC-MS/MS) in crude P. striolata extracts, but (E,E)-FPPS activity was detected in vitro in crude protein extracts of both sexes. This finding is expected because (E,E)-FPP is the precursor for insect juvenile hormone biosynthesis and is required for basic processes such as prenylation of proteins and ubiquitin biosynthesis in all insect orders studied (4). What was unexpected in the context of previous work was our detection of (Z,E)-FPPS activity in crude beetle protein extracts, which was present at ∼23-fold higher levels in males than females (Fig. 1B).
Two out of the three P. striolata IDS genes, PsIDS1 and PsIDS2, encode enzymes with high sequence identity (>48%) to characterized FPPS/GPPS from other Coleoptera. PsIDS1 was shown to produce (E,E)-FPP (Fig. S3A) and its position in the phylogenetic tree (Fig. 2) suggests that this enzyme is the P. striolata representative of the ubiquitous (E,E)-FPP synthase involved in the formation of juvenile hormone, ubiquitin, and prenylated proteins in insects. In PsIDS2, the second aspartate-rich motif is altered to KDxxN, which might explain the lack of activity in our assays. Interestingly, PsIDS3 converted IPP and DMAPP into NerylPP (Fig. S4) and (Z,Z)-FPP, but produced the PsTPS1 substrate (Z,E)-FPP when supplied with (E)-GPP and IPP (Fig. S4C). The production of (Z,E)-FPP is not unprecedented. However, previous reports implicate a separate IDS family in this catalysis, the cis-IDSs. A cis-IDS from Myobacterium tuberculosis was described to generate (Z,E)-FPP from (E)-GPP and IPP, which in turn is used as substrate by a decaprenyl diphosphate synthase to synthesize components of the cell wall (36, 37). All cis-IDS enzymes known to date exhibit large sequence and structural differences from trans-IDS (28, 38). However, PsIDS3 shares no sequence similarity with known cis-IDS enzymes and possesses both aspartate-rich motifs characteristic of trans-IDS (Fig. S2), which are absent in cis-IDS enzymes. Thus, PsIDS3 apparently represents (to our knowledge) the first cis-IDS that evolved from a trans-IDS.
Because we could not detect any (Z,Z)-FPPS activity in the beetle (Fig. S1B), and PsIDS3 was significantly more expressed in males than in females (about 15-fold; n = 5, t test, t = 28.9, P < 0.001), we hypothesize that PsIDS3 might provide the (Z,E)-FPP substrate for sesquiterpene pheromone production in P. striolata by catalyzing a head-to-tail condensation of (E)-GPP and IPP. However, none of the other P. striolata trans-IDS enzymes identified in this study produced significant amounts of (E)-GPP under our assay conditions. It was previously shown that product chain length or activity of plant and insect IDSs can be influenced by heterooligomerization, which may also play a role in (E)-GPP and (Z,E)-FPP synthesis in P. striolata (9, 39–41). Moreover, IDS product chain length may also be influenced by the IPP/DMAPP ratio (8), and different metal ion cofactors as recently demonstrated in the mustard leaf beetle, Phaedon cochleariae (5). The putative role of PsIDS and PsIDS-like enzymes in the biosynthesis of (Z,E)-FPP are subjects of ongoing research.
Outlook.
The male-specific aggregation pheromones of Phyllotreta spp. mediate mass attacks on economically important crucifer crops, and pheromone-baited traps or lures may prove an effective approach to reducing the damage caused by these major insect pests. In P. striolata, the full pheromone blend consists of several compounds (22, 23) that may require the activity of additional enzymes besides PsTPS1, such as monooxygenases. However, because the PsTPS1 product (6R,7S)-himachala-9,11-diene was shown to act as the major aggregation pheromone component of several Phyllotreta species (23, 24), use of this substance may have promise in pest control. Because chemical synthesis of (6R,7S)-himachala-9,11-diene is quite complex (42, 43), recombinant PsTPS1 may offer a viable method for producing large quantities of this sesquiterpene. Further progress in understanding pheromone biosynthesis and its regulation in Phyllotreta spp. could open up other ways for controlling these important pest species.
Materials and Methods
Crude protein extracts were prepared from male and female P. striolata adults and analyzed for TPS and IDS activity. For TPS activity assays, protein extracts were incubated with different FPP isomers, and assay products were detected by solid-phase microextraction coupled with gas chromatography–mass spectrometry (GC-MS). IDS activity assays using IPP and DMAPP as substrates were analyzed with LC-MS/MS. Nine putative trans-IDS transcripts were identified in the P. striolata transcriptome, and the corresponding full-length sequences were obtained using RACE-PCR. The candidate genes were amplified as N-terminally truncated ORFs from cDNAs, cloned into the expression vector pET100/D-TOPO or pET200/D-TOPO, and expressed as N-terminal His-tag fusions in Escherichia coli. Recombinant proteins were partially purified via the His-tag and tested for TPS and IDS activity. PsIDS1, PsIDS3, and PsTPS1 were additionally amplified from genomic DNA of P. striolata and sequenced to determine intron positions. The function of PsTPS1 in vivo was investigated in RNAi experiments by injecting male P. striolata adults with dsRNA of PsTPS1 or gfp (control), followed by quantitative RT-PCR and quantification of sesquiterpenes emitted by feeding males. Details on organisms, phylogenetic analyses, experiments, analytical procedures, and data analyses are provided in SI Materials and Methods. All sequences reported in this manuscript have been deposited in the GenBank database (accession nos. KT959237–KT959261).
Supplementary Material
Acknowledgments
We thank Michael Reichelt for help with LC-MS/MS analyses, Nathalie Gatto and Wilhelm Boland for providing (Z,E)-FPP, Allard Cossé for providing trans-α-himachalene and (6R,7S)-2,2,6-trimethyl-10-methylenebicyclo[5.4.0]undec-1(11)-ene as reference compounds, Zhi-ling Yang for help with the selection analysis, Yannick Pauchet and Roy Kirsch for scientific discussion, Domenica Schnabelrauch for DNA-sequencing services, Susanne Donnerhacke for technical support, Lin Mei-ying and Hu Ruey-chuan from AVRDC–The World Vegetable Center in Taiwan for providing P. striolata, and the greenhouse team of the Max Planck Institute for Chemical Ecology for taking care of plants. Financial support was provided by the Max-Planck-Gesellschaft.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database (accession nos. KT959237–KT959261).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1523468113/-/DCSupplemental.
References
- 1.Blomquist GJ, et al. Pheromone production in bark beetles. Insect Biochem Mol Biol. 2010;40(10):699–712. doi: 10.1016/j.ibmb.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 2.Gershenzon J, Dudareva N. The function of terpene natural products in the natural world. Nat Chem Biol. 2007;3(7):408–414. doi: 10.1038/nchembio.2007.5. [DOI] [PubMed] [Google Scholar]
- 3.Vandermoten S, Mescher MC, Francis F, Haubruge E, Verheggen FJ. Aphid alarm pheromone: An overview of current knowledge on biosynthesis and functions. Insect Biochem Mol Biol. 2012;42(3):155–163. doi: 10.1016/j.ibmb.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 4.Bellés X, Martín D, Piulachs MD. The mevalonate pathway and the synthesis of juvenile hormone in insects. Annu Rev Entomol. 2005;50:181–199. doi: 10.1146/annurev.ento.50.071803.130356. [DOI] [PubMed] [Google Scholar]
- 5.Frick S, et al. Metal ions control product specificity of isoprenyl diphosphate synthases in the insect terpenoid pathway. Proc Natl Acad Sci USA. 2013;110(11):4194–4199. doi: 10.1073/pnas.1221489110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gilg AB, Bearfield JC, Tittiger C, Welch WH, Blomquist GJ. Isolation and functional expression of an animal geranyl diphosphate synthase and its role in bark beetle pheromone biosynthesis. Proc Natl Acad Sci USA. 2005;102(28):9760–9765. doi: 10.1073/pnas.0503277102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gonzalez-Caballero N, Valenzuela JG, Mc Ribeiro J, Cuervo P, Brazil RP. 2013. Transcriptome exploration of the sex pheromone gland of Lutzomyia longipalpis (Diptera: Psychodidae: Phlebotominae). Parasit Vectors 6:56. [DOI] [PMC free article] [PubMed]
- 8.Keeling CI, et al. Frontalin pheromone biosynthesis in the mountain pine beetle, Dendroctonus ponderosae, and the role of isoprenyl diphosphate synthases. Proc Natl Acad Sci USA. 2013;110(47):18838–18843. doi: 10.1073/pnas.1316498110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sen SE, et al. Purification, properties and heteromeric association of type-1 and type-2 lepidopteran farnesyl diphosphate synthases. Insect Biochem Mol Biol. 2007;37(8):819–828. doi: 10.1016/j.ibmb.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 10.Vandermoten S, et al. Characterization of a novel aphid prenyltransferase displaying dual geranyl/farnesyl diphosphate synthase activity. FEBS Lett. 2008;582(13):1928–1934. doi: 10.1016/j.febslet.2008.04.043. [DOI] [PubMed] [Google Scholar]
- 11.Degenhardt J, Köllner TG, Gershenzon J. Monoterpene and sesquiterpene synthases and the origin of terpene skeletal diversity in plants. Phytochemistry. 2009;70(15-16):1621–1637. doi: 10.1016/j.phytochem.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 12.Yamada Y, et al. Terpene synthases are widely distributed in bacteria. Proc Natl Acad Sci USA. 2015;112(3):857–862. doi: 10.1073/pnas.1422108112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Quin MB, Flynn CM, Schmidt-Dannert C. Traversing the fungal terpenome. Nat Prod Rep. 2014;31(10):1449–1473. doi: 10.1039/c4np00075g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilg AB, Tittiger C, Blomquist GJ. Unique animal prenyltransferase with monoterpene synthase activity. Naturwissenschaften. 2009;96(6):731–735. doi: 10.1007/s00114-009-0521-1. [DOI] [PubMed] [Google Scholar]
- 15.Wawrzyn GT, Quin MB, Choudhary S, López-Gallego F, Schmidt-Dannert C. Draft genome of Omphalotus olearius provides a predictive framework for sesquiterpenoid natural product biosynthesis in Basidiomycota. Chem Biol. 2012;19(6):772–783. doi: 10.1016/j.chembiol.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garms S, Köllner TG, Boland W. A multiproduct terpene synthase from Medicago truncatula generates cadalane sesquiterpenes via two different mechanisms. J Org Chem. 2010;75(16):5590–5600. doi: 10.1021/jo100917c. [DOI] [PubMed] [Google Scholar]
- 17.Li G, et al. Nonseed plant Selaginella moellendorffi [corrected] has both seed plant and microbial types of terpene synthases. Proc Natl Acad Sci USA. 2012;109(36):14711–14715. doi: 10.1073/pnas.1204300109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakano Y, et al. Characterization of the casbene synthase homolog from Jatropha (Jatropha curcas L.) Plant Biotechnol. 2012;29(2):185–189. [Google Scholar]
- 19.Wawrzyn GT, Bloch SE, Schmidt-Dannert C. Discovery and characterization of terpenoid biosynthetic pathways of fungi. Methods Enzymol. 2012;515:83–105. doi: 10.1016/B978-0-12-394290-6.00005-7. [DOI] [PubMed] [Google Scholar]
- 20.Zilkowski BW, Bartelt RJ, Cossé AA, Petroski RJ. Male-produced aggregation pheromone compounds from the eggplant flea beetle (Epitrix fuscula): Identification, synthesis, and field biossays. J Chem Ecol. 2006;32(11):2543–2558. doi: 10.1007/s10886-006-9163-3. [DOI] [PubMed] [Google Scholar]
- 21.Bartelt RJ, Cossé AA, Zilkowski BW, Weisleder D, Momany FA. Male-specific sesquiterpenes from Phyllotreta and Aphthona flea beetles. J Chem Ecol. 2001;27(12):2397–2423. doi: 10.1023/a:1013667229345. [DOI] [PubMed] [Google Scholar]
- 22.Bartelt RJ, et al. Male-specific sesquiterpenes from Phyllotreta flea beetles. J Nat Prod. 2011;74(4):585–595. doi: 10.1021/np100608p. [DOI] [PubMed] [Google Scholar]
- 23.Beran F, et al. Male Phyllotreta striolata (F.) produce an aggregation pheromone: Identification of male-specific compounds and interaction with host plant volatiles. J Chem Ecol. 2011;37(1):85–97. doi: 10.1007/s10886-010-9899-7. [DOI] [PubMed] [Google Scholar]
- 24.Tóth M, Csonka E, Bartelt RJ, Cossé AA, Zilkowski BW. Similarities in pheromonal communication of flea beetles Phyllotreta cruciferae Goeze and Ph. vittula Redtenbacher (Coleoptera, Chrysomelidae) J Appl Entomol. 2012;136(9):688–697. [Google Scholar]
- 25.Tóth M, et al. Pheromonal activity of compounds identified from male Phyllotreta cruciferae: Field tests of racemic mixtures, pure enantiomers, and combinations with allyl isothiocyanate. J Chem Ecol. 2005;31(11):2705–2720. doi: 10.1007/s10886-005-7621-y. [DOI] [PubMed] [Google Scholar]
- 26.Lamb RJ. Entomology of oilseed Brassica crops. Annu Rev Entomol. 1989;34:211–229. [Google Scholar]
- 27.Beran F, et al. Phyllotreta striolata flea beetles use host plant defense compounds to create their own glucosinolate-myrosinase system. Proc Natl Acad Sci USA. 2014;111(20):7349–7354. doi: 10.1073/pnas.1321781111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gao Y, Honzatko RB, Peters RJ. Terpenoid synthase structures: A so far incomplete view of complex catalysis. Nat Prod Rep. 2012;29(10):1153–1175. doi: 10.1039/c2np20059g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Köllner TG, et al. A maize (E)-beta-caryophyllene synthase implicated in indirect defense responses against herbivores is not expressed in most American maize varieties. Plant Cell. 2008;20(2):482–494. doi: 10.1105/tpc.107.051672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Köllner TG, Schnee C, Gershenzon J, Degenhardt J. The variability of sesquiterpenes emitted from two Zea mays cultivars is controlled by allelic variation of two terpene synthase genes encoding stereoselective multiple product enzymes. Plant Cell. 2004;16(5):1115–1131. doi: 10.1105/tpc.019877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kinjoh T, et al. Control of juvenile hormone biosynthesis in Bombyx mori: Cloning of the enzymes in the mevalonate pathway and assessment of their developmental expression in the corpora allata. Insect Biochem Mol Biol. 2007;37(8):808–818. doi: 10.1016/j.ibmb.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 32.Sen SE, Trobaugh C, Béliveau C, Richard T, Cusson M. Cloning, expression and characterization of a dipteran farnesyl diphosphate synthase. Insect Biochem Mol Biol. 2007;37(11):1198–1206. doi: 10.1016/j.ibmb.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 33.Sallaud C, et al. A novel pathway for sesquiterpene biosynthesis from Z,Z-farnesyl pyrophosphate in the wild tomato Solanum habrochaites. Plant Cell. 2009;21(1):301–317. doi: 10.1105/tpc.107.057885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gonzales-Vigil E, Hufnagel DE, Kim J, Last RL, Barry CS. Evolution of TPS20-related terpene synthases influences chemical diversity in the glandular trichomes of the wild tomato relative Solanum habrochaites. Plant J. 2012;71(6):921–935. doi: 10.1111/j.1365-313X.2012.05040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nabeta K, Fujita M, Komuro K, Katayama K, Takasawa T. In vitro biosynthesis of cadinanes by cell-free extracts of cultured cells of Heteroscyphus planus. J Chem Soc Perk T. 1997;1(14):2065–2070. [Google Scholar]
- 36.Schulbach MC, Brennan PJ, Crick DC. Identification of a short (C15) chain Z-isoprenyl diphosphate synthase and a homologous long (C50) chain isoprenyl diphosphate synthase in Mycobacterium tuberculosis. J Biol Chem. 2000;275(30):22876–22881. doi: 10.1074/jbc.M003194200. [DOI] [PubMed] [Google Scholar]
- 37.Wang W, et al. The structural basis of chain length control in Rv1086. J Mol Biol. 2008;381(1):129–140. doi: 10.1016/j.jmb.2008.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takahashi S, Koyama T. Structure and function of cis-prenyl chain elongating enzymes. Chem Rec. 2006;6(4):194–205. doi: 10.1002/tcr.20083. [DOI] [PubMed] [Google Scholar]
- 39.Orlova I, et al. The small subunit of snapdragon geranyl diphosphate synthase modifies the chain length specificity of tobacco geranylgeranyl diphosphate synthase in planta. Plant Cell. 2009;21(12):4002–4017. doi: 10.1105/tpc.109.071282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Burke CC, Wildung MR, Croteau R. Geranyl diphosphate synthase: Cloning, expression, and characterization of this prenyltransferase as a heterodimer. Proc Natl Acad Sci USA. 1999;96(23):13062–13067. doi: 10.1073/pnas.96.23.13062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang G, Dixon RA. Heterodimeric geranyl(geranyl)diphosphate synthase from hop (Humulus lupulus) and the evolution of monoterpene biosynthesis. Proc Natl Acad Sci USA. 2009;106(24):9914–9919. doi: 10.1073/pnas.0904069106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Muto S, Bando M, Mori K. Synthesis and stereochemistry of the four himachalene-type sesquiterpenes isolated from the flea beetle (Aphthona flava) as pheromone candidates. Eur J Org Chem. 2004;2004(9):1946–1952. [Google Scholar]
- 43.Jimenez-Aleman GH, Schoner T, Montero-Alejo AL, Brandt W, Boland W. Improved synthesis of the chrysomelid pheromone (6R,7S)-(+)-himachala-9,11-diene via spontaneous bromination and didehydrobromination of 2,6,6,9-tetramethyl-bicyclo[5.4.0]undec-8-ene. ARKIVOC. 2012;2012(3):371–378. [Google Scholar]
- 44.Cane DE, Yang G, Xue Q, Shim JH. Trichodiene synthase. Substrate specificity and inhibition. Biochemistry. 1995;34(8):2471–2479. doi: 10.1021/bi00008a010. [DOI] [PubMed] [Google Scholar]
- 45.Black WC, DuTeau NM. RAPD-PCR and SSCP analysis for insect population genetic studies. In: Crampton JM, Beard CB, Louis C, editors. The Molecular Biology of Insect Disease Vectors: A Methods Manual. Chapman and Hall; London: 1997. pp. 361–373. [Google Scholar]
- 46.Yang Z. PAML: A program package for phylogenetic analysis by maximum likelihood. Comput Appl Biosci. 1997;13(5):555–556. doi: 10.1093/bioinformatics/13.5.555. [DOI] [PubMed] [Google Scholar]
- 47.Yang Z. A likelihood ratio test of speciation with gene flow using genomic sequence data. Genome Biol Evol. 2010;2:200–211. doi: 10.1093/gbe/evq011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gasteiger E, et al. Protein identification and analysis tools on the ExPASy server. In: Walker JM, editor. The Proteomics Protocols Handbook. Humana; Totowa, NJ: 2005. pp. 571–607. [Google Scholar]
- 49.Emanuelsson O, Nielsen H, Brunak S, von Heijne G. Predicting subcellular localization of proteins based on their N-terminal amino acid sequence. J Mol Biol. 2000;300(4):1005–1016. doi: 10.1006/jmbi.2000.3903. [DOI] [PubMed] [Google Scholar]
- 50.Bannai H, Tamada Y, Maruyama O, Nakai K, Miyano S. Extensive feature detection of N-terminal protein sorting signals. Bioinformatics. 2002;18(2):298–305. doi: 10.1093/bioinformatics/18.2.298. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.