Significance
Amyotrophic lateral sclerosis (ALS) is a fatal disease leading to motor neuron degeneration and progressive paralysis. Other studies have revealed defects in skeletal muscle even in the absence of motor neuron anomalies, focusing on acetylcholine receptors (AChRs) and supporting the so-called “dying-back” hypothesis. Our results indicate that the endocannabinoid palmitoylethanolamide (PEA) reduces the rundown of AChRs currents in ALS muscle and can clinically improve patients’ pulmonary function. This study strengthens the important role of muscle in ALS pathogenesis and the idea that AChRs can be therapeutic targets.
Keywords: ALS, muscle AChR, Xenopus oocytes, microtransplantation, qPCR
Abstract
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disease affecting motor neurons that leads to progressive paralysis of skeletal muscle. Studies of ALS have revealed defects in expression of acetylcholine receptors (AChRs) in skeletal muscle that occur even in the absence of motor neuron anomalies. The endocannabinoid palmitoylethanolamide (PEA) modified the clinical conditions in one ALS patient, improving muscle force and respiratory efficacy. By microtransplanting muscle membranes from selected ALS patients into Xenopus oocytes, we show that PEA reduces the desensitization of acetylcholine-evoked currents after repetitive neurotransmitter application (i.e., rundown). The same effect was observed using muscle samples from denervated (non-ALS) control patients. The expression of human recombinant α1β1γδ (γ-AChRs) and α1β1εδ AChRs (ε-AChRs) in Xenopus oocytes revealed that PEA selectively affected the rundown of ACh currents in ε-AChRs. A clear up-regulation of the α1 subunit in muscle from ALS patients compared with that from non-ALS patients was found by quantitative PCR, but no differential expression was found for other subunits. Clinically, ALS patients treated with PEA showed a lower decrease in their forced vital capacity (FVC) over time as compared with untreated ALS patients, suggesting that PEA can enhance pulmonary function in ALS. In the present work, data were collected from a cohort of 76 ALS patients and 17 denervated patients. Our results strengthen the evidence for the role of skeletal muscle in ALS pathogenesis and pave the way for the development of new drugs to hamper the clinical effects of the disease.
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disease marked by the degeneration of motor neurons. It leads to progressive paralysis ending with the death of the patient 3–5 y after diagnosis. Although most ALS cases are sporadic, about 10% are familial and are linked to monogenic mutations in different genes such as the gene for superoxide dismutase 1 (SOD1), genes encoding RNA-binding proteins such as TARDBP and FUS, or the noncoding region of the poorly characterized C9ORF72 gene (1). These mutations have been used to develop mammalian animal models that mimic some of the symptomatology and to investigate some of the molecular mechanisms of the disease. In mice, as well as in patients, the first observed disease event is the destruction of the neuromuscular junction (NMJ) (2). Studies have uncovered defects in the skeletal muscle that occur even in the absence of motor neuron anomalies, supporting the “dying-back” hypothesis in which distal motor endplate degeneration plays a key role in the progression of the disease (2–4). NMJs of ALS patients consistently show electrophysiological properties distinctly different from those of patients with pure denervation (5, 6). Using muscle biopsies from sporadic ALS patients, we previously characterized the nicotinic acetylcholine currents resulting from the activation of functional acetylcholine receptors (AChRs) (5). Upon denervation, receptors formed by the α1β1γδ (γ-AChR) instead of the α1β1εδ (ε-AChR) subunits appear widely distributed over the entire sarcolemma (7, 8). In ALS patients, in whom muscle denervation and abnormal reinnervation are pathological hallmarks of the disease, both γ- and ε-AChRs are present. In addition, we showed that riluzole, the only approved drug available against ALS, may affect AChR function in ALS muscle (6). These findings strengthen the hypothesis that pathogenic events target the NMJ directly and suggest that its protection could be a useful therapeutic strategy.
The cannabinoid system, consisting of cannabinoid (CB) receptors, endocannabinoids (eCBs), and enzymes involved in the synthesis and degradation of these eCBs, has been shown to be a key player in ALS etiology (9, 10). The eCB-related molecule palmitoylethanolamide (PEA) induces anti-inflammatory effects through the PPARα receptor without binding to CB receptors and consequently without side effects (11, 12). Notably, PEA was able to improve pulmonary function and clinical conditions in a single case of ALS (13). Here, taking advantage of the microtransplantation technique by which cell membranes isolated from human muscle are injected into Xenopus oocytes (14), we investigated whether PEA can modulate AChR currents using voltage-clamp intracellular recordings in oocytes transplanted with membranes from ALS muscle. We measured the composition of AChR subunits in ALS muscle compared with non-ALS denervated muscle by quantitative PCR (qPCR) analysis. In addition, we studied the effect of PEA in a cohort of ALS patients to determine whether this compound can improve the clinical characteristics of patients, with particular attention to muscle force and respiratory capacity.
Results
PEA Affects the Desensitization of ACh Currents in Oocytes Transplanted with Muscle Membranes from ALS Patients.
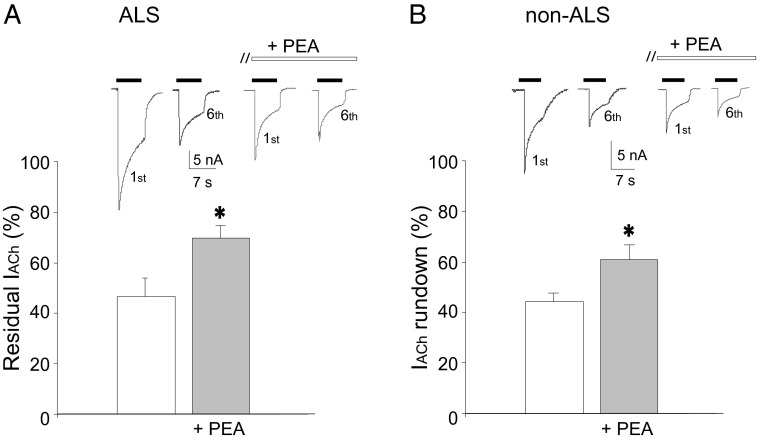
We first investigated whether PEA modulates AChR function in Xenopus oocytes microtransplanted with human muscle membranes from patients with the spinal form of ALS and from non-ALS denervated controls (Table S1). Functional AChRs from these samples are incorporated into the oocyte’s membrane, and application of ACh (5 μM to 1 mM) elicited an inward current with a peak amplitude that depended on transmitter concentration and was sensitive to the α-bungarotoxin (αBuTX) block (5 μM; 30 min of pretreatment; six oocytes; two frogs). For some ALS patients the needle biopsy did not render samples of sufficient quality or quantity to record functional ACh currents (Table S1). In oocytes injected with muscle membranes from 10 ALS patients, ACh (500 μM; holding potential, −60 mV) elicited a current (IACh) with amplitudes ranging between −9 and −65 nA (40 oocytes; 12 frogs; patients #1–4, 14, 20, and 39–42) (Figs. 1 and 2A and Table S1). Similar amplitudes were recorded in oocytes injected with muscle membranes from nine non-ALS denervated control patients (range, −5 to −40 nA; 35 oocytes; 10 frogs; patients #79, 80, 82–84, and 90–93) (Fig. 2B and Table S1). Acute coapplication of ACh with PEA (1–100 µM) did not affect IACh amplitude in either ALS or control membranes (10 oocytes; two frogs; patients #2 and 79) (Table S1). On the other hand, prolonged exposure (30 s to 60 min) to 10 µM PEA produced a time- and dose-dependent decrease in IACh amplitude, reaching a plateau after 10 min of exposure (IACh decrease: 56 ± 5.2%; 50 oocytes; 12 frogs; patients #1–4, 14, 20, and 39–42) (Fig. 1 and Table S1). We did not observe a further decrease in IACh amplitude when the PEA concentration was increased to 100 μM (IACh decrease: 50 ± 7.2%; eight oocytes; two frogs). This effect was reversible and was not influenced by holding potential (IACh decrease: 51.6 ± 7% at −100 mV; 51.7 ± 6% at −60 mV; 49.8 ± 3% at −40 mV; five oocytes; one frog; patient #14) (Table S1). Furthermore, the decrease in IACh was accompanied by a decrease in current decay time (T0.5: 6.5 ± 1.1 s before PEA vs. 3.2 ± 0.8 s after PEA; P < 0.05) (Fig. 1, Inset), suggesting that PEA, unlike riluzole (5), can influence the rate of IACh inactivation. To investigate whether PEA affects current desensitization, we studied the effect of PEA on use-dependent current decrease (i.e., IACh rundown) during repetitive ACh applications (500 µM, 7-s duration, 60-s interval). In oocytes injected with ALS muscle membranes the percentage of remaining IACh was 46.3 ± 7.2% (40 oocytes; 12 frogs; patients #1–4, 14, 20, and 39–42) (Table S1), a value very similar to that observed in oocytes injected with non-ALS denervated muscle membranes (44.2 ± 3.6%; 35 oocytes; 10 frogs; patients #79, 80, 82–84, and 90–93) (Table S1). However, after pretreatment for 10 min with 10 μM PEA, we observed a marked increase in remaining IACh in both ALS and non-ALS patients (ALS, 70.4 ± 2%; non-ALS, 61.1 ± 6.2%; P < 0.05) (Fig. 2), suggesting that PEA can reduce the rate of receptor desensitization during repetitive ACh applications. This effect was not related to a change in ACh sensitivity, because PEA treatment did not modify ACh dose–response relationships (EC50: 50.1 ± 0.54 µM before PEA vs. 54.4 ± 1.38 µM after PEA; 10 oocytes; two frogs; patients #1, 14, and 20) (Table S1). In addition, to reproduce the therapeutic regimen of the patients recruited in this study, we performed further experiments to evaluate whether cotreatment with PEA and riluzole would modify this effect. We found that additional exposure to riluzole did not produce a significant difference in the remaining IACh compared with PEA alone [PEA: 67.1 ± 2.8%; PEA + riluzole: 68.9 ± 4.1%; 10 oocytes; two frogs; patients #14 and 20 (Table S1); P > 0.05].
Table S1.
Clinical characteristics of the population of ALS and non-ALS denervated control patients
| Patient no. | Sex | Age, y | Pathology (phenotype) | Disease duration | ALSFRS-R score | Site of biopsy | Denervation grade |
| 1* | F | 60 | ALS (spinal) | 45 mo | 27 | Deltoid | 1 |
| 2* | F | 77 | ALS (spinal) | 19 mo | 20 | Deltoid | 2 |
| 3* | M | 70 | ALS (spinal) | 60 mo | 31 | Deltoid | 2 |
| 4* | F | 54 | ALS (spinal) | 17 mo | 41 | Deltoid | 1 |
| 5† | M | 67 | ALS (spinal) | 32 mo | 40 | Anterior tibialis | 4 |
| 6 | F | 79 | ALS (spinal) | 60 mo | 31 | Anterior tibialis | 1 |
| 7† | M | 67 | ALS (spinal) | 10 mo | 37 | Anterior tibialis | 4 |
| 8† | F | 52 | ALS (spinal) | 21 mo | 24 | Anterior tibialis | 1 |
| 9 | M | 55 | ALS (bulbar) | 5 mo | 33 | Anterior tibialis | 1 |
| 10† | M | 71 | ALS (spinal) | 18 mo | 43 | Anterior tibialis | 3 |
| 11 | M | 45 | ALS (spinal) | 11 mo | 43 | Anterior tibialis | 2 |
| 12 | M | 70 | ALS (bulbar) | 19 mo | 35 | Anterior tibialis | 1 |
| 13 | F | 48 | ALS (spinal) | 14 mo | 29 | Anterior tibialis | 5 |
| 14* | M | 43 | ALS (spinal) | 17 mo | 42 | Anterior tibialis | 5 |
| 15 | M | 46 | ALS (pseudobulbar) | 11 mo | 44 | Anterior tibialis | 3 |
| 16† | M | 79 | ALS (spinal) | 25 mo | 27 | Anterior tibialis | 5 |
| 17† | M | 70 | ALS (spinal) | 10 mo | 38 | Anterior tibialis | 3 |
| 18† | M | 57 | ALS (spinal) | 21 mo | 33 | Anterior tibialis | 3 |
| 19 | F | 64 | ALS (bulbar) | 14 mo | 43 | Anterior tibialis | 1 |
| 20* | F | 66 | ALS (spinal) | 32 mo | 39 | Anterior tibialis | 4 |
| 21 | F | 70 | ALS (spinal) | 21 mo | 23 | Anterior tibialis | 3 |
| 22† | M | 40 | ALS (spinal) | 28 mo | 31 | Anterior tibialis | 5 |
| 23 | M | 80 | ALS (spinal) | 57 mo | 39 | Anterior tibialis | 4 |
| 24 | M | 76 | ALS (spinal) | 18 mo | 33 | Quadriceps | 3 |
| 25 | F | 68 | ALS (bulbar) | 19 mo | 34 | Anterior tibialis | 1 |
| 26 | M | 62 | ALS (bulbar) | 23 mo | 38 | Quadriceps | 1 |
| 27 | M | 61 | ALS (spinal) | 82 mo | 37 | Anterior tibialis | 2 |
| 28† | M | 48 | ALS (spinal) | 18 mo | 45 | Anterior tibialis | 3 |
| 29† | M | 59 | ALS (spinal) | 9 mo | 44 | Anterior tibialis | 5 |
| 30 | F | 64 | ALS (pseudobulbar) | 11 mo | 39 | Anterior tibialis | 2 |
| 31† | M | 76 | ALS (spinal) | 60 mo | 30 | Anterior tibialis | 1 |
| 32† | F | 64 | ALS (spinal) | 34 mo | 38 | Anterior tibialis | 2 |
| 33 | M | 66 | ALS (bulbar) | 39 mo | 27 | Anterior tibialis | 2 |
| 34 | M | 49 | ALS (bulbar) | 17 mo | 27 | Quadriceps | 2 |
| 35 | F | 61 | ALS (bulbar) | 14 mo | 44 | Anterior tibialis | 3 |
| 36 | M | 74 | ALS (bulbar) | 54 mo | 20 | Anterior tibialis | 2 |
| 37 | M | 72 | ALS (bulbar) | 30 mo | 42 | Anterior tibialis | 3 |
| 38 | F | 72 | ALS (bulbar) | 20 mo | 42 | Anterior tibialis | 1 |
| 39* | F | 57 | ALS (spinal) | 33mo | 17 | Deltoid | 2 |
| 40* | F | 79 | ALS (spinal) | 36mo | 20 | Deltoid | 4 |
| 41* | M | 73 | ALS (spinal) | 4mo | 33 | Quadriceps | 2 |
| 42* | M | 70 | ALS (spinal) | 32mo | 19 | Deltoid | 4 |
| 43 | F | 58 | ALS (spinal) | 81 mo | 29 | — | 2 |
| 44 | F | 53 | ALS (spinal) | 17 mo | 38 | — | 3 |
| 45 | M | 54 | ALS (spinal) | 21 mo | 25 | — | 3 |
| 46 | M | 55 | ALS (spinal) | 57 mo | 28 | — | 4 |
| 47 | M | 73 | ALS (spinal) | 21 mo | 24 | — | 3 |
| 48 | M | 56 | ALS (spinal) | 17 mo | 42 | — | 1 |
| 49 | M | 60 | ALS (spinal) | 21 mo | 43 | — | 2 |
| 50 | M | 60 | ALS (spinal) | 39 mo | 34 | — | 1 |
| 51 | M | 79 | ALS (spinal) | 20 mo | 43 | — | 2 |
| 52 | M | 53 | ALS (spinal) | 53 mo | 29 | — | 3 |
| 53 | M | 59 | ALS (spinal) | 81 mo | 28 | — | 2 |
| 54 | M | 61 | ALS (spinal) | 39 mo | 35 | — | 1 |
| 55 | M | 52 | ALS (spinal) | 19 mo | 39 | — | 2 |
| 56 | M | 76 | ALS (spinal) | 25 mo | 28 | — | 4 |
| 57 | F | 74 | ALS (spinal) | 33 mo | 39 | — | 3 |
| 58 | F | 53 | ALS (spinal) | 38 mo | 32 | — | 1 |
| 59 | F | 72 | ALS (spinal) | 33 mo | 40 | — | 3 |
| 60 | F | 43 | ALS (spinal) | 17 mo | 43 | — | 2 |
| 61 | F | 59 | ALS (spinal) | 39 mo | 28 | — | 3 |
| 62 | F | 55 | ALS (spinal) | 38 mo | 33 | — | 1 |
| 63 | M | 54 | ALS (spinal) | 34 mo | 29 | — | 1 |
| 64 | M | 60 | ALS (spinal) | 27 mo | 43 | — | 3 |
| 65 | M | 81 | ALS (spinal) | 69 mo | 40 | — | 3 |
| 66 | M | 75 | ALS (spinal) | 18 mo | 34 | — | 4 |
| 67 | M | 59 | ALS (spinal) | 45 mo | 28 | — | 1 |
| 68 | F | 72 | ALS (spinal) | 59 mo | 35 | — | 2 |
| 69 | F | 76 | ALS (spinal) | 36 mo | 37 | — | 3 |
| 70 | F | 55 | ALS (spinal) | 34 mo | 30 | — | 2 |
| 71 | F | 73 | ALS (spinal) | 83 mo | 36 | — | 1 |
| 72 | M | 61 | ALS (spinal) | 27 mo | 42 | — | 1 |
| 73 | F | 75 | ALS (spinal) | 36 mo | 36 | — | 3 |
| 74 | F | 59 | ALS (spinal) | 11 mo | 42 | — | 3 |
| 75 | F | 59 | ALS (spinal) | 21 mo | 34 | — | 1 |
| 76 | F | 60 | ALS (spinal) | 11 mo | 41 | — | 3 |
| 77† | M | 60 | Cauda syndrome | 4 mo | — | Quadriceps | 2 |
| 78† | F | 74 | Diabetic mononeuropathy | 4 wk | — | Anterior tibialis | 3 |
| 79* | M | 40 | Polytrauma | 3 wk | — | Anterior tibialis | 4 |
| 80* | M | 35 | Polytrauma | 3 wk | — | Quadriceps | 4 |
| 81† | F | 40 | Cauda syndrome | 5 mo | — | Gastrocnemius | 5 |
| 82*,† | F | 55 | Axonal neuropathy | 36 mo | — | Anterior tibialis | 4 |
| 83*,† | M | 29 | Axonal neuropathy | 20 mo | — | Anterior tibialis | 5 |
| 84*,† | M | 80 | Axonal neuropathy | 5 y | — | Anterior tibialis | 3 |
| 85† | F | 30 | Cauda syndrome | 4 mo | — | Gastrocnemius | 2 |
| 86† | M | 54 | Cauda syndrome | 3 mo | — | Anterior tibialis | 1 |
| 87† | F | 53 | CIDP | 12 mo | — | Anterior tibialis | 3 |
| 88† | M | 81 | Radiculopathy | 6 mo | — | Anterior tibialis | 3 |
| 89† | F | 56 | Polytrauma | 7 mo | — | Deltoid | 5 |
| 90*,† | F | 66 | CMT1 | 16 y | — | Anterior tibialis | 2 |
| 91* | F | 44 | CMT1 | 12 y | — | Anterior tibialis | 4 |
| 92* | M | 65 | ASMAN | 3 wk | — | Anterior tibialis | 5 |
| 93* | M | 34 | Axonal neuropathy | 36 mo | — | Deltoid | 4 |
ALS, amyotrophic lateral sclerosis; ALSFRS-R, Amyotrophic Lateral Sclerosis Functional Rating Scale-Revised, 1–48; ASMAN, Acute Sensory Motor Axonal Neuropathy, denervation grade, 1–5; CIDP, chronic inflammatory demyelinating polyneuropathy; CMT1, Charcot Marie Tooth type 1.
Electrophysiological recordings in oocytes. For some patients in this table the tissue sample was too small or too atrophic to be used for recordings.
qPCR. For some patients in this table the tissue sample was too small or too atrophic to be used for qPCR.
Fig. 1.
PEA affects ACh currents in oocytes injected with ALS muscle membranes. Bar graphs show the percentage of ACh amplitude remaining before and after 10 μM PEA treatment (10 min, 50 oocytes), as indicated. (Inset) Sample current traces elicited during prolonged ACh application (500 μM for 60 s) in one oocyte (representative of 10) injected with ALS muscle membranes before and after PEA treatment. In this and subsequent figures, horizontal filled bars indicate the timing of ACh applications, and horizontal open bars indicate the drug applications. *P < 0.05. Holding potential, −60 mV.
Fig. 2.
Effect of PEA on IACh rundown. (A) Bar graphs of residual IACh (i.e., the ratio between sixth and first IACh amplitude) before and after treatment with 10 µM PEA (10 min) in oocytes injected with ALS muscle membranes. (Inset) Representative currents elicited by the first and sixth ACh application (500 µM; horizontal filled bar) in one oocyte injected with ALS muscle membranes before and after PEA (open bar), as indicated. (B) Bar graphs represent IACh remaining in oocytes injected with non-ALS denervated muscle membranes before and after PEA, as in A. (Inset) Representative currents elicited in one oocyte injected with non-ALS denervated muscle membranes, as in A. Data are the means ± SEM of 40 oocytes (ALS patients #1–4,14, 20, and 39–42) (Table S1) and 35 oocytes (non-ALS patients #79, 80, 82–84, and 90–93) (Table S1). *P < 0.05. Holding potential, −60 mV.
PEA Affects Only ε-AChRs.
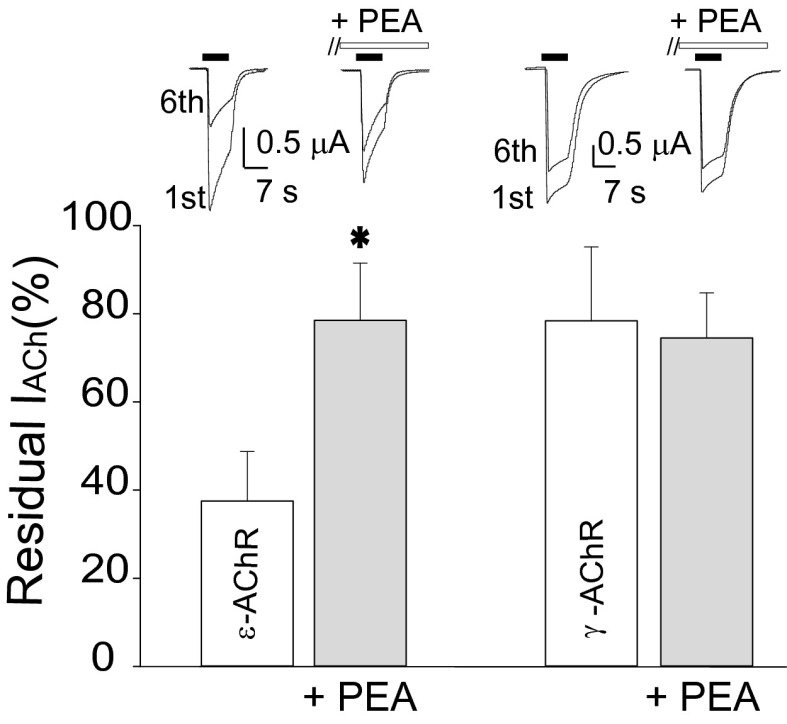
To elucidate PEA action at the molecular level, we examined the effect of PEA on both ε- and γ-AChR isoforms expressed in Xenopus oocytes. In oocytes expressing human α1β1εδ AChRs we found that 10 μM PEA induced a statistically significant increase in remaining IACh (37.5 ± 11.2% before PEA and 75.5 ± 13% after PEA; ACh = 50 μM; 10 oocytes; two frogs; P < 0.05) (Fig. 3). This increase was accompanied by a decrease in IACh amplitude (79.4 ± 7%). In contrast, oocytes expressing human α1β1γδ AChRs were only weakly sensitive to the same PEA treatment, and neither the remaining IACh (78.4 ± 10% before PEA and 75.4 ± 16% after PEA; ACh = 50 μM; 10 oocytes; two frogs; P > 0.05) (Fig. 3) nor the IACh peak amplitude (90.3 ± 5.7%) was significantly modified.
Fig. 3.
Effect of PEA on oocytes expressing ε-AChRs or γ-AChRs. Bar graphs of residual IACh (i.e., the ratio between sixth and first IACh amplitude) before and after 10 µM PEA treatment (10 min) in oocytes expressing ε-AChRs (Left) or γ-AChRs (Right), as indicated. (Inset) Representative superimposed currents elicited by the first and sixth ACh application (500 µM; horizontal filled bar) in oocytes expressing ε-AChRs or γ-AChRs before and after PEA (open bar), as indicated. *P < 0.05. Holding potential, −60 mV.
Altered Expression of the α1 AChR Subunit in ALS Patients.
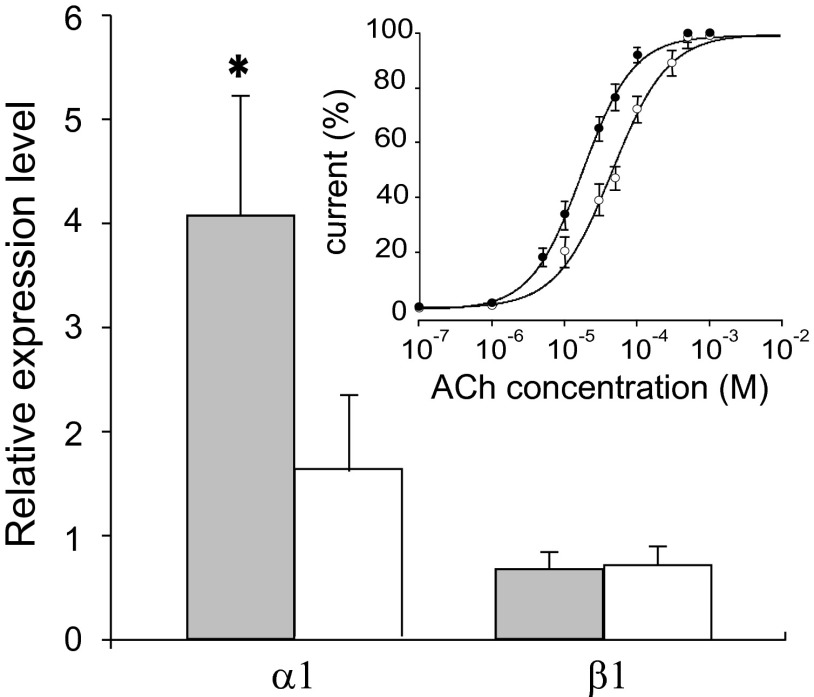
Our previous studies suggested that ACh sensitivity is decreased significantly in muscle obtained from ALS patients as compared with muscle from non-ALS patients (5, 6). To determine whether an altered AChR subunit composition is related to this change of affinity, we performed qPCR analysis using tissue samples obtained from muscle of ALS and non-ALS patients. Interestingly, we found a fourfold increase in α1 subunit expression in ALS muscle (patients #5, 7, 8, 10, 16–18, 22, 28, 29, 31, and 32) (Fig. 4 and Table S1) compared with non-ALS muscle (patients #77, 78, and 81–90) (Table S1). We did not find statistically significant differential expression of the β1 (Fig. 4) or γ, δ, and ε subunits.
Fig. 4.
The α1 AChR subunit is increased in ALS patients. A statistically significant difference (P = 0.0058) was found in the relative expression level of the α1 ACh subunit in ALS (gray bars) compared with non-ALS (white bars) patients (twelve for each group; Table S1). In contrast, no significant change was found in the expression of the β1 subunit. (Inset) Amplitude of currents evoked by various ACh concentrations, expressed as a percentage of the maximal current evoked by 1 mM ACh, plotted as mean ± SEM and best fitted with Hill curves. Averaged EC50 values and nH in oocytes injected with α1β1εδ cDNAs (1:1:1:1 ratio) were 18.4 ± 3.8 μM and 1.2 ± 0.01 (●); in oocytes injected with α1β1εδ cDNA (5:1:1:1 ratio), the values were 56 ± 7 μM and 1 ± 0.01 (○).
To determine whether this α1 up-regulation modified ACh sensitivity, ACh dose–current response experiments were performed with oocytes injected with cDNAs of α1β1εδ AChR subunits (1:1:1:1 cDNA ratio) yielding half-maximal effect (EC50) and Hill number (nH) values of 18.4 ± 0.8 μM and 1.2 ± 0.01, respectively (Fig. 4, Inset; 10 oocytes, two frogs). In oocytes expressing α1β1εδ AChRs with an increased α1 cDNA ratio (5:1:1:1), the EC50 was significantly greater (56 ± 7 μM; 10 oocytes; two frogs; P < 0.05), but nH was unchanged (1.0 ± 0.01).
PEA Treatment of ALS Patients Slows the Decline of Pulmonary Function.
To test the clinical applicability of these findings, we investigated the effect of PEA treatment on ALS patients. As shown in Table S2, 28 patients (16 male and 12 female, four with bulbar-onset ALS and 24 with spinal-onset ALS) were randomly selected to receive 50 mg riluzole twice daily plus 600 mg PEA twice daily (PEA-treated patients). The remaining 36 patients (19 male and 17 female, six with bulbar-onset and 30 with spinal-onset ALS) received riluzole only (untreated patients). No adverse events occurred during the study, and all patients completed the protocol with complete compliance. The analysis was performed in all the randomized patients and by intention-to-treat for the patients stratified by severity and onset (SI Text).
Table S2.
Clinical trial: Baseline characteristics of ALS patients
| Characteristic | PEA-treated group (n = 28) | Untreated group (n = 36) | P |
| Male/female | 16/12 | 19/17 | >0.05 |
| Spinal/bulbar | 24/4 | 30/6 | >0.05 |
| Mean age at onset, y ± SD | 62.3 ± 9.2 | 60.1 ± 9.9 | >0.05 |
| Mean age at enrollment, y ± SD | 65.4 ± 10.0 | 62.0 ± 9.3 | >0.05 |
| Mean FVC, % predicted ± SD | 73.5 ± 16.3 | 77.1 ± 19.3 | >0.05 |
| Disease duration, months ± SD | 35.4 ± 21.6 | 28.5 ± 17.2 | >0.05 |
| Mean ALSFRS-R score ± SD | 36.6 ± 5.1 | 35.0 ± 6.8 | >0.05 |
| Mean MRC score ± SD | 28.3 ± 10.0 | 31.1 ± 8.4 | >0.05 |
| Mean ulnar nerve CMAP ± SD | 6.4 ± 4.3 | 6.4 ± 4.5 | >0.05 |
| Mean phrenic nerve CMAP amplitude ± SD | 0.5 ± 0.3 | 0.6 ± 0.4 | >0.05 |
ALSFRS-R, Amyotrophic Lateral Sclerosis Functional Rating Scale–revised; CMAP, compound muscle action potentials; FVC, forced vital capacity; MRC, Medical Research Council; PEA, palmitoylethanolamide.
The demographic data of ALS patients included in this study are given in Table S2. Clinical and electrophysiological data are summarized in Table S3. There were no significant differences in demographic variables between groups at baseline: mean age in the PEA-treated group was 65.3 (±10.2) y vs. 62.1 (±9.5) y in the control group; mean age at the onset symptoms was 62.0 (±9.1) y vs. 60.1 (±9.9) y. No significant differences in baseline values between groups were detected for the ALS Functional Rating Scale–revised (ALSFRS-R) and its subscores (15), the percentage of predicted forced vital capacity (FVC%), the Medical Research Council (MRC) scale for muscle strength, or the compound muscle action potential (CMAP) amplitude of ulnar and phrenic nerves (Materials and Methods, SI Text, and Table S3).
Table S3.
Clinical trial: Clinical and electrophysiological data (mean ± SD) of ALS patients at baseline and at 12 and 24 wk
| Baseline | 12 wk | 24 wk | |
| PEA-treated group (n = 28) | |||
| FVC% predicted | 73.5 ± 16.3 | 72.2 ± 16.3 | 70.7 ± 19.0 |
| ALSFRS-R total score | 36.6 ± 5.1 | 34.4 ± 5.2 | 31.7 ± 6.2 |
| Bulbar subscore | 10.5 ± 2.6 | 10.2 ± 3.1 | 10.2 ± 3.1 |
| Spinal subscore | 15.1 ± 4.7 | 13.3 ± 4.7 | 10.8 ± 4.3 |
| Respiratory subscore | 11.0 ± 1.3 | 10.9 ± 1.3 | 10.7 ± 1.3 |
| MRC score | 28.3 ± 10.0 | 24.3 ± 11.4 | 21.9 ± 11.2 |
| Ulnar nerve CMAP amplitude | 6.4 ± 4.3 | 6.2 ± 5.1 | 5.6 ± 5.1 |
| Phrenic nerve CMAP amplitude | 0.49 ± 0,33 | 0.56 ± 0.29 | 0.49 ± 0.27 |
| Untreated group (n = 36) | |||
| FVC% predicted | 77.1 ± 19.3 | 70.3 ± 18.8 | 65.8 ± 18.8 |
| ALSFRS-R total score | 35.0 ± 6.8 | 32.0 ± 7.9 | 29.3 ± 9.9 |
| Bulbar subscore | 9.8 ± 2.5 | 9.0 ± 3.1 | 8.4 ± 3.2 |
| Spinal subscore | 15.0 ± 5.0 | 13.8 ± 5.4 | 12.6 ± 5.6 |
| Respiratory subscore | 10.3 ± 2.7 | 9.3 ± 3.3 | 8.4 ± 4.1 |
| MRC score | 31.1 ± 8.4 | 29.3 ± 9.1 | 26.8 ± 10.1 |
| Ulnar nerve CMAP amplitude | 6.4 ± 4.5 | 5.8 ± 4.2 | 5.1 ± 4.6 |
| Phrenic nerve CMAP amplitude | 0.56 ± 0.38 | 0.52 ± 0.34 | 0.50 ± 0.32 |
ALSFRS-R, Amyotrophic Lateral Sclerosis Functional Rating Scale–revised; CMAP, compound muscle action potentials; FVC, forced vital capacity; MRC, Medical Research Council; PEA, palmitoylethanolamide.
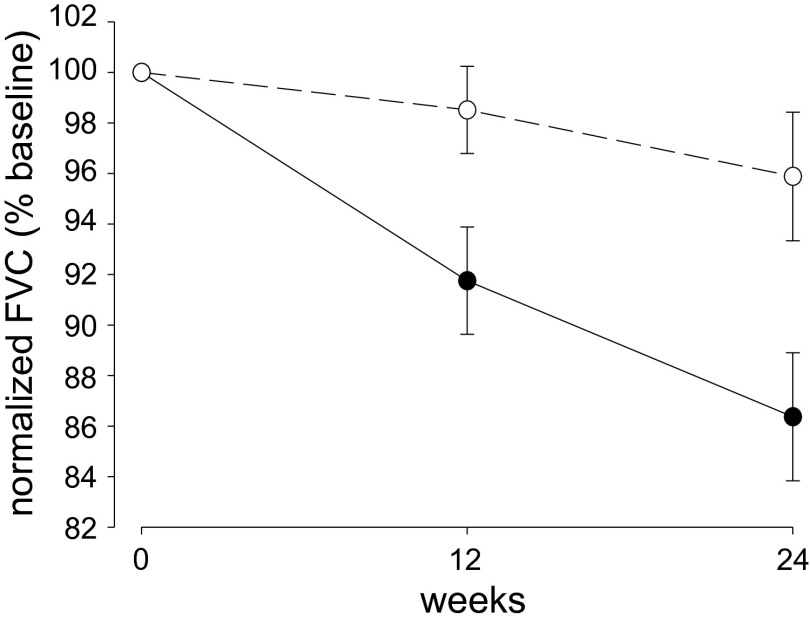
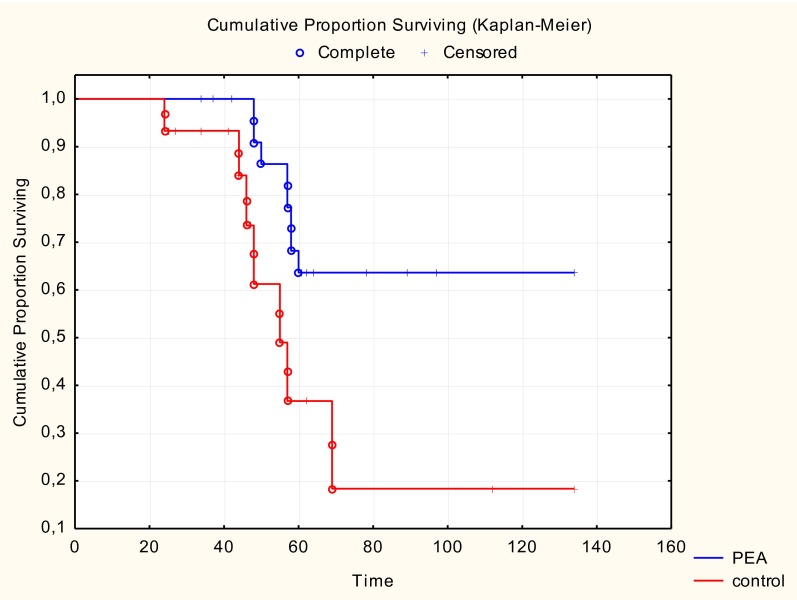
For all clinical and neurophysiological variables the repeated-measures ANOVA revealed a significant decrease in the average values over time (P < 0.0001). Notably, the decrease in FVC% over time was higher in untreated than in PEA-treated patients (repeated-measure ANOVA, F = 446, P = 0.0154 for time × treatment effect) (Fig. 5). Post hoc tests indicated a significant reduction in FVC% among untreated patients between the first and second visits (Tukey–Kramer, P = 0.0003), between the second and third visits (Tukey–Kramer, P = 0.0019), and between the first and third visits (Tukey–Kramer, P < 0.0001) (Table S3). On the contrary, no significant reductions in FVC% were detected by pairwise comparisons during the post hoc analysis in PEA-treated patients. These data indicate that disease progressed more slowly in subjects receiving PEA during the 6-mo follow-up. No significant time × treatment effect was observed for the MRC scale (F = 2.49, P = 0.0910), ulnar (F = 0.75, P = 0.4775) and phrenic nerve (F = 1.79, P = 0.1761) CMAP amplitudes, or the ALSFRS-R scale (F = 0.48, P = 0.6211; Table S3). However, similar to the FVC%, the repeated-measures ANOVA revealed a significant time × treatment effect for the bulbar (F = 4.51, P = 0.0148) and respiratory (F = 5.36, P = 0.0071) subscores of the ALSFRS-R scale. Therefore, both bulbar functions and respiratory function are significantly influenced by the administration of PEA, achieving a slowdown in the worsening of symptoms over time compared with the untreated group (Table S3). Analysis of the cumulative proportion of survivors in need of respiratory assistance showed that death and tracheotomy occurred more frequently in untreated than in PEA-treated patients (F = 2.01; P = 0.05) (Fig. S1) in the 6-mo follow-up period after 6 mo of PEA treatment.
Fig. 5.
Effects of PEA treatment on pulmonary function in ALS patients. Predicted FVC% of untreated (●, Table S2) and PEA–treated (○, Table S2) patients at baseline (0) and after 12 and 24 wk of treatment. Data are normalized to the baseline value in each patient; values are expressed as mean ± SEM. The decrease in FVC% was higher in untreated patients than in PEA-treated patients (repeated measures ANOVA, F = 4.46, P = 0.0154 for time × treatment effect), indicating that the disease progressed more slowly during the 24-wk follow-up in patients receiving PEA.
Fig. S1.
Effects of PEA treatment on tracheotomy and death. Cumulative proportion surviving related to tracheotomy and death in untreated (red trace, 36 patients) and PEA-treated (blue trace, 28 patients) patients in the 6-mo follow-up period after 6 mo of PEA treatment. The cumulative proportion surviving is the proportion of ALS patients who survived without tracheotomy (days). Note that death and tracheotomy occurred more frequently in untreated than in PEA-treated patients (P = 0.05).
SI Text
Biopsy Study: Patients.
Forty-two ALS patients (16 females, 26 males, mean age 63.8 ± 10.9 y) (Table S1) were recruited from the ALS Center of Policlinico Umberto I, Sapienza University, Rome. All patients met the established criteria for the diagnosis of probable or definite ALS (27); 29 patients presented with a spinal onset of disease, and 13 patients presented with a bulbar onset of disease. Mean time from the onset of symptoms to the time of muscle biopsy was 26.7 ± 17.6 mo. The mean ALSFRS-R score at the time of muscle biopsy was 33.9 ± 8.1.
Seventeen non-ALS denervated control patients (eight females, nine males, mean age 52.7 ± 16.8 y) (Table S1) had neurogenic lesions resulting from trauma or peripheral neuropathies; mean duration of disease was variable, ranging from 3 wk to 16 y. Both ALS and control denervated patients underwent needle electromyography testing of several muscles to determine denervation grade before the muscle biopsy was completed; the muscle in which biopsy was performed was decided accordingly. Five muscle sites were sampled, and one point was assigned for each insertion site where denervation potentials could be observed (maximum = five points). Thus, biopsy samples were characterized consistently among patients according to the degree of denervation, as reported in Table S1.
Clinical Study: Patients.
In the cohort of 64 ALS patients, 54 presented with the spinal form of the disease; the remaining 10 presented with bulbar or pseudobulbar onset. The group of recruited patients included 30 of the previously described patients who underwent needle biopsy. No sample size determination was needed because of the pilot nature of the proposed study.
The study was authorized by the Ethical Committee of Policlinico Umberto I, Sapienza University of Rome and was conducted in accordance with the guidelines of the Declaration of Helsinki. Patients provided written informed consent. Inclusion criteria were age between 18 and 85 y; disease duration ≤60 mo; moderate disability, as indicated by an ALSFRS-R score ≥20, with FVC ≥30% predicted; documented disease progression in the last 3 mo; being on a stable dose of riluzole (50 mg), twice daily for at least 2 mo before screening; and the patient’s understanding and acceptance of the protocol requirements. Exclusion criteria were a history of intolerance to riluzole; use of mechanical ventilation or percutaneous endoscopic gastrostomy feeding; severe mental deterioration; if female, pregnancy or breast-feeding or, if, of childbearing age, with a positive pregnancy test; and any other comorbid condition that would make completion of the trial unlikely. No family history of ALS, dementia, Parkinson disease, or other relevant neurodegenerative disorder could be present.
The study was designed to last 6 mo. At each visit the ALSFRS-R, the FVC%, the MRC score for muscle strength limited to the right upper limbs, and the CMAP from right ulnar and phrenic nerves were assessed.
The ALSFRS-R is a validated measure of functional impairment in ALS (15). It is a questionnaire-based functional scale divided into three subscores: bulbar (12 points maximum), motor (24 points maximum), and respiratory (12 points maximum), with normal function defined by a score of 48 and a locked-in condition with respiratory assistance and artificial nutrition defined by a score of 0.
Muscle strength of lower limbs was assessed with the MRC score, an ordinal scale ranging from 0 (absence of movement) to 5 (contraction against full resistance) that quantifies muscle weakness in isolated muscle or muscle groups (32). In this test, eight muscle groups in the upper limbs and seven muscle groups in the lower limbs are tested. The maximum scores are 40 for each upper limb and 35 for each lower limb.
After the end of the treatment period, the patients were seen clinically every 3 mo at the ALS Centre of Policlinico Umberto I for clinical assistance.
Electrophysiological Evaluations.
We recorded CMAP from the right abductor digiti minimi muscle by ulnar nerve stimulation at the wrist. The level of the stimulus intensity was increased slowly until supramaximal stimulation was obtained. The amplitudes of the initial negative peaks of the CMAPs were measured, and the changes in amplitude were analyzed.
To record CMAPs from the right phrenic nerve, stimuli were delivered by a standard monopolar needle electrode inserted medially from the lateral aspect of the neck (posterior to the lateral border of the sternocleidomastoid muscle). A surface electrode placed on the manubrium served as anode. The recording electrodes were placed on the xiphoid process and at the eighth intercostal space near the costochondral junction. Phrenic nerve stimulation always was performed at the end-of-expiration movement. The response with the highest peak-to-peak amplitude was selected for analysis.
The methods used for the electrophysiological evaluation followed the recommendations of experts of the International Federation of Clinical Neurophysiology (33, 34).
Pulmonary Function Tests.
Patients underwent pulmonary function tests according to the American Thoracic Society (ATS) and European Respiratory Society recommendations (35–37). FVC was selected from the standard respiratory function tests for the present study. The best of three expiratory maneuvers, each obtained after a maximal inspiratory effort with the patients sitting, was used to obtain the FVC% for each patient. Predicted values were calculated by normalizing to the reference values proposed by the ATS (38) and the European Community for Coal and Steel (39).
Data Analysis.
The case analysis used all subjects entered in the protocol study. Normal distributions of demographic, clinical, and electrophysiological parameters were tested with the Shapiro–Wilk normality test. Measures were compared using an ANOVA for repeated measures with Tukey–Kramer post hoc tests. Comparisons between the two groups at baseline were performed by using the unpaired t test and Fischer’s exact t test. The statistical significance limit was accepted as P ≤ 0.05. Data were analyzed using SAS 9.3 software (SAS Institute, Inc.). The delay between the onset of disease and poor prognosis, described by tracheotomy or death in PEA and in no-PEA patients, was measured with a Kaplan–Meyer curve. The cumulative proportion surviving metric was the proportion of surviving ALS patients without tracheotomy (months). Statistical differences between curves were calculated by using Cox’s F test.
Discussion
This study suggests that muscle AChRs can be a target for ALS pharmacological therapy. Our concept is supported by evidence of the role of AChRs in the degeneration of NMJs. Particularly noteworthy are the observations that alterations in neuromuscular transmission can be found before the onset of motor symptoms (4) and that AChR blockade with αBuTX produces changes in motoneuron excitability equivalent to those observed following axotomy (16). In the light of this evidence, the possibility of targeting AChRs in the therapy of neuromuscular disorders becomes an interesting research topic and strongly implies the need for a deeper knowledge of the molecular mechanisms that underlie impairment of AChRs in human muscle. Nevertheless, the study of ALS patients has remained a very difficult issue to tackle, mainly because of technical difficulties and ethical concerns.
To overcome these limitations, we took advantage of the microtransplantation technique, injecting human muscle membranes into Xenopus oocytes. This approach allowed us to study functional AChRs isolated from muscle biopsies of ALS patients and non-ALS denervated controls. We show that (i) PEA, an eCB-related molecule, can reduce the desensitization of AChRs in both ALS and non-ALS human samples; (ii) this compound is more effective on human ε-AChRs than on γ-AChRs; (iii) the α1 AChR subunit is up-regulated in ALS patients compared with non-ALS individuals, suggesting a different AChR composition in ALS muscle; (iv) PEA treatment of a selected group of ALS patients for 6 mo slowed their respiratory impairment, affecting the FVC and delaying the need for tracheotomy. Indeed, although numerous articles have been published on the role of eCBs (i.e., lipid mediators and derivatives) in the therapy of neurological diseases (17), this study is the first, to our knowledge, to investigate whether the eCB PEA can affect muscle function both at the molecular level and in clinical practice using a large cohort of patients.
We demonstrate that PEA affects AChR function, decreasing desensitization of ACh evoked-currents after repetitive neurotransmitter application. Excessive receptor desensitization can be detrimental for its function; thus the observed effect can be relevant because it promotes the stability of AChR function that in turn may contribute to the conservation of muscle excitability. Because we did not find a difference between the application of PEA and the coapplication of PEA and riluzole, we exclude the possibility that the two drugs have a synergistic effect on AChR desensitization. PEA is a fatty acid ethanolamide formed by membrane phospholipids, and it is well known that fatty acid can bind to ligand-gated channels on allosteric sites, inducing a modulation of the receptors’ activated currents (18). Our data show that PEA can induce a reversible decrease in ACh-evoked currents, changing the rate of current inactivation. A similar current decrease has been reported previously for the cannabinoid anandamide on neuronal nicotinic AChRs (19). Therefore, we hypothesize that PEA can decrease ACh current rundown by stabilizing a portion of the ACh current that desensitizes when the AChRs are repetitively stimulated. Further experiments will be necessary to elucidate this point better. Moreover, whether the effects of eCBs are mediated by binding to the ion channels or by affecting the properties of the lipid membrane remains an open question (18).
In innervated muscle fibers, AChRs are formed by α1β1εδ subunits and are densely packed at the endplate (7), whereas in denervated muscle cells AChRs are formed also by α1β1γδ subunits with an almost uniform distribution over the entire sarcolemma (8, 20, 21). Therefore, in muscle from ALS patients, variable proportions of γ- and ε-AChRs are present, reflecting cycles of denervation/reinnervation (5, 22). Interestingly, we found a selective PEA effect on oocytes expressing ε-AChRs, suggesting that it is not mediated by “microtransplanted” accessory proteins and/or signaling molecules. These data are particularly relevant because γ-AChRs activity can impair motor neuron survival (23–25). Thus, it is reasonable to suppose that selective PEA action on ε-AChRs could contribute to the maintenance of functional NMJs.
We found a fourfold increase in the expression of the α1 AChR subunit in ALS muscle by qPCR. We previously reported a significant decrease in ACh sensitivity in oocytes injected with ALS muscle membranes compared with those injected with non-ALS membranes (5). When we mimicked this increase by expressing AChR subunits in Xenopus oocytes, we found reduced ACh sensitivity comparable to that found on microtransplanted ALS membranes (5). This evidence supports the hypothesis that ALS denervation induces changes at the NMJ that are not present after denervation resulting from other neuropathies or trauma.
PEA was shown previously to be able to improve the pulmonary function and clinical condition of a single ALS patient (13). In our study we observed that PEA treatment for 6 mo in a large cohort of ALS patients slowed their decline in pulmonary function as measured by FVC%. Typically, the diaphragm and, to a lesser extent, the intercostal muscles of ALS patients are the last muscles to be paralyzed: symptoms of respiratory defect do not appear until the ultimate stages of the disease (26). PEA may have a selective effect on diaphragmatic muscle because it continues to be active until death. In our study CMAP amplitudes recorded by phrenic and ulnar nerve stimulation were not different between untreated and PEA-treated patients over time. This finding suggests that the PEA effect reported here is determined mainly by direct action on NMJs of muscle membrane rather than a nerve-mediated effect leading to regeneration and collateral sprouting of peripheral nerve fibers. Taken together, our molecular, electrophysiological, and clinical observations pave the way for new research opportunities that may lead to a better understanding of ALS-mediated mechanisms and to the development of new therapeutic approaches.
Materials and Methods
Patients.
All patients were recruited and examined by the neurologists at the Rare Neuromuscular Disease Unit of the Policlinico Umberto I, Sapienza University, Rome, and provided written informed consent. See SI Text for further details.
The clinical characteristics derived from the patients' medical records are summarized in Table S1. The number assigned to each patient is reported in the text for each experiment using the # symbol and is referred to Table S1. All patients provided informed consent to use part of the biopsy material for our experiments. Tissue was obtained and used in accordance with the Declaration of Helsinki, and the human subjects Ethical Committee of Sapienza University approved the selection process and technical procedures (reference 3314/25.09.14; protocol no. 1186/14). The study is registered on ClinicalTrials.gov, https://clinicaltrials.gov, ID: NCT02645461.
We conducted a 6-mo, randomized, single-blinded study of patients with probable or definite ALS according to El Escorial diagnostic criteria (27). We screened 120 patients and recruited 64 patients (29 females, 35 males) that were analyzed at the ALS Centre of Policlinico Umberto I, University of Rome Sapienza.
To preserve blindness, each patient was followed by two physicians: a treating physician, who was aware of the treatment allocation, administered the dose of PEA, and observed for adverse events, and an evaluating physician, who was not aware of treatment allocation and performed all clinical assessments.
Patients were randomly assigned to one of two groups: to receive 50 mg riluzole plus 600 mg PEA (ultramicronized) twice daily or to receive riluzole only. Visits were performed at 0 (randomization), 3, and 6 mo. The main outcome analysis was performed in all randomized patients and by intention-to-treat for the patients stratified by severity and onset. Additional details are given in SI Text.
Membrane Preparation, Injection Procedures, and Electrophysiological Recordings from Oocytes.
Human muscle specimens (10–50 mg) were frozen in liquid nitrogen immediately after biopsy and stored at −80 °C. Membranes were prepared as previously described (14, 28). Preparation of Xenopus laevis oocytes and cytoplasmic injection procedures were as described elsewhere (28). The use of female Xenopus laevis frogs conformed to institutional policies and the guidelines of the Italian Ministry of Health (authorization no. 78/2015-PR).
In other experiments human recombinant α1, β1, ε, δ, or α1, β1, γ, δ cDNAs (pCDNA plasmids) were intranuclearly injected as previously reported (6). Membrane currents were recorded from voltage-clamped oocytes 24–48 h after injection using two microelectrodes filled with 3 M KCl. The oocytes were placed in a recording chamber (0.1 mL volume) and were perfused continuously, 9–10 mL/min, with oocyte Ringer’s solution (OR) (5) at room temperature (20–22 °C). Unless otherwise specified the oocytes were voltage-clamped at −60 mV. Decreases in IACh during repetitive ACh applications (500 μM for 7 s at 60-s intervals) were quantified by expressing the peak amplitude of the sixth response as a percent of the peak amplitude of the first response.
The ACh concentrations producing half-maximal effect (EC50) were estimated by fitting the data to Hill equations, using least-square routines as previously described (5).
α-BuTX, ACh, PEA, and riluzole were dissolved in OR just before use. Where not indicated, drugs were purchased from Sigma. Data were analyzed using Sigma Plot software and are given as means ± SEM; datasets are considered statistically different when P < 0.05 (ANOVA test).
RNA Isolation and qPCR.
Muscle samples of 12 ALS patients and 12 denervated controls were used for RNA isolation (Table S1). Samples were resuspended in 600 µL Qiagen RLT buffer, were placed in a tube containing Lysing Matrix M (MP Biomedicals, catalog no. 116923050) and were homogenized in a Fast Prep apparatus (MP Biomedicals) for 40 s at a speed of 6.5 m/s. RNA isolation was performed following the manufacturer’s instructions (Protocol: Purification of Total RNA from Animal Tissues; RNeasy catalog no. 74104). A DNase-treatment step was included to eliminate any traces of genomic DNA. Total RNA was eluted in 35 µL of RNase-free water. In accordance with the Minimum Information for publication of Quantitative real-time PCR Experiments (MIQE) guidelines (29), 5 µL was used to calculate the concentration by nano-drop and RNA integrity number (RIN) (30). Only samples with a RIN of 7 or higher were considered for subsequent steps. Relative expression levels of AChR subunit α1, β1, γ, δ, and ε mRNAs were evaluated by qPCR. iScript (Bio-Rad) was used to obtain cDNA from 2 μg of RNA. The resulting cDNA was used as a template to perform qPCR in triplicate with the SYBR Green mix from Bio-Rad and an iCycler real-time PCR machine (two-step qPCR, 94C 15 s, 68C 30 s, for 40 cycles). For data analysis, threshold cycles obtained by qPCR were normalized against GAPDH and used to calculate relative amounts of neurotransmitter receptor transcripts using the method described by Thomsen et al. (31), and its statistical significance was calculated by t test. These experiments were performed in a blind procedure by J.M.R.-R.
Acknowledgments
We thank all the patients who made this study possible by donating muscle tissue; Mr. Giorgio Tartaglia for technical assistance in carrying out the neurophysiological examinations performed in this study; and Count Giampiero Auletta Armenise for his support. Ultramicronized PEA for the clinical study was a kind gift from the Epitech Group SpA. This work was supported by grants from Associazione Italiana contro l'epilessia (AICE-FIRE) and UCB Pharma, Bruxelles (to E.P.) and from the Ri.MED Foundation (to P.C.) and by the Italian Ministry of Health for Institutional Research and PRIN Project 2010 (C.R.). G.R., D.L. and E.O. were supported by the PhD program in Clinical/Experimental Neuroscience and Psychiatry at Sapienza University, Rome.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1600251113/-/DCSupplemental.
References
- 1.Robberecht W, Philips T. The changing scene of amyotrophic lateral sclerosis. Nat Rev Neurosci. 2013;14(4):248–264. doi: 10.1038/nrn3430. [DOI] [PubMed] [Google Scholar]
- 2.Dupuis L, Loeffler JP. Neuromuscular junction destruction during amyotrophic lateral sclerosis: Insights from transgenic models. Curr Opin Pharmacol. 2009;9(3):341–346. doi: 10.1016/j.coph.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 3.Musarò A. Understanding ALS: New therapeutic approaches. FEBS J. 2013;280(17):4315–4322. doi: 10.1111/febs.12087. [DOI] [PubMed] [Google Scholar]
- 4.Rocha MC, Pousinha PA, Correia AM, Sebastião AM, Ribeiro JA. Early changes of neuromuscular transmission in the SOD1(G93A) mice model of ALS start long before motor symptoms onset. PLoS One. 2013;8(9):e73846. doi: 10.1371/journal.pone.0073846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palma E, et al. Physiological characterization of human muscle acetylcholine receptors from ALS patients. Proc Natl Acad Sci USA. 2011;108(50):20184–20188. doi: 10.1073/pnas.1117975108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deflorio C, et al. Riluzole blocks human muscle acetylcholine receptors. J Physiol. 2012;590:2519–2528. doi: 10.1113/jphysiol.2012.230201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Katz B, Miledi R. Further observations on the distribution of acetylcholine –reactive sites in skeletal muscle. J Physiol. 1964;170:379–388. doi: 10.1113/jphysiol.1964.sp007338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Witzemann V, Brenner HR, Sakmann B. Neural factors regulate AChR subunit mRNAs at rat neuromuscular synapses. J Cell Biol. 1991;114(1):125–141. doi: 10.1083/jcb.114.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rossi S, Bernardi G, Centonze D. The endocannabinoid system in the inflammatory and neurodegenerative processes of multiple sclerosis and of amyotrophic lateral sclerosis. Exp Neurol. 2010;224(1):92–102. doi: 10.1016/j.expneurol.2010.03.030. [DOI] [PubMed] [Google Scholar]
- 10.Moreno-Martet M, Espejo-Porras F, Fernández-Ruiz J, de Lago E. Changes in endocannabinoid receptors and enzymes in the spinal cord of SOD1(G93A) transgenic mice and evaluation of a Sativex(®) -like combination of phytocannabinoids: Interest for future therapies in amyotrophic lateral sclerosis. CNS Neurosci Ther. 2014;20(9):809–815. doi: 10.1111/cns.12262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.André A, Gonthier MP. The endocannabinoid system: Its roles in energy balance and potential as a target for obesity treatment. Int J Biochem Cell Biol. 2010;42(11):1788–1801. doi: 10.1016/j.biocel.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 12.Scuderi C, et al. Palmitoylethanolamide exerts neuroprotective effects in mixed neuroglial cultures and organotypic hippocampal slices via peroxisome proliferator-activated receptor-α. J Neuroinflammation. 2012;9:49. doi: 10.1186/1742-2094-9-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clemente S. Amyotrophic lateral sclerosis treatment with ultramicronized palmitoylethanolamide: A case report. CNS Neurol Disord Drug Targets. 2012;11(7):933–936. doi: 10.2174/1871527311201070933. [DOI] [PubMed] [Google Scholar]
- 14.Eusebi F, Palma E, Amici M, Miledi R. Microtransplantation of ligand-gated receptor-channels from fresh or frozen nervous tissue into Xenopus oocytes: A potent tool for expanding functional information. Prog Neurobiol. 2009;88(1):32–40. doi: 10.1016/j.pneurobio.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 15.Cedarbaum JM, et al. BDNF ALS Study Group (Phase III) The ALSFRS-R: A revised ALS functional rating scale that incorporates assessments of respiratory function. J Neurol Sci. 1999;169(1-2):13–21. doi: 10.1016/s0022-510x(99)00210-5. [DOI] [PubMed] [Google Scholar]
- 16.Nakanishi ST, Cope TC, Rich MM, Carrasco DI, Pinter MJ. Regulation of motoneuron excitability via motor endplate acetylcholine receptor activation. J Neurosci. 2005;25(9):2226–2232. doi: 10.1523/JNEUROSCI.5065-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mattace Raso G, Russo R, Calignano A, Meli R. Palmitoylethanolamide in CNS health and disease. Pharmacol Res. 2014;86:32–41. doi: 10.1016/j.phrs.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 18.Oz M. Receptor-independent effects of endocannabinoids on ion channels. Curr Pharm Des. 2006;12(2):227–239. doi: 10.2174/138161206775193073. [DOI] [PubMed] [Google Scholar]
- 19.Oz M, Ravindran A, Diaz-Ruiz O, Zhang L, Morales M. The endogenous cannabinoid anandamide inhibits alpha7 nicotinic acetylcholine receptor-mediated responses in Xenopus oocytes. J Pharmacol Exp Ther. 2003;306(3):1003–1010. doi: 10.1124/jpet.103.049981. [DOI] [PubMed] [Google Scholar]
- 20.Miledi R. Junctional and extra-junctional acetylcholine receptors in skeletal muscle fibres. J Physiol. 1960;151:24–30. [PMC free article] [PubMed] [Google Scholar]
- 21.Brenner HR, Rudin W. On the effect of muscle activity on the end-plate membrane in denervated mouse muscle. J Physiol. 1989;410:501–512. doi: 10.1113/jphysiol.1989.sp017546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsujihata M, et al. The motor end-plate fine structure and ultrastructural localization of acetylcholine receptors in amyotrophic lateral sclerosis. Muscle Nerve. 1984;7(3):243–249. doi: 10.1002/mus.880070310. [DOI] [PubMed] [Google Scholar]
- 23.Oppenheim RW, et al. Reduction of neuromuscular activity is required for the rescue of motoneurons from naturally occurring cell death by nicotinic-blocking agents. J Neurosci. 2000;20(16):6117–6124. doi: 10.1523/JNEUROSCI.20-16-06117.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oppenheim RW, et al. The rescue of developing avian motoneurons from programmed cell death by a selective inhibitor of the fetal muscle-specific nicotinic acetylcholine receptor. Dev Neurobiol. 2008;68(7):972–980. doi: 10.1002/dneu.20636. [DOI] [PubMed] [Google Scholar]
- 25.Terrado J, et al. Motoneuron survival is enhanced in the absence of neuromuscular junction formation in embryos. J Neurosci. 2001;21(9):3144–3150. doi: 10.1523/JNEUROSCI.21-09-03144.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Silani V, et al. The diagnosis of Amyotrophic lateral sclerosis in 2010. Arch Ital Biol. 2011;149(1):5–27. doi: 10.4449/aib.v149i1.1260. [DOI] [PubMed] [Google Scholar]
- 27.Brooks BR. El Escorial World Federation of Neurology criteria for the diagnosis of amyotrophic lateral sclerosis. Subcommittee on Motor Neuron Diseases/Amyotrophic Lateral Sclerosis of the World Federation of Neurology Research Group on Neuromuscular Diseases and the El Escorial “Clinical limits of amyotrophic lateral sclerosis” workshop contributors. J Neurol Sci. 1994;124(Suppl):96–107. doi: 10.1016/0022-510x(94)90191-0. [DOI] [PubMed] [Google Scholar]
- 28.Miledi R, Palma E, Eusebi F. Microtransplantation of neurotransmitter receptors from cells to Xenopus oocyte membranes: New procedure for ion channel studies. Methods Mol Biol. 2006;322:347–355. doi: 10.1007/978-1-59745-000-3_24. [DOI] [PubMed] [Google Scholar]
- 29.Bustin SA, et al. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin Chem. 2009;55(4):611–622. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- 30.Schroeder A, et al. The RIN: An RNA integrity number for assigning integrity values to RNA measurements. BMC Mol Biol. 2006;7:3. doi: 10.1186/1471-2199-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thomsen R, Sølvsten CA, Linnet TE, Blechingberg J, Nielsen AL. Analysis of qPCR data by converting exponentially related Ct values into linearly related X0 values. J Bioinform Comput Biol. 2010;8(5):885–900. doi: 10.1142/s0219720010004963. [DOI] [PubMed] [Google Scholar]
- 32.Medical Research Council 1976. Aids to examination of the peripheral nervous system. Memorandum no. 45. (London: Her Majesty’s Stationary Office)
- 33.Kimura J. Peripheral Nerve Diseases. Handbook of Clinical Neurophysiology. Elsevier; Amsterdam: 2006. [Google Scholar]
- 34.Inghilleri M, Iacovelli E. Clinical neurophysiology in ALS. Arch Ital Biol. 2011;149(1):57–63. doi: 10.4449/aib.v149i1.1264. [DOI] [PubMed] [Google Scholar]
- 35.Miller MR, et al. ATS/ERS Task Force General considerations for lung function testing. Eur Respir J. 2005a;26(1):153–161. doi: 10.1183/09031936.05.00034505. [DOI] [PubMed] [Google Scholar]
- 36.Miller MR, et al. ATS/ERS Task Force Standardisation of spirometry. Eur Respir J. 2005b;26(2):319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 37.Pellegrino R, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26(5):948–968. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 38.American Thoracic Society Standardization of spirometry. Am J Respir Crit Care Med. 1995;152(3):1107–1136. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 39.Quanjer PH, et al. Official Statement of the European Respiratory Society Lung volumes and forced ventilatory flows. Report working party standardization of lung function tests. European Community for Steel and Coal. Official statement of the European Respiratory Society. Eur Respir J Suppl. 1993;16(Suppl.):5–40. [PubMed] [Google Scholar]