Significance
The hormone leptin homeostatically maintains long-term fat stores in mammals. Made by adipocytes in proportion to total adipose mass, leptin functions by regulating behavioral, autonomic, and endocrine circuits in the CNS to control energy intake and expenditure. As leptin signals nutritional sufficiency, it also acts as a gating factor for reproductive maturation and competence. Defective leptin signaling in mammals results in hyperphagia, obesity, diabetes, and infertility. Much less is known about leptin in nonmammalian vertebrates; however, the teleost leptin homologue is not primarily expressed in adipocytes. Here we show that zebrafish leptin is not required for adipostasis, food intake, or reproduction. However, we show here that, as in mammals, zebrafish leptin retains a role in the regulation of glucose homeostasis.
Keywords: leptin, zebrafish, glucose homeostasis, adipostasis
Abstract
Leptin is the primary adipostatic factor in mammals. Produced largely by adipocytes in proportion to total adipose mass, the hormone informs the brain regarding total energy stored as triglycerides in fat cells. The hormone acts on multiple circuits in the brain to regulate food intake, autonomic outflow, and endocrine function to maintain energy balance. In addition to regulating adipose mass, mammalian leptin also plays a role in the regulation of glucose homeostasis and as a gating factor in reproductive competence. Leptin-deficient mice and people exhibit early onset profound hyperphagia and obesity, diabetes, and infertility. Although leptin and the leptin receptor are found in fish, the hormone is not expressed in adipose tissue, but is found in liver and other tissues. Here, we show that adult zebrafish lacking a functional leptin receptor do not exhibit hyperphagia or increased adiposity, and exhibit normal fertility. However, leptin receptor-deficient larvae have increased numbers of β-cells and increased levels of insulin mRNA. Furthermore, larval zebrafish have been shown to exhibit β-cell hyperplasia in response to high fat feeding or peripheral insulin resistance, and we show here that leptin receptor is required for this response. Adult zebrafish also have increased levels of insulin mRNA and other alterations in glucose homeostasis. Thus, a role for leptin in the regulation of β-cell mass and glucose homeostasis appears to be conserved across vertebrates, whereas its role as an adipostatic factor is likely to be a secondary role acquired during the evolution of mammals.
The hormone leptin was identified in mammalian adipocytes (1) and well characterized in mice and humans as an adipostatic hormone. It is secreted into the serum in proportion to adipose mass and homeostatically regulates adipose mass primarily via binding to a distinct leptin receptor expressed in behavioral, endocrine, and autonomic control circuits in the central nervous system (2, 3). Failure of leptin signaling, due to mutations in leptin or leptin receptor genes, results in hyperphagia and hypometabolism to produce extreme obesity, diabetes, and infertility. Leptin and leptin receptor are highly conserved across mammalian species. Mouse and human leptin proteins are 83% identical, and leptin receptor proteins are 75% identical. However, the mammalian leptin and leptin receptor amino acid sequences are less well conserved with those of lower vertebrates. Indeed, the use of primary sequence homology failed to identify the leptin gene in fish or birds; chromosomal synteny was ultimately used to identify the gene in these vertebrate classes (4). For example, the zebrafish leptin protein is only 19% identical to the human protein.
Although the amino acid sequences are divergent, the basic structural features and intracellular signaling mechanisms of leptin and its receptor appear to be conserved throughout vertebrates (4). Furthermore, administration of mammalian leptin in birds and fish caused an anorexigenic effect, suggesting conservation of function of the leptin system (5). However, although leptin in mammals is predominantly expressed in adipose tissue (6), leptin expression in fish and birds appears to be negligible in adipose tissue (7). The expression profile varies widely, with many studies reporting the liver as a site of expression in fish (4). Interestingly, multiple studies report a rise in plasma leptin upon fasting (5), a response diametrically opposite to the leptin decrease observed in fasting mice (8). In fish, hepatic leptin expression rises upon fasting (7) and the fish liver can secrete leptin (9). Deletion of the leptin receptor in medaka produced a modest increase in food intake, and juvenile but not adult growth, and a modest increase in visceral but not total body fat (10). These studies cast doubt as to the role of leptin as an adipostatic factor in fish and, thus, roles for leptin in nonmammalian vertebrates remains to be established. We report here on the role of leptin signaling in the zebrafish, Danio rerio, using a zebrafish mutant for the leptin receptor, and using clustered regularly interspaced short palindromic repeats (CRISPR) gene editing to mutate both leptin receptor and leptin genes.
Results
Mutation of the Leptin Receptor in Adult Zebrafish Has Limited Effect on Body Size, Weight, Adiposity, and Feeding.
The zebrafish genome contains one leptin receptor gene (lepr) (11), and two leptin genes, lepa and lepb (12). To study the leptin system in teleosts, we obtained a zebrafish line expressing a mutant form of the leptin receptor (13). The sa1508 allele, obtained through screening for mutations after N-ethyl-N-nitrosourea (ENU) mutagenesis, is a C > A nonsense mutation that leads to a premature stop codon after the start of the cytoplasmic domain of the receptor (Fig. S1A). This mutant is comparable to the nonsignaling truncated leptin receptor isoform in db/db mice (14) and rainbow trout (15).
Fig. S1.
Protein alignments for predicted mutations. (A) Indicated in bold on the mouse leprb sequence are cytoplasmic amino acids 13–24 and 31–36, which have been shown to be essential for Jak2 recruitment and activation. Surrounded by a box are the amino acids comprising the transmembrane domain and the Box1 motif. (B) Alignment of zebrafish WT and Mutant lepa alleles tested for β-cell number. We identified two alleles in heterozygous F1 fish that led to identical early stop codons between helix A and B of lepa. Stop codons are indicated with an asterisk; remaining C-terminal amino acids are indicated at the end of the sequence. AA, amino acid; DaRe, Danio rerio; MuMu, Mus musculus; OnMy, Oncorhynchus mykiss.
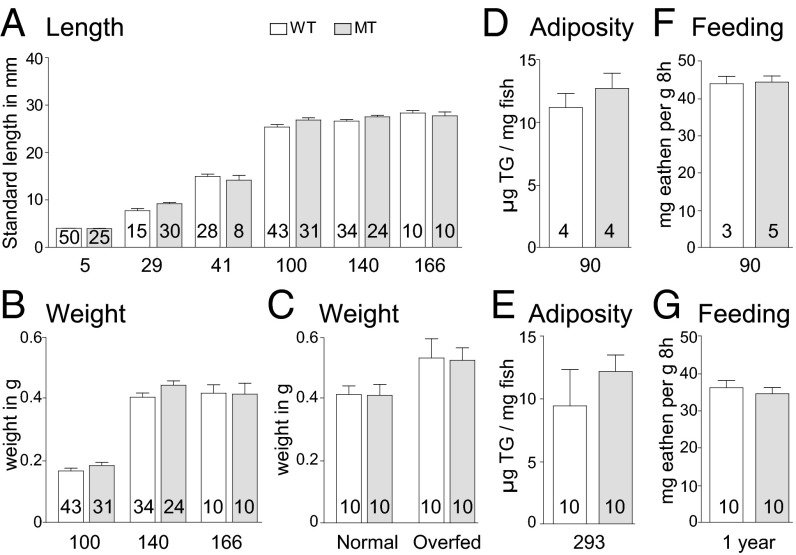
Given the role of the mammalian leptin receptor in the adipostat, we first looked at size, weight, and body composition in the homozygous leprsa1508/sa1508 mutant compared with wild-type (WT) sibling animals, all produced from leprsa1508/+ heterozygous in-crosses, and raised as a mixed genotype population to prevent density effects. We measured standard length (SL) 5 d after fertilization (dpf), and SL and weight at 29 dpf, 41 dpf, and 100 dpf, and found no effect of genotype upon SL or weight (Fig. 1 A and B). We separated animals by genotype, keeping them at the same density and scored SL after maturity at 4 and 5.5 mo (140 and 166 dpf, respectively), with no differences observed. Stratifying the data by gender from 100 dpf onward also did not identify any effects of genotype. We also examined WT and homozygous leprsa1508/sa1508 fish by using an overfeeding paradigm demonstrated to produce obesity in the zebrafish (16). As expected, the “overfed” fish gained a significant amount of weight compared with normal fed fish (Fig. 1C). However, we observed no significant effect of genotype. We next investigated whole body adiposity in 3-mo-old male fish and again saw no significant difference between genotypes (Fig. 1D). To ascertain that there was no long-term adiposity effect of the leprsa1508/sa1508 genotype, we kept two clutches of fish until 293 dpf, but saw no significant effect of genotype (Fig. 1E). The leptin-deficient ob/ob and leptin receptor-deficient db/db mice also exhibit profound hyperphagia (1). We trained male animals to recognize a commercially available fish food (Betta Bits, TopFin) and tested food intake for 16 d with 8 h of exposure to excess food, each followed by a day of rest. There was no effect of genotype on food intake either in 3-mo or 1-y-old sibling animals (Fig. 1 F and G).
Fig. 1.
Length, weight, adipose mass, and food intake in normal and leptin receptor mutant zebrafish. Heterozygous leprsa1508/+ mutant fish were crossed to provide WT (open bars) and homozygous leprsa1508/sa1508 (gray bars) offspring, which were grown together at similar densities, then characterized for growth, body composition, and feeding behavior, with the numbers (inside bars) and ages in days after fertilization (under bars) indicated. (A) Length of WT and leprsa1508/sa1508 mutant fish from 5 to 166 dpf. Two-way ANOVA shows a significant effect of age [F(5, 304)=1,284, P < 0.0001] but not genotype [F(1, 304) = 2.104, P > 0.05]. (B) Weight of WT and leprsa1508/sa1508 mutant fish from 100 to 166 dpf. Two-way ANOVA shows a significant effect of age [F(2, 151) = 227.1, P < 0.0001] but not genotype [F(1, 151) = 1.706, P > 0.05]. (C) Weight of WT and leprsa1508/sa1508 mutant fish following 30 d of high fat feeding. Two-way ANOVA shows significant increase in weight as a function of diet [F(1, 39) = 4.687 P < 0.05] but not of genotype [F(1, 39) = 0.03255, P > 0.05]. (D and E) Total triglyceride content of WT and leprsa1508/sa1508 mutant fish at 90 and 293 d of age, respectively. There was no significant difference at either age t (6) = 0.8631, P > 0.4213 (D) and t (18) = 0.9246, P > 0.3673 (E); two-tailed t test. (F and G) Food consumption of 3-mo-old male (F) and 1 y old female WT and leprsa1508/sa1508 mutant (G) fish over 16 trials. A repeated-measures ANOVA shows no significant effect of genotype in males [F(1, 127) = 0.00476, P > 0.05] or females [F(1, 319) = 1.013, P > 0.05]. Data shown as means ± SEM.
Mutation of the Leptin Receptor in Zebrafish Has No Effect on Fertility.
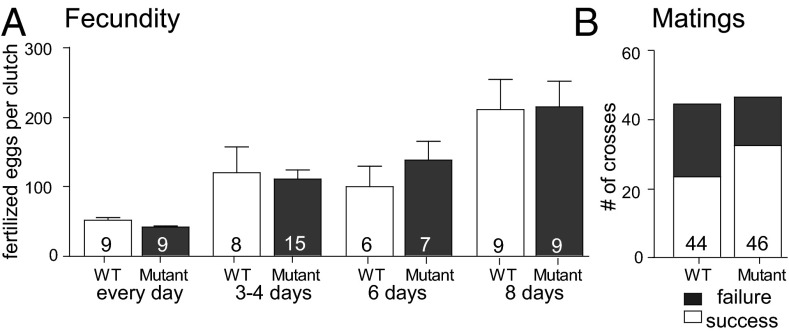
An additional phenotype of leptin deficiency in mice and humans is infertility (17, 18). We therefore investigated reproductive competency by crossing wild-type siblings as well as leprsa1508/sa1508 mutant adults and scoring the productivity and efficiency of breeding events (Fig. 2). Reproductive productivity was determined by counting individual clutch sizes of five couples of each genotype with variable periods of separation (daily breeding, 3–4 d, 6 d or ≥8 d of separation). Clutch size increases based on the time of parental separation, but no effect of genotype on the number of fertilized eggs laid (Fig. 2A) or frequency of successful breedings (Fig. 2B) was observed.
Fig. 2.
Fecundity and mating efficiency in normal and leptin receptor mutant zebrafish. (A) Fertilized eggs from age-matched WT and leprsa1508/sa1508 mutant couples bred daily or with variable times of separation between breeding, with days of separation indicated. Two-way ANOVA shows a significant effect for days of separation [F(3, 71) = 13.29, P < 0.0001] but not genotype [F(1, 71) = 0.09, P > 0.05] or an interaction of the two [F(1, 71) = 0.29, P > 0.05]. (B) Mating efficiency for WT and leprsa1508/sa1508 mutant mating for fish of 3–6 mo of age. Data shown as means ± SEM (P > 0.05, Fisher’s exact test); number of attempted matings indicated.
Mutation of the Leptin Receptor in Larval Zebrafish Increased Insulin and Glucagon Gene Expression, and β-Cell Mass.
The leptin receptor-deficient diabetes (db/db) mouse exhibits hyperglycemia by 3–4 wk of age (19). Hepatic mRNA levels for leptin have been shown to change upon fasting in zebrafish, common carp, Atlantic salmon, and Arctic charr (5), and recombinant leptin was shown to induce hepatic glucose mobilization in tilapia (20). Thus, we next sought to examine effects of leptin receptor deficiency on glucose homeostasis in the zebrafish (Fig. 3).
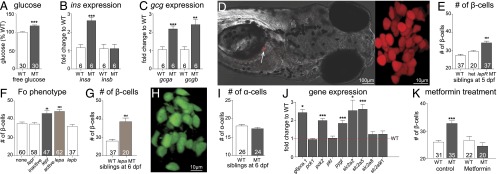
Fig. 3.
Glucose homeostasis phenotypes in normal and leptin receptor mutant larval zebrafish. (A) Whole-body free glucose in WT and leprsa1508/sa1508 mutant in-crosses collected at 6 dpf. A t test shows a significant difference between genotypes, t(58) = 5.179, P < 0.0001. (B) Expression of insa and insb mRNA at 6 dpf, determined by quantitative RT-PCR (qRTPCR). insa shows significant up-regulation t (10) = 6.058, P < 0.001, whereas insb does not t(10) = 0.016, P > 0.05, t tests. (C) Expression of gcga and gcgb mRNA levels, determined by qRTPCR. gcga t(10) = 4.973, P < 0.001 and gcgb t (10) = 4.232, P < 0.01 are up-regulated, t tests. (D) A representative image of a zebrafish carrying the β-cell marker [Tg(1.2ins:H2BmCherry)], and an enlarged image showing individual β-cells. (E) Numbers of β-cells in offspring of an in-cross of heterozygous leprsa1508/+ mutant animals at 5 dpf. Homozygous leprsa1508/sa1508 mutants exhibit a significant increase in β-cell number by one-way ANOVA, F(2, 93) = 18.69, P < 0.001, Bonferroni’s MCT. (F) Number of β-cells in animals at 6 dpf, following injection of embryos with Cas9 and guide RNA directed against lepr, lepa, or lepb genes; ANOVA F(4, 263) = 11.67, P < 0.0001, Bonferroni’s MCT. (G) Number of β-cells is significantly increased in larvae homozygous for a mutation in lepa compared with their WT siblings at 6 dpf. t test t(41) = 4.738, P < 0.001. (H) A representative picture of α-cells in fish carrying the transgene [Tg(gcg:GFP)]. (I) Numbers of α-cells observed in WT and leprsa1508/sa1508 mutant siblings from heterozygous leprsa1508/+ in-crosses at 6 dpf. There was no effect of genotype [t test t(24) = 0.96, P = 0.34]. (J) Data showing gene expression analysis of fry at 6 dpf compared with WT controls. t tests show an up-regulation of glucose 6 phosphatase (g6pca.1; t (9) = 7.059, P < 0.001), mitochondrial phosphoenolpyruvate carboxykinase (pck2; t (9) = 8.601, P < 0.001), liver glycogen phosphorylase [pygl; t(9) = 4.423, P < 0.01], glucose transporters 2 (scl2a2; t (9) = 2.808, P < 0.05) and 5 [slc2a5; t(10) = 5.160, P < 0.001], but not cytoplasmic phosphoenolpyruvate carboxykinase [pck1; t(9) = 0.4822, P > 0.05], liver pyruvate kinase (pklr; t (10) = 0.2263, P > 0.05), glucose transporters 8 [slc2a8; t(10) = 0.903 P > 0.05] or 9 [slc2a9l1; t(10) = 1.31, P > 0.05). (K) Effect of metformin on the developmental increase in β-cell number. Two-factor ANOVA shows an effect of genotype [F(1,107) = 4.785, P < 0.05] and treatment [F(1,107) = 12.56, P < 0.001]. A Bonferroni post test showed a significant elevation in the DMSO-treated leprsa1508/sa1508 mutant group. Data shown as means ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001.
Total glucose levels were measured in zebrafish fry at 6 dpf, a timepoint at which fry have consumed their yolk. We found a small but significant increase in the glucose content of homozygous leprsa1508/sa1508 mutant fry compared with WT fry (Fig. 3A). We next investigated insulin mRNA levels at 6 dpf by quantitative RT-PCR (qPCR). Zebrafish has two preproinsulin genes, insa and insb, due to a genome duplication event (21), with both isoforms being expressed in the pancreas (22). We found that insa but not insb is significantly up-regulated at 6 dpf in leprsa1508/sa1508 mutant fry (Fig. 3B). We also looked at glucagon transcript levels and found that both glucagon a and b were up-regulated at 6 dpf in leprsa1508/sa1508 fry (Fig. 3C).
db/db mice exhibit altered β-cell numbers and hyperglycemia (23, 24). We therefore crossed the leprsa1508 allele into a zebrafish line carrying a β-cell marker [Tg(-1.2ins:H2BmCherry)] (25) and counted β-cells in 5-dpf fry (Fig. 3D). We found that the number of β-cells was significantly increased by ∼25% in leprsa1508/sa1508 animals compared with their WT and heterozygous siblings (Fig. 3E). To confirm that this phenotype is specific to defective leptin receptor function, we injected Tg(-1.2ins:H2BmCherry) embryos at the one-cell stage with Cas9 mRNA and guide RNA against the lepr gene to disrupt the lepr gene by using CRISPR (26). We used a mutagenic or a nonmutagenic control guide RNA against exons in lepr, and also controlled for DNA damage by using a guide RNA against the tyrosinase gene. Using a heteroduplex mobility shift assay, we confirmed mutagenesis in the tyrosinase gene and one of the two targeted sites in the lepr gene. We found a significant 14% increase in β-cells for the functional guide RNA against lepr but not in the groups injected with the tyrosinase guide RNA or the nonmutagenic lepr guide RNA, confirming results in leprsa1508/sa1508 fish (Fig. 3F). Furthermore, we found that a guide RNA against lepa, but not lepb, also led to a 17% increase in the number of β-cells, further confirming a role for leptin signaling in regulation of β-cell mass in the larval zebrafish (Fig. 3F). We followed up on the involvement of lepa by establishing germ-line founders carrying mutations in lepa and testing their offspring by using the β-cell marker [Tg(-1.2ins:H2BmCherry)]. We found an increase in the number of β-cells at 6 dpf by ∼38% (Fig. 3G). We also crossed Tg(gcga:eGFP), a transgene driving GFP expression in α-cells (25), into leprsa1508/sa1508 fish, to look for effects of lepr deficiency on α-cells (Fig. 3H). No significant effect of genotype on the number of α-cells at 6 dpf (Fig. 3I) was observed.
Mutation of the Leptin Receptor in Larval Zebrafish Alters Expression of Genes Involved in Hepatic Glucose Metabolism.
Next, we investigated transcript levels of key enzymes in hepatic glucose metabolism at 6 dpf (Fig. 3J). Using qPCR, we found an up-regulation of mitochondrial but not cytosolic phosphoenolpyruvate carboxykinase (pck2 and pck1, respectively) in leprsa1508/sa1508 mutant fry. We tested for liver-specific transcripts of glycogen phosphorylase (pygl) and pyruvate kinase (pklr) and found the former to be up-regulated in leprsa1508/sa1508 mutant fry. Lastly, we looked at glucose 6 phosphatase (g6ca.1), and glucose transporter (glut) isoforms expressed in the zebrafish liver (2, 5, 8, and 9a; ref. 27). We found a significant up-regulation of g6pase, slc2a2, and slc2a5 in leprsa1508/sa1508 mutant fry, but not slc2a8 or slc2a9l1 (Fig. 3J). We exposed larvae to metformin, a drug thought to have beneficial effects in diabetes patients because of effects on hepatic glucose homeostasis and insulin sensitivity (28). Exposure of larvae to metformin from 3 to 5 dpf completely abolished the increase seen in leprsa1508/sa1508 mutant fry at 5 dpf (Fig. 3K).
Mutation of the Leptin Receptor in the Adult Zebrafish Leads to Altered Glucose Tolerance and Hepatic Gene Expression.
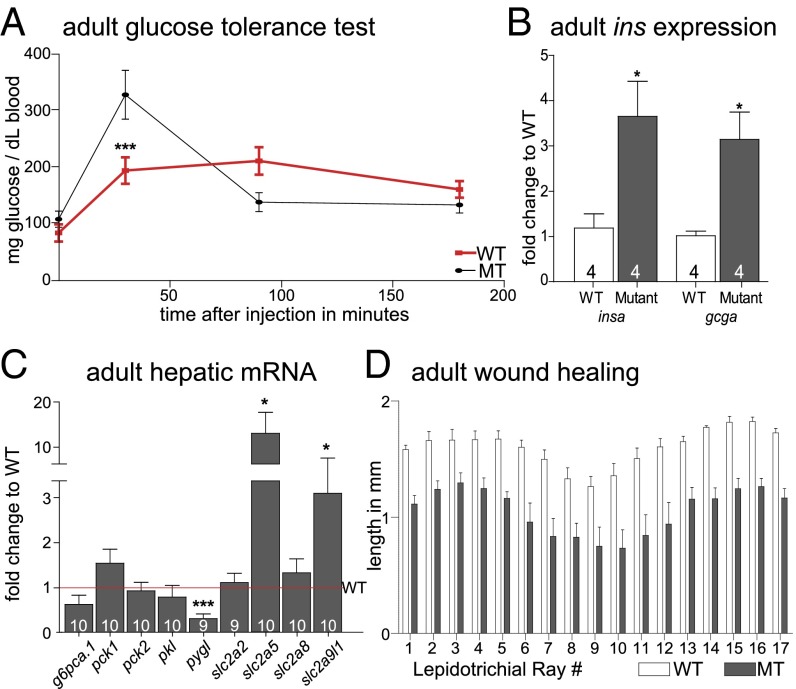
We next investigated aspects of glucose homeostasis in adult zebrafish. We saw no differences in fasting blood glucose between WT and homozygous leprsa1508/sa1508 fish. Challenging the fish with 0.5 mg of glucose per gram of fish, however, we saw improved glucose disposal at 30 min in homozygous leprsa1508/sa1508 fish (Fig. 4A), consistent with increased capacity for insulin release. The zebrafish β-cells are diffusely distributed along the intestine, whereas the primary islet in the pancreas is attached to the intestine. We thus examined insa and gcga transcript levels in whole visceral tissue, ∼1–2 h after a meal of brine shrimp. We found a threefold higher expression of both genes in homozygous adult leprsa1508/sa1508 fish relative to WT levels (Fig. 4B), paralleling larval results. We next tested hepatic transcript levels by using qPCR and found changes in pygl, slc2a5, and slc2a9l1 but not pck1, pck2, g6ca.1, pkl, slc2a2, or slc2a8 (Fig. 4C). Despite no evidence for elevated blood glucose or reduced glucose tolerance, we tested for a diabetic-like phenotype in adult fish, reduced wound healing. Streptozotocin-treated adult zebrafish have been shown to exhibit abnormalities in fin regeneration (29). We therefore clipped fins of 7-mo-old WT and homozygous leprsa1508/sa1508 fish and imaged these fins after 3 d of growth. Regrowth of the lepidotrichial rays was impaired, indicative of a defect in wound healing (Fig. 4D).
Fig. 4.
Glucose homeostasis phenotypes in normal and leptin receptor mutant adult zebrafish. (A) Glucose tolerance test in 1-y-old WT and homozygous leprsa1508/sa1508 fish. A two-way ANOVA post hoc test shows a significant decrease of blood glucose in homozygous leprsa1508/sa1508 fish at 30 min with a significant interaction between time and genotype [F(3,102) = 7.266, ***P < 0.0001]. (B) insa and gcga mRNA expression in adult visceral samples is increased [insa t(6) = 2.947, *P < 0.05 and gcga t (6) = 3.459, *P < 0.05]. (C) Adult hepatic gene expression analysis shows a down-regulation in liver glycogen phosphorylase [pygl; t (17) = 4.650, ***P < 0.001], and up-regulation in glucose transporters 5 [slc2a5; t (16) = 2.289, *P < 0.05] and 9 [slc2a9l1; t(17) = 2.616, *P < 0.05] and no change in glucose 6 phosphatase [g6pca.1; t(18) = 1.984, P = 0.0627], cytoplasmic phosphoenolpyruvate carboxykinase [pck1; t(17) = 0.3637, P = 0.72], mitochondrial phosphoenolpyruvate carboxykinase [pck2; t(18) = 0.6966, P = 0.49], liver pyruvate kinase [pklr; t(17) = 0.9630, P = 0.35], glucose transporters 2 [scl2a2; t(17) = 0.1563, P = 0.88] or 8 [slc2a8; t(18) = 0.9041 P = 0.38], all t tests. (D) Tissue regeneration 3 d after fin-clipping. A two-way ANOVA shows a significant effect of genotype [F(1, 186) = 169.7, P < 0.0001]. Data shown as means ± SEM.
Mutation of the Leptin Receptor in Larval Zebrafish Blocks Nutrient-Induced β-Cell Compensation.
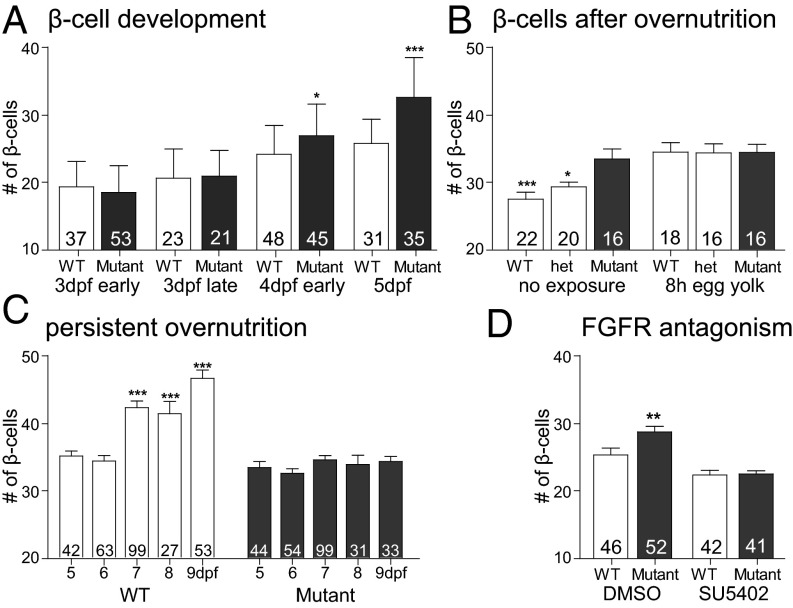
In zebrafish, nutrient excess leads to an increase in larval β-cell neogenesis (25). Because we saw a similar increase in the leprsa1508/sa1508 mutant fry, we wished to test whether chronic nutrient excess would be ineffective in further increasing β-cell numbers in leprsa1508/sa1508 mutant fry. First, we determined when, during development, leptin receptor deficiency led to an increase in β-cell mass. We saw a slight trend at 3 dpf and a significant increase in β-cell number at 4 dpf (Fig. 5A). By exposing the larvae to 5% egg yolk by volume (26.5% lipid and 15.8% protein content by weight) for 8 h at 5 dpf, a timepoint at which most of the larvae’s own yolk is consumed, we found an increase in β-cell number in overfed wild-type animals but not in mutant leprsa1508/sa1508 siblings. Indeed, nutrient excess seemed to equalize the number of β-cells between wild-type and homozgous leprsa1508/sa1508 mutants (Fig. 5B). Persistent daily exposure to egg yolk leads to a further increase in β-cell mass during days 7–9 in WT larvae, but this late effect of nutrition was also completely blocked in leprsa1508/sa1508 mutants (Fig. 5C). Thus, leptin receptor deficiency increases β-cell mass early in development, but at the same time, blocks nutrient-induced compensation. FGF1 mediates overnutrition-induced β-cell differentiation in the fish (30). We therefore tested whether an FGF signal mediates the early β-cell increase in leprsa1508/sa1508 mutant larvae. We exposed larvae late at 3 dpf to SU5402 and scored β-cell number at 4 dpf. We found that the FGF inhibitor also blocks the early increase in β-cell number seen in leprsa1508/sa1508 mutants (Fig. 5D), implicating FGF in the increase in β-cells resulting from either overnutrition or lepr deficiency.
Fig. 5.
β-cell development and regulation in normal and leptin receptor mutant zebrafish. (A) β-cell number in WT and leprsa1508/sa1508 mutants develops between 3 and 5 dpf. A two-factor analysis showed a significant effect of genotype at 4 dpf [F(1,254) = 6.4, P < 0.05, Bonferroni post hoc test] and developmental time [F(2, 254) = 59.83, P < 0.0001]. (B) β-cell number increases following 8 h of high fat feeding (egg yolk) in 5 dpf animals produced from a leprsa1508/+ heterozygous mutant in-cross [F(1,106) = 19.38, P < 0.0001, Bonferroni post hoc test]. leprsa1508/sa1508 mutant animals already show an elevation in β-cell number and are nonresponsive to high-fat feeding [two-way ANOVA effect of genotype F(2,106) = 3.3, P < 0.05]. (C) Chronic high-fat feeding, with 8-h exposure to egg yolk each day from 5 to 9 dpf. Two-way ANOVA shows an effect of genotype [F(1,544) = 79.72, P < 0.001] and feeding [F(4,544) = 14.59, P < 0.001]. Bonferroni post hoc tests showed a compensatory β-cell increase in WT but not leprsa1508/sa1508 animals. (D) Effect of FGFR inhibitor SU5402 on the developmental increase in β-cell number from 3 to 4 dpf. Two-factor ANOVA shows an effect of genotype [F(1,180) = 4.935, P < 0.05] as well as treatment [F(1,180) = 32.73, P < 0.001]. A Bonferroni post hoc test showed a significant elevation in the DMSO-treated leprsa1508/sa1508 mutant group. Data shown as means ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001.
Conclusions
The identification of leptin and leptin receptor gene homologs in lower vertebrates, such as birds and fish, has only recently allowed investigators to begin to address the comparative endocrinology of leptin action. A leptin receptor knockout reported in medaka (Oryzias latipes) (10) measured a 1.6- to twofold increase in postjuvenile body weight, but no increase in juvenile or adult body weight, and a 1.5- to 1.7-fold increase in postjuvenile and adult food intake. A small increase in visceral fat was reported, but without a detectable change in liver, muscle, or plasma triacylglycerol level. This increased adiposity is a relatively mild phenotype compared with leptin receptor signaling deficiency in mammals, which exhibit severe hyperphagia, and morbid obesity in which total triacylglyceride levels can reach 50% of total body weight (31). For analysis of growth and body composition in lepr mutant zebrafish, we kept WT and mutant siblings of an in-cross of leprsa1508/+ heterozygotes at the same density for half a year, and then scored weight and standard length at selected times. This approach, not used in the reported medaka experiments, is essential when working with fish to rule out effects of background genetics and tank densities. At no point did we see a difference in genotypes. Together with the limited phenotypes observed in medaka, these data argue that leptin does not act as an adipostatic factor in fish. In mammals, leptin has also acquired a role as a gating factor for reproductive competence, presumably informing the organism that adequate levels of energy are present to sustain a pregnancy. We found no difference in reproductive competency between WT and leprsa1508/sa1508 mutant fish, arguing against a role for leptin in communicating information about long-term energy stores to reproductive circuits in the CNS.
Leptin has effects on glucose homeostasis in mice, independent of its effects on body weight or adiposity. For example, low-dose leptin can make ob/ob mice normoglycemic without affecting food intake or body weight (32). Remarkably, leptin has recently been demonstrated to normalize blood glucose, even in insulin-deficient mice (33). This regulatory action of leptin has been demonstrated to be mediated by leptin receptors in the CNS, and other effects have been reported in the periphery (for review, see ref. 34). Based on these findings, and our lack of evidence for adipostatic regulation by fish leptin, we examined effects of leptin signaling on glucose homeostasis in the zebrafish. When we looked at free glucose levels in fry, we observed a small increase in total body glucose, along with elevated insulin and glucagon mRNA expression levels. It was, of course, not possible to measure blood glucose in larvae. We proceeded to score the number of α- and β-cells and found an early increase in the number of β-cells, but no difference in α-cells, in leprsa1508/sa1508 mutant fry. In mice, lack of the leptin receptor leads to persistent hyperglycemia, hyperglucagonemia and hyperinsulinemia (35), concomitant with an increase in β-cell as well as α-cell mass (36). Both α- and β-cells are known to be able to significantly increase peptide hormone levels on a per-cell basis (37), thus there is clearly a differential response of these two islet cell types to a deficiency of leptin signaling in the zebrafish.
To confirm that these islet phenotypes resulted from defective leptin signaling, we used CRISPR mutagenesis in zebrafish embryos and characterized the resulting fry at a comparable age following mutagenesis of lepr, lepa, or lepb genes in a zebrafish line carrying a β-cell marker. Previous data show that this method can be used to characterize genotype–phenotype relationships in the F0 generation, because biallelic mutagenesis at efficiencies of up to 80% can be readily achieved (26). We found that mutagenesis of lepr and lepa, but not lepb, exhibited increased β-cell number. We also replicated an increase in β-cell number in F2 sibling larvae mutant for lepa, providing independent support of the validity of using CRISPR in the F0 generation and the role of lepr and lepa in the islet phenotype. These data support the hypothesis that leptin signaling regulates β-cell mass in the larval zebrafish.
Genes involved in glucose metabolism in liver were also found to be dysregulated. We found that the mRNA for the mitochondrial (pck2) but not cytosolic form (pck1) of PEPCK is elevated. Although the cytosolic form has a more canonical role in gluconeogenesis, hepatic mPEPCK also plays a role in gluconeogenesis and silencing of the gene lowers blood glucose in mice (38). We furthermore saw an increase in hepatic glycogen phosphorylase expression (glycogenolysis) and no change in pyruvate kinase expression (glycolysis). Additionally, we observed an increase in glucose 6 phosphatase (g6ca.1) and glucose transporters 2 and 5. Taken together, these results are suggestive of increased gluconeogenesis and glycogenolysis in larvae. When we looked at adult hepatic expression ∼5 h after a meal, however, we found transcript changes in glycogen phosphorylase and glucose transporters 5 and 9a, but none of the other transcripts. Together, the observed expression changes argue for a dysregulation in multiple glucose homeostasis pathways. Interestingly, metformin treatment normalized the number of β-cells in leprsa1508/sa1508 mutant fry, providing some support for the idea that reduced leptin signaling exerts its effects through the liver and/or other peripheral tissues.
In contrast to larvae, adult fish exhibited fasting euglycemia, and improved glucose disposal after 30 min in a glucose tolerance test, suggestive of increased β-cell mass, but not insulin resistance. insa and gcga expression were increased in leprsa1508/sa1508 mutant adults, paralleling the finding in larvae. These findings are similar to mouse models in which growth factor expression is targeted to β-cells. In these models, increased β-cell number and improved glucose tolerance are seen in the absence of obesity or insulin resistance (e.g., ref. 39). Mice with either liver- or pancreas-specific knockout of lepr show a similar phenotype, with euglycemia, and improved glucose clearance in a GTT due to an enhanced early phase insulin secretion (40, 41). The liver-specific lepr knockout mice also show an increase in β-cell mass (41). A speculative suggestion is that the conserved function of leptin seen in the fish parallels that documented for peripheral leptin action in the mouse: regulation of islet development and function. Despite the absence of obesity or glucose intolerance in adult leprsa1508/sa1508 fish, these animals exhibited a defect in wound healing, a finding previously validated in a fish model of diabetes (29). Additional work will be needed to determine whether this is a true diabetic phenotype or altered function of one or more growth factors unrelated to blood glucose levels.
High-fat feeding in zebrafish leads to a compensatory increase in the number of β-cells (25). A similar response to defective leptin signaling, shown here, suggested that the nutritional signal leading to an increase in β-cell number may require leptin. Indeed, leprsa1508/sa1508 animals did not respond to either an acute or sustained nutrient challenge by increasing the number of β-cells. This result suggests that leprsa1508/sa1508 mutant animals lack a signaling pathway critical for compensatory increase in β-cell mass in response to overnutrition. Data in the zebrafish (30) show that nutrient excess leads β-cells to secrete FGF, which then leads to β-cell differentiation. The FGF receptor (FGFR) antagonist SU5402 also blocks the increase in β-cell number due to defective leptin signaling. Therefore, FGF signaling appears to act downstream of leptin in the regulation of β-cell mass.
In conclusion, the data reported here (Table 1), particularly in the context of the limited expression of leptin in adipose tissue in fish, support the hypothesis that leptin is not an adipostatic factor in fish. The data show a sustained effect of lepr deficiency on total insulin and glucagon transcript levels, and dysregulated hepatic gene expression in larval and adult fish. Data from larvae shows clear effects on developmental and nutritional regulation of β-cell number. Further studies are needed to determine effects of zebrafish leptin on hepatic glucose production, glucose action, and insulin action. Because regulation of components of glucose homeostasis appears to be a conserved function of leptin in fish and mammals, these data suggest that this function has been conserved throughout vertebrate evolution, and that the role of leptin in adipostasis developed subsequently in mammals, or was lost and supplanted by an as-yet unknown signaling pathway in fish.
Table 1.
Comparison of leptin action in mammals and larval zebrafish
| Phenotype | Mammal | Zebrafish |
| Hyperphagia | Yes | No |
| Hypometabolism | Yes | Not tested |
| Obesity | Yes | No |
| Hyperinsulinemia | Yes | Yes* |
| Hyperglucagonemia | Yes | Yes* |
| Diabetes | Yes | Yes† |
| Infertility | Yes | No |
| Immune dysfunction | Yes | Not tested |
Suggested by increased total gene expression.
In larvae.
Methods
Zebrafish Strains and Maintenance.
Larvae were raised in 28 °C incubators on 14:10h light-dark cycles until 5 dpf when they were placed on a standard diet and maintained in an Aquatic Habitats system on a 14:10h light-dark cycle. The lepr mutant strain sa1508 was obtained from the Zebrafish International Resource Center. Tg(−1.2ins:H2BmCherry) and Tg(gcgA:eGPF) were obtained from the W.C. laboratory (25). The lepa mutant line was established by using a gRNA to the target (AATCTCTGGATAATGTCCTGG) as described (26). All procedures were approved by the Vanderbilt Institutional Animal Care and Use Committee.
Body Composition.
Animals were isolated, anesthetized with tricaine solution and standard length measured. Animals were then blotted dry, weighed, and allowed to recover before being returned to their home tank. Whole-body adiposity was analyzed by the Vanderbilt Hormone Assay and Analytical Services core. For all body composition experiments, animals were kept at the same tank density (42).
Feeding.
Standard feeding protocol was two meals of live artemia and two meals of Tetra-Min flakes (Tetra). For overfeeding, control fish were fed a reduced diet of one meal of artemia a day, whereas overfed animals were fed ad libitum artemia from approximately 9:00 AM until about 4:00 PM. Uneaten and/or dead artemia were removed before each feeding. For food consumption, animals were fed betta bit pellets (TopFin), exclusively off the system with frequent water changes for a month. Fish were then weighed and individually kept in 3-L tanks. Animals were fed every other day with excess food pellets, and the remaining pellets were counted and removed after 8 h of overfeeding with yolk, as described (25).
RNA Extraction and qPCR.
RNA was extracted from pools of 10 fry for larvae and from dissected male viscera or liver for adults by using TRIzol (Invitrogen). RNA was measured, and 1 µg of RNA with a 260/280 ratio >1.8 was used for reverse transcription by using a high-capacity cDNA kit (ABI). Gene expression was measured with SYBR green (ABI/Promega) by using a CFX96 cycler (Bio-Rad). Relative expression compared with WT was calculated based on primer efficiency by using a pooled sample of the relative dataset’s cDNA, then standardized to eef1a1l1 expression. Primer sequences are sent upon request.
α- and β-Cell Counting.
As described (25), representative pictures were taken with a Zeiss LSM710 META by using the Vanderbilt Cell Imaging Shared Resource core.
F0 CRISPR Experiment.
Offspring of Tg(−1.2ins:H2BmCherry) animals were injected at the one-cell stage into the cell with ∼1 nL of a solution containing zebrafish 150 µg/µL Cas9 mRNA (26) and 125 µg/µL gRNA. Efficiency was analyzed by using heteroduplex mobility assay analysis. Target sequences were lepr (inactive) AGCATGATGAAGACAGACCTAGG; lepr (active) GGAGCGCCAGTAAAGCCGTGTGG; lepa GGAATCTCTGGATAATGTCCTGG, lepb ACAGAACTGAGACCATCAATGGG; and tyr (26).
Free Glucose Assay.
Whole-body glucose was determined by using an Amplex Red glucose assay kit (Life Technologies). Briefly, sets of five fry were homogenized as a pool in sample buffer on ice, the homogenate was cleared, and the supernatant measured according to the included instructions.
Glucose Tolerance Test.
The test was performed according to refs. 43 and 44. Briefly, zebrafish were starved overnight, anesthetized with 3-aminobenzoic acid ethyl ester methanesulfonate, and injected i.p. with 0.5 mg of glucose per gram of fish weight in Hanks’ buffered salt solution (Cellgro). Blood was collected from the tail and immediately diluted with assay buffer and snap frozen until measured (Amplex Red, Life Technologies).
Fin Regeneration.
Fin regeneration was carried out as described in ref. 29. Briefly, fins were cut proximal to the proximal branch point of the dermal rays and allowed to recover for 3 d in the system before a picture was taken. Growth from the cut site was measured using ImageJ.
Compound Exposure.
SU5402 (Tocris) was dissolved in DMSO and added to 0.3× Danieau solution at a final concentration of 15 µM for 24 h from 3 dpf to 4 dpf (30). Metformin (Sigma) was dissolved to 100 mM in 0.3× Danieau solution and used at 250 µM (45), with exposure from 3 dpf until 5 dpf. Solution was changed twice daily.
Acknowledgments
We thank Lisette Maddison for assistance in glucose measurements in the zebrafish. M.M. was supported by National Institute of Mental Health T32MH065215-11. The Vanderbilt Hormone Assay and Analytical Services Core is supported by NIH Grants DK059637 and DK020593.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. S.A.F. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1513212113/-/DCSupplemental.
References
- 1.Halaas JL, et al. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995;269(5223):543–546. doi: 10.1126/science.7624777. [DOI] [PubMed] [Google Scholar]
- 2.Elmquist JK, Ahima RS, Maratos-Flier E, Flier JS, Saper CB. Leptin activates neurons in ventrobasal hypothalamus and brainstem. Endocrinology. 1997;138(2):839–842. doi: 10.1210/endo.138.2.5033. [DOI] [PubMed] [Google Scholar]
- 3.Elmquist JK, Bjørbaek C, Ahima RS, Flier JS, Saper CB. Distributions of leptin receptor mRNA isoforms in the rat brain. J Comp Neurol. 1998;395(4):535–547. [PubMed] [Google Scholar]
- 4.Denver RJ, Bonett RM, Boorse GC. Evolution of leptin structure and function. Neuroendocrinology. 2011;94(1):21–38. doi: 10.1159/000328435. [DOI] [PubMed] [Google Scholar]
- 5.Londraville RL, et al. Comparative endocrinology of leptin: Assessing function in a phylogenetic context. Gen Comp Endocrinol. 2014;203:146–157. doi: 10.1016/j.ygcen.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Green ED, et al. The human obese (OB) gene: RNA expression pattern and mapping on the physical, cytogenetic, and genetic maps of chromosome 7. Genome Res. 1995;5(1):5–12. doi: 10.1101/gr.5.1.5. [DOI] [PubMed] [Google Scholar]
- 7.Huising MO, et al. Increased leptin expression in common Carp (Cyprinus carpio) after food intake but not after fasting or feeding to satiation. Endocrinology. 2006;147(12):5786–5797. doi: 10.1210/en.2006-0824. [DOI] [PubMed] [Google Scholar]
- 8.Ahima RS, et al. Role of leptin in the neuroendocrine response to fasting. Nature. 1996;382(6588):250–252. doi: 10.1038/382250a0. [DOI] [PubMed] [Google Scholar]
- 9.Douros JD, et al. Prolactin is a major inhibitor of hepatic Leptin A synthesis and secretion: Studies utilizing a homologous Leptin A ELISA in the tilapia. Gen Comp Endocrinol. 2014;207:86–93. doi: 10.1016/j.ygcen.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 10.Chisada S-i, et al. Leptin receptor-deficient (knockout) medaka, Oryzias latipes, show chronical up-regulated levels of orexigenic neuropeptides, elevated food intake and stage specific effects on growth and fat allocation. Gen Comp Endocrinol. 2013;195:9–20. [PubMed] [Google Scholar]
- 11.Liu Q, et al. Expression of leptin receptor gene in developing and adult zebrafish. Gen Comp Endocrinol. 2010;166(2):346–355. doi: 10.1016/j.ygcen.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gorissen M, Bernier NJ, Nabuurs SB, Flik G, Huising MO. Two divergent leptin paralogues in zebrafish (Danio rerio) that originate early in teleostean evolution. J Endocrinol. 2009;201(3):329–339. doi: 10.1677/JOE-09-0034. [DOI] [PubMed] [Google Scholar]
- 13.Kettleborough RN, et al. A systematic genome-wide analysis of zebrafish protein-coding gene function. Nature. 2013;496(7446):494–497. doi: 10.1038/nature11992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee GH, et al. Abnormal splicing of the leptin receptor in diabetic mice. Nature. 1996;379(6566):632–635. doi: 10.1038/379632a0. [DOI] [PubMed] [Google Scholar]
- 15.Gong N, Björnsson BT. Leptin signaling in the rainbow trout central nervous system is modulated by a truncated leptin receptor isoform. Endocrinology. 2014;155(7):2445–2455. doi: 10.1210/en.2013-2131. [DOI] [PubMed] [Google Scholar]
- 16.Oka T, et al. Diet-induced obesity in zebrafish shares common pathophysiological pathways with mammalian obesity. BMC Physiol. 2010;10:21. doi: 10.1186/1472-6793-10-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chehab FF, Lim ME, Lu R. Correction of the sterility defect in homozygous obese female mice by treatment with the human recombinant leptin. Nat Genet. 1996;12(3):318–320. doi: 10.1038/ng0396-318. [DOI] [PubMed] [Google Scholar]
- 18.Mounzih K, Lu R, Chehab FF. Leptin treatment rescues the sterility of genetically obese ob/ob males. Endocrinology. 1997;138(3):1190–1193. doi: 10.1210/endo.138.3.5024. [DOI] [PubMed] [Google Scholar]
- 19.Hummel KP, Dickie MM, Coleman DL. Diabetes, a new mutation in the mouse. Science. 1966;153(3740):1127–1128. doi: 10.1126/science.153.3740.1127. [DOI] [PubMed] [Google Scholar]
- 20.Baltzegar DA, Reading BJ, Douros JD, Borski RJ. Role for leptin in promoting glucose mobilization during acute hyperosmotic stress in teleost fishes. J Endocrinol. 2014;220(1):61–72. doi: 10.1530/JOE-13-0292. [DOI] [PubMed] [Google Scholar]
- 21.Irwin DM. A second insulin gene in fish genomes. Gen Comp Endocrinol. 2004;135(1):150–158. doi: 10.1016/j.ygcen.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 22.Papasani MR, Robison BD, Hardy RW, Hill RA. Early developmental expression of two insulins in zebrafish (Danio rerio) Physiol Genomics. 2006;27(1):79–85. doi: 10.1152/physiolgenomics.00012.2006. [DOI] [PubMed] [Google Scholar]
- 23.Baetens D, et al. Alteration of islet cell populations in spontaneously diabetic mice. Diabetes. 1978;27(1):1–7. doi: 10.2337/diab.27.1.1. [DOI] [PubMed] [Google Scholar]
- 24.Coleman DL. Obese and diabetes: Two mutant genes causing diabetes-obesity syndromes in mice. Diabetologia. 1978;14(3):141–148. doi: 10.1007/BF00429772. [DOI] [PubMed] [Google Scholar]
- 25.Maddison LA, Chen W. Nutrient excess stimulates β-cell neogenesis in zebrafish. Diabetes. 2012;61(10):2517–2524. doi: 10.2337/db11-1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jao LE, Wente SR, Chen W. Efficient multiplex biallelic zebrafish genome editing using a CRISPR nuclease system. Proc Natl Acad Sci USA. 2013;110(34):13904–13909. doi: 10.1073/pnas.1308335110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tseng YC, et al. Specific expression and regulation of glucose transporters in zebrafish ionocytes. Am J Physiol Regul Integr Comp Physiol. 2009;297(2):R275–R290. doi: 10.1152/ajpregu.00180.2009. [DOI] [PubMed] [Google Scholar]
- 28.Shaw RJ, et al. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science. 2005;310(5754):1642–1646. doi: 10.1126/science.1120781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Olsen AS, Sarras MP, Jr, Intine RV. Limb regeneration is impaired in an adult zebrafish model of diabetes mellitus. Wound Repair Regen. 2010;18(5):532–542. doi: 10.1111/j.1524-475X.2010.00613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li M, Page-McCaw P, Chen W. FGF1 mediates overnutrition-induced compensatory β-cell differentiation. Diabetes. 2015;65(1):96–109. doi: 10.2337/db15-0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yen TT, Allan JA, Yu PL, Acton MA, Pearson DV. Triacylglycerol contents and in vivo lipogenesis of ob/ob, db/db and Avy/a mice. Biochim Biophys Acta. 1976;441(2):213–220. doi: 10.1016/0005-2760(76)90164-8. [DOI] [PubMed] [Google Scholar]
- 32.Pelleymounter MA, et al. Effects of the obese gene product on body weight regulation in ob/ob mice. Science. 1995;269(5223):540–543. doi: 10.1126/science.7624776. [DOI] [PubMed] [Google Scholar]
- 33.Yu X, Park BH, Wang MY, Wang ZV, Unger RH. Making insulin-deficient type 1 diabetic rodents thrive without insulin. Proc Natl Acad Sci USA. 2008;105(37):14070–14075. doi: 10.1073/pnas.0806993105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fujikawa T, Coppari R. Living without insulin: The role of leptin signaling in the hypothalamus. Front Neurosci. 2015;9:108. doi: 10.3389/fnins.2015.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stearns SB, Benzo CA. Glucagon and insulin relationships in genetically diabetic (db/db) and in streptozotocin-induced diabetic mice. Horm Metab Res. 1978;10(1):20–23. doi: 10.1055/s-0028-1093473. [DOI] [PubMed] [Google Scholar]
- 36.Puff R, et al. Reduced proliferation and a high apoptotic frequency of pancreatic beta cells contribute to genetically-determined diabetes susceptibility of db/db BKS mice. Horm Metab Res. 2011;43(5):306–311. doi: 10.1055/s-0031-1271817. [DOI] [PubMed] [Google Scholar]
- 37.Thorel F, et al. Normal glucagon signaling and β-cell function after near-total α-cell ablation in adult mice. Diabetes. 2011;60(11):2872–2882. doi: 10.2337/db11-0876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stark R, et al. A role for mitochondrial phosphoenolpyruvate carboxykinase (PEPCK-M) in the regulation of hepatic gluconeogenesis. J Biol Chem. 2014;289(11):7257–7263. doi: 10.1074/jbc.C113.544759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.García-Ocaña A, et al. Transgenic overexpression of hepatocyte growth factor in the beta-cell markedly improves islet function and islet transplant outcomes in mice. Diabetes. 2001;50(12):2752–2762. doi: 10.2337/diabetes.50.12.2752. [DOI] [PubMed] [Google Scholar]
- 40.Morioka T, et al. Disruption of leptin receptor expression in the pancreas directly affects beta cell growth and function in mice. J Clin Invest. 2007;117(10):2860–2868. doi: 10.1172/JCI30910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huynh FK, et al. Disruption of hepatic leptin signaling protects mice from age- and diet-related glucose intolerance. Diabetes. 2010;59(12):3032–3040. doi: 10.2337/db10-0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leibold S, Hammerschmidt M. Long-term hyperphagia and caloric restriction caused by low- or high-density husbandry have differential effects on zebrafish postembryonic development, somatic growth, fat accumulation and reproduction. PLoS One. 2015;10(3):e0120776. doi: 10.1371/journal.pone.0120776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zang L, Shimada Y, Nishimura Y, Tanaka T, Nishimura N. A novel, reliable method for repeated blood collection from aquarium fish. Zebrafish. 2013;10(3):425–432. doi: 10.1089/zeb.2012.0862. [DOI] [PubMed] [Google Scholar]
- 44.Eames SC, Philipson LH, Prince VE, Kinkel MD. Blood sugar measurement in zebrafish reveals dynamics of glucose homeostasis. Zebrafish. 2010;7(2):205–213. doi: 10.1089/zeb.2009.0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jiménez-Amilburu V, Jong-Raadsen S, Bakkers J, Spaink HP, Marín-Juez R. GLUT12 deficiency during early development results in heart failure and a diabetic phenotype in zebrafish. J Endocrinol. 2015;224(1):1–15. doi: 10.1530/JOE-14-0539. [DOI] [PubMed] [Google Scholar]