Abstract
Kiwifruit bacterial canker, an economically important disease caused by Pseudomonas syringae pv. actinidiae (Psa), has caused severe losses in all major areas of kiwifruit cultivation. Using a GFPuv-labeled strain of Psa, we monitored the invasion, colonization, and movement of the pathogen in kiwifruit twigs, leaves and veins. The pathogen can invade twigs through both wounds and natural openings; the highest number of Psa is obtained in cut tissues. We determined that, following spray inoculation, Psa-GFPuv could infect leaves and cause lesions in the presence and absence of wounds. Light and transmission electron microscopic observations showed that bacterial cells colonize both phloem and xylem vessels. Bacterial infection resulted in marked alterations of host tissues including the disintegration of organelles and degeneration of protoplasts and cell walls. Furthermore, low temperature was conducive to colonization and movement of Psa-GFPuv in kiwifruit tissues. Indeed, the pathogen migrated faster at 4°C than at 16°C or 25°C in twigs. However, the optimum temperature for colonization and movement of Psa in leaf veins was 16°C. Our results, revealing a better understanding of the Psa infection process, might contribute to develop more efficacious disease management strategies.
Introduction
Kiwifruit (Actinidia deliciosa, the green-fleshed kiwifruit and A. chinensis, the yellow-fleshed kiwifruit), native to China, is an economically important fruit crop. In recent years a bacterial canker of kiwifruit, caused by Pseudomonas syringae pv. actinidiae (Psa), has caused severe losses in all major areas of kiwifruit cultivation. The pathogen was first reported and described in Japan in 1989 [1]; then, it was detected in Korea [2], Italy [3] and China [4]. In recent years, Psa re-emerged in all countries where A. deliciosa and A. chinensis are cultivated, such as in Japan, Korea, Italy, France, Portugal, Chile, Spain, Turkey and New Zealand [5]. In Italy, the yield losses caused by Psa infection were estimated to cost up to 2 million Euros in the spring of 2008 [6]. In New Zealand, bacterial canker caused by the virulent strain of Psa, has seen predicted to cause yield losses between $310 and $410 million over the next five years, which will probably increase to $740-$885 million over the next 15 years [7]. In China, Psa was first described in Sichuan province [4], then the pathogen spread soon to other regions of China where kiwifruits were grown, such as in the provinces of Shaanxi, Sichuan, Hunan and Hubei. In these areas kiwifruit plantations have also been severely affected by the disease. In Shaanxi, the largest kiwifruit producer in China, the Psa infected area expanded from 0.13 to 311.5 ha between 1992 and 2002 [8]. In 2012, up to 80% of the orchards were affected, and the portion of diseased plants at each site ranged from 30% to 50% [9].
Psa can infect the above ground organs of kiwifruit trees. In green-fleshed and yellow-fleshed kiwifruit cultivars, the symptoms include canker formation on the trunk and vines with whitish to red exudates, brown discoloration of twigs, leader and cane dieback, reddening of lenticels, brown-black leaf spots with yellow halos, and blossom necrosis [6]. A survey of previous Psa studies revealed that they can be divided into two categories. Firstly, in the 1990s, studies were carried out mainly in Japan and China and focused on isolation and identification of the pathogen in kiwifruit orchards [1,2,4,10], as well as on disease prevalence and possible effects of environmental factors on the disease development [1,11,12,13,14,15]. Secondly, during this period the efficacy of different chemicals, including copper compounds and streptomycin, were investigated to control Psa [16,17]. Since the “global outbreak of Psa in 2008”, great efforts have been made to better understand the nature of the disease. For example, PCR-based methods have been used to detect the survival and transmission of Psa [18,19,20,21,22]. Additionally, the global populations of Psa have been redefined based on pathogenic, molecular, and phenotypic characteristics [23,24,25,26,27]. Whole genome sequencing analyses have been performed to understand specific traits of Psa determining processes and factors of its prevalence and virulence [28,29,30]. These studies have made important contributions to our understanding of the re-emergence of Psa, and have suggested strategies for protection of Psa-threatened crops [31].
However, our knowledge on mechanisms determining infection, colonization and movement processes of Psa are still insufficient. In a previous study, we engineered a Psa strain expressing the fluorescent protein, GFPuv [32]; this approach greatly facilitated monitoring of Psa during colonization and movement in plant tissues of kiwifruit. In the present study, we combined the fluorescence monitoring method with electron microscopy, in order to elucidate in more detail infection colonization and spread of Psa at different temperatures in various plant tissues of kiwifruit.
Materials and Methods
Bacterial strain, inoculum preparation, and plant material
Strain Psamx7 was isolated in Mei county of Shaanxi province from infected leaves of A. chinensis ‘Hongyang’, which is widely cultivated in China and susceptible to Psa. No specific permissions were required for collecting sample, because ‘Hongyang’ is widely cultivated in Mei county of Shaanxi province and Psa infected leaves were observed in every kiwifruit orchard. Furthermore, we confirm that the field studies did not involve endangered or protected species. The strain was identified as Psa biovar 3 according to the concatenated 16S-gyrB-dnaA sequence [22]. This strain was successfully transformed with GFPuv by electroporation [32]. Compared to the wild Psa strain, the modified strain Psamx7-GFPuv1 (named as Psa-GFPuv) showed no significant differences regarding morphology, growth pattern and pathogenicity; since expression level of GFPuv in the modified strain is also stable and strong during cell multiplication [32], the GFPuv-labeled strain is suitable not only for studying colonization and movement of Psa but also to trace formation and spread of lesions in kiwifruit leaves and veins.
The modified strain, was routinely grown for 48 h at 28°C on beef peptone agar (BPA) medium (Joel High-Tech Co., Ltd., Dalian, China) (containing tryptone 1%, beef extract 0.3%, yeast extract 0.1%, sucrose 1%, agar 1.5%, pH 7.0) and supplemented with kanamycin (KAN, 50 μg·mL−1). Then, single colonies were picked and cultured in BP liquid medium supplemented with KAN for 16–20 h at 28°C on a rotary shaker (180 RPM). For inoculation, the culture was centrifuged at 8000× g for 20 min and the bacterial cells were re-suspended in phosphate buffer solution (PBS, pH 7.0). Bacterial suspensions, diluted to 2×107 CFU·mL-1, were used to inoculate kiwifruit twigs and leaves.
Two-year old kiwifruit trees of A. deliciosa ‘Xuxiang’ were grown and maintained in the greenhouse. Temperature was maintained at 25°C for 14 h by light and at 15°C for 10 h in the dark; relative humidity (RH) was maintained between 60 and 70%.
Inoculation in kiwifruit tissues with Psa
Twig inoculation
To ascertain whether the method of wounding affects invasion and colonization of Psa-GFPuv, we created different injuries prior to inoculation, including inoculation by smearing unwounded lenticels, inoculation by dripping twigs after causing slight injury (to simulate natural rubbing), inoculation by smearing leaf scars, and inoculation by dripping after cutting. Approximately 1 cm above the inoculation sites, twigs were covered with moist sterile cotton and plastic wraps for 3 day. Each plant was inoculated once at a single inoculation site, and twigs treated correspondingly with PBS were used as controls. Each experiment consisted of three replicates of the different inoculation types and the experiment was repeated three times. The treated plants were kept in growth chambers with cycles of 14 h light and 10 h darkness. During the experiments, plants were maintained at 95% RH.
Inoculation of leaves and veins
Before inoculation, fully expanded kiwifruit leaves were rinsed three times by spraying with sterile water. Then, the bacterial suspension (2×107 CFU mL-1) was sprayed onto the upper and lower surface of non-wounded kiwifruit leaves using a hand-held sprayer. Leaf veins were inoculated through wounds using a sterile needle and then a drop of the bacterial suspension applied on the wound sites. In each experiment the different inoculation types were repeated five times and the experiment was repeated three times.
Quantitative analysis of Psa in kiwifruit twigs
In order to determine colonization of Psa-GFPuv in twigs, samples were collected at 3, 6, 24, and 72 HAI (hours after inoculation). For isolation, samples were surface sterilized by dipping in 70% ethanol for 5 min followed by immersion in 1% (wt/vol) Clorox for further 5 min and then washed thrice in sterile water and blotted with sterile filter paper. Samples were thoroughly ground in 5 mL of 0.1 M PBS. Serial dilutions (2 × 10−4, 10−5, 10−6, and 10−7) of bacterial suspensions (100 μL) were placed directly on BPA plates supplemented with KAN. Three plates per dilution were used. After incubation at 25°C for 2 d, the plates were illuminated with UV light and GFP-expressing colonies were counted. Bacterial cell numbers were calculated on the basis of the fresh weights of twig tissues (CFU·g-1 tissue).
Microscopic observation
For microscopy studies, leaves, veins and twigs were similarly inoculated as described above. The kiwifruit plants with inoculated twigs were cultivated in a chamber at 4°C and those plants with inoculated leaves were kept of 16°C. To observe Psa-GFPuv colonization in kiwifruit tissues by light and transmission electron microscopy (TEM), from each inoculation type 5 samples of twigs were collected 7, 15 and 25 DAI (days after inoculation), and leaf samples were taken 5 and 10 DAI. Samples were cut into small pieces (5 × 3 mm2) and processed for light microscopy and TEM as described by Kang and Buchenauer [33] and Ke et al. [34]. For TEM analysis, ultrathin sections of samples cut with a diamond knife were collected on copper grids. The grids were examined with a TEM 1230 microscope (JEOL) after contrasting with uranyl acetate and lead citrate.
Effects of temperature on the spread of Psa
Twig inoculation and treatment
The inoculation method followed the procedure described by Zhao et al. [9] with minor modifications. The twigs were subjected to one oblique cut with a sterile scalpel, and the wound was inoculated with a 40-μL droplet of bacterial suspension. To determine the effect of temperature on colonization and spread of Psa-GFPuv, the treated kiwifruit plants were placed at 4°C, 16°C, and 25°C in growth chambers and RH was maintained at 95%. The samples were collected every 3 for 24 HAI, then each day for the next 10 d, and thereafter every second day until 30 d. Starting at the inoculation site, tissues were cut in 2 cm sections for a total distance of 15 cm. The methods of isolation and quantification were the same as described above.
Leaf vein inoculation and treatment
Leaf veins were inoculated as described above. After inoculation plants were cultured in chambers at 16°C, 20°C, and 24°C at RH>95%. To visualize bacterial infection and migration in leaf veins, the treated leaves were directly examined and photographed under a fluorescence stereomicroscope (Leica MZ12) in a dark room each day after inoculation.
Results
Invasion and colonization of Psa-GFPuv in twigs kiwifruit tissues
Twigs
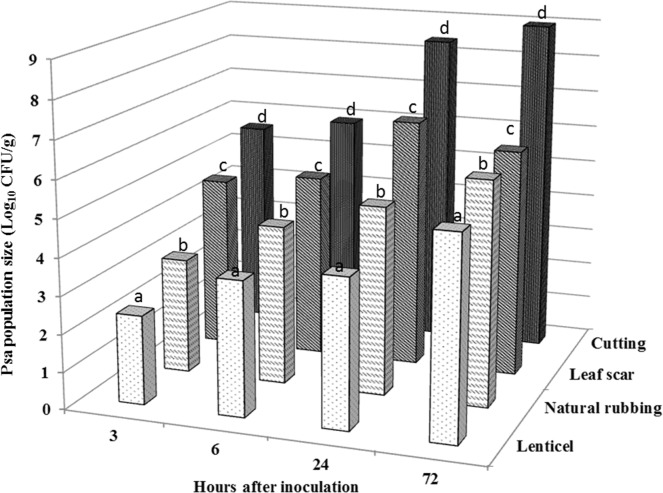
Although the pathogen was isolated from all experimental samples, the relative number of fluorescent colonies varied according to the inoculation site and/or wound type (Fig 1). In lenticels, 3 HAI 2.4±0.5×102 CFU·g-1 were determined; the number of bacterial colonies was significantly lower than in samples of leaf scar and in cut tissues (4.2±0.3×104 CFU·g-1 and 3.8±0.3×105 CFU·g-1, respectively). Between 3 and 72 HAI, the number of fluorescent colonies in the plant tissues exponentially increased. The highest number of colonies was obtained in cut tissues 72 HAI (9.9±0.2×108 CFU·g-1). At the same incubation period (72 HAI), the relative number of colonies in samples of lenticels, naturally rubbed tissues, and leaf scars were 2.2±0.4×105, 9.4±0.2×105 and 1.2±0.5×107 CFU·g-1, respectively. The results suggest that GFUuv-labeled Psa strain can enter via different wound types and natural openings.
Fig 1. Population size of the GFPuv-labeled Psa in kiwifruit twigs after inoculation of different wound types.
The GFPuv-labeled Psa were isolated and cultured in BPA plates supplemented with KAN (50 μg·mL−1) and colonies were counted under the fluorescence microscope. Values in columns followed by different letters were significantly different according to Fisher’s protected LSD test among lenticel, natural rubbing, leaf scar and cutting (P = 0.05).
Population dynamics of Psa-GFPuv in twigs
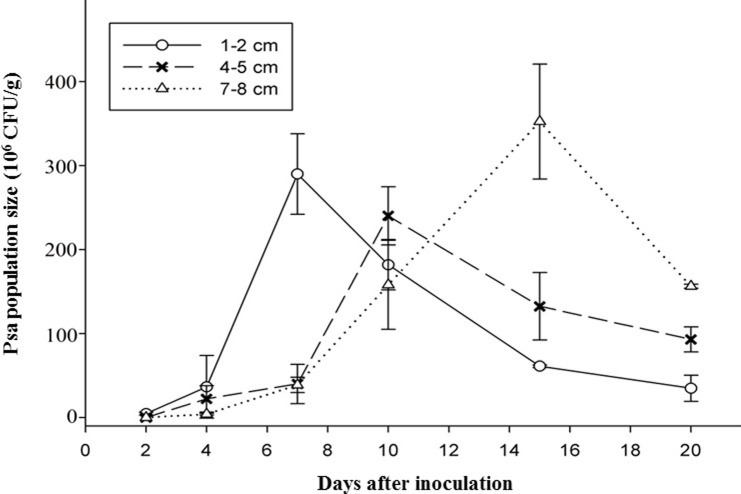
Population of the labeled Psa strain was quantified in different sections (1–8 cm) from the cut inoculation site of twigs during an incubation time of 2 to 20 days. In each twig section, bacterial cells were detected (Fig 2). After inoculation, bacterial colonies increased more rapidly in the section close to the inoculation site, whereas, increase in colony numbers was delayed in the middle and more remove twig section. At 1–2 cm from the inoculation site, the number of Psa-GFPuv reached 3.1×106 CFU·g-1 at 2 DAI; then, a constant increase until a maximum of 2.9×108 CFU·g-1 was reached 7 DAI. Similar population dynamics were observed in section 4–5 cm and 7–8 cm from the inoculation site; the highest population numbers in the middle and more distant sections were reached 10 and 15 DAI. After maximum of bacterial population was reached, colony numbers in each section decreased.
Fig 2. Quantification of the GFPuv-labeled Psa in kiwifruit twigs using the plating method (in BPA plates supplemented with 50 μg·mL−1KAN) following cutting inoculation.
Invasion and colonization of Psa in kiwifruit leaves
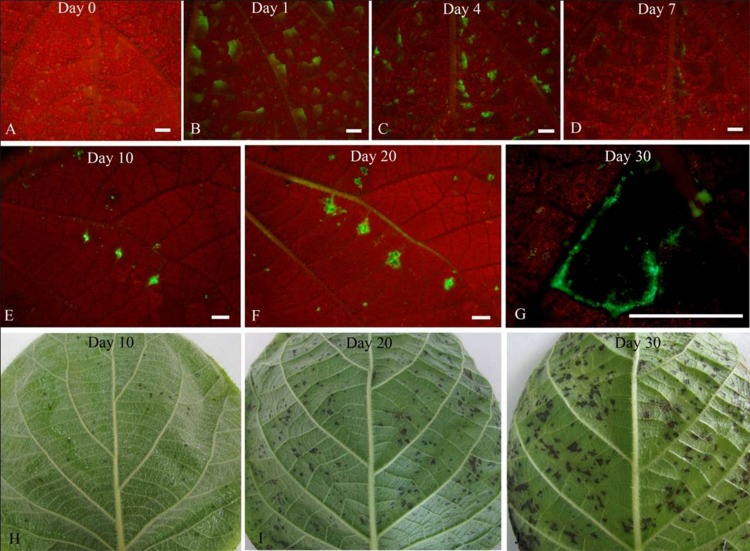
The colonization and spread of Psa-GFPuv was observed in kiwifruit leaves by using fluorescence stereomicroscopy (Fig 3). After spraying bacterial cells onto non-wounded leaf surfaces, green fluorescence (Psa-GFPuv strain) was clearly observed in the inoculated leaves; the fluorescence intensity gradually increased and reached a maximum at 4 DAI. At 7 DAI, green fluorescence became steadily weaker. Concomitant with the appearance of brown lesions at 10 DAI, there was an increase in the size of green fluorescent zones surrounding the lesions. Following extension of lesions, the size of the green fluorescence area increased and was even more intense than in veins at 20 DAI. However, at 30 DAI, the intensity of green fluorescence at the margin of lesions clearly decreased.
Fig 3. Formation and spread of the leaf lesions by spraying the GFPuv-labeled Psa.
The inoculated leaves were observed under a fluorescence stereomicroscope (Leica MZ12) at different days after inoculation. Bar = 2 mm
Microscopical observations
Infection of twigs by Psa
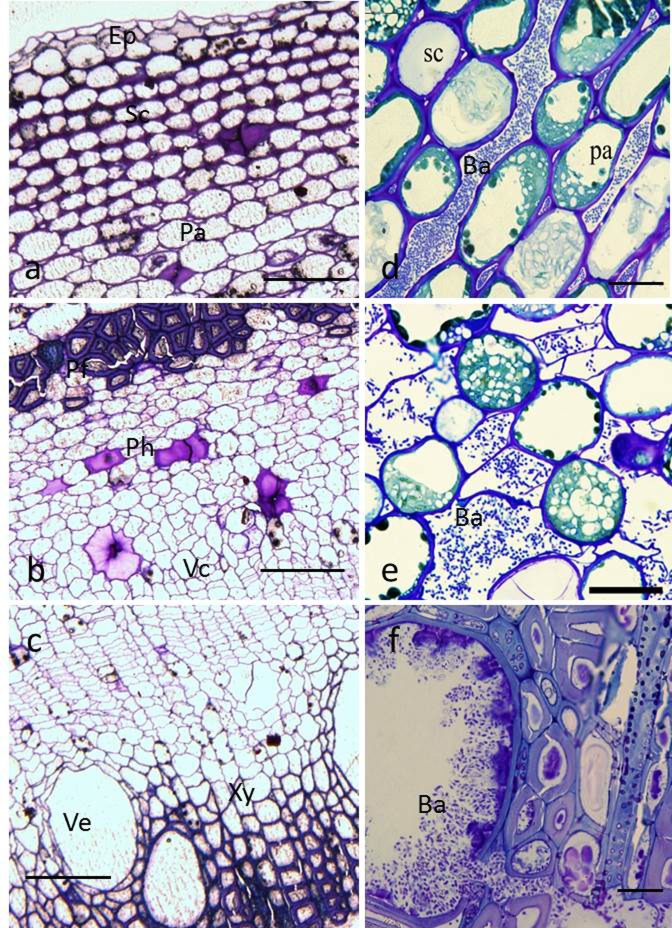
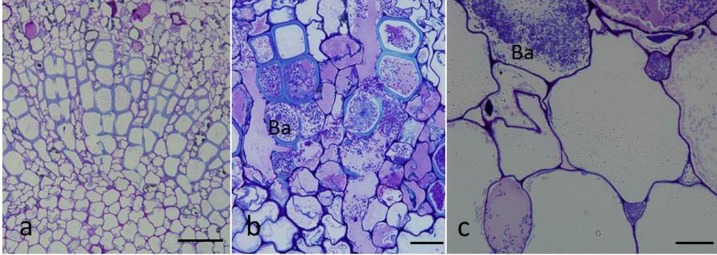
Light microscopic observations of thin sections of the non-infected kiwifruit twigs revealed the typical anatomy of kiwifruit bark tissue, consisting of epidermis, sclerenchyma, parenchyma (Fig 4A), phloem fiber, phloem, vascular cambium (Fig 4B), and xylem and xylem vessel (Fig 4C). Seven DAI of cut wounds, the pathogen had extensively colonized the intercellular parenchymal spaces (Fig 4D). Accompanied with lesion development, high bacterial cell numbers were observed in phloem cells and intercellular spaces (Fig 4E), and also in xylem vessels at 15 DAI (Fig 4F).
Fig 4. Light micrographs of healthy and infected kiwifruit bark.
(a–c), Transverse sections of healthy bark, showing typical anatomical tissues consisting of a typical epidermis (Ep), sclerenchyma (Sc), parenchyma (Pa), phloem fiber (Pf), phloem (Ph), vascular cambium (Vc), xylem (Xy), and vessel (Ve). Bar = 100 μm. (d–f), transverse sections of inoculated bark. (d), bacterial cells (Ba) in intercellular spaces of parenchyma; (e), bacterial cells in phloem cells and intercellular spaces; (f), bacterial cells tightly packed within xylem vessels. Bar = 20 μm.
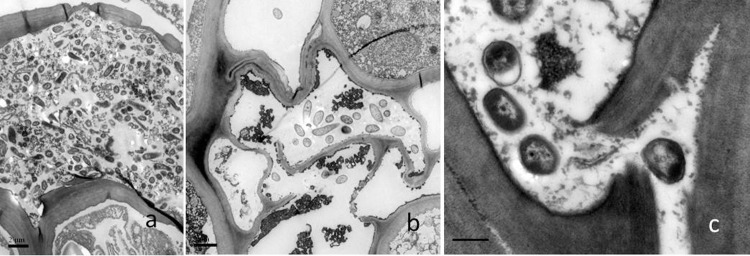
TEM observation of infected kiwifruit bark showed that host cells were aggressively colonized by GFPuv-labeled Psa. Five DAI, bacterial cells have been predominantly located in the intercellular space between cortical and parenchyma cells (Fig 5A). During Psa-GFPuv colonization, host cells tended to rupture and exhibited signs of organelle disintegration (Fig 5B and 5C).
Fig 5. Transmission electron micrographs of cross sections of kiwifruit twigs infected with the GFPuv-labeled Psa 5 DAI.
(a), Bacterial cells in intercellular spaces of the parenchyma. Bar = 2 μm; (b), bacterial colonization in parenchyma cells where the cell walls are ruptured and organelles have disintegrated. Bar = 2 μm; (c), bacterial colonization in intercellular spaces where the middle lamella tended to be ruptured. Bar = 0.5 μm.
Detection of Psa-GFPuv in leaves and veins
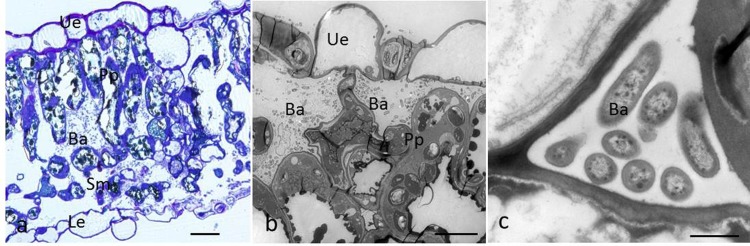
Sections of leaves inoculated by spraying demonstrated that bacterial cells massively accumulated in mesophyll cells of the foliage at 5 DAI (Fig 6A). High-magnification images revealed that bacterial cells were embedded within epidermis cells, palisade parenchyma, and the intercellular space (Fig 6B and 6C). During leaf infection, morphology of mesophyll cells changed due to the presence of very high numbers of bacteria that invaded the intercellular space (Fig 6B and 6C).
Fig 6. Light and transmission electron micrographs of a cross-section of kiwifruit leaf infected by the GFPuv-labeled Psa.
(a), Intercellular bacteria in the mesophyll cells at 5 dai. Bar = 50 μm; (b), bacterial colonization in the palisade parenchyma where the cells were abnormal. Bar = 10 μm. (c), bacteria that have colonized the intercellular space. Bar = 1 μm. Abbreviations: upper epidermis (Ue); palisade parenchyma (Pp); spongy mesophyll (Sm); lower epidermis (Le); bacteria (ba).
The vessels of the vascular bundle, cortex cells, and intercellular spaces were infected by Psa (Fig 7B and 7C). In contrast to the findings for healthy veins (Fig 7A), pathogen-induced morphological alterations, such as ruptured cell walls, were seen in infected host cells (Fig 7B).
Fig 7. Light micrographs of leaf veins colonized by GFPuv-labeled Psa cells.
(a), Cross-section of the healthy vein. Bar = 50 μm; (b), bacterial colonization of vessels in the vascular bundle. Bar = 20 μm; c, bacterial colonization of the cortex cells and intercellular spaces. Bar = 20 μm.
Effects of temperature on the spread of Psa-GFPuv in kiwifruit tissues
Effects of temperature on spread of bacterial cells in twigs
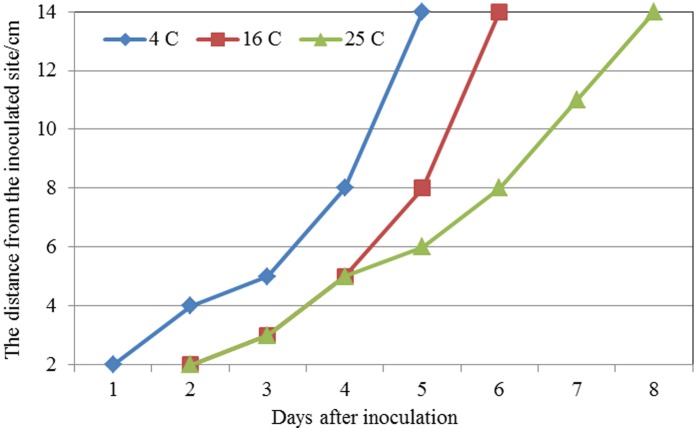
The data showed that temperature had a significant effect on the movement of the pathogen in kiwifruit twigs (Fig 8). Specifically, the rate of spreading is faster at 4°C compared to inoculation at 16°C or 25°C. Fluorescent colonies were isolated at a distance of 2 cm from the inoculation site 1 DAI when the inoculated kiwifruit twigs were incubated at 4°C; by contrast, it took 2 d for colonies to reach this distance when tissues were placed at 16°C and 25°C. A similar effect of temperature was noticed at the more distal sites (14 cm from the inoculation site). For example, Psa-GFPuv reached this distance in 5 DAI at 4°C, but took 6 d or 8 d when tissues were kept at 16°C or 25°C, respectively.
Fig 8. The migration of the GFPuv-labeled Psa in kiwifruit twigs in different temperature treatments by cutting inoculation.
Samples were taken 2 cm from the inoculation site. The GFPuv-labeled Psa were isolated and cultured in BPA plates supplemented with KAN (50 μg·mL−1) and colonies were counted under the fluorescence microscope.
Effects of temperature on the spread of Psa-GFPuv in leaf veins
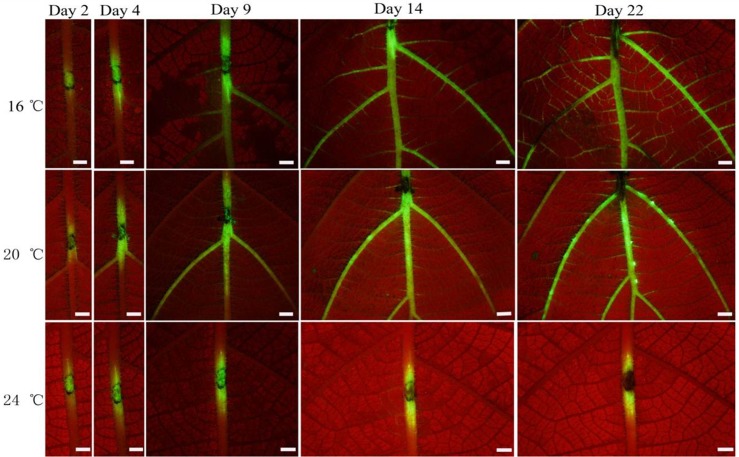
Bacterial cells could invade and colonize veins after wounding with a sterile needle; however, the incubation temperature influenced the degree of bacterial cells spreading. While green fluorescence was observed in the entire main vein and lateral veins 14 DAI at 16°C, it was present only in the main vein at 20°C (Fig 9). Increasing the temperature to 24°C, colonization of veins by the pathogen was clearly restricted to a narrow distant of the inoculation sites.
Fig 9. The GFPuv-labeled Psa colonization and migration in kiwifruit leaf veins.
The inoculated leaves were observed under a fluorescence stereomicroscope (Leica MZ12) at different days after inoculation. Bar = 2 mm.
Discussion
Weather conditions that favor bacterial infection are usually sporadic, being confined to particular seasons and are associated with rainy, wet and humid conditions. Once plants are infected, disease development is largely determined by temperature [35]. Psa is reported to cause disease at relatively low temperatures in kiwifruit [13,14]. Cankers in trunks and leaders are the most serious symptoms, which develop most rapidly in late winter and spring, because they girdle whole limbs and kill entire vines [15,36]. Serizawa et al. (1993) tested the effect of temperature from 10°C to 25°C on the disease development on new canes. These results suggested that the optimum temperature for growth of Psa on new canes was in the range of 10 to 20°C [14]. Psa populations in infected host tissues decreased when temperatures increased and, at temperatures above 25°C, Psa inoculations did not lead to exudate formation or infection in canes [14]. In Italy, Psa in cankers of kiwifruit trees usually die out in spring to summer when temperatures rise above 20°C [35]. This is not the case in New Zealand, which has a mild maritime, practically frost-free, climate; disease in canes is practically unchecked, the disease spreading in canes throughout the summer [35]. In our study, we found that the GFPuv-labeled Psa strain could invade and grow in the temperature range between 4 and 25°C in twigs. Epidemics occur under precisely prescribed conditions, usually appearing regionally or sub-regionally. According to our results, several important findings about the behavior of Psa could be found in terms of Chinese seasonal conditions compared with those in other countries. Only when the pathogen is distributed more widely in areas with a wider climatic range, it will be to establish possible in these areas for future planting and those to avoid.
However, to emphasize an important point, the result of the growth curve of Psa showed that the pathogen could grow in vitro in a temperature range from 4 to 35°C, the optimum temperature was at 25°C [32]. Obviously there is a great temperature difference between growth of Psa in vitro and in vivo. The main reason is that growth of the bacterium in the host tissue was abruptly inhibited at high temperature by the formation of wound-healing tissue surrounding the infected area. Serizawa et al. [15] reported that formation of wound healing tissue was highest in mid-summer (with a mean temperature of 25°C). Formation of wound-healing tissue gradually ceased when the temperature dropped gradually from 22 to 20°C; and no healing of the affected tissue was observed when the temperature dropped to 15°C or below. In New Zealand, researchers [37] found a similar relationship between spread of Psa and temperature in kiwifruit tissues. More rapid and complete healing occurred on pruning cuts made in late spring and summer than on early spring cuts. This study also showed that bacterial lesions were confined where callus formation rapidly occurred.
Several researchers reported that dark angular necrotic spots in leaves were often accompanied by yellow chlorotic halos around the outer edge of the spots, especially in early spring and summer [35,38,39]. In Japan, Serizawa and Ichikawa [14] found that bacterial populations in leaf lesions were highest in late spring and autumn; when the mean temperature over a period of 10 days prior to isolation ranged between 20°C and 24°C, but, Psa populations dropped rapidly during summer. Once the temperature increased over 25°C, Psa could either not be detected in some lesions or in other lesions bacterial population was very low. To date, there is very little information about the effect of temperature on the spread of Psa in kiwifruit leaf veins. Using fluorescence stereomicroscopy, we visualized spread of bacteria in veins at 16 to 25°C. At the lower temperature (16°C), Psa rapidly spread from the inoculated site to the main and lateral veins. At 25°C, fluorescence was restricted close to the inoculated site.
Psa appeared to be capable colonizing the host all year round and to migrate systemically from young leaves to twigs. Unprotected lenticels, fruit stalks and leaf scars, as well as pruning wounds and tissues surrounding the elastic laces were easily colonized by the pathogen [40]. Consistent with previous reports on the routes by which pathogenic bacteria enter plants [37,41], we found that Psa could enter the twigs though natural openings and wounds. Strikingly, however, we found that the type of wound has a remarkable influence on the amount of bacterial colonization. Of all wound types studied, cutting was the most favorable for Psa to colonize the host tissues. Within this context, some agronomical techniques (i.e., pruning, tying of young twigs) causing wounds to the tree greatly contribute to increase the possibility of colonizing of the plant [37].
Using microscopic techniques, we were able to localize the pathogen and to observe multiplication and accumulation of Psa cells in the twig, leaf, and vein tissues; this approach also allowed us to monitor the host response. Through microscopic examination, Psa cells were present in all regions of the cane down to the cambium layer in two A. chinensis cultivars; but no bacterial cells were detected in the xylem or woody parts of the cane, however, in heavily infected canes in some samples bacterial cells were found [37]. We also showed that the GFPuv-labeled Psa strain colonized twigs intensively after wound inoculation; especially, high bacterial cell numbers were observed in xylem vessels. Colonization of xylem and phloem vessels may explain that the pathogen can spread systemically to young twigs within few minutes after penetration. Furthermore, Psa-GFPuv could effectively colonize internal portions of kiwifruit twigs and, subsequently, migrate to the leader and main trunk during the following season [42]. In addition, Psa bacterial cells also colonized the intra- and intercellular spaces, and this activity was associated with rupture of the cell walls of the plant tissue [43–44]. The pathogenesis factors of Psa causing cell wall ruptures will be investigated in future studies.
In conclusion, we suggest that prevention of wounds or protecting them as well as a temperature-controlled environment should form part of new strategies aimed at better management of kiwifruit orchards. Our fluorescent tracking system can be further exploited to test other environmental or man-made factors for the effects on the pathogenicity of bacterial plant pathogens or resistance of new cultivars.
Author Contributions: conceived and designed the experiments: XG LH
Author Contributions: performed the experiments: XG QLH ZZ HQ
Author Contributions: analyzed the data: XG QMH
Author Contributions: contributed reagents/materials/analysis tools: QLH XK
Author Contributions: wrote the manuscript: XG QLH LH
Acknowledgments
We are grateful to Prof. Heinrich Buchenauer for review of the manuscript.
Data Availability
All relevant data are within the paper.
Funding Statement
This study was supported by the Fundamental Research Funds for the Central Universities of China (No. QN2013007), Natural Science Basic Research Plan in Shaanxi Province of China (No. 2014JQ2-3011), and the 111 project from the Education Ministry of China (No. B07049).
References
- 1.Takikawa Y, Serizawa S, Ichikawa T, Tsuyumu S, Goto M. Pseudomonas syringae pv. actinidiae pv. nov.: the causal bacterium of canker of kiwifruit in Japan. Ann. Phytopathol. Soc. Japan. 1989; 55(4): 437–444. [Google Scholar]
- 2.Koh YJ, Chung HJ, Cha BJ, Lee DH. Outbreak and spread of bacterial canker in kiwifruit. Korean Journal of Plant Pathology. 1994; 10: 68–72. [Google Scholar]
- 3.Scortichini M. Occurrence of Pseudomonas syringae pv. actinidiae on kiwifruit in Italy. Plant Pathology. 1994; 43(6): 1035–1038. [Google Scholar]
- 4.Wang ZS, Tang XZ, Liu SJ. Identifcaton of the pathogenic bacterium for bacterial canker on actinidia in Sichuan. Journal of Southwest Agricultural University. 1992; 14(6): 500–503. (in chinese) [Google Scholar]
- 5.Scortichini M, Marcelletti S, Ferrante P, Petriccione M, Firrao G. Pseudomonas syringae pv. actinidiae: a reemerging, multifaceted, pandemic pathogen. Molecular plant pathology. 2012; 13(7): 631–640. 10.1111/j.1364-3703.2012.00788.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balestra GM, Mazzaglia A, Quattrucci A, Renzi M, Rossetti A. Current status of bacterial canker spread on kiwifruit in Italy. Australasian Plant Disease Notes. 2009; 4(1): 34–36. [Google Scholar]
- 7.Vanneste JL. Pseudomonas syringae pv. actinidiae (Psa): a threat to the New Zealand and global kiwifruit industry. New Zealand Journal of Crop and Horticultural Science. 2012; 40(4): 265–267. [Google Scholar]
- 8.Liu LZ, Chu D, Li YZ, Wang TW, Song XB. Risk analysis of kiwifruit bacterial canker. Journal of Catan Strophology. 2007; 22: 91–94. (in chinese) [Google Scholar]
- 9.Zhao ZB, Gao XN, Huang QL, Huang LL, Qin HQ, Kang ZS. Identification and characterization of the causal agent of bacterial canker of kiwifruit in the Shaanxi province of China. Journal of Plant Pathology. 2013; 95(1):155–162. [Google Scholar]
- 10.Wang ZS, Tang XF, Liu SJ. Identification of the pathogenic bacterium for bacterial canker on Actinidia in Sichuan. Journal of Southwest Agricultural University. 1992; 14(6): 500–503. (in chinese) [Google Scholar]
- 11.Serizawa S, Ichikawa T. Epidemiology of bacterial canker of kiwifruit, 1: Infection and bacterial movement in tissue of new canes. Ann. Phytopathol. Soc. Japan. 1993a; 59: 452–459. [Google Scholar]
- 12.Serizawa S, Ichikawa T. Epidemiology of bacterial canker of kiwifruit. 2: The most suitable times and environments for infection on new canes. Ann. Phytopathol. Soc. Japan. 1993b; 59: 460–468. [Google Scholar]
- 13.Serizawa S, Ichikawa T. Epidemiology of bacterial canker of kiwifruit. 3: The seasonal changes of bacterial population in lesions and its exudation from lesion. Ann. Phytopathol. Soc. Japan. 1993c; 59: 469–476. [Google Scholar]
- 14.Serizawa S, Ichikawa T. Epidemiology of bacterial canker of kiwifruit. 4: Optimum temperature for disease development on new canes. Ann. Phytopathol. Soc. Japan. 1993d; 59: 694–701. [Google Scholar]
- 15.Serizawa S, Ichikawa T, Suzuki H. Epidemiology of bacterial canker of kiwifruit. 5: Effect of infection in fall to early winter on the disease development in branches and trunk after winter. Ann. Phytopathol. Soc. Japan. 1993e; 60: 237–244. [Google Scholar]
- 16.Serizawa S, Ichikawa T, Takikawa Y, Tsuyumu S, Goto M. Occurrence of bacterial canker of kiwifruit in Japan: Description of symptoms, isolation of the pathogen and screening of bactericides. Ann. Phytopathol. Soc. Japan. 1989; 55: 427–443. [Google Scholar]
- 17.Koh YJ, Park S, Lee D. Characteristics of bacterial canker of kiwifruit occurring in Korea and its control by trunk injection. Korean Journal of Plant Pathology. 1996; 12: 324–330. [Google Scholar]
- 18.Rees-George J, Vanneste JL, Cornish DA, Pushparajah IPS, Yu J, Templeton MD, et al. Detection of Pseudomonas syringae pv. actinidiae using polymerase chain reaction (PCR) primers based on the 16S-23S rDNA intertranscribed spacer region and comparison with PCR primers based on other gene regions. Plant Pathol. 2010; 59: 453–464. [Google Scholar]
- 19.Gallelli A, L’Aurora A, Loreti S. Gene sequence analysis for the molecular detection of Pseudomonas syringae pv. actinidiae: developing diagnostic protocols. Journal of Plant Pathology. 2011; 93: 425–35. [Google Scholar]
- 20.Biondi E, Galeone A, Kuzmanovic N, Ardizzi S, Lucchese C, Bertaccini A. Pseudomonas syringae pv. actinidiae detection in kiwifruit plant tissue and bleeding sap. Annals of Applied Biology. 2013; 162: 60–70. [Google Scholar]
- 21.Gallelli A, Talocci S, Pilotti M, Loreti S. Real-time and qualitative PCR for detecting Pseudomonas syringae pv. actinidiae isolates causing recent outbreaks of kiwifruit bacterial canker. Plant Pathology. 2014; 63(2): 264–276. [Google Scholar]
- 22.Zhao Z, Gao XN, Yang DH, Huang LL, Qin HQ, Kang ZS. Field detection of canker-causing bacteria on kiwifruit trees: Pseudomonas syringae pv. actinidiae is the major causal agent. Crop Protection. 2015; 75: 55–62. [Google Scholar]
- 23.Mazzaglia A, Renzi M, Balestra GM. Comparison and utilization of different PCR-based approaches for molecular typing of Pseudomonas syringae pv. actinidiae strains from Italy. Canadian Journal of Plant Pathology. 2011; 33(1): 8–18. [Google Scholar]
- 24.Vanneste JL, Yu J, Cornish DA. Molecular characterisations of Pseudomonas syringae pv. actinidiae strains isolated from the recent outbreak of bacterial canker on kiwifruit in Italy. New Zealand Plant Protection. 2010; 63: 7–14. [Google Scholar]
- 25.Vanneste JL, Yu J, Cornish DA, Tanner DJ, Windner R, Chapman JR, et al. Identification, virulence and distribution of two biovars of Pseudomonas syringae pv. actinidiae in New Zealand. Plant Disease. 2013. 97: 708–719. [DOI] [PubMed] [Google Scholar]
- 26.Ferrante P, Scortichini M. Redefining the global populations of Pseudomonas syringae pv. actinidiae based on pathogenic, molecular and phenotypic characteristics. Plant Pathology. 2015; 64: 51–62. [Google Scholar]
- 27.Ferrante P, Takikawa Y, Scortichini M. Pseudomonas syringae pv. actinidiae strains isolated from past and current epidemics to Actinidia spp. reveal a diverse population structure of the pathogen. European Journal of Plant Pathology. 2015; 10.1007/s10658-015-0643-6 [DOI] [Google Scholar]
- 28.Mazzaglia A, Studholme DJ, Taratufolo MC, Cai R, Almeida NF, Goodman T, et al. Pseudomonas syringae pv. actinidiae (PSA) isolates from recent bacterial canker of kiwifruit outbreaks belong to the same genetic lineage. PLoS One. 2012; 7(5): e36518 10.1371/journal.pone.0036518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Butler MI, Stockwell PA, Black MA, Day RC, Lamont IL, Poulter RT. Pseudomonas syringae pv. actinidiae from recent outbreaks of kiwifruit bacterial canker belong to different clones that originated in China. PloS one. 2013; 8(2): e57464 10.1371/journal.pone.0057464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCann HC, Rikkerink EH, Bertels F, Fiers M, Lu A, Rees-George J, et al. Genomic analysis of the kiwifruit pathogen Pseudomonas syringae pv. actinidiae provides insight into the origins of an emergent plant disease. PLoS pathogens. 2013; 9(7): e1003503 10.1371/journal.ppat.1003503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carmeron A, Sarojini V. Pseudomonas syringae pv. actinidiae: chemical control, resistance mechanisms and possible alternatives. Plant Pathology. 2014; 63: 1–11. [Google Scholar]
- 32.Huang QL, Gao XN, Zhao ZB, Qin HQ, Huang LL. Transformed GFPuv into Pseudomonas syringae pv. actinidiae and its biological characteristics and colonization in soil and roots of kiwifruit. Scientia Agricultura Sinica. 2013; 46(2): 282–291. (in chinese) [Google Scholar]
- 33.Kang Z, Buchenauer H. Cytology and ultrastructure of the infection of wheat spikes by Fusarium culmorum. Mycol. Res. 2000; 104(9): 1083–1093. [Google Scholar]
- 34.Ke XW, Huang LL, Han QM, Gao XN, Kang ZS. Histological and cytological investigations of the infection and colonization of apple bark by Valsa mali var. mali. Australasian Plant Pathology. 2013; 42(1): 85–93. [Google Scholar]
- 35.Young JM. Pseudomonas syringae pv. actinidiae in New Zealand. Journal of Plant Pathology. 2012; 94(1, Supplement), S1.5–S1.10. [Google Scholar]
- 36.Qin HQ, Gao XN, Zhao ZB, Zhu SC, Li JM. The prevalence dynamics and rules of bacterial canker of kiwi fruit in Shaanxi. Acta Phytophylacica Sinica. 2013; 40(3): 225–230. (in chinese) [Google Scholar]
- 37.Froud KJ, Everett KR, Tyson JL, Beresford RM, Cogger N. Review of the risk factors associated with kiwifruit bacterial canker caused by Pseudomonas syringae pv. actinidiae. New Zealand Plant Protection. 2015; 68: 313–327. [Google Scholar]
- 38.Everett KR, Taylor RK, Romberg MK, Rees-George J, Fullerton RA, Vanneste JL, et al. First report of Pseudomonas syringae pv. actinidiae causing kiwifruit bacterial canker in New Zealand. Australasian Plant Disease Notes 2011; 6: 67–71. [Google Scholar]
- 39.Donati I, Buriani G, Cellini A, Mauri S, Costa G, Spinelli F. New insights on the bacterial canker of kiwifruit (Pseudomonas syringae pv. actinidiae). Journal of Berry Research 2014; 4: 53–67. [Google Scholar]
- 40.Ferrante P, Fiorillo E, Marcelletti S, Marocchi F, Mastroleo M, Simeoni S, et al. The importance of the main colonization and penetration sites of Pseudomonas syringae pv. actinidiae and prevailing weather conditions in the development of epidemics in yellow kiwifruit, recently observed in central Italy. Journal of Plant Pathology. 2012; 94(2): 455–461. [Google Scholar]
- 41.Renzi M, Copini P, Taddei AR, Rossetti A, Gallipoli L, Mazzaglia A, et al. Bacterial canker on kiwifruit in Italy: anatomical changes in the wood and in the primary infection sites. Phytopathology. 2012; 102(9): 827–840. 10.1094/PHYTO-02-12-0019-R [DOI] [PubMed] [Google Scholar]
- 42.Spinelli F, Donati I, Vanneste JL, Costa M, Costa G. Real time monitoring of the interactions between Pseudomonas syringae pv. actinidiae and Actinidia species. Acta Horticulturae. 2011; 913: 461–466. [Google Scholar]
- 43.Collmer A, Keen NT. The role of pectic enzymes in plant pathogenesis. Annual review of phytopathology. 1986; 24(1): 383–409. [Google Scholar]
- 44.von Bodman SB, Bauer WD, Coplin DL. Quorum sensing in plant-pathogenic bacteria. Annual review of phytopathology. 2003; 41(1): 455–482. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.