Abstract
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is a valuable therapeutic strategy for a wide variety of diseases. Acute graft-versus-host disease (aGVHD) is a major complication in up to 75% of allo-HSCT. The absence of a reliable predicative marker for aGVHD onset prevents preemptive treatment and impedes widespread and successful application of this therapy. In this study we found that after allo-HSCT, the levels of miR-181a were reduced significantly prior to the onset of aGVHD. More importantly, the degree of its reduction correlated with the severity of aGVHD. Mechanistically, miR-181a affects the function of T lymphocytes by down-regulating IFN-γ in a dose-dependent manner. Meanwhile, we confirmed that miR-181a can effectively preserve the anti-leukemic effect in vitro. Using a murine allo-HSCT model, we demonstrated that murine miR-181b, the human miR-181a homolog, served as an effective predictor of aGVHD. Moreover, expression of this microRNA ameliorated the severity of aGVHD. Collectively, these results show that the level of miR-181a may serve as a reliable marker for the diagnosis and prognosis the onset of aGVHD.
Keywords: stem cell transplantation, graft-versus-host disease, haematological malignancy, T cells
Introduction
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is one of the most effective therapies for a variety of diseases. Unfortunately, up to 75% of patients develop acute graft-versus-host disease (aGVHD) after allo-HSCT [1]. Consequently, this is one of the major barriers to more widespread and successful application of allo-HSCT. It is known that donor-derived T lymphocytes are the primary effector cells responsible for triggering aGVHD, and mechanistically an enhanced Th1 response is considered the leading cause of aGVHD [2-4]. However, the exact mechanisms involved in aGVHD development remain largely unknown. Currently, the diagnosis and treatment of the aGVHD patients rely almost entirely on the presence of clinical symptoms which arise after organ damage has already occurred. The absence of reliable markers for the prognosis of aGVHD impedes effective interventions to those patients who may later develop aGVHD. Therefore, the identification and application of such predicative markers may facilitate use of preemptive treatment to prevent the development of aGVHD, thus rendering allo-HSCT a more applicable and valuable therapy.
MicroRNAs (miRNAs) regulate gene expression by binding to the 3’ untranslated region (UTR) of target genes [5-6]. Multiple lines of evidence indicate that altered levels of miRNAs are involved in the development of different diseases. For instance, miR-146a is related to Th1 response-mediated immune damage [7]; miR-142-3p is correlated with the occurrence of systemic sclerosis [8]; and miR-126 is significantly up-regulated in patients with systemic lupus erythematosus [9]. Additionally, miRNAs are involved in regulation of T lymphocyte function in immune response. MiR-326 can promote the differentiation of Th0 cells into Th17 cells [10]; miR-155 promotes the proliferation of Treg cells [11]; miR-146a is important for the function of regulatory T cells (Treg) [7]; and miR-181a can inhibit negative regulatory factors in the T-cell receptor signaling pathway [12]. During the differentiation and maturation of T and B cells, miR-150 has been shown to play a role in regulating the lifespan of mature T and B cells [13, 14]. The miR-17-92 cluster regulates the maturation of T lymphocytes and thymic selection [15, 16]. The miR-146 family may play negative roles in the regulation of immune response [17, 18]. Most importantly, some miRNAs are used as reliable biomarkers for the diagnosis and prognosis of diseases. The down-regulation of miR-15 and miR-16 is a characteristic marker in the diagnosis of chronic lymphocytic leukemia whereas high levels of miR-155 and low levels of let-7a-2 are important diagnostic factors in patients with lung cancer [19, 20].
In this study, we found that miR-181a regulates the levels of cytokines which are directly involved in the development of aGVHD, with plasma levels significantly reduced prior to the onset of the disease. Most importantly we demonstrated that the degree of the reduction of miR-181a correlated with the severity of aGVHD. In addition, we also confirmed that miR-181a can preserve anti-leukemic effect while controlling aGVHD. These findings were further confirmed in a mouse allo-HSCT model. To our knowledge, this is the first report that miR-181a can serve as a predicative marker for aGVHD, potentially providing a window for the preemptive treatment of aGVHD. In addition, results from the experiments conducted in the mouse model indicate that manipulation of miR-181a levels may be a potential strategy in prevention of aGVHD.
Methods and Materials
Human study protocols were approved by the Institutional Review Board of the Affiliated Hospital of Xuzhou Medical College (XYFY2800211). The signed consents were obtained from patients and donors or their surrogates. All animal experimental procedures were approved by the Laboratory Animal Ethics Committee of Xuzhou Medical College (2012023).
Samples
Peripheral blood samples were obtained once a week from 27 patients, without or with grades II, III and IV aGVHD, who were recipients of allo-HSCT in the Affiliated Hospital of Xuzhou Medical College from 2007 to 2011. Ten healthy age-matched donors serve as control subjects. Plasma and PBMCs were separated from the peripheral blood within 6 hours centrifuged in Lymphocyte Separation Medium (SCR, Shanghai, China) in a volume ratio of 1:1 for 15 min at 400g and room temperature. CD4+ T lymphocytes were then purified via magnetic cell sorting (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufactures’ instructions. All samples were collected and stored at −80°C for future usage.
Quantitative Real-time PCR (qRT-PCR)
Total RNA was isolated from human CD4+ T lymphocytes and plasma using TRIZOL (Invitrogen, Carlsbad, CA, USA) following the manufacturer's instructions. Reverse transcriptions were performed using miScript Reverse Transcription kit (Qiagen, Hilden, Germany). QuantiTect SYBR RT-PCR Kit (Qiagen) was used for qRT-PCR analyses according to the manufacturer's instructions. U6 small RNA (TaKaRa, Japan) served as the endogenous control. Fold changes were calculated using the 2(-Delta Delta C(T)) method according to the manufacturer's protocol. The PCR primer sequences are listed in Supplemental Table 1.
Mice and cells
C57BL/6 (H2b) and BABL/c (H2d) mice, 8 to 10 weeks old, were purchased from the Shanghai Laboratory Animal Center and maintained in pathogen-free conditions. For experiments in vitro, naïve CD4+CD62L+ T helper cells of human and mice were purified by magnetic cell sorting from PBMCs of age-matched healthy volunteers and spleens/PBMCs of C57BL/6 (H2b). Isolated cells were maintained in 1640 medium, 10% FBS and specific reagent were added in different experiment settings.
aGVHD murine models
All mice were 8-10 wk of age. Recipient mice (BABL/c (H2d)) were irradiated with 8.0 Gy. The first group of mice received irradiation only (TBI group), the second group received T cell-depleted BM cells only (1×107) (BM group), the third group received both T cell-depleted BM cells (1×107) and splenocytes (2×107) from C57BL/6 (H2b) donors via tail vein injection (BM+S group). For other allo-HSCT transplantation experiments, lentivirus vector expressing pre-miR-181b (LV-181b) was infected along with T cell-depleted BM cells and splenocytes (BM+S (LV-181b) group), empty lentivirus vector (LV-Ctrl) was used as control (BM+S (LV-Ctrl) group). We were interested in determining whether reductions in miR-181b levels could be associated with development of aGVHD in this animal model. Consequently, levels of miR-181b were sampled individually in a group of 50 mice during the time predicted to be at maximum risk of aGVHD onset (days 5-10 post transplant). Since these results were similar to those seen in our patients, we studied an additional set of 50 mice to investigate the possible clinical utility of reduction of miR-181b levels to guide preemptive therapy aimed at preventing aGHVD occurrence. Similar to the experimental design above, individual mice were sampled once from days 5 to 10 post transplant. Samples obtained at day 2 post transplant served as control. Mice determined to have significant reduction in miR-181-b levels were then treated with dexamethasone (2.5 ug/per gram of body weight daily for 3 days by intraperitoneal injection). These mice were assigned to the BM+S(DM) group. The aGVHD score was assessed according to the Cooke Criteria [21], which includes 5 parameters: weight loss, activity, posture, fur texture, and skin integrity. Each mouse was ear tagged and graded every two days. Mice having a score of more than or equal to 7 were thought to be very sick, then they were euthanized and the tissues were harvested.
Cytotoxic assay in vitro
PBMCs or CD4+ T cells isolated from health donors were transfected with either LV-Ctrl, LV-181a, or mock transfection. 1×104 cells/well of the target cells (HEL 60 and KU812) (ATCC, Manassas, VA, USA) were co-cultured with effector cells at E/T (effector/target) ratio of 40:1, 20:1, 10:1 and 1:1 at 37°C for 12 hours before 10 μl of CCK-8 (DIJINDO, Japan) was added to each well and incubated for another 3 hours. The OD490nm were measured using a Thermo Scientific Microplate Reader. The cytotoxicity was determined by the formula: % cytotoxicity = 1 - [(absorbance at 450 nm of effector + target cells) - (absorbance at 450 nm of effector cells)]/[absorbance at 450 nm of target cells] ×100%. After 48h of co-culturing, the morphological alterations were analyzedunder a light phase contrast microscope (Axiovert 40®, Carl Zeiss Argentina). The apoptosis cells were detected by FACS (San Diego, CA,USA) with the target cells labeled with anti-CD33-FITC (BioLegend, California, USA) and apoptosis cells labeled with 7-AAD(eBioscience, San Diego, CA).
Lentivirus-mediated overexpression of miR-181a/b and infection
Lentiviral Production and Transduction Virus particles carrying the has-pre-miR-181a precursor, mmu-pre-miR-181b precursor and respective controls were purchased from GENECHEM Company (GENECHEM, Shanghai, China). The package of recombinant lentiviruses was carried out according to the suggested procedure from GENECHEM. The expression levels of miR-181a and miR-181b in cells were validated by qRT-PCR. For lentivirus infection experiments, concentration gradient experiment in vitro, naïve CD4+CD62L+ helper T cells (2×105, human) activated by anti-human CD3 (OKT3), (Biolegend, San Diego, CA, USA) and anti-human CD28 (CD28.2, Biolegend) were infected with 6μl polybrene, centifugated at 150g for 4h and incubated at 37°C for 48h. Transfection of LV-empty was used as negative control and transfection without vector served as a mock control. Different transform units (0, 5, 10, 20, 30×106) of LV-181a were assigned as LV-181a. Co-trasfection of LV-181a and lentivirus expressing INF-γ was assigned as LV-181a+IFN-γ. For murine assays, about 2×107 transforming units of LV-181a and LV-181b were injected through tail vein of C57BL/6 (H2b) mice, respectively. LV-empty was injected as controls.
Th1/Th2/Th17 Cytokine Secretion Assays
Cytokines (IFN-γ, IL-2, IL-4, IL-6, IL-10, IL17A and TNF) in plasma from human and mice were quantified by BD Cytometric Bead Array (CBA) Human/mouse Th1/Th2/Th17 Cytokine Kit (BD Bioscience, San Jose, California, USA) following the manufacturers’ instructions. Briefly, Th1/Th2/Th17 Cytokine Standards were prepared; samples were mixed with Th1/Th2/Th17 Cytokine Capture beads at dilution of 1:4. The Th1/Th2/Th17 Cytokine Assay was then performed, with samples analyzed using BD FACSCalibur by flow cytometry with gating on FSC/SSC.
Luciferase reporter assays
HEK293T cells (ATCC, Manassas, VA, USA) were maintained in DMEM (GIBCO, Gaithersburg, MD, USA) and cells of 80% confluence in 24-well plates were transfected with vector GV268 containing sequences of miR-181a (human)/181b (mice) or empty vector as control and lipofectamine 2000 (Invitrogen). A firefly luciferase reporter gene vector containing either wild-type or mutated 3'UTR sequences of IFN-γ (human and mice, respectively) were co-transfected. Cell extracts were prepared 24 h after transfection and luciferase activity was measured with the Dual-Luciferase Reporter Assay system (Promega, Madison, WI, USA) according to the manufacturers’ instructions.
Intracellular staining and flow cytometry
For CD4+ T helper lymphocytes subsets (Th1, Th2, and Th17) assays, naive CD4+CD62L+ helper T cells isolated from healthy donors or mice (BABL/c) were activated using PMA (50 ng/ml) (Sigma, St.Louis, MO, USA) and ionomycin (750 ng/ml; Sigma) and fixed with brefeldin A (10 μg/ml; Sigma). Cell surface staining was conducted with anti-human CD3-PerCP/Cy5.5 (Biolegend) or CD4-FITC (Beckman) and anti-mouse CD3-PerCP/Cy5.5 or CD4-FITC (Biolegend), respectively. After surface staining, cells were resuspended in Fixation/Permeabilization solution and intracellular cytokine staining (Abs: APC anti-human IFN-γ (B27), PE anti-human IL-4 (8D4-8), PE anti-human IL-17A (BL168), PE anti- mouse IFN-γ (XMG1.2 ), Biolegend) was conducted according to the manufacturer's protocol. For Foxp3 Staining, activated cells were subsequently fixed and permeabilized using the Foxp3/Transcription Factor Staining Buffers (cat. 00-5523) and stained with PE anti-human Foxp3(206D, Biolegend), Mouse IgG1 K Isotype Control PE (cat. 11-4714) was used as control. Th1, Th2, Th17 and Treg subsets were analyzed using a FACS Calibur software for flow cytometry, gated on anti-CD3-PerCP/Cy5.5 and anti- CD4-FITC.
Proliferation and Apoptosis assays
Cells from in vitro culture were harvested and assessed for proliferation by [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt; MTS] (Promega) in 96 well plate, The OD490nm were measured using a Thermo Scientific Microplate Reader. For apoptosis, the Annexin V and 7-AAD (eBioscience, San Diego, CA, USA) were used to differentiate apoptotic cells from normal and necrotic cells using the 490nm wavelength of the flow cytometer. Each assay was performed according to the manufacturer's instructions and repeated three times independently.
Cell and tissue histopathology
Human CD4+ T cells activated by PHA (Sigma) for 24h and 48h were collected and stained with H&E. Liver and small bowel specimens of recipient mice were cut to appropriate size and fixed in 10% formalin, dehydrated in an ethylalcohol series, embedded in paraffin, sectioned at 6 um and stained with H&E. The severity of GVHD was graded from o (absence of GVHD) to 4 (severe GVHD) by detailed histopathology analysis of liver and bowel according to previously reported scoring system [22].
Western blotting
The protein of cell lysates (treated with brefeldin A before lysis) was quantified using a BCA Protein Assay Kit (TIANGEN, Beijing, China) and subjected to SDS-PAGE. Labeled bands were detected using the ECL chemiluminescent kit (TIANGEN). Anti-human IFN-γ monoclonal (clone B27; Biolegend), anti-mouse IFN-γ monoclonal (clone XMG1.2; Biolegend) were used. Monoclonal anti-β-actin (1:5000), (Kangchen, Shanghai, China) was used for loading control.
ELISA
Secreted IFN-γ, IL-4 and IL-17 in cell culture media after in vitro stimulation with PHA (human) and ConA (mice) (sigma) for 48h was quantified by commercial sandwich ELISA kits (eBioscience) according to the manufacturer's instructions. The OD450nm of samples was measured using the Thermo Scientific Microplate Reader for ELISA. Each group was made in triplicate using standard curves and IFN-γ levels were expressed in pg/ml.
Statistical considerations
All values are expressed as the mean±SD. A two side-χ2 test (Fisher exact test) was used to compare the difference of the clinical characteristics of patients with aGVHD and non-aGVHD. Spearman correlate analysis was used to test whether the expression levels of miRNAs were related to the degree of aGVHD severity. The Wilcoxon rank-test was performed in order to analyze survival data. Other statistical comparisons were completed using Student's t test with p < 0.05 considered statistically significant.
Results
Differential expressions of selected miRNAs in individuals with aGVHD
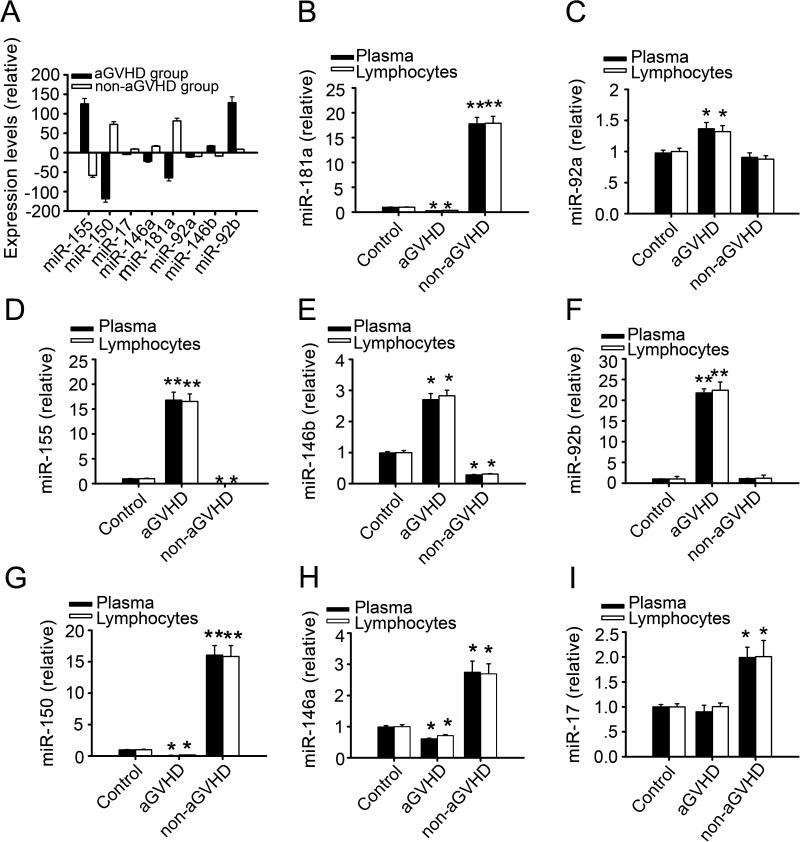
Healthy volunteers and patients without or with grade II, III and IV aGVHD as categorized according to the classic Glucksberg-Seattle criteria (GSC) were selected within 100 days post allo-HSCT (Table1) to participate in this study [23, 24]. qRT-PCR assays were conducted in order to evaluate miRNA levels in CD4+ T lymphocytes purified from the peripheral blood of eight candidate. Four miRNAs in the aGVHD group were differentially expressed (Figure 1A); miR-150 and miR-181a were significantly down-regulated, while miR-155 and miR-92b were markedly up-regulated. In the non-aGVHD group, miR-181a expression levels were significantly increased and miR-155 expression levels were greatly down-regulated. No significant differential expressions of miR-92b or miR-92a were evident. We also examined the expression levels of these miRNAs in the plasma of patients (Figure 1 B-I) and found the patterns similar to those observed in the CD4+ T lymphocytes (p < 0.05).
Table 1.
Patient characteristics
| Characteristic | Controls | Patients |
P | |
|---|---|---|---|---|
| Non-GVHD | GVHD | |||
| Sample size | 10 | 27 | ||
| Median age | 29 years(19-48) | 32 years(16-52) | ||
| Sex | ||||
| Male | 6(60%) | 16(59.3%) | ||
| Female | 4(40%) | 11(40.7%) | ||
| Type of transplant | 0.616 | |||
| Volunteer unrelated donor | - | 3 | 16 | |
| Sibling allogeneic | - | 2 | 6 | |
| Infection | 0.636 | |||
| Yes | 1 | 8 | ||
| No | 4 | 14 | ||
| Source of stem cells | 1.000 | |||
| Peripheral blood | - | 4 | 17 | |
| Bone marrow and peripheral blood | - | 1 | 5 | |
| Conditioning | ||||
| BU/CY2 | - | 27 | ||
| aGVHD | ||||
| II | 9 | |||
| III | 7 | |||
| IV | 6 | |||
| aGVHD prophylaxis | ||||
| CSA and MTX and MMF | - | 27 | ||
Relevant information about human subjects in this study. Sample size is total number of subjects; Sex is total number (with percent of group in parentheses); “-” indicates “not applicable”; Abbreviations: BU (busulfan) CY (cyclophosphamide), CSA (cyclosporin A), MTX (methotrexate), MMF (mycophenolate mofetil).
FIGURE 1. Different expression levels of selected miRNAs in individuals.
Blood samples were obtained from patients either nine weeks after allo-HSCT without aGVHD or the day of the aGVHD onset. The isolated CD4+ T lymphocytes and plasma were used for RNA purification and micoRNA quantification. (A) Quantification of the eight selected miRNAs by qRT-PCR. The 2(-Delta Delta CT) method was used to quantitate miRNAs expression levels and the levels of the miRNAs from healthy volunteers served as controls. All data were normalized to controls and had statistical significance (p < 0.05). (B-I) Normalized relevant expression levels of eight miRNAs in plasma and CD4+ T lymphocytes of patients with or without aGVHD. * p < 0.05, ** p < 0.01. All values represented are mean±SD.
Th1 responses play a pivotal role in aGVHD
The enhanced Th1 immune response and reduced immune tolerance mediated by Th2 and Treg are the main mechanisms implicated in the development of aGVHD [25-28]. We examined the expression of relevant cytokines in the plasma from patients to further explore the potential mechanisms involved in the development of aGVHD (Supplemental Figure 1). Our results showed that IL-2, IFN-γ, TNF and IL-17A were significantly up-regulated in the aGVHD group. Furthermore, the levels of cytokines such as IL-10 and IL-4 were significantly decreased, while the level of IL-6 showed no significant change. In the non-aGVHD group, IL-2, IFN-γ and TNF were significantly down-regulated, but no significant changes in the levels of IL-10, IL-4, IL-17A and IL-6 were observed. However, the protein levels of different cytokines (i.e., IFN-γ, IL-2, TNF and IL-10) were not changed in patients prior to the onset of aGVHD (Supplemental Figure 2A).
Specific miRNA expression profiles could be potential predictors of the aGVHD onset
Our results suggest that the levels of some cytokines correlated to the severity of aGVHD. Further experiments were performed in order to assess whether the plasma levels of miRNAs correlated with the severity of aGVHD. We found that miR-150 and miR-181a were up-regulated in the non-aGVHD group and that their levels negatively correlated with aGVHD occurrence. No significant change in miR-92b expression in the non-aGVHD group was observed and its levels positively correlated with aGVHD severity. MiR-155 was significantly down-regulated in the non-aGVHD group and its levels also positively correlated with aGVHD severity (Supplemental Figure 2B). Importantly, we found that the levels of the miRNAs were significantly altered prior to the onset of aGVHD (Supplemental Figure 2C). Of all 27 patients included in the group, the 5 patients who did not develop aGVHD revealed no change of their levels of miR-181a. Reduction of the miR-181a was observed in 86% (19 of 22) of patients who developed aGVHD (Supplemental Figure 2C and Supplemental Table 2). Reduction of these levels occurred ranging from days −1 to day −7 prior to aGVHD onset (Supplemental Table 2). Since blood draws were performed on a weekly schedule, it is not possible to determine precisely when reduced levels of miR-181a occur prior to development of aGVHD. Of note was that the three patients who developed aGVHD but had no observable reduction in miR-181a levels had samples collected at days −5, −6, and −7. It is conceivable that these levels might have dropped later, at a time closer to the onset of aGVHD. This is a potential explanation for the three false negative results. These findings indicate that the levels of miRNAs, but not cytokines, could be used as potential biomarkers in prognosis of the onset of aGVHD.
MiR-181a directly targets IFN-γ
Based on the search of three databases (i.e. miRbase, miRranda and Targetscan), we selected 51 genes, present in all three, for pathway and gene ontology analyses. We found that 19 miR-181a target genes may be potentially involved in transplantation immunity-related signaling pathways such as the T-cell receptor pathway, antigen presentation and GVHD (data not shown). Fluorescence quantitative PCR was conducted to assess the mRNA levels of these target genes. We found that five of them, including IFN-γ, were significantly down-regulated (data not shown). We had previously confirmed that the levels of IFN-γ, a Th1 cytokine, are positively correlated with aGVHD severity. As shown in Supplemental Figure 3A, the 3'UTR of the human IFN-γ gene is a perfect match for the miR-181a seed sequence. Co-transfection of a vector expressing miR-181a with the luciferase reporter vector containing the wide-type IFN-γ 3'UTR decreased the luciferase activity significantly. However, overexpression of miR-181a had no significant effect on the luciferase activity when the reporter contained the mutated 3'UTR of the IFN-γ gene (Supplemental Figure 3B). Furthermore, infection of the activated human CD4+ T lymphocytes with lentivirus encoding pre-miR-181a (LV-181a) (Supplemental Figure 3C) had no effect on the level of IFN-γ mRNA (Supplemental Figure 3D), but did significantly decrease the protein level of this cytokine in both CD4+ T cells and culture media, as quantified by Western blot and ELISA, respectively (Supplemental Figure 3 E-F). These data collectively demonstrate that IFN-γ is directly targeted by miR-181a.
Up-regulation of miR-181a controls Th1 response in vitro
A series of experiments were conducted to confirm that IFN-γ is regulated by miR-181a as well as to further assess its subsequent effect on cellular function. First, we infected the activated CD4+ T lymphocytes with LV-181a and evaluated the population of each T cell subset (Figure 2A). Compared to those in the control group, the Th1- and Th17-cell subsets in the cells infected with LV-181a were significantly reduced while the Th2- and Treg-cell subsets were markedly increased. Likewise, we found the levels of secreted IFN-γ and Il-17 to be reduced whereas levels of IL-4 were significantly increased in the LV-181a group (Figure 2B). Furthermore, overexpression of miR-181a significantly decreased cell proliferation and increased apoptosis in CD4+ T cells. However, after restoration of the IFN-γ expression in CD4+ T cells, no significant differences in the differentiation, proliferation, or apoptosis of T cell subsets were evident (Figure 2C). These findings, reduced cell numbers and increased apoptosis in the LV-181a group with rescue by IFN-γ, were confirmed by cytomorphology (Figure 2D). These results collectively suggest that miR-181a affects the differentiation of naïve CD4+ T lymphocytes by targeting IFN-γ, inhibiting cell proliferation, and promoting apoptosis, therefore affecting T lymphocyte function.
FIGURE 2. Up-regulation of miR-181a effectively controls Th1 responses in vitro.
(A) Intracellular staining of IFN-γ, IL-4, IL-17 and Foxp3 in CD4+ T cells infected with LV-Ctrl, LV-181a, and LV-181a+IFN-γ. Mock infected CD4+ T cells served as negative control. Cells were examined by intracellular staining 3 hours after stimulation with PMA and ionomycin after fixation by brefeldin A. Data are representive of three independent experiments. (B) ELISA analysis of cytokines (IFN-γ, IL-4 and IL-17) in culture media of CD4+ T cells according to the protocol described in Materials and Methods. Levels of IFN-γ and IL-17 were significantly decreased while IL-4 was significantly increased. (C) Analysis of proliferation (MTS) and apoptosis (Annexin V and 7-AAD) of CD4+ T cells 3 hours post-stimulation with PMA, ionomycin and fixation with brefeldin A. In cells overexpressing miR-181a, the OD value measured at 490nm of CD4+ T cells was significantly lower while Annexin V and 7-AAD positive cells was higher; these changes returned to normal when IFN-γ was re-expressed. (D) Representative H&E straining of human CD4+ T cells activated by PHA for 24 and 48 hours. Final magnification 100× (Olympus, DP-2-BSW). ** p < 0.01, *** p < 0.001. All values represent mean±SD. Data are from three independent experiments.
MiR-181a-mediated down regulation of IFN-γ is concentration-dependent
The concentration and abundance of miRNAs determine their cellular functions [16, 29, 30]. Since the concentrations of miR-181a and cytokines were associated with the severity of aGVHD, we decided to investigate whether miR-181a-mediated down regulation of IFN-γ is concentration-dependent. A defined amount of CD4+ T lymphocytes were infected with different titers of LV-181a and the IFN-γ levels were estimated (Supplemental Figure 4A). Increasing levels of miR-181a had no effect on the levels of IFN-γ mRNA (Supplemental Figure 4B); however, protein levels of IFN-γ in cells and in culture media were incrementally decreased and negatively correlated with the levels of the miR-181a (Supplemental Figure 4 C-D). This indicates that the miR-181a-mediated post-transcriptional regulation of IFN-γ is indeed concentration-dependent.
MiR-181a preserved anti-leukemic effect of lymphocytes in vitro
To determine the effect of miR-181a on anti-leukemia effect in vitro, we co-cultured the leukemic cells (HEL60 cells and KU812 cells) with either CD4+T cells or PBMCs at different ratio of E/T. We found that with the increased ratio of E/T, the cytotoxicity increased accordingly. Since the observed effect plateaued with the E/T ratio at or above 40:1 (Supplemental Figure. 5A), the E/T ratio of 20:1 was used in the subsequent study of the anti-leukemic effect of CD4+T cells and PBMC infected with LV-181a. Results from this experiment showed that miR-181a decreased the lymphocyte-induced toxicity markedly, and reduced the PBMC-induced cytotoxicity marginally (Supplemental Figure. 5B). As shown in Supplemental Figure 5C and 5D that miR-181a preserved the anti-leukemic effects of both CD4+ T cells and PBMC, albeit the effect on CD4+ T cells is lesser. The apoptosis rate of the leukemia cells were estimated when the cells were co-cultured with CD4+T cells or PBMCs infected with LV-181a. The results showed that miR-181a partially reduced both CD4+ T cell- and PBMC-induced leukemic cell apoptosis, although this enhancement on the PBMC is less significant (Supplemental Figure 5E). However, these data collectively demonstrated that miR-181a effectively preserved the anti-leukemic effects.
MiR-181b down regulating IFN-γ in mice
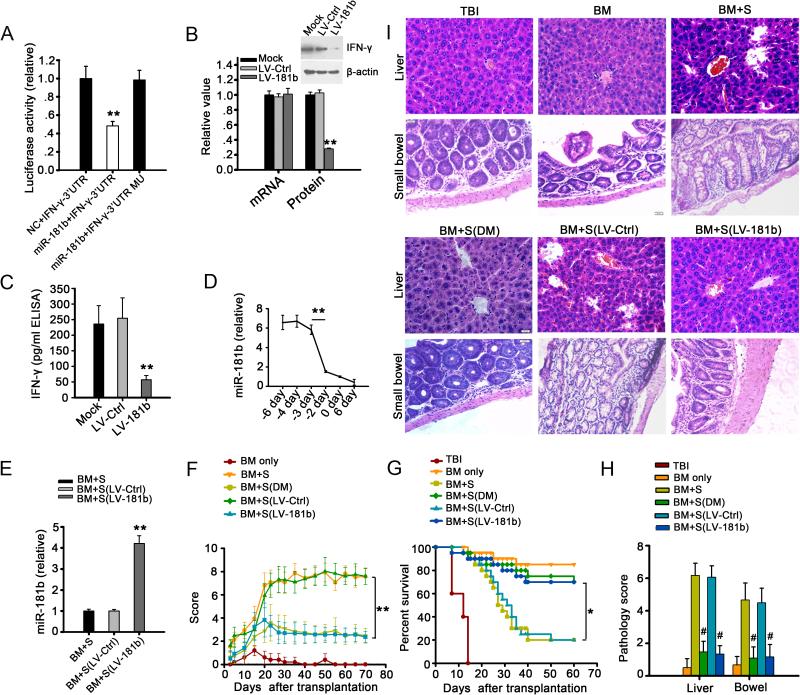
After demonstrating that miR-181a regulates IFN-γ and observing the effects of miR-181a on cell function in vitro, we decided to further confirm these findings in vivo. A mouse model of allo-HSCT was established by transplanting the spleen lymphocytes and T lymphocyte-depleted bone marrow cells from donor mice (C57BL/6 (H-2b)) to the recipient mice (BABL/c (H-2d)). The mouse and human mature miR-181a sequences are identical (supplemental Figure 6A) and we examined whether miR-181a also targeted IFN-γ in mice. LV-181a (2×107 TU) was injected into mice via the tail vein, and spleen cells were collected seven days later. The CD4+ T lymphocytes were sorted from the recipient mice and the levels of IFN-γ mRNA and protein were determined. Unexpectedly, we found that neither the mRNA nor the protein levels of IFN-γ were affected (supplemental Figure 6 B-C). Luciferase reporter assays also failed to show that the mouse IFN-γ mRNA was targeted by miR-181a (supplemental Figure 6 D-E). Further bioinformatic analysis showed that the 3'UTR of the mouse IFN-γ gene perfectly matches the seed sequence of miR-181b and miR-181d, but not miR-181a and miR-181c (supplemental Figure 6F). Moreover, the luciferase activity decreased significantly only when the cells were co-transfected with both the vector expressing miR-181b and the vector containing the luciferase sequence cloned in front of the 3'UTR of the mouse IFN-γ gene (supplemental Figure 6G, Figure 3A). Additionally, when the mice were infected with the vector expressing pre-miR-181b (LV-181b) only the expression of endogenous IFN-γ protein, but not its mRNA, was significantly down-regulated in both CD4+ T cells and plasma (Figure 3 B-C). These data demonstrate that the murine miR-181b targeted IFN-γ in mice as miR-181a did in patients.
FIGURE 3. MiR-181b alleviates aGVHD by targeting IFN-γ in mice.
(A) Luciferase activity of reporter bearing wide type or mutant (MU) 3'UTR of IFN-γ co-transfected into HEK293T cells with murine miR-181b. (B) Relative levels of IFN-γ mRNA and protein in activated CD4+ T cells isolated from spleens of mice infected with LV-181b or LV-Ctrl; CD4+ T cells were activated by PMA and ionomycin. The mRNA and protein levels in mock-infected cells as negative control were set to 1. ** p < 0.01. (C) Expression of IFN-γ protein in mice was quantified by ELISA. ** p < 0.01. (D) Relative expression levels of miR-181b in plasma of recipient mice with aGVHD on days around the onset of aGVHD. Day 0 represented the day of aGVHD onset. The level of miR-181b was significantly decreased prior to development of aGVHD. ** p < 0.01. (E) Expression level of miR-181b in plasma of mice receiving T lymphocyte-depleted bone marrow cells and splenocytes (BM+S), LV-Ctrl (BM+S (LV-Ctrl)) or LV-181b infected splenocytes (BM+S (LV-181b)). ** p < 0.01. (F) Clinical scores of different groups of recipient mice after transplantation. ** p < 0.01. (G) Survival rate of different groups of mice after transplantation. * p < 0.05. Scores (H) and H&E stained sections (I) of the liver and bowel samples from different groups of mice at day 12 after transplantation. ** p < 0.01, # p < 0.001, compared to group BM+S. All values (A-H) represent mean±SD. Data are from three independent experiments, representing 6 mice per group (B-H).
MiR-181b, but not Th1 cytokines, predicts the development of aGVHD in vivo
Th1 cytokines are the major causative factors of aGVHD [25, 28]. We measured the levels of Th1 cytokines in the mouse aGVHD model. The levels of plasma cytokines IFN-γ, IL-2, and TNF were significantly increased and the number of Th1 cells was much higher in the mice transplanted with both T cell-depleted BM cells and splenocytes (supplemental Figure 7 A-B). Reduction of levels of miR-181b was seen in 27 of 50 mice starting at three days prior to the onset of aGVHD (Figure 3 D, Supplemental Table 3). However, changes in the level of cytokines were minimal at the same time points (supplemental Figure 7 C). Of note, 19 of 23 mice developing aGVHD but without reduction in levels of miR-181b were sampled at time points earlier than day −3 (ranging from day −4 to day −11). These data suggest that such false negatives may be the result of early sampling. Furthermore, these results demonstrate a similar timeframe for detecting decreased levels of miR-181b in relation to the onset of aGVHD as that observed in human aGVHD patients.
Overexpression of miR-181b eliminates Th1 response and ameliorates aGVHD in vivo
Since miR-181b targets IFN-γ and thereby affects T lymphocyte function in the mouse model, we determined to examine whether overexpression of miR-181b could effectively downregulate IFN-γ and prevent aGVHD. Co-transplanting spleen T lymphocytes from the donor mice (C57BL/6 (H2b)) that were infected with LV-181b and T lymphocyte-depleted bone marrow cells of the donor mice to the recipient mice (BABL/c (H2d)) increased the level of miR-181b approximately 4-fold (Figure 3 E). Severity of aGVHD and overall survival rate improved markedly in this group of mice (Figures 3 F-G). We were interested in investigating the clinical utility of predicative marker studies. Therefore, we tested whether preemptive treatment could be an important strategy to effectively reduce the complications associated with aGVHD. Dexamethasone was given to mice when they showed reduced miR-181b levels. Importantly, we found decreased aGVHD scores (Figure 3 F) and improved survival (Figure 3 G) comparable to that observed in mice overexpressing miR-181b. Pathologic studies confirmed these observations. Liver sections from the group receiving T cell-depleted BM cells plus splenocytes showed more lymphoplasmacytic infiltration, apoptosis of liver cells, and even structural damage of the hepatic lobule. Small bowel sections from the same group showed markedly diminished number of goblet cells in the crypts and lymphocyte infiltration. In contrast, liver and bowel tissue from groups treated with dexamethasone (BM+S(DM)) or LV-181b (BM+S(LV-181b)) appeared fairly normal (Figure 3 H-I). These results altogether demonstrate that a sudden reduction in miR-181b levels can be used as accurate predictor for the onset of aGVHD, and the overexpression of miR-181b can effectively ameliorate aGVHD in vivo.
Discussion
Acute GVHD has a significant impact on the success of allogeneic transplantation. The ability to accurately predict which patients will develop aGVHD prior to its clinical onset might provide a window for preemptive treatment, effectively preventing aGVHD occurrence. Steroid or other immunosuppressive treatments administered even one day before the onset of clinical symptoms may have the potential to effectively control the immunologic injury or at least ameliorate the extent of aGVHD [31,32]. Due to this potential, great efforts have been exerted in the identification and validation of aGVHD biomarkers, unfortunately, with little success so far [33-35].
Our results suggest that miR-181a levels may be a valuable predicative marker for the prognosis of aGVHD development. In each of 5 patients we studied who never developed aGVHD there was no change in the levels of miR-181a. In contrast, a sudden reduction of mR-181a was observed in 19 of 22 patients (86%) prior to development of aGVHD. Others have also investigated the potential value of predictive biomarkers for development of aGVHD. Werrmke M et al. suggested that the IL-23 receptor and bactericidal permeability increasing protein expression could be used as predictive indicators of aGVHD [36]. It has been reported that combination of three plasma factors sIL-2R, sTNFR1 and sCD8 could predict aGVHD onset with 77% accuracy [33], and a similar panel of four plasma biomarkers had similar findings [37]. These same studies also found that IL-6 and IL-12 concentration correlated with aGVHD, but some of them are also released in all types of transplantation-related complications [38, 39]. Although some high-throughput based studies such as mass spectrometry (MS)-based and non-MS-based proteotmic approaches are sensitive and more accurate for biomarker discovery, they have shown little clinical value because of the time required for analyses [40, 41]. The method used in our study was relatively simple and can be easily adapted to clinical labs.
It is well known that miRNAs play pivotal roles in immune responses by regulating the function of T lymphocytes [42-44], which are involved in the development of aGVHD. In this study, we confirmed that miRNAs are involved in the regulation of T cell function during the development of aGVHD and that unlike cytokines levels which increased concurrently with the development of aGVHD symptoms, the levels of miR-181a changed significantly prior to the onset of aGVHD with high sensitivity (19/22, 86%) and specificity (24/27, 89%). Mechanistically, the results from our in vitro and in vivo experiments showed that over-expressed miR-181a in CD4+ T lymphocytes enhanced their differentiation toward Th2 and Treg by targeting IFN-γ. In addition, miR-181a also affected the differentiation of Th17 cells. However, we noticed that miR-181a is not included on the lists of microRNAs shown to be deregulated before the onset of aGVHD in two recent publications. Xiao et al. used a miRNA microarray to generate a panel of up-regulated plasma miRNAs that have the potential to be used as early biomarkers for aGVHD in humans. However, out of the nearly 2000 miRNAs that have been identified in the human genome, the authors selectivity targeted the 345 most well-characterized human miRNAs and it is unclear if miR-181a was included in this specific microarray assay[45]. Additionally, Sun et al. conducted a modified high-throughput sequencing of RNA isolated by cross-linking immunoprecipitation and identified a uniquely expressed pattern of 44 miRNAs following the allostimulation of T cells. They further demonstrated that two previously unidentified molecules, Wapal and Synij1, were regulated by the mmu-miRNA network. Again miR-181a/b did not show on their miRNA list and it is very likely due to the specific procedure they chose. Nevertheless, it will be interesting to see if miR-181a is down-regulated in the above mentioned experimental settings[46].
IL-6 is a major pathogenic cytokine, which becomes deregulated after clinical allo-HSCT and reaches its peak at day around 15 [38,47,48]. In our results, no statistical difference of the levels of IL-6 between the non-GVHD and GVHD group maybe due to the median time of onset being about 37 days and reduced intensity conditioning. However, the underlying mechanisms of these actions warrant further investigation. Nevertheless, down-regulation of miR-181a is one of the main causes of the elevated levels of cytokines which are directly involved in the development of aGVHD. Although our attention was mainly focused on miR-181a, we noticed that the levels of some other miRNAs also varied significantly before the onset of aGVHD, suggesting that miRNAs other than miR-181a may also play pivotal role in the development of aGVHD. Therefore, the role of miRNAs in immune response demands further exploration.
Since the reduction of miR-181a levels are correlated with the severity of aGVHD, we speculated that the effect of miR-181a on lymphocyte function is concentration-dependent. We further demonstrated that the levels of miR-181a in lymphocytes were inversely related to the protein levels but not the mRNA levels of its target gene, IFN-γ. These results indicate that miR-181a regulates the expressions of its target genes not only depending on its sequence specificity but also the availability of the miRNA itself, supporting the notion that miRNA regulates the expression of its target genes though a fine-tuned mechanism. Little is known with regard to mechanisms controlling the expression of miR-181a in vivo. However, we speculate that manipulation of miR-181a levels may not only affect the levels of cytokines but also which cytokines are regulated, such that fine-tuned manipulation of miR-181a levels could become a valuable strategy towards preventing of the onset of aGVHD. Meanwhile, the results from co-culturing the effective cells expressing miR-181a with different leukemia cell lines further demonstrated that miR-181a effectively reduced aGVHD and preserved the anti-leukemic effect. By targeting IFN-γ, MiR-181a can efficiently down-regulate Th1 differentiation and enhance their immune deviation toward Th2 and Treg, which play important roles in anti-leukemic effects [49, 50]. In addition, and also by targeting IFN-γ, miR-181a can inhibit Th1 immune response by weakening CD4+ T cell function without affecting the other cell lineages such as CD8+T cells, NK cells and BMMSC (bone marrow mesenchymal stem cells), which have been proved to preserve anti-leukemic effects while controlling aGVHD[51-53].
Most importantly, we were able to confirm the clinical utility of monitoring miRNA levels using a murine model of allogeneic transplantation. Similar to human aGVHD, reduced levels of murine miR-181b, the homolog of human miR-181a, occurred starting at three days prior to the onset of aGVHD in the allo-HSCT animal model. Importantly, by monitoring the level of miR-181b, we utilized timely application of dexamethasone to effectively ameliorate aGVHD organ damages with decreased severity of aGVHD and longer survival. Furthermore, overexpression of miR-181b in the mouse allo-HSCT model not only reduced the incidence and severity of aGVHD but also led to a higher survival rate. Pathological examination confirmed that elevated levels of miR-181b in mice ameliorated aGVHD organ damage. These data suggest that manipulation of miRNA levels could be a promising approach to reduce morbidity and mortality in allo-HSCT recipients.
In summary, our study suggests that levels of miR-181a may be a reliable predictive marker of aGVHD. We observed a sudden and significant drop in the levels of miR-181a prior to the onset of aGVHD. Provocative clinical findings in a small series of patients indicated 86% sensitivity and 89% specificity for prognosis of aGVHD. These results need confirmation in a larger clinical trial. Importantly, however, our murine studies validated the biological relevance observed in patients. Results from the animal model showed that overexpression of the miRNA not only reduced the incidence and the severity of the disease but also increased the overall survival rate in allo-HSCT, suggesting that manipulation of the levels of miR-181a could be a suitable strategy in prevention of aGVHD after allo-HSCT.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (Grant No. 2010-30971281), National Institutes of Health (Grant No. CA 94872), Natural Science Foundation of Jiangsu Province (Grant No. BK2012572) and Foundation of Public Health Department of Jiangsu Province (Grant No. H201216). We acknowledge the Department of Experimental Animal Center of Xuzhou Medical College for technical help in animal experiments. In addition, the authors want to thank Donald Hasenmayer for his help in final language/grammar proof. Finally, the authors reported no competing interest in this manuscript.
References
- 1.Ferrara JL, Levine JE, Reddy P, et al. Graft-versus-host disease. Lancet. 2009;373(9674):1550–1561. doi: 10.1016/S0140-6736(09)60237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Korngold R, Sprent J. Lethal graft-versus-host disease after bone marrow transplantation across minor histocompatibility barriers in mice. Prevention by removing mature T cells from marrow. J Exp Med. 1978;148(6):1687–1698. doi: 10.1084/jem.148.6.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Korngold R, Sprent J. Features of T cells causing H-2-restricted lethal graft-vs-host disease across minor histocompatibility barriers. J Exp Med. 1982;155(3):872–883. doi: 10.1084/jem.155.3.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Socié G, Blazar BR. Acute graft-versus-host disease: from the bench to the bedside. Blood. 2009;114(20):4327–4336. doi: 10.1182/blood-2009-06-204669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lagos-Quintana M, Rauhut R, Lendeckel W, et al. Identification of novel genes coding for small expressed RNAs. Science. 2001;294(5543):853–858. doi: 10.1126/science.1064921. [DOI] [PubMed] [Google Scholar]
- 6.Guo H, Ingolia NT, Weissman JS, et al. Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature. 2010;466(7308):835–840. doi: 10.1038/nature09267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu LF, Boldin MP, Chaudhry A, et al. Function of miR-146a in controlling Treg cell-mediated regulation of Th1 responses. Cell. 2010;142(6):914–929. doi: 10.1016/j.cell.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Makino K, Jinnin M, Kajihara I, et al. Circulating miR-142-3p levels in patients with systemic sclerosis. Clin Exp Dermatol. 2012;37(1):34–39. doi: 10.1111/j.1365-2230.2011.04158.x. [DOI] [PubMed] [Google Scholar]
- 9.Zhao S, Wang Y, Liang Y, et al. MicroRNA-126 Regulates DNA Methylation in CD4+ T Cells and Contributes to Systemic Lupus Erythematosus by Targeting DNA Methyltransferase 1. Arthritis Rheum. 2011;63(5):1376–1386. doi: 10.1002/art.30196. [DOI] [PubMed] [Google Scholar]
- 10.Du C, Liu C, Kang J, et al. MicroRNA miR-326 regulates TH-17 differentiation and is associated with the pathogenesis of multiple sclerosis. Nat Immunol. 2009;10(12):1252–1259. doi: 10.1038/ni.1798. [DOI] [PubMed] [Google Scholar]
- 11.Lu LF, Thai TH, Calado DP, et al. Foxp3-dependent microRNA155 confers competitive fitness to regulatory T cells by targeting SOCS1 protein. Immunity. 2009;30(1):80–91. doi: 10.1016/j.immuni.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lykken EA, Li QJ. MicroRNAs at the regulatory frontier: an investigation into how microRNAs impact the development and effector functions of CD4 T cells. Immunol Res. 2011;49(1-3):87–96. doi: 10.1007/s12026-010-8196-4. [DOI] [PubMed] [Google Scholar]
- 13.Zhou B, Wang S, Mayr C, et al. MiR-150, a microRNA expressed in mature B and T cells, blocks early B cell development when expressed prematurely. Proc Natl Acad Sci U S A. 2007;104(17):7080–7085. doi: 10.1073/pnas.0702409104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xiao C, Calado DP, Galler G, et al. MiR-150 controls B cell differentiation by targeting the transcription factor c-Myb. Cell. 2007;131(1):146–159. doi: 10.1016/j.cell.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 15.Xiao C, Srinivasan L, Calado DP, et al. Lymphoproliferative disease and autoimmunity in mice with increased miR-17-92 expression in lymphocytes. Nat Immunol. 2008;9(4):405–414. doi: 10.1038/ni1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ventura A, Young AG, Winslow MM, et al. Targeted deletion reveals essential and overlapping functions of the miR-17 through 92 family of miRNA clusters. Cell. 2008;132(5):875–886. doi: 10.1016/j.cell.2008.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang Y, Luo X, Cui H, et al. MicroRNA-146A contributes to abnormal activation of the type I interferon pathway in human lupus by targeting the key signaling proteins. Arthritis Rheum. 2009;60(4):1065–1075. doi: 10.1002/art.24436. [DOI] [PubMed] [Google Scholar]
- 18.Curtale G, Citarella F, Carissimi C, et al. An emerging player in the adaptive immune response: microRNA-146a is a modulator of IL-2 expression and activation-induced cell death in T lymphocytes. Blood. 2010;115(2):265–273. doi: 10.1182/blood-2009-06-225987. [DOI] [PubMed] [Google Scholar]
- 19.Calin GA, Dumitru CD, Shimizu M, et al. Frequent deletions and down-regulation of micro-RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc Natl Acad Sci U S A. 2002;99(24):15524–15529. doi: 10.1073/pnas.242606799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilmott JS, Zhang XD, Hersey P, et al. The emerging important role of microRNAs in the pathogenesis, diagnosis and treatment of human cancers. Pathology. 2011;43(6):657–671. doi: 10.1097/PAT.0b013e32834a7358. [DOI] [PubMed] [Google Scholar]
- 21.Cooke KR, Kobzik L, Martin TR, et al. An experimental model of idiopathic pneumonia syndrome after bone marrow transplantation: I. The roles of minor H antigens and endotoxin. Blood. 1996;88(8):3230–3239. [PubMed] [Google Scholar]
- 22.Kaplan DH, Anderson BE, McNiff JM, et al. Target antigens determine graft-versus-host disease phenotype. J Immunol. 2004;173(9):5467–5475. doi: 10.4049/jimmunol.173.9.5467. [DOI] [PubMed] [Google Scholar]
- 23.Glucksberg H, Storb R, Fefer A, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HLA matched sibling donors. Transplantation. 1974;18(4):295–304. doi: 10.1097/00007890-197410000-00001. [DOI] [PubMed] [Google Scholar]
- 24.Martino R, Romero P, Subirá M, et al. Comparison of the classic Glucksberg criteria and the IBMTR Severity Index for grading acute graft-versus-host disease following HLA-identical sibling stem cell transplantation. International Bone Marrow Transplant Registry. Bone Marrow Transplant. 1999;24(3):283–287. doi: 10.1038/sj.bmt.1701899. [DOI] [PubMed] [Google Scholar]
- 25.Antin JH, Ferrara JL. Cytokin disregulation and acute graft versus-host-disease. Blood. 1992;80(12):2964–2968. [PubMed] [Google Scholar]
- 26.Nikolic B, Lee S, Bronson RT, et al. Th1 and Th2 mediate acute graft-versus-host disease, each with distinct end-organ targets. J Clin Invest. 2000;105(9):1289–1298. doi: 10.1172/JCI7894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rieger K, Loddenkemper C, Maul J, et al. Mucosal FOXP31 regulatory T cells are numerically deficient in acute and chronic GVHD. Blood. 2006;107(4):1717–1723. doi: 10.1182/blood-2005-06-2529. [DOI] [PubMed] [Google Scholar]
- 28.Murphy WJ, Welniak LA, Taub DD, et al. Differential effects of the absence of interferon-gamma and IL-4 in acute graft-versus host disease after allogeneic bone marrow transplantation in mice. J Clin Invest. 1998;102(9):1742–1748. doi: 10.1172/JCI3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O'Connell RM, Rao DS, Chaudhuri AA, et al. Sustained expression of microRNA-155 in hematopoietic stem cells causes a myeloproliferative disorder. J Exp Med. 2008;205(3):585–594. doi: 10.1084/jem.20072108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuchen S, Resch W, Yamane A, et al. Regulation of microRNA expression and abundance during lymphopoiesis. Immunity. 2010;32(6):828–839. doi: 10.1016/j.immuni.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferrara JL, Harris AC, Greenson JK, et al. Regenerating islet-derived 3-alpha is biomarker of gastrointestinal graft-versus-host disease. Blood. 2011;118(25):6702–6708. doi: 10.1182/blood-2011-08-375006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martin PJ, Rizzo JD, Wingard JR, et al. First- and second-line systemic treatment of acute graft-versus-host disease: recommendations of the American Society of Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2012;18(8):1150–1163. doi: 10.1016/j.bbmt.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.August KJ, Chiang KY, Bostick RM, et al. Biomarkers of immune activation to screen for severe, acute GVHD. Bone Marrow Transplant. 2011;46(4):601–604. doi: 10.1038/bmt.2010.165. [DOI] [PubMed] [Google Scholar]
- 34.Cho BS, Min CK, Kim HJ, et al. High levels of B cell activating factor during the peritransplantation period are associated with a reduced incidence of acute graft-versus-host disease following myeloablative allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2010;16(5):629–638. doi: 10.1016/j.bbmt.2009.11.023. [DOI] [PubMed] [Google Scholar]
- 35.Choi SW, Kitko CL, Braun T, et al. Change in plasma tumor necrosis factor receptor 1 levels in the first week after myeloablative allogeneic transplantation correlates with severity and incidence of GVHD and survival. Blood. 2008;112(4):1539–1542. doi: 10.1182/blood-2008-02-138867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wermke M, Maiwald S, Schmelz R, et al. Genetic variations of interleukin-23R (1143A>G) and BPI (A645G), but not of NOD2, are associated with acute graft-versus-host disease after allogeneic transplantation. Biol Blood Marrow Transplant. 2010;16(12):1718–1727. doi: 10.1016/j.bbmt.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 37.Paczesny S, Krijanovski OI, Braun TM, et al. A biomarker panel for acute graft-versus-host disease. Blood. 2009;113(2):273–278. doi: 10.1182/blood-2008-07-167098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schots R, Kaufman L, Van Riet I, et al. Proinflammatory cytokines and their role in the development of major transplant-related complications in the earlyphase after allogeneic bone marrow transplantation. Leukemia. 2003;17(6):1150–1156. doi: 10.1038/sj.leu.2402946. [DOI] [PubMed] [Google Scholar]
- 39.Mohty M, Blaise D, Faucher C, et al. Inflammatory cytokines and acute graft-versus-host disease after reduced-intensity conditioning allogeneic stem cell transplantation. Blood. 2005;106(13):4407–4411. doi: 10.1182/blood-2005-07-2919. [DOI] [PubMed] [Google Scholar]
- 40.Srinivasan R, Daniels J, Fusaro V, et al. Accurate diagnosis of acute graft-versus-host disease using serum proteomic pattern analysis. Exp Hematol. 2006;34(6):796–801. doi: 10.1016/j.exphem.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 41.Imanguli MM, Atkinson JC, Harvey KE, et al. Changes in salivary proteome following allogeneic hematopoietic stem cell transplantation. Exp Hematol. 2007;35(2):184–192. doi: 10.1016/j.exphem.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taganov KD, Boldin MP, Baltimore D. MicroRNAs and immunity: tiny players in a big field. Immunity. 2007;26(2):133–137. doi: 10.1016/j.immuni.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 43.Zhang J, Jima DD, Jacobs C, et al. Patterns of microRNA expression characterize stages of human B-cell differentiation. Blood. 2009;113(19):4586–4594. doi: 10.1182/blood-2008-09-178186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Belver L, Papavasiliou FN, Ramiro AR. MicroRNA control of lymphocyte differentiation and function. Curr Opin Immunol. 2011;23(3):368–373. doi: 10.1016/j.coi.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xiao B, Wang Y, Li W, et al. Plasma microRNA signature as a noninvasive biomarker for acute graft-versus-host disease. Blood. 2013;122(19):3365–3375. doi: 10.1182/blood-2013-06-510586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sun Y, Tawara I, Zhao M, et al. Allogeneic T cell responses are regulated by a specific miRNA-mRNA network. J Clin Invest. 2013;123(11):4739–4754. doi: 10.1172/JCI70013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kennedy GA, Varelias A, Vuckovic S, et al. Addition of interleukin-6 inhibition with tocilizumab to standard graft-versus-host disease prophylaxis after allogeneic stem-cell transplantation: a phase 1/2 trial. Lancet Oncol. 2014;15(13):1451–1459. doi: 10.1016/S1470-2045(14)71017-4. [DOI] [PubMed] [Google Scholar]
- 48.Fuji S, Kim SW, Fukuda T, et al. Preengraftment serum C-reactive protein (CRP) value may predict acute graft-versus host disease and nonrelapse mortality after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2008;14(5):510–517. doi: 10.1016/j.bbmt.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 49.Eguchi J, Kuwashima N, Hatano M, et al. IL-4-transfected tumor cell vaccines activate tumor-infiltrating dendritic cells and promote type-1 immunity. J Immunol. 2005 Jun 1;174(11):7194–201. doi: 10.4049/jimmunol.174.11.7194. [DOI] [PubMed] [Google Scholar]
- 50.Wang H, Nemoto-Sasaki Y, Kondo T, et al. Potential involvement of monocyte chemoattractant protein (MCP)-1/CCL2 in IL-4-mediated tumor immunity through inducing dendritic cell migration into the draining lymph nodes. Int Immunopharmacol. 2003 May;3(5):627–42. doi: 10.1016/S1567-5769(02)00251-5. [DOI] [PubMed] [Google Scholar]
- 51.Leen AM, Rooney CM, Foster AE. Improving T cell therapy for cancer. Annu Rev Immunol. 2007;25:243–65. doi: 10.1146/annurev.immunol.25.022106.141527. [DOI] [PubMed] [Google Scholar]
- 52.Ruggeri L1, Capanni M, Casucci M, et al. Role of natural killer cell alloreactivity in HLA-mismatched hematopoietic stem cell transplantation. Blood. 1999 Jul 1;94(1):333–9. [PubMed] [Google Scholar]
- 53.Caimi PF, Reese J, Lee Z, et al. Emerging therapeutic approaches for multipotent mesenchymal stromal cells. Curr Opin Hematol. 2010 Nov;17(6):505–13. doi: 10.1097/MOH.0b013e32833e5b18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.