Abstract
Introduction
Studies have reported associations between serum anticholinergic activity (SAA) and decline in cognitive performance, delirium, and functional impairment. The aim of this meta-analysis was to explore and quantify associations between SAA and adverse cognitive and functional outcomes in older people.
Materials and Methods
A literature search in Ovid MEDLINE, EMBASE, PsycINFO and IPA from 1946–2014 was completed. The primary outcomes of interest were cognitive and functional adverse outcomes associated with SAA in older people aged 55 years and above. The Cochrane Risk-Bias assessment tool was used to assess bias in randomised controlled trials (RCTs). The Newcastle-Ottawa Scale was used to assess the quality of non-RCTs. Meta-analyses were conducted for RCTs and cohort studies separately. Heterogeneity was assessed using I2 tests.
Results
The primary electronic literature search identified a total of 1559 records in the 4 different databases. On the basis of full-text analysis, 33 studies that met the inclusion criteria. The review included 4 RCTs, 5 prospective cohort studies, 3 longitudinal cohort studies, 17 cross-sectional studies, and 4 case-control studies. Twenty-four of the retrieved studies examined an association between SAA and cognitive outcomes, 2 studies examined an association with SAA and functional outcomes and 8 studies examined associations between SAA and both cognitive, and functional outcomes. The meta-analysis on 4 RCTs showed no association with higher SAA and cognitive performance (I2 = 89.38%, H2 = 25.53 and p-value = <0.05) however, the pooled data from 4 observational studies showed elevated SAA was associated with reduced cognitive performance (I2 = 0.00%, H2 = 3.37 and p-value = 0.34).
Conclusion
This systematic review summarises the limitations of the SAA on predicting cognitive and functional outcomes in older people. SAA measured by receptor bioassay is flawed and its use in older people with multimorbidity and polypharmacy is questionable.
Introduction
Medicines with anticholinergic properties are often prescribed to older people for various medical conditions [1, 2]. Anticholinergic burden refers to the cumulative exposure to multiple medicines with anticholinergic properties [3–5]. In early 1980s, a radioreceptor assay, now commonly referred to as serum anticholinergic activity (SAA), was developed by Tune and colleagues to quantify an individual’s overall anticholinergic burden contributed by the cumulative effect of drugs and their metabolites, and potentially by unknown endogenous factors [6, 7]. SAA is generally measured in terms of atropine equivalents (pmol/ml) and ranges from the lowest detectable limit of 0.25 pmol/ml to 25.00 pmol/ml [6, 8–10].
The evidence between association of SAA and adverse outcomes is mixed and mostly derived from case-control or cohort studies. Higher SAA levels have shown to be positively correlated with cognitive impairment in older presurgical patients [11], Alzheimer's patients [12], and in nursing home residents [13, 14]. A study by Chew et al. [8] reported a correlation between SAA and cognitive decline even in moderately to severely demented patients. Rovner et al. reported that higher SAA scores were associated with lower Mini Mental State Examination (MMSE) scores of 24 or less [14]. A community-based cross-sectional study by Mulsant et al. found an association between SAA and decline in MMSE scores with varying degrees of SAA in 90% of the study population [7]. In contrast, Nishtala et al. also found that high SAA medicines were not often associated with neuropsychiatric events [15]. A recent study found no significant difference in SAA levels measured in cerebrospinal fluid and serum of participants with and without delirium [16] A recent cross-sectional study conducted in Finland also found no association between SAA levels and MMSE scores, even though the study reported relatively higher SAA levels [17].
SAA is recognised as a biomarker for cognitive impairment, but concerns whether peripheral SAA measurements predict central nervous system (CNS) effects have been debated [18, 19]. Importantly, no definite threshold level of SAA has been identified that predicts delirium or cognitive dysfunction [20, 21]. There have also been concerns about the variability in SAA bioassay methods, Gerresten and Pollock [19] suggested the use of human cloned selective muscarinic receptor subtypes to improve the specificity and reliability of the bioassay for predicting CNS effects. SAA reflects the state in the peripheral blood and is not necessarily associated with conditions in the central nervous system [22]. Literature shows only limited studies are available on finding associations between SAA and functional outcomes [23, 17] and there is a lack of systematic review that has been identified in this area. Functional impairment may be caused by peripheral adverse effects like accommodation difficulties, tachycardia and gait disturbance [24]. Hence, in this review we have included and assessed the functional outcomes such as activity of daily living, physical function, and psychomotor function to answer the existing gap.
Objectives
The validity of Serum Anticholinergic Activity (SAA) as a biomarker for cognitive and functional impairment in older people is a subject of incessant debate [20, 21, 25]. Therefore, a systematic review is needed to appraise and summarise the current evidence regarding SAA and associations with adverse cognitive and functional outcomes such as change in cognition, delirium, and activities of daily living in older people.
We therefore conducted (1) a systematic review of published studies of randomised and non-randomised controlled trials that assessed the association between SAA and adverse outcomes in older people; and (2) a meta-analysis to quantify the association between elevated SAA and its impact on cognition.
Materials and Methods
Data sources and search strategy
A literature search in Ovid MEDLINE, EMBASE, PsycINFO and International Pharmaceutical Abstracts (IPA) covering the period 1946—September 2014 was completed to identify SAA and adverse outcomes in older people using the keywords; (anticholinergic*.mp), AND (cogniti#.mp) AND ("aged/ or elder*.mp. or frail.mp. or geriatric*.mp. or seniors.mp. or “old#.mp "). The search was then limited to English language AND humans. The MEDLINE search strategy is presented in S1 Table.
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) criteria was employed to report this systematic review and meta-analyses [26]. A protocol was not registered and ethics review was not required for conducting this study. A PRISMA checklist for systematic review is depicted as supplementary information.
Following the primary systematic search to identify relevant studies, the reference list from each study was searched to identify potentially relevant articles examining the association between SAA and adverse outcomes. Web of Science and Google Scholar were used to track prospective citing of references of selected articles. Potentially relevant articles identified were then reviewed according to the predefined inclusion and exclusion criteria.
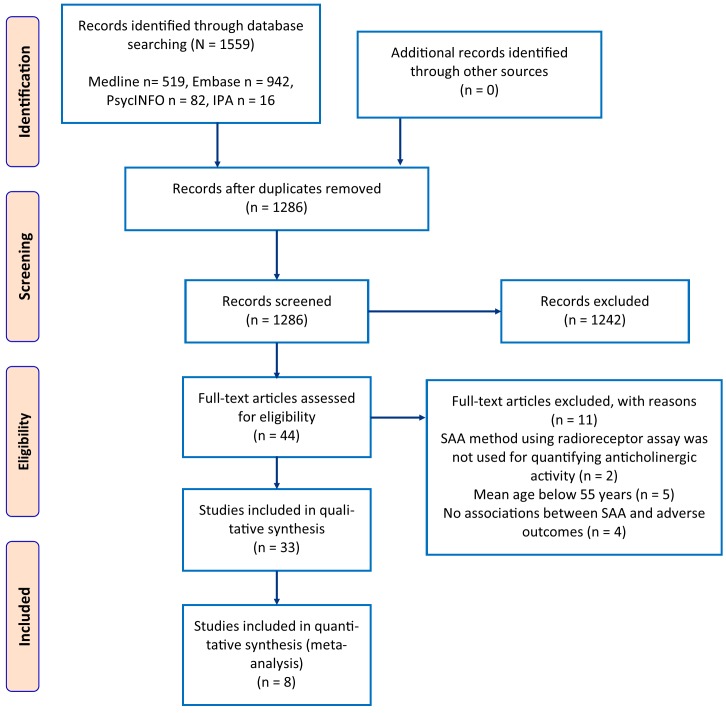
A PRISMA flowchart of study selection process is depicted in Fig 1.
Fig 1. PRISMA flow diagram of study selection process.
Study screening and selection
The title and abstract of the publication were screened by two independent reviewers for its eligibility for inclusion in the review process (M.S.S. and P.S.N.). The eligible studies were subject to a thorough full text analysis for relevance and pre-defined inclusion criteria. Studies that met the following criteria were included in the final review.
Studies that include participants of either sex, of mean age 55 years or older and living in community or primary care or nursing homes or hospital settings.
Studies that reported the use of SAA as quantification method either using a radioreceptor assay technique or in vitro measurement of individual muscarinic receptor activity.
Studies that included a tool to assess cognitive or/and functional outcomes.
Any intervention that employed SAA as a quantification tool to measure adverse clinical outcomes.
We excluded articles in languages other than English, as well as case reports, commentaries, letters and editorials from the primary search and citation analysis. Anticholinergic rating scales based predominantly on SAA were also excluded from the review.
Eleven studies [27–37] were excluded from the analysis as they failed to meet the inclusion criteria. The studies excluded considered age less than 55 years (n = 5), where SAA was not the primary method employed to quantify anticholinergic burden (n = 2) and where adverse outcomes were not examined (n = 4). A detailed summary of excluded studies is depicted in S2 Table.
The primary aim of this review was to evaluate cognitive and functional adverse outcomes such as change in cognition, delirium, and activities of daily living associated with SAA in older people. In this study, association between delirium and SAA was also assessed as a primary outcome measure.
Risk of bias assessment
The quality of the included studies was critically appraised by two authors (M.S.S. and P.S.N.). The Cochrane Risk of Bias tool [38] was used to assess the methodological quality of RCTs. The Newcastle-Ottawa scale [39] which consists of three broad criterions on selection, comparability and study outcome (cohort studies) or based on exposure (case-control studies) was used to assess the quality of the non-RCTs. Differences between review authors concerning eligibility were reviewed by the third author (T.Y.C.) and decisions were made by consensus.
Data extraction and synthesis
Two reviewers (M.S.S. and P.S.N.) compiled data onto standardised format based on study population, study design, sample size, study duration, mean age, mean SAA and adverse outcome measures. The primary outcomes of interest were cognitive and functional adverse outcomes including change in cognition, delirium, and activities of daily living associated with SAA quantified by using radioreceptor assay or in vitro muscarinic receptor activity assay.
A citation analysis was performed to identify and compare the clinical utility of SAA and to evaluate its association with adverse outcomes in older people. Studies that used the SAA for assessing the adverse outcomes in older people aged 55 years and above are reported in this review.
Statistical analysis
For meta-analysis, the required standard deviations (SD) and mean values were extracted from the included studies. We contacted the authors for information that were not shown or derivable from the original publication. From the extracted study information, statistical analysis was pooled for doing a meta-analysis, if there were minimum three studies assessing the same outcome measure.
The data was meta-analysed using the package METAFOR in R 3.1.2. The data from 3 RCT studies were pooled to quantify the impact of SAA on cognitive outcomes. A separate meta-analysis was completed for observational studies that reported the same outcome measures. The primary outcome for measure of cognitive performance was change in MMSE scores. The means and standard deviations (SD) of MMSE scores in the intervention and control groups were converted into a standardised effect size. A random effects model was used to combine the standardised effect sizes with 95% confidence interval. Heterogeneity was assessed using I2 statistics. A statistically significant I2 suggests that variation of standardised effect sizes among the included studies is due to the uniqueness of each study (i.e. a significant heterogeneity) rather than random variation.
Funnel plot (scatterplot of the intervention effect estimate of individual studies against outcome measure of each study size, is a visual aid for detecting bias or systematic heterogeneity) was used to identify studies that were potential outliers and over-presented in the random effect modelling. All data were distributed symmetrically in the funnel plot and therefore, publication bias was not evident. MMSE scores outside the funnel-shaped region were excluded, and the combined standardised effect size was recalculated without the influential data by random effect modelling. Studies were also excluded if the MMSE scores were not reported as means and SD, or information provided was incomplete.
Results
Search results
The primary search using four databases identified a total of 33 studies as being relevant to this systematic review. A qualitative description of the included studies is shown in Table 1.
Table 1. Qualitative summary of included study characteristics between serum anticholinergic activity and cognitive and functional outcomes.
SAA = Serum Anticholinergic Activity; MMSE = Mini Mental State Examination; IQCODE = Informant questionnaire on Cognitive Decline in the Elderly; SPMSQ = Short Portable Mental Status Questionnaire; SIB = Severe Impairment Battery; CAM = Confusion Assessment Method; BI = Barthel Index; AD = Alzheimer’s disease; FAST = Functional Assessment Staging; BEHAVE-AD = Behavioural Pathology in Alzheimer’s Disease Rating Scale; BARS = Brief Agitation Rating Scale; POCD = Postoperative cognitive decline; CAM-ICU = Confusion Assessment Method for critically ill patients in Intensive Care Unit; MCI = Mild Cognitive Impairment; DI = Delirium Index; SDM = Symbol Digit Modalities; SDC = Saskatoon Delirium Checklist; IQR = Interquartile range; PGDRS = Psychogeriatric Dependency Rating Scale; DRS = Dementia Rating Scale; WMH = White Matter Hyperintensities; ICU = Intensive care unit; RCT = Randomised Controlled Trial; ADL = Activities of Daily Living; IADL = Instrumental Activities of Daily Living; CERAD = Consortium to Establish a Registry for Alzheimer Disease; GDS = Geriatric Depression Scale; SD = Standard Deviation; DSM = Diagnostic and Statistical Manual of Mental Disorders.
| Studies used SAA | Study design | Study setting / participants | Mean (SD) age (years) | Study duration | Adverse outcome(s) studied | Significant association |
|---|---|---|---|---|---|---|
| RCT | ||||||
| Kersten et al., Norway 2013 [41] | RCT, single-blinded | nursing home residents with AD, N = 87 | 86.0 ± 5.6 | 8 weeks | mouth dryness (whole-mouth resting saliva flow) | – |
| cognitive function (MMSE, CERAD) | – | |||||
| Lackner et al., USA 2008 [40] | RCT, double-blinded | nursing home, N = 50 | 88.6 ± 6.2 | 4 weeks | cognitive functions, (MMSE, SIB) | – |
| delirium (CAM, BARS) | – | |||||
| Miller et al., Canada 1988 [11] | RCT, double blinded | inpatients, N = 36 | 67.0 ± 5.9 | unclear | cognitive performance; MMSE | – |
| SDM | – | |||||
| SDC (delirium) | + | |||||
| Rey Auditory-verbal learning test | + | |||||
| Tollefson et al., USA 1991 [13] | RCT, parallel-arm | nursing home, N = 34 | 79.0 ± 9.7 | 4 weeks | cognitive performance (MMSE) | – |
| Longitudinal cohort | ||||||
| Golinger et al., USA 1987 [46] | Longitudinal cohort | surgical ICU, N = 25 | 58.1 (29–76) | 3 months | Delirium (DSM) | + |
| Kashyap et al., Canada 2014 [48] | Longitudinal cohort | outpatient clinic, N = 121 | 71 ± 7.2 | 1-year | change in cognition | – |
| van Munster et al., Netherlands 2012 [47] | Longitudinal study | hospitalised pts, N = 142 | 83.9 ± 6.9 | 3 ½ years | delirium (CAM) | – |
| Cross-sectional | ||||||
| Chew et al., USA 2005 [8] | Cross-sectional | geropsychiatric ward, N = 26 (demented pts) | 83.6 ± 5.8 | 2 year and 2 months | cognitive function (MMSE) | + |
| SIB | – | |||||
| Flacker et al., USA 1998 [51] | Cross-sectional | medical unit, N = 67 | 85.5 ± 6.1 | 3 ½ months | ADL | – |
| Flacker et al., USA 1999 [52] | Cross-sectional | nursing home, N = 22 | 88.0 ± 4.5 | 1 year | cognitive performance scale | + |
| SAA level for acute illness | + | |||||
| Hori et al., Japan 2011 [57] | Cross-sectional | hospital visited AD patients, N = 76 | 74.3 ± 8.1 | 1 year 11 months | cognitive and psychiatric symptoms; MMSE, BEHAVE-AD | + |
| FAST | + | |||||
| Kersten et al., Norway 2013 [58] | Cross-sectional | nursing home residents, N = 87 | 86.0 ± 5.6 | 1 year | mouth dryness | + |
| MMSE, CERAD | – | |||||
| functional outcome (ADL) | – | |||||
| Konishi et al., Japan 2010 [55] | Cross-sectional | psychogeriatric inpatients with AD, N = 76 | 78.9 ± 7.2 (SAA > 1.95 nmol); 77.9 ± 7.1 (SAA ≤1.95 nmol) | 1 year and 10 months | cognitive functions (MMSE) | + |
| FAST score | + | |||||
| Lampela et al., Finland 2013 [17] | Cross-sectional | community-dwelling, N = 621 | 81.7 ± 4.9 | 3 years | adverse events | – |
| cognitive function (MMSE, GDS) | – | |||||
| functional outcomes (ADL, IADL) | – | |||||
| Mangoni et al., Netherlands 2013 [59] | Cross-sectional | hospitalised patients with hip fracture, N = 71 | 84 ± 6 | 3 ½ years | cognitive impairment (IQCODE/ MMSE) | + |
| physical function (Katz ADL) | + | |||||
| Mulsant et al., USA 2003 [7] | Cross-sectional | community patients, N = 201 | 78.2 ± 5.2 | 2 ½ years | cognitive performance (MMSE) | + |
| Mussi et al., Italy 1999 [53] | Cross-sectional | geriatric inpatients, N = 61 | 79.2 ± 11.6 | 2 months | delirium (CAM) with elevated SAA | + |
| Nebes et al., USA 1997 [50] | Cross-sectional | geropsychiatric ward, N = 36 | 69 (6) | 1 year | cognitive performance; DRS | – |
| total immediate recall | – | |||||
| delayed recall | + | |||||
| percent retention | + | |||||
| Nebes et al., USA 2005 [54] | Cross-sectional | community-based, N = 134 | 73.3 ± 3.8 | not mentioned in the study | cognitive decrements based on WMH volume | + |
| Nebes et al., USA 2007 [23] | Cross-sectional | community-dwelling, N = 90 | 72.1 ± 4.1 | not mentioned in the study | functional outcomes; psychomotor function (gait speed, simple response time) to predict falls | + |
| Nebes et al., USA 2011 [56] | Cross-sectional | community-based, N = 152 | 72.0 ± 4 | not mentioned in the study | cognitive function, processing-speed | + |
| working-memory | – | |||||
| Rovner et al., USA 1988 [14] | Cross-sectional | demented nursing home patients, N = 22 | 80.8 ± 9.6 | unclear | MMSE | – |
| self-care capacity (PGDRS) | + | |||||
| Thomas et al., Germany 2008 [18] | Cross-sectional | hospitalised pts, N = 61 | 86.2 ± 4.5 | 7 months | delirium (DI), MMSE, IQCODE, SPMSQ | + |
| functional outcome (BI) | + | |||||
| Tune et al., USA 1993 [49] | Cross-sectional | surgical ICU pts, N = 25 | 58.1 | 3 weeks | delirium (DSM) | + |
| Case-control | ||||||
| Mach Jr et al., USA 1995 [10] | Case-control | hospital medical ward, N = 22 | 71.3 ± 7.0 | 1-year | delirium (DSM) | + |
| Plaschke et al., Germany 2010 [60] | Case-control | surgical patients, N = 30 | 64.5 ± 13 | 8 months | cognitive function (neuropsychological tests) | – |
| Plaschke et al., Germany 2013 [61] | Case Control | hospitalised pts, N = 117 | 69.3 ± 8.0 | 1 year and 3 months | cognitive function (neuropsychometric testing), IQCODE | – |
| subjective memory complaints | – | |||||
| Thienhaus et al., USA 1990 [12] | Case-control | geropsychiatric inpatients, N = 28 | 65 (9) | unclear | cognitive performance (MMSE, Digit Retention Span, word recognition category retrieval, Self-rated Memory Scale) | – |
| Prospective cohort | ||||||
| Plaschke et al., Germany 2007 [44] | Prospective, cohort | intensive care unit patients, N = 37 | 63.6 ± 11.6 | 5 months | delirium using electroencephalographic data (CAM-ICU) | – |
| Remillard et al., Canada 1994 [43] | Prospective cohort | nursing home, N = 31 | 85.1 ± 7.0 | unclear | MMSE | – |
| SDM | + | |||||
| Rossi et al., Switzerland 2014 [45] | Prospective cohort | surgical inpatients, N = 70 | 72 (67–77) | 1 week | cognitive function (MMSE, CERAD) | – |
| Tune et al., USA 1981 [42] | Prospective cohort | postcardiotomy patients, N = 29 | 55 | 2 weeks | delirium (MMSE) | + |
| Watne et al., Norway & UK 2014 [16] | Prospective cohort | from 2 hospitals, N = 148; n = 52, hospital 1 (Edinburgh) | 84.0 (median) | 1 year and 8 months | IQCODE | + |
| Katz ADL | – | |||||
| n = 96, hospital 2 (Oslo) | 84.0 (median) | 1 year and 8 months | IQCODE | + | ||
| Barthel ADL | + | |||||
The primary electronic literature search identified a total of 1559 articles from 4 different databases such as Ovid MEDLINE, EMBASE, PsycINFO, and IPA. EndNote was used to eliminate duplicates and we considered 1286 articles for screening. Out of 1286 screened articles based on title and abstract, only 44 were eligible for full-text analysis. From the eligible 44 studies, 11 were excluded on full text analysis according to the set inclusion and exclusion criteria. Hence, in total, 33 studies were included in this review that considered SAA in the estimation of anticholinergic burden. The complete study selection process is portrayed in Fig 1.
Summary of study findings
The 33 studies retrieved from 1981 through 2014 comprised of 4 RCTs (12%) [11, 13, 40, 41], 5 prospective cohort studies (15%) [16, 42–45], 3 longitudinal cohorts (9%) [46–48], 17 cross-sectional studies (52%) [7, 8, 14, 17, 18, 23, 49–59], and 4 case-control studies (12%) [10, 12, 60, 61] that validated SAA and association with adverse outcomes.
From the aforementioned studies, majority examined (n = 24) an association between SAA and cognitive outcomes [7, 8, 10–13, 40–50, 52–54, 56, 57, 60, 61] using mostly MMSE as a standard measure of cognitive performance. Limited studies (n = 8) reported an association between SAA and both cognitive, and functional outcomes [14, 16–18, 55, 57–59]. Only 2 studies [62, 63] examined an association between SAA and functional outcomes. Combined, twenty-seven per cent (n = 10) of the studies included in this review showed an association between elevated SAA and functional limitations in Activities of Daily Living (ADL) [16, 51, 58, 59], Barthel Index (BI) [18] and Functional Assessment Staging (FAST) [55, 57].
The study settings varied, and included hospitalised patients (n = 19, 58%), nursing home residents (n = 7, 21%), community people (n = 5, 15%) and ambulatory care patients (n = 2, 6%). Thirty per cent (n = 10) of included studies showed an association between SAA and delirium. In these studies, delirium was assessed using several tools and a meta-analysis was not undertaken due to heterogeneity of the study designs, diversity in the interventions and differences in outcome measures. Summary of the heterogeneity of the included studies between SAA and delirium is depicted in S3 Table. Limited studies examined SAA and functional outcomes and in light of heterogeneity, a meta-analysis was not undertaken instead, a descriptive approach was commenced due to methodological limitations. Overall, the studies included in this review had a variable study duration ranging from one week up to three and half years. Study characteristics describing SAA and association with adverse outcomes in older people are shown in Tables 1 and 2.
Table 2. Quantitative summary of included study characteristics between serum anticholinergic activity and cognitive and functional outcomes.
SAA = Serum Anticholinergic Activity; MMSE = Mini Mental State Examination; IQCODE = Informant questionnaire on Cognitive Decline in the Elderly; SPMSQ = Short Portable Mental Status Questionnaire; SIB = Severe Impairment Battery; CAM = Confusion Assessment Method; BI = Barthel Index; AD = Alzheimer’s disease; FAST = Functional Assessment Staging; BEHAVE-AD = Behavioural Pathology in Alzheimer’s Disease Rating Scale; BARS = Brief Agitation Rating Scale; POCD = Postoperative cognitive decline; CAM-ICU = Confusion Assessment Method for critically ill patients in Intensive Care Unit; MCI = Mild Cognitive Impairment; DI = Delirium Index; SDM = Symbol Digit Modalities; IQR = Interquartile range; PGDRS = Psychogeriatric Dependency Rating Scale; DRS = Dementia Rating Scale; WMH = White Matter Hyperintensities; ICU = Intensive care unit; RCT = Randomised Controlled Trial; ADL = Activities of Daily Living; IADL = Instrumental Activities of Daily Living; CERAD = Consortium to Establish a Registry for Alzheimer Disease; GDS = Geriatric Depression Scale; CI = Cognitive impairment; MANCOVA = Multivariate Analysis of Covariance; ANOVA = Analysis of Variance; ANCOVA = Analysis of Covariance; N.S = Not Significant; pmol = Picomol
| Studies used SAA | Study design | Outcome measure | Sample size (n) | Mean SAA (pmol/mL) | Outcome of interest | Statistical test | p value | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Control group | Intervention group | Control/ Pre | Intervention/ Post | Control/pre | Intervention/post | |||||
| RCTs | ||||||||||
| Kersten et al., Norway 2013 [41] | RCT, single-blinded | CERAD | 31 | 37 | 3.80 (2.29–8.0) (median), (n = 26) | 4.27 (2.19–6.39) (median), (n = 35) | 13.06 ± 6.26 | 14.46 ± 5.92 | ANCOVA | .48 |
| MMSE | 30 | 34 | 3.80 (2.29–8.0) (median), (n = 26) | 4.27 (2.19–6.39) (median), (n = 35) | 19.7 ± 5.21 | 20.68 ± 5.18 | ANCOVA | .57 | ||
| saliva flow (g/min) | 27 | 34 | 3.80 (2.29–8.0) (median), (n = 26) | 4.27 (2.19–6.39) (median), (n = 35) | 0.27 (0.16–0.49) | 0.25 (0.09–0.60) | ANCOVA | .34 | ||
| Lackner et al., USA 2008 [40] | RCT, double-blinded | MMSE | 24 | 26 | 1.15 (0.0–5.05) (median), (n = 24) | 0.95 (0.0–6.20) (median), (n = 26) | 13.7 ± 0.9 | 15.2 ± 0.8 | Correlation analysis | .25 |
| SIB | 24 | 26 | 1.15 (0.0–5.05) (median), (n = 24) | 0.95 (0.0–6.20) (median), (n = 26) | 87.0 ± 1.6 | 87.1 ± 1.6 | Correlation analysis | .72 | ||
| CAM | 24 | 26 | 1.15 (0.0–5.05) (median), (n = 24) | 0.95 (0.0–6.20) (median), (n = 26) | 1.8 ± 0.3 | 2.0 ± 0.3 | Correlation analysis | .96 | ||
| BARS | 24 | 26 | 1.15 (0.0–5.05) (median), (n = 24) | 0.95 (0.0–6.20) (median), (n = 26) | 16.1 ± 1.8 | 16.9 ± 1.9 | Correlation analysis | .75 | ||
| Miller et al., Canada 1988 [11] | RCT, double blinded | MMSE | 16 | 16 | 11.6 ± 18.2 (n = 16) | 121.1 ± 85.5 (n = 14) | 28.3 ± 2.2 | 26.7 ± 3.5 | ANCOVA | N.S |
| SDM | 17 | 16 | 11.6 ± 18.2 (n = 16) | 121.1 ± 85.5 (n = 14) | 36 ± 12.1 | 28.4 ±13.3 | ANCOVA | N.S | ||
| Delirium | 17 | 16 | 11.6 ± 18.2 (n = 16) | 121.1 ± 85.5 (n = 14) | 37 ± 2.7 | 33.3 ± 4.1 | ANCOVA | .02 | ||
| Rey audio-verbal learning test | 16 | 16 | 11.6 ± 18.2 (n = 16) | 121.1 ± 85.5 (n = 14) | 4.4 ± 0.7 | 3.2 ± 2.0 | ANCOVA | < .01 | ||
| Tollefson et al., USA 1991 [13] | RCT, parallel-arm | MMSE | 19 | 15 | 2.49 ± 3.9 | 1.89 ± 3.4 | 22.59 ± 4.76 | 23.03 ± 4.92 | Correlation analysis | < .01 |
| Longitudinal cohort | ||||||||||
| Golinger et al., USA 1987 [46] | Longitudinal cohort | drug-risk number for delirium | 16 | 9 | 0.81 ± 1.0 (delirium) | 4.67 ± 3.3 (no delirium) | 12.0 ± 9.2 (delirium) | 8.3 ± 6.4 (no delirium) | t-test | .3 (N.S) |
| Kashyap et al., Canada 2014 [48] | Longitudinal cohort | change in cognition (SAA) | 68 | 21, 20, 12 | 1.03 ± 0.75 | 0.08 ± 1.2 | 0.83 (median) | 0.65, 0.76, 0.50 (median) | Kruskal-Wallis one-way ANOVA | .87 |
| van Munster et al., Netherlands 2012 [47] | Longitudinal study | effect of delirium onset on SAA level as effect size, adjusted for CI | 70 | 72 | 3.4 | 4.2 | 28.8 (SD not reported) | Mixed-model regression | < .05 | |
| Cross-sectional | ||||||||||
| Chew et al., USA 2005 [8] | Cross-sectional | Correlation study; MMSE and SAA | 25 in total | 1.06 ± 1.2 (overall) | r = -0.398 | Spearman correlation | .049 | |||
| SIB and SAA | 28 in total | r = -0.405 | Spearman correlation | 095 | ||||||
| Flacker et al., USA 1998 [51] | Cross-sectional | ADL | 47 | 20 | 0.7 ± 0.8 | 1.8 ± 1.6 | 1.5 ± 2.2 | 4.0 ± 2.6 | t-test | < .001 |
| Flacker et al., USA 1999 [52] | Cross-sectional | changes in SAA level in individuals with febrile illness between study entry and at one-month follow-up | 14 | 8 | 0.65 ± 0.51 (entry, delirium) | 0.69 ± 0.85 (entry, no delirium) | 0.08 ± 0.12 (follow-up, delirium) | 0.10 ± 0.16 (follow-up, no delirium) | ANOVA | < .01 for overall change |
| Hori et al., Japan 2011 [57] | Cross-sectional | MMSE | 50 | 26 | <1.95 | 4.14 ± 2.70 | 13.16 ± 8.27 | 8.89 ± 8.40 | Student t-test | .0367 |
| FAST | 50 | 26 | <1.95 | 4.14 ± 2.70 | 4.78 ± 0.98 | 5.46 ± 1.21 | Student t-test | .0096 | ||
| BEHAVE-AD: delusion | 50 | 26 | <1.95 | 4.14 ± 2.70 | 1.2 ± 1.7 | 3.4 ± 1.3 | Student t-test | < .0001 | ||
| BEHAVE-AD: hallucination | 50 | 26 | <1.95 | 4.14 ± 2.70 | 0.7 ± 1.0 | 1.9 ± 1.0 | Student t-test | < .0001 | ||
| BEHAVE-AD: activity disturbance | 50 | 26 | <1.95 | 4.14 ± 2.70 | 2.1 ± 2.2 | 2.3 ± 2.2 | Student t-test | .7162 | ||
| BEHAVE-AD: aggressiveness | 50 | 26 | <1.95 | 4.14 ± 2.70 | 1.1 ± 1.7 | 1.9 ± 2.1 | Student t-test | .0714 | ||
| BEHAVE-AD: rhythm disturbance | 50 | 26 | <1.95 | 4.14 ± 2.70 | 0.6 ± 0.8 | 1.7 ± 0.7 | Student t-test | < .0001 | ||
| BEHAVE-AD: affection | 50 | 26 | <1.95 | 4.14 ± 2.70 | 1.0 ± 1.2 | 0.6 ± 0.8 | Student t-test | .1590 | ||
| BEHAVE-AD: anxiety | 50 | 26 | <1.95 | 4.14 ± 2.70 | 1.4 ± 1.8 | 1.7 ± 1.8 | Student t-test | .6278 | ||
| Kersten et al., Norway 2013 [58] | Cross-sectional | MMSE | 72 (EM, extensive metaboliser) | 8 (PM, poor metaboliser) | 4.2 (2.4, 7.0) for EM | 10.3 (5.7, 39.9) for PM | 19.5 (17,22) | 24 (16,25.5) | Mann-Whitney test | N.S |
| CERAD | 72 (EM, extensive metaboliser) | 8 (PM, poor metaboliser) | 4.2 (2.4, 7.0) for EM | 10.3 (5.7, 39.9) for PM | 12 (9,14) | 9.5 (7.25,14.3) | Mann-Whitney test | N.S | ||
| ADL | 72 (EM, extensive metaboliser) | 8 (PM, poor metaboliser) | 4.2 (2.4, 7.0) for EM | 10.3 (5.7, 39.9) for PM | 4 (3,5) | 4 (3,5) | Mann-Whitney test | N.S | ||
| mouth dryness (whole mouth resting salivary flow) | 72 (EM, extensive metaboliser) | 8 (PM, poor metaboliser) | 4.2 (2.4, 7.0) for EM | 10.3 (5.7, 39.9) for PM | 0.7 (0.4,1.2) (EM) | 1.34 (0.1,2.3) (PM) | Mann-Whitney test | N.S | ||
| Konishi et al., Japan 2010 [55] | Cross-sectional | MMSE | 50 | 26 | <1.95 | 4.14 ± 2.7 | 13.16 ± 8.27 | 8.89 ± 8.40 | t-test | .0367 |
| FAST | 50 | 26 | <1.95 | 4.14 ± 2.7 | 4.78 ± 0.98 | 5.46 ± 1.21 | t-test | .0096 | ||
| Lampela et al., Finland 2013 [17] | Cross-sectional | MMSE, GDS, ADL, IADL, short distance vision | 12/609 with and without dementia | median 9.3 (2.27–82.7) Overall | numerical data not shown | Kruskall-Wallis one-way ANOVA | N.S | |||
| Mangoni et al., Netherlands 2013 [59] | Cross-sectional | Regression analysis: SAA vs ADL | 71 in total | median 2.8 (Range 1.1–4.9) | β = 0.39 | Linear regression and Cox regression | 0.001 | |||
| 3-month mortality vs SAA | 71 in total | median 2.8 (Range 1.1–4.9) | HR = 0.07 | Linear regression and Cox regression | 0.07 | |||||
| 1-year mortality vs SAA | 71 in total | median 2.8 (Range 1.1–4.9) | HR = 1.10 | Linear regression and Cox regression | 0.11 | |||||
| Mulsant et al., USA 2003 [7] | Cross-sectional | MMSE (≤24) | n = 21 with undetectable SAA | n = 159 with low SAA; n = 21 with high SAA | <0.25 (undetectable) | 0.25–2.79 (detectable); ≥2.80 (high) | 4.8% | 7.6%; 28.6% | Pearson χ2 test | .006 |
| Mussi et al., Italy 1999 [53] | Cross-sectional | SAA level for delirious and non-delirious individuals | 49 | 12 | 3.9 ± 8.4 | 23.0 ± 15.5 | 3.9 ± 8.4 | 23.0 ± 15.5 | t-test | < .004 |
| Nebes et al., USA 1997 [50] | Cross-sectional | DRS | 17 | 19 | 0.0 | 0.28 ± 0.26 | 138.6 ± 3.0 | 135.6 ± 3.6 | ANOVA | N.S |
| total immediate recall | 17 | 19 | 0.0 | 0.28 ± 0.26 | 26.1 ± 7.2 | 21.9 ± 7.1 | ANOVA | .24 | ||
| delayed recall | 17 | 19 | 0.0 | 0.28 ± 0.26 | 6.5 ± 2.5 | 4.2 ± 2.5 | ANOVA | < .05 | ||
| percent retention | 17 | 19 | 0.0 | 0.28 ± 0.26 | 86% ± 35% | 58% ± 25% | ANOVA | < .05 | ||
| Nebes et al., USA 2005 [54] | Cross-sectional | cognitive decrements based on WMH volume | n = 35, no SAA | n = 69, moderate; SAA; n = 30, high SAA | 0.0 | 1.96 ± 1.0; 6.1 ± 1.7 | 6.2 ± 7.7 | 6.3 ± 11.9; 9.2 ± 15.2 | ANOVA | < .005 (high SAA group correlated with WHM volume) |
| Nebes et al., USA 2007 [23] | Cross-sectional | gait speed | n = 29, low SAA | n = 33, medium SAA; n = 26, high SAA | 0.36 ± 0.34 | 1.36 ± 0.31 | 4.32 ± 0.78 | 4.77 ± 1.06; 5.08 ± 0.88 | MANCOVA | .0109 |
| simple response time | n = 29, low SAA | n = 33, medium SAA; n = 26, high SAA | 0.36 ± 0.34 | 3.42 ± 2.33 | 244.4 ± 40.0 | 276.4 ± 56.2; 285.7 ± 63.4 | MANCOVA | .0078 | ||
| Nebes et al., USA 2011 [56] | Cross-sectional | processing-speed (n = 75); perceptual comparison | n = 76, low-paraxanthine | n = 76, high-paraxanthine | 1.72 ± 2.03 | 1.35 ± 1.37 | 781.4 ± 158.9 | 755.2 ± 133.5 | Pearson correlation | < .009 |
| processing-speed (n = 75); conceptual comparison | n = 76, low-paraxanthine | n = 76, high-paraxanthine | 1.72 ± 2.03 | 1.35 ± 1.37 | 799.5 ± 147.3 | 795.6 ± 134.1 | Pearson correlation | < .017 | ||
| processing-speed (n = 75); working-memory (N Back) | n = 76, low-paraxanthine | n = 76, high-paraxanthine | 1.72 ± 2.03 | 1.35 ± 1.37 | 32.6 ± 13.5 | 34.1 ± 12.6 | Pearson correlation | N.S | ||
| Rovner et al., USA 1988 [14] | Cross-sectional | MMSE | 11 | 11 | <0.83 | >0.83 | 6.3 ± 9.6 | 5.2 ± 2.1 | t-test | .7 |
| self-care capacity (PGDRS) | 11 | 11 | <0.83 | >0.83 | 13.2 ± 8.3 | 20.1 ± 1.5 | t-test | .03 | ||
| Thomas et al., Germany 2008 [18] | Cross-sectional | delirium (DI) | n = 15, cognitively unimpaired | n = 31, with dementia; n = 15, delirium with dementia | 9.33 ± 4.44 | 11.03 ± 6.15; 12.25 ± 10.53 | 2.5 ± 0.7 | 6.2 ± 4.0; 8.7 ± 4.5 | ANOVA and Duncan’s post-hoc-tests | < .02 |
| MMSE | n = 15, cognitively unimpaired | n = 31, with dementia; n = 15, delirium with dementia | 9.33 ± 4.44 | 11.03 ± 6.15; 12.25 ± 10.53 | 28.8 ± 1.8 | 16.7 ± 7.5; 14.4 ± 6.0 | ANOVA and Duncan’s post-hoc-tests | < .001 | ||
| IQCODE | n = 15, cognitively unimpaired | n = 31, with dementia; n = 15, delirium with dementia | 9.33 ± 4.44 | 11.03 ± 6.15; 12.25 ± 10.53 | 3.1 ± 0.2 | 4.2 ± 0.6; 4.2 ± 0.7 | ANOVA and Duncan’s post-hoc-tests | < .004 | ||
| SPMSQ | n = 15, cognitively unimpaired | n = 31, with dementia; n = 15, delirium with dementia | 9.33 ± 4.44 | 11.03 ± 6.15; 12.25 ± 10.53 | 0.6 ± 0.9 | 4.7 ± 2.8; 6.4 ± 2.7 | ANOVA and Duncan’s post-hoc-tests | < .001 | ||
| BI | n = 15, cognitively unimpaired | n = 31, with dementia; n = 15, delirium with dementia | 9.33 ± 4.44 | 11.03 ± 6.15; 12.25 ± 10.53 | 62.5 ± 31.2 | 33.7 ± 24.3; 45.0 ± 18.1 | ANOVA and Duncan’s post-hoc-tests | < .005 | ||
| Tune et al., USA 1993 [49] | Cross-sectional | SAA level for delirious and non-delirious individuals | 16 | 9 | 5.0 ± 2.41 | 7.09 ± 2.10 | 5.0 ± 2.41 | 7.09 ± 2.10 | t-test | .045 |
| Case-control | ||||||||||
| Mach Jr et al., USA 1995 [10] | Case-control | MMSE | 11 | 11 | 3.38 ± 2.49 | 6.05 ± 2.97 | 28 ± 1.3 | 26 ± 2.7 | paired t-test | < .05 |
| Plaschke et al., Germany 2010 [60] | Case-control | cognitive function (neuropsychological tests), cortisol and SAA correlation | 23, low SAA group | 7, high SAA group | 3.3 ± 2.2 | 6.1 ± 3.9 | not reported | not reported | ANOVA | N.S for both studies |
| Plaschke et al., Germany 2013 [61] | Case Control | IQCODE | n = 87, no POCD | n = 30, with POCD | 4.5 ± 3.9 | 1.6 ± 1.7 | 86 (98.9) | 28 (93.3) | χ2 test | .099 |
| subjective memory complaints | n = 87, no POCD | n = 30, with POCD | 4.5 ± 3.9 | 1.6 ± 1.7 | 76 (87.4) | 22 (73.3) | χ2 test | .073 | ||
| Thienhaus et al., USA 1990 [12] | Case-control | MMSE | 18 | 18 | 4.09 ± 4.83 | 6.66 ± 6.23 | 27.7 ± 1.2 | 27.9 ± 1.9 | Paired t-test | N.S |
| Digit Retention Span | 18 | 18 | 4.09 ± 4.83 | 6.66 ± 6.23 | 6.2 ± 2.1 | 6.1 ± 1.7 | Paired t-test | N.S | ||
| word recognition | 18 | 18 | 4.09 ± 4.83 | 6.66 ± 6.23 | 12.4 ± 5.3 | 14.0 ± 6.1 | Paired t-test | N.S | ||
| category retrieval | 18 | 18 | 4.09 ± 4.83 | 6.66 ± 6.23 | 10.4 ± 4.0 | 10.5 ± 4.9 | Paired t-test | N.S | ||
| self-rated Memory | 18 | 18 | 4.09 ± 4.83 | 6.66 ± 6.23 | 26.0 ± 38.4 | 27.2 ± 43.3 | Paired t-test | N.S | ||
| Prospective cohort | ||||||||||
| Plaschke et al., Germany 2007 [44] | Prospective, cohort | Correlation of SAA and cerebral spinal fluid anticholinergic activity. | n = 20 | n = 17, with delirium | 2.6 ± 2.3 | 2.8 ± 2.5 | r = 0.861 | Correlation between two numerical variables | >.05 | |
| Remillard, Canada 1994 [43] | Prospective cohort | MMSE | 23, non-detectable SAA | 8, detectable SAA | <1.8 | >1.8 | 23.9 ± 3.7 | 25.2 ± 3.8 | unpaired t-test | N.S |
| SDM | 23, non-detectable SAA | 8, detectable SAA | <1.8 | >1.8 | 15.2 ± 6.4 | 24.2 ± 8.7 | unpaired t-test | < .001 | ||
| Rossi et al., Switzerland 2014 [45] | Prospective cohort | MMSE | n = 38, no POCD | n = 32, with POCD | 0.97 (.65, 1.83) | 1.32 (.68, 2.59) | 29 (28, 30) | 27.5 (25, 29) | χ2 test | .004 |
| CERAD | n = 38, no POCD | n = 32, with POCD | 0.97 (.65, 1.83) | 1.32 (.68, 2.59) | 99 (93, 105) | 93 (85, 101) | t-test | .007 | ||
| Tune et al., USA 1981 [42] | Prospective cohort | Correlation between delirium (MMSE) and SAA level. | 19 controls | 10 patients with delirium | Not clearly mentioned, but a SAA level >1.5 pmol is defined as high-SAA | r = -0.83 | Correlation between two numerical variables | < .001 | ||
| Watne et al., Norway & UK 2014 [16] | Prospective cohort | IQCODE | 32, no delirium (Edinburg) | 20, with delirium | 1.62 (0.81–2.45) (IQR) | 1.35 (0.76–2.46) (IQR) | 0 | 5 | Mann-Whitney test/ Chi-square test | .01 |
| Katz ADL | 32, no delirium (Edinburg) | 20, with delirium | 1.62 (0.81–2.45) (IQR) | 1.35 (0.76–2.46) (IQR) | 31 | 16 | Mann-Whitney test/ Chi-square test | .07 | ||
| IQCODE | 44, no delirium (Oslo) | 52, with delirium | 6.08 (4.08–9.86) (IQR) | 7.02 (4.24–9.73) (IQR) | 10 | 42 | Mann-Whitney test/ Chi-square test | < .001 | ||
| Barthel ADL | 44, no delirium (Oslo) | 52, with delirium | 6.08 (4.08–9.86) (IQR) | 7.02 (4.24–9.73) (IQR) | 29 | 11 | Mann-Whitney test/ Chi-square test | < .001 | ||
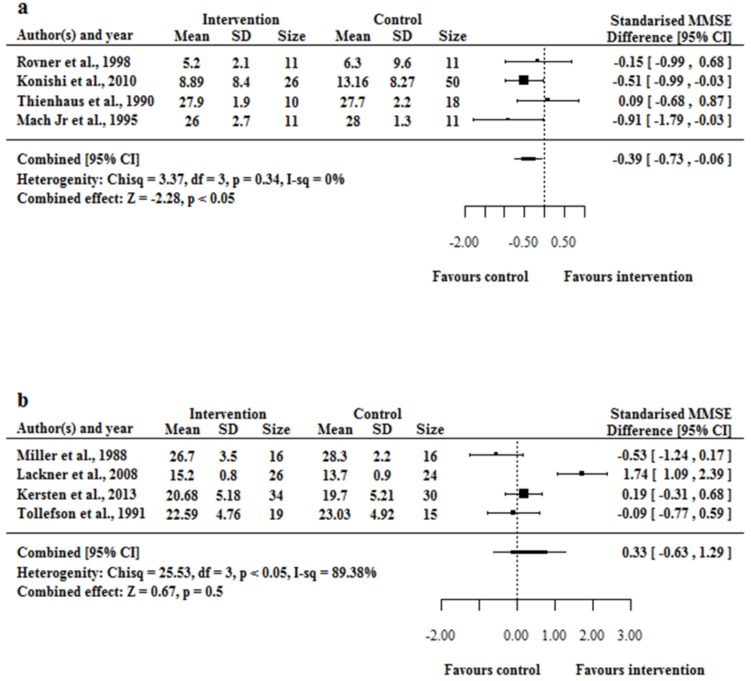
Data from 4 cross-sectional and case-control studies [10, 12, 14, 55] were pooled for a meta-analysis. An initial random-effect modelling included all 5 studies (data not shown). The study by Hori et al. [57] was excluded from the analysis, as the funnel plot analysis revealed this study as a potential outlier that could bias the pooled estimate and the MMSE score mentioned in their article was adopted from Konishi et al. [55]. Pooled data from 4 observational studies using random effect modelling showed elevated SAA was associated with reduced cognitive performance (I2 = 0.00%, H2 = 3.37 and p-value = 0.34) (Fig 2A). The funnel-plot illustrates no outliers and excludes large-study bias (see S1 Fig).
Fig 2.
(a and b) Forest plot of standardised mean difference between increased SAA and a reduced MMSE score (positive favours MMSE reduction). Forest plot, using data from (a) 4 observational studies and (b) 4 randomised controlled trails following interventions that increase SAA and a decline in MMSE score. A) The result support SAA lowers the MMSE score (p < 0.05) for observational studies. B) The result did not support a conclusion that SAA lowers the MMSE score (p = 0.5) for RCTs.
Four RCTs met our inclusion criteria. One study that reported median MMSE scores with interquartile ranges was excluded from the analyses [41]. In the meta-analysis of RCT studies, the heterogeneity is statistically significant. A potential outlier [40] from the funnel plot analysis (see S2 Fig) was noted as the confidence interval does not overlap at all with the rest. After excluding the study by Lackner et al., the heterogeneity is reduced greatly and no longer statistically significant. Regardless of whether the study by Lackner et al. [40] is included or not, the conclusion remains unchanged (i.e. an increase in SAA level does not change MMSE score) (S3 Fig). In the final analyses, data from 4 RCTs were pooled and meta-analysed using random-effect modelling [11, 13, 40]. In Tollefson’s work [13], the treatment was designed to reduce SAA, so in random effects model, we considered ‘non-treatment’ as the intervention that increases SAA and the standardised effect size was relative to the post-treatment MMSE scores. The pooled data from 4 RCTs using random effect modelling found no association between elevated SAA and cognitive performance (I2 = 89.38%, H2 = 25.53 and p-value = <0.05) (Fig 2B).
A sensitivity analysis for the combined standardised MMSE differences was performed to verify that the observed effect size was not influenced by a particular study. This was accomplished by dropping out one study at a time and the standardised MMSE differences recalculated with confidence intervals. For the observational studies, the result favours the trend that an increase in SAA level lowers the MMSE score, but the changes were not always statistically significant. The study by Konishi et al. [55] demonstrated the adverse effect of high-SAA on MMSE scores, and their study size is much larger than the rest. This may have biased the result that an increase in SAA level changed MMSE scores. Finding from the RCT meta-analysis remained unchanged regardless of which study was excluded. The summary of sensitivity analysis findings is reported in S4 Table.
Quality assessment
The qualities of the included RCT studies were critically appraised using the Cochrane risk assessment tool, highlighted in S5 Table. Two studies [11, 13] did not report the randomisation process, and there was general lack of adequate blinding, between participants and health professionals, and between outcomes and assessors.
The quality of the included case-control and cross-sectional studies were critically appraised using Newcastle Ottawa scale and the assessments are shown in S6 and S7 Tables. The qualities of included individuals in these studies were adequate, but in some of them, potential confounders were not discussed that may have lowered the reliability of the outcomes.
Discussion
To our knowledge, this is the first systematic review that conducted a meta-analysis to quantify the association between SAA and adverse outcomes in older people.
The evidence from pooled analyses from 4 RCTs failed to confirm an association between SAA and impaired cognitive performance (Fig 2B). However, evidence from 4 observational studies shows an association between SAA and cognitive performance (Fig 2A). There were several methodological limitations that hindered inclusion of studies into the meta-analysis. Several studies failed to blind the participant and the health professional [41], and blinding of outcomes and the assessments were not undertaken [13]. The observed relationship between the intervention and change in MMSE scores may be confounded by participant selection and different outcome assessments. Included studies considered reporting of MMSE scores before and after the intervention, but very limited details were provided with respect to participant’s follow-up and information on when MMSE measurements were undertaken.
SAA and cognitive outcomes
The MMSE scale was widely employed to measure cognitive performance followed by the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE), Consortium to Establish a Registry for Alzheimer Disease (CERAD), Confusion Assessment Method (CAM). MMSE is one of the globally accepted tools for measuring cognitive impairment in older people, however a recent systematic review highlighted that the sensitivity of MMSE for measuring cognitive improvements may be low in nursing home residents and MMSE as a tool has not been evaluated for measuring changes caused by drug-induced cognitive impairment [4]. In addition, studies reported that MMSE may not be an optimal method to detect mild drug-induced cognitive changes in older people [11, 17, 64].
The studies included in this review confirm a consistent correlation between higher SAA and worsening cognitive performance [7, 53, 60, 65]. However, negative association between cognitive performance and SAA were identified as well. A study by Remillard [43] reported similar findings to those of Rovner et al. [14] regarding negative associations between SAA and MMSE scores. Thomas and colleagues [18] failed to show a positive correlation between higher SAA and cognitive function using the cerebral cholinergic function measured with electroencephalography. Findings from this study suggests a poor correlation between peripheral SAA levels and cognitive effects.
The qualitative and quantitative findings from this review showed that SAA is associated with an increased risk of delirium, assessed using the Diagnostic and Statistical Manual of Mental Disorders (DSM) criteria [10, 46, 49] and also from other derivatives such as the CAM [40, 47, 53], CAM-ICU [44] for critically-ill individuals, delirium index (DI) [18] and Saskatoon Delirium Checklist (SDC) [11]. A study by Plaschke et al. [44] evaluated the correlation of SAA with delirium in critically-ill patients. However, the study findings showed higher SAA value in the delirious cohort was not correlated with a risk of developing delirium.
SAA and functional outcomes
Findings from a recent cross-sectional study [18] conducted in Germany reported a moderate correlation of SAA with functional outcomes, indicating an inverse relationship of the anticholinergic burden on functional capacity in non-delirious individuals and cognitively unimpaired however lack of correlation was reported in individuals with dementia and delirium indicating the role of additional mechanisms that leads to functional deficits. Another cross-sectional study by Nebes et al. [23] reported higher SAA was associated with slowing of psychomotor tasks including gait speed and simple manual response times. A cross-sectional study involving 67 older medical inpatients failed to find an association between SAA levels and ADLs among older people [51]. Methodological limitations hampered the completion of meta-analyses of data from studies that examined a relationship between SAA and functional outcomes.
Variations in SAA levels
A RCT study conducted by Miller et al. [11] in presurgical older patients found a huge variation in mean SAA values ranging from 9.1 ± 17.7 pmol/ml atropine equivalents and increased to 121.1 ± 85.5 pmol/ml atropine equivalents after administration of scopolamine. A cross-sectional study [17] reported detectable SAA of 2.27 pmol/ml in the absence of anticholinergic medicines. Another study conducted by Tune et al. reported absolute mean SAA level greater than 7.5 pmol/mL in surgical patients experiencing delirium, compared with less than 7.5 pmol/mL in surgical patients without delirium [42]. SAA levels associated with delirium or cognitive decline varied considerably in these studies [20]. A detailed summary of variations in SAA measurement is highlighted in Table 2.
SAA is an important biomarker to understand cognitive impairment, but has several limitations. Findings of this systematic review failed to confirm a threshold level of SAA that predicts delirium or cognitive decline. SAA measures anticholinergic activity in the blood rather than in the central nervous system and there is a poor correlation between peripheral anticholinergic activity measured by SAA and central nervous system effects [25, 37, 66]. Endogenous substances in addition to anticholinergic medicines and their metabolites have also shown to affect SAA measurements [21]. The standardisation of bioassay reported in the studies is also uncertain [20] and the reasons could be intra-laboratory variability in bioassay methods and heterogeneity of study populations [25]. The summary of potential limitations of SAA measurement is depicted in S8 Table.
Strengths and limitations
This systematic review was comprehensive in that the electronic search conducted in 4 different databases endeavoured to identify all potential studies that met our eligibility criteria. We used the PICO (population, intervention, comparison and outcome) criteria to frame research questions. The population mean age was set to 55 years to capture potential studies, especially from the USA, to be included in this review. The review explicitly looked into SAA measurement and its association with adverse outcomes. The objectives were clearly stated and the search methodology including the citation analysis were robust. A systematic approach was used to synthesise and characterise the findings of this review followed by a meta-analysis.
The exact relationship between SAA and cognitive functions in older people remains unclear [9]. Only a small number of medications were assessed using SAA method and the metabolites of these medications remain unexamined [8, 9]. The variation in study methodologies prevented several case-control studies, longitudinal studies and prospective cohorts to be excluded from the meta-analyses. The inconsistent measure of cognitive and functional outcomes reported in the studies limited the meta-analysis. The review found associations between SAA and cognitive adverse outcomes, but not with functional outcomes. Larger well designed experimental studies are needed to confirm these associations. Notwithstanding, there are several technical concerns for the utility of SAA [19,20] and further discussions are needed to determine the utility of SAA in clinical practice.
Conclusions
This systematic review and meta-analysis examined studies that used SAA as a method to quantify anticholinergic burden and examined associations with adverse outcomes in older people. The complexity of assessing anticholinergic burden using the SAA method limits its widespread acceptance as a biomarker to assess anticholinergic effects. The evidence from pooled analyses from 4 RCTs failed to confirm an association between SAA and impaired cognitive performance. Though, evidence from 4 observational studies shows an association between SAA and cognitive performance. SAA measured by receptor bioassay is flawed and its use in older people with multimorbidity and polypharmacy is questionable. In conclusion, SAA has a number of limitations as a biomarker for predicting cognitive impairment in older people.
Supporting Information
Funnel plot shows the validity of the meta-analysis on the cross-sectional and case-control studies. All data falls within the allowable region of the funnel plot, indicating that the analysis does not involve outliers that are overrepresented in the analysis
(TIF)
Funnel plot shows the validity of the meta-analysis on the RCT studies. All data falls within the allowable region of the funnel plot, indicating that the analysis does not involve outliers that are overrepresented in the analysis.
(TIF)
The result did not support a conclusion that SAA lowers the MMSE score for RCTs.
(TIF)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability
Data are available from supplementary information of the paper.
Funding Statement
The authors have no support or funding to report.
References
- 1.Salahudeen MS, Hilmer SN, Nishtala PS. Comparison of anticholinergic risk scales and associations with adverse health outcomes in older people. J Am Geriatr Soc. 2015;63(1):85–90. Epub 2015/01/20. 10.1111/jgs.13206 . [DOI] [PubMed] [Google Scholar]
- 2.Roe CM, Anderson MJ, Spivack B. Use of anticholinergic medications by older adults with dementia. J Am Geriatr Soc. 2002;50(5):836–42. . [DOI] [PubMed] [Google Scholar]
- 3.Tune LE. Anticholinergic effects of medication in elderly patients. J Clin Psychiatry. 2001;62(Suppl21):11–4. . [PubMed] [Google Scholar]
- 4.Salahudeen MS, Duffull SB, Nishtala PS. Impact of anticholinergic discontinuation on cognitive outcomes in older people: a systematic review. Drugs Aging. 2014;31(3):185–92. 10.1007/s40266-014-0158-4 . [DOI] [PubMed] [Google Scholar]
- 5.Salahudeen MS, Nishtala PS, Duffull SB. The Influence of Patient Characteristics on Anticholinergic Events in Older People. Dement Geriatr Cogn Dis Extra. 2015;5(3):530–41. 10.1159/000441718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tune L, Coyle JT. Serum levels of anticholinergic drugs in treatment of acute extrapyramidal side effects. Arch Gen Psychiatry. 1980;37(3):293–7. Epub 1980/03/01. . [DOI] [PubMed] [Google Scholar]
- 7.Mulsant BH, Pollock BG, Kirshner M, Shen C, Dodge H, Ganguli M. Serum anticholinergic activity in a community-based sample of older adults: relationship with cognitive performance. Arch Gen Psychiatry. 2003;60(2):198–203. . [DOI] [PubMed] [Google Scholar]
- 8.Chew ML, Mulsant BH, Pollock BG. Serum anticholinergic activity and cognition in patients with moderate-to-severe dementia. Am J Geriatr Psychiatry. 2005;13(6):535–8. . [DOI] [PubMed] [Google Scholar]
- 9.Chew ML, Mulsant BH, Pollock BG, Lehman ME, Greenspan A, Mahmoud RA, et al. Anticholinergic activity of 107 medications commonly used by older adults. J Am Geriatr Soc. 2008;56(7):1333–41. 10.1111/j.1532-5415.2008.01737.x [DOI] [PubMed] [Google Scholar]
- 10.Mach JR Jr, Dysken MW, Kuskowski M, Richelson E, Holden L, Jilk KM. Serum anticholinergic activity in hospitalized older persons with delirium: A preliminary study. J Am Geriatr Soc. 1995;43(5):491–5. . [DOI] [PubMed] [Google Scholar]
- 11.Miller PS, Richardson JS, Jyu CA, Lemay JS, Hiscock M, Keegan DL. Association of low serum anticholinergic levels and cognitive impairment in elderly presurgical patients. Am J Psychiatry. 1988;145(3):342–5. Epub 1988/03/01. . [DOI] [PubMed] [Google Scholar]
- 12.Thienhaus OJ, Allen A, Bennett JA, Chopra YM, Zemlan FP. Anticholinergic serum levels and cognitive performance. Eur Arch Psychiatry Clin Neurosci. 1990;240(1):28–33. Epub 1990/01/01. . [DOI] [PubMed] [Google Scholar]
- 13.Tollefson GD, Montague-Clouse J, Lancaster SP. The relationship of serum anticholinergic activity to mental status performance in an elderly nursing home population. J Neuropsychiatry Clin Neurosci. 1991;3(3):314–9. Epub 1991/01/01. . [DOI] [PubMed] [Google Scholar]
- 14.Rovner BW, David A, Lucas-Blaustein MJ, Conklin B, Filipp L, Tune L. Self-care capacity and anticholinergic drug levels in nursing home patients. Am J Psychiatry. 1988;145(1):107–9. Epub 1988/01/01. . [DOI] [PubMed] [Google Scholar]
- 15.Nishtala PS, Fois RA, McLachlan AJ, Bell JS, Kelly PJ, Chen TF. Anticholinergic activity of commonly prescribed medications and neuropsychiatric adverse events in older people. J Clin Pharmacol. 2009;49(10):1176–84. Epub 2009/09/29. 10.1177/0091270009345690 . [DOI] [PubMed] [Google Scholar]
- 16.Watne LO, Hall RJ, Molden E, Raeder J, Frihagen F, MacLullich AMJ, et al. Anticholinergic activity in cerebrospinal fluid and serum in individuals with hip fracture with and without delirium. J Am Geriatr Soc. 2014;62(1):94–102. 10.1111/jgs.12612 [DOI] [PubMed] [Google Scholar]
- 17.Lampela P, Lavikainen P, Garcia-Horsman JA, Bell JS, Hartikainen S, et al. Anticholinergic drug use, serum anticholinergic activity, and adverse drug events among older people: a population-based study. Drugs Aging. 2013;30(5):321–30. 10.1007/s40266-013-0063-2 [DOI] [PubMed] [Google Scholar]
- 18.Thomas C, Hestermann U, Kopitz J, Plaschke K, Oster P, Driessen M, et al. Serum anticholinergic activity and cerebral cholinergic dysfunction: An EEG study in frail elderly with and without delirium. BMC Neurosci. 2008;9(1):86 10.1186/1471-2202-9-86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gerretsen P, Pollock BG. Drugs with anticholinergic properties: a current perspective on use and safety. Expert Opin Drug Saf. 2011;10(5):751–65. Epub 2011/06/04. 10.1517/14740338.2011.579899 . [DOI] [PubMed] [Google Scholar]
- 20.Carnahan RM, Lund BC, Perry PJ, Pollock BG. A critical appraisal of the utility of the serum anticholinergic activity assay in research and clinical practice. Psychopharmacol Bull. 2002;36(2):24–39. Epub 2002/10/26. . [PubMed] [Google Scholar]
- 21.Cox EA, Kwatra SG, Shetty S, Kwatra MM. Flaws in the serum anticholinergic activity assay: Implications for the study of delirium. J Am Geriatr Soc. 2009;57(9):1707–8. 10.1111/j.1532-5415.2009.02411.x [DOI] [PubMed] [Google Scholar]
- 22.Lampela P, Paajanen T, Hartikainen S, Huupponen R. Central Anticholinergic Adverse Effects and Their Measurement. Drugs Aging. 2015. Epub 2015/11/01. 10.1007/s40266-015-0321-6 . [DOI] [PubMed] [Google Scholar]
- 23.Nebes RD, Pollock BG, Halligan EM, Kirshner MA, Houck PR. Serum anticholinergic activity and motor performance in elderly persons. J Gerontol A Biol Sci Med Sci. 2007;62A(1):83–5. . [DOI] [PubMed] [Google Scholar]
- 24.Remillard AJ. A pilot project to assess the association of anticholinergic symptoms with anticholinergic serum levels in the elderly. Pharmacotherapy. 1994;14(4):482–7. . [PubMed] [Google Scholar]
- 25.Kersten H, Wyller TB. Anticholinergic drug burden in older people's brain—how well is it measured? Basic Clin Pharmacol Toxicol. 2014;114(2):151–9. Epub 2013/10/12. 10.1111/bcpt.12140 . [DOI] [PubMed] [Google Scholar]
- 26.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement2009 2009-July-21 10:46:49. [PMC free article] [PubMed] [Google Scholar]
- 27.Bottiggi KA, Salazar JC, Yu L, Caban-Holt AM, Ryan M, Mendiondo MS, et al. Long-term cognitive impact of anticholinergic medications in older adults. Am J Geriatr Psychiatry. 2006;14(11):980–4. . [DOI] [PubMed] [Google Scholar]
- 28.Landi F, Russo A, Liperoti R, Cesari M, Onder G, et al. Anticholinergic drugs and physical function among frail elderly population. Clin Pharmacol Ther. 2007;81(2):235–41. [DOI] [PubMed] [Google Scholar]
- 29.Tune LE, Strauss ME, Lew MF, Breitlinger E, Coyle JT. Serum levels of anticholinergic drugs and impaired recent memory in chronic schizophrenic patients. Am J Psychiatry. 1982;139(11):1460–2. Epub 1982/11/01. . [DOI] [PubMed] [Google Scholar]
- 30.Mondimore FM, Damlouji N, Folstein MF, Tune L. Post-ECT confusional states associated with elevated serum anticholinergic levels. Am J Psychiatry. 1983;140(7):930–1. Epub 1983/07/01. . [DOI] [PubMed] [Google Scholar]
- 31.Richardson JS, Keegan DL, Bowen RC, Blackshaw SL, Cebrian-Perez S, Dayal N, et al. Verbal learning by major depressive disorder patients during treatment with fluoxetine or amitriptyline. Int Clin Psychopharmacol. 1994;9(1):35–40. . [DOI] [PubMed] [Google Scholar]
- 32.Brecht S, Reiff J, Vock U, Voget J, Ley L, Boning A, et al. Serum anticholinergic activity in patients following cardiac surgery and healthy individuals following amitriptyline application. Methods Find Exp Clin Pharmacol. 2007;29(3):223–30. . [DOI] [PubMed] [Google Scholar]
- 33.Vinogradov S, Fisher M, Warm H, Holland C, Kirshner MA, Pollock BG. The cognitive cost of anticholinergic burden: decreased response to cognitive training in schizophrenia. Am J Psychiatry. 2009;166(9):1055–62. Epub 2009/07/03. 10.1176/appi.ajp.2009.09010017 ; PubMed Central PMCID: PMCPmc3735363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tune L, Carr S, Hoag E, Cooper T. Anticholinergic effects of drugs commonly prescribed for the elderly: potential means for assessing risk of delirium. Am J Psychiatry. 1992;149(10):1393–4. Epub 1992/10/01. . [DOI] [PubMed] [Google Scholar]
- 35.Carnahan RM, Lund BC, Perry PJ, Pollock BG, Gulp KR. The anticholinergic drug scale as a measure of drug-related anticholinergic burden: Associations with serum anticholinergic activity. J Clin Pharmacol. 2006;46(12):1481–6. . [DOI] [PubMed] [Google Scholar]
- 36.Ancelin ML, Artero S, Portet F, Dupuy AM, Touchon J, Ritchie K. Non-degenerative mild cognitive impairment in elderly people and use of anticholinergic drugs: longitudinal cohort study. BMJ. 2006;332(7539):455–9. Epub 2006/02/03. 10.1136/bmj.38740.439664.DE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Plaschke K, Thomas C, Engelhardt R, Teschendorf P, Hestermann U, Weigand MA, et al. Significant correlation between plasma and CSF anticholinergic activity in presurgical patients. Neurosci Lett. 2007;417(1):16–20. . [DOI] [PubMed] [Google Scholar]
- 38.Higgins JPT AD. Assessing risk of bias in included studies Higgins JPT, Green S, eds Cochrane Handbooks for Systematic Reviews of Interventions. Chichester, UK: Wiley-Blackwell; 2011. p. 187–242. [Google Scholar]
- 39.GA Wells BS, D O'Connell, J Peterson, V Welch, M Losos, P Tugwell. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analysis. Available from: www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 40.Lackner TE, Wyman JF, McCarthy TC, Monigold M, Davey C. Randomized, placebo-controlled trial of the cognitive effect, safety, and tolerability of oral extended-release oxybutynin in cognitively impaired nursing home residents with urge urinary incontinence. J Am Geriatr Soc. 2008;56(5):862–70. 10.1111/j.1532-5415.2008.01680.x [DOI] [PubMed] [Google Scholar]
- 41.Kersten H, Molden E, Tolo IK, Skovlund E, Engedal K, Wyller TB. Cognitive effects of reducing anticholinergic drug burden in a frail elderly population: A randomized controlled trial. J Gerontol A Biol Sci Med Sci. 2013;68A(3):271–8. . [DOI] [PubMed] [Google Scholar]
- 42.Tune LE, Damlouji NF, Holland A, Gardner TJ, Folstein MF, Coyle JT. Association of postoperative delirium with raised serum levels of anticholinergic drugs. Lancet. 1981;2(8248):651–3. Epub 1981/09/26. . [DOI] [PubMed] [Google Scholar]
- 43.Remillard AJ. Pilot project to assess the association of anticholinergic symptoms with anticholinergic serum levels in the elderly. Pharmacotherapy. 1994;14(4):482–7. [PubMed] [Google Scholar]
- 44.Plaschke K, Hill H, Engelhardt R, Thomas C, von Haken R, Scholz M, et al. EEG changes and serum anticholinergic activity measured in patients with delirium in the intensive care unit. Anaesthesia. 2007;62(12):1217–23. . [DOI] [PubMed] [Google Scholar]
- 45.Rossi A, Burkhart C, Dell-Kuster S, Pollock BG, Strebel SP, Monsch AU, et al. Serum anticholinergic activity and postoperative cognitive dysfunction in elderly patients. Anesth Analg. 2014;119(4):947–55. 10.1213/ane.0000000000000390 . [DOI] [PubMed] [Google Scholar]
- 46.Golinger RC, Peet T, Tune LE. Association of elevated plasma anticholinergic activity with delirium in surgical patients. Am J Psychiatry. 1987;144(9):1218–20. Epub 1987/09/01. . [DOI] [PubMed] [Google Scholar]
- 47.van Munster BC, Thomas C, Kreisel SH, Brouwer JP, Nanninga S, Kopitz J, et al. Longitudinal assessment of serum anticholinergic activity in delirium of the elderly. J Psychiatr Res. 2012;46(10):1339–45. 10.1016/j.jpsychires.2012.06.015 [DOI] [PubMed] [Google Scholar]
- 48.Kashyap M, Mulsant BH, Tannenbaum C. Small longitudinal study of serum anticholinergic activity and cognitive change in community-dwelling older adults. Am J Geriatr Psychiatry. 2015;23(3):326–9. 10.1016/j.jagp.2014.09.009 [DOI] [PubMed] [Google Scholar]
- 49.Tune L, Carr S, Cooper T, Klug B, Golinger RC. Association of anticholinergic activity of prescribed medications with postoperative delirium. J Neuropsychiatry Clin Neurosci. 1993;5(2):208–10. Epub 1993/01/01. . [DOI] [PubMed] [Google Scholar]
- 50.Nebes RD, Pollock BG, Mulsant BH, Kirshner MA, Halligan E, Zmuda M, et al. Low-level serum anticholinergicity as a source of baseline cognitive heterogeneity in geriatric depressed patients. Psychopharmacol Bull. 1997;33(4):715–20. . [PubMed] [Google Scholar]
- 51.Flacker JM, Cummings V, Mach JR Jr, Bettin K, Kiely DK, Wei J. The association of serum anticholinergic activity with delirium in elderly medical patients. Am J Geriatr Psychiatry. 1998;6(1):31–41. . [PubMed] [Google Scholar]
- 52.Flacker JM, Lipsitz LA. Serum anticholinergic activity changes with acute illness in elderly medical patients. J Gerontol A Biol Sci Med Sci. 1999;54A(1):M12–M6. . [DOI] [PubMed] [Google Scholar]
- 53.Mussi C, Ferrari R, Ascari S, Salvioli G. Importance of serum anticholinergic activity in the assessment of elderly patients with delirium. J Geriatr Psychiatry Neurol. 1999;12(2):82–6. . [DOI] [PubMed] [Google Scholar]
- 54.Nebes RD, Pollock BG, Meltzer CC, Saxton JA, Houck PR, Halligan EM, et al. Serum anticholinergic activity, white matter hyperintensities, and cognitive performance. Neurology. 2005;65(9):1487–9. . [DOI] [PubMed] [Google Scholar]
- 55.Konishi K, Hori K, Uchida H, Watanabe K, Tominaga I, Kimura M, et al. Adverse effects of anticholinergic activity on cognitive functions in Alzheimer's disease. Psychogeriatrics. 2010;10(1):34–8. 10.1111/j.1479-8301.2010.00310.x [DOI] [PubMed] [Google Scholar]
- 56.Nebes RD, Pollock BG, Halligan EM, Houck P, Saxton JA. Cognitive slowing associated with elevated serum anticholinergic activity in older individuals is decreased by caffeine use. Am J Geriatr Psychiatry. 2011;19(2):169–75. 10.1097/JGP.0b013e3181e4490d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hori K, Konishi K, Watanabe K, Uchida H, Tsuboi T, Moriyasu M, et al. Influence of anticholinergic activity in serum on clinical symptoms of Alzheimer's disease. Neuropsychobiology. 2011;63(3):147–53. 10.1159/000321591 [DOI] [PubMed] [Google Scholar]
- 58.Kersten H, Molden E, Willumsen T, Engedal K, Wyller TB. Higher anticholinergic drug scale (ADS) scores are associated with peripheral but not cognitive markers of cholinergic blockade. Cross sectional data from 21 Norwegian nursing homes. Br J Clin Pharmacol. 2013;75(3):842–9. 10.1111/j.1365-2125.2012.04411.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mangoni AA, Munster BCV, Woodman RJ, Rooij SED. Measures of anticholinergic drug exposure, serum anticholinergic activity, and all-cause postdischarge mortality in older hospitalized patients with hip fractures. Am J Geriatr Psychiatry. 2013;21(8):785–93. 10.1016/j.jagp.2013.01.012 [DOI] [PubMed] [Google Scholar]
- 60.Plaschke K, Kopitz J, Mattern J, Martin E, Teschendorf P. Increased cortisol levels and anticholinergic activity in cognitively unimpaired patients. J Neuropsychiatry Clin Neurosci. 2010;22(4):433–41. 10.1176/appi.neuropsych.22.4.433 [DOI] [PubMed] [Google Scholar]
- 61.Plaschke K, Hauth S, Jansen C, Bruckner T, Schramm C, Karck M, et al. The influence of preoperative serum anticholinergic activity and other risk factors for the development of postoperative cognitive dysfunction after cardiac surgery. J Thorac Cardiovasc Surg. 2013;145(3):805–11. 10.1016/j.jtcvs.2012.07.043 [DOI] [PubMed] [Google Scholar]
- 62.Flacker JM, Cummings V, Mach JR Jr, Bettin K, Kiely DK, Wei J. The association of serum anticholinergic activity with delirium in elderly medical patients. Am J Geriatr Psychiatry. 1998;6(1):31–41. . [PubMed] [Google Scholar]
- 63.Nebes RD, Pollock BG, Halligan EM, Kirshner MA, Houck PR. Serum anticholinergic activity and motor performance in elderly persons. J Gerontol A Biol Sci Med Sci. 2007;62A(1):83–5. . [DOI] [PubMed] [Google Scholar]
- 64.Lechevallier-Michel N, Molimard M, Dartigues JF, Fabrigoule C, Fourrier-Reglat A. Drugs with anticholinergic properties and cognitive performance in the elderly: results from the PAQUID Study. Br J Clin Pharmacol. 2005;59(2):143–51. Epub 2005/01/29. 10.1111/j.1365-2125.2004.02232.x ; PubMed Central PMCID: PMCPmc1884748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Campbell N, Boustani M, Limbil T, Ott C, Fox C, Maidment I, et al. The cognitive impact of anticholinergics: a clinical review. Clin Interv Aging. 2009;4:225–33. Epub 2009/06/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bostock CV, Soiza RL, Mangoni AA. Association between prescribing of antimuscarinic drugs and antimuscarinic adverse effects in older people. Expert Rev Clin Pharmacol. 2010;3(4):441–52. Epub 2010/07/01. 10.1586/ecp.10.34 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Funnel plot shows the validity of the meta-analysis on the cross-sectional and case-control studies. All data falls within the allowable region of the funnel plot, indicating that the analysis does not involve outliers that are overrepresented in the analysis
(TIF)
Funnel plot shows the validity of the meta-analysis on the RCT studies. All data falls within the allowable region of the funnel plot, indicating that the analysis does not involve outliers that are overrepresented in the analysis.
(TIF)
The result did not support a conclusion that SAA lowers the MMSE score for RCTs.
(TIF)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
Data are available from supplementary information of the paper.