ABSTRACT
In experimental mouse models of cancer, increasingly compelling evidence point toward a contribution of tumor associated macrophages (TAM) to tumor lymphangiogenesis. Corresponding experimental observations in human cancer remain scarce although lymphatic metastasis is widely recognized as a predominant route for tumor spread. We previously showed that, in malignant tumors of untreated breast cancer (BC) patients, TIE-2-expressing monocytes (TEM) are highly proangiogenic immunosuppressive cells and that TIE-2 and VEGFR signaling pathways drive TEM immunosuppressive function. We report here that, in human BC, TEM express the canonical lymphatic markers LYVE-1, Podoplanin, VEGFR-3 and PROX-1. Critically, both TEM acquisition of lymphatic markers and insertion into lymphatic vessels were observed in tumors but not in adjacent non-neoplastic tissues, suggesting that the tumor microenvironment shapes both TEM phenotype and spatial distribution. We assessed the lymphangiogenic activity of TEM isolated from dissociated primary breast tumors in vitro and in vivo using endothelial cells (EC) sprouting assay and corneal vascularization assay, respectively. We show that, in addition to their known hemangiogenic function, TEM isolated from breast tumor display a lymphangiogenic activity. Importantly, TIE-2 and VEGFR pathways display variable contributions to TEM angiogenic and lymphangiogenic activities across BC patients; however, combination of TIE-2 and VEGFR kinase inhibitors abrogated these activities and overcame inter-patient variability. These results highlight the direct contribution of tumor TEM to the breast tumor lymphatic network and suggest a combined use of TIE-2 and VEGFR kinase inhibitors as a therapeutic approach to block hem- and lymphangiogenesis in BC.
KEYWORDS: Angiogenesis, breast cancer, lymphatics, TIE-2-expressing monocytes, tumor microenvironment
Abbreviations
- ANG-1–4
angiopoietins 1–4
- BC
breast cancer
- BEC
blood endothelial cells
- BMDC
bone marrow-derived cells
- EC
endothelial cells
- HUVEC
Human Umbilical Vein Endothelial Cells
- LEC
lymphatic endothelial cell
- LYVE-1
lymphatic vessel endothelial hyaluronan receptor
- PlGF
Placenta Growth Factor
- PROX-1
prospero-related homeobox 1
- SMA
Alpha-smooth muscle actin
- TAM
tumor associated macrophages
- TEM
TIE-2-expressing monocytes
- TIE-2
tunica interna endothelial cell kinase
- TNF-α
Tumor Necrosis Factor
- VEGF
Vascular endothelial growth factor
- VEGFR
Vascular endothelial growth factor receptor
Introduction
Several lines of evidence suggest that the recruitment of bone marrow-derived cells (BMDC) to malignant tumors is crucial for the angiogenic switch and metastasis.1 BMDC circulating in the peripheral blood can differentiate within the tumor microenvironment into tumor-associated macrophages (TAM) which are believed to exert both protumoral and tumoricidal functions. In this respect, TAM appear to be responsible for conflicting roles of the immune system in cancer.2 This functional dichotomy probably reflects TAM plasticity and notably their adaptation to a local tumor microenvironment, which contains a complex network of proinflammatory and angiogenic mediators.3-5
TAM are proposed to carry out their protumoral activity via three different mechanisms; (i) release of angiogenic factors that trigger an increase in tumor vasculature via EC sprouting from pre-existing vessels, (ii) secretion of proteases that mediate breakdown of the extracellular matrix, a key step in the metastatic spread of tumor cells and (iii) secretion of immunosuppressive mediators that impair T-cell cytotoxic activity and proliferation.4 The contribution of TAM to tumor angiogenesis has been investigated as a potential target for cancer therapy.3 Recent evidence suggests that, in addition to their hemangiogenic activity, TAM also participate in tumor lymphangiogenesis.6, 7 Unfortunately, investigations into the role of TAM in tumor lymphangiogenesis are complicated by overlapping phenotypes with other macrophages8 and EC, as well as phenotype plasticity.9 In a murine model of cancer, TAM were found to carry the canonical lymphatic marker LYVE-1 (lymphatic vessel endothelial hyaluronan receptor).10-12 Studies in murine models of inflammation13,14 have confirmed the trans-differentiation of macrophages into EC and their expression of other canonical lymphatic markers such as VEGFR-3,15,16 prospero-related homeobox 1 (PROX-1) or Podoplanin.12
De Palma and coworkers identified a subset of TAM expressing the tunica interna endothelial cell kinase (TIE-2 or CD202b), a receptor tyrosine kinase (RTK) expressed by EC.17,18 TEM have been documented in murine and human peripheral blood19,20 as well as in tumor tissues where they act as paracrine inducers of angiogenesis.21 TEM were found to account for most, if not all angiogenic activity of BMDC in a murine experimental model of cancer.22 TIE-2 is a RTK that binds angiopoietins 1–4 (ANG-1–4) and is critically involved in vascular embryogenesis and adult angiogenesis.23 In mouse models of BC, TIE-2 signaling upon ANG-2 binding was proposed to contribute to tumor growth, metastasis and TEM angiogenic activity mice.24,25 We reported that in human BC TEM are angiogenic26 and suppressive cells.27 Higher TAM number was associated with a reduced relapse-free and overall survival,28 a higher tumor grade, estrogen and progesterone receptors negativity, HER-2 positivity and a basal phenotype.29
However, corresponding evidence for the contribution of TAM or TEM to human cancer lymphangiogenesis remains scarce. To the best of our knowledge, observations are limited to cervical squamous carcinoma where TAM expressing VEGFR-3 have been linked to the density of lymphatic microvessels.30 Herein, we examined the contribution of TEM to tumor lymphangiogenesis in human invasive breast carcinoma. We report that TEM display a lymphatic endothelial cell (LEC) phenotype and associate with tumor lymphatics, but not with lymphatics of adjacent non-neoplastic tissue where they show drastically reduced TIE-2 expression. Furthermore, we show that tumor TEM are lymphangiogenic in vitro and in vivo. TEM hem- and lymphangiogenic activities are controlled by both TIE-2 and VEGFR kinase activities as shown by using specific kinase inhibitors of these receptors. In light of the crucial role of the lymphatic system in BC metastasis,31 a better understanding of the signaling pathways controlling TEM angiogenesis may enable the design of efficient anti-angiogenic therapies.
Results
TEM can be identified in breast tumors solely based on CD14 immunostaining
The presence of TEM within the haematopoietic infiltrate of human solid tumors has been demonstrated in various malignant human tumors, including BC.21 However, an in-depth characterization of TEM present in breast tumor tissues is missing. We determined by FACS the frequency of TEM in freshly dissociated primary tumors of BC patients (n = 10) who were untreated at time of surgery (clinicopathological characteristics of all 31 patients from this study are listed in Table 1). Fresh tumor specimens were collected from the viable (non-necrotic) tumor center and excluded most of the peritumoral non-neoplastic tissues (see material and methods). Tumor specimens were freshly dissociated with collagenase and TEM were characterized by FACS using a combination of specific antibodies for the pan-haematopoietic marker protein tyrosine phosphatase receptor type C (CD45), the myeloid cell-specific leucine-rich glycoprotein (CD14), integrin α M (CD11b) and TIE-2, as previously established21 (see supplemental material and method section and Fig. S1B). We observed that TEM represent a substantial fraction of the haematopoietic infiltrate (22% ± 2.7% of CD45+ leukocytes), comparable to the frequency of CD8+ T lymphocytes (24% ± 10%). Using confocal microscopy and flow cytometry, we observed that TEM in breast tumor tissues are CD14+, CD45+, CD11c-, HLA-DR+, CD68+ (Fig. S1), TIE-2+ (Figs. 1A, S1B and S2A). In addition, TEM were heterogeneously distributed within the tumor. They were almost excluded from the peritumoral area (where CD3+ and CD45+ cell counts were higher) and enriched in specific area of the tumor center and tumor invasive edges (Fig. S3C).
Table 1.
Clinical and pathological features of tumors and patients (n = 31).
| Patient characteristics | % (nb of patients) | |
|---|---|---|
| Age (median, range) years | ||
| <50 | 52 (16) | |
| ≥50 | 48 (15) | |
| Surgical treatment | ||
| Mastectomy | 39 (12) | |
| Tumorectomy | 61 (19) | |
| Lymph node status | ||
| Negative | 61 (19) | |
| Positive | 39 (12) | |
| Tumor | ||
| T1 | 48 (15) | |
| T2< 3cm | 52 (16) | |
| Histology | ||
| Ductal | 81 (25) | |
| Lobular | 13 (4) | |
| Others | 6 (2) | |
| Grade | ||
| I | 13 (5) | |
| II | 42 (13) | |
| III | 45 (14) | |
Figure 1.
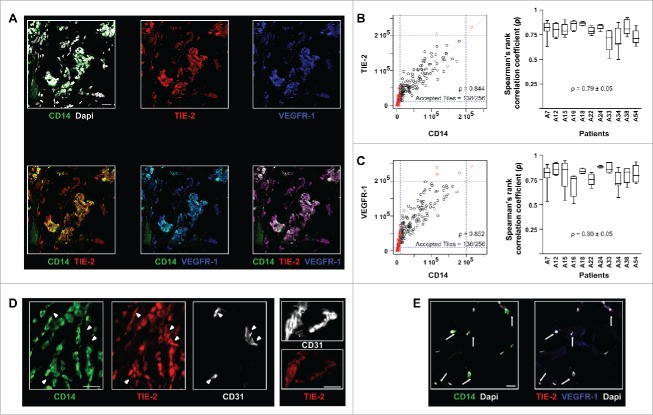
In human breast tumors, TEM can be identified solely based on CD14 immunostaining. (A) Immunofluorescence staining and confocal analysis of human BC tumor sections showing CD14, TIE-2 and VEGFR-1 triple positive cells. Representative image from 16 patients; (B) CD14 and TIE-2 expression correlation analysis. The dot plot (left panel) represents all tiles for one representative image; the box plot (right panel) shows the Spearman’s rank correlation coefficient (ρ) for CD14 and TIE-2 expression in 11 patients. In the dot plot, 54% of CD14+ tiles and 70% of TIE-2+ tiles were above the threshold. 100% of CD14+ tiles were TIE-2+ which means that the high correlation (ρ = 0.844) applied to all CD14+ tiles; (C) CD14 and VEGFR-1 expression correlation analysis for 11 patients. In the dots plot, 55% of CD14+ tiles and 75% of VEGFR-1+ tiles were above the threshold. 100% of CD14+ tiles are VEGFR-1+ which means that the high correlation (ρ = 0.852) applied to all CD14+ tiles; (D) Example of TIE-2 expression in TEM, clusters of BEC (arrow heads) and blood vessels (right panel) shown in confocal microscopy images of BC sections. TEM and blood endothelial structures were stained with CD14 and CD31, respectively; (E) Example of immunofluorescence labeling of CD14, VEGFR-1 and TIE-2 in sections containing non-neoplastic breast tissue adjacent to tumor tissues. CD14+ cells (arrows) are VEGFR- and TIE-2-. Non-neoplastic (panel E) and tumor tissue (panel A) were stained and imaged simultaneously under the same conditions and thus intensities of the expression of TIE-2 and VEGFR-1 signals can be compared. Representative images from four patients. Scale bars: 25 μm.
Further, confocal microscopy analysis revealed that the vast majority (≥ 95%) of CD14+ cells express both TIE-2 and VEGFR-1 (Figs. 1A, 1B, S1 and S2A). A quantitative analysis of CD14, TIE-2 and VEGFR-1 expression levels was carried out on immunofluorescence images (Fig. 1A, control stainings and HE stainings are shown in Figs. S2B and C) and a Spearman’s rank correlation coefficient was calculated (Methods) for individual confocal microscopy fields (n = 6 ± 2 for each patient) across all patients (n = 11). A high Spearman’s rank correlation was found between CD14 and TIE-2 expression levels (ρ = 0.79 ± 0.05, Fig. 1B), as well as CD14 and VEGFR-1 levels (ρ = 0.80 ± 0.05, Fig. 1C), indicating that CD14, VEGFR-1 and TIE-2 expression is phenotypically linked. In order to validate our methodology, the correlation between VEGFR-1 and TIE-2 expression levels was considered as a positive control (ρ = 0.88 ± 0.05, Fig. S3A) since these two receptors are known to be co-expressed by blood endothelial cells (BEC).32 As previously described,21 TEM did not express detectable levels of the platelet endothelial cell adhesion molecule (CD31, Fig. 4A), a prototypical BEC marker and, accordingly CD14 and CD31 expression levels did not correlate (Fig. S3B). Finally, TEM were found to express comparable levels of TIE-2 relative to tumor blood vessels (Fig. 1D).
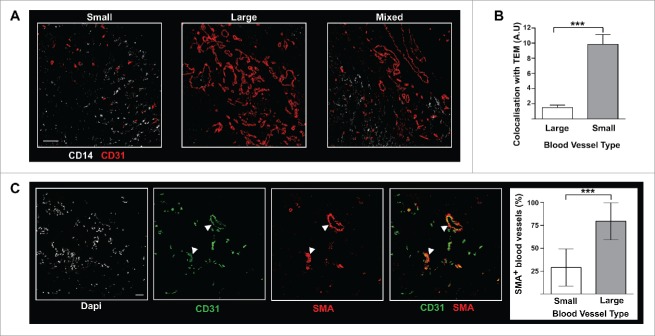
Figure 4.
In BC tumors, TEM are associated with patchy and poorly structured blood vessels. (A) TEM spatial relationship to small and large vessels. Scale bar: 100 μm, representative image from 16 patients; (B) TEM were found located in the proximity of small blood vessels, rather than large vessels (t test, *** p ≤ 0.0001); (C) In BC tumors, the vast majority of large vessels (arrow heads) stain positive for α-SMA while small vessels do not. Scale bar: 50 μm.
These results suggest that in human BC tissues TEM can be reliably identified using CD14 as a single marker, based on the observation that CD14 expression correlates with that of TIE-2 and VEGFR-1 (Figs. 1B and C).
TEM are highly angiogenic cells promoting tumor growth
In BC, more than 95% of CD14+ cells are TEM (i.e. express TIE-2, Figs. 1A, 1B, S1 and S2A). Importantly, we observed that CD14+ cells from non-neoplastic tissues adjacent to the tumor (Fig. 1E) show low levels of TIE-2 and VEGFR-1 expression suggesting that the tumor microenvironment markedly shapes TEM phenotype. In BC, CD14 expression levels were heterogeneous and not significantly different in the tumor center or the tumor leading edges (n = 6, p > 0.05 and Fig. S3C). Further, CD14 levels correlated with VEGFR-1 and TIE-2 expression levels in these two tumor zones (ρ = 0.79 ± 0.05; ρ = 0.79 ± 0.05, respectively).
However, this tumor TEM phenotype may not hold true for other types of cancer where TIE-2 was only detected in 37%–72% of all CD14+ cells.21 We have recently shown that TIE-2 and VEGFR-1 co-expression control TEM angiogenic activity.26 Since CD14 expression correlates with the expression of TIE-2 and VEGFR-1 (Figs. 1B and C), monitoring CD14 infiltrate across BC tissues may be sufficient to evaluate their angiogenic activity. Consistent with this observation, we found that CD14 expression levels per mg of tumor tissue correlated with the size of the tumor26 suggesting that TEM contribute to tumor growth by inducing tumor vascularization. In order to check this hypothesis, we used a patient-derived BC xenograft (PDX) mouse model. Three months post-tumor engraftment, pairs of PDX mice engrafted with the same primary patient tumor fragments and showing comparable tumor size were selected. TEM were isolated by CD14 immunomagnetic selection from autologous primary tumors. Tumor TEM or as a control magnetic beads only, were injected in the tumor vicinity of each mouse. Two weeks later, the mice were sacrificed and the tumor volume and the total surface area covered by blood vessels in the tumor quantified. Mice injected with TEM showed a dramatic increase of their tumor size and total blood vascular network relative to control mice (Fig 2) showing that tumor TEM support tumor growth and vascularization.
Figure 2.
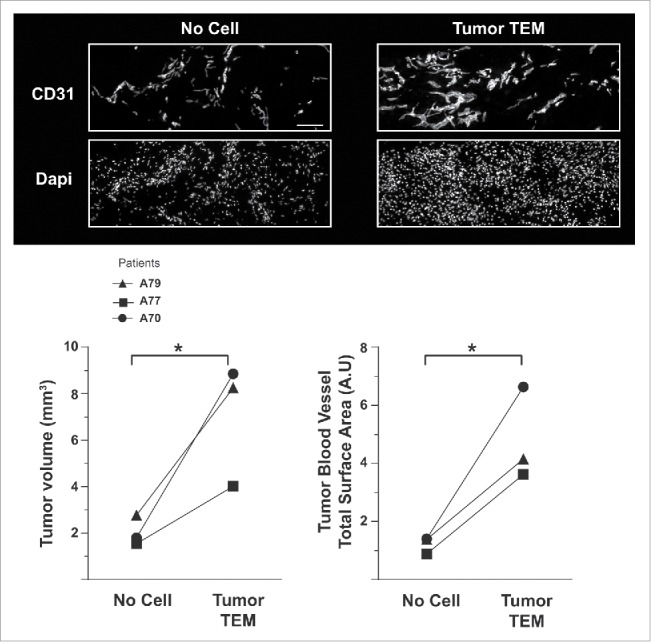
TEM are highly angiogenic cells supporting breast tumor growth. Quantification of the volume and of the vascularization of PDX tumors two weeks post-injection in the tumor vicinity of autologous tumor TEM (Tumor TEM mice) or buffer and magnetic beads (No cell, control mice). Pairs of mice showing comparable tumor volume have been used. Scale bar: 50 μm.
In human breast tumors, TEM express LEC markers
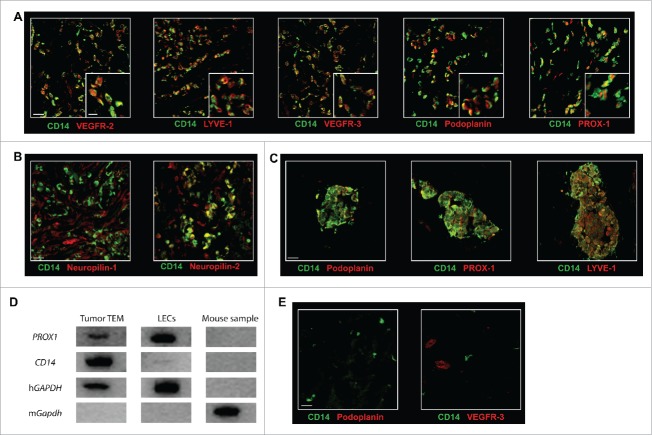
In various murine experimental models of cancer, macrophages have been reported to display LEC traits.15,33 To date, the corresponding evidence for the expression of a LEC phenotype in human TAM has been scarce. This prompted us to examine the expression of LEC markers, as well as the VEGFR co-receptors Neuropilin-1 and Neuropilin-2,34 on TEM associated with BC tissues. Confocal microscopy analysis of breast tumor sections revealed that TEM express the canonical lymphatic markers VEGFR-2, VEGFR-3, LYVE-1, Podoplanin and PROX-1 (Fig. 3A and S4). However, while the expression of Podoplanin, VEGFR-3 and LYVE-1 is homogeneous, VEGFR-2 and PROX-1 expression displayed a higher degree of variability in TEM (Fig. 3A). When analyzing for VEGFR co-receptor expression, we found expression of Neuropilin-2 but no detectable expression of Neuropilin-1 (Fig. 3B). The expression of the VEGFR-2, VEGFR-3, LYVE-1, Podoplanin, PROX-1 and Neuropilin-2 suggests a putative role for TEM in cancer lymphangiogenesis, in addition to their characterized proangiogenic activity (Fig. 2 and 26). Since lymphatics express very low levels of CD31 in contrast to blood vessels, the absence of observable CD31 expression on TEM (vide supra) is also consistent with a lymphatic phenotype. The majority of TEM (LYVE-1+/Podoplanin+/VEGFR-3+/PROX-1+) were cells scattered across the tumor with no apparent degree of structural organization and displayed variable expression levels for lymphatic markers (Fig. 3A). Some TEM were also found to form small aggregates of various shapes and sizes (10–50 cells, Fig. 3C and Fig. S5A). In the latter case, CD14 expression was high and showed good correlation with lymphatic marker expression (Fig. S5B), suggesting that these aggregates are composed of a homogeneous population. We confirmed TEM lymphatic phenotype by gene expression analysis of TEM (CD14+, CD45+) sorted ex vivo from collagenase-dissociated breast tumors. PROX1 expression was assessed by RT-PCR in eight independent samples of 10-cell aliquots sorted from four distinct tumors. A representative example of gene expression profile is shown in Fig. 3D. PROX1 was expressed in the four tumors and in 75% of the cell aliquots (24 positive samples/32 samples in total) examined.
Figure 3.
TEM carry a lymphatic phenotype in human breast tumor. The expression of (A) the lymphatic markers VEGFR-2, LYVE-1, VEGFR-3, Podoplanin, and PROX-1 and (B) the VEGFR co-receptors Neuropilin-1 and Neuropilin-2 was examined in breast tumor sections by confocal microscopy. Representative images from minimum five patients; (C) Some TEM formed small cell aggregates (10–100 cells); (D) PROX1 expression analysis was performed by RT-PCR from mRNA isolated from mouse cells (negative control), LEC in culture (positive control) and from TEM (CD14+CD45+ cells) sorted by flow cytometry from dissociated breast tumor. One representative gene expression profile from 10-cell samples from one patient is shown out of 32 cell samples from four patients (8 samples/patient of 10 cells each); (E) Immunofluorescence labeling in sections of non-neoplastic breast tissue adjacent to tumor tissues shows no detectable expression of Podoplanin and VEGFR-3. Non-neoplastic (panel E) and tumor tissue (panel A and C) were stained and imaged simultaneously under the same conditions and thus intensities of the expression of Podoplanin and VEGFR-3 signals can be compared. Scale bars (A-C and E): 25 μm, (A) higher magnification, scale bar: 10 μm.
Finally and importantly, the observation that CD14+ cells from non-neoplastic tissues adjacent to the tumor did not express detectable levels of Podoplanin and VEGFR-3 (Fig. 3E) further supports a phenotypic switch of CD14+ cells in the tumor microenvironment. Finally and consistent with these findings, when we expressed TEM angiogenic activity as the percentage of tumor area covered by CD14+ cells multiplied by the mean fluorescence intensity of CD14+ cells, we observed a correlation of this angiogenic activity with the ratio of CD14 to lymphocyte infiltrate (Fig. S3D). This ratio reflects the balance of TEM immunosuppression27 to effective antitumor immune response.35
TEM accumulate in tumor areas that are enriched in small immature blood vessels
Because TEM isolated from BC tissues are highly proangiogenic (Fig. 2 and 26), we examined the spatial relationship between TEM distribution and the tumoral vascular network. To this end, blood vessels and TEM were identified in breast tumor cryosections by CD31 and CD14 dual immunofluorescence using confocal microscopy. The CD31+ structures were categorized into small and large vessels based on their surface area. Of the total vessel area, 88% were either small or large vessels present in similar proportions (53% and 47% respectively). Blood vessels of intermediate size accounted for the remaining 12% of the total vessel area and were excluded from the analysis. Small vessels, in contrast to large vessels, failed to stain for α-smooth muscle actin (SMA) and represent immature vessels (Fig. 4C). Small vessels displayed a chaotic architecture (Fig. 4A, first panel) and likely represent newly formed blood vessels36 while large vessels were organized in a more structured vascular network (Fig. 4A, second panel). Using a modification of the quantification method used for the determination of expression levels correlation (Materials and Methods) the proximity of TEM with large or small vessels was examined in 945 fields. We found a markedly higher spatial relationship (p ≤ 0.001) between TEM and small vessels compared to large vessels (Fig. 4B). The trend showing association of TEM with small vessels was also observed in zones where both types of vascular networks overlap (Fig. 4A, third panel). Thus, TEM are highly proangiogenic monocytes (Fig. 2) enriched in tumor areas of intense neo-vascularization (Fig. 4B).
TEM are found associated with lymphatic vessels
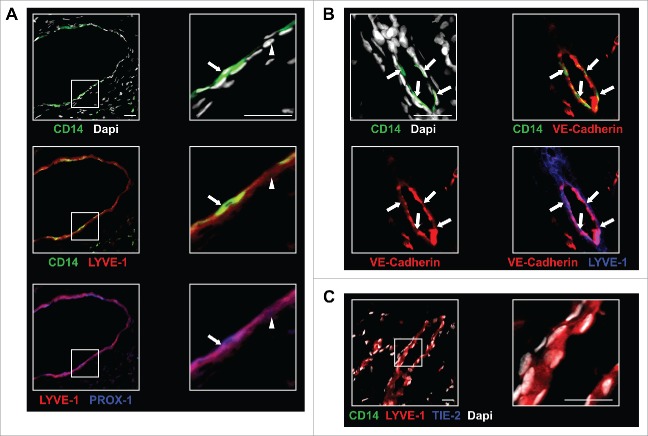
We also examined TEM distribution relative to lymphatic vessels. Since TEM and LEC seem to share canonical lymphatic markers (Fig. 3A), CD14 labeling was used to discriminate between cells of myeloid and endothelial character. Triple immunostaining experiments using CD14- and PROX-1-specific antibodies in combination with either LYVE-1 or Podoplanin were carried out and the sections analyzed by confocal microscopy. TEM were inserted into circular or elongated structures composed of a single layer of overlapping cells, an architectural feature characteristic of lymphatic vessels (Fig. 5A, Figs. S6A and B).34 These LYVE-1+/Podoplanin+/PROX-1+/CD14- (Fig. 5A, Figs. S6A and B) structures appeared mostly VEGFR-3+ and Neuropilin-2+ but TIE-2low (Fig. S6A), with insertion of TEM expressing variable levels of CD14, TIE-2 and LEC markers. It is worth noting that few intact lymphatic structures were present in the tumor sections investigated and that most TEM were observed either scattered across the tumor or aggregated into small clusters (Figs. 3A and 3C). The observation that TEM are associated with tumor lymphatics may reflect either an ongoing transmigration of TEM through the lymphatic endothelium, a stable association of TEM with lymphatics. Two lines of evidences suggest that TEM are indeed inserted into lymphatics. First, TEM consistently display an elongated shape when observed in lymphatics (Fig. 5A), contrasting with their round and bulky shape when aggregated into clusters (Fig. 3C) or scattered across the tumor (Fig. 3A). Second, co-staining with the endothelial cell-specific adherens junction protein VE-Cadherin shows a continuous expression along the lymphatic structure, including the site of TEM interaction (Fig. 5B). Comparable observations were made for BMDC incorporated into lymphatics in mouse experimental model of cancer12 and may reflect a similar cellular process.
Figure 5.
In BC tumors, TEM are associated with lymphatic structures. (A) TEM (arrow, CD14+LYVE-1+PROX-1+ cells) were associated with lymphatic vessels (arrowhead, CD14-LYVE-1+PROX-1+ cells); (B) Immunofluorescence labeling of TEM-containing lymphatics (arrows) with VE-cadherin; (C) Immunofluorescence labeling in sections of non-neoplastic breast tissue adjacent to tumor tissues shows no TEM association with LYVE-1+ lymphatic vessels. Scale bars: 25 μm. Representative image from seven patients.
In order to investigate the relevance of the association of TEM with lymphatic vessels, we examined sections of non-neoplastic breast tissue adjacent to tumor tissues within the surgical margin in the same patients (n = 7). We observed that Podoplanin+ and LYVE-1+ lymphatic vessels at the tumor periphery did not express any detectable levels of CD14 (Fig. 5C and Fig. S6C) although CD14+TIE-2- myeloid cells were present in these areas (Fig. 1E). These observations suggest that TEM association with lymphatics occurs specifically within the BC microenvironment. Because BC spreads primarily via lymphatics and regional lymph nodes are usually the first metastatic sites to be involved,31 we examined the relationship between lymph node status and TEM association with tumor lymphatics. Interestingly, all patients (100%) with metastasis to the LN (n = 10 ) were found to have TEM associated with tumor lymphatics whereas this was only the case for 57% of the patients (n = 12 ) without LN metastasis. These observations suggest that TEM associated to lymphatics may contribute to the spreading of tumor cells to proximal LN through a yet unknown mechanism.
TEM isolated from human breast tumors are lymphangiogenic
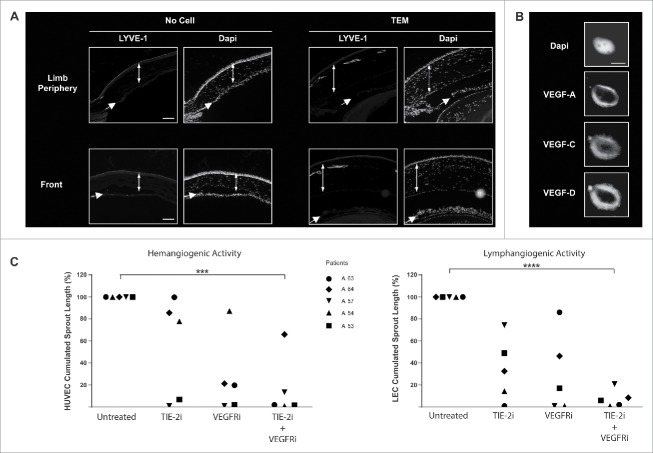
The observed TEM association with cancerous lymphatic structures (Fig. 5A, B and Figs. S6A, B) together with their expression of lymphatic markers (Figs. 3A and B) strongly suggest a role for TEM in tumoral lymphangiogenesis. TEM lymphangiogenic activity was assessed by the in vivo corneal vascularization assay. The cornea is avascular and the formation of blood or lymphatic vessels sprouting from the peripheral limbal vasculature can be taken as a qualitative measure of TEM angiogenic and lymphangiogenic activity, respectively.37 Due to the limited number of TEM that can be isolated from primary BC tissues, mice were preferred to rabbits for corneal vascularization assays because of the smaller size of their eyes. Immunocompromised NOD-scid IL2Rγnull mice were used to prevent the rejection of patient TEM by the mouse immune system. Mouse corneas were injected with CD14+ cells immunoselected from freshly dissociated BC tissue and 3–4 weeks later sagittal cryosections of the mouse eyes were stained with antibodies specific for LYVE-1 to assess de novo growth of lymphatic vessels. The surface area covered by lymphatic vessels was > 800-fold higher in the cornea of mice injected with TEM relative to the one of mice injected with buffer and beads only (p < 0.01, Fig. 6A). These results indicate that tumor TEM induce corneal lymphatics vascularization i.e., are lymphangiogenic cells. Consistent with these observations, the majority of TEM sorted from dissociated breast carcinoma by flow cytometry (CD45+, CD14+) express VEGF-A, -C and -D (Fig. 6B). Thus, in addition to their hemangiogenic activity (Fig. 2 and26), TEM isolated from human breast tumor tissues are lymphangiogenic in vivo.
Figure 6.
TEM lymphangiogenic activity is supported by cross-talk between VEGFR and TIE-2 pathways. (A) In vivo corneal vascularization assay was used to assess the lymphangiogenic activity of TEM isolated from human breast tumors. Lymphatic vessels emerging from the peripheral limbal vasculature were labeled with LYVE-1-specific antibodies in sagittal sections of mouse eyes. Double-headed and single arrows depict cornea and iris respectively. Scale bar: 100 μm; (B) Confocal microscopy images of TEM sorted from dissociated breast tumor showing expression of VEGF-A, -C and -D. Scale bar: 5μm; (C) TEM isolated from dissociated breast tumors were exposed to TIE-2- and VEGFR-specific kinase inhibitors and their hem- and lymphangiogenic activities were measured in vitro using a sprouting assay of HUVEC and LEC respectively (t test, *** p <0.001, **** p < 0.0001).
TIE-2 and VEGFR kinase activities contribute to TEM hem- and lymphangiogenic activities
We next examined the contribution of TIE-2 and VEGFR kinase activities to TEM angiogenic activity. To this end, TEM isolated from freshly dissociated breast carcinoma were treated for 2 h with kinase inhibitors specific for TIE-2 and VEGFR-1–3, extensively washed and then added to human umbilical vein endothelial cells (HUVEC)- or LEC-covered microcarrier beads embedded in a 3D fibrin gel (Supplemental Material and Methods). In the absence of inhibitors, both HUVEC and LEC were found to form sprouts when co-cultured with TEM (Fig. 6C), confirming TEM dual hem- (Fig. 2 and26) and lymphangiogenic (Fig. 6A) activities. Individually, TIE-2 or VEGFR inhibitor treatments showed a highly variable effect on TEM hemangiogenic and lymphangiogenic activities among different patients (Fig. 6C). However, TEM-induced hemangiogenic and lymphangiogenic activities were almost completely impaired in all patients by a combined treatment. These observations indicate that the dual hem- and lymphangiogenic activities are under the control of a synergistic action between TIE-2 and VEGFR pathways.26 Furthermore, this synergy shows inter-patient variability, but a combined blockade of TIE-2 and VEGFR kinase activities shows a consistent effect, overcoming patient heterogeneity.
Discussion
We show in this study that the levels of CD14 expression correlates with that of TIE-2 and VEGFR-1 for TEM in primary tumors of untreated BC patients (Figs. 1B and C). Since we show that the expression levels of TIE-2 and VEGFR-1 reflect TEM proangiogenic activity,26 we thus propose that CD14 can be considered an indicator of TEM-induced angiogenic activity in BC. Furthermore, we show that TEM account for the majority (> 95%) of the CD14highCD11b+HLA-DR+ cells (Figs. 1 and S1) which constitute a substantial portion (> 20%) of the CD45+ infiltrate in breast carcinomas. In this respect, our results contrast with reports suggesting that CD14highCD11b+HLA-DR+ cells account for only 4% of the CD45+ population in breast carcinomas.38 While Coussens and coworkers did not investigate TIE-2 expression,38 this discrepancy may be due to a sampling artifact,39 and more precisely the proximity with the resection margins. Our results are based on the analysis of viable center of tumors, where the density of total haematopoietic infiltrate might be lower, as suggested by a low T-cell infiltration.
Importantly, we show that in breast tumors, TEM systematically express lymphatic markers (Figs. 3A and S4) as well as Neuropilin-2 (Fig. 3B), VEGF-A, -C and -D (Fig. 6B). Based on this observation, we propose that in BC, TEM can be discriminated from LEC solely based on their CD14 expression. The infiltration of tumor tissues by macrophages displaying canonical LEC markers has been reported in murine experimental models of cancer.15,33 To our knowledge, there is only one comparable observation in humans describing that TAM expressing VEGFR-3, VEGF-C and -D are present in cervical squamous carcinomas.30
The insertion of BMDC into lymphatic vessels was reported in murine models of inflammation10,12,15,40,41 or cancer11 in mice. To the best of our knowledge, our results support for the first time that TEM have a similar behavior in human cancer. The significance and the mechanism of TEM association with lymphatics predominantly in patients showing regional LN metastasis remain to be elucidated. Regional lymph node status is the single most important prognostic factor in BC and patients with axillary metastasis at the time of diagnosis have a much worse prognosis than those without metastasis. The extent of sentinel LN lymphangiogenesis and lymphatic tumor invasion was correlated with non-sentinel LN metastasis in BC patients.42,43 Thus, given the lymphangiogenic activity of BC TEM and the role of lymphatics in metastasis,31 it is reasonable to suggest that TEM not only participate to cytokine-mediated lymphangiogenesis (44 and Fig. 6B) but also contribute to the spreading of tumor cells to regional LN. Indeed, the observed association of tumor TEM with tumor lymphatics (Figs. 5A and B) and LN lymphangiogenesis7 prior to the onset of LN metastasis align wells with the concept of a creation of a premetastatic niche by the primary tumor.7,45 Breast tumors were reported to contain few intratumoral lymphatics46,47 and hyperplasia of pre-existing lymphatic vessels, rather than de novo formation of lymphatic vessels was reported to be associated with metastasis in BC.48,49 We make the assumption that TEM might contribute to lymphatics hyperplasia through their association with lymphatic vessels (Fig. 5), their lymphangiogenic activity and their aptitude to produce VEGF-C and VEGF-D (Fig. 6).
The insertion into lymphatics of BDMC displaying a LEC phenotype was observed in humans under various inflammatory conditions, for example following kidney transplantation,50 nematode infection51 or pulmonary fibrosis.52 By contrast, although TEM display a hemangiogenic character (26 and Fig. 2), they were not found to be inserted into the blood vessels of breast tumor. TEM are enriched into tumor zones containing newly formed and poorly structured blood vascular network (Figs. 4A and B), thus emphasizing their role in the early phase of tumor vascularization and growth (Fig. 2).
We propose that the breast tumor microenvironment may shape TEM phenotype and function. Indeed, as compared to tumor-infiltrating TEM, CD14+ cells in adjacent non-neoplastic breast tissue expressed much lower levels of TIE-2 and VEGFR-1 (Fig. 1E), and no detectable levels of Podoplanin and VEGFR-3 (Fig. 3E). This observation suggests that the LEC phenotype and TIE-2 expression are acquired by TEM following their infiltration into the tumor. The acquisition of a TEM phenotype coincided with the association of TEM with tumor lymphatics (Figs. 5A, B and Figs. S6A and B), while TEM were absent from lymphatic vessels in adjacent non-neoplastic tissue (Fig. 5C and Fig. S6C), suggesting that the breast tumor microenvironment shapes both TEM phenotype and spatial distribution. In cervix cancer, TAM displaying traits of LEC were observed in close proximity but not inserted into lymphatic vessels30 supporting the hypothesis that distinct tumor microenvironments induce different TAM phenotype and spatial distribution.
Our study comprised a low number of BC, the majority of which were of luminal histological subtype (Table 2). We found that the levels of TEM infiltration tended to increase in high histological grade tumors and correlated with tumor size.26 Our observations are consistent with previous studies where larger numbers of BC were examined and that reported that higher levels of monocyte infiltration is associated with poor prognosis, high histological tumor grade53-55 and LN metastasis.56
Table 2.
Pathological features of the tumors used for each experiment.
| Parameter examined | Figures | Luminal A | Luminal B | Triple Neg. | HER2Enriched | Total |
|---|---|---|---|---|---|---|
| Correlation CD14_TIE2_VEGFR1 | 1, S1 and S3 | 4 | 5 | 1 | 1 | 11 |
| TEM angiogenic activity in PDX models | 2 | 3 | 0 | 0 | 0 | 3 |
| TEM express LEC markers | 2, 3, S4 and S5 | 4 | 6 | 1 | 0 | 11 |
| TEM and blood Vascular network | 4 | 6 | 7 | 2 | 1 | 16 |
| TEM association with lymphatics(among which LN metastasis) | 5 and S6 | 12(6/12) | 14(5/14) | 5(3/5) | 00 | 22 |
| Lymphangiogenic activity | 6 | 1 | 4 | 2 | 0 | 7 |
Finally, TEM dual hem- and lymphangiogenic activities are synergistically controlled by TIE-2 and VEGFR pathways27 and impaired by combined treatment with TIE-2 and VEGFR kinase inhibitors (Fig 6C). This result may explain the limited efficacy of VEGF-A neutralizing antibody in TEM hemangiogenic activity27 and in the treatment of BC.57 Furthermore, our results suggest rather that combined inhibition of VEGFR and TIE-2 kinase activities consistently overcame TEM intrinsic resistance mechanisms in untreated BC patients (Fig. 6C). Importantly, we previously reported that BC-associated TEMs are plastic cells that can be reverted from suppressive, hemangiogenic cells into cells that are able to mediate an antitumoral immune response by inhibition of the TIE-2 and VEGFR pathways.27 Taken together, our results show that lymphangiogenic, hemangiogenic and suppressive functions of TEMs are similarly driven by TIE-2 and VEGFR kinase pathways which may represent attractive therapeutic targets in BC.
Materials and methods
Detailed material and methods are reported in supplementary material and methods.
Cells and tissue specimens
This study was approved by the ethics committee of the University Hospital (CHUV) of Lausanne, Switzerland. Patient tissue specimens were obtained according to the declaration of Helsinki and upon written informed consent. Invasive ductal breast carcinomas were resected from untreated patients (Table 1). All characterization and assays were performed using tumor specimens from the viable (non-necrotic) tumor center, and excluding most of the peritumoral non-neoplastic tissues. Tumor tissues were freshly dissociated enzymatically with 0.1% collagenase I (Worthington, Lakewood, NJ) for 40 min at 37°C. HUVEC (Human Umbilical Vein Endothelial Cells) and LEC (human dermal lymphatic microvascular endothelial cells) from adult were from Lonza (Basel, Switzerland).
Reagents and antibodies
Chemicals were from Sigma-Aldrich (St. Louis, MO) unless indicated otherwise. TIE-2 inhibitor compound 7 was from Alexis Biochemicals (San Diego, CA) and VEGFR inhibitor PTK787 from Novartis Institutes for BioMedical Research (Basel, Switzerland). Antibodies are described in supplementary material and methods.
In vivo and in vitro angiogenic assays
The assays were performed as described.26,27, 58 Detailed procedure can be found in supplementary material and methods.
Animals and establishment of patient-derived BC xenografts
About 12 fragments of patient tumor (3.35 mm3) were embedded in matrigel and grafted subcutaneously into NOD-scid IL2Rγnull (NSG) mice. Mice received β-estradiol (8.5 mg/mL) diluted in drinking water and xenografts larger than 1mm3 developed 3 months later in 20% of the mice. Mice with comparable tumor size were used and 20,000 tumor TEM isolated by positive immunomagnetic selection (from frozen viable dissociated tumors of the same patient) or beads only were injected in the tumor vicinity of test and control mice, respectively. Two weeks later, the mice were sacrificed, the tumors were embedded in OCT and sectioned every 0.2 mm. All the sections were stained for CD31 and the vascular network quantified by epifluorescence microscopy.
Histology, confocal microscopy and image quantification
Frozen primary BC specimen were cut in 6 μm sections, stained and imaged as previously described.27 Receptors colocalization was examined using a custom algorithm written in C as detailed in supplemental methods and available upon request.
Statistical analyses
Statistical analyses (two-tailed Student's t test) were performed using GraphPad Prism version 4.00 for Windows (GraphPad Software, San Diego, CA). Results were considered to be significant with * p < 0.05; ** p < 0.01; *** p < 0.001, **** p < 0.0001. Spearman’s rank correlation coefficients were calculated using R.
Authors’ contributions
S.B. and L.H. performed research, analyzed and interpreted the data and wrote the manuscript. E.F.v.H., E. M. I., and R.T. performed research, analyzed and interpreted the data. I.X, N.G., A.S., E. M. I., N.R. and D.V. contributed to the development of methodologies, analyzed and interpreted the data. H.-A. L., J.-F.D. and A. I.-T. managed patients and interpreted the data. G.C and M.-A. D. designed and performed research, interpreted the data and wrote the manuscript.
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was disclosed.
Acknowledgments
We thank Antonio Cassara (University of Lausanne) for technical help and Novartis (Basel, Switzerland) for providing the VEGFR kinase inhibitor PTK787.
Funding
This work was supported by grants from Oncosuisse (M.-A. D. project 02069–04–2007), the Swiss National Foundation (M.-A. D. project 310030–120473 and J.-F. D. project CR32I3_135073), the Medic foundation (M.-A. D.) and the Experimental Network for Functional Integration FP6 Program (I.X. project LSHG-CT-2005–518254).
Supplemental Material
Supplemental Material may be downloaded here: publisher's website
References
- 1.Lin EY, Pollard JW. Tumor-associated macrophages press the angiogenic switch in breast cancer. Cancer Res 2007; 67:5064-6; PMID:17545580; http://dx.doi.org/ 10.1158/0008-5472.CAN-07-0912. [DOI] [PubMed] [Google Scholar]
- 2.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell 2010; 141:39-51; PMID:20371344; http://dx.doi.org/ 10.1016/j.cell.2010.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biswas SK, Sica A, Lewis CE. Plasticity of macrophage function during tumor progression: regulation by distinct molecular mechanisms. J Immunol 2008; 180:2011-7; PMID:18250403; http://dx.doi.org/ 10.4049/jimmunol.180.4.2011 [DOI] [PubMed] [Google Scholar]
- 4.Ruffell B, Affara NI, Coussens LM. Differential macrophage programming in the tumor microenvironment. Trends Immunol 2012; 33:119-26; PMID:22277903; http://dx.doi.org/ 10.1016/j.it.2011.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Motz GT, Coukos G. The parallel lives of angiogenesis and immunosuppression: cancer and other tales. Nat Rev Immunol 2011; 11:702-11; PMID:21941296; http://dx.doi.org/ 10.1038/nri3064 [DOI] [PubMed] [Google Scholar]
- 6.Ji RC. Macrophages are important mediators of either tumor- or inflammation-induced lymphangiogenesis. Cell Mol Life Sci 2012; 69:897-914; PMID:21984600; http://dx.doi.org/ 10.1007/s00018-011-0848-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karaman S, Detmar M. Mechanisms of lymphatic metastasis. J Clin Invest 2014; 124:922-8; PMID:24590277; http://dx.doi.org/ 10.1172/JCI71606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol 2005; 5:953-64; PMID:16322748; http://dx.doi.org/ 10.1038/nri1733 [DOI] [PubMed] [Google Scholar]
- 9.Coffelt SB, Lewis CE, Naldini L, Brown JM, Ferrara N, De Palma M. Elusive identities and overlapping phenotypes of proangiogenic myeloid cells in tumors. Am J Pathol 2010; 176:1564-76; PMID:20167863; http://dx.doi.org/ 10.2353/ajpath.2010.090786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schledzewski K, Falkowski M, Moldenhauer G, Metharom P, Kzhyshkowska J, Ganss R, Demory A, Falkowska-Hansen B, Kurzen H, Ugurel S et al. Lymphatic endothelium-specific hyaluronan receptor LYVE-1 is expressed by stabilin-1+, F4/80+, CD11b+ macrophages in malignant tumours and wound healing tissue in vivo and in bone marrow cultures in vitro: implications for the assessment of lymphangiogenesis. J Pathol 2006; 209:67-77; PMID:16482496; http://dx.doi.org/ 10.1002/path.1942 [DOI] [PubMed] [Google Scholar]
- 11.Zumsteg A, Baeriswyl V, Imaizumi N, Schwendener R, Ruegg C, Christofori G. Myeloid cells contribute to tumor lymphangiogenesis. PLoS One 2009; 4:e7067; PMID:19759906; http://dx.doi.org/ 10.1371/journal.pone.0007067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zumsteg A, Christofori G. Myeloid cells and lymphangiogenesis. Cold Spring Harb Perspect Med 2012; 2:a006494; PMID:22675661; http://dx.doi.org/ 10.1101/cshperspect.a006494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim H, Kataru RP, Koh GY. Inflammation-associated lymphangiogenesis: a double-edged sword? J Clin Invest 2014; 124:936-42; PMID:24590279; http://dx.doi.org/ 10.1172/JCI71607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cursiefen C, Chen L, Borges LP, Jackson D, Cao J, Radziejewski C, D'Amore PA, Dana MR, Wiegand SJ, Streilein JW. VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J Clin Invest 2004; 113:1040-50; PMID:15057311; http://dx.doi.org/ 10.1172/JCI20465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maruyama K, Ii M, Cursiefen C, Jackson DG, Keino H, Tomita M, Van Rooijen N, Takenaka H, D'Amore PA, Stein-Streilein J et al. Inflammation-induced lymphangiogenesis in the cornea arises from CD11b-positive macrophages. J Clin Invest 2005; 115:2363-72; PMID:16138190; http://dx.doi.org/ 10.1172/JCI23874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hall KL, Volk-Draper LD, Flister MJ, Ran S. New model of macrophage acquisition of the lymphatic endothelial phenotype. PLoS One 2012; 7:e31794; PMID:22396739; http://dx.doi.org/ 10.1371/journal.pone.0031794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Palma M, Venneri MA, Roca C, Naldini L. Targeting exogenous genes to tumor angiogenesis by transplantation of genetically modified hematopoietic stem cells. Nat Med 2003; 9:789-95; PMID:12740570; http://dx.doi.org/ 10.1038/nm871 [DOI] [PubMed] [Google Scholar]
- 18.De Palma M, Venneri MA, Galli R, Sergi Sergi L, Politi LS, Sampaolesi M, Naldini L. Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer Cell 2005; 8:211-26; PMID:16169466; http://dx.doi.org/ 10.1016/j.ccr.2005.08.002 [DOI] [PubMed] [Google Scholar]
- 19.Nowak G, Karrar A, Holmen C, Nava S, Uzunel M, Hultenby K, Sumitran-Holgersson S. Expression of vascular endothelial growth factor receptor-2 or Tie-2 on peripheral blood cells defines functionally competent cell populations capable of reendothelialization. Circulation 2004; 110:3699-707; PMID:15381639; http://dx.doi.org/ 10.1161/01.CIR.0000143626.16576.51 [DOI] [PubMed] [Google Scholar]
- 20.Murdoch C, Tazzyman S, Webster S, Lewis CE. Expression of Tie-2 by human monocytes and their responses to angiopoietin-2. J Immunol 2007; 178:7405-11; PMID:17513791; http://dx.doi.org/ 10.4049/jimmunol.178.11.7405 [DOI] [PubMed] [Google Scholar]
- 21.Venneri MA, De Palma M, Ponzoni M, Pucci F, Scielzo C, Zonari E, Mazzieri R, Doglioni C, Naldini L. Identification of proangiogenic TIE2-expressing monocytes (TEMs) in human peripheral blood and cancer. Blood 2007; 109:5276-85; PMID:17327411; http://dx.doi.org/ 10.1182/blood-2006-10-053504 [DOI] [PubMed] [Google Scholar]
- 22.De Palma M, Murdoch C, Venneri MA, Naldini L, Lewis CE. Tie2-expressing monocytes: regulation of tumor angiogenesis and therapeutic implications. Trends Immunol 2007; 28:519-24; PMID:17981504; http://dx.doi.org/ 10.1016/j.it.2007.09.004 [DOI] [PubMed] [Google Scholar]
- 23.Jones N, Iljin K, Dumont DJ, Alitalo K. Tie receptors: new modulators of angiogenic and lymphangiogenic responses. Nat Rev Mol Cell Biol 2001; 2:257-67; PMID:11283723; http://dx.doi.org/ 10.1038/35067005 [DOI] [PubMed] [Google Scholar]
- 24.Coffelt SB, Tal AO, Scholz A, De Palma M, Patel S, Urbich C, Biswas SK, Murdoch C, Plate KH, Reiss Y et al. Angiopoietin-2 regulates gene expression in TIE2-expressing monocytes and augments their inherent proangiogenic functions. Cancer Res 2010; 70:5270-80; PMID:20530679; http://dx.doi.org/ 10.1158/0008-5472.CAN-10-0012 [DOI] [PubMed] [Google Scholar]
- 25.Mazzieri R, Pucci F, Moi D, Zonari E, Ranghetti A, Berti A, Politi LS, Gentner B, Brown JL, Naldini L et al. Targeting the ANG2/TIE2 axis inhibits tumor growth and metastasis by impairing angiogenesis and disabling rebounds of proangiogenic myeloid cells. Cancer Cell 2011; 19:512-26; PMID:21481792; http://dx.doi.org/ 10.1016/j.ccr.2011.02.005 [DOI] [PubMed] [Google Scholar]
- 26.Guex N, Crespo I, Bron S, Ifticene-Treboux A, Faes-Van't Hull E, Kharoubi S, Liechti R, Werffeli P, Ibberson M, Majo F et al. Angiogenic activity of breast cancer patients’ monocytes reverted by combined use of systems modeling and experimental approaches. PLoS Comput Biol 2015; 11:e1004050; PMID:25768678; http://dx.doi.org/ 10.1371/journal.pcbi.1004050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ibberson M, Bron S, Guex N, Faes-van't Hull E, Ifticene-Treboux A, Henry L, Lehr HA, Delaloye JF, Coukos G, Xenarios I et al. TIE-2 and VEGFR kinase activities drive immunosuppressive function of TIE-2-expressing monocytes in human breast tumors. Clin Cancer Res 2013; 19:3439-49; PMID:23649001; http://dx.doi.org/ 10.1158/1078-0432.CCR-12-3181 [DOI] [PubMed] [Google Scholar]
- 28.Leek RD, Lewis CE, Whitehouse R, Greenall M, Clarke J, Harris AL. Association of macrophage infiltration with angiogenesis and prognosis in invasive breast carcinoma. Cancer Res 1996; 56:4625-9; PMID:8840975 [PubMed] [Google Scholar]
- 29.Mahmoud SM, Lee AH, Paish EC, Macmillan RD, Ellis IO, Green AR. Tumour-infiltrating macrophages and clinical outcome in breast cancer. J Clin Pathol 2012; 65:159-63; PMID:22049225; http://dx.doi.org/ 10.1136/jclinpath-2011-200355 [DOI] [PubMed] [Google Scholar]
- 30.Schoppmann SF, Birner P, Stockl J, Kalt R, Ullrich R, Caucig C, Kriehuber E, Nagy K, Alitalo K, Kerjaschki D. Tumor-associated macrophages express lymphatic endothelial growth factors and are related to peritumoral lymphangiogenesis. Am J Pathol 2002; 161:947-56; PMID:12213723; http://dx.doi.org/ 10.1016/S0002-9440(10)64255-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cunnick GH, Jiang WG, Gomez KF, Mansel RE. Lymphangiogenesis and breast cancer metastasis. Histol Histopathol 2002; 17:863-70; PMID:12168797 [DOI] [PubMed] [Google Scholar]
- 32.Pober JS, Sessa WC. Evolving functions of endothelial cells in inflammation. Nat Rev Immunol 2007; 7:803-15; PMID:17893694; http://dx.doi.org/ 10.1038/nri2171 [DOI] [PubMed] [Google Scholar]
- 33.Skobe M, Hamberg LM, Hawighorst T, Schirner M, Wolf GL, Alitalo K, Detmar M. Concurrent induction of lymphangiogenesis, angiogenesis, and macrophage recruitment by vascular endothelial growth factor-C in melanoma. Am J Pathol 2001; 159:893-903; PMID:11549582; http://dx.doi.org/ 10.1016/S0002-9440(10)61765-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Choi I, Lee S, Hong YK. The new era of the lymphatic system: no longer secondary to the blood vascular system. Cold Spring Harb Perspect Med 2012; 2:a006445; PMID:22474611; http://dx.doi.org/ 10.1101/cshperspect.a006445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salgado R, Denkert C, Demaria S, Sirtaine N, Klauschen F, Pruneri G, Wienert S, Van den Eynden G, Baehner FL, Penault-Llorca F et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol 2015; 26:259-71; PMID:25214542; http://dx.doi.org/ 10.1093/annonc/mdu450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res 2006; 66:605-12; PMID:16423985; http://dx.doi.org/ 10.1158/0008-5472.CAN-05-4005 [DOI] [PubMed] [Google Scholar]
- 37.Gimbrone MA Jr., Cotran RS, Leapman SB, Folkman J. Tumor growth and neovascularization: an experimental model using the rabbit cornea. J Natl Cancer Inst 1974; 52:413-27; PMID:4816003; http://dx.doi.org/ 10.1093/jnci/52.2.413 [DOI] [PubMed] [Google Scholar]
- 38.Ruffell B, Au A, Rugo HS, Esserman LJ, Hwang ES, Coussens LM. Leukocyte composition of human breast cancer. Proc Natl Acad Sci U S A 2012; 109:2796-801; PMID:21825174; http://dx.doi.org/ 10.1073/pnas.1104303108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van der Auwera I, Van den Eynden GG, Colpaert CG, Van Laere SJ, van Dam P, Van Marck EA, Dirix LY, Vermeulen PB. Tumor lymphangiogenesis in inflammatory breast carcinoma: a histomorphometric study. Clin Cancer Res 2005; 11:7637-42; PMID:16278382; http://dx.doi.org/ 10.1158/1078-0432.CCR-05-1142 [DOI] [PubMed] [Google Scholar]
- 40.Bailey AS, Willenbring H, Jiang S, Anderson DA, Schroeder DA, Wong MH, Grompe M, Fleming WH. Myeloid lineage progenitors give rise to vascular endothelium. Proc Natl Acad Sci U S A 2006; 103:13156-61; PMID:16920790; http://dx.doi.org/ 10.1073/pnas.0604203103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Religa P, Cao R, Bjorndahl M, Zhou Z, Zhu Z, Cao Y. Presence of bone marrow-derived circulating progenitor endothelial cells in the newly formed lymphatic vessels. Blood 2005; 106:4184-90; PMID:16141354; http://dx.doi.org/ 10.1182/blood-2005-01-0226 [DOI] [PubMed] [Google Scholar]
- 42.Kerjaschki D, Bago-Horvath Z, Rudas M, Sexl V, Schneckenleithner C, Wolbank S, Bartel G, Krieger S, Kalt R, Hantusch B et al. Lipoxygenase mediates invasion of intrametastatic lymphatic vessels and propagates lymph node metastasis of human mammary carcinoma xenografts in mouse. J Clin Invest 2011; 121:2000-12; PMID:21540548; http://dx.doi.org/ 10.1172/JCI44751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van den Eynden GG, Vandenberghe MK, van Dam PJ, Colpaert CG, van Dam P, Dirix LY, Vermeulen PB, Van Marck EA. Increased sentinel lymph node lymphangiogenesis is associated with nonsentinel axillary lymph node involvement in breast cancer patients with a positive sentinel node. Clin Cancer Res 2007; 13:5391-7; PMID:17875768; http://dx.doi.org/ 10.1158/1078-0432.CCR-07-1230 [DOI] [PubMed] [Google Scholar]
- 44.Kerjaschki D. The crucial role of macrophages in lymphangiogenesis. J Clin Invest 2005; 115:2316-9; PMID:16138185; http://dx.doi.org/ 10.1172/JCI26354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hirakawa S, Kodama S, Kunstfeld R, Kajiya K, Brown LF, Detmar M. VEGF-A induces tumor and sentinel lymph node lymphangiogenesis and promotes lymphatic metastasis. J Exp Med 2005; 201:1089-99; PMID:15809353; http://dx.doi.org/ 10.1084/jem.20041896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Agarwal B, Saxena R, Morimiya A, Mehrotra S, Badve S. Lymphangiogenesis does not occur in breast cancer. Am J Surg Pathol 2005; 29:1449-55; PMID:16224211; http://dx.doi.org/ 10.1097/01.pas.0000174269.99459.9d [DOI] [PubMed] [Google Scholar]
- 47.Williams CS, Leek RD, Robson AM, Banerji S, Prevo R, Harris AL, Jackson DG. Absence of lymphangiogenesis and intratumoural lymph vessels in human metastatic breast cancer. J Pathol 2003; 200:195-206; PMID:12754740; http://dx.doi.org/ 10.1002/path.1343 [DOI] [PubMed] [Google Scholar]
- 48.Ran S, Volk L, Hall K, Flister MJ. Lymphangiogenesis and lymphatic metastasis in breast cancer. Pathophysiology 2010; 17:229-51; PMID:20036110; http://dx.doi.org/ 10.1016/j.pathophys.2009.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stacker SA, Williams SP, Karnezis T, Shayan R, Fox SB, Achen MG. Lymphangiogenesis and lymphatic vessel remodelling in cancer. Nat Rev Cancer 2014; 14:159-72; PMID:24561443; http://dx.doi.org/ 10.1038/nrc3677 [DOI] [PubMed] [Google Scholar]
- 50.Kerjaschki D, Huttary N, Raab I, Regele H, Bojarski-Nagy K, Bartel G, Krober SM, Greinix H, Rosenmaier A, Karlhofer F et al. Lymphatic endothelial progenitor cells contribute to de novo lymphangiogenesis in human renal transplants. Nat Med 2006; 12:230-4; PMID:16415878; http://dx.doi.org/ 10.1038/nm1340 [DOI] [PubMed] [Google Scholar]
- 51.Attout T, Hoerauf A, Denece G, Debrah AY, Marfo-Debrekyei Y, Boussinesq M, Wanji S, Martinez V, Mand S, Adjei O et al. Lymphatic vascularisation and involvement of Lyve-1+ macrophages in the human onchocerca nodule. PLoS One 2009; 4:e8234; PMID:20011036; http://dx.doi.org/ 10.1371/journal.pone.0008234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.El-Chemaly S, Malide D, Zudaire E, Ikeda Y, Weinberg BA, Pacheco-Rodriguez G, Rosas IO, Aparicio M, Ren P, MacDonald SD et al. Abnormal lymphangiogenesis in idiopathic pulmonary fibrosis with insights into cellular and molecular mechanisms. Proc Natl Acad Sci U S A 2009; 106:3958-63; PMID:19237567; http://dx.doi.org/ 10.1073/pnas.0813368106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gwak JM, Jang MH, Kim DI, Seo AN, Park SY. Prognostic value of tumor-associated macrophages according to histologic locations and hormone receptor status in breast cancer. PLoS One 2015; 10:e0125728; PMID:25884955; http://dx.doi.org/ 10.1371/journal.pone.0125728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Laoui D, Movahedi K, Van Overmeire E, Van den Bossche J, Schouppe E, Mommer C, Nikolaou A, Morias Y, De Baetselier P, Van Ginderachter JA. Tumor-associated macrophages in breast cancer: distinct subsets, distinct functions. Int J Dev Biol 2011; 55:861-7; PMID:22161841; http://dx.doi.org/ 10.1387/ijdb.113371dl [DOI] [PubMed] [Google Scholar]
- 55.Zhang Y, Cheng S, Zhang M, Zhen L, Pang D, Zhang Q, Li Z. High-infiltration of tumor-associated macrophages predicts unfavorable clinical outcome for node-negative breast cancer. PLoS One 2013; 8:e76147; PMID:24098773; http://dx.doi.org/ 10.1371/journal.pone.0076147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tang X. Tumor-associated macrophages as potential diagnostic and prognostic biomarkers in breast cancer. Cancer Lett 2013; 332:3-10; PMID:23348699; http://dx.doi.org/ 10.1016/j.canlet.2013.01.024 [DOI] [PubMed] [Google Scholar]
- 57.Derleth C, Mayer IA. Antiangiogenic therapies in early-stage breast cancer. Clin Breast Cancer 2010; 10 Suppl 1:E23-31; PMID:20587404; http://dx.doi.org/ 10.3816/CBC.2010.s.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Van't Hull EF, Bron S, Henry L, Ifticene-Treboux A, Turrini R, Coukos G, Delaloye JF, Doucey MA. Bone marrow-derived cells are implicated as a source of lymphatic endothelial progenitors in human breast cancer. Oncoimmunology 2014; 3:e29080; PMID:25101222; http://dx.doi.org/ 10.4161/onci.29080 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.