Abstract
Aim
The goal of this study was to compare associations between clinical and ECG predictors of cardiac resynchronization therapy (CRT) response with electrical dyssynchrony.
Methods
Body-surface potentials were recorded using a 120-lead system in 4 patients (age 62 ± 12 y, left ventricular ejection fraction (LVEF) 29 ± 5 %; QRS duration 154 ± 19 ms) with post-myocardial infarction scar and left bundle branch block before CRT implantation. A patient-specific heart-torso model derived from MRI with 291 heart-surface nodes was developed. Electrical dyssynchrony index (EDI) was computed as the standard deviation of activation times on the epicardium while uncoupling index (UI) was measured as the difference between the activation times.
Results
QRS duration correlated with mean activation time (r = 0.977; P = 0.023), but did not correlate with EDI or UI. LVEF inversely correlated with activation time at the lowest 20th percentile (r = −0.960; P = 0.040). Sum absolute QRST integral (SAI QRST), measured on orthogonal XYZ ECG, correlated with EDI (r = 0.955; P = 0.045), and characterized late-activated area of the left ventricle.
Conclusion
SAI QRST is a measure of electrical dyssynchrony on ECG.
1. Introduction
Cardiac resynchronization therapy (CRT) improves outcomes in heart failure patients with electrical dyssynchrony. However, about a third of CRT recipients do not improve with bi-ventricular pacing optimally. Assessment of electrical, rather than mechanical, dyssynchrony is needed to identify appropriate CRT candidates. Electrical dyssynchrony is traditionally defined by QRS duration and morphology [1]. However, both QRS duration and QRS morphology are imperfect markers of electrical dyssynchrony.
1.1. Sum Absolute QRST integral (SAI QRST) on surface ECG
Recently Tereshchenko et al [2] showed that a simple surface ECG measure, sum absolute QRST integral (SAI QRST) was independently (after adjustment for QRS duration, bundle branch block morphology, left ventricular ejection fraction, type of cardiomyopathy, gender, and age) associated with CRT response in the SMART-AV randomized clinical trial.
1.2. Electrical Dyssynchrony
Noninvasive mapping of ventricular activation can quantify electrical dyssynchrony in detail. In a small pilot study, electrical dyssynchrony measured by non-invasive mapping of ventricular activation on epicardium predicted clinical CRT response better than QRS duration or morphology [3]. However, correlation between non-invasively mapped ventricular activation dyssynchrony and traditional clinical and ECG predictors of CRT response have not been studied. The goal of this pilot study was to determine the association between clinical and ECG predictors of CRT response and electrical dyssynchrony. We hypothesized that SAI QRST measured on surface ECG is associated with electrical dyssynchrony.
2. Methods
The study conformed to principles outlined in the Declaration of Helsinki and was approved by the Johns Hopkins Institution Review Board. Each participant provided written informed consent.
Heart failure patients with left bundle branch block (LBBB) and currently approved indications (per ACC/AHA/HRS guidelines) for CRT device implantation were enrolled at the Johns Hopkins Hospital, as previously described [4]. In this study we analyzed baseline data in sinus rhythm prior to CRT device implantation.
2.1. Body Surface Mapping
Cardiac Magnetic Resonance Imaging (MRI) was performed on 1.5 Tesla MRI scanners (MAGNETOM Avanto Syngo MR B17 Siemens, Erlangen, Germany and INTERA, Phillips, Amsterdam, The Netherlands) with gadolinium contrast.
Body-surface potentials were recorded using a 128-lead system (BioSemi, Amsterdam, The Netherlands) at 2048 Hz with 24-bit resolution. Disposable Ag/AgCl surface electrodes with MRI skin markers were placed on the torso. A custom program (MAPPER, Dalhousie University, Halifax, Canada) was used for data recording. A patient-specific heart-torso model with 291 heart-surface nodes was used, as previously described [4]. The inverse procedure was performed as developed by Dr. Horáček [5]. Body surface ECG signals were transformed into unipolar epicardial electrogram signals by Dr. Dawoud as previously described [4]. Subsequent analysis of reconstructed epicardial electrograms and construction of activation maps was performed in the Tereshchenko laboratory.
2.2. Electrical Dyssynchrony Assessment
Median sinus beats were analyzed. The ventricular activation time was measured as the time from the surface ECG QRS onset to the time of the steepest downward slope on unipolar epicardial electrogram. This activation time was mapped at 291 heart-surface nodes to construct an activation map.
Electrical dyssynchrony on epicardial activation map was quantified by the following parameters. An electrical dyssynchrony index (EDI) was computed as the standard deviation of activation times throughout the epicardium. Uncoupling index (UI) was measured as the difference between activation times. Regions of late activation were defined as sites where the activation time was above 80th percentile of QRS duration. The percentage of the area activating late was computed by dividing the number of late nodes by the total number of nodes used to image ventricular epicardium. Descriptive statistics of ventricular activation time were also calculated.
2.3. Sum Absolute QRST integral
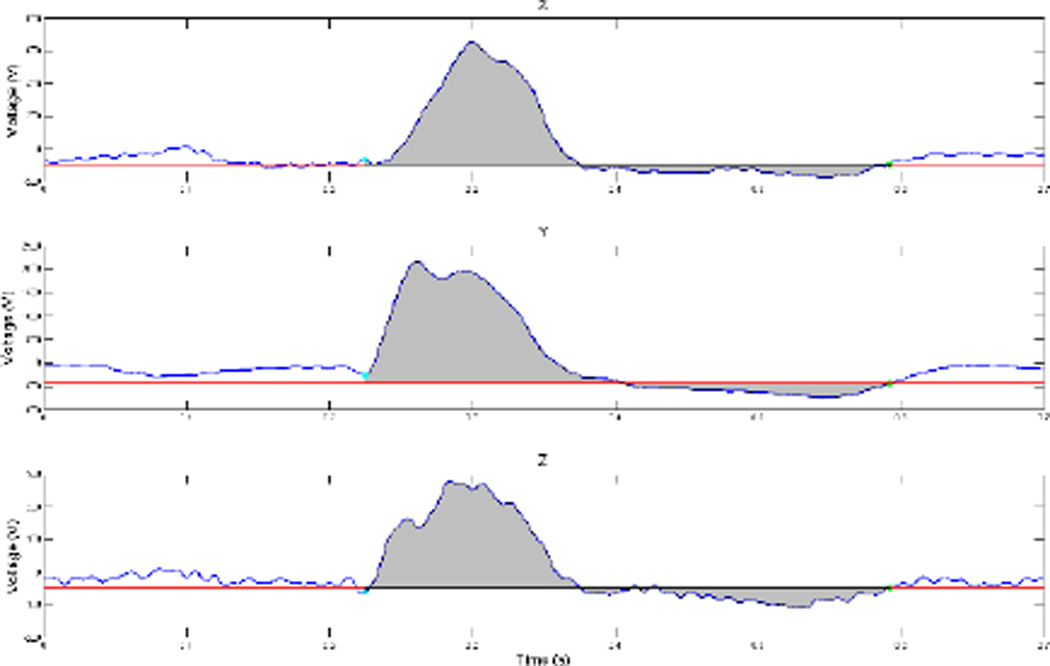
Orthogonal XYZ surface ECG signals were computed from the appropriate body surface leads (Figure 1). Median sinus beats were constructed and analyzed. Onset of the QRS complex and end of the T wave on median sinus cardiac beats, on each XYZ lead were defined by a customized MATLAB (the MathWorks, Inc, Natick, MA) software application. The absolute value of the area under the entire QRST waveform was calculated on each orthogonal lead (X, Y, and Z). Absolute QRST integral values on X, Y, and Z leads were then added together to obtain SAI QRST, as previously described [6–9] (Figure 1).
Figure 1.
Representative (patient DH) median sinus beat on theorthogonal surface ECG leads X (upper panel), Y (middle panel), and Z (lower panel). The area under the QRST waveform is colored gray. A zero value of baseline is assigned at the level of the end of the T wave.
2.4. Statistical analysis
Due to the small sample size of this pilot study, only unadjusted pairwise correlation coefficients with significance levels were calculated to determine the association between electrical dyssynchrony quantified on epicardial activation map and SAI QRST on surface ECG, left ventricular ejection fraction (LVEF), and QRS duration.
3. Results
3.1. Patient Population
Clinical characteristics of study participants are summarized in Table 1.
Table 1.
Clinical characteristics of study participants.
| Characteristic | N = 4 |
|---|---|
| Age (SD), y | 62.25 (11.67) |
| Males, n (%) | 4 (100) |
| Whites, n (%) | 4 (100) |
| LVEF (SD), % | 28.75 (4.79) |
| NYHA II–III class | 4 (100) |
| Ischemic Cardiomyopathy, n (%) | 3 (75) |
| Chronic kidney disease, n (%) | 1 (25) |
| QRS duration (SD), ms | 153.5 (19.07) |
| Left bundle branch block, n (%) | 4 (100) |
| SAI QRST (SD), mV*ms | 290.75 (90.94) |
3.2. Electrical Dyssynchrony on Activation Map
Activation maps of study participants are presented in Figure 2.
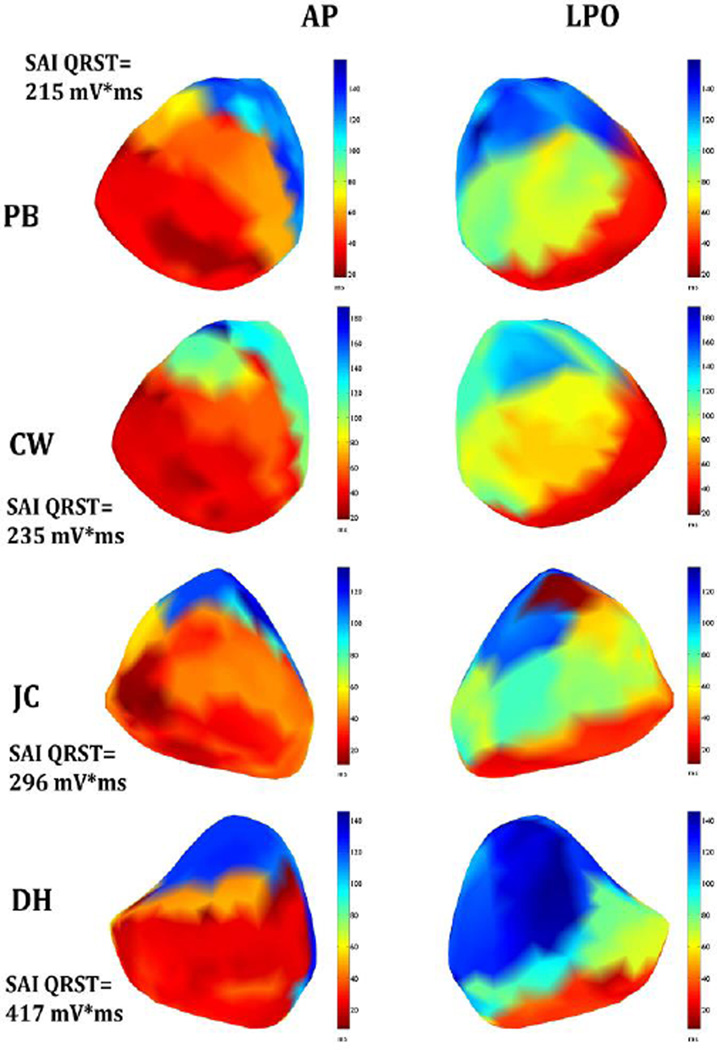
Figure 2.
Activation map. Epicardial maps of activation in the left anterior-posterior (AP) and left posterior oblique (LPO) views in 4 patients with LBBB, placed in order by increasing values of SAI QRST.
In all 4 patients, epicardial activation started with right ventricle (RV) breakthrough 12.8±9.5 ms after onset of body surface QRS. RV epicardial breakthroughs occurred at anterolateral free wall sites and then epicardial activation spread radially. (Figure 2). A line of conduction block (indicated by a sharp transition from red to blue color on anterior view; difference in activation time > 40ms) was located between the epicardial aspect of the septum and the left ventricular (LV) lateral wall, causing activation to turn around the apex and resulting in late activation (dark blue, seen on left posterior oblique (LPO) view) of a large portion of the lateral and inferior wall. LV activation sequences were varied. The patient with the largest value of SAI QRST (DH) had the biggest late-activated area of the left ventricle. LV activation was delayed (mean delay, 64±16 ms). Parameters of ventricular activation are reported in Table 2.
Table 2.
Parameters of epicardial ventricular activation.
| Characteristic | N = 4 |
|---|---|
| Mean ventricular activation time (SD), ms | 64.2(16.0) |
| Median ventricular activation (SD), ms | 55.4(17.7) |
| Maximum ventricular activation (SD), ms | 135.3(17.8) |
| Electrical Dyssynchrony Index (SD), ms | 37.4(9.1) |
| Uncoupling Index (SD), ms | 122.4 (19.0) |
| Activation time at the lowest 20th percentile (SD), ms | 25.6 (10.1) |
| Activation time at the highest 80th percentile (SD), ms | 101.4 (35.4) |
| Percentage of the area activating early at 20% of way through QRS (SD), % | 17.1(3.4) |
| Percentage of the area activating late at 80% of way through QRS (SD), % | 18.7 (1.3) |
3.3. Correlations of the Electrical Dyssynchrony with Clinical and ECG Metrics
QRS duration correlated with mean activation time (r = 0.977; P = 0.023), but did not correlate with EDI or UI. LVEF inversely correlated with activation time at the lowest 20th percentile (r = −0.960; P = 0.040) and trended towards correlation with an area, occupied by delayed activation (> 80th percentile). Out of all clinical predictors of CRT response, only SAI QRST correlated with EDI (r = 0.955; P = 0.045) and therefore, characterized the degree of electrical dyssynchrony.
4. Discussion
In this pilot study we observed the correlation of the simple surface ECG parameter SAI QRST with EDI, measured as a standard deviation of ventricular activation times on the epicardium. Thus, SAI QRST reflects the degree of electrical dyssynchrony. SAI QRST correlated with electrical dyssynchrony while neither LVEF nor QRS duration correlated with electrical dyssynchrony. QRS duration correlated with mean activation time.
SAI QRST was developed by Tereshchenko and colleagues [10, 11] as a risk marker of ventricular arrhythmias. In a prospective cohort study (PROSE-ICD) of systolic heart failure patients with implanted primary prevention cardioverter-defibrillators (ICD) or CRT-defibrillators (CRT-D) low or intermediate SAI QRST (≤145 mV*ms) was associated with increased risk of sustained ventricular arrhythmias, which was especially noticeable in patients with LBBB and CRT-D [11]. Very likely, PROSE-ICD participants with implanted CRT-D and high SAI QRST (>145 mV*ms) responded well to the CRT, exhibiting antiarrhythmic effect of CRT with decreased rates of ventricular arrhythmias. In contrast, CRT-D participants with low SAI QRST were more likely to be non-responders. CRT in patients with low SAI QRST was associated with 4-fold increased risk of sustained ventricular arrhythmias and appropriate shocks or anti-tachycardia pacing. Therefore, baseline low SAI QRST identified CRT non-responders in PROSE-ICD cohort, which suggested that SAI QRST is a measure of electrical dyssynchrony.
Recently this hypothesis was proven in a blinded analysis of the SMART-AV randomized controlled trial [2]. SAI QRST was independently (beyond QRS duration, bundle branch block morphology, LVEF, cardiomyopathy type, gender, age) associated with CRT response in the SMART-AV randomized clinical trial. Moreover, baseline SAI QRST remained associated with CRT response even after adjustment for LV lead position. Results of this study help to explain our recent SMART-AV findings. The study participant (DH) with the largest SAI QRST had the biggest late-activated area of the LV. Thus, LV pacing from any location on epicardial surface within this large late-activated area of LV likely would be beneficial. Having high SAI QRST makes CRT response less dependent on LV lead placement location and increases chances of success. In contrast, patients with low SAI QRST have relatively small late-activated LV regions. Obtaining CRT response in patients with low SAI QRST would require precise LV lead placement in the latest-activated area, which might be technically challenging, decreasing chance of success.
Importantly, our study revealed that QRS duration correlated with mean activation time but did not correlate with electrical dyssynchrony. This finding highlights the limitation of QRS duration as a main criterion for selection of CRT candidates.
5. Conclusion
Surface ECG SAI QRST is a measure of electrical dyssynchrony. SAI QRST strongly correlates with EDI, measured as a standard deviation of ventricular activation times on the epicardium. QRS duration correlates with mean activation time, but not with EDI.
Supplementary Material
Acknowledgments
Authors thank Fady Dawoud, PhD, David Spragg, MD, and Albert C Lardo, PhD, for providing data of the study.
This work was partially supported by the NIH 1R01HL118277 (LGT).
References
- 1.Gold MR, Thebault C, Linde C, Abraham WT, Gerritse B, Ghio S, et al. Effect of QRS duration and morphology on cardiac resynchronization therapy outcomes in mild heart failure: results from the Resynchronization Reverses Remodeling in Systolic Left Ventricular Dysfunction (REVERSE) study. Circulation. 2012;126(7):822–829. doi: 10.1161/CIRCULATIONAHA.112.097709. [DOI] [PubMed] [Google Scholar]
- 2.Tereshchenko LG, Cheng A, Park J, Wold N, Meyer TE, Gold MR, et al. Novel Measure Of Electrical Dyssynchrony Predicts Response In Cardiac Resynchronization Therapy: Results From The SMART-AV Trial. Heart Rhythm. 2015 doi: 10.1016/j.hrthm.2015.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ploux S, Lumens J, Whinnett Z, Montaudon M, Strom M, Ramanathan C, et al. Noninvasive Electrocardiographic Mapping to Improve Patient Selection for Cardiac Resynchronization Therapy: Beyond QRS Duration and Left Bundle Branch Block Morphology. Journal of the American College of Cardiology. 2013;61(24):2435–2443. doi: 10.1016/j.jacc.2013.01.093. [DOI] [PubMed] [Google Scholar]
- 4.Dawoud F, Spragg D, Schuleri KH, Horáček BM, Halperin H, Lardo AC. Inverse electrocardiographic imaging to assess electrical dyssynchrony in cardiac resynchronization therapy patients. Computing in cardiology. 2012;39:993–996. [Google Scholar]
- 5.Horacek BM, Clements JC. The inverse problem of electrocardiography: a solution in terms of single- and double-layer sources of the epicardial surface. Math Biosci. 1997;144(2):119–154. doi: 10.1016/s0025-5564(97)00024-2. [DOI] [PubMed] [Google Scholar]
- 6.Sur S, Han L, Tereshchenko LG. Comparison of sum absolute QRST integral, and temporal variability in depolarization and repolarization, measured by dynamic vectorcardiography approach, in healthy men and women. PLoS One. 2013;8(2):e57175. doi: 10.1371/journal.pone.0057175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tereshchenko LG, Cheng A, Fetics BJ, Butcher B, Marine JE, Spragg DD, et al. A new electrocardiogram marker to identify patients at low risk for ventricular tachyarrhythmias: sum magnitude of the absolute QRST integral. J Electrocardiol. 2011;44(2):208–216. doi: 10.1016/j.jelectrocard.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tereshchenko LG, Cheng A, Fetics BJ, Marine JE, Spragg DD, Sinha S, et al. Ventricular arrhythmia is predicted by sum absolute QRST integralbut not by QRS width. J Electrocardiol. 2010;43(6):548–552. doi: 10.1016/j.jelectrocard.2010.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tereshchenko LG, McNitt S, Han L, Berger RD, Zareba W. ECG marker of adverse electrical remodeling post-myocardial infarction predicts outcomes in MADIT II study. PLoS One. 2012;7(12):e51812. doi: 10.1371/journal.pone.0051812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tereshchenko LG, Cheng A, Fetics BJ, Marine JE, Spragg DD, Sinha S, et al. Ventricular arrhythmia is predicted by sum absolute QRST integral but not by QRS width. Journal of Electrocardiology. 2010;43(6):548–552. doi: 10.1016/j.jelectrocard.2010.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tereshchenko LG, Cheng AA, Fetics BJ, Butcher B, Marine JE, Spragg DD, et al. A new electrocardiogram marker to identify patients at low risk for ventricular tachyarrhythmias: sum magnitude of the absolute QRST integral. Journal of Electrocardiology. 2011;44(2):208–216. doi: 10.1016/j.jelectrocard.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.