Significance
In humans and genetically engineered mouse models (GEMs), the development of pancreatic ductal adenocarcinoma (PDAC) is accompanied by intimate neural–tumor interactions. Using a PDAC GEM that phenocopies the human disease, we found that many changes in peripheral and central nervous systems, indicative of injury and inflammation, arise at time points prior to overt tumor formation. Ablation of sensory neurons that innervate the pancreas, via neonatal capsaicin treatment, prevented neurogenic inflammation and delayed tumor formation. The slowing of PDAC in capsaicin-treated mice suggests the nervous system is not a bystander with respect to disease progression. Further studies are warranted to examine nervous system–tumor interactions and to identify potential targets for early detection, prevention, and treatment.
Keywords: sensory neuron, pancreatic ductal adenocarcinoma, tumorigenesis, inflammation, PanIN
Abstract
Pancreatic ductal adenocarcinoma (PDAC) is characterized by an exuberant inflammatory desmoplastic response. The PDAC microenvironment is complex, containing both pro- and antitumorigenic elements, and remains to be fully characterized. Here, we show that sensory neurons, an under-studied cohort of the pancreas tumor stroma, play a significant role in the initiation and progression of the early stages of PDAC. Using a well-established autochthonous model of PDAC (PKC), we show that inflammation and neuronal damage in the peripheral and central nervous system (CNS) occurs as early as the pancreatic intraepithelial neoplasia (PanIN) 2 stage. Also at the PanIN2 stage, pancreas acinar-derived cells frequently invade along sensory neurons into the spinal cord and migrate caudally to the lower thoracic and upper lumbar regions. Sensory neuron ablation by neonatal capsaicin injection prevented perineural invasion (PNI), astrocyte activation, and neuronal damage, suggesting that sensory neurons convey inflammatory signals from Kras-induced pancreatic neoplasia to the CNS. Neuron ablation in PKC mice also significantly delayed PanIN formation and ultimately prolonged survival compared with vehicle-treated controls (median survival, 7.8 vs. 4.5 mo; P = 0.001). These data establish a reciprocal signaling loop between the pancreas and nervous system, including the CNS, that supports inflammation associated with oncogenic Kras-induced neoplasia. Thus, pancreatic sensory neurons comprise an important stromal cell population that supports the initiation and progression of PDAC and may represent a potential target for prevention in high-risk populations.
Pancreatic ductal adenocarcinoma (PDAC) is one of the most lethal cancers with a median survival of ∼6 mo from diagnosis (National Cancer Institute). A number of unique features distinguish PDAC from other carcinomas, but the most striking is the exuberant desmoplastic infiltrate within tumors. This compartment exhibits an array of cell types, including activated myofibroblasts and myeloid-derived cells. Indeed, this inflammatory infiltrate is present at the inception of neoplasia and accumulates at a near exponential rate during progression to carcinoma and tumor formation. It provides a complex balance of pro- and antitumorigenic signals to neoplastic cells (and also to each other) that is a focus of intense investigation. The pancreas tumor microenvironment has been studied previously, but new tools [genetically engineered mouse models (GEMs) that faithfully recapitulate the salient features of human PDAC] now allow for a careful dissection of the stroma. Using these models, we showed that generalized inflammation is required for the development of precancerous pancreatic intraepithelial neoplasias (1) and that Hedgehog-dependent stromal elements, including activated myofibroblasts, serve to constrain tumor growth and spread (2). Other cellular components of the pancreatic inflammatory stroma have not been examined, and it is possible they also contribute to the complex balance of inputs, supportive and inhibitory, which underlie tumorigenesis and progression.
Previous reports indicate sensory neurons have a central role in benign inflammatory disease of the pancreas. Like most abdominal organs, the pancreas is innervated by sensory fibers from both the nodose (via the vagal nerve) and spinal ganglia (via splanchnic nerves) (3–7). In rodent models of acute or chronic pancreatitis, blockade of primary afferents from both nodose and spinal ganglia can moderate or prevent inflammation (8, 9) as well as associated pathology, even if done after the inciting injury (10, 11). In addition, autonomic neurons (sympathetic, parasympathetic, and enteric) (12) innervate the pancreas and interact with sensory fibers.
Because of the documented role of sensory neurons in the pathogenesis of pancreatitis (8–11, 13–15), a known contributor to the pathogenesis of PDAC, we hypothesized that sensory neurons innervating the pancreas provide key proinflammatory inputs that support the early stages of tumorigenesis. Here, using GEMs of PDAC, we provide evidence that bidirectional communication between the pancreas and sensory neurons is active well before the establishment of tumors. We detected inflammation in the spinal cord when histologically only precancerous lesions (pancreatic intraepithelial neoplasia, PanIN) were present. This was accompanied by perineural invasion (PNI) of sensory ganglia and spinal cord and evidence of injury to pancreatic sensory and sympathetic neurons. Ablation of sensory neurons at postnatal days 1–2 prevented injury to peripheral neurons and spinal cord inflammation. Surprisingly, sensory neuron ablation was associated with a dose-dependent and dramatic prolongation of survival in PDAC mice. The animals with the greatest degree of capsaicin-induced neuronal ablation (>80%) did not develop cancer (up to 18 mo when they were euthanized for analysis), whereas more than 90% of untreated mice succumbed to PDAC within 6 mo. These studies indicate that sensory neurons contribute significantly to the initiation and progression of PDAC and may be required for the development of this disease.
Results
Benign and Oncogene-Induced Pancreas Inflammation Elicits Activation of CNS Glia and Injury-Related Genes.
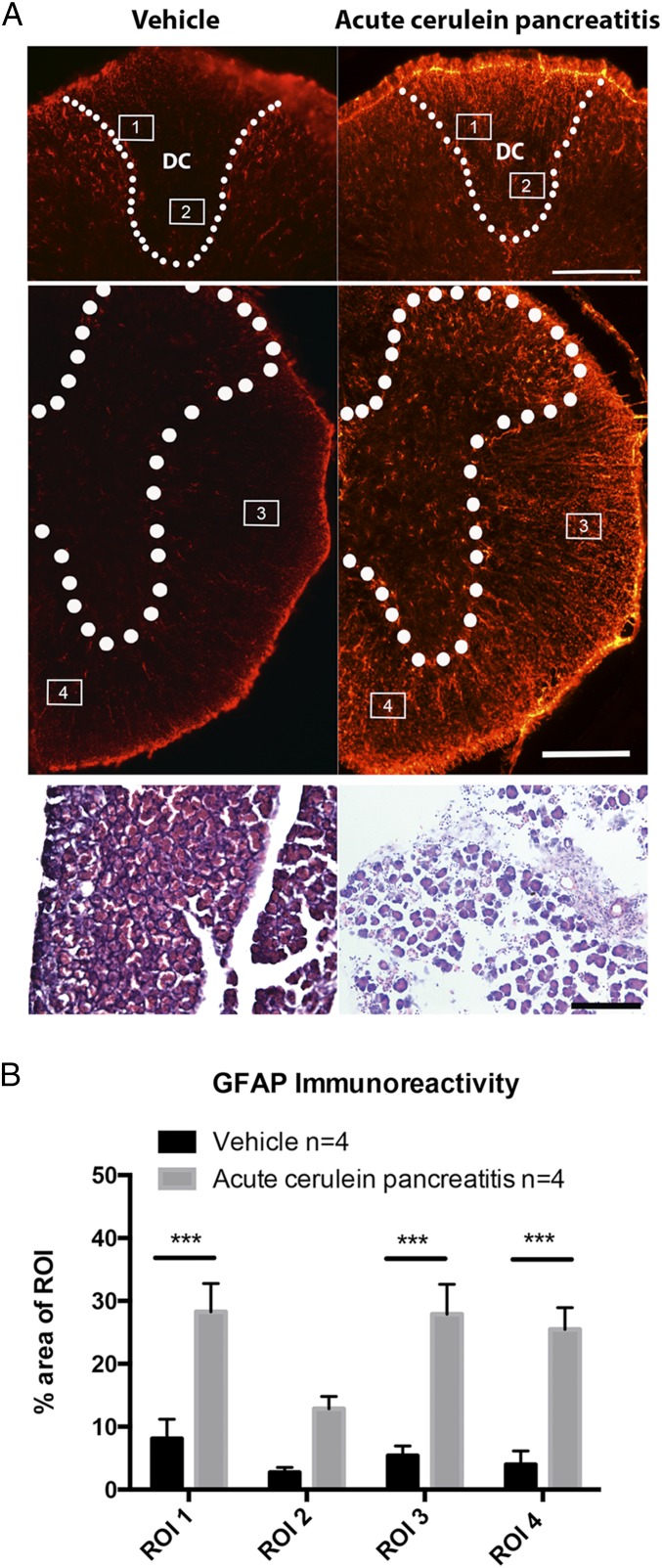
In humans and the two mouse models used here (PKCT and PKCY mice, Fig. S1), pancreatitis is required for the initiation and development of PDAC (1, 16). Pancreatitis has also been shown to induce injury-like responses in sensory neurons (11). To further understand the relationship between tissue inflammation, tumor formation, disease progression, and the nervous system, we compared how benign acute pancreatitis, induced by repeated injections of cerulein, and Kras-driven precancerous inflammatory neoplastic disease (PanIN) affected the peripheral and central nervous systems. We first examined activation of spinal glial cells using neurochemical markers associated with CNS inflammation. Four regions of interest (ROIs), encompassing the dorsal columns, the canonical pain-transmitting anterolateral spinothalamic tract, and a medioventral nonpain-related region, were analyzed to determine the percent area covered by GFAP immunoreactivity, an indicator of astrocyte activation. Measures were made at the level of the thoracic spinal cord, which receives input from primary afferents that innervate the pancreas. Acute pancreatitis induced by cerulein (Fig. 1A) caused a significant increase in GFAP immunoreactivity in ROIs overlying the dorsal columns, anterolateral, and medioventral white matter tracts (F = 73.06, P < 0.0001, Fig. 1B).
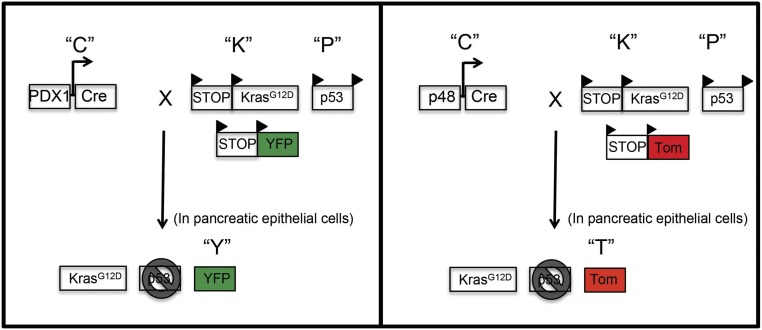
Fig. S1.
Generation of PDAC transgenic mice (PKCT and PKCY). PDAC mice express a conditional activated (mutant) Kras allele targeted to the endogenous Kras locus under Lox-Stop-Lox control (KrasG12D) and a conditional TP53 allele with LoxP sites in intron 1 and intron 10 of the Tp53 gene (p53). PDAC mice were crossed with PDX1-Cre (PKC) or Ptf1a-p48-Cre (p48-Cre) mice. PKC mice expressing PDX1-Cre were crossed with a ROSA-YFP mouse to make PKCY mice (Left). PKC mice expressing p48-Cre were crossed with a ROSA Tdtomato reporter strain to make PKCT mice (Right) (1, 29).
Fig. 1.
Cerulein-induced acute pancreatitis causes spinal inflammation. (A) The thoracic spinal cord has increased GFAP immunoreactivity in cerulein-treated mice. H&E staining (Lower) shows cerulein-induced disruption in pancreatic histology. (B) Quantification of GFAP immunoreactivity across ROIs encompassing the dorsal columns, anterolateral, and medioventral white matter tracts of vehicle and cerulein-treated groups. ***P < 0.001, n = 4 per group. DC, dorsal columns. (Scale bar, 200 µm.)
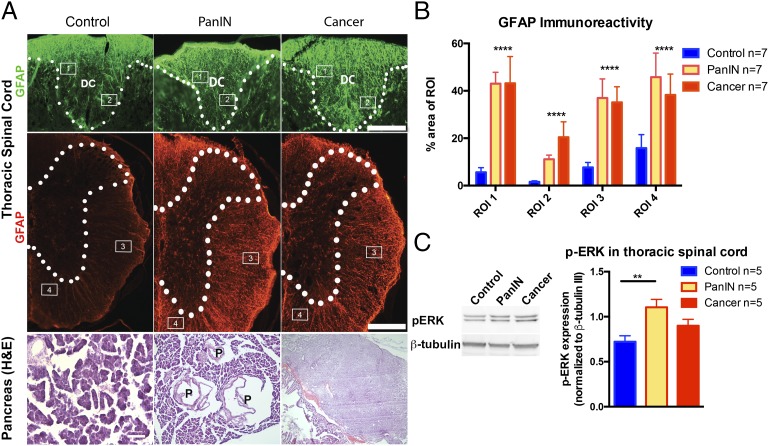
Markers of spinal cord inflammation were also elevated in PKCT and PKCY mice at the PanIN and PDAC stages. The pattern of GFAP immunoreactivity was almost identical in PKCT (tomato) and PKCY (YFP) mice (Fig. 2A) and increased as mice aged and developed PDAC (F = 21.45, P < 0.0001, Fig. 2B). Phospho-ERK (p-ERK), another marker of inflammation (17–21), was also elevated in the thoracic spinal cord of PKCT mice (Fig. 2C). Thus, similar to cerulein-induced acute pancreatitis, Kras-induced neoplasia leads to increased inflammation of the spinal cord.
Fig. 2.
Spinal inflammation is detected in early phases of PDAC. (A) Disease-stage–specific changes occur in GFAP staining in the thoracic spinal cord and pancreatic histology in PKCT and PKCY mice. (B) Astrocyte reactivity increases in regions of the spinal cord at the PanIN and cancer stage. (C) p-ERK is up-regulated in thoracic spinal cord of PKCT mice at the PanIN stage. ****P < 0.0001, n = 5–7 per group. DC, dorsal columns; P, PanIN lesion. (Scale bar, 200 µm.)
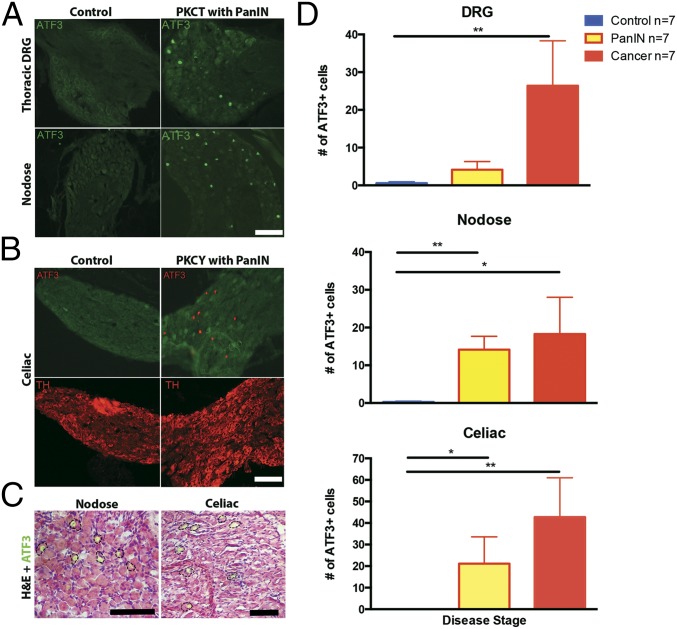
Chronic tissue inflammation, proinflammatory cytokine release, and physiological stress all change primary afferent gene expression. A particularly sensitive marker is activating transcription factor 3 (ATF3), a host defense transcription factor (22). We used ATF3 immunolabeling to determine if damage in the pancreas of either cerulein-treated or PKC animals caused an injury response in afferents of celiac, nodose, and T9–T12 dorsal root ganglia (DRG) (22–25). Whereas vehicle-treated mice lacked ATF3 expression in all ganglia, cerulein-treated mice showed a few positive cells in all ganglia examined. In contrast, PKC mice at the PanIN stage exhibited a significant increase in ATF3 immunoreactivity in nodose and DRG that was maintained throughout cancer progression (Kruskal–Wallis, P = 0.002, Fig. 3 A and C). ATF3 labeling was also increased in sympathetic celiac ganglia at the PanIN and PDAC stages (Kruskal–Wallis, P = 0.003, Fig. 3 B and C). Thus, both sensory and sympathetic postganglionic neuron injury occurs at precancerous PanIN stages and increases with progression to PDAC (Fig. 3D).
Fig. 3.
Pancreatic disease increases ATF3 in sensory afferents. (A) Nuclear localization of ATF3 immunoreactivity in DRG (Top) and nodose ganglia (Bottom) of PKCT mice at the PanIN stage. (B) ATF3 is also elevated in celiac ganglia of PKCY mice. (C) H&E staining shows ATF3-IR in neurons of nodose and celiac ganglia. (D) Summary of increases in neuronal ATF3 expression in sensory and autonomic ganglia during PanIN and cancer stages. *P < 0.05, **P < 0.01, n = 7 per group. (Scale bar, 100 µm.)
Pancreatic Cells Invade the Nervous System at PanIN Stages.
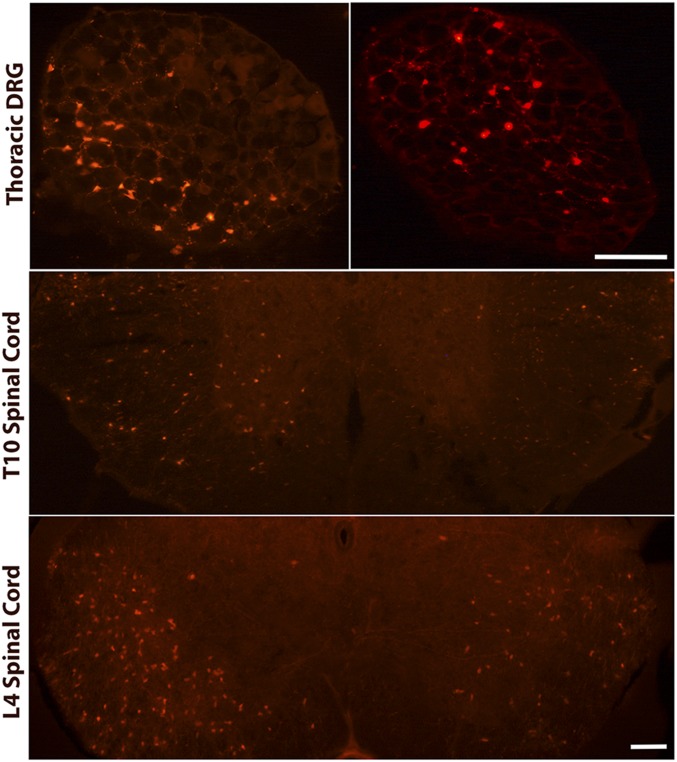
PNI is a common clinical feature of PDAC that coincides with tumor and metastasis formation (26). Interestingly, a similar correlation occurs in PKCT mice where cells of pancreatic origin migrate to the DRG during PanIN and early cancer stages (Fig. 4). Tdtomato (Tdt)-positive pancreatic cells were also present in thoracic and lumbar (Fig. 4) spinal cord. Invasion into the lumbar spinal cord was observed only in mice that exhibited cell migration into the thoracic segment, suggesting Tdt cells enter at thoracic levels and migrate along the rostrocaudal axis to lumbar regions. Tdt cells were present in five of six mice examined at the PanIN stage and in seven of eight mice with primary tumors without metastases. The presence of pancreas-derived cells, which may activate glial defense mechanisms, could explain why regions of the spinal cord unrelated to pancreatic sensory innervation (i.e., ventral white matter) exhibit increased GFAP immunoreactivity. This migration may also underlie the increased ATF3 expression in PKC mice relative to mice with cerulein-induced inflammation.
Fig. 4.
Pancreatic cells invade the DRG and spinal cord before PDAC development. Individual Tdtomato positive cells are present in thoracic DRG and thoracic and lumbar regions of the spinal cord. (Scale bar, 200 µm for DRG and 500 µm for spinal cord sections.)
Neonatal Ablation of Sensory Fibers Prevents CNS Inflammation in PKC Mice and Slows PanIN Progression.
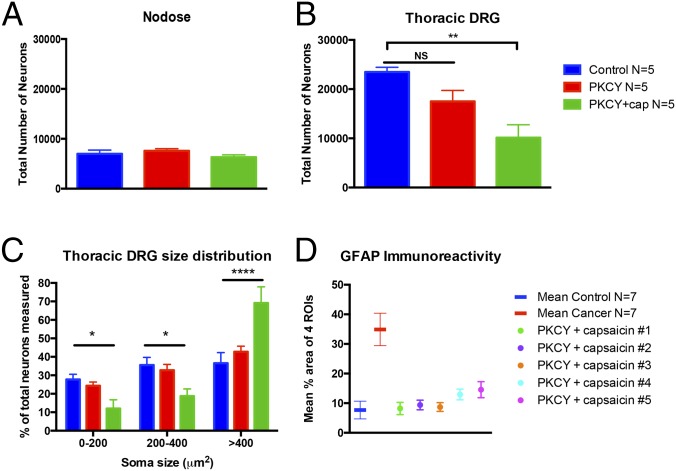
Because primary afferents contribute to inflammation in mouse models of pancreatitis, we examined whether sensory neuron ablation inhibited activation of spinal glial cells in PKCY mice. Postnatal days 1–2 (P2) mice treated with capsaicin had no loss of nodose neurons (Fig. 5A) but did have a reduction in neurons in thoracic DRG (Fig. 5B). A shift in the percentage of neurons with larger soma size also occurred (Fig. 5C), suggesting successful ablation of small diameter C fibers. Importantly, PKCY mice treated with capsaicin exhibited normal levels of GFAP immunoreactivity in the spinal cord (Fig. 5D), suggesting that activation of spinal astrocytes requires intact pancreatic sensory neurons.
Fig. 5.
Neonatal capsaicin ablates DRG neurons. (A) The total number of nodose ganglion neurons is unchanged in adult mice treated with capsaicin at P2. (B) In contrast, capsaicin causes a significant loss of thoracic DRG neurons. (C) A rightward shift in somal diameters of the remaining neurons indicates selective reduction of small diameter afferents. (D) Neonatal capsaicin prevents spinal inflammation. The mean percent area of the four ROIs (designated in Fig. 2A) covered by GFAP immunoreactivity is plotted as a mean (n = 7 per group) and compared with the mean of the four ROIs for individual animals treated with neonatal capsaicin. PKCY mice treated with capsaicin exhibit GFAP immunoreactivity levels similar to control. *P < 0.05, **P < 0.01, ****P < 0.0001.
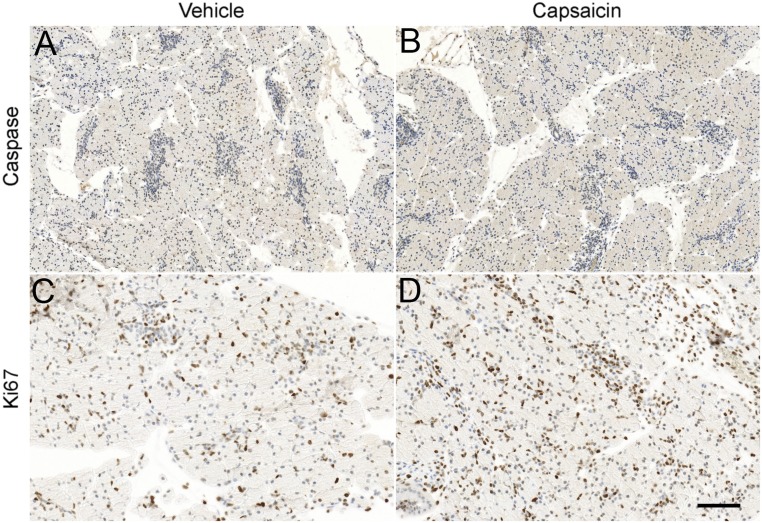
Sensory neuron ablation attenuates inflammation in models of pancreatitis, suggesting these neurons provide proinflammatory signals (8, 10, 11, 13, 14, 27). To examine how sensory neurons impact PDAC initiation and progression, we first determined if neonatal capsaicin had direct effects on pancreatic cells because previous studies using cancer-derived cell lines showed it affected apoptosis and proliferation (28–34). The neurotoxic effects of neonatal capsaicin treatment are assumed to be via binding to TRPV1, the vanilloid receptor specific for capsaicin. At the dose used in this and the majority of studies, a single treatment ablates unmyelinated fibers and a subset of Aδ-myelinated afferents (35–39). This specificity of action correlates to what is known about the developmental expression of TRPV1, i.e., that it is expressed embryologically in virtually all C fibers and a subset of Aδ fibers and then becomes restricted to a subset of these fibers in adulthood (40). To determine whether the P2 pancreas is affected by capsaicin treatment via a receptor-mediated mechanism, we used semiquantitative real-time PCR to measure TRPV1 transcript level. Although highly expressed in the thoracic DRG (ΔCT = 6.61 ± 0.23, n = 8 normalized to GAPDH), where significant neuron loss is measured in adults, (Fig. 5B), TRPV1 was undetectable in the P2 pancreas (n = 7). The absence of TRPV1 in the P2 pancreas suggests that neonatal capsaicin could not affect pancreatic cells via a receptor-specific mechanism. However, as noted by Diaz-Laviada and Rodriguez-Henche (41), for many tumor cell lines, capsaicin effects, especially with respect to alterations in apoptosis or proliferation, are TRPV1 independent (33, 42, 43). We therefore examined pancreata from P2 vehicle- and capsaicin-treated mice stained for markers of apoptosis (anticaspase 3) and proliferation (anti-Ki67) 2 h posttreatment. There was no significant difference for either marker in pancreata from mice treated with capsaicin compared with vehicle-treated controls (Fig. S2).
Fig. S2.
Six P2 pups were euthanized 2 h post injection of capsaicin or vehicle. Vehicle-treated pancreata were processed for cleaved caspase 3 (A) or Ki67 (C) immunodetection as previously described (79, 80). Slides were counterstained with hematoxylin. Neonatal capsaicin treatment had no effect on cleaved caspase 3 (B) or Ki67 (D) expression. (Scale bar, 200 μm for A and B and 100 µm for C and D.)
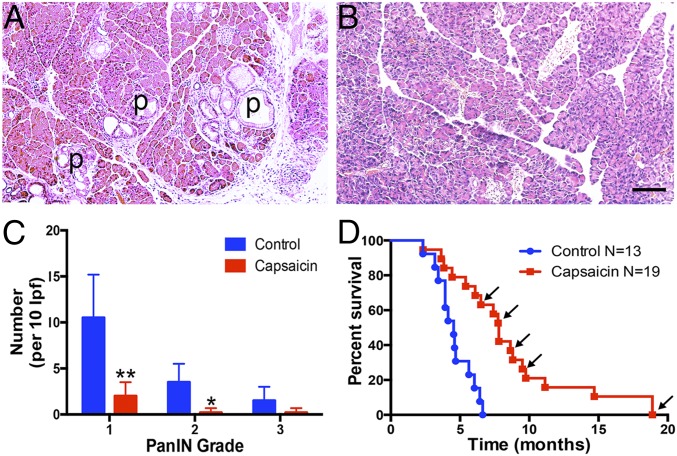
To examine the long-term effect of neonatal sensory neuron ablation on cancer progression, we assessed the presence of precancerous PanIN lesions in pancreata from 10-wk-old PKC mice that were treated with vehicle or capsaicin at P2. At 10 wk, there were significantly fewer grade 1 and 2 PanINs in pancreata from mice treated with capsaicin (Fig. 6 A–C). There was a trend for a decrease in grade 3 PanINs. We next performed a survival study of PKCY mice treated with neonatal capsaicin and found that capsaicin treatment significantly prolonged survival of PKCY mice compared with vehicle-treated controls (median survival, 7.80 vs. 4.53 mo, P = 0.0001, Fig. 6D). Moreover, this increase in survival is likely an underestimate as a number of capsaicin-treated mice were euthanized to allow histological analysis of disease progression even though no tumors were detected by ultrasound (these mice are indicated by arrows in Fig. 6D). In some of the longest-lived cases (two of the mice euthanized at 18.9 mo) only benign metaplasia with low-grade inflammation was observed. These data suggest that sensory innervation of the pancreas is required for both the initiation and progression of PanINs and that ablation of innervation can significantly increase survival.
Fig. 6.
Neonatal capsaicin slows development of PanIN lesions and prolongs survival of PKCY mice. Pancreata of 10-wk-old PKC mice were treated with vehicle (A) or capsaicin (B). Vehicle-treated mice exhibit numerous PanIN lesions (P), whereas pancreata from capsaicin-treated mice do not. (C) There were significantly fewer total PanIN lesions and virtually no lesions above grade 1 in capsaicin-treated mice; *P < 0.05, **P < 0.001. (D) Capsaicin-treated mice survive longer than vehicle-treated PKCY mice. Arrows indicate mice that were euthanized for histological analysis while otherwise healthy (no tumors upon ultrasound).
Discussion
PDAC is characterized by a complex and exuberant desmoplastic inflammatory stroma. Whereas some aspects of the tumor microenvironment support tumor growth, others can inhibit tumorigenesis. In humans, pancreatic inflammation is one of the most significant risk factors for the development of PDAC. The data presented here demonstrate that at the PanIN stage of PDAC in the PKC model, spinal cord inflammation and PNI of both the spinal cord and peripheral ganglia are already well under way. Stopczynski et al. (26), showed that in the PanIN stage, sprouting of sensory fibers and increases in neurotrophic factors accompany an increase in mRNAs encoding CGRP, TRPV1, and TRPA1 in DRG, all hallmarks of pancreatic inflammation driven by increased activity in pancreatic sensory neurons, i.e., neurogenic inflammation. Growth-factor–related changes, as well as chemokine release, support PNI (44–47), a common clinical feature of several cancers including up to 100% of PDAC cases (48–50).
In mouse models of both acute and chronic pancreatitis, silencing of pancreatic afferents can block neurogenic inflammation (10, 11). Thus, the present study was formulated to test the hypothesis that ablation of sensory neurons (to silence primary afferents throughout PDAC progression) might slow tumorigenesis. Results indicate that loss of sensory neurons slows the development of PanIN lesions and significantly increases overall survival, and both of these phenomena are accompanied by the absence of spinal cord inflammation.
That the peripheral nervous system plays a role in tumor progression has been reported recently for two types of visceral tumors; however, the focus of these studies was on the autonomic portion of the peripheral nervous system. Magnon et al. (51), used a mouse model of prostate cancer and found that chemical or surgical ablation of hypogastric nerves was associated with decreased tumorigenesis. They proposed that sympathetic postganglionic neurons regulated the initiation of the disease, whereas postganglionic parasympathetic neurons were implicated in advanced disease. Zhao et al. (52), used three models of gastric cancer and found that surgical vagotomy slowed and/or decreased tumor progression and increased the effectiveness of chemotherapy. Parasympathetic postganglionic neurons were also implicated based on changes in signaling pathways downstream of the type 3 muscarinic receptor expressed by these neurons, and that botox was able to slow tumor growth. Unfortunately, the role of sensory neurons was not examined in this study despite the fact that 85% of vagal fibers are from sensory neurons originating in the nodose ganglia (53).
The potential role of sensory neurons and their association with sympathetic and parasympathetic neurons remains to be defined. Mantyh and colleagues have documented in detail that autonomic nerves, especially sympathetic postganglionic fibers, are affected in cancer progression (5, 54–56). Moreover, both sympathetic and parasympathetic neurons release molecules that can activate sensory neurons; sympathetic postganglionic neurons release three molecules of ATP for every molecule of norepinephrine. Sensory neurons express both ionotropic and metabotropic receptors for ATP and its breakdown products (e.g., ADP). Sensory neurons also express nicotinic receptors (e.g., α3, β4, α7) (57, 58) that can be activated by acetylcholine released from postganglionic parasympathetic neurons. That sensory neurons play a direct role in tumor formation has also been reported for basal cell carcinoma (59). In these studies, tamoxifen-inducible Cre drivers were used to delete the hedgehog suppressor Ptch1 in adult mouse skin. This strategy was particularly effective at producing tumors in touch domes, sensory structures containing specialized epithelium (Merkel cells) innervated by large, low-threshold, mechanically responsive sensory fibers. Once tumor formation was underway (timed by application of tamoxifen), denervation dramatically suppressed tumorigenesis, suggesting that sensory neurons directly contribute to an environment that supports tumor production.
The data reported here also indicate that sensory neurons and the spinal cord are extremely sensitive to pancreatic disease progression. Although at the early PanIN stage the pancreas does not exhibit major inflammation, astrocyte activation in the spinal cord was equivalent to that seen in response to acute pancreatitis. The activation of spinal cord glia affected all regions of the white matter although pancreatic sensory fibers run primarily in the dorsal columns (60–63) and anterolateral regions of the spinal cord (64). In patients with pancreatic cancer, peripheral nerve damage associated with PNI has been linked to spinal cord glial activation in T10–L1 (65). Thus, the widespread appearance of activated astrocytes is likely related to the migration of pancreatic cells from the pancreas to the peripheral nervous system where they track into the thoracic and lumbar spinal cord. It should be noted that the tumorigenic potential of these cells is unknown; however, spinal metastases are rare in patients with PDAC. At later disease stages, tumors can be found in both peripheral nerves and spinal cord in the PKC model (26), but at the time in which spinal inflammation is first observed, no obvious tumors were detected. Importantly, the migration of cells is not restricted to animal models. Circulating pancreatic cells have been identified even in patients with pancreatitis and precancerous lesions, but no cancer diagnosis (1).
A central role of primary afferents in pancreatic disease has been reported for models of pancreatitis and diabetes, where sensory neuron-released CGRP played a role in initiation and prevention of islet cell inflammation (10, 11, 66). In these cases, the link between sensory neurons and pancreatic cells relates to the ability of the sensory system to regulate neurogenic inflammation. But, could there be a more direct and fundamental interaction between these two cell types? It is well documented that sensory neurons have efferent function through the wide range of small molecules they release in the periphery such as glutamate, ATP, CGRP, and SP (27, 67–69). Moreover, sensory, sympathetic, and parasympathetic neurons express receptors for these molecules that when stimulated, release additional substances that modulate pancreatic cellular function. These interactions are present in virtually all tissues (e.g., prostate, stomach, skin) in which recent studies have invoked a role for the peripheral nervous system in tumorigenesis. These convergent observations suggest a common mechanism by which predisposing genetic mutations hijacks the normal homeostatic process regulated by the peripheral nervous system to produce an environment that supports tumorigenesis.
Methods
Animals were cared for and studies were performed in accordance with guidelines of the Institutional Animal Care and Use Committee at the University of Pittsburgh/University of Michigan and the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Two mouse models of PDAC, PKCY, and PKCT were generated (1, 26) (Fig. S1). Disease stage was assessed using H&E staining. Pancreatitis was induced using repeated cerulein injections (1, 10, 70). Sensory neurons were ablated via neonatal capsaicin treatment (20 μL, 50 mg/kg, i.p.) at 1–2 d of age (P2). More detailed information including the protocols for assessing spinal inflammation can be found in SI Methods (23, 26, 71–81).
SI Methods
Mouse Models of PDAC.
Two mouse models of PDAC: PKCY and PKCT, were generated as previously described (1, 26) (Fig. S1). PDAC mice express a conditional Cre-activated mutant Kras allele targeted to the endogenous Kras locus (LSL-KrasG12D) and a conditional Trp53 allele with LoxP sites in intron 1 and intron 10 of the Trp53 gene (p53Lox). These mice were crossed with Cre-driver lines, Ptf1a-p48-Cre (p48-Cre) or PDX1-Cre, to generate PKC mice (1, 26). Mice lacking the Cre allele or the oncogenic alleles were used as littermate controls. To visualize cells of pancreatic origin, p48-Cre mice were crossed with mice carrying the ROSA-Tdtomato allele and PDX1-Cre mice were crossed with mice expressing ROSA-YFP (both from The Jackson Laboratory) to produce PKCT or PKCY mice, respectively. Male and female mice were used in these studies. In a separate set of experiments, wild-type 6- to 8-wk-old male C57/Bl6 mice (The Jackson Laboratory) were used to assess neuroplastic changes associated with acute pancreatitis.
Animals were housed in the Association for Assessment and Accreditation of Laboratory Animal Care-accredited Division of Laboratory Animal Resources at the University of Pittsburgh or the University of Michigan. They were maintained in a 12-h light/dark cycle and temperature-controlled environment with ad libitum access to water and food. Animals were cared for and studies were performed in accordance with guidelines of the Institutional Animal Care and Use Committee at the University of Pittsburgh/University of Michigan and the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Induction of Acute Pancreatitis.
Pancreatitis was induced as previously described (1, 10, 70). Briefly, 6- to 8-wk-old C57BL/6 mice were injected every hour for 8 h on 2 consecutive days with cerulein (Sigma), a cholecystokinin analog, dissolved in 0.01 M phosphate buffer (PB; total 50 μg/kg, i.p.). Twenty-four hours after the final injection, mice were euthanized and tissue was collected and processed.
H&E Staining.
Disease stage was assessed through histological analysis of pancreata and stage of metastases to other tissues. Tissue sections were dehydrated in serial alcohols as follows: 2× water for 5 min, 2× 50% EtOH for 30 s, 2× 75% EtOH for 30 s, 2× 95% EtOH for 1 min, 2× 100% EtOH for 2 min, and 2× Fisherbrand Citrisolv clearing agent (Fisher Scientific) for 5 min. Sections were then rehydrated through alcohols in the reverse order, dipped in hematoxylin (Thermo Scientific) for 30 s, rinsed in water, dipped 10 times in eosin (Thermo Scientific), and rinsed in water. Sections were again dehydrated through serial alcohols, coverslipped with DPX mounting medium (Electron Microscopy Sciences), and viewed and photographed on a Leica DM 4000B microscope using Leica Application Suite (LAS) software (Leica Microsystems). Pancreas sections (10 μm) were scored by Douglas J. Hartman and Andrew D. Rhim. PanIN lesions were quantified in pancreata from 10-wk-old PKC mice (7 vehicle-treated and 10 capsaicin-treated) embedded in paraffin, sectioned, and H&E stained. Ten random low-powered (10×) fields were captured using Olympus DS software. PanIN lesions were counted by grade in a blinded manner. Data were analyzed by a two-way ANOVA followed by Sidak’s multiple comparison test.
Immunohistochemistry.
Animals were euthanized via an overdose of inhaled isoflurane and perfused transcardially with 4% (wt/vol) paraformalyehyde. The pancreas, stomach, and duodenum were dissected en bloc and additional (e.g., lung, liver) organs removed if metastases were grossly visible. Spinal cord, nodose, celiac, and DRG were also collected. Tissues were postfixed for 2–4 h and cryoprotected in 25% (wt/vol) sucrose in 0.1 M PB at 4 °C. Celiac ganglia and spinal cords were embedded in gelatin and sectioned on a sliding microtome. All other tissues were embedded in Tissue-Tek OCT compound (Sakura Finetek). Pancreata were sectioned at 10 μm and mounted serially on Superfrost Plus slides (Fisher Scientific). Spinal cords (30 μm), celiac ganglia (20 μm), DRG, and nodose (14 μm) were sectioned and analyzed via systematic random sampling (every fifth section). Sections were washed, blocked with 5% (vol/vol) normal horse serum in 0.1 M PB containing 0.25% Triton X-100, and incubated overnight with primary antibodies in the blocking buffer. Primary antibodies used are: ATF3 (C-19, 1:300, Santa Cruz SC-188) targeted to the C terminus of human ATF3 (23, 74); GFAP (GA5 monoclonal, 1:500, Cell Signaling 3670) made against porcine GFAP (26, 73), cleaved caspase 3 (Cell Signaling, 1:10) (71), and anti-Ki67 (Abcam, ab15580; 1:2,500) (72). Sections were washed and immunoreactivity was detected using dye-conjugated secondary antibodies (1:500, Jackson ImmunoResearch Laboratories). Sections were photographed using LAS software and a Leica DM 4000B microscope.
Estimation of Ganglionic Cell Number and Size.
The number of neurons in celiac, nodose, and T8–T12 spinal ganglia were estimated by counting the number of neuronal nuclei in every fifth section. This number was multiplied by the fraction of sections represented of the total number of sections [total number of cells = (no. of cells in sections examined) × (total no. of sections/no. sections analyzed)]. Neurons were counted by the presence of a defined nucleus; nucleoli of small neurons are not often detectable (75). Soma sizes were determined by measuring the profiles of 25 random neurons per section (four sections per animal). Cell number and size were compared using a one-way ANOVA. To determine the number of ATF3-positive neurons, NIH ImageJ software was used to threshold the staining intensity in one section. This setting was used for all subsequent sections across all experimental groups. For ATF3, cell counts were analyzed by Kruskal–Wallis test followed by Dunn’s post hoc analysis.
Estimation of Spinal Cord GFAP Immunoreactivity.
GFAP staining in spinal cord astrocytes has been validated as a measure of spinal cord inflammation, especially in relation to peripheral nerve injury and neuropathic and inflammatory pain (76–81). To compare GFAP immunoreactivity in the spinal cord, the percent of staining in four regions of interest (perimeter = 470 µm) in four sections per animal (n = 7 per group) was determined. NIH ImageJ was used to threshold each section at the same setting and the area filled by GFAP staining was compared using a two-way ANOVA followed by Tukey’s post hoc analysis.
Estimation of Spinal Cord p-ERK.
The expression of p-ERK protein was compared using standard Western blot analyses. Thoracic spinal cord segments were harvested from control, PanIN, and tumor-bearing mice. Total protein was extracted on ice by homogenization in 50 mM Tris⋅HCl lysis buffer (pH 7.4) containing 0.5% SDS and protease inhibitors. Protein concentration was determined via bicinchoninic acid (BCA) assay (Thermo Fisher Scientific). Aliquots containing 40 µg total protein were separated on 12% SDS/PAGE gels and transferred to PDVF membranes using the Bio-Rad Transblot system. Nonspecific binding was blocked using 5% (wt/vol) BSA, and membranes were incubated overnight in primary antibodies directed against p-ERK p44/42 (1:1,000; Cell Signaling). Protein bands were detected using HRP secondary antibody (1:5,000). Membranes were also probed with β-tubulin III (1:1,000) as a loading control. Densitometry readings were performed using SuperSignal Chemilumescent Detection reagents (Thermofisher) and an LAS3000 imager (Fujifilm).
Ablation of Sensory Fibers.
Sensory neurons were ablated via neonatal capsaicin treatment. At 1–2 d of age (P2), 20 μL of capsaicin (50 mg/kg, i.p.) in absolute ethanol/Tween-80/isotonic saline (10:10:80) was administered. At times after tumor development (8–18 mo), capsaicin-treated mice were euthanized and tissue was collected for histological and biochemical analyses. Survival data were analyzed via Kaplan–Meier log rank test.
Acknowledgments
A.D.R. was supported by the Wicha Family Fund, the American Gastroenterological Association, a Department of Defense Career Development Award, and NIH Grants K08-DK088945, R01-CA177857, P30-DK050306, and P30-DK034933.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1512603113/-/DCSupplemental.
References
- 1.Rhim AD, et al. EMT and dissemination precede pancreatic tumor formation. Cell. 2012;148(1-2):349–361. doi: 10.1016/j.cell.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rhim AD, et al. Detection of circulating pancreas epithelial cells in patients with pancreatic cystic lesions. Gastroenterology. 2014;146(3):647–651. doi: 10.1053/j.gastro.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carobi C. Capsaicin-sensitive vagal afferent neurons innervating the rat pancreas. Neurosci Lett. 1987;77(1):5–9. doi: 10.1016/0304-3940(87)90597-0. [DOI] [PubMed] [Google Scholar]
- 4.Fasanella KE, Christianson JA, Chanthaphavong RS, Davis BM. Distribution and neurochemical identification of pancreatic afferents in the mouse. J Comp Neurol. 2008;509(1):42–52. doi: 10.1002/cne.21736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lindsay TH, et al. Pancreatic cancer pain and its correlation with changes in tumor vasculature, macrophage infiltration, neuronal innervation, body weight and disease progression. Pain. 2005;119(1-3):233–246. doi: 10.1016/j.pain.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 6.Sharkey KA, Williams RG. Extrinsic innervation of the rat pancreas: Demonstration of vagal sensory neurones in the rat by retrograde tracing. Neurosci Lett. 1983;42(2):131–135. doi: 10.1016/0304-3940(83)90395-6. [DOI] [PubMed] [Google Scholar]
- 7.Won MH, Park HS, Jeong YG, Park HJ. Afferent innervation of the rat pancreas: Retrograde tracing and immunohistochemistry in the dorsal root ganglia. Pancreas. 1998;16(1):80–87. doi: 10.1097/00006676-199801000-00013. [DOI] [PubMed] [Google Scholar]
- 8.Nathan JD, et al. Primary sensory neurons: A common final pathway for inflammation in experimental pancreatitis in rats. Am J Physiol Gastrointest Liver Physiol. 2002;283(4):G938–G946. doi: 10.1152/ajpgi.00105.2002. [DOI] [PubMed] [Google Scholar]
- 9.Romac JM, McCall SJ, Humphrey JE, Heo J, Liddle RA. Pharmacologic disruption of TRPV1-expressing primary sensory neurons but not genetic deletion of TRPV1 protects mice against pancreatitis. Pancreas. 2008;36(4):394–401. doi: 10.1097/MPA.0b013e318160222a. [DOI] [PubMed] [Google Scholar]
- 10.Schwartz ES, et al. Synergistic role of TRPV1 and TRPA1 in pancreatic pain and inflammation. Gastroenterology. 2011;140(4):1283–1291.e1, 2. doi: 10.1053/j.gastro.2010.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwartz ES, et al. TRPV1 and TRPA1 antagonists prevent the transition of acute to chronic inflammation and pain in chronic pancreatitis. J Neurosci. 2013;33(13):5603–5611. doi: 10.1523/JNEUROSCI.1806-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kirchgessner AL, Gershon MD. Innervation of the pancreas by neurons in the gut. J Neurosci. 1990;10(5):1626–1642. doi: 10.1523/JNEUROSCI.10-05-01626.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nathan JD, et al. Capsaicin vanilloid receptor-1 mediates substance P release in experimental pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2001;281(5):G1322–G1328. doi: 10.1152/ajpgi.2001.281.5.G1322. [DOI] [PubMed] [Google Scholar]
- 14.Toma H, et al. Characterization of the neurotrophic response to acute pancreatitis. Pancreas. 2002;25(1):31–38. doi: 10.1097/00006676-200207000-00009. [DOI] [PubMed] [Google Scholar]
- 15.Liddle RA. The role of Transient Receptor Potential Vanilloid 1 (TRPV1) channels in pancreatitis. Biochim Biophys Acta. 2007;1772(8):869–878. doi: 10.1016/j.bbadis.2007.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whitcomb DC, Applebaum S, Martin SP. Hereditary pancreatitis and pancreatic carcinoma. Ann N Y Acad Sci. 1999;880:201–209. doi: 10.1111/j.1749-6632.1999.tb09524.x. [DOI] [PubMed] [Google Scholar]
- 17.Horvath RJ, Landry RP, Romero-Sandoval EA, DeLeo JA. Morphine tolerance attenuates the resolution of postoperative pain and enhances spinal microglial p38 and extracellular receptor kinase phosphorylation. Neuroscience. 2010;169(2):843–854. doi: 10.1016/j.neuroscience.2010.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Romero-Sandoval EA, Horvath R, Landry RP, DeLeo JA. Cannabinoid receptor type 2 activation induces a microglial anti-inflammatory phenotype and reduces migration via MKP induction and ERK dephosphorylation. Mol Pain. 2009;5:25. doi: 10.1186/1744-8069-5-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ji RR, Baba H, Brenner GJ, Woolf CJ. Nociceptive-specific activation of ERK in spinal neurons contributes to pain hypersensitivity. Nat Neurosci. 1999;2(12):1114–1119. doi: 10.1038/16040. [DOI] [PubMed] [Google Scholar]
- 20.Ji RR, Befort K, Brenner GJ, Woolf CJ. ERK MAP kinase activation in superficial spinal cord neurons induces prodynorphin and NK-1 upregulation and contributes to persistent inflammatory pain hypersensitivity. J Neurosci. 2002;22(2):478–485. doi: 10.1523/JNEUROSCI.22-02-00478.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ji RR, Gereau RW, 4th, Malcangio M, Strichartz GR. MAP kinase and pain. Brain Res Brain Res Rev. 2009;60(1):135–148. doi: 10.1016/j.brainresrev.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hai T, Wolford CC, Chang YS. ATF3, a hub of the cellular adaptive-response network, in the pathogenesis of diseases: Is modulation of inflammation a unifying component? Gene Expr. 2010;15(1):1–11. doi: 10.3727/105221610x12819686555015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hyatt Sachs H, et al. Activating transcription factor 3 induction in sympathetic neurons after axotomy: Response to decreased neurotrophin availability. Neuroscience. 2007;150(4):887–897. doi: 10.1016/j.neuroscience.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 24.Ohtori S, et al. Differences in damage to CGRP immunoreactive sensory nerves after two lumbar surgical approaches: Investigation using humans and rats. Spine. 2012;37(3):168–173. doi: 10.1097/BRS.0b013e31821258f7. [DOI] [PubMed] [Google Scholar]
- 25.Wang R, et al. Artemin induced functional recovery and reinnervation after partial nerve injury. Pain. 2014;155(3):476–484. doi: 10.1016/j.pain.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stopczynski RE, et al. Neuroplastic changes occur early in the development of pancreatic ductal adenocarcinoma. Cancer Res. 2014;74(6):1718–1727. doi: 10.1158/0008-5472.CAN-13-2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liddle RA, Nathan JD. Neurogenic inflammation and pancreatitis. Pancreatology. 2004;4(6):551–559, discussion 559–560. doi: 10.1159/000082180. [DOI] [PubMed] [Google Scholar]
- 28.Díaz-Laviada I. Effect of capsaicin on prostate cancer cells. Future Oncol. 2010;6(10):1545–1550. doi: 10.2217/fon.10.117. [DOI] [PubMed] [Google Scholar]
- 29.Ito K, et al. Induction of apoptosis in leukemic cells by homovanillic acid derivative, capsaicin, through oxidative stress: Implication of phosphorylation of p53 at Ser-15 residue by reactive oxygen species. Cancer Res. 2004;64(3):1071–1078. doi: 10.1158/0008-5472.can-03-1670. [DOI] [PubMed] [Google Scholar]
- 30.Jin J, et al. Capsaicin mediates cell cycle arrest and apoptosis in human colon cancer cells via stabilizing and activating p53. Int J Biol Sci. 2014;10(3):285–295. doi: 10.7150/ijbs.7730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pramanik KC, Boreddy SR, Srivastava SK. Role of mitochondrial electron transport chain complexes in capsaicin mediated oxidative stress leading to apoptosis in pancreatic cancer cells. PLoS One. 2011;6(5):e20151. doi: 10.1371/journal.pone.0020151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramos-Torres Á, Bort A, Morell C, Rodríguez-Henche N, Díaz-Laviada I. The pepper’s natural ingredient capsaicin induces autophagy blockage in prostate cancer cells. Oncotarget. 2015;7(2):1569–1583. doi: 10.18632/oncotarget.6415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sánchez AM, Sánchez MG, Malagarie-Cazenave S, Olea N, Díaz-Laviada I. Induction of apoptosis in prostate tumor PC-3 cells and inhibition of xenograft prostate tumor growth by the vanilloid capsaicin. Apoptosis. 2006;11(1):89–99. doi: 10.1007/s10495-005-3275-z. [DOI] [PubMed] [Google Scholar]
- 34.Zhang JH, et al. Involvement of the phosphoinositide 3-kinase/Akt pathway in apoptosis induced by capsaicin in the human pancreatic cancer cell line PANC-1. Oncol Lett. 2013;5(1):43–48. doi: 10.3892/ol.2012.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lawson SN. The morphological consequences of neonatal treatment with capsaicin on primary afferent neurones in adult rats. Acta Physiol Hung. 1987;69(3-4):315–321. [PubMed] [Google Scholar]
- 36.Ribeiro-da-Silva A, Coimbra A. Capsaicin causes selective damage to type I synaptic glomeruli in rat substantia gelatinosa. Brain Res. 1984;290(2):380–383. doi: 10.1016/0006-8993(84)90961-2. [DOI] [PubMed] [Google Scholar]
- 37.Nagy JI, Iversen LL, Goedert M, Chapman D, Hunt SP. Dose-dependent effects of capsaicin on primary sensory neurons in the neonatal rat. J Neurosci. 1983;3(2):399–406. doi: 10.1523/JNEUROSCI.03-02-00399.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jancsó G, Király E. Sensory neurotoxins: Chemically induced selective destruction of primary sensory neurons. Brain Res. 1981;210(1-2):83–89. doi: 10.1016/0006-8993(81)90886-6. [DOI] [PubMed] [Google Scholar]
- 39.Cuello AC, Gamse R, Holzer P, Lembeck F. Substance P immunoreactive neurons following neonatal administration of capsaicin. Naunyn Schmiedebergs Arch Pharmacol. 1981;315(3):185–194. doi: 10.1007/BF00499834. [DOI] [PubMed] [Google Scholar]
- 40.Cavanaugh DJ, et al. Restriction of transient receptor potential vanilloid-1 to the peptidergic subset of primary afferent neurons follows its developmental downregulation in nonpeptidergic neurons. J Neurosci. 2011;31(28):10119–10127. doi: 10.1523/JNEUROSCI.1299-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Díaz-Laviada I, Rodríguez-Henche N. The potential antitumor effects of capsaicin. Prog Drug Res. 2014;68:181–208. doi: 10.1007/978-3-0348-0828-6_8. [DOI] [PubMed] [Google Scholar]
- 42.Kim CS, et al. Capsaicin, a spicy component of hot pepper, induces apoptosis by activation of the peroxisome proliferator-activated receptor gamma in HT-29 human colon cancer cells. J Med Food. 2004;7(3):267–273. doi: 10.1089/jmf.2004.7.267. [DOI] [PubMed] [Google Scholar]
- 43.Zhang R, Humphreys I, Sahu RP, Shi Y, Srivastava SK. In vitro and in vivo induction of apoptosis by capsaicin in pancreatic cancer cells is mediated through ROS generation and mitochondrial death pathway. Apoptosis. 2008;13(12):1465–1478. doi: 10.1007/s10495-008-0278-6. [DOI] [PubMed] [Google Scholar]
- 44.Gil Z, et al. Paracrine regulation of pancreatic cancer cell invasion by peripheral nerves. J Natl Cancer Inst. 2010;102(2):107–118. doi: 10.1093/jnci/djp456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.He S, et al. GFRα1 released by nerves enhances cancer cell perineural invasion through GDNF-RET signaling. Proc Natl Acad Sci USA. 2014;111(19):E2008–E2017. doi: 10.1073/pnas.1402944111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.He S, et al. The chemokine (CCL2-CCR2) signaling axis mediates perineural invasion. Mol Cancer Res. 2015;13(2):380–390. doi: 10.1158/1541-7786.MCR-14-0303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cavel O, et al. Endoneurial macrophages induce perineural invasion of pancreatic cancer cells by secretion of GDNF and activation of RET tyrosine kinase receptor. Cancer Res. 2012;72(22):5733–5743. doi: 10.1158/0008-5472.CAN-12-0764. [DOI] [PubMed] [Google Scholar]
- 48.Pour PM, Bell RH, Batra SK. Neural invasion in the staging of pancreatic cancer. Pancreas. 2003;26(4):322–325. doi: 10.1097/00006676-200305000-00002. [DOI] [PubMed] [Google Scholar]
- 49.Ceyhan GO, et al. Neural invasion in pancreatic cancer: A mutual tropism between neurons and cancer cells. Biochem Biophys Res Commun. 2008;374(3):442–447. doi: 10.1016/j.bbrc.2008.07.035. [DOI] [PubMed] [Google Scholar]
- 50.Liu B, Lu KY. Neural invasion in pancreatic carcinoma. Hepatobiliary Pancreat Dis Int. 2002;1(3):469–476. [PubMed] [Google Scholar]
- 51.Magnon C, et al. Autonomic nerve development contributes to prostate cancer progression. Science. 2013;341(6142):1236361. doi: 10.1126/science.1236361. [DOI] [PubMed] [Google Scholar]
- 52.Zhao CM, et al. Denervation suppresses gastric tumorigenesis. Sci Transl Med. 2014;6(250):250ra115. doi: 10.1126/scitranslmed.3009569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Berthoud HR, Neuhuber WL. Functional and chemical anatomy of the afferent vagal system. Auton Neurosci. 2000;85(1-3):1–17. doi: 10.1016/S1566-0702(00)00215-0. [DOI] [PubMed] [Google Scholar]
- 54.Ghilardi JR, et al. Sustained blockade of neurotrophin receptors TrkA, TrkB and TrkC reduces non-malignant skeletal pain but not the maintenance of sensory and sympathetic nerve fibers. Bone. 2011;48(2):389–398. doi: 10.1016/j.bone.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jimenez-Andrade JM, Ghilardi JR, Castañeda-Corral G, Kuskowski MA, Mantyh PW. Preventive or late administration of anti-NGF therapy attenuates tumor-induced nerve sprouting, neuroma formation, and cancer pain. Pain. 2011;152(11):2564–2574. doi: 10.1016/j.pain.2011.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mantyh WG, et al. Blockade of nerve sprouting and neuroma formation markedly attenuates the development of late stage cancer pain. Neuroscience. 2010;171(2):588–598. doi: 10.1016/j.neuroscience.2010.08.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Albers KM, et al. Artemin growth factor increases nicotinic cholinergic receptor subunit expression and activity in nociceptive sensory neurons. Mol Pain. 2014;10:31. doi: 10.1186/1744-8069-10-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dussor GO, et al. Potentiation of evoked calcitonin gene-related peptide release from oral mucosa: A potential basis for the pro-inflammatory effects of nicotine. Eur J Neurosci. 2003;18(9):2515–2526. doi: 10.1046/j.1460-9568.2003.02935.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Peterson SC, et al. Basal cell carcinoma preferentially arises from stem cells within hair follicle and mechanosensory niches. Cell Stem Cell. 2015;16(4):400–412. doi: 10.1016/j.stem.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Houghton AK, Kadura S, Westlund KN. Dorsal column lesions reverse the reduction of homecage activity in rats with pancreatitis. Neuroreport. 1997;8(17):3795–3800. doi: 10.1097/00001756-199712010-00028. [DOI] [PubMed] [Google Scholar]
- 61.Houghton AK, Wang C-C, Westlund KN. Do nociceptive signals from the pancreas travel in the dorsal column? Pain. 2001;89(2-3):207–220. doi: 10.1016/s0304-3959(00)00364-x. [DOI] [PubMed] [Google Scholar]
- 62.Willis WD, Al-Chaer ED, Quast MJ, Westlund KN. A visceral pain pathway in the dorsal column of the spinal cord. Proc Natl Acad Sci USA. 1999;96(14):7675–7679. doi: 10.1073/pnas.96.14.7675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang CC, Westlund KN. Responses of rat dorsal column neurons to pancreatic nociceptive stimulation. Neuroreport. 2001;12(11):2527–2530. doi: 10.1097/00001756-200108080-00047. [DOI] [PubMed] [Google Scholar]
- 64.Rucker HK, Holloway JA, Keyser GF. Response characteristics of cat spinothalamic tract neurons to splanchnic nerve stimulation. Brain Res. 1984;291(2):383–387. doi: 10.1016/0006-8993(84)91274-5. [DOI] [PubMed] [Google Scholar]
- 65.Imoto A, et al. Neural invasion induces cachexia via astrocytic activation of neural route in pancreatic cancer. Int J Cancer. 2012;131(12):2795–2807. doi: 10.1002/ijc.27594. [DOI] [PubMed] [Google Scholar]
- 66.Razavi R, et al. TRPV1+ sensory neurons control beta cell stress and islet inflammation in autoimmune diabetes. Cell. 2006;127(6):1123–1135. doi: 10.1016/j.cell.2006.10.038. [DOI] [PubMed] [Google Scholar]
- 67.Chiu IM, von Hehn CA, Woolf CJ. Neurogenic inflammation and the peripheral nervous system in host defense and immunopathology. Nat Neurosci. 2012;15(8):1063–1067. doi: 10.1038/nn.3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Miller KE, Hoffman EM, Sutharshan M, Schechter R. Glutamate pharmacology and metabolism in peripheral primary afferents: Physiological and pathophysiological mechanisms. Pharmacol Ther. 2011;130(3):283–309. doi: 10.1016/j.pharmthera.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vera-Portocarrero L, Westlund KN. Role of neurogenic inflammation in pancreatitis and pancreatic pain. Neurosignals. 2005;14(4):158–165. doi: 10.1159/000087654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Siveke JT, et al. Notch signaling is required for exocrine regeneration after acute pancreatitis. Gastroenterology. 2008;134(2):544–555. doi: 10.1053/j.gastro.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 71.Ardito CM, et al. EGF receptor is required for KRAS-induced pancreatic tumorigenesis. Cancer Cell. 2012;22(3):304–317. doi: 10.1016/j.ccr.2012.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Delgiorno KE, et al. Identification and manipulation of biliary metaplasia in pancreatic tumors. Gastroenterology. 2014;146(1):233–44.e5. doi: 10.1053/j.gastro.2013.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Koh BH, et al. Platelet-derived growth factor receptor-α cells in mouse urinary bladder: A new class of interstitial cells. J Cell Mol Med. 2012;16(4):691–700. doi: 10.1111/j.1582-4934.2011.01506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Young BA, Girard BM, Parsons RL. Neurturin suppresses injury-induced neuronal activating transcription factor 3 expression in cultured guinea pig cardiac ganglia. J Comp Neurol. 2008;508(5):795–805. doi: 10.1002/cne.21711. [DOI] [PubMed] [Google Scholar]
- 75.Schmalbruch H. The number of neurons in dorsal root ganglia L4-L6 of the rat. Anat Rec. 1987;219(3):315–322. doi: 10.1002/ar.1092190313. [DOI] [PubMed] [Google Scholar]
- 76.Coyle DE. Partial peripheral nerve injury leads to activation of astroglia and microglia which parallels the development of allodynic behavior. Glia. 1998;23(1):75–83. [PubMed] [Google Scholar]
- 77.Cao H, Zhang YQ. Spinal glial activation contributes to pathological pain states. Neurosci Biobehav Rev. 2008;32(5):972–983. doi: 10.1016/j.neubiorev.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 78.Honore P, et al. Murine models of inflammatory, neuropathic and cancer pain each generates a unique set of neurochemical changes in the spinal cord and sensory neurons. Neuroscience. 2000;98(3):585–598. doi: 10.1016/s0306-4522(00)00110-x. [DOI] [PubMed] [Google Scholar]
- 79.Ren K, Dubner R. Neuron-glia crosstalk gets serious: Role in pain hypersensitivity. Curr Opin Anaesthesiol. 2008;21(5):570–579. doi: 10.1097/ACO.0b013e32830edbdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Thacker MA, Clark AK, Marchand F, McMahon SB. Pathophysiology of peripheral neuropathic pain: immune cells and molecules. Anesth Analg. 2007;105(3):838–847. doi: 10.1213/01.ane.0000275190.42912.37. [DOI] [PubMed] [Google Scholar]
- 81.Zhuang ZY, Gerner P, Woolf CJ, Ji RR. ERK is sequentially activated in neurons, microglia, and astrocytes by spinal nerve ligation and contributes to mechanical allodynia in this neuropathic pain model. Pain. 2005;114(1-2):149–159. doi: 10.1016/j.pain.2004.12.022. [DOI] [PubMed] [Google Scholar]