Abstract
INTRODUCTION
The developing fetus relies on the maternal blood supply to provide the choline it requires for making membrane lipids, synthesizing acetylcholine, and performing important methylation reactions. It is vital, therefore, that the placenta is efficient at transporting choline from maternal to fetal circulation. Although choline transporters have been found in term placenta samples, little is known about what cell types express specific choline transporters and how expression of the transporters may change over gestation. The objective of this study was to characterize choline transporter expression levels and localization in the human placenta throughout placental development.
METHODS
We analyzed CTL1 and −2 expression over gestation in human placental biopsies from 6 to 40 weeks gestation (n=6–10 per gestational window) by immunoblot analysis. To determine the cellular expression pattern of the choline transporters throughout gestation, immunofluorescence analysis was then performed.
RESULTS
Both CTL1 and CTL2 were expressed in the chorionic villi from 6 weeks gestation to term. Labor did not alter expression levels of either transporter. CTL1 localized to the syncytial trophoblasts and the endothelium of the fetal vasculature within the chorionic villous structure. CTL2 localized mainly to the stroma early in gestation and by the second trimester co-localized with CTL1 at the fetal vasculature.
DISCUSSION
The differential expression pattern of CTL1 and CTL2 suggests that CTL1 is the key transporter involved in choline transport from maternal circulation and both transporters are likely involved in stromal and endothelial cell choline transport.
Keywords: Choline transport, CTL1, CTL2, chorionic villi, trophoblasts, fetal endothelium
INTRODUCTION
Choline is an essential nutrient that must be obtained through the diet for maintenance of proper health. The synthesis of phosphatidylcholine and sphingomyelin, major constituents of cell membranes that comprise the majority of total membrane lipid content, require choline [1]. Choline is also a critical precursor of the synthesis of the neurotransmitter acetylcholine [2]. Additionally, choline provides a source of methyl groups for the synthesis of S-adenosylmethionine (SAM), the primary methyl donor for cellular methylation reactions [3]. With choline playing roles in such critical pathways in cellular biology, it is not surprising that a choline deficient diet would have adverse effects on health. In fact, several investigators have shown that lack of adequate choline levels can lead to development of fatty liver [4, 5], liver damage [6], muscle damage [7], and increased sensitivity to carcinogens [8] in adult humans. Additionally, Niculescu [9] and Mehedint [10] have used rodent models to demonstrate a loss in fetal hippocampal progenitor cell proliferation and angiogenesis, respectively, resulting from a maternal diet deficient in choline during pregnancy. These results demonstrate that choline not only plays an important role in adult health, but also plays an essential role in fetal brain development. In 1998, the National Academy of Sciences recognized choline as an essential nutrient and recommended daily intake levels (550 mg/day for men, 425 mg/day for non-pregnant and 450 mg/day for pregnant women).
Enriching adult diets with choline has been shown to improve cognition [11–13]. Importantly, enriching the diet of pregnant mothers with choline has been shown to have beneficial consequences for offspring that can last well into adulthood. Rodent models have demonstrated that supplementing the diets of dams with choline can improve spatial and temporal memory in offspring [14] and can improve cognitive function for years after birth [15]. Recently, this phenomenon has been demonstrated in humans. Infants from mothers supplemented with phosphatidylcholine, as a choline source, during pregnancy showed improved cerebral inhibition as measured by electroencephalographic P50 ratio [16]. These observations have important implications in understanding the origins of psychotic disorders. Abnormal development of GABA-related inhibitory neurocircuitry has been implicated in a variety of neuropsychiatric illnesses including externalizing disorders (ADHD and conduct disorder), anxiety disorders (including PTSD), psychotic illnesses (including schizophrenia and bipolar), depressive disorders, and substance use disorders [17–22]. Toward the end of gestation, stimulation of the α7 nicotinic acetylcholingergic receptor facilitates the transition of the neurotransmitter GABA from excitatory to inhibitory. During fetal development, acetylcholinergic innervation of most brain regions has not yet fully developed; choline itself is the primary prenatal agonist at the alpha 7-nicotinic acetylcholine receptor and thus sufficient choline is critical for proper development of cerebral inhibitory neurons. Choline availability during fetal development may have a crucial role in brain development and deficiencies in choline may lead to lasting effects in the offspring.
During development, the fetus depends on maternal supply of choline for its metabolic needs [23]. The placenta plays a vital role in the active transfer of choline from the maternal blood supply to the fetus. It was demonstrated in 1985 [24], through perfusion studies in the guinea pig, that choline transport in the placenta is rapid, unidirectional against a concentration gradient, occurs at both the maternal and fetal interface of the placenta, and is sodium-independent. In 1994 [25], a similar choline transport mechanism was demonstrated in brush border vesicles isolated from human term placenta and in 2005 [26], a similar mechanism was demonstrated in human BeWo and JEG-3 trophoblast cell lines derived from choriocarcinomas. Further characterization of choline transport in the placenta has been limited.
Choline transport is known to occur through three different protein-mediated transport systems: (1) the high affinity choline-specific CHT1 transporter (SLC5A7) that is expressed in specific regions of the brain rich in cholinergic neurons, (2) the intermediate affinity choline transporter-like CTL proteins (SLC44A1–5) found in multiple tissues, and (3) the low affinity organic cation OCT transporters found in various tissues [27]. The objective of this study is to characterize choline transporter expression levels and localization in the placenta over gestation. The chorionic villi are the primary sites of placental nutrient transport and were, therefore, the focus of our studies. Choline transporter expression levels were examined from chorionic villi lysates at various time points throughout gestation. Corresponding tissue sections were examined by immunofluorescence to determine specific cell types expressing the transporters.
METHODS
Placenta Samples
Chorionic villi were collected from human placenta at various gestational time points and grouped into 6–8 weeks (n=6), 10–12 weeks (n=6), 18–24 weeks (n=6), 28–32 weeks (n=6) and, 37–40 weeks both with (n=10) and without (n=10) labor. Collection of placenta was approved by the Institutional Review Board of the University of Colorado and informed consent was obtained. Subjects were excluded if mother had chronic disease, was a smoker, or abused alcohol or drugs. First and second trimester placenta were collected from uncomplicated elective terminations. Third trimester preterm placentas were collected from subjects with preterm labor that were normotensive and did not have preterm premature rupture of membranes (PPROM) or chorioamnionitis. Term placentas were collected from uncomplicated vaginal delivery and unlabored C-section. Tissue was immediately snap frozen in liquid nitrogen or placed in formalin (described in more detail below) for analysis of protein expression and localization.
Western Blot Analysis
Tissue was briefly washed in cold phosphate buffered saline (PBS) before being snap frozen in liquid nitrogen and stored in −80°C. Frozen tissue was placed in ice-cold lysis buffer (Tris-HCl, pH 7.4 50 mM, NaCl 150 mM, NP-40 1%, Sodium deoxycholate 0.5%, SDS 0.1%, EDTA 5 mM; RIP A Buffer with EDTA, Boston Bioproducts, Ashland, MA) with protease inhibitors (Complete EDTA-free Protease Inhibitor Cocktail, Roche, Mannheim, Germany) and homogenized with a Brinkman Instruments Polytron, centrifuged at 14,000 rpm for 10 minutes at 4°C, and supernatant collected. Tissue lysates (15 μg total protein) were denatured, resolved on 10% SDS-PAGE, and transferred to a polyvinylidene difluoride (PVDF) membrane (Bio Rad, Hercules, CA, USA). Membranes were blocked with 5% nonfat dry milk in Tris-buffered saline with 0.1% Tween-20 (TBST) for one hour at room temperature (RT) before treatment with either rabbit anti-human SLC44A1/CTL1 (1:1000, Bioss Inc., Woburn, MA, USA), mouse anti-human SLC44A2/CTL2 (1:500; Sigma, St. Louis, MO, USA), or rabbit anti-human β-actin (1:10,000; Abcam, Cambridge, MA, USA) overnight at 4°C. Membr anes were then washed 4 times with TBST for 15 minutes before treatment with horseradish peroxidase-conjugated goat anti-rabbit (1:10,000; GE Healthcare, Buckinghamshire, UK) or goat anti-mouse (1:10,000; Jackson ImmunoResearch Laboratories, West Grove, PA, USA) antibodies for 1 hour at room temperature. Membranes were washed with TBST as described above and then visualized using an ECL Prime Western Blotting Detection Reagent (GE Healthcare) and X-ray film (CL-XPosure Film, Thermo Scientific, Rockford, IL, USA). Densitometry was performed using ImageJ software (Wayne Rasband, National Institutes of Health), normalizing relative band density to the loading control.
Immunofluorescence
Chorionic villi were collected from human placenta at the gestational time points described above. Tissue was fixed in 10% buffered formalin at RT for 24–48 hours before being embedded in paraffin and sectioned. Tissue sections were hydrated with washes in xylene (2 twenty minute washes) followed by decreasing amounts of ethanol (2 washes in 100% ethanol, one wash in 70% ethanol, and one wash in 30% ethanol) for three minutes each. Sections were then washed in PBS two times for 5 minutes each before antigen retrieval, by boiling sections in citrate-based Antigen Unmasking Solution (Vector Laboratories, Inc., Burlingame, CA, USA) for 10 seconds followed by a 45 second rest and repeated 10 times. Sections were cooled for 10 minutes before washing with PBS and permeabilizing with 1% Triton X-100 for 15 minutes. After two washes with PBS, sections were blocked for one hour with 10% normal donkey serum plus 10 μM/ml saponin. Sections were then treated with rabbit anti-human SLC44A1/CTL1 (1:200, Bioss Inc., Woburn, MA, USA), mouse anti-human SLC44A2/CTL2 (1:100; Sigma, St. Louis, MO, USA), mouse anti-human CK7 (1:50, Dako, Carpinteria, CA, USA), rat anti-human CK7 (1:50, kind gift from M. McMaster, University of California, San Francisco, USA), mouse anti-human CD31 (1:100, Abcam, Cambridge, MA, USA), and/or rabbit anti-human CD31 (1:200, Abcam) overnight at 4°C. After being washe d five times with PBS, five minutes each, sections were treated with donkey anti-rabbit-CY3 (1:100, Jackson ImmunoResearch), donkey anti-mouse-488 (1:100, Jackson ImmunoResearch), and/or donkey anti-rat-FITC (Jackson ImmunoResearch) for 45 minutes in the presence of DAPI (DAPI (5 μg/ml, MP Biochemicals, Solon, OH, USA). Sections were then washed with PBS (5 times, 5 min each) before application of OPDA (20 mg/ml, o-phenylenediamine dihydrochloride in 1M Tris, pH 8.5) to preserve fluorescence and coverslip applied. Specificity of antibodies was tested as described above and results are shown in Supplementary Figures 2 & 3. Fluorescence was imaged using an Olympus Spinning Disk confocal microscope located in the University of Colorado Advanced Light Microscopy Core Facility. Images were analyzed using Slidebook Software (Intelligent Imaging Innovations, Inc., Denver, CO, USA).
STATISTICS
Data are presented as Mean ± Standard Error of the Mean (s.e.m.). A one-way ANOVA was used to determine variance among multiple gestational groups, with a Tukey’s Multiple Comparison post-test to determine significance between individual groups. A p value of < 0.05 was considered significant.
RESULTS
Choline transporter gene expression in human term placenta
To determine the major choline transporters expressed in the human placenta, we first analyzed the gene expression profiles of the known choline transporters in an established microarray database [28]. This analysis revealed that CTL1, CTL2, and OCT3 were the only transporters that showed Affymetrix intensity values above background. CTL1 and CTL2 showed the highest intensity values, suggesting these are the most highly expressed choline transporters (Supplementary Figure 1). Although OCT3 is expressed in the placenta, Kekuda and colleagues [29] have shown that this organic cation transporter does not transport choline to any significant level, and likely plays a role in the placenta independent of choline uptake. Therefore, we focused on characterizing CTL1 and CTL2 expression in the human placenta.
CTL1 and CTL2 protein expression levels over gestation
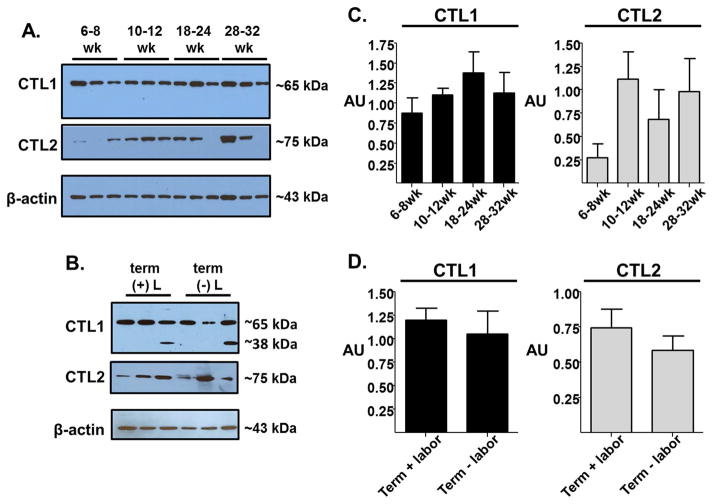
To determine whether CTL1 and CTL2 protein expression changes over gestation, we performed Western blot analysis on tissue lysates from chorionic villi isolated from placenta collected at specific gestational windows (6–8 weeks, 10–12 weeks, 18–24 weeks, 28–32 weeks, term with labor, term without labor). CTL1 protein expression appears to be consistently expressed throughout gestation (Figure 1), with no drastic changes in expression levels. Importantly, it appears CTL1 expression levels maintain consistency even when the placenta is exposed to the dynamic environment of labor. In the term placenta, the presence of a lower molecular weight band (~38 kDa) appears in a few samples regardless of labor status. Although this lower molecular weight band has been observed by other investigators [30, 31] and did not appear in our IgG and antigen peptide controls (see Supplementary Figure 2), the significance of this band is unkonwn. Therefore, we did not include this band in our densitometry analysis. CTL2 protein expression is not as consistent as CTL1. Early in gestation, CTL2 expression level is low and is higher after 10 weeks gestation, although the increase in protein expression is not significant with the current sample size. At term, CTL2 protein expression does not seem to alter significantly with the presence or absence of labor.
Figure 1. Placental CTL1 and CTL2 expression levels over gestation.
Western blot analysis of CTL1 and CTL2 protein expression was performed using human placenta lysates of the chorionic villi region at 6–8, 10–12, 18–24, 28–32, and 37–40 (labored and unlabored) week gestation. (A & B) Representative blots for CTL1 and CTL2 protein expression levels for three samples at each gestational time point with β-actin serving as a loading control. (C & D) Western blot densitometry from multiple western blot analyses was performed using ImageJ. Normalization across blots was done on identical placental sample present on each blot. Mean ± s.e.m., n=5–13 per gestational group.
Localization of CTL1 and CTL2
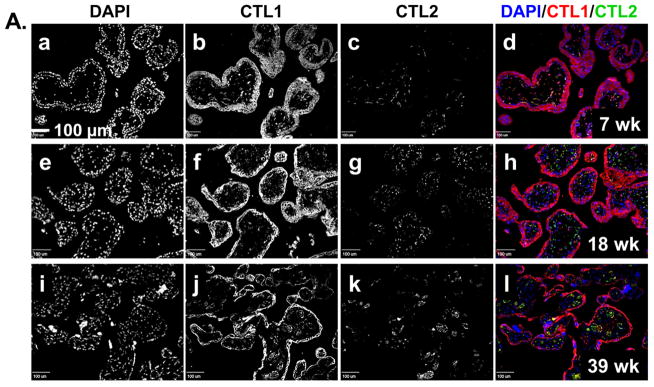
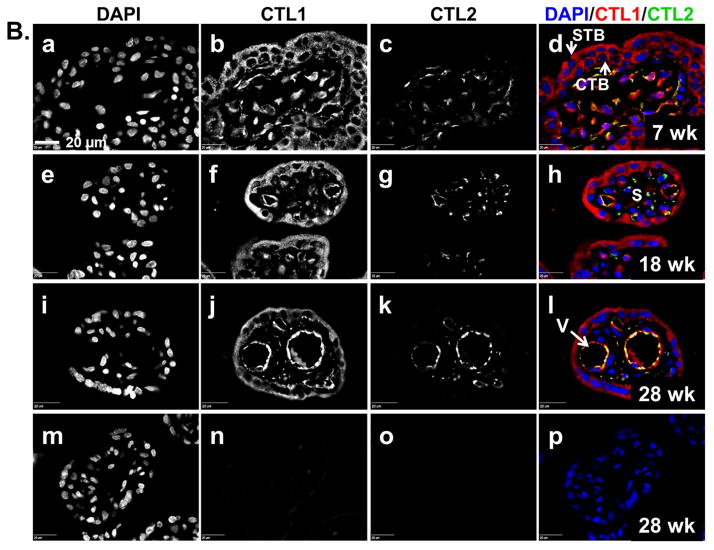
To determine where CTL1 and CTL2 are localized in the placenta and whether localization changes during development, we performed immunofluorescence on placenta tissue sections. The uptake of choline by the placenta from the maternal blood supply is at the chorionic villi. Therefore, the localization of CTL1 and CTL2 within the chorionic villous structure was examined by immunofluorescence on placenta tissue collected at specific time points throughout gestation. Figure 2A shows the general localization of CTL1 and CTL2 in representative samples from early (6–8 weeks), mid (18–24 weeks), and late (39–40 weeks) gestation. CTL1 appears to localize most strongly to the syncytial trophoblasts that form the outer layer (adjacent to the maternal blood) of the chorionic villi. There is also a consistent presence of CTL1 in the stroma. As pregnancy progresses, CTL1 expression is noted in ring-like structures which appear to correspond with the fetal vasculature. CTL2, on the other hand, does not appear in the trophoblast layer(s). Instead, CTL2 appears to localize most strongly within the stroma and as pregnancy progresses appears to localize within the vascular-like structures within the villi. The pattern of localization for both CTL1 and CTL2 is consistent during all gestational windows examined (6–8 weeks, 10–12 weeks, 18–24 week, 28–32 weeks, 39–40 weeks, data not shown for 10–12, 28–32 week time points).
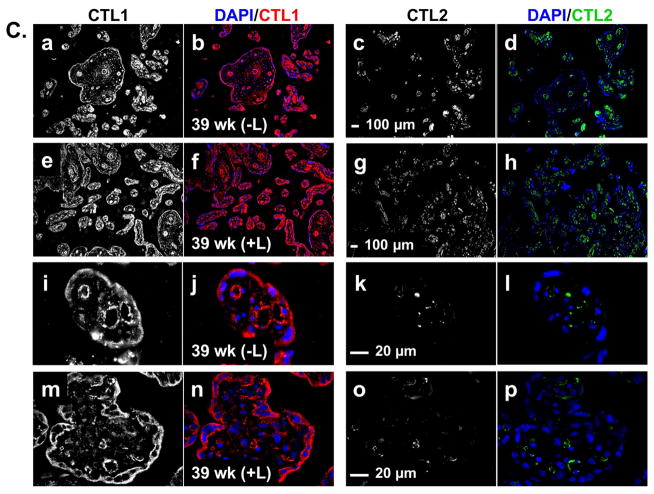
Figure 2. CTL1 and CTL2 localization in chorionic villi.
Immunofluorescence was performed on paraffin embedded tissue sections from the chorionic villi region of human placenta collected at 6–8, 10–12, 18–24, 28–32, 37–40 (labored and unlabored) week gestational time points. Representative images for gestational windows are displayed. Fluorescence from antibodies directed to CTL1 (red) and CTL2 (green) was imaged with a confocal microscope. Nuclei are stained with DAPI (blue). (A) Lower magnification (scale bar: 100 μm) representative images showing localization of nuclei (a, e, i), CTL1 (b, f, j), and CTL2 (c, g, k) individually and together (d, h, l) at 7 (a–d), 18 (e–h), and 39 (i–l) weeks gestation are displayed. (B) Higher magnification (scale bar: 20 μm) representative images showing localization of nuclei (a, e, i, m), CTL1 (b, f, j), and CTL2 (c, g, k) individually and together (d, h, l, p) at 7 (a–d), 18 (e–h), and 28 (i–l) weeks of gestation are displayed. A no primary antibody control is shown (m–p) for the 28 week gestation time point. (C) Lower (scale bar: 100 μm) and higher (scale bar: 20 μm) magnification representative images showing localization of CTL1 (a,e,i,m), CTL2 (c,g,k,o) individually and together with nuclei (b,f,j,n,d,h,l,p) in term placentas (39 weeks) that did not experienced labor (a–d, i–l) or did experience labor (e–h, m–p) are displayed. STB=syncytial trophoblasts, CTB=cytotrophoblasts, S=stroma, V=fetal vasculature, yellow represents co-localization of CTL1 and CTL2, n=6 for each gestational time point (representative images shown).
To determine how CTL1 and CTL2 were distributed within the distinct villous structures indicated above, we examined the chorionic villi at higher magnification (Figure 2B & C). At the early gestational time points, CTL1 is diffusely distributed throughout the trophoblast cells that make up both the cytotrophoblast and snycytiotrophoblast layers of the villi. By 18–24 weeks, CTL1 expression remains high in the trophoblasts and is now also clearly localized to vascular-like structures within the villi. At 28–32 weeks, CTL1 shows a strong localization within the vascular-like structures. Interestingly, CTL1 expression in the syncytial trophoblasts becomes strongly associated with the apical region/membrane at this time point. Throughout gestation, CTL1 can also be found in stroma of the chorionic villi. In contrast to CTL1, CTL2 does not localize to trophoblasts at any time during gestation. Early in gestation, CTL2 is found mainly in the stroma. At 18–24 weeks, CTL2 starts to appear in the vascular-like structures, where co-localization with CTL1 can begin to be visualized. By 28–32 weeks, CTL2 shows a strong localization in the vasculature. At term, CTL1 and CTL2 localization remains as seen at 28–32 weeks, with CTL1 mainly in the trophoblasts and vascular-like structures and CTL2 mainly in the vascular-like structures and stroma. When localization was compared between term chorionic villi of placentas from labored versus non-labored subjects, neither transporter changes localization with the physiologic process of labor (Figure 2C).
Co-localization with trophoblasts and endothelial markers
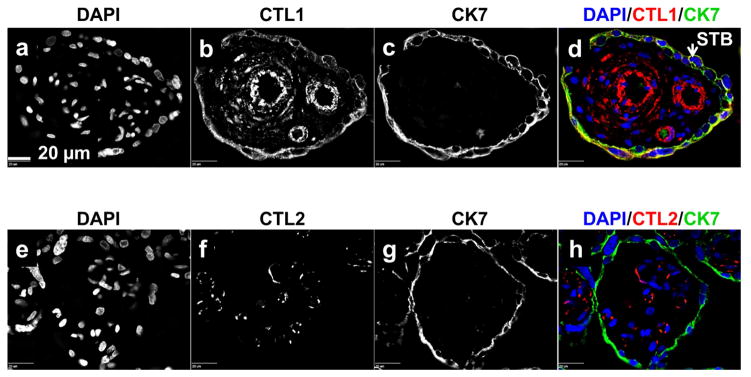
To confirm that CTL1 is localizing with trophoblasts, sections of chorionic villi, at all gestational time points were treated simultaneously with antibody directed to CTL1 and antibody directed to the trophoblast marker CK7. Fluorescence from the secondary antibodies revealed that, indeed, CTL1 co-localized with CK7 (Figure 3). CTL2, however, did not co-localize with CK7 at any gestational time points.
Figure 3. CTL1 localizes to trophoblasts.
Immunofluorescence was performed on paraffin embedded tissue sections from the chorionic villi region of human placenta collected at 6–8, 10–12, 18–24, 28–32, 37–40 (labored and unlabored) week gestational time points. Representative images are displayed (shown here: 39 weeks, no labor). Fluorescence from antibodies directed to CTL1 [b, d (red)], CTL2 [f, h (red)], and CK7 [c, d (green), f, h (green)] was imaged with a confocal microscope. STB=syncytial trophoblasts, scale bar: 20 μm, n=6 for each gestational window.
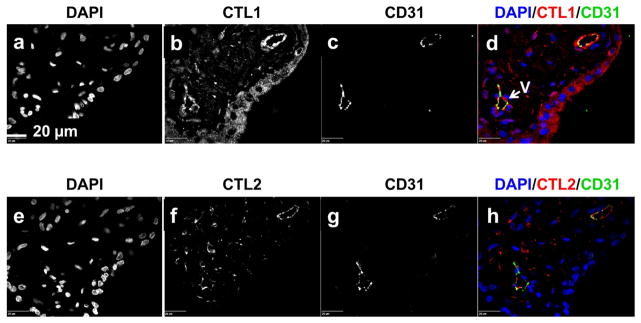
To confirm that CTL1 and CTL2 localize to the fetal vasculature, sections of chorionic villi, at all gestational time points, were treated simultaneously with antibody directed to either CTL1 or CTL2 and antibody directed to the endothelial cell marker CD31. Figure 4 shows that both transporters co-localize with CD31 in the chorionic villi at gestational time points where fetal vasculature is present.
Figure 4. CTL1 and CTL2 localize to fetal endothelial cells.
Immunofluorescence was performed on paraffin embedded tissue sections from the chorionic villi region of human placenta collected at 6–8, 10–12, 18–24, 28–32, 37–40 (labored and non-labored) week gestational time points. Representative images are displayed (shown here: 11 weeks). Fluorescence from antibodies directed to CTL1 [b, d (red)], CTL2 [f, h (red)], and CD31 [c, d (green), f, h (green)] was imaged with a confocal microscope. V=fetal vasculature, scale bar: 20 μm, n=6 for each gestational window.
DISCUSSION
This is the first study that has examined human placental choline transporter, specifically CTL1 and CTL2, expression and localization over gestation. We observed constitutive expression of CTL1 throughout placental development. CTL1 was found in the cytotrophoblasts and syncytiotrophoblasts of the chorionic villi from early gestation (around 8 weeks) through term (around 40 weeks). Toward the end of the first trimester, CTL1 also showed a strong expression in the endothelium of the fetal vasculature. CTL2 showed a more variable expression pattern. Early in gestation, CTL2 was found mainly in the stroma of the chorionic villi. By 18 weeks, CTL2 could be found in the endothelium of the fetal vasculature of the chorionic villi and became strongly expressed in the endothelium by 28 weeks. These observations suggest that CTL1, being present in the syncytial trophoblasts, is likely the main choline transporter responsible for placental uptake of choline from the maternal blood supply. CTL2 expression levels correspond to the growing vasculature of the placenta and the strict localization of CTL2 to the endothelium suggests that CTL2 may play a specific role in endothelial choline transport.
CTL1 was localized to the apical region of the syncytial trophoblasts, as would be expected for choline uptake from the maternal blood. However, CTL1 was also found in the cytosol. This pattern of expression could represent cycling of the transporter between the plasma membrane and the cytosol for regulation of choline transport. It has been reported that movement of CTL1 out of the membrane reduces choline transport activity in macrophages [32]. Yuan and colleagues [30], when characterizing the promoter region of the CTL1 isoforms, also suggested that CTL1 may be found in the membranes surrounding intracellular organelles for the shuttling of choline from the plasma membrane to the mitochondria, for oxidation of betadine, and the nucleus, for utilization in the phosphatidylcholine cycle. Therefore, it is expected that intracellular expression of CTL1 could have a functional role in choline transport.
Constitutive expression of CTL1 in the syncytial trophoblasts of the chorionic villi is in agreement with work from Lee and colleagues [33] where they showed that CTL1 was the main choline transporter in the rat syncytial trophoblast cell line TR-TBT. They were able to demonstrate that the CTL1 transport activity in TR-TBT cells was similar to what had been previously described for human placenta, weakly sodium-dependent, saturable, and inhibited by hemicholinium-3 (HC-3). Of note, it has been demonstrated that CTL1 has 2 splice variants, CTL1a and CTL1b, which have been detected in human term placenta tissue lysates. Our studies, however, are unable to determine if the ratio of splice variants change over gestation.
In contrast to our observations, Yara and colleagues [34] have recently shown expression of CTL2 in the trophoblastic cell line JEG-3 and suggest a potential role for CTL2 in the low-affinity choline transport found in these cells [26]. The discrepancy between results is likely due to the approach of analysis. We examined choline transport in native tissue from normal placentas and Yara investigated choline transporters in a choriocarcinoma cell line. Cancer cells are known to have aberrant choline transporter expression and activity [27] and, therefore, may explain differences in CTL2 expression seen in JEG-3 cells and our normal placenta tissue. Alternatively, it may be that expression of CTL2 in the trophoblasts of the tissue samples examined in this study may be too low to detect by our Western and IP techniques.
Although this is the first report of the CTL2 expression pattern in the placenta, others have shown that CTL2 has 2 isoforms driven off of different promoters. It is interesting to note that only the P2 isoform of CTL2 (CTL2-P2) has choline transport activity in other tissues [35]. Our studies were not able to distinguish the 2 isoforms, CTL2-P1 and CTL2-P2, so how much CTL2 contributes to choline transport in the placenta remains to be determined. Since the expression pattern of CTL2 does not follow the same pattern as CTL1, CTL2 may serve an independent role from CTL1 and is not just simply a redundant molecule.
To our knowledge this is the first report of CTL proteins localizing to the placental vasculature. CTL1 and CTL2 are both strongly expressed in endothelial cells, suggesting a significant need for choline in these cells. Whether this is unique to the fetal vasculature of the placenta is an interesting question. Kommareddi [35] has shown both membrane and cytosolic staining of CTL2 in other tissues (in rodent), including keratinocytes, nerve cells, kidney and spleen, but their analysis did not include the placenta.
Western blot analysis of CTL expression and immunofluorescence analysis of CTL localization at term showed that the process of labor does not significantly change CTL1 or CTL2 expression levels or localization. The placenta is exposed to oxidative and inflammatory stresses during labor [36] and microarray analysis of placenta from women who delivered vaginally (labored) shows over 300 genes that differed in expression pattern from placentas from women who underwent elective cesarean section (unlabored) [37]. Although labor produces significant changes in the placenta, our data suggests that CTL transporters are not regulated or altered by these changes. CTL transporters, however, have been shown to be increased by glucocorticoids. Nakamura et al. [38] demonstrated that treatment of human alveolar type II cells, A549, with dexamethasone can increase CTL1 and CTL2 expression and can enhance choline uptake in these cells. Our placenta samples that were exposed to the corticosteroid betamethasone (preterm samples) did not suggest a dramatic increase in choline transporter expression, but number of samples and variable time from betamethasone exposure are not adequate to fully address this relationship in the current study.
In conclusion, we have demonstrated that CTL1 and CTL2 are expressed in the chorionic villi of human placenta throughout gestation. CTL1 is expressed in the syncytial trophoblasts and cytotrophoblasts and the endothelium of the fetal vasculature. CTL2 is expressed in the stroma during early gestation and can be found strongly expressed by the fetal endothelium as placental development progresses. This change in expression may reflect, at least in part, the increase in fetal vascular development. It will be important for future studies to determine the contribution of each transporter, particularly each isoform of the transporters, to choline transport in both syncytial trophoblasts and endothelial cells. The CTL1 and CTL2 transporters have known coding region SNPs (rs3199966;S644A, rs2288904;Q154R, respectively), but the functional significance of these variants is unknown. Determining the impact of these SNPs on transport capacity will be needed to assess the predictive power genetic analysis would have related to fetal choline availability. Understanding how the placenta contributes to the uptake and fetal delivery of choline, an essential and critical nutrient for fetal growth, development and health outcome, during gestation holds promise in defining potential opportunities for interventions that could improve fetal outcomes.
Supplementary Material
Using GEO GSE9984, the expression values for the known members of the 3 families of choline transporters were extracted, normalized using RMA, and plotted as mean ±SD intensity values. The dash line is at 3.5 as intensity values below this typically represent background.
Western blot analysis (A) and immunohistochemical analysis (B) of CTL1 antibody specificity was tested with normal IgG (rabbit), CTL1 antibody pre-incubated with antigen peptide or CTL1 antibody applied to PDVF membrane or tissue sections. For immunofluorescence, nuclei are seen in blue (DAPI) and IgG or CTL1 is seen in red (CY3 conjugated secondary antibody). The white arrows are pointing to the syncytial trophoblasts (a, b, c).
Western blot analysis (A) and immunohistochemical analysis (B) of CTL2 antibody specificity was tested with normal IgG (mouse) or CTL1 primary antibody applied to PVDF membrane or placenta tissue sections. For immunofluorescence, nuclei are seen in blue (DAPI) and IgG or CTL2 is seen in green (Alexa 488 conjugated secondary antibody). The white arrows are pointing to vessels (a, b).
HIGHLIGHTS.
CTL1 and CTL2 show different expression patterns in the placenta over pregnancy.
CTL1 localizes to syncytiotrophoblasts and endothelium of chorionic villi.
CTL2 localizes to fetal endothelium of chorionic villi.
Labor does not affect expression levels or localization of CTL1 or CTL2.
Acknowledgments
The authors would like to thank Anita Kramer and Emily Busta for their assistance and technical support. The authors would like to thank Dr. Thomas Jansson for critical review of the manuscript. This work was supported by the Institute for Children’s Mental Disorders, University of Colorado, and the Department of Obstetrics and Gynecology, University of Colorado (Research Award to HKB). Placenta tissue collection was supported by NIH/NCATS Colorado CTSA Grant Number UL1 TR001082. Contents are the authors’ sole responsibility and do not necessarily represent official NIH views. We would like to acknowledge that imaging was made possible by the University of Colorado Advanced Light Microscopy Core, supported in part by NIH/NCRR CCTSI grant UL1 RR025780.
Footnotes
AUTHOR CONTRIBUTIONS
HKB and VDW designed and directed experiments, with significant intellectual input from RGR. HKB and KMT performed Western blot, immunofluorescence, and image analysis. CEG, and AP performed immunofluorescence. TP performed the microarray database analysis. VDW provided tissue samples and expertise in the interpretation of the data. HKB wrote the manuscript, with critical review and edits by VDW and RGR. All authors approved the final version of the manuscript.
CONFLICT OF INTEREST
All authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Heidi K. Baumgartner, Email: Heidi.Wilson@ucdenver.edu.
Kinsey M. Trinder, Email: Kinsey.Trinder@Colorado.edu.
Carly E. Galimanis, Email: Carly.Galimanis@ucdenver.edu.
Annalisa Post, Email: Annalisa.Post@ucdenver.edu.
Tzu Phang, Email: Tzu.Phang@ucdenver.edu.
Virginia D. Winn, Email: vwinn@stanford.edu.
References
- 1.Zeisel SH. Choline: essential for brain development and function. Adv Pediatr. 1997;44:263–295. [PubMed] [Google Scholar]
- 2.Blusztajn JK, Wurtman RJ. Choline and cholinergic neurons. Science. 1983;221(4611):614–620. doi: 10.1126/science.6867732. [DOI] [PubMed] [Google Scholar]
- 3.Zeisel SH, Blusztajn JK. Choline and human nutrition. Annu Rev Nutr. 1994;14:269–296. doi: 10.1146/annurev.nu.14.070194.001413. [DOI] [PubMed] [Google Scholar]
- 4.Buchman AL, Dubin MD, Moukarzel AA, Jenden DJ, Roch M, Rice KM, Gornbein J, Ament ME. Choline deficiency: a cause of hepatic steatosis during parenteral nutrition that can be reversed with intravenous choline supplementation. Hepatology. 1995;22(5):1399–1403. [PubMed] [Google Scholar]
- 5.Zeisel SH, Da Costa KA, Franklin PD, Alexander EA, Lamont JT, Sheard NF, Beiser A. Choline, an essential nutrient for humans. FASEB J. 1991;5(7):2093–2098. [PubMed] [Google Scholar]
- 6.Albright CD, Liu R, Bethea TC, Da Costa KA, Salganik RI, Zeisel SH. Choline deficiency induces apoptosis in SV40-immortalized CWSV-1 rat hepatocytes in culture. FASEB J. 1996;10(4):510–516. doi: 10.1096/fasebj.10.4.8647350. [DOI] [PubMed] [Google Scholar]
- 7.da Costa KA, Gaffney CE, Fischer LM, Zeisel SH. Choline deficiency in mice and humans is associated with increased plasma homocysteine concentration after a methionine load. Am J Clin Nutr. 2005;81(2):440–444. doi: 10.1093/ajcn.81.2.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zeisel SH. Choline: an essential nutrient for humans. Nutrition. 2000;16(7–8):669–671. doi: 10.1016/s0899-9007(00)00349-x. [DOI] [PubMed] [Google Scholar]
- 9.Niculescu MD, Craciunescu CN, Zeisel SH. Dietary choline deficiency alters global and gene-specific DNA methylation in the developing hippocampus of mouse fetal brains. FASEB J. 2006;20(1):43–49. doi: 10.1096/fj.05-4707com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mehedint MG, Craciunescu CN, Zeisel SH. Maternal dietary choline deficiency alters angiogenesis in fetal mouse hippocampus. Proc Natl Acad Sci U S A. 107(29):12834–12839. doi: 10.1073/pnas.0914328107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang Y, Liu Z, Cermak JM, Tandon P, Sarkisian MR, Stafstrom CE, Neill JC, Blusztajn JK, Holmes GL. Protective effects of prenatal choline supplementation on seizure-induced memory impairment. J Neurosci. 2000;20(22):RC109. doi: 10.1523/JNEUROSCI.20-22-j0006.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garner SC, Mar MH, Zeisel SH. Choline distribution and metabolism in pregnant rats and fetuses are influenced by the choline content of the maternal diet. J Nutr. 1995;125(11):2851–2858. doi: 10.1093/jn/125.11.2851. [DOI] [PubMed] [Google Scholar]
- 13.Cermak JM, Holler T, Jackson DA, Blusztajn JK. Prenatal availability of choline modifies development of the hippocampal cholinergic system. FASEB J. 1998;12(3):349–357. doi: 10.1096/fasebj.12.3.349. [DOI] [PubMed] [Google Scholar]
- 14.Meck WH, Williams CL. Simultaneous temporal processing is sensitive to prenatal choline availability in mature and aged rats. Neuroreport. 1997;8(14):3045–3051. doi: 10.1097/00001756-199709290-00009. [DOI] [PubMed] [Google Scholar]
- 15.Stevens KE, Adams CE, Yonchek J, Hickel C, Danielson J, Kisley MA. Permanent improvement in deficient sensory inhibition in DBA/2 mice with increased perinatal choline. Psychopharmacology (Berl) 2008;198(3):413–420. doi: 10.1007/s00213-008-1170-3. [DOI] [PubMed] [Google Scholar]
- 16.Ross RG, Hunter SK, McCarthy L, Beuler J, Hutchison AK, Wagner BD, Leonard S, Stevens KE, Freedman R. Perinatal choline effects on neonatal pathophysiology related to later schizophrenia risk. Am J Psychiatry. 170(3):290–298. doi: 10.1176/appi.ajp.2012.12070940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arrúe A, Dávila R, Zumárraga M, Basterreche N, González-Torres M, Goienetxea B, Zamalloa M, Anguiano J, Guimón J. Neurochem Res Neurochemical Research: 2010. Springer US; 2010. GABA and homovanillic acid in the plasma of schizophrenic and bipolar I patients; pp. 247–253. [DOI] [PubMed] [Google Scholar]
- 18.Edden RECD. Reduced GABA concentration in attention-deficit/hyperactivity disorder. Archives of general psychiatry. 2012;69(7):750–753. doi: 10.1001/archgenpsychiatry.2011.2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gao SF, Bao AM. Corticotropin-Releasing Hormone, glutamate, and g-Aminobutyric Acid in Depression. The Neuroscientist. 2011;17(1):124–144. doi: 10.1177/1073858410361780. [DOI] [PubMed] [Google Scholar]
- 20.Malcolm R. GABA systems, benzodiazepine, and substance dependence. Journal of Clinical Psychiatry. 2003;64(suppl 3):36–40. [PubMed] [Google Scholar]
- 21.Mòhler H. The GABA system in anxiety and depression and its therapeutic potential. Neuropharmacology Anxiety and Depression: 1/2012. 2012:42–53. doi: 10.1016/j.neuropharm.2011.08.040. [DOI] [PubMed] [Google Scholar]
- 22.Vaiva, Boss, Ducrocq, Fontaine, Devos P, Brunet P, Laffargue, Goudemand, Thomas Relationship Between Posttrauma GABA Plasma Levels and PTSD at 1-Year Follow-Up. American Journal of Psychiatry. 2006;163(8):1446–1448. doi: 10.1176/ajp.2006.163.8.1446. [DOI] [PubMed] [Google Scholar]
- 23.Zeisel SH, Mar MH, Zhou Z, da Costa KA. Pregnancy and lactation are associated with diminished concentrations of choline and its metabolites in rat liver. J Nutr. 1995;125(12):3049–3054. doi: 10.1093/jn/125.12.3049. [DOI] [PubMed] [Google Scholar]
- 24.Sweiry JH, Yudilevich DL. Characterization of choline transport at maternal and fetal interfaces of the perfused guinea-pig placenta. J Physiol. 1985;366:251–266. doi: 10.1113/jphysiol.1985.sp015795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grassl SM. Choline transport in human placental brush-border membrane vesicles. Biochim Biophys Acta. 1994;1194(1):203–213. doi: 10.1016/0005-2736(94)90221-6. [DOI] [PubMed] [Google Scholar]
- 26.Muller J, Born I, Neubert RH, Brandsch M. Apical uptake of choline and cationic drugs in epithelial cell lines derived from human placenta. Placenta. 2005;26(2–3):183–189. doi: 10.1016/j.placenta.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 27.Inazu M. Choline transporter-like proteins CTLs/SLC44 family as a novel molecular target for cancer therapy. Biopharm Drug Dispos. 2014;35(8):431–449. doi: 10.1002/bdd.1892. [DOI] [PubMed] [Google Scholar]
- 28.Mikheev AM, Nabekura T, Kaddoumi A, Bammler TK, Govindarajan R, Hebert MF, Unadkat JD. Profiling gene expression in human placentae of different gestational ages: an OPRU Network and UW SCOR Study. Reprod Sci. 2008;15(9):866–877. doi: 10.1177/1933719108322425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kekuda R, Prasad PD, Wu X, Wang H, Fei YJ, Leibach FH, Ganapathy V. Cloning and functional characterization of a potential-sensitive, polyspecific organic cation transporter (OCT3) most abundantly expressed in placenta. The Journal of biological chemistry. 1998;273(26):15971–15979. doi: 10.1074/jbc.273.26.15971. [DOI] [PubMed] [Google Scholar]
- 30.Yuan Z, Tie A, Tarnopolsky M, Bakovic M. Genomic organization, promoter activity, and expression of the human choline transporter-like protein 1. Physiol Genomics. 2006;26(1):76–90. doi: 10.1152/physiolgenomics.00107.2005. [DOI] [PubMed] [Google Scholar]
- 31.Michel V, Yuan Z, Ramsubir S, Bakovic M. Choline transport for phospholipid synthesis. Exp Biol Med (Maywood) 2006;231(5):490–504. doi: 10.1177/153537020623100503. [DOI] [PubMed] [Google Scholar]
- 32.Fullerton MD, Wagner L, Yuan Z, Bakovic M. Impaired trafficking of choline transporter-like protein-1 at plasma membrane and inhibition of choline transport in THP-1 monocyte-derived macrophages. Am J Physiol Cell Physiol. 2006;290(4):C1230–1238. doi: 10.1152/ajpcell.00255.2005. [DOI] [PubMed] [Google Scholar]
- 33.Lee NY, Choi HM, Kang YS. Choline transport via choline transporter-like protein 1 in conditionally immortalized rat syncytiotrophoblast cell lines TR-TBT. Placenta. 2009;30(4):368–374. doi: 10.1016/j.placenta.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 34.Yara M, Iwao B, Hara N, Yamanaka T, Uchino H, Inazu M. Molecular and functional characterization of choline transporter in the human trophoblastic cell line JEG-3 cells. Placenta. 2015;36(6):631–637. doi: 10.1016/j.placenta.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 35.Kommareddi PK, Nair TS, Thang LV, Galano MM, Babu E, Ganapathy V, Kanazawa T, McHugh JB, Carey TE. Isoforms, expression, glycosylation, and tissue distribution of CTL2/SLC44A2. Protein J. 2010;29(6):417–426. doi: 10.1007/s10930-010-9268-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cindrova-Davies T, Yung HW, Johns J, Spasic-Boskovic O, Korolchuk S, Jauniaux E, Burton GJ, Charnock-Jones DS. Oxidative stress, gene expression, and protein changes induced in the human placenta during labor. Am J Pathol. 2007;171(4):1168–1179. doi: 10.2353/ajpath.2007.070528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee KJ, Shim SH, Kang KM, Kang JH, Park DY, Kim SH, Farina A, Shim SS, Cha DH. Global gene expression changes induced in the human placenta during labor. Placenta. 31(8):698–704. doi: 10.1016/j.placenta.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 38.Nakamura T, Fujiwara R, Ishiguro N, Oyabu M, Nakanishi T, Shirasaka Y, Maeda T, Tamai I. Involvement of choline transporter-like proteins, CTL1 and CTL2, in glucocorticoid-induced acceleration of phosphatidylcholine synthesis via increased choline uptake. Biol Pharm Bull. 2010;33(4):691–696. doi: 10.1248/bpb.33.691. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Using GEO GSE9984, the expression values for the known members of the 3 families of choline transporters were extracted, normalized using RMA, and plotted as mean ±SD intensity values. The dash line is at 3.5 as intensity values below this typically represent background.
Western blot analysis (A) and immunohistochemical analysis (B) of CTL1 antibody specificity was tested with normal IgG (rabbit), CTL1 antibody pre-incubated with antigen peptide or CTL1 antibody applied to PDVF membrane or tissue sections. For immunofluorescence, nuclei are seen in blue (DAPI) and IgG or CTL1 is seen in red (CY3 conjugated secondary antibody). The white arrows are pointing to the syncytial trophoblasts (a, b, c).
Western blot analysis (A) and immunohistochemical analysis (B) of CTL2 antibody specificity was tested with normal IgG (mouse) or CTL1 primary antibody applied to PVDF membrane or placenta tissue sections. For immunofluorescence, nuclei are seen in blue (DAPI) and IgG or CTL2 is seen in green (Alexa 488 conjugated secondary antibody). The white arrows are pointing to vessels (a, b).