Abstract
Herbivore-induced plant volatiles (HIPVs) are clues that help predatory insects search for food. The hypothesis that entomopathogenic fungi, which protect plants, benefit from the release of HIPVs was tested. The plant Arabidopsis thaliana was used as the source of HIPVs. The insect herbivore Lipaphis erysimi (Kaltenbach) was used as the inducer, and the fungal pathogen of the aphid Lecanicillium lecanii was exposed to HIPVs to test our hypothesis. When exposed to aphid-induced A. thaliana volatiles, the mortality of aphids pre-treated with a conidial suspension of L. lecanii, the conidial germination and the appressorial formation were significantly increased compared with the control. The decan-3-ol and 4-methylpentyl isothiocyanate that were detected in the headspace seemed to have positive and negative affection, respectively. Moreover, HIPVs generated from groups of eight aphids per plant promoted significantly increased conidial germination and appressorial formation compared with HIPVs from groups of one, two and four aphids per plant. Our results demonstrated that the pathogenicity of the entomopathogenic fungus L. lecanii was enhanced when exposed to HIPVs and that the HIPVs were affected by the number of insect herbivores that induced them.
Introduction
Most plants produce toxic compounds and emit herbivore-induced plant volatiles (HIPVs) for protection when attacked by various insect herbivores [1–7]. As an indirect defensive strategy of the plant, HIPVs can “alert enemies of insect herbivores for help” [7–9]. Some of the compounds emitted as HIPVs that attract or impact predator behavior have been chemically separated and analyzed in several studies. For example, the predatory mite Neoseiulus womersleyi exhibited a significant preference for a mixture of three compounds [(E)-β-ocimene, (E)-4,8-dimethyl-1,3,7-nonatriene, and (E,E)-α-farnesene] in tea leaves [10], whereas tachinid flies were highly attracted to cis-3-hexen-1-ol [11]. Maize that was damaged by lepidopteran pests produced (E)-β-caryophyllene from roots and leaves to attract parasitic wasps that deposit their eggs into the larvae, thus incapacitating the larvae and minimizing plant damage [12]. Methyl salicylate, a major compound emitted in HIPVs, was the key factor that attracted lacewings (Chrysoperla externa) to aphid-infested rose plants [13].
Natural enemies can use HIPVs to locate their hosts or prey [14]. For example, in an experiment conducted using a Y-tube olfactometer, nymphs and adults of experienced mirid bugs, Macrolophus pygmaeus (Rambur), were attracted by HIPVs, which were emitted from prey-infested plants [15]. In another study, some chemical components of HIPVs regulated electrophysiological characteristics and behaviors of wasps [16]. In addition, diamond back moth larvae-induced cabbage-plant volatiles were an important factor that affected the response of the parasitoid, Cotesia vestalis, to different colored lights [17]. HIPVs can also act as a distress signal cue from the damaged plant to the surrounding vegetation [18–20]. For example, HIPVs emitted from blueberry leaves fed upon by the gypsy moth triggered undamaged leaves to accumulate increased amounts of endogenous cis-jasmonic acid compared with non-exposed leaves [21], and it should be the reason that Spodoptera exigua were repelled by the Arabidopsis which primed with HIPVs [22]. Moreover, several resistance marker genes (PATHOGENESIS-RELATED [PR] 1, 2 and 4) in susceptible common bean (Phaseolus vulgaris) were activated by volatile organic compounds (VOCs) from resistant plants [23].
In addition, some research has demonstrated that microorganisms, including fungal pathogens, can be impacted by plant VOCs. Trans-2-hexenal and cis-3-hexenal, green-odor compounds emitted by the green leaves of rice plants have demonstrated a remarkable ability to suppress disease attributed to the rice blast fungus, Magnaporthe oryzae, under laboratory conditions [24]. More recently, VOCs induced from the common bean leaves, including limonene, linalool, nonanal, methyl-salicylate and methyl-jasmonate, directly inhibit conidial germination of the fungal pathogen Colletotrichum lindemuthianum [23]. The germination of the conidia of the entomopathogenic fungus Metarhizium anisopliae was inhibited by the VOCs emitted from highly insect-tolerant cowpea leaves [25]. The effects of HIPVs emitted from plants on entomopathogenic fungi and the potential role of HIPVs as protectants is currently being investigated [26,27]. For example, Brown et al. [28] reported that the germination of conidia of the entomophthoralean pathogen Pandora neoaphidis was inhibited by HIPVs induced by tobacco aphids feeding on tobacco plants. Hountondji et al. [29] demonstrated that HIPVs induced from cassava by the cassava green mite Mononychellus tanajoa influence conidia and capilliconidia production of the fungal entomophthoralean, Neozygites tanajoae. Few studies have investigated the effect of HIPVs or VOCs on Hypocreales [25,30]. In a literature review, Elliot et al. [26] introduced the hypothesis that plants may utilize fungal entomopathogens as protectants. The question of whether plants can increase their own fitness has not been tested in tritrophic systems and remains to be demonstrated [27]. Therefore, research investigating the effect of HIPVs on the pathogenicity of the fungal entomopathogens against any herbivore must be conducted.
A structure that is a precursor to the infection process of pathogenic fungi is the formation of the appressoria, which are swollen, dome-shaped cells [31–33]. In the presence of nutrients and water, conidia of fungi form germ tubes, and the germ tubes form appressoria on the host and non-host cuticle [34]. These structures can also be formed on the surface of glass slides and hydrophobic membranes [35,36]. When conidia are placed on these artificial surfaces, such as glass or plastic, it typically requires 36 to 40 h to complete appressorial formation [37]. However, differentiation in the rate of appressorial formation is affected by various factors, including the substrate surface and abiotic conditions (temperature, humidity, pH value, etc.) [34,35,38]. For example, Spence et al. [39] discovered that the volatile, hydrogen cyanide produced by rice rhizospheric bacteria inhibited appressoria formation in Magnaporthe oryzae.
In this study, we investigated two systems: 1) the HIPV emitting system in which the volatiles were induced by aphids feeding on A. thaliana plants and 2) an entomopathogenic fungal infection system in which the aphid was treated with Lecanicillium lecanii prior to exposure to the aphid-induced HIPVs [40,41]. To evaluate the influence of HIPVs on entomopathogenic fungi, it was hypothesized that A. thaliana would modify its VOCs and emit HIPVs when infested by aphids [42]. Lastly, we compared the performance of the conidia and appressorial formation with or without exposure to aphid-induced HIPVs. Overall, the aim of this study was to understand whether HIPVs enhance or deter the pathogenicity processes of entomopathogenic fungi-infecting herbivores.
Materials and Methods
Organisms
Arabidopsis thaliana was planted at 25°C and 75% relative humidity (RH), under a 16 h light:8 h dark (L:D) photoperiod. Forty-day-old plants were used for the experiments.
The aphid Lipaphis erysimi Kaltenbach was collected from cabbage fields on the Fujian Agriculture and Forestry University (FAFU) campus and bred in netted cages (50 cm × 50 cm × 50 cm) in the laboratory at 25°C, 75% RH, and a 16 h:8 h L:D photoperiod on A. thaliana. Two-day-old apterous adult aphids were used in the experiments.
The fungus Lecanicillium lecanii strain V3450 was isolated from Siphoninus phillyreae Haliday and was stored and maintained at 4°C with Czapek's medium [34] in our laboratory at FAFU until needed.
HIPV-inducing systems and fungal infection testing protocols
Two systems were utilized in the experiment: the HIPV-inducing system and the fungal-infecting system. The systems were used to test the sub-lethal time (LT50) values (d) of the fungus for infection of the insect in multiple HIPVs scenarios (Fig 1). In the first system, adult aphids were used to induce HIPVs. Different numbers of aphids (0, 1, 2, 4, 8 and 16) were transferred to a forty-day-old A. thaliana plant to produce HIPVs. In the second system, a conidial suspension of L. lecanii was used to infect the aphids. Conidia of L. lecanii were cultured with modified Czapek’s medium for 14 d [34], and then the conidia were scraped using an inoculating loop in sterile water containing 0.05% Triton X-80 [43]. The conidial suspension was filtered by cotton in a 20-ml syringe to remove the hyphal debris and then diluted to 5 × 107conidia·mL-1 by using a Neubauer hemocytometer with a sterile solution of 0.1% Tween-80 for infection. All suspensions were shaken using a vortex mixer (Lab dancer, IKA®, India Private Limited, Bangalore, Kamataka, INDIA) for 5 min prior to use. Groups of apterous adult aphids were immersed in the conidial suspension for 10 seconds to inoculate the insects with the fungus. As a control, aphids were treated with 0.05% Triton X-80 alone. Following inoculation, all the aphid groups and controls were air dried for approximately 5 min and then separately transferred into a netted bag (50 mesh, 5 cm × 5 cm). The mortality of the aphids in each treatment was recorded every 24 h, and the experiment was replicated thrice.
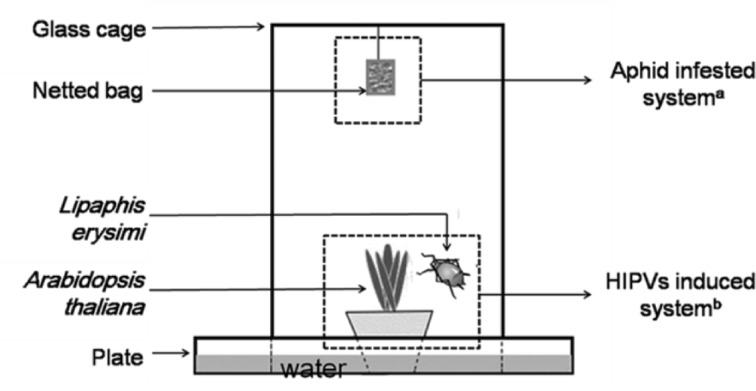
Fig 1. Side view of the HIPVs induced and aphid fungi-infected systems.
a Lecanicillium lecanii infected aphids (Lipaphis erysimi) inside of the netted bags were suspended from the top of the cage while being exposed to HIPVs (aphid-infected system) over time. b Different numbers (0, 1, 2, 4, 8, and 16) of aphids feeding on a potted Arabidopsis thaliana plant inside a glass cage induced the HIPVs (HIPV-induced system).
Four infested plants with the same number (0, 1, 2, 4, 8, or 16) of aphids were transferred into a glass cage (20 cm × 20 cm × 30 cm) to produce different concentrations of HIPVs. A single netted bag containing 50 aphids treated at one of the five fungal concentrations or with Triton X-80 only (control) was hung from the top of the cage (Fig 1). Each HIPV-induced plant containing a specific number of aphids was tested as described above. The individual netted bags per concentration were suspended such that they did not come into contact with the leaves of the A. thaliana plant below them. After a 24-h exposure time inside the cage, all the aphids in the netted bags for each treatment were removed and then transferred to a non-infested A. thaliana plant. The experiment was conducted at 25°C with a 75% RH and 16 h:8 h L:D photoperiod in the laboratory. Three replicate bags were used per concentration and control to determine the LT50 for each treatment. The entire experiment was repeated on four separate occasions.
The effect of plant HIPVs on the germination of conidia
The experiment using the HIPV system was conducted as described above by placing a different number of healthy aphids on an A. thaliana plant. However, instead of attaching a netted bag as described above, a 2.5 μL spore suspension (1 × 108 conidia·mL-1) prepared in Czapek’s-Dox liquid medium was placed on a concave glass slide (concave at both ends of the slide) according to the protocol of Gonzalez et al. [44]. The sample was allowed to dry, and then the flat side of the slide was attached to the top of the glass cage for each HIPV system. The control slide was attached in a Petri dish (90 mm). The conidial germination of L. lecanii was determined every 6 h for the duration of each HIPV system exposure time. Every 6 h, slides were quickly removed, and the conidial germination was observed with a light microscope at 1600X magnification (see Fig 2). Three random areas in each concavity in the slide were observed. Three replicates were performed for each serial treatment (aphid number per plant), and the experiment was conducted on four separate occasions.
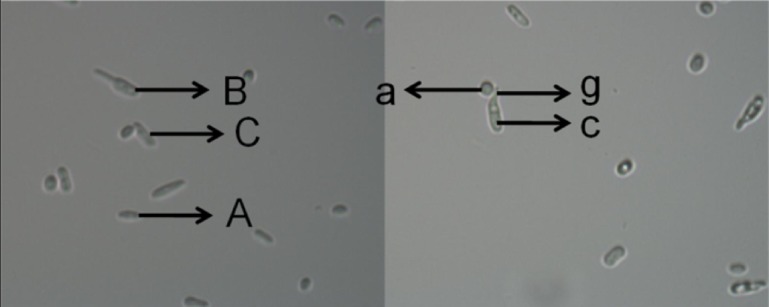
Fig 2. Germination and appressorial formation of L. lecanii conidia as observed on a concave slide after exposure to different concentrations of HIPVs for 12 hours.
i) Non-germinated conidia (A), ii) germinated conidia with no appressoria (B), iii) germinated conidia with appressoria (C), iv) germ tube (g), appressoria (a) and conidia (c).
The effect of plant HIPVs on the formation of appressorium
The evaluation of the HIPV system was conducted as described above by placing a different number of healthy aphids on an A. thaliana plant. A 2.5-μL spore suspension (1 × 108 conidia·mL-1) prepared in Czapek’s-Dox liquid medium was placed into a concave glass slide and then attached to the top of the glass cage as described above. The control slide was pasted in a petri dish (90 mm). The appressorial formation of L. lecanii was determined every 6 h for the duration of each HIPV system exposure time as described above (see Fig 2) to obtain the germination rate. Three replicates for each serial treatment (aphid number plant-1) were conducted per experiment, and the experiment was conducted on four separate occasions.
Headspace collection and analysis of volatiles
Headspace collection was conducted from infested A. thaliana plants during the first 24 h and collected in a system modified from Pineda et al. [45]. Plants were placed into an empty glass jar (12 cm diameter, 30 cm high), and air was forced into the jar after being filtered by activated carbon and silica gel. HIPVs were collected for 6 h by drawing air out of the jars at a rate of 200 to 250 mL∙min−1 by a pump through a glass tube filled with 200 mg Tenax TA. Immediately after collection, the HIPVs that were trapped by Tenax TA were eluted by methenyl trichloride and maintained at -20°C.
HIPVs were analyzed with a gas chromatograph (Agilent Technologies 7890B GC System, United States)-mass spectrometer (Agilent Technologies 5977A MSD, United States) (GC-MS) with HP-5 column (30 m × 0.25 mm i.d., 1.0 μm film thickness, Agilent). The GC oven temperature was programmed to hold at 40°C for 2 min followed by a linear thermal gradient of 10°C∙min−1. The temperature was then increased to 220°C at 5°C∙min−1 and held for 2 min in a column 3 mL∙min−1. The column effluent was ionized by electron impact ionization at 70 eV. Mass spectra were acquired by scanning from 35 to 350 m/z with a scan rate of 5.38 scans∙sec−1.
Compounds were identified by using the deconvolution software AMDIS (version 2.64, NIST, USA) in combination with NIST 05 and Wiley 7th edition spectral libraries and by comparing their retention indices with those from the literature. The relative quantification of each compound is indicated by its peak area [46].
Statistical analysis
The sub-lethal time (LT50) values were analyzed by comparing the longevity of infected aphids and the controls in the same HIPV system using linear regression. For calculations of the conidial germination, appressorium rates and the peak area of each compound, the data were analyzed using analysis of variance (ANOVA), and the significance of mean differences between the treatments was analyzed using the Least Significance Difference test (α = 0.05) in SPSS. Analysis of correlation between the LT50 values of both the germination and the appressorial formation rate of L. lecanii was also determined. All data analyses were conducted using SPSS (2010) (Release 21.0).
Results
Infection of Lecanicillium lecanii on aphids as influenced by HIPVs
Laboratory test results of L. lecanii strain V3450 used against the aphid L. erysimi exposed to different concentrations of HIPVs for 8 d are summarized in Table 1. The natural control mortality was 10% after 8 days. In treatment 1, 2 and 4, mortalities (55.33± 3.06, 60.00 ± 6.00 and 60.67 ± 3.05, respectively) did not show significant differences with each other and significantly higher than that of treatment 0 (48.00 ± 7.21), but significantly lower than that of treatment 8 (77.33 ± 5.78). The mean values of LT50 in all treatments were arranged from low to high, 8 (6.15) < 16 (6.46) < 4 (6.91) <1 (7.09) < 2 (7.11) < 0 (7.87).
Table 1. LT50 and mortality of infected adult L. erysimi exposed to HIPVs for 8 days.
| Treatmentsa | LT50(d)b | 95% Confidence limits | Regression equation | Mortalityc |
|---|---|---|---|---|
| 0 | 7.87 | 7.26~8.76 | y = 7.142x - 13.31 | 48.00 ± 7.21c |
| 1 | 7.09 | 6.61~7.72 | y = 8.865x - 15.64 | 55.33 ± 3.06b |
| 2 | 7.11 | 6.60~7.80 | y = 8.642x - 13.97 | 60.00 ± 6.00b |
| 4 | 6.91 | 6.39~7.61 | y = 8.714x - 11.71 | 60.67 ± 3.05b |
| 8 | 6.15 | 5.77~6.50 | y = 11.12x - 17.90 | 77.33 ± 5.78a |
| 16 | 6.46 | 6.03~6.98 | y = 10.11x - 15.61 | 69.33 ± 7.02ab |
a Six different HIPV concentrations were produced from 0, 1, 2, 4, 8, and 16 aphids feeding on one plant for each group.
b Median lethal time
c Mean ± standard error (SE) of total mortality in 8 days
Germination rate of conidia as influenced by HIPVs
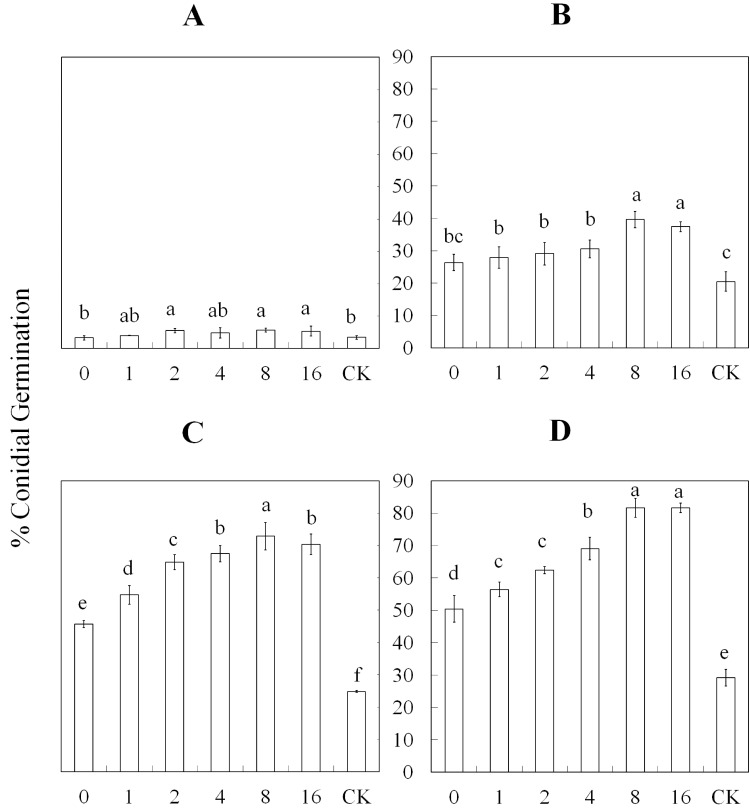
The conidial germination rate after exposure to different concentrations of HIPVs induced by a different number of aphids varied greatly over time (Fig 2, Fig 3 and S1 Table). The germination of conidia in treatment 2, 8, and 16 were increased (F2, 6 = 2.51; P = 0.0728) compared with treatments 0 and 1 and the control (CK) after a 6-h exposure. After a 12-h exposure, treatments 8 and 16 exhibited a significantly increased (F2, 6 = 14.97; P = 0.0001) conidial germination rate compared with the other treatments, including the control and treatment 0 which was slower than all the other treatments. The germination rate of conidia was fastest in treatment 8 and slowest in treatment 0. However, all treatments were significantly faster (F2, 6 = 126.94; P = 0.0001) than the control after an 18-h exposure. Additionally, treatments 4–16 were not significantly different, but they were significantly faster than treatments 0–2. Treatments 8 and 16 exhibited significantly increased (F2, 6 = 130.04; P = 0.0001) germination rates compared with the other treatments and the control after a 24-h exposure to the HIPVs. In general, the germination rates of the conidia after a 12-h exposure to the different concentrations of HIPVs were faster than the control, which did not include aphids.
Fig 3. Percent (± SE) germination rate of L. lecanii conidia after exposure to different concentrations of HIPVs over time.
The HIPVs produced from A. thaliana infested by 0, 1, 2, 4, 8, and 16 aphids, and conidia incubated in clean petri dishes were used as control. The control was abbreviated as CK. Treatments included different exposure times (6, 12, 18 and 24 h) to the induced HIPVs. The germination rate was analyzed after A) 6-, B) 12-, C) 18-, and D) 24-h exposures to the HIPVs. The concentration of L. lecanii was 1×108 conidia·mL-1.
Appressorium formation as influenced by HIPVs
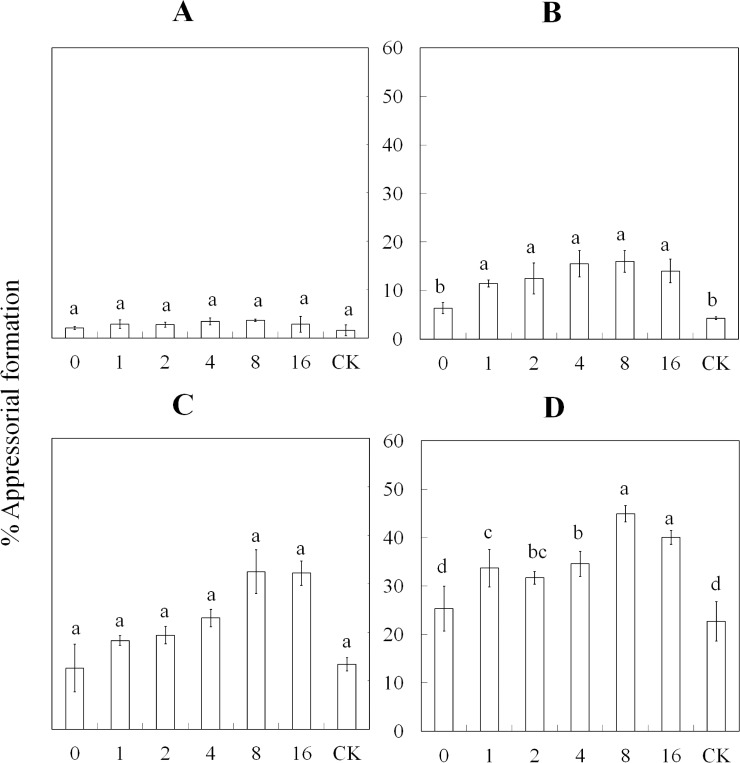
The results of the percent appressorial formation with exposure to HIPVs induced by the different numbers of aphids are presented in Fig 2, Fig 4 and S2 Table. After a 6-h exposure to the HIPVs, no significant differences were noted among all treatments. In treatments 1–16, the percent appressorial formation was significantly increased (F2, 6 = 9.29; P = 0.0003) compared with treatment 0 and the control after a 12-h exposure. At 18 h post-exposure, the appressorium developed faster (F2, 6 = 27.29; P = 0.0001) in treatments 8 and 16 compared with treatments 1–4, which all exhibited similar percent formation but were faster than treatment 0 and the control. Treatments 8–16 were similar, and the percent formation of the appressoria was significantly increased (F2, 6 = 18.44; P = 0.0001) compared with the other treatments after being exposed to HIPVs for 24 h. In addition, the percent appressorial formation was increased in treatments 1–4 compared with treatments 0 and the control; only treatments 0 and the control were similar and had the lowest percent formation compared with the other treatments. In general, after an 18-h exposure to the HIPVs, treatments 8–16 had the highest percentage of appressoria formed compared with the other treatments.
Fig 4. Percent (± SE) appressorial formation of L. lecanii conidia after exposure to different concentrations of HIPVs over time.
The HIPVs produced from A. thaliana infested by 0, 1, 2, 4, 8, and 16 aphids, and conidia incubated in clean petri dishes were used as control. The control was abbreviated as CK. The treatments had different exposure times (6, 12, 18 and 24 h) to the HIPVs. The appressorial rate of formation was analyzed after A) 6; B) 12; C) 18; D) or 24 h exposure. The supernatant concentration of L. lecanii was diluted to 1×108 conidia·mL-1.
Aphid densities modified the profile of HIPVs from A. thaliana
Twelve major compounds were detected in the headspace of A. thaliana that were fed on by aphids. The results indicate that the area peaks of decan-3-ol, benzaldehyde, phenylacetaldehyde, 4-methylpentyl isothiocyanate and α-terpineol could not be detected in treatment 0, whereas 1-octen-3-ol, 6-methyl hept-5-en-2-one, 2,2-dimethyl-1-butanol, limonene and menthol did not exhibit significant differences among the treatments (Table 2 and S1A:, S1B: S1C: S1D: S1E: and S1F Fig). Decan-3-ol was only detected in treatment 16, and the peak area was 119052.67 ± 19982.46 (F2, 0 = 106.48). The peak area of the benzaldehyde detected in treatment 8, was significantly increased compared with treatment 2 and 4 (F2, 3 = 5.73; P = 0.0216). The peak area of phenylacetaldehyde did not exhibit significant differences in the treatments in which phenylacetaldehyde was detected (F2, 4 = 2.328; P = 0.127). The peak area of 4-methylpentyl isothiocyanate in treatment 8 (F2, 3 = 11.96; P = 0.0025) was significantly increased compared with the other treatments. α-Terpineol was only detected in treatments 8 and 16, and no significant difference was noted between the peak areas (F2, 1 = 0.48; P = 0.53). The peak area of 4-methyl-1-penten-3-ol in treatment 0 (F2, 5 = 28.28; P = 0.001) was significantly increased compared with the others treatments, whereas the area of 5-methyl-3-hexanol in treatment 8 (F2, 5 = 11.55; P = 0.001) was significantly increased compared with the other treatments.
Table 2. Major compounds emitted from A. thaliana infested by different densities of L. erysimi in 24 hours.
Data are reported as the mean peak area ± standard deviation (SD) of values of three replicates. Means followed by different letters are significantly different (P < 0.05, one-way ANOVA, Least Significance Difference test), ‘nd’ means undetected.
| Compounds | Insect density | |||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 4 | 8 | 16 | |
| Decan-3-ol | nd | nd | nd | nd | nd | 119052.67±19982.46 |
| Benzaldehyde | nd | nd | 14182.33±4596.23b | 13530.67±3974.81b | 32993.67±11120.47a | 26550.33±5557.76ab |
| Phenylacetaldehyde | nd | 9443.00±2273.06a | 13347.00±5693.95a | 5033.66±3694.87a | 8696.33±1693.89a | 8128.67±1823.38a |
| 4-methylpentyl isothiocyanate | nd | nd | 4973.00±2469.20b | 27511.33±15142.41b | 389790.33±161978.90a | 129853.00±69043.61b |
| α-Terpineol | nd | nd | nd | nd | 44969.33±25078.87a | 34517.67±7737.01a |
| 6-Methyl hept-5-en-2-one | 566773.67±178447.29a | 492802.33±195227.37a | 564150.33±223597.04a | 474091.00±168735.92a | 459241.00±128623.00a | 319327.33±153739.46a |
| 1-Octen-3-ol | 35962±17399.88a | 40329.33±26260.96a | 43139±16189.12a | 48815.33±17158.75a | 49225.33±29073.80a | 41623.67±22858.13a |
| 2,2-Dimethyl-1-butanol | 88603.33±32255.92a | 70132±27931.15a | 73059.67±16764.1a | 83442±9951.98a | 55480.67±22865.95a | 66119±29749.43a |
| Limonene | 5771.67±2402.66a | 5181±2524.47a | 4937.33±3512.66a | 5743±2828.1a | 5373±3179.86a | 11338±7671.69a |
| Menthol | 5113.67±2105.34a | 8152±3329.9538a | 3409.67±1970.45a | 6697.33±3318.06a | 39310±1365.90a | 5405.00±3648.37a |
| 4-Methyl-1-penten-3-ol | 3020657.67±636263.76a | 557273.00±188672.61b | 582240±126145.6903b | 624548.00±253567.94b | 582294.00±142243.97b | 547386.00±303443.08b |
| 5-Methyl-3-hexanol | 5109.33±3700.81c | 14622.33±3945.65bc | 27295.00±17257.87bc | 61919.33±18132.80bc | 294275.33±131252.95a | 117880.33±28869.94b |
Correlation analysis for LT50 and conidia performance
The correlation between pathogenicity and conidial performance of V. lecanii as influenced by HIPVs is summarized in Table 3. The correlation coefficients for both biological processes after exposure to HIPVs were less than -0.5, indicating a significant negative correlation trend over time. The highest negative correlation coefficient between LT50 values and the percent conidial germination was -0.90 after an 18-h exposure to the HIPVs. However, between the LT50 and the formation of the appressorium, the correlation coefficient was much higher (-96) after only a 12-h exposure to the HIPVs.
Table 3. Analysis of correlation between the LT50 values and performance of conidia as influenced by HIPVs.
| Correlation analysis for LT50 and germination rate | Correlation analysis for LT50 and % appressorial formation | |||||||
|---|---|---|---|---|---|---|---|---|
| 6 h | 12 h | 18 h | 24 h | 6 h | 12 h | 18 h | 24 h | |
| Correlation coefficient | -0.69 | -0.92 | -0.97 | -0.95 | -0.86 | -0.92 | -0.97 | -0.99 |
Discussion
The emission of HIPVs are one of the strategies that plants utilize to relieve herbivorous stress by attracting insect enemies and giving warning to nearby plants [47–52]. HIPVs can act as a bridge in the tri-trophic system of the plant-herbivore-predator community [26]. In addition to predators, some microorganisms, which are important natural enemies, can have a positive impact by infecting herbivores that are feeding on the plant. Although some evidence has suggested that the performance and pathogenicity of microorganisms is impacted by the emission of VOCs from plants [23–25], the interaction of entomopathogenic fungi and plants in a tri-trophic system of plant-herbivore-entomopathogenic fungi has not been reported. In our experiment, HIPVs were induced by aphids feeding on a plant, and the volatiles were exposed to contaminated adult aphids that acquired conidia of entomopathogenic fungi. Our results indicated that the percent germination rate and appressorial formation of L. lecanii against L. erysimi within the HIPV induced system was significantly increased compared with that without HIPVs. Significant negative correlations were noted between LT50 values and both the conidial germination rate and appressorium formation of L. lecanii, suggesting that HIPVs can increase the pathogenicity of entomopathogenic fungi by promoting conidial germination or appressorial formation [30,53]. Benzaldehyde and 4-methylpentyl isothiocyanate are probably the chemicals that encourage entomopathogenic fungi according to the headspace analysis.
Insects are the key factor that induce HIPVs; therefore, the composition and emission rate of HIPVs might be influenced by the number, species, age, sex, and physical condition of each insect. Rodriguez-Saona et al. [22] observed that as the perennial shrub Vaccinium corymbosum became gradually infested by gypsy moth (Lymantria dispar) caterpillars over time, the plant VOC emission rate also increased concomitantly. Our results revealed that the exposure of HIPVs emitted from A. thaliana to L. lecanii increased with the subsequent increase in the number of aphids on the plant. In addition, it appears that the concentration of HIPVs did not increase when the number of aphids reached the critical value or asymptote of ≥ 8 aphids per plant for the optimum threshold production of VOCs from this plant. Therefore, it could be hypothesized that the emission rate of HIPVs is proportional to the number of insects present on the plant until the critical number of insect density is reached. Decan-3-ol was detected only in the headspace in the treatment with 16 aphids per plant, and the amount of 4-methylpentyl isothiocyanate in the treatment with 8 aphids per plant was significantly higher than the other treatments. It appears that the emergence of decan-3-ol and/or the decrease of 4-methylpentyl isothiocyanate led to the down-regulation of the activity of the HIPVs inducing the conidial germination of entomopathogenic fungi.
The appearance of an optimum aphid density that is proportional to the emission rate of induced HIPVs may be explained by some physiological processes a plant may employ in response to an ever increasing population of feeding insects. Plants may down-regulate the emission rate of HIPVs or modify the constitution of HIPVs when the critical insect number has reached the VOC production threshold. In addition to the density of insects, the reduction in HIPV emissions may be regulated when a sufficient number of symbiotic bacteria from the oral secretions or honeydew production from these insects deceive the plant into reducing its defensive production of VOCs [54,55]. These hypotheses warrant more research to be confirmed. The emission rate and constitution of HIPVs induced by the different number of insects per plant as well as the mechanism by which conidia are influenced by HIPVs was not investigated in this study.
In conclusion, we provided direct evidence that HIPVs derived from insects functioning as inductors promoted the performance and pathogenicity of conidia of entomopathogenic fungi. In addition, a critical optimal threshold level was determined for aphid density that produced the optimum amount of HIPVs to promote the highest conidial performance of the entomopathogenic fungi. Our results provide important clues that HIPVs play a significant role and provide a bridge in the plant-herbivore-entomopathogenic fungi tri-trophic system. This study also adds a unique dimension to our understanding of how plants provide feedback to their ‘bodyguards’.
Supporting Information
(DOCX)
(DOCX)
A: (Mass spectrogram of the headspace collected from 0-aphid-induced Arabidopsis). B: (Mass spectrogram of the headspace collected from 1-aphid-induced Arabidopsis). C: (Mass spectrogram of the headspace collected from 2-aphids-induced Arabidopsis). D: (Mass spectrogram of the headspace collected from 4-aphids-induced Arabidopsis). E: (Mass spectrogram of the headspace collected from 8-aphids-induced Arabidopsis). F: (Mass spectrogram of the headspace collected from 16-aphids-induced Arabidopsis).
(ZIP)
Acknowledgments
We are grateful to Weibin Fang, Xiaolin Zhang, Mei Wu, Xiaoli Lin and Dr. Baohua Wang for their assistance with collecting and rearing insects, growing A. thaliana, culturing L. lecanii at FAFU and technical assistance, respectively. Reviews by Dr. David Hall (USDA-ARS) and Dr. David Perovic (Institute of Applied Ecology, FAFU) provided constructive criticism for improving the manuscript.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
Funding provided by National Nature Science Foundation of China (NSFC) with grant number 31371998(http://www.nsfc.gov.cn/), LDW received the funding and Research Fund for the Doctoral Program of Higher Education of China with grant number 20123515110003(http://www.cutech.edu.cn/cn/kyjj/gdxxbsdkyjj/A010301index_1.htm), LDW received the funding. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Bramley GN, Waas JR. Laboratory and field evaluation of predator odors as repellents for kiore (Rattus exulans) and ship rats (R. Rattus). J Chem Ecol. 2001;27: 1029–1047. 10.1023/A:1010399322861 [DOI] [PubMed] [Google Scholar]
- 2.James DG. Further field evaluation of synthetic herbivore-induced plan volatiles as attractants for beneficial insects. J Chem Ecol. 2005;31: 481–495. 10.1007/s10886-005-2020-y [DOI] [PubMed] [Google Scholar]
- 3.Tan X, Liu T. Aphid-induced plant volatiles affect the attractiveness of tomato plants to Bemisia tabaci and associated natural enemies. Entomol Exp Appl. 2014;151: 259–269. 10.1111/eea.12190 [DOI] [Google Scholar]
- 4.Erb M, Veyrat N, Robert CAM, Xu H, Frey M, Ton J, et al. Indole is an essential herbivore-induced volatile priming signal in maize. Nat Commun. 2015;6: 6273 10.1038/ncomms7273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pérez-Hedo M, Bouagga S, Jaques JA, Flors V, Urbaneja A. Tomato plant responses to feeding behavior of three zoophytophagous predators (Hemiptera: Miridae). Biol Contr. 2015;86: 46–51. 10.1016/j.biocontrol.2015.04.006 [DOI] [Google Scholar]
- 6.van Veen FF. Plant-modified trophic interactions. Curr Opin. Insect Sci. 2015;8: 29–33. [DOI] [PubMed] [Google Scholar]
- 7.Venkatesan R. Biosynthesis and regulation of herbivore-induced plant volatile emission. J Indian Inst Sci. 2015;95: 25–34. [Google Scholar]
- 8.Frost CJ, Mescher MC, Carlson JE, De Moraes CM. Plant defense priming against herbivores: getting ready for a different battle. Plant Physiol. 2008;146: 818–824. 10.1104/pp.107.113027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rodriguez-Saona CR, Frost CJ. New evidence for a multi-functional role of herbivore-induced plant volatiles in defense against herbivores. Plant Signal Behav. 2010;5: 58–60. 10.4161/psb.5.1.10160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ishiwari H, Suzuki T, Maeda T. Essential compounds in herbivore-induced plant volatiles that attract the predatory mite Neoseiulus womersleyi. J Chem Ecol. 2007;33: 1670–1681. 10.1007/s10886-007-9344-8 [DOI] [PubMed] [Google Scholar]
- 11.Braasch J, Wimp GM, Kaplan I. Testing for phytochemical synergism: arthropod community responses to induced plant volatile blends across crops. J Chem Ecol. 2012;38: 1264–1275. 10.1007/s10886-012-0202-y [DOI] [PubMed] [Google Scholar]
- 12.Farquharson KL. A sesquiterpene distress signal transmitted by maize. Plant Cell. 2008;20: 244 10.1105/tpc.108.200210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salamanca J, Pareja M, Rodriguez-Saona C, Resende ALS, Souza B. Behavioral responses of adult lacewings, Chrysoperla externa, to a rose-aphid-coriander complex. Biol Contr. 2015;80: 103–112. [Google Scholar]
- 14.Vet LEM, Dicke M. Ecology of infochemical use by natural enemies in a tritrophic context. Annu Rev Entomol. 1992;37: 141–172. 10.1146/annurev.en.37.010192.001041 [DOI] [Google Scholar]
- 15.Lins JC, van Loon JJA, Bueno VHP, Lucas-Barbosa D, Dicke M, van Lenteren JC. Response of the zoophytophagous predators Macrolophus pygmaeus and Nesidiocoris tenuis to volatiles of uninfested plants and to plants infested by prey or conspecifics. BioControl. 2014;59: 707–718. 10.1007/s10526-014-9602-y [DOI] [Google Scholar]
- 16.Tamiru A, Bruce TJA, Woodcock CM, Birkett MA, Midega CAO, Pickett JA, et al. Chemical cues modulating electrophysiological and behavioural responses in the parasitic wasp Cotesia sesamiae. Can J Zool. 2015;93: 281–287. 10.1139/cjz-2014-0266 [DOI] [Google Scholar]
- 17.Uefune M, Kugimiya S, Shimoda T, Takabayashi J. Starvation and herbivore-induced plant volatiles affect the color preferences of parasitic wasps. BioControl. 2013;58: 187–193. 10.1007/s10526-012-9483-x [DOI] [Google Scholar]
- 18.Baldwin IT, Kessler A, Halitschke R. Volatile signaling in plant-plant-herbivore interactions: what is real? Curr Opin Plant Biol. 2002;5: 351–354. 10.1016/S1369-5266(02)00263-7 [DOI] [PubMed] [Google Scholar]
- 19.Pierik R, Ballaré CL, Dicke M. Ecology of plant volatiles: taking a plant community perspective. Plant Cell Environ. 2014;37: 1845–1853. 10.1111/pce.12330 [DOI] [PubMed] [Google Scholar]
- 20.Yoneya K, Takabayashi J. Plant-plant communication mediated by airborne signals: ecological and plant physiological perspectives. Plant Biotechnol. 2014;31: 409–416. 10.5511/plantbiotechnology.14.0827a [DOI] [Google Scholar]
- 21.Rodriguez-Saona CR, Rodriguez-Saona LE, Frost CJ. Herbivore-induced volatiles in the perennial shrub, Vaccinium corymbosum, and their role in inter-branch signaling. J Chem Ecol. 2009;35: 163–175. 10.1007/s10886-008-9579-z [DOI] [PubMed] [Google Scholar]
- 22.Tatyana S, Pearse IS, Laura I, Richard K, Katayoon D. Insect herbivores selectively suppress the hpl branch of the oxylipin pathway in host plants. Plant J, 2013;73: 653–662. 10.1111/tpj.12064 [DOI] [PubMed] [Google Scholar]
- 23.Quintana-Rodriguez E, Morales-Vargas AT, Molina-Torres J, Ádame-Alvarez RM, Acosta-Gallegos JA, Heil M. Plant volatiles cause direct, induced and associational resistance in common bean to the fungal pathogen Colletotrichum lindemuthianum. J Ecol. 2015;103: 250–260. 10.1111/1365-2745.12340 [DOI] [Google Scholar]
- 24.Tajul MI, Motoyama T, Hatanaka A, Sariah M, Osada H. Green-odour compounds have antifungal activity against the rice blast fungus Magnaporthe oryzae. Eur J Plant Pathol. 2012;132: 91–100. 10.1007/s10658-011-9851-x [DOI] [Google Scholar]
- 25.Ekesi S, Maniania NK, Lwande W. Susceptibility of the legume flower thrips to Metarhizium anisopliae on different varieties of cowpea. BioControl. 2000;45: 79–95. 10.1023/A:1009927302916 [DOI] [Google Scholar]
- 26.Elliot LS, Sabelis MW, Janssen A, van der Geest LPS, Beerling EAM, Fransen J. Can plants use entomopathogens as bodyguards? Ecol Lett. 2000;3: 228–235. 10.1046/j.1461-0248.2000.00137.x [DOI] [Google Scholar]
- 27.Cory JS, Hoover K. Plant-mediated effects in insect-pathogen interactions. Trends Ecol Evol. 2006;21: 278–286. 10.1016/j.tree.2006.02.005 [DOI] [PubMed] [Google Scholar]
- 28.Brown GC, Prochaska GL, Hildebrand DF, Nordin GL, Jackson DM. Green leaf volatiles inhibit conidial germination of the entompathogen Pandora neoaphidis (Entomophthorales: Entomophthoraceae). Environ Entomol. 1995;24: 1637–1643. 10.1093/ee/24.6.1637 [DOI] [Google Scholar]
- 29.Hountondji FCC, Sabelis MW, Hanna R. The role of infochemicals in the interaction between cassava green mite and its fungal pathogen Neozygites tanajoae In: Sabelis MW and Bruin J, editors. Trends in acarology. Dordrecht: Springer; 2009. pp. 249–253. [Google Scholar]
- 30.Butt TM, Ibrahim L, Clark SJ, Beckett A. The germination behaviour of Metarhizium anisopliae on the surface of aphid and flea beetle cuticles. Mycol Res. 1995;99: 945–950. 10.1016/S0953-7562(09)80754-5 [DOI] [Google Scholar]
- 31.Gachomo EW, Seufferheld MJ, Kotchoni SO. Melanization of appressoria is critical for the pathogenicity of Diplocarpon rosae. Mol Biol Rep. 2010;37: 3583–3591. 10.1007/s11033-010-0007-4 [DOI] [PubMed] [Google Scholar]
- 32.Takano Y, Komeda K, Kojima K, Okuno T. Proper regulation of cyclic AMP-dependent protein kinase is required for growth, conidiation, and appressorium function in the anthracnose fungus Colletotrichum lagenarium. Mol Plant Microbe Interact. 2001;14: 1149–1157. 10.1094/MPMI.2001.14.10.1149 [DOI] [PubMed] [Google Scholar]
- 33.Wang C, St. Leger RJ. The Metarhizium anisopliae perilipin homolog MPL1 regulates lipid metabolism, appressorial turgor pressure, and virulence. J Biol Chem. 2007;282: 21110–21115. 10.1074/jbc.M609592200 [DOI] [PubMed] [Google Scholar]
- 34.Wang L, Huang J, You M, Guan X, Liu B. Effects of toxins from two strains of Verticillium lecanii (Hyphomycetes) on bioattributes of a predatory ladybeetle, Delphastus catalinae (Col., Coccinellidae). J Appl Entomol. 2005;129: 32–38. 10.1111/j.1439-0418.2005.00929.x [DOI] [Google Scholar]
- 35.Hatzipapas P, Kalosak K, Dara A, Christias C. Spore germination and appressorium formation in the entomopathogenic alternaria alternata. Mycol Res. 2002;106: 1349–1359. 10.1017/S0953756202006792 [DOI] [Google Scholar]
- 36.Gourgues M, Brunet-Simon A, Lebrun M, Levis C. The tetraspanin BcPls1 is required for appressorium-mediated penetration of Botrytis cinerea into host plant leaves. Mol Microbiol. 2003;51: 619–629. 10.1046/j.1365-2958.2003.03866.x [DOI] [PubMed] [Google Scholar]
- 37.Weber RWS, Wakley GE, Thines E, Talbot NJ. The vacuole as central element of the lytic system and sink for lipid droplets in maturing appressoria of Magnaporthe grisea. Protoplasma. 2001;216: 101–112. 10.1007/BF02680137 [DOI] [PubMed] [Google Scholar]
- 38.Chakraborty U, Das G, Chakraborty BN. Factors influencing spore germination, appressoria formation and disease development in Camellia sinensis by Glomerella cingulata. Folia Microbiol. 1995;40: 159–164. 10.1007/BF02815415 [DOI] [Google Scholar]
- 39.Spence C, Alff E, Johnson C, Ramos C, Donofrio N, Sundaresan V, et al. Natural rice rhizospheric microbes suppress rice blast infections. BMC Plant Biol. 2014;14: 130 10.1186/1471-2229-14-130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hall RA. A bioassay of the pathogenicity of Verticillium lecanii conidiospores on the aphid, Macrosiphoniella sanborni. J Invertebr Pathol. 1976;27: 41–48. 10.1016/0022-2011(76)90026-4 [DOI] [Google Scholar]
- 41.Askary H, Benhamou N, Brodeur J. Ultrastructural and cytochemical characterization of aphid invasion by the hyphomycete Verticillium lecanii. J Invertebr Pathol. 1999;74: 1–13. 10.1006/jipa.1999.4857 [DOI] [PubMed] [Google Scholar]
- 42.Truong D, Delory BM, Vanderplanck M, Brostaux Y, Vandereycken A, Heuskin S, et al. Temperature regimes and aphid density interactions differentially influence VOC emissions in Arabidopsis. Arthropod Plant Interact. 2014;8: 317–327. 10.1007/s11829-014-9311-6 [DOI] [Google Scholar]
- 43.Ganassi S, Grazioso P, Moretti A, Sabatini MA. Effects of the fungus Lecanicillium lecanii on survival and reproduction of the aphid Schizaphis graminum. BioControl. 2010;55: 299–312. 10.1007/s10526-009-9250-9 [DOI] [Google Scholar]
- 44.Gonzalez LC, Nicao MEL, Muino BL, Perez RH, Sanchez DG, Martinez VL. Effect of six fungicides on Lecanicillium (Verticillium) lecanii (Zimm.) Zare & Gams. J Food Agric Environ. 2012;10: 1142–1145. [Google Scholar]
- 45.Pineda A, Soler R, Weldegergis BT, Shimwela MM, Van loon JJA, Dicke M, et al. Non-pathogenic rhizobacteria interfere with the attraction of parasitoids to aphid-induced plant volatiles via jasmonic acid signalling. Plant Cell Environ. 2013;36: 393–404. 10.1111/j.1365-3040.2012.02581.x [DOI] [PubMed] [Google Scholar]
- 46.Hountondji FC, Sabelis MW, Hanna R, Janssen A. Herbivore-induced plant volatiles trigger sporulation in entomopathogenic fungi: the case of Neozygites tanajoae infecting the cassava green mite. J Chem Ecol. 2005;31: 1303–1021. [DOI] [PubMed] [Google Scholar]
- 47.Arimura G, Kost C, Boland W. Herbivore-induced, indirect plant defences. Biochim Biophys Acta. 2005;1734: 91–111. 10.1016/j.bbalip.2005.03.001 [DOI] [PubMed] [Google Scholar]
- 48.Khan ZR, James DG, Midega CAO, Pickett JA. Chemical ecology and conservation biological control. Biol Contr. 2008;45: 210–224. 10.1016/j.biocontrol.2007.11.009 [DOI] [Google Scholar]
- 49.Dicke M, Baldwin IT. The evolutionary context for herbivore-induced plant volatiles: beyond the 'cry for help'. Trends Plant Sci. 2010;15: 167–175. 10.1016/j.tplants.2009.12.002 [DOI] [PubMed] [Google Scholar]
- 50.Orre GUS, Wratten SD, Jonsson M, Hale RJ. Effects of an herbivore-induced plant volatile on arthropods from three trophic levels in brassicas. Biol Contr. 2010;53: 62–67. 10.1016/j.biocontrol.2009.10.010 [DOI] [Google Scholar]
- 51.Jones VP, Steffan SA, Wiman NG, Horton DR, Miliczky E, Zhang Q, et al. Evaluation of herbivore-induced plant volatiles for monitoring green lacewings in Washington apple orchards. Biol Contr. 2011;56: 98–105. 10.1016/j.biocontrol.2010.10.001 [DOI] [Google Scholar]
- 52.Kaplan I. Attracting carnivorous arthropods with plant volatiles: the future of biocontrol or playing with fire? Biol Contr. 2012;60: 77–89. 10.1016/j.biocontrol.2011.10.017 [DOI] [Google Scholar]
- 53.Hassan AEM, Dillon RJ, Charnley AK. Influence of accelerated germination of conidia on the pathogenicity of Metarhizium anisopliae for Manduca sexta. J Invertebr Pathol. 1989;54: 277–279. 10.1016/0022-2011(89)90040-2 [DOI] [Google Scholar]
- 54.Chung SH, Rosa C, Scully ED, Peiffer M, Tooker JF, Hoover K, et al. Herbivore exploits orally secreted bacteria to suppress plant defenses. Proc Natl Acad Sci U S A. 2013;110: 15728–15733. 10.1073/pnas.1308867110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schwartzberg EG, Tumlinson JH. Aphid honeydew alters plant defence responses. Funct Ecol. 2014;28: 386–394. 10.1111/1365-2435.12182 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
A: (Mass spectrogram of the headspace collected from 0-aphid-induced Arabidopsis). B: (Mass spectrogram of the headspace collected from 1-aphid-induced Arabidopsis). C: (Mass spectrogram of the headspace collected from 2-aphids-induced Arabidopsis). D: (Mass spectrogram of the headspace collected from 4-aphids-induced Arabidopsis). E: (Mass spectrogram of the headspace collected from 8-aphids-induced Arabidopsis). F: (Mass spectrogram of the headspace collected from 16-aphids-induced Arabidopsis).
(ZIP)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.