Significance
In flowering plants, pollen tubes deliver paired sperm cells into ovules for double fertilization by perceiving and responding to multiple attractants secreted from the ovules. Ca2+ gradients in pollen tube tips are essential for pollen tube guidance, and plasma membrane Ca2+ channels in the pollen tube tips are the core components for the regulation of the Ca2+ gradients by mediating and regulating external Ca2+ influx. Therefore, the Ca2+ channels are essential for pollen tube guidance. However, the molecular identities of the Ca2+ channels remain to be addressed. In this research, we found that cyclic nucleotide-gated channel 18, out of eight Ca2+ channels present in pollen tubes, is the main one essential for pollen tube guidance in Arabidopsis.
Keywords: CNGC18, pollen tube guidance, Ca2+ gradient, Ca2+ channel, Arabidopsis
Abstract
In flowering plants, pollen tubes are guided into ovules by multiple attractants from female gametophytes to release paired sperm cells for double fertilization. It has been well-established that Ca2+ gradients in the pollen tube tips are essential for pollen tube guidance and that plasma membrane Ca2+ channels in pollen tube tips are core components that regulate Ca2+ gradients by mediating and regulating external Ca2+ influx. Therefore, Ca2+ channels are the core components for pollen tube guidance. However, there is still no genetic evidence for the identification of the putative Ca2+ channels essential for pollen tube guidance. Here, we report that the point mutations R491Q or R578K in cyclic nucleotide-gated channel 18 (CNGC18) resulted in abnormal Ca2+ gradients and strong pollen tube guidance defects by impairing the activation of CNGC18 in Arabidopsis. The pollen tube guidance defects of cngc18-17 (R491Q) and of the transfer DNA (T-DNA) insertion mutant cngc18-1 (+/−) were completely rescued by CNGC18. Furthermore, domain-swapping experiments showed that CNGC18’s transmembrane domains are indispensable for pollen tube guidance. Additionally, we found that, among eight Ca2+ channels (including six CNGCs and two glutamate receptor-like channels), CNGC18 was the only one essential for pollen tube guidance. Thus, CNGC18 is the long-sought essential Ca2+ channel for pollen tube guidance in Arabidopsis.
Pollen tubes deliver paired sperm cells into ovules for double fertilization, and signaling communication between pollen tubes and female reproductive tissues is required to ensure the delivery of sperm cells into the ovules (1). Pollen tube guidance is governed by both female sporophytic and gametophytic tissues (2, 3) and can be separated into two categories: preovular guidance and ovular guidance (1). For preovular guidance, diverse signaling molecules from female sporophytic tissues have been identified, including the transmitting tissue-specific (TTS) glycoprotein in tobacco (4), γ-amino butyric acid (GABA) in Arabidopsis (5), and chemocyanin and the lipid transfer protein SCA in Lilium longiflorum (6, 7). For ovular pollen tube guidance, female gametophytes secrete small peptides as attractants, including LUREs in Torenia fournieri (8) and Arabidopsis (9) and ZmEA1 in maize (10, 11). Synergid cells, central cells, egg cells, and egg apparatus are all involved in pollen tube guidance, probably by secreting different attractants (9–15). Additionally, nitric oxide (NO) and phytosulfokine peptides have also been implicated in both preovular and ovular pollen tube guidance (16–18). Thus, pollen tubes could be guided by diverse attractants in a single plant species.
Ca2+ gradients at pollen tube tips are essential for both tip growth and pollen tube guidance (19–27). Spatial modification of the Ca2+ gradients leads to the reorientation of pollen tube growth in vitro (28, 29). The Ca2+ gradients were significantly increased in pollen tubes attracted to the micropyles by synergid cells in vivo, compared with those not attracted by ovules (30). Therefore, the Ca2+ gradients in pollen tube tips are essential for pollen tube guidance. The Ca2+ gradients result from external Ca2+ influx, which is mainly mediated by plasma membrane Ca2+ channels in pollen tube tips. Thus, the Ca2+ channels are the key components for regulating the Ca2+ gradients and are consequently essential for pollen tube guidance. Using electrophysiological techniques, inward Ca2+ currents were observed in both pollen grain and pollen tube protoplasts (31–36), supporting the presence of plasma membrane Ca2+ channels in pollen tube tips. Recently, a number of candidate Ca2+ channels were identified in pollen tubes, including six cyclic nucleotide-gated channels (CNGCs) and two glutamate receptor-like channels (GLRs) in Arabidopsis (37–40). Three of these eight channels, namely CNGC18, GLR1.2, and GLR3.7, were characterized as Ca2+-permeable channels (40, 41) whereas the ion selectivity of the other five CNGCs has not been characterized. We hypothesized that the Ca2+ channel essential for pollen tube guidance could be among these eight channels.
In this research, we first characterized the remaining five CNGCs as Ca2+ channels. We further found that CNGC18, out of the eight Ca2+ channels, was the only one essential for pollen tube guidance in Arabidopsis and that its transmembrane domains were indispensable for pollen tube guidance.
Results
CNGC7, -8, -9, -10, and -16 Are Ca2+ Channels.
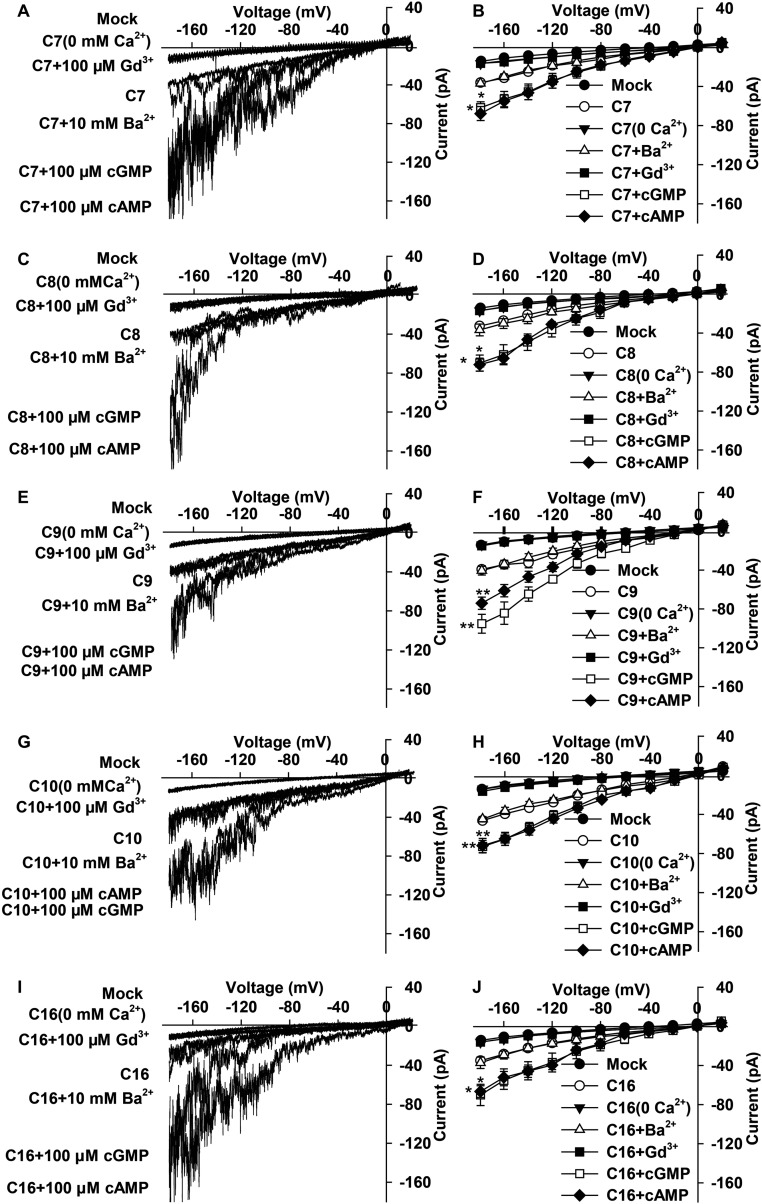
We characterized CNGC7, -8, -9, -10, and -16 using a patch-clamping technique by transiently expressing the CNGCs in HEK293T cells. Large inward currents were observed in HEK293T cells expressing each of the five CNGCs (Fig. S1). The CNGC-mediated inward currents were strongly decreased upon removal of external Ca2+ or after application of either Gd3+ (100 µM) or La3+ (100 µM) (Fig. S1). The reversal potentials shifted to a more negative potential direction upon removal of external 10 mM Ca2+ (Fig. S1 B, D, F, H, and J). We also observed large inward currents using a 10-mM Ba2+ bath solution, and the current amplitudes were similar to that with 10-mM Ca2+ bath conditions (Fig. S1). These data demonstrate that these five CNGCs are all Ca2+-permeable channels. We also tested their permeability to monovalent cation K+. Large inward K+ currents were observed in HEK293T cells expressing the typical inward K+ channel KAT1 (Fig. S2), as reported (41, 42), but only background conductance was observed in the mock control or in HEK293T cells expressing any of the five CNGCs (Fig. S2). Thus, the five CNGCs have no obvious permeability to K+. CNGC18, GLR1.2, and GLR3.7 have been characterized as Ca2+ channels (40, 41). Thus, the eight channels present in pollen tubes are all Ca2+-permeable channels.
Fig. S1.
CNGC7, CNGC8, CNGC9, CNGC10, and CNGC16 are all Ca2+-permeable channels. (A and B) Typical whole-cell recordings (A) and average current-voltage curves (B) for CNGC7. Nine HEK293T cells were tested for CNGC7, CNGC7 + 0 mM Ca2+, CNGC7 + 10 mM Ba2+, and CNGC7 + 100 μM 8Br-cAMP; 6 cells for CNGC7 + 100 μM Gd3+; and 5 cells for CNGC7 + 100 μM 8Br-cGMP. (C and D) Typical whole-cell recordings (C) and average current-voltage curves (D) for CNGC8. Ten HEK293T cells were tested for CNGC8, 8 cells for CNGC8 + 0 mM Ca2+, 6 cells for CNGC8 + 10 mM Ba2+, 3 cells for CNGC8 + 100 μM Gd3+, 7 cells for CNGC8 + 100 μM 8Br-cGMP, and 6 cells for CNGC8 + 100 μM 8Br-cAMP. (E and F) Typical whole-cell recordings (E) and average current-voltage curves (F) for CNGC9. Seven HEK293T cells were tested for CNGC9, 6 cells for CNGC9 + 0 mM Ca2+, 4 cells for CNGC9 + 10 mM Ba2+, 6 cells for CNGC9 + 100 μM Gd3+, and 5 cells for CNGC9 + 100 μM 8Br-cGMP and CNGC9 + 100 μM 8Br-cAMP. (G to H) Typical whole-cell recordings (G) and average current-voltage curves (H) for CNGC10. Eight HEK293T cells were tested for CNGC10, 6 cells for CNGC10 + 0 mM Ca2+, 7 cells for CNGC10 + 10 mM Ba2+, 6 cells for CNGC10 + 100 μM Gd3+, 5 cells for CNGC10 + 100 μM 8Br-cGMP, and 3 cells for CNGC10 + 100 μM 8Br-cAMP. (I and J) Typical whole-cell recordings (I) and the average current-voltage curves (J) for CNGC16. Eleven HEK293T cells were tested are for CNGC16, 9 cells for CNGC16 + 0 mM Ca2+, 5 cells for CNGC16 + 10 mM Ba2+, 6 cells for CNGC16 + 100 μM Gd3+, 9 cells for CNGC16 + 100 μM 8Br-cGMP, and 8 cells for CNGC16 + 100 μM 8Br-cAMP. C7, C8, C9, C10, and C16 denote CNGC7, CNGC8, CNGC9, CNGC10, and CNGC16, respectively. Nineteen HEK293T cells were tested for mock control, and the same control data were used to plot the average current-voltage curve in B, D, F, H, and J. Error bars depict means ± SEM. *Significant difference (P < 0.05) and **significant difference (P < 0.01), relative to cyclic nucleotide-free conditions.
Fig. S2.
CNGC7, CNGC8, CNGC9, CNGC10, and CNGC16 exhibit no obvious permeability to K+ relative to the typical inward K+ channel KAT1. (A and B) Typical whole-cell recordings (A) and the average current-voltage curves (B). Eight HEK293T cells were tested for mock control, 10 cells for CNGC7, 9 cells for CNGC8, 7 cells for CNGC9, 8 cells for CNGC10, 9 cells for CNGC16, and 13 cells for KAT1. (C) The amplitudes of average steady-state whole-cell currents at −160 mV as shown in B. Error bars depict means ± SEM. **Significant difference from mock control (P < 0.01).
The CNGC18 Point Mutations R491Q and R578K Lead to Male Sterility in Arabidopsis.
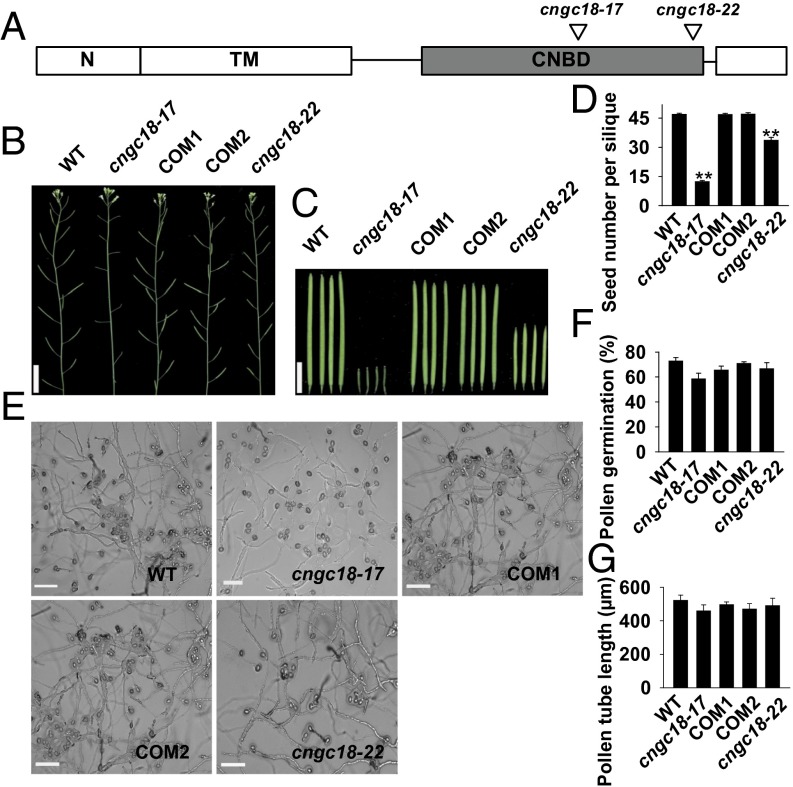
To investigate the functions of the Ca2+ channels in pollen tube guidance, we collected homozygous transfer DNA (T-DNA) insertion mutants of all eight Ca2+ channels, except CNGC18 (Fig. S3 and Table S1). Homozygous knockout mutant cngc18-1 is not available because of its complete male sterility (37). As an alternative, we screened a pool of 23 point mutants and identified two point mutants, cngc18-17 (R491Q) and cngc18-22 (R578K) (Fig. 1A), that had shorter siliques, fewer seeds per silique, and slightly lower in vitro pollen germination rates than in WT (Fig. 1 B–F). However, the pollen tubes of the two point mutants looked normal and were comparable with WT with respect to their shape and length (Fig. 1 E and G). We generated two complementation lines, complemented line 1 (COM1) and complemented line 2 (COM2), by expressing WT CNGC18 driven by its native promoter in the cngc18-17 mutant background (Fig. S4 and Table S1). The phenotypes of cngc18-17 were rescued in COM1 and COM2 (Fig. 1 B–F).
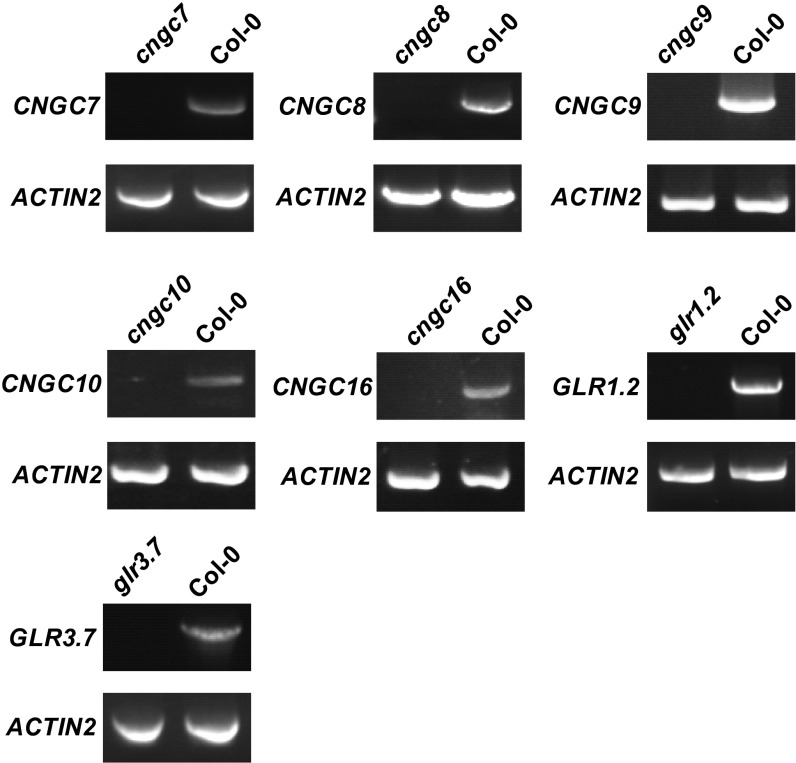
Fig. S3.
RT-PCR to demonstrate that cngc7 (CS870639), cngc8 (CS409627), cngc9 (Salk_026086), cngc10 (Salk_015952C), cncg16 (Salk_065792C), glr1.2 (Salk_031498), and glr3.7 (Salk_103942C) are T-DNA insertion knock-out mutants.
Table S1.
Primers for gene cloning, vector construction, and qRT-PCR
| Name | Sequence | Purpose |
| CNGC7 Kpn+ | GGGGTACCATGATGATGCAGAGAAACTGTTTCG | Cloning of CNGC7 |
| CNGC7 Pst− | AACTGCAGTTCAGCATCAAAATCAGGTTCCGG | |
| CNGC8 Kpn+ | GGGGTACCATGTACAAATCTCAATATATAAGTGGCCA | Cloning of CNGC8 |
| CNGC8 Pst− | AACTGCAGGTTCAAGTCATCTGTATCAAGAGCCTC | |
| CNGC9 Kpn+ | GGGGTACCATGTTAGACTGTGGCAAAAAAGCAG | Cloning of CNGC9 |
| CNGC9 Pst− | AACTGCAGACTAGTATCATCAGCAGAGAAATC | |
| CNGC10 Kpn+ | GGGGTACCATGGCATTTAGCCATGATAATCG | Cloning of CNGC10 |
| CNGC10 Pst− | AACTGCAGAGGGTCAGTTGTATGATTGGCG | |
| CNGC16 Kpn+ | GGGGTACCATGAGCAACCTCCACCTCTAC | Cloning of CNGC16 |
| CNGC16 Pst− | AACTGCAGGAAGAATCCTGGATCCTCTGG | |
| CNGC18 Kpn+ | GGGGTACCATGAATAAAATCCGGTCTCTCCGCTGCC | Cloning of CNGC18 |
| CNGC18 Pst− | AACTGCAGAACGTCTTCTTTATCTATAGAGAA | |
| AtGLR1.2 Kpn+ | GGGGTACCATGGTGAGAATTTGTATTCAAACC | Cloning of GLR1.2 |
| AtGLR1.2 Sal− | ACGCGTCGACTCGTCTGTTACGTTGAGCCATTTG | |
| AtGLR3.7 Eco+ | GGAATTCATGGGACTGGGCATTGAC | Cloning of GLR3.7 |
| AtGLR3.7 BstEII− | GGGTWACCATTTCGTGGTACCTCAGTATCAG | |
| proCNGC18 Eco+ | GGAATTCTGAATGGGTTTTGTATCTAAAGATCAAAGGAAT | Cloning of CNGC18 promoter |
| proCNGC18 Kpn− | GGGGTACCCTTCCATGGCGGATTCCGGTGTTAAATCGG | |
| eGFP Nco+ | CATGCCATGGATGGTGAGCAAGGGCGAGGAG | Cloning of eGFP |
| eGFP BstEII− | GGGTWACCCTTGTACAGCTCGTCCATG | |
| CNGC18-R491Q+ | GCTATTTGTGATCCAAGGACAAATAG | Point mutation of CNGC18-R491Q |
| CNGC18-R491Q− | CTCTCTATTTGTCCTTGGATCACAAA | |
| CNGC18-R578K+ | CAAAAAGCTTCAACACGCTTTCAAGTACTACTCACATCA | Point mutation of CNGC18-R578K |
| CNGC18-R578K− | TGATGTGAGTAGTACTTGAAAGCGTGTTGAAGCTTTTTG | |
| qACTIN2+ | CCAACAGAGAGAAGATGACT | qRT-PCR |
| qACTIN2− | ATGTCTCTTACAATTTCCCG | |
| qeGFP+ | TGAAGGGCATCGACTTCAAG | qRT-PCR |
| qeGFP− | ATGGGGGTGTTCTGCTGGTA | |
| C16-18N− | GAGGCATGTGATGAGGAAAATGTGGTTCCAATAGGTGACG | For the construction of CNGC16-18N |
| C16-18N+ | CGTCACCTATTGGAACCACATTTTCCTCATCACATGCCTC | |
| C16-18TM1− | ATTGAGGTAATGAGAAAGACGTGGTTCCATCTCGTGATTA | For the construction of CNGC16-18TM |
| C16-18TM1+ | TAATCACGAGATGGAACCACGTCTTTCTCATTACCTCAAT | |
| C16-18TM2− | GTCGATTGCAGATAGTTCTGCATGTTACCAATCAGGAGTG | |
| C16-18TM2+ | CACTCCTGATTGGTAACATGCAGAACTATCTGCAATCGAC | |
| C16-18C− | ATCGATTGCAGAGAAGATTGAACATTTCCAATGAGATGAG | For the construction of CNGC16-18C |
| C16-18C+ | CTCATCTCATTGGAAATGTTCAATCTTCTCTGCAATCGAT | |
| C18-16TM1+ | TCGTCACCTATTGGAACCACATTTTCCTCATCACATGCCT | For the construction of CNGC18-16TM |
| C18-16TM1− | AGGCATGTGATGAGGAAAATGTGGTTCCAATAGGTGACGA | |
| C18-16TM2+ | CTCATCTCATTGGAAATGTTCAATCTTCTCTGCAATCGAT | |
| C18-16TM2− | ATCGATTGCAGAGAAGATTGAACATTTCCAATGAGATGAG |
Fig. 1.
cngc18-17 and cngc18-22 show severe male sterility. (A) Schematic model showing the cngc18-17 (R491Q) and cngc18-22 (R578K) point mutations. N, TM, and CNBD denote the N-terminal domain, transmembrane domains, and cyclic nucleotide binding domain, respectively. (B, C, and E) Images of plants (B), siliques (C), and in vitro-grown pollen tubes (E). (Scale bars: B, 2 cm; C, 0.5 cm; and E, 100 μm.) (D, F, and G) Average seed number per silique (D), pollen germination rate (F), and average pollen tube length (G). Error bars depict means ± SEM. **Significant differences from WT (P < 0.01).
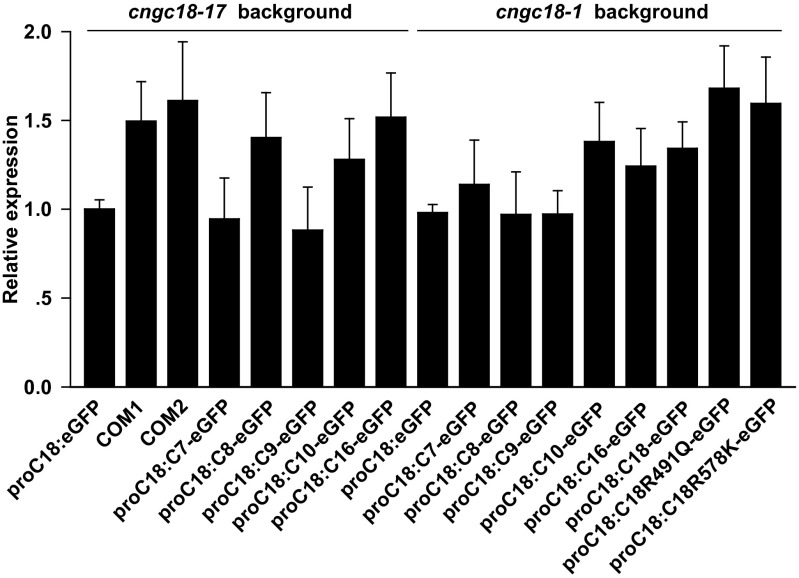
Fig. S4.
qRT-PCR to assess relative gene expression levels in transgenic Arabidopsis lines. Expression of proCNGC18:eGFP in transgenic line proCNGC18-eGFP/cngc18-17 was set to 1.0.
Among the Eight Ca2+ Channels Present in Pollen Tubes, CNGC18 Is the only One Critical for Pollen Tube Guidance.
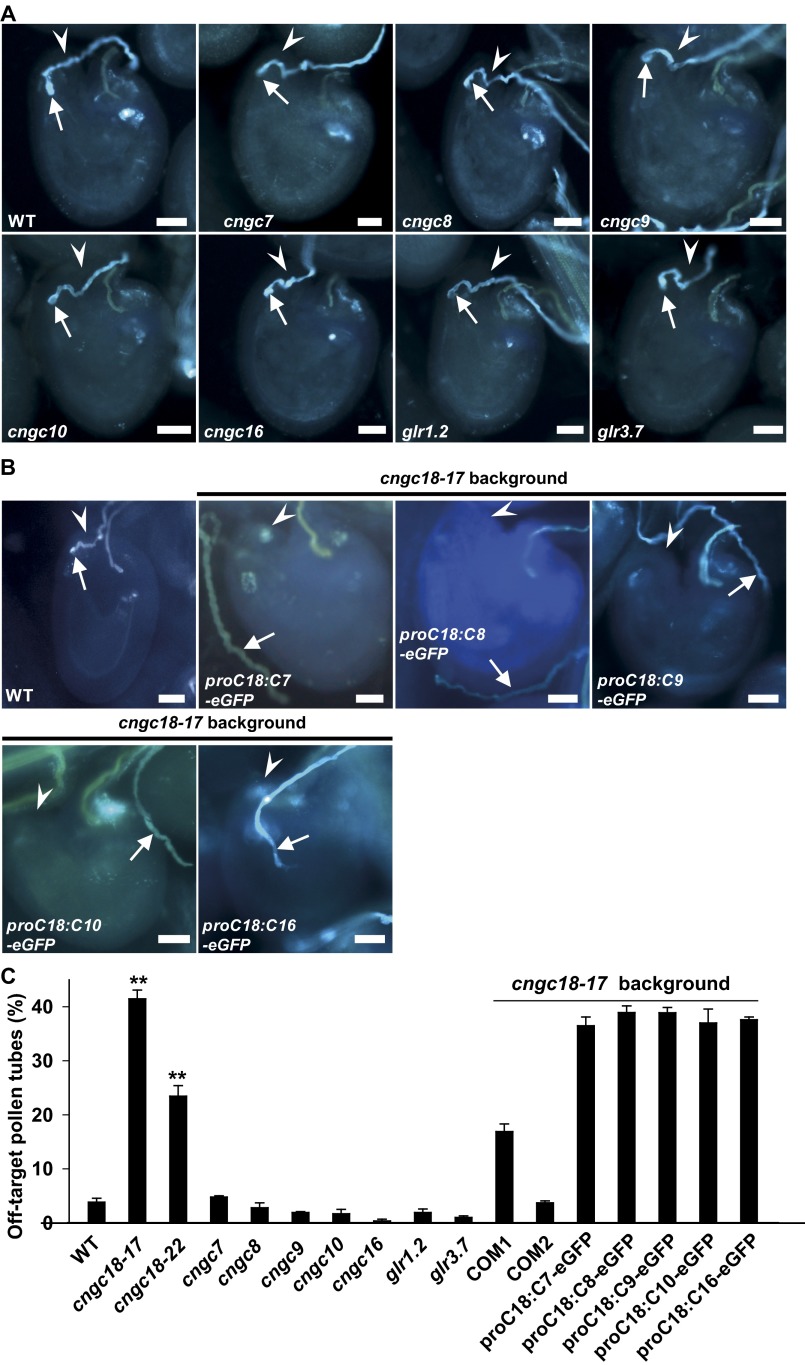
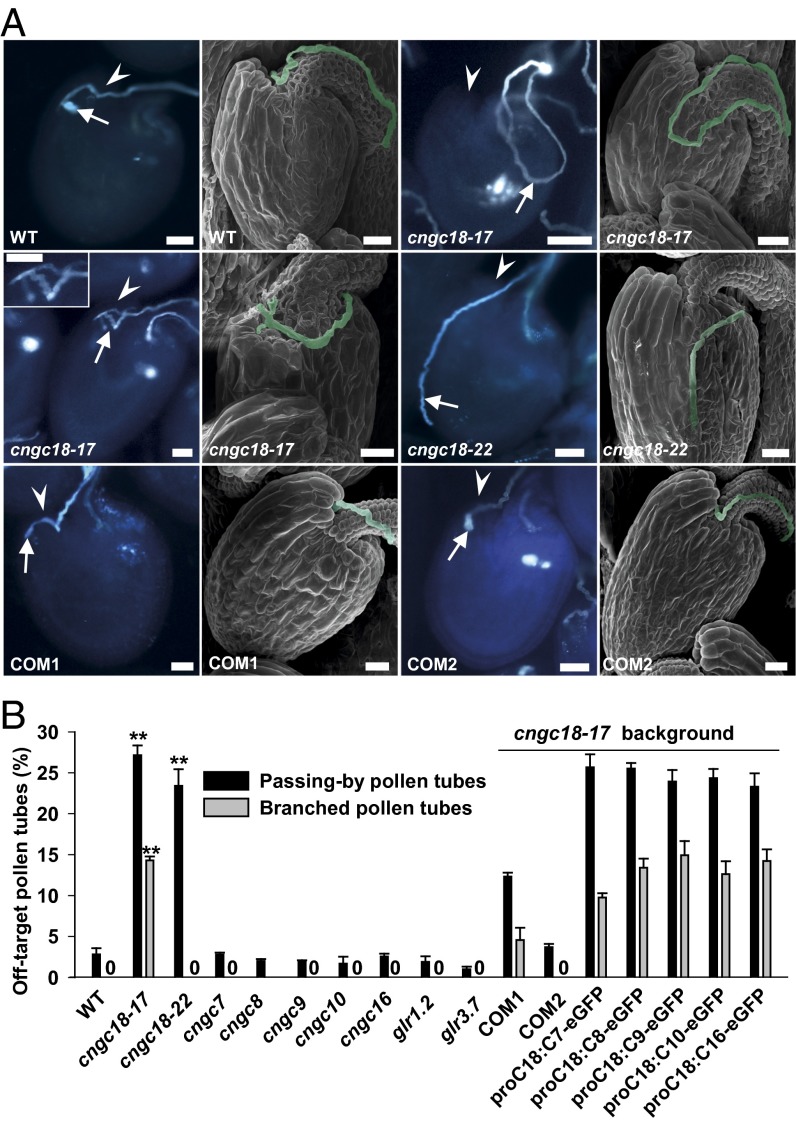
We conducted limited pollination experiments using male sterility 1 (ms1) plants (43) as the female parent and the cngc and glr mutants as male parents. We found that ∼96% of the pollen tubes of seven mutants (cngc7, cngc8, cngc9, cngc10, cngc16, glr1.2, and glr3.7) and WT successfully entered into ms1 micropyles (hereafter termed on-target pollen tubes) (Fig. S5A) whereas less than 5% of pollen tubes failed to do so (hereafter termed off-target pollen tubes) (Fig. S5C). Interestingly, there were 41.9% and 24.1% off-target pollen tubes for cngc18-17 and cngc18-22, respectively, significantly more than in WT and in the other seven mutants (Fig. S5C). Further observation revealed that the off-target pollen tubes fell into two groups. The first group was termed passing-by pollen tubes, which either wandered around a micropyle or passed very close to a micropyle (Fig. 2A). The second group was termed branched pollen tubes, which branched and stopped near micropyles (Fig. 2A). There were 27.5% and 24.1% passing-by pollen tubes for cngc18-17 and cngc18-22, respectively, much more than the 2.8% in WT (Fig. 2B). There were 14.4% branched pollen tubes for cngc18-17, but no branched pollen tubes in cngc18-22 or WT (0%) (Fig. 2B). All other cngc and glr mutants showed no branched pollen tubes, except for cngc8 (a single branched pollen tube out of 155) (Fig. 2B). These data demonstrate that CNGC18 is the main Ca2+ channel essential for pollen tube guidance in Arabidopsis. CNGCs are downstream targets of cyclic nucleotides (cAMP and cGMP), and cAMP has been reported to function as a second messenger in pollen tube orientation by regulating Ca2+ channel activity in pollen tubes (34, 44, 45). Then, we focused on the six CNGCs, especially CNGC18, in further experiments.
Fig. S5.
Pollen tube growth analysis. (A and B) Aniline blue staining images showing typical on-target pollen tubes of WT and seven channel mutants (cngc7, -8, -9, -10, and -16 and glr1.2 and -3.7) (A) and a typical on-target pollen tube of WT and off-target pollen tubes of transgenic lines expressing either of the five CNGCs (CNGC7, -8, -9, -10, and -16) in the cngc18-17 background (B). Pollen tubes are indicated using white arrows in A and B. (C) The percentages of total off-target pollen tubes, including passing-by and branched pollen tubes. Pollen tubes and micropyles were indicated by white arrows and arrowheads, respectively. (Scale bars: 20 µm.) proC18:C7-eGFP denotes proCNGC18:CNGC7-eGFP, and the others are similarly represented.
Fig. 2.
The R491Q and R578K point mutations in CNGC18 resulted in severe pollen tube growth defects whereas the T-DNA insertion mutations in the other seven Ca2+ channels had no pollen tube growth defects. (A) In vivo aniline blue staining and scanning electron microscopy images, showing typical on-target pollen tubes of WT, COM1, and COM2, examples of passing-by pollen tubes of cngc18-17 and cngc18-22, and an example of branched pollen tubes of cngc18-17. Pollen tubes and micropyles are indicated by white arrows and arrowheads, respectively. (Scale bars: 20 µm.) (B) Percentages of passing-by and branched pollen tubes. A “0” indicates that no branched pollen tubes were observed. **Significant difference from WT (P < 0.01). Error bars depict means ± SEM.
We generated transgenic Arabidopsis lines by expressing enhanced green fluorescent protein (eGFP)-tagged versions of CNGC7, CNGC8, CNGC9, CNGC10, and CNGC16 in cngc18-17, under the CNGC18 promoter, to ensure that these genes were expressed at a level similar to CNGC18. We failed to observe an eGFP signal in the transgenic plants because of a weak CNGC18 promoter (38). However, qRT-PCR (quantitative real-time PCR) data verified the expression of eGFP fused to the C terminus of the CNGCs (Fig. S4 and Table S1). We then performed further limited pollination experiments with these transgenic lines. The percentages of off-target pollen tubes (passing-by pollen tubes plus branched pollen tubes) were 36.2% (25.7% + 10.5%), 38.5% (25.5% + 13%), 39.1% (23.9% + 15.2%), 36.8% (24.3% + 12.5%), and 37.3% (23.3% + 14%) for cngc18-17 mutants expressing CNGC7, -8, -9, -10, and -16, respectively (Fig. 2B and Fig. S5 B and C), similar to the cngc18-17 mutant. However, the percentages of off-target pollen tubes were 16.9% for COM1 (12.7% passing-by pollen tubes and 4.2% branched pollen tubes) and 3.4% for COM2 (including only passing-by pollen tubes and no branched pollen tubes), in comparison with 2.8% for WT and 41.9% for the cngc18-17 mutant (Fig. 2 A and B and Fig. S5C). These data demonstrate that the pollen tube guidance defects of cngc18-17 were rescued by CNGC18, but not by the other five CNGCs.
Among the Six CNGCs Present in Pollen Tubes, only CNGC18 Is Able to Completely Rescue the Male Transmission Defect of cngc18-1 (+/−).
To further verify the role of CNGC18 in pollen tube guidance, we expressed eGFP-tagged versions of CNGC7, -8, -9, -10, -16, and -18 and two point-mutated CNGC18, CNGC18-R491Q and CNGC18-R578K, in T-DNA insertion mutant cngc18-1 (+/−) under the CNGC18 promoter (Fig. S4 and Table S1) and then analyzed whether the male transmission defect of heterozygous cngc18-1 (+/−) could be rescued by any of the CNGCs. The male transmission defect of cngc18-1 (+/−) (0%) was completely rescued by CNGC18-eGFP (53%); strongly rescued by CNGC18-R578K-eGFP (22.0%); poorly rescued by CNGC18-R491Q-eGFP (0.83%), CNGC7-eGFP (3.58%), CNGC10-eGFP (0.86%), and CNGC16-eGFP (0.63%); and not rescued by CNGC8-eGFP (0%) and CNGC9-eGFP (0%) (Table 1). The poor rescue of the male transmission defect of cngc18-1 (+/−) by CNGC18-R491Q-eGFP is consistent with the strong male transmission and pollen tube guidance defects of cngc18-17 whereas the strong rescue of the male transmission defect of cngc18-1 (+/−) by CNGC18-R578K-eGFP is consistent with the much weaker male transmission and pollen tube guidance defects of the cngc18-22 mutant (Figs. 1 and 2 and Table 1). Similarly, the rescue of the transmission defect of cngc18-1 (+/−) by the other five CNGCs is in agreement with the normal pollen tube guidance in their T-DNA insertion knockout mutants (Fig. 2 and Fig. S5). This genetic evidence clearly demonstrates that CNGC18 is an essential Ca2+ channel for pollen tube guidance in Arabidopsis whereas the other five CNGCs and two GLRs play no role or negligible roles in this process.
Table 1.
Transmission analysis of WT, mutants, and transgenic Arabidopsis lines
| Parent, female × male | Segregation of resistance genes (resistant/sensitive) | ||
| Expected | Observed | Transmission, % | |
| ms1 × cngc18-1 (+/−) | 298: 298 | 0:596 | 0** |
| ms1 × proC18:CNGC7-eGFP/cngc18-1 (+/−) | 223: 223 | 16:431 | 3.58** |
| ms1 × proC18:CNGC8-eGFP/cngc18-1 (+/−) | 711: 711 | 0:1,423 | 0** |
| ms1 × proC18:CNGC9-eGFP/cngc18-1 (+/−) | 183: 183 | 0:366 | 0** |
| ms1 × proC18:CNGC10-eGFP/cngc18-1 (+/−) | 347: 347 | 6:688 | 0.86** |
| ms1 × proC18:CNGC16-eGFP/cngc18-1 (+/−) | 79: 79 | 1:157 | 0.63** |
| ms1 × proC18:CNGC18-eGFP/cngc18-1 (+/−) | 231: 231 | 245: 217 | 53.0 |
| ms1 × proC18:CNGC18-R491Q-eGFP/cngc18-1 (+/−) | 60: 60 | 1:120 | 0.83** |
| ms1 × proC18:CNGC18-R578K-eGFP/cngc18-1 (+/−) | 250:250 | 110:390 | 22.0** |
| ms1 × proC18:eGFP/cngc18-1 (+/−) | 62:62 | 0:124 | 0 ** |
| ms1 × proC18:CNGC16-18NT/cngc18-1 (+/−) | 64:64 | 1:127 | 0.781** |
| ms1 × proC18:CNGC16-18TM/cngc18-1 (+/−) | 77:77 | 69:85 | 44.8 |
| ms1 × proC18:CNGC16-18CT/cngc18-1 (+/−) | 68:68 | 2:135 | 1.46** |
| ms1 × proC18:CNGC18-16TM/cngc18-1 (+/−) | 58:58 | 1:115 | 0.862** |
proC18, the promoter of CNGC18. **Significant difference from the expected Mendelian segregation ratio (1:1, e.g., 50%) (χ2 test, χ2, P < 0.01).
The Transmembrane Domains of CNGC18 Are Essential for Pollen Tube Guidance.
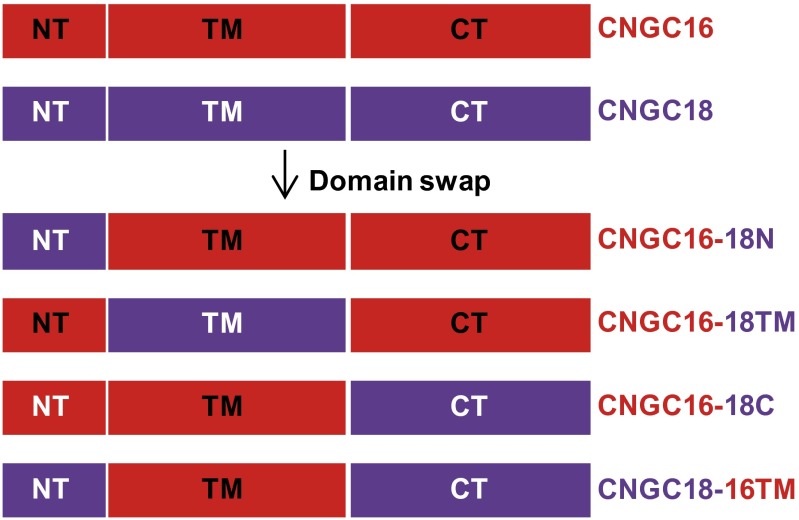
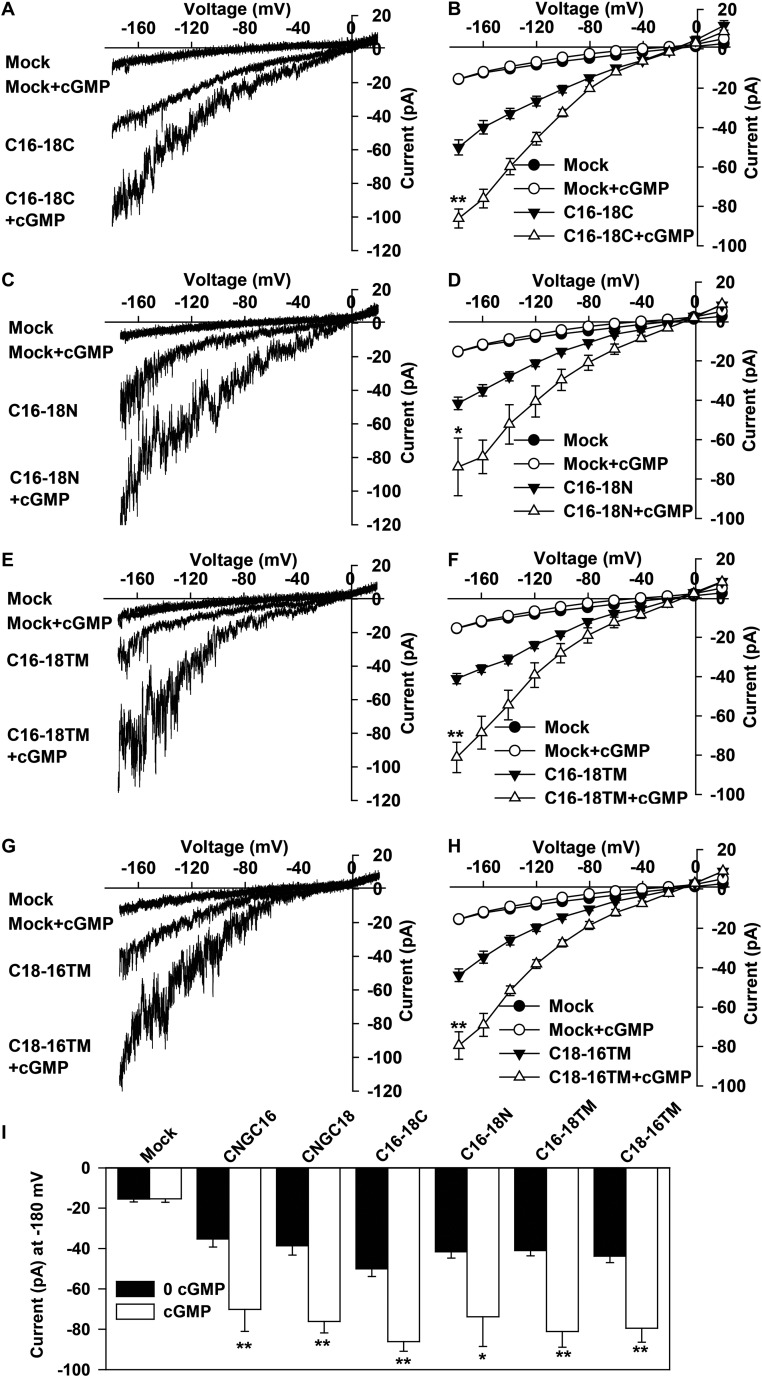
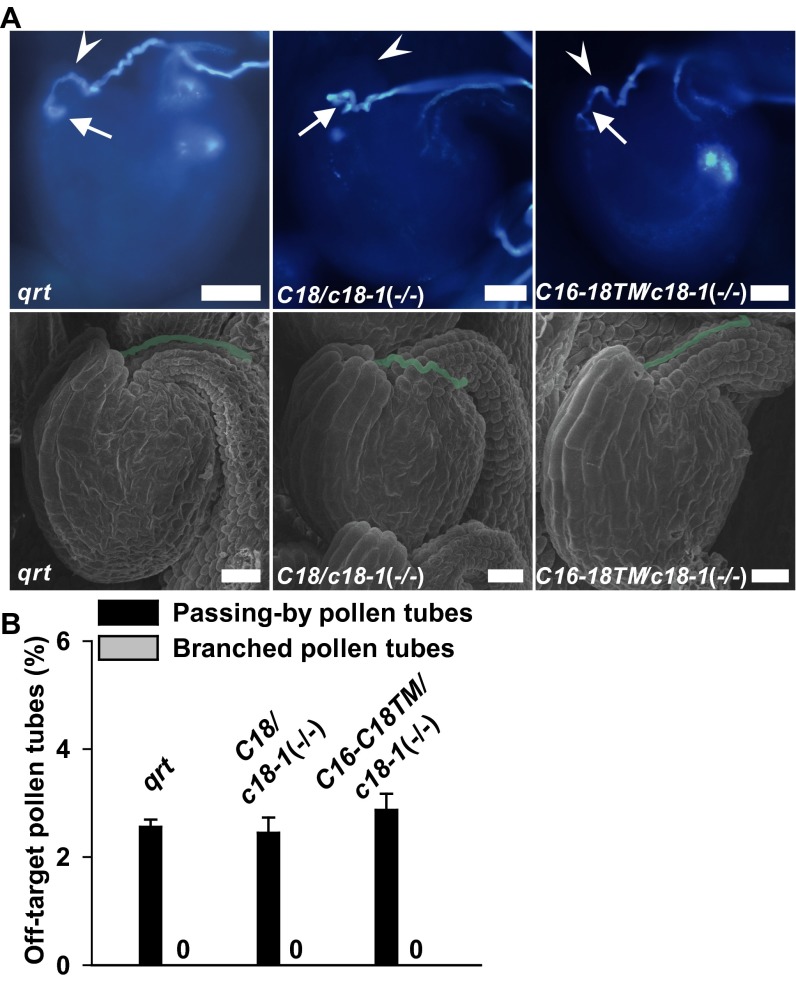
CNGC16 is a close homolog of CNGC18, but with no role or a negligible role in pollen tube guidance (Fig. 2, Fig. S5, and Table 1). To investigate which domain of CNGC18 is essential for pollen tube guidance, we exchanged domains between CNGC18 and CNGC16 (Fig. S6). We tested the Ca2+ channel activity of the chimeric channels using a patch-clamping technique after expressing the chimeric channels in HEK293T cells. We observed similar Ca2+ channel currents and cGMP-induced channel currents in HEK293T cells expressing the chimeric channels (Fig. S7) as for WT CNGC16 (Fig. S1 I and J) or WT CNGC18, as previously reported (41). These data demonstrate that the chimeric channels have normal Ca2+ channel activity and can be activated normally by cyclic nucleotides. We then generated transgenic Arabidopsis lines by expressing the chimeric channels driven by the CNGC18 promoter in the cngc18-1 (+/−) background. The male transmission defect of cngc18-1 (+/−) was poorly rescued by CNGC16-18N (0.781%), CNGC16-18C (1.46%), and CNGC18-16TM (0.862%), but was rescued by CNGC16-18TM nearly completely (44.8%), as for WT CNGC18 (53%) (Table 1). We carried out limited pollination experiments and found that the homozygous transgenic plants CNGC16-18TM/cngc18-1 (−/−) showed no obvious defect in pollen tube guidance compared with the control qrt plants (Fig. S8). These data demonstrate that the transmembrane domains of CNGC18 are indispensable domains for its essential role in pollen tube guidance in Arabidopsis.
Fig. S6.
Schematic for domain swapping between CNGC18 and CNGC16. NT, TM, and CT denote N-terminal domain, transmembrane domains, and C-terminal domain, respectively.
Fig. S7.
The chimeric channels CNGC16-18C, CNGC16-18N, CNGC16-18TM, and CNGC18-16TM are all Ca2+-permeable channels and can be activated by cyclic nucleotide cGMP. (A and B) Typical whole-cell recordings (A) and the average current-voltage curves (B) for CNGC16-18CT. Seven HEK293T cells were tested for CNGC16-18CT and CNGC16-18CT + 100 μM 8Br-cGMP. (C and D) Typical whole-cell patch-clamping recordings (C) and the average current-voltage curves (D) for CNGC16-18NT. Eight HEK293T cells were tested for CNGC16-18N and 6 cells for CNGC16-18NT + 100 μM 8Br-cGMP. (E and F) Typical whole-cell recordings (E) and the average current-voltage curves (F) for CNGC16-18TM. Ten HEK293T cells were tested for CNGC16-18TM and 6 cells for CNGC16-18TM + 100 μM 8Br-cGMP. (G and H) Typical whole-cell recordings (G) and the average current-voltage curves (H) for the CNGC18-16TM. Eleven HEK293T cells were tested for CNGC18-16TM and 8 cells for CNGC18-16TM + 100 μM 8Br-cGMP. (I) The amplitudes of average steady-state whole-cell currents recorded at −180 mV as shown in B, D, F, H, Fig. S1J, and Fig. 4B. Seven HEK293T cells were tested for Mock and Mock + 8Br-cGMP, and the same Mock and Mock + 8Br-cGMP data were used to plot the average current-voltage curves in B, D, F, and H. Error bars depict means ± SEM. *Significant difference relative to cyclic nucleotide-free conditions (P < 0.05); **significant difference relative to cyclic nucleotide-free conditions (P < 0.01).
Fig. S8.
The transmembrane domains of CNGC18 are essential for pollen tube growth and guidance. (A) Aniline blue staining (Upper) and scanning electron microscopy observation (Lower) of pollen tubes attracted by ovules, showing that the pollen tubes of qrt, CNGC18/cngc18-1 (−/−), and CNGC16-18TM/cngc18-1 (−/−) precisely entered micropyles. Pollen tubes and micropyles are indicated by white arrows and arrowheads, respectively. (Scale bars: 20 µm.) (B) The proportion of pollen tubes that precisely entered an ovule. Three replicates were performed. Error bars depict means ± SEM. Note that the background of cngc18-1 is qrt and that the qrt mutant was used as a positive control, as reported (37).
The Point Mutations R491Q and R578K Impaired the Activation of CNGC18 by Cyclic Nucleotides Both in Vitro and in Vivo.
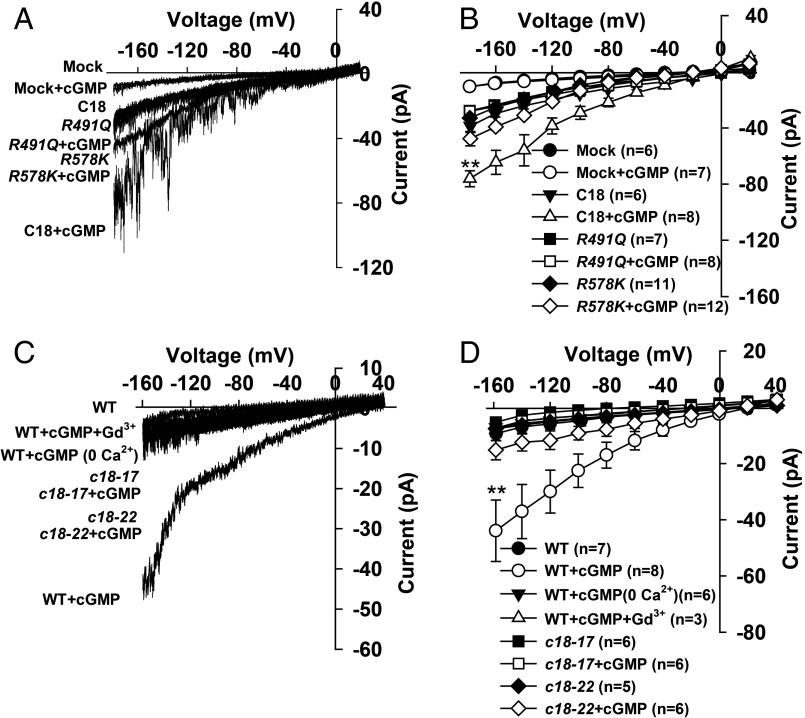
To investigate how the two point mutations affected the channel activity of CNGC18, we carried out patch-clamping experiments in HEK293T cells via transient expression. We observed obvious inward Ca2+ channel currents in HEK293T cells expressing either CNGC18-R491Q, CNGC18-R578K, or WT CNGC18 (Fig. 3 A and B), showing no significant impairment of the channel activity by the two point mutations in CNGC18. Interestingly, we found that both CNGC18-R491Q and CNGC18-R578K failed to be further activated by the application of 100 μM 8Br-cGMP whereas WT CNGC18 was significantly activated (Fig. 3 A and B), as previously reported (41). To test whether the point mutations R491Q and R578K impaired the activation of CNGC18 in vivo, we isolated pollen tube protoplasts (46) and measured the whole-cell inward Ca2+ currents using a patch-clamping technique (32). In comparison with background currents in a cGMP-free condition, large currents were triggered by the intracellular application of 100 μM cGMP in WT (Fig. 3 C and D). These cGMP-activated currents were abolished by removal of extracellular Ca2+ or by application of the Ca2+ channel blockers La3+ (100 μM) or Gd3+ (100 μM) (Fig. 3 C and D). The reversal potential shifted from +21.5 mV to +2.9 mV upon removing extracellular Ca2+ (Fig. 3D). These results demonstrate that the cGMP-activated inward currents in Arabidopsis pollen tube protoplasts are Ca2+ channel currents. Further patch-clamping results showed that the cGMP-activated Ca2+ currents were impaired in cngc18-17 and cngc18-22 relative to WT (Fig. 3 C and D). Together, the patch-clamping data in both HEK293T cells and pollen tube protoplasts demonstrate that the two point mutations R491Q and R578K impaired the cGMP activation of the Ca2+ channel CNGC18.
Fig. 3.
The CNGC18 point mutations R491Q and R578K impair activation of CNGC18 by cGMP. (A and B) Typical whole-cell recordings (A) and the average current-voltage curves (B) of whole-cell currents recorded in HEK293T cells. (C and D) Typical whole-cell recordings (C) and the average current-voltage curves (D) of whole-cell currents recorded in Arabidopsis pollen tube protoplasts. Extracellular 8Br-cGMP and intracellular cGMP treatments were used to activate channel currents in HEK293T cells and pollen tube protoplasts, respectively. C18, R491Q, R578K, c18-17, and c18-22 denote WT CNGC18, CNGC18-R491Q, CNGC18-R578K, cngc18-17, and cngc18-22, respectively. Error bars depict means ± SEM. **Significant difference from WT (P < 0.01).
cngc18-17 and cngc18-22 Mutants Showed Abnormal Cytosolic Ca2+ Oscillation in Pollen Tube Tips.
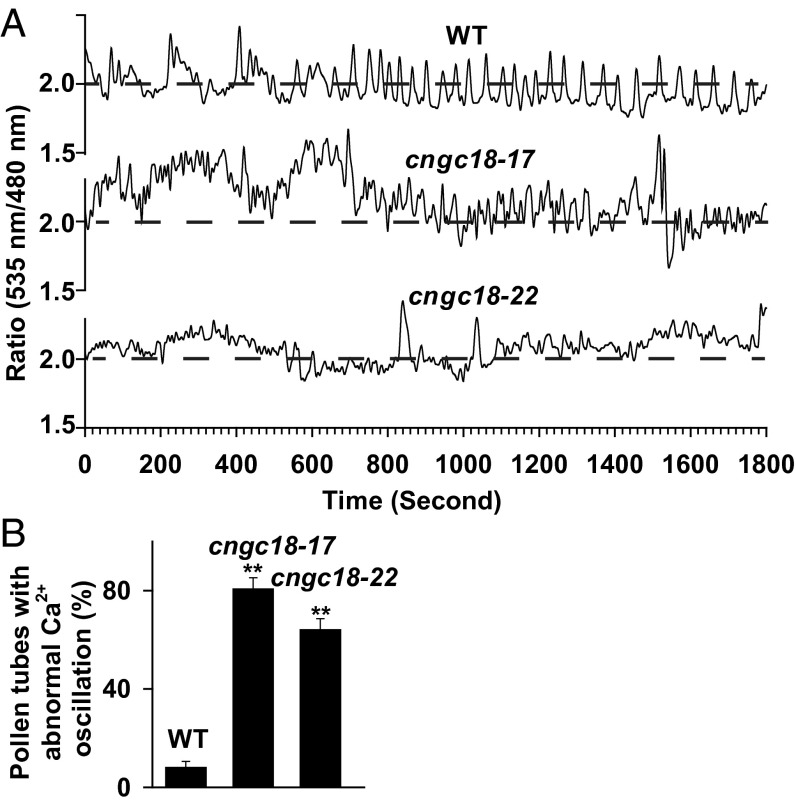
We tested whether the guidance defect of pollen tubes in cngc18-17 and cngc18-22 resulted from an abnormal Ca2+ gradient at the pollen tube tips. We monitored the dynamic fluorescence of the Ca2+ sensor yellow cameleon version 3.6 (YC3.6) and observed regular and normal [Ca2+]cyt (cytosolic Ca2+ concentration) oscillation at different frequencies around a relatively stable [Ca2+]cyt level in growing pollen tube tips of WT (Fig. 4A), similar to previous reports (25, 47). However, most of the growing pollen tube tips of cngc18-17 (Fig. 4A) and cngc18-22 (Fig. 4A) manifested abnormal Ca2+ oscillation patterns, especially irregular drifts of basal [Ca2+]cyt levels, whereas, in WT, there were relatively stable [Ca2+]cyt levels (Fig. 4A). There were 80.6% and 64.1% pollen tubes showing abnormal drifts of basal [Ca2+]cyt for cngc18-17 and cngc18-22, respectively, compared with 8% for WT (Fig. 4B). It has been reported that the [Ca2+]cyt gradients, rather than high frequent [Ca2+]cyt oscillation, are required for pollen tube growth and guidance (24, 30). Therefore, the irregular drift of the basal [Ca2+]cyt levels (i.e., the disturbance of the Ca2+ gradients in pollen tube tips) might be the main cause of pollen tube guidance defects in cngc18-17 and cngc18-22.
Fig. 4.
CNGC18 point mutations R491Q and R578K lead to irregular drifts of basal [Ca2+]cyt levels in Arabidopsis pollen tube tips. (A) Time course of typical [Ca2+]cyt oscillation in the pollen tube tips of WT, cngc18-17, and cngc18-22. (B) Fractions of pollen tubes showing irregular drifts of basal [Ca2+]cyt levels. Thirty-five pollen tubes were tested for WT, 33 for cngc18-17, and 39 for cngc18-22. Dashed lines indicate the expected relatively stable basal [Ca2+]cyt levels. Error bars depict means ± SEM. **Significant difference from WT (P < 0.01).
Discussion
A number of candidate channel families for Ca2+ channels were identified in Arabidopsis, including ligand-gated channels, such as CNGC and GLR, and stretch-activated Ca2+ channels OSCA (reduced hyperosmolality-induced [Ca2+]i increase 1) and MCA (MID1-complementing activity 1) families (20, 48, 49). Although eight are present in pollen tubes, only CNGC18 is essential for pollen tube guidance. Therefore, CNGC18 could be the main Ca2+ channel for pollen tube guidance in Arabidopsis.
It is striking that CNGC18 is essential for pollen tube guidance whereas the other five CNGCs tested apparently have a negligible role or no such role. CNGC18 is mainly localized in the tips of pollen tubes (37) whereas CNGC7 and CNGC8 are mainly localized at the pollen tube flanks (38). A T-DNA insertion in CNGC16 showed no phenotype in normal plant growth conditions (39), and CNGC9 and CNGC10 had no function in pollen tube guidance (Fig. 2 and Fig. S5). Thus, it is plausible that the subcellular localization of CNGC18 might explain its essential function in pollen tube growth and guidance.
CNGC18 may play dual roles in pollen tube tips. First, CNGC18 mediates external Ca2+ influx to establish and maintain the Ca2+ gradient, which allows the formation and tip growth of pollen tubes. This role of CNGC18 may account for the lethal male sterile phenotype of T-DNA insertion mutant cngc18-1 (37) because the absence of the Ca2+ channel activity and Ca2+ gradient is lethal for pollen tubes. Second, CNGC18 is critical for pollen tube guidance. Both R491Q and R578K did not abolish the Ca2+ channel activity of CNGC18 but impaired its further activation by cyclic nucleotides (Fig. 3). The Ca2+ channel activity of the two point-mutated CNGC18 allows external Ca2+ influx to establish the Ca2+ gradient and further allows the formation and tip growth of pollen tubes. But the two point mutations in CNGC18 could reduce the sensitivity of CNGC18 to upstream regulators, by impairing its further activation, and consequently lead to abnormal Ca2+ gradients and pollen tube guidance defects. The upstream regulators of CNGC18 could be cyclic nucleotides and/or protein kinases (50).
The transmembrane domains of CNGC18 are indispensable for pollen tube guidance (Table 1 and Fig. S8). However, the importance of CNGC18's transmembrane domains does not exclude the importance of its N terminus and especially the C terminus. The free C terminus of CNGC18 might be the main regulatory domain for its Ca2+ channel activity because both the cyclic nucleotide binding domain (CNBD) and calmodulin-binding domain are in the C terminus. Furthermore, both the point mutations R491Q and R578K are in the CNBD domain (Fig. 1). These point mutations did not abolish the Ca2+ channel activity of CNGC18 (Fig. 3) but impaired its further activation by cyclic nucleotides (Fig. 3) and led to sterility and a pollen tube guidance defect (Figs. 1 and 2 and Fig. S5), supporting the importance of the C terminus for pollen tube guidance. The rescue of the male transmission and pollen tube guidance defects of cngc18-1 (+/−) by CNGC16-18TM (Table 1 and Fig. S8) might be explained by two possible scenarios. First, the transmembrane domains of CNGC18 might have caused a localization of the chimeric channel at pollen tube tips. Secondly, the C terminus of CNGC16 is similar to that of CNGC18 and thus might have allowed the chimeric channel to be regulated by upstream regulators of CNGC18.
We cannot exclude the involvement of GLRs in pollen tube guidance, but these two GLRs could play only minor roles, if any, considering the normal pollen tube guidance in glr1.2 and glr3.7 mutants (Fig. 2 and Fig. S5). GLR and CNGC are quite different Ca2+ channels, including their amino acid residue sequences and activating ligands (40, 41). Therefore, it is reasonable to assume that GLRs respond to different upstream attractants than CNGC18.
Taken together, CNGC18 might be the main Ca2+ channel for pollen tube guidance and tip growth in Arabidopsis.
Materials and Methods
In Vitro Pollen Germination and Tube Growth Assay.
The medium for in vitro pollen germination and tube culturing was modified from a previous report (51) and contained 18% (wt/vol) sucrose, 1.6 mM boric acid, 10 mM CaCl2, 1 mM Ca(NO3)2, 1 mM MgSO4, and 0.5% (wt/vol) agarose, adjusted to pH 6.4 with KOH. In vitro pollen germination and tube culturing experiments were conducted at 22 °C under a 100% saturated relative humidity for 6 h (52) and then photographed using an inverted microscope (model Axio observer D1; Carl Zeiss) equipped with a neo CMOS CCD camera (Andor Technology). In each replicate, about 300 pollen grains were counted for pollen germination analysis, and about 100 pollen tubes were used for pollen tube length analysis. Pollen tube length was measured using Image J (imagej.nih.gov/ij/, 1997–2012). Three replicates were conducted.
In Vivo Pollen Tube Growth Observations.
Pollen tube growth was analyzed using limited pollination methods. About 10 pollen grains were manually placed on each ms1 pistil. After 16 h to allow pollen grains to germinate and pollen tubes to grow, the pistils were cut off and fixed in 3:1 ethanol:acetic acid for 24 h as described (53).
For decolorized aniline blue staining, the fixed pistils were rehydrated in a series of ethanol solutions (70%, 50%, 30%) and then with distilled water, softened in 8 M NaOH for 12 h, and washed five times with distilled water. The pistils were stained overnight in 0.1% decolorized aniline blue (pH 11 in 50 mM K3PO4), and pictures were taken under a fluorescence microscope (Model DM6000B; Leica). Three replicates were performed, and no fewer than 40 pollen tubes were analyzed for each assay in each replicate.
For scanning electron microscopy observations, the pistils were fixed in FAA solution (50% ethanol, 5% acetic acid, 3.7% formaldehyde) for 24 h and dehydrated with a series of ethanol solutions (30%, 50%, 70%, 85%, 95%, and 100%) (54). The pistils were subsequently CO2 critical point-dried, coated with sputtering gold, and then observed using a scanning electron microscope (JSM-6360LV; JEOL).
Whole Cell Patch-Clamping Experiments.
CNGC-mediated Ca2+ and K+ current recordings in HEK293T cells were performed as described (41). For whole-cell Ca2+ current recordings in Arabidopsis pollen tube protoplasts, protoplast isolation was carried out as described (46). cGMP was freshly added each day. A ramp voltage protocol of 2 s duration from −180 mV to +20 mV was applied 1 min after accessing to whole-cell configuration (32). Whole-cell Ca2+ currents were recorded every 30 s for 10 min. See SI Materials and Methods for bath and pipette solutions for patch-clamping experiments in HEK293T cells and Arabidopsis pollen tube protoplasts.
Cytosolic Ca2+ Imaging in Pollen Tube Tips.
Ca2+ imaging in pollen tube tips was performed by monitoring the ratio (535 nm/480 nm) of YC3.6 using an inverted microscope (Model IX71; Olympus). The interval of image acquisition was 5 s, and the exposure time for excitation light was 200 ms for both 535 nm and 480 nm. Software MAG Biosystems 7.5 (MetaMorph) was used for data acquisition and analysis.
SI Materials and Methods
Plant Material and Growth Conditions.
Arabidopsis thaliana plants (Columbia-0 ecotype) were grown in soil (Sunrise) in a growth room under a 16-h-light/8-h-dark cycle at a photon fluence rate of approximate 80 mmol⋅m−2⋅s−1 during the day, with a temperature of 22 ± 0.5 °C, at a relative humidity of ≥70%.
Arabidopsis Mutant Collection and Identification.
Twenty-three TILLING alleles containing point mutations in CNGC18 (55) were obtained from ABRC (Arabidopsis Biological Resource Center), two of which (Salk line no. CS91890, R491Q; and CS87733, R578K) showed strong but partial male sterility. The two lines were designated cngc18-17 (R491Q) and cngc18-22 (R578K) according to the full mutant list, in which the first line was the T-DNA insertion mutant cngc18-1 (37), and the 23 point mutants were named as cngc18-2 to cngc18-24. These two point mutants were backcrossed to WT three times to clean their genetic background, and the point mutations were reconfirmed by DNA sequencing.
T-DNA insertion mutants cngc7, cngc8, cngc9, cngc10, cngc16, glr1.2, and glr3.7 were obtained from ABRC. Bloomed flowers were used for RNA extraction and RT-PCR for the identification of these mutants. The mutants were backcrossed to WT two times to clean their genetic background before further experiments.
Vector Construction and Plant Transformation.
The constructs were prepared in pCambia1304 with eGFP fused to the C terminus of the CNGCs. The CNGC18 promoter was PCR-amplified and cloned at the EcoRI and KpnI sites. Then, full-length cDNAs of each CNGC were amplified and cloned downstream of the CNGC18 promoter at the KpnI and PstI sites. For the eGFP fusion, the coding sequence of eGFP was amplified and cloned downstream of the CNGCs at the NcoI and BstEII sites. Transformation of Arabidopsis was carried out with Agrobacterium tumefaciens (strain GV3101) using the floral dip method (56). To express the CNGCs in HEK293T cells, full-length cDNAs of CNGC7, CNGC8, CNGC9, CNGC10, CNGC16, and CNGC18 were cloned into pCI-neo (Promega) as described (41). For domain swapping between CNGC16 and CNGC18, an overlap extension PCR-based method for site-directed mutagenesis was used (57).
Primers for gene cloning and vector construction are listed in Table S1.
HEK293T Cell Culture and Transfection.
HEK293T cells were cultured in Dulbecco’s modified Eagle's medium supplemented with 10% FBS, penicillin (100 IU/mL), and streptomycin (100 μg/mL) in a water-injected incubator (Thermo) with 5% CO2 at 37 °C in a moist atmosphere, as described (41). Transfections were performed using a Lipofectamine 2000 Transfection Reagent Kit (Invitrogen). Plasmids for transfection were extracted from Escherichia coli (DH5α) using a QIAGEN Plasmid Midi Kit, and 2 μg of plasmids were added into each well of a six-well plate. HEK293T cells were used for patch-clamping experiments 48 h after transfection. Each of the CNGCs (CNGC7, -8, -9, -10, -16, and -18) was cotransfected with peGFP-C1 (Clontech) at a 10:1 plasmid ratio (CNGC/eGFP), as described (41). HEK293T cells showing bright green fluorescence were used for patch-clamping experiments.
Generating Transgenic Arabidopsis Lines Expressing Yellow Cameleon for Ca2+ Imaging in Pollen Tube Tips.
A transgenic Arabidopsis line expressing yellow cameleon (Version 3.6) (YC3.6) under a LAT52 promoter (58) was generated and then crossed with cngc18-17 and cngc18-22 mutants. cngc18-17 and cngc18-22 mutants expressing YC3.6 were selected from F2 plants. Pollen tubes were germinated and incubated in vitro on solid medium at 22 °C for 6 h (52).
Bath and Pipette Solutions for Patch-Clamping Experiments.
To record Ca2+ currents in HEK293T cells, the standard bath solution contained 120 mM NaCl, 2.8 mM KCl, 10 mM CsCl, 2 mM MgCl2, 10 mM CaCl2, and 10 mM Hepes, adjusted to pH 7.2 with NaOH, as reported (41). The standard pipette solution contained 120 mM Cs-glutamate, 8 mM NaCl, 6.7 mM EGTA, 3.35 mM CaCl2, 3 mM MgCl2, and 10 mM Hepes, adjusted to pH 7.2 with CsOH, as reported (41). Osmolality was adjusted to 313 mmol/kg using d-glucose for both the bath and the pipette solutions. Free [Ca2+] in the pipette solution was 175 nM, as calculated using the Webmaxc Standard (web.stanford.edu/∼cpatton/webmaxc/webmaxcS.htm). Then, 10 mM Ca2+ in the bath solution was removed to attain 0 mM Ca2+ or substituted with 10 mM Ba2+ as indicated.
For inward K+ current recordings in HEK293T cells, the bath solution contained 120 mM NaCl, 14.5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 10 mM glucose, and 10 mM Hepes, adjusted to pH 7.2 with NaOH. The pipette solution contained 145 mM K-glutamate, 8 mM Na-glutamate, 4 mM Mg-glutamate, 3.35 mM EGTA, 1.675 mM CaCl2, and 10 mM Hepes, adjusted to pH 7.2 with NaOH, as reported (41). The free Ca2+ concentration in the pipette solution was 100 nM, as calculated using the Webmaxc Standard. The osmolality for both standard bath and pipette solutions was 313.3 mmol/kg adjusted with d-glucose. To activate CNGC channel currents in HEK293T cells, 8Br-cGMP and 8Br-cAMP were applied extracellularly by perfusion.
To record Ca2+ currents in Arabidopsis pollen tube protoplasts, the standard bath solution contained 50 mM CaCl2, and 5 mM Mes, adjusted to pH 5.8 with Tris. The standard pipette solution contained 2 mM BAPTA, 0.05 mM CaCl2, 0.09 mM calcium gluconate, 92 mM potassium glutamate, and 5 mM Hepes, adjusted to pH 7.2 with Tris. The osmolality was adjusted to 1.5 mol/kg and 1.55 mol/kg with d-sorbitol for the standard bath and pipette solutions, respectively (32). The free Ca2+ concentration in the pipette solution was 100 nM, as calculated using the Webmaxc Standard.
Quantitative RT-PCR.
Expression levels of CNGCs were determined by qRT-PCR by testing the expression levels of eGFP fused to the C terminus of the CNGCs, with ACTIN2 as a normalization standard. Bloomed flowers were used as the material for qRT-PCR experiments. Primers are listed in Table S1.
Accession Numbers.
The gene sequence information in this article can be found in GenBank databases using accession numbers AT5G14870 (CNGC18), AT1G15990 (CNGC7), AT1G19780 (CNGC8), AT4G30560 (CNGC9), AT1G01340 (CNGC10), AT3G48010 (CNGC16), AT5G48400 (GLR1.2), AT2G32400 (GLR3.7), and AT3G18780 (ACTIN2).
Acknowledgments
We thank Sheila McCormick (University of California, Berkeley) for helpful discussion about the research and for editing the English, Zhenbiao Yang (University of California, Riverside) for suggestions for the manuscript writing, and Xiaoshu Gao and Jiqin Li (SIPPE) for assistance in aniline blue staining and scanning electron microscopy experiments. This research was supported by Hundred Talents Program of the Chinese Academy of Sciences Grant 2010OHTP06 and National Natural Science Foundation of China Grant 31071239, and was partially supported by China Postdoctoral Science Foundation Grant 2015M571608.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1524629113/-/DCSupplemental.
References
- 1.Higashiyama T, Takeuchi H. The mechanism and key molecules involved in pollen tube guidance. Annu Rev Plant Biol. 2015;66:393–413. doi: 10.1146/annurev-arplant-043014-115635. [DOI] [PubMed] [Google Scholar]
- 2.Dresselhaus T, Márton ML. Micropylar pollen tube guidance and burst: Adapted from defense mechanisms? Curr Opin Plant Biol. 2009;12(6):773–780. doi: 10.1016/j.pbi.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 3.Dresselhaus T, Franklin-Tong N. Male-female crosstalk during pollen germination, tube growth and guidance, and double fertilization. Mol Plant. 2013;6(4):1018–1036. doi: 10.1093/mp/sst061. [DOI] [PubMed] [Google Scholar]
- 4.Wu HM, Wang H, Cheung AY. A pollen tube growth stimulatory glycoprotein is deglycosylated by pollen tubes and displays a glycosylation gradient in the flower. Cell. 1995;82(3):395–403. doi: 10.1016/0092-8674(95)90428-x. [DOI] [PubMed] [Google Scholar]
- 5.Palanivelu R, Brass L, Edlund AF, Preuss D. Pollen tube growth and guidance is regulated by POP2, an Arabidopsis gene that controls GABA levels. Cell. 2003;114(1):47–59. doi: 10.1016/s0092-8674(03)00479-3. [DOI] [PubMed] [Google Scholar]
- 6.Chae K, Kieslich CA, Morikis D, Kim SC, Lord EM. A gain-of-function mutation of Arabidopsis lipid transfer protein 5 disturbs pollen tube tip growth and fertilization. Plant Cell. 2009;21(12):3902–3914. doi: 10.1105/tpc.109.070854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim S, et al. Chemocyanin, a small basic protein from the lily stigma, induces pollen tube chemotropism. Proc Natl Acad Sci USA. 2003;100(26):16125–16130. doi: 10.1073/pnas.2533800100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Okuda S, et al. Defensin-like polypeptide LUREs are pollen tube attractants secreted from synergid cells. Nature. 2009;458(7236):357–361. doi: 10.1038/nature07882. [DOI] [PubMed] [Google Scholar]
- 9.Takeuchi H, Higashiyama T. A species-specific cluster of defensin-like genes encodes diffusible pollen tube attractants in Arabidopsis. PLoS Biol. 2012;10(12):e1001449. doi: 10.1371/journal.pbio.1001449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Márton ML, Cordts S, Broadhvest J, Dresselhaus T. Micropylar pollen tube guidance by egg apparatus 1 of maize. Science. 2005;307(5709):573–576. doi: 10.1126/science.1104954. [DOI] [PubMed] [Google Scholar]
- 11.Márton ML, Fastner A, Uebler S, Dresselhaus T. Overcoming hybridization barriers by the secretion of the maize pollen tube attractant ZmEA1 from Arabidopsis ovules. Curr Biol. 2012;22(13):1194–1198. doi: 10.1016/j.cub.2012.04.061. [DOI] [PubMed] [Google Scholar]
- 12.Alandete-Saez M, Ron M, McCormick S. GEX3, expressed in the male gametophyte and in the egg cell of Arabidopsis thaliana, is essential for micropylar pollen tube guidance and plays a role during early embryogenesis. Mol Plant. 2008;1(4):586–598. doi: 10.1093/mp/ssn015. [DOI] [PubMed] [Google Scholar]
- 13.Chen YH, et al. The central cell plays a critical role in pollen tube guidance in Arabidopsis. Plant Cell. 2007;19(11):3563–3577. doi: 10.1105/tpc.107.053967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li H-J, et al. Arabidopsis CBP1 is a novel regulator of transcription initiation in central cell-mediated pollen tube guidance. Plant Cell. 2015;27(10):2880–2893. doi: 10.1105/tpc.15.00370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shimizu KK, Ito T, Ishiguro S, Okada K. MAA3 (MAGATAMA3) helicase gene is required for female gametophyte development and pollen tube guidance in Arabidopsis thaliana. Plant Cell Physiol. 2008;49(10):1478–1483. doi: 10.1093/pcp/pcn130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prado AM, Porterfield DM, Feijó JA. Nitric oxide is involved in growth regulation and re-orientation of pollen tubes. Development. 2004;131(11):2707–2714. doi: 10.1242/dev.01153. [DOI] [PubMed] [Google Scholar]
- 17.Prado AM, Colaço R, Moreno N, Silva AC, Feijó JA. Targeting of pollen tubes to ovules is dependent on nitric oxide (NO) signaling. Mol Plant. 2008;1(4):703–714. doi: 10.1093/mp/ssn034. [DOI] [PubMed] [Google Scholar]
- 18.Sauter M. Phytosulfokine peptide signalling. J Exp Bot. 2015;66(17):5161–5169. doi: 10.1093/jxb/erv071. [DOI] [PubMed] [Google Scholar]
- 19.Guan Y, Guo J, Li H, Yang Z. Signaling in pollen tube growth: Crosstalk, feedback, and missing links. Mol Plant. 2013;6(4):1053–1064. doi: 10.1093/mp/sst070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hepler PK, Kunkel JG, Rounds CM, Winship LJ. Calcium entry into pollen tubes. Trends Plant Sci. 2012;17(1):32–38. doi: 10.1016/j.tplants.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 21.Kroeger J, Geitmann A. The pollen tube paradigm revisited. Curr Opin Plant Biol. 2012;15(6):618–624. doi: 10.1016/j.pbi.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 22.Qu LJ, Li L, Lan Z, Dresselhaus T. Peptide signalling during the pollen tube journey and double fertilization. J Exp Bot. 2015;66(17):5139–5150. doi: 10.1093/jxb/erv275. [DOI] [PubMed] [Google Scholar]
- 23.Holdaway-Clarke TL, Feijo JA, Hackett GR, Kunkel JG, Hepler PK. Pollen tube growth and the intracellular cytosolic calcium gradient oscillate in phase while extracellular calcium influx is delayed. Plant Cell. 1997;9(11):1999–2010. doi: 10.1105/tpc.9.11.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iwano M, et al. Fine-tuning of the cytoplasmic Ca2+ concentration is essential for pollen tube growth. Plant Physiol. 2009;150(3):1322–1334. doi: 10.1104/pp.109.139329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Messerli MA, Créton R, Jaffe LF, Robinson KR. Periodic increases in elongation rate precede increases in cytosolic Ca2+ during pollen tube growth. Dev Biol. 2000;222(1):84–98. doi: 10.1006/dbio.2000.9709. [DOI] [PubMed] [Google Scholar]
- 26.Pierson ES, et al. Pollen tube growth is coupled to the extracellular calcium ion flux and the intracellular calcium gradient: Effect of BAPTA-type buffers and hypertonic media. Plant Cell. 1994;6(12):1815–1828. doi: 10.1105/tpc.6.12.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pierson ES, et al. Tip-localized calcium entry fluctuates during pollen tube growth. Dev Biol. 1996;174(1):160–173. doi: 10.1006/dbio.1996.0060. [DOI] [PubMed] [Google Scholar]
- 28.Malho R, Read ND, Trewavas AJ, Pais MS. Calcium channel activity during pollen tube growth and reorientation. Plant Cell. 1995;7(8):1173–1184. doi: 10.1105/tpc.7.8.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Malho R, Trewavas AJ. Localized apical increases of cytosolic free calcium control pollen tube orientation. Plant Cell. 1996;8(11):1935–1949. doi: 10.1105/tpc.8.11.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iwano M, et al. Cytoplasmic Ca2+ changes dynamically during the interaction of the pollen tube with synergid cells. Development. 2012;139(22):4202–4209. doi: 10.1242/dev.081208. [DOI] [PubMed] [Google Scholar]
- 31.Dutta R, Robinson KR. Identification and characterization of stretch-activated ion channels in pollen protoplasts. Plant Physiol. 2004;135(3):1398–1406. doi: 10.1104/pp.104.041483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang YF, Fan LM, Zhang WZ, Zhang W, Wu WH. Ca2+-permeable channels in the plasma membrane of Arabidopsis pollen are regulated by actin microfilaments. Plant Physiol. 2004;136(4):3892–3904. doi: 10.1104/pp.104.042754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu Y, et al. Heterotrimeric G-protein participation in Arabidopsis pollen germination through modulation of a plasmamembrane hyperpolarization-activated Ca2+-permeable channel. New Phytol. 2007;176(3):550–559. doi: 10.1111/j.1469-8137.2007.02214.x. [DOI] [PubMed] [Google Scholar]
- 34.Wu J, et al. cAMP activates hyperpolarization-activated Ca2+ channels in the pollen of Pyrus pyrifolia. Plant Cell Rep. 2011;30(7):1193–1200. doi: 10.1007/s00299-011-1027-9. [DOI] [PubMed] [Google Scholar]
- 35.Wu J, et al. Long-chain base phosphates modulate pollen tube growth via channel-mediated influx of calcium. Plant J. 2014;79(3):507–516. doi: 10.1111/tpj.12576. [DOI] [PubMed] [Google Scholar]
- 36.Yu G-H, et al. Exogenous γ-aminobutyric acid (GABA) affects pollen tube growth via modulating putative Ca2+-permeable membrane channels and is coupled to negative regulation on glutamate decarboxylase. J Exp Bot. 2014;65(12):3235–3248. doi: 10.1093/jxb/eru171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Frietsch S, et al. A cyclic nucleotide-gated channel is essential for polarized tip growth of pollen. Proc Natl Acad Sci USA. 2007;104(36):14531–14536. doi: 10.1073/pnas.0701781104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tunc-Ozdemir M, et al. Cyclic nucleotide gated channels 7 and 8 are essential for male reproductive fertility. PLoS One. 2013;8(2):e55277. doi: 10.1371/journal.pone.0055277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tunc-Ozdemir M, et al. A cyclic nucleotide-gated channel (CNGC16) in pollen is critical for stress tolerance in pollen reproductive development. Plant Physiol. 2013;161(2):1010–1020. doi: 10.1104/pp.112.206888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Michard E, et al. Glutamate receptor-like genes form Ca2+ channels in pollen tubes and are regulated by pistil D-serine. Science. 2011;332(6028):434–437. doi: 10.1126/science.1201101. [DOI] [PubMed] [Google Scholar]
- 41.Gao QF, Fei CF, Dong JY, Gu LL, Wang YF. Arabidopsis CNGC18 is a Ca²⁺-permeable channel. Mol Plant. 2014;7(4):739–743. doi: 10.1093/mp/sst174. [DOI] [PubMed] [Google Scholar]
- 42.Schachtman DP, Schroeder JI, Lucas WJ, Anderson JA, Gaber RF. Expression of an inward-rectifying potassium channel by the Arabidopsis KAT1 cDNA. Science. 1992;258(5088):1654–1658. doi: 10.1126/science.8966547. [DOI] [PubMed] [Google Scholar]
- 43.van der Veen JH, Wirtz P. EMS-induced genic male sterility in Arabidopsis thaliana: A model selection experiment. Euphytica. 1968;17(3):371–377. [Google Scholar]
- 44.Gehring C. Adenyl cyclases and cAMP in plant signaling: Past and present. Cell Commun Signal. 2010;8:15. doi: 10.1186/1478-811X-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moutinho A, Hussey PJ, Trewavas AJ, Malhó R. cAMP acts as a second messenger in pollen tube growth and reorientation. Proc Natl Acad Sci USA. 2001;98(18):10481–10486. doi: 10.1073/pnas.171104598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhao LN, et al. Ca2+-dependent protein kinase11 and 24 modulate the activity of the inward rectifying K+ channels in Arabidopsis pollen tubes. Plant Cell. 2013;25(2):649–661. doi: 10.1105/tpc.112.103184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Messerli M, Robinson KR. Tip localized Ca2+ pulses are coincident with peak pulsatile growth rates in pollen tubes of Lilium longiflorum. J Cell Sci. 1997;110(Pt 11):1269–1278. doi: 10.1242/jcs.110.11.1269. [DOI] [PubMed] [Google Scholar]
- 48.Nakagawa Y, et al. Arabidopsis plasma membrane protein crucial for Ca2+ influx and touch sensing in roots. Proc Natl Acad Sci USA. 2007;104(9):3639–3644. doi: 10.1073/pnas.0607703104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yuan F, et al. OSCA1 mediates osmotic-stress-evoked Ca2+ increases vital for osmosensing in Arabidopsis. Nature. 2014;514(7522):367–371. doi: 10.1038/nature13593. [DOI] [PubMed] [Google Scholar]
- 50.Zhou L, Lan W, Jiang Y, Fang W, Luan S. A calcium-dependent protein kinase interacts with and activates a calcium channel to regulate pollen tube growth. Mol Plant. 2014;7(2):369–376. doi: 10.1093/mp/sst125. [DOI] [PubMed] [Google Scholar]
- 51.Li H, Lin Y, Heath RM, Zhu MX, Yang Z. Control of pollen tube tip growth by a Rop GTPase-dependent pathway that leads to tip-localized calcium influx. Plant Cell. 1999;11(9):1731–1742. doi: 10.1105/tpc.11.9.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Boavida LC, McCormick S. Temperature as a determinant factor for increased and reproducible in vitro pollen germination in Arabidopsis thaliana. Plant J. 2007;52(3):570–582. doi: 10.1111/j.1365-313X.2007.03248.x. [DOI] [PubMed] [Google Scholar]
- 53.Muschietti J, Dircks L, Vancanneyt G, McCormick S. LAT52 protein is essential for tomato pollen development: Pollen expressing antisense LAT52 RNA hydrates and germinates abnormally and cannot achieve fertilization. Plant J. 1994;6(3):321–338. doi: 10.1046/j.1365-313x.1994.06030321.x. [DOI] [PubMed] [Google Scholar]
- 54.Liu J, et al. Membrane-bound RLCKs LIP1 and LIP2 are essential male factors controlling male-female attraction in Arabidopsis. Curr Biol. 2013;23(11):993–998. doi: 10.1016/j.cub.2013.04.043. [DOI] [PubMed] [Google Scholar]
- 55.Till BJ, et al. Large-scale discovery of induced point mutations with high-throughput TILLING. Genome Res. 2003;13(3):524–530. doi: 10.1101/gr.977903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Clough SJ, Bent AF. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998;16(6):735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- 57.Higuchi R, Krummel B, Saiki RK. A general method of in vitro preparation and specific mutagenesis of DNA fragments: Study of protein and DNA interactions. Nucleic Acids Res. 1988;16(15):7351–7367. doi: 10.1093/nar/16.15.7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Twell D, Wing R, Yamaguchi J, McCormick S. Isolation and expression of an anther-specific gene from tomato. Mol Gen Genet. 1989;217(2-3):240–245. doi: 10.1007/BF02464887. [DOI] [PubMed] [Google Scholar]