ABSTRACT
Dendritic cell (DC)-based immunotherapy has yielded promising results against high-grade glioma (HGG). However, the efficacy of DC vaccines is abated by HGG-induced immunosuppression and lack of attention toward the immunogenicity of the tumor lysate/cells used for pulsing DCs. A literature analysis of DC vaccination clinical trials in HGG patients delineated the following two most predominantly applied methods for tumor lysate preparation: freeze-thaw (FT)-induced necrosis or FT-necrosis followed by X-ray irradiation. However, from the available clinical evidence, it is unclear which of both methodologies has superior immunogenic potential. Using an orthotopic HGG murine model (GL261-C57BL/6), we observed that prophylactic vaccination with DCs pulsed with irradiated FT-necrotic cells (compared to FT-necrotic cells only) prolonged overall survival by increasing tumor rejection in glioma-challenged mice. This was associated, both in prophylactic and curative vaccination setups, with an increase in brain-infiltrating Th1 cells and cytotoxic T lymphocytes (CTL), paralleled by a reduced accumulation of regulatory T cells, tumor-associated macrophages (TAM) and myeloid-derived suppressor cells (MDSC). Further analysis showed that irradiation treatment of FT-necrotic cells considerably increased the levels of carbonylated proteins — a surrogate-marker of oxidation-associated molecular patterns (OAMPs). Through further application of antioxidants and hydrogen peroxide, we found a striking correlation between the amount of lysate-associated protein carbonylation/OAMPs and DC vaccine-mediated tumor rejection capacity thereby suggesting for the first time a role for protein carbonylation/OAMPs in at least partially mediating antitumor immunity. Together, these data strongly advocate the use of protein oxidation-inducing modalities like irradiation for increasing the immunogenicity of tumor lysate/cells used for pulsing DC vaccines.
KEYWORDS: Antitumor immunity, dendritic cell vaccination, high-grade glioma, oxidation-associated molecular patterns (OAMPs), protein carbonylation, whole tumor lysate
Abbreviations
- CD
cluster of differentiation
- CTL
cytotoxic T lymphocyte
- DC
dendritic cell
- ELISA
enzyme-linked immunosorbent assay
- FCS
fetal calf serum
- FT
freeze-thaw
- GBM
glioblastoma multiforme
- GM-CSF
granulocyte/macrophage colony-stimulating factor
- HGG
high-grade glioma
- ICD
immunogenic cell death
- IFN
interferon
- IL
interleukin
- i.p.
intraperitoneal
- IR
irradiation
- L-Hist
L-Histidine
- LPS
lipopolysaccharide
- MDSC
myeloid-derived suppressor cell
- mAb
monoclonal antibody
- MHC
major histocompatibility complex
- NAC
N-acetylcysteine
- OAMP
oxidation-associated molecular pattern
- ROS
reactive oxygen species
- TAM
tumor-associated macrophage
- TGF
transforming growth factor
- Tregs
regulatory T cells
- WHO
world health organization
Introduction
Gliomas are a group of central nervous system tumors of glial cell origin, accounting for at least 50% of all primary intrinsic brain tumors. Among gliomas, glioblastoma multiforme (GBM) is the most aggressive and most frequently occurring primary brain tumor in adults. According to the World Health Organization (WHO) it is classified as a grade IV tumor.1 Together with WHO grade III anaplastic astrocytoma, anaplastic oligodendroglioma and anaplastic oligoastrocytoma, GBM are categorized as HGG. Despite multidisciplinary treatment consisting of surgical resection and radiochemotherapy, median overall survival of GBM patients is restricted to approximately 14.6 mo.2 Hence, there is an urgent need for novel, effective treatment strategies with minimal off-target effects against normal brain parenchyma.3 In this regard, DC-based immunotherapy can be an attractive option, because of its relative tumor-specific nature and its ability to elicit immune memory responses crucial for controlling or eliminating residual cancer cells.4,5 A recent meta-analysis revealed that DC-based immunotherapy in HGG patients markedly prolonged the overall and the progression-free survival compared with a non-DC group.6 However, there is a consensus that DC vaccines have not yet reached their full potential. This is in part due to the selection of advanced disease stage patients and the presence of tumor-induced immunosuppressive mechanisms.7,8 In addition, the lack of optimization and standardization of the different steps in the production process of DC vaccines, especially those pertaining to the overall immunogenicity of the tumor cells/lysate itself, also contributes notably to the limited DC vaccine efficacy.9
HGG-induced immunosuppression is associated with a profound infiltration of anti-inflammatory and pro-tumorigenic immune cells like T regulatory cells (Tregs), MDSCs and TAMs.10–14 Therefore, an immunotherapeutic strategy that activates antitumor immunity and simultaneously tackles the infiltration of these immunosuppressive immune cell populations is highly desirable.4,15
DCs pulsed with whole tumor lysate tend to have superior efficacy over DCs pulsed with selected cancer-associated antigen peptide(s).16 Moreover, the tumor specificity of the antitumor immune response elicited by whole tumor cell-based DC vaccination has been demonstrated in the preclinical GL261 HGG model.17 Interestingly, multiple preclinical studies in different cancer models have convincingly demonstrated that the methodology utilized for tumor lysate preparation can influence its immunogenicity and in turn the overall efficacy of antitumor immunity elicited by tumor lysate pulsed DCs.18–21 There is, however, no consensus yet on the methodology of whole tumor lysate preparation; most clinical reports involving DC vaccinated HGG patients describe the use of multiple FT cycles to induce necrosis of tumor cells (i.e., FT-necrosis) or a combination of FT-necrosis and subsequent high-dose X-ray irradiation. The latter preparation methodology offers interesting, but as-yet-untested avenues in light of the fact that radiotherapy is a known enhancer of oxidation-based immunogenicity.15,22 More specifically, radiotherapy is known to generate reactive oxygen species (ROS)23 that have the ability to induce OAMPs like carbonylated proteins.24-29 This is important because protein carbonylation, a surrogate indicator of irreversible protein oxidation, has been shown to improve cancer cell immunogenicity and facilitate formation of neo-immunogenic antigens.26 In addition, OAMPs like protein carbonylation can facilitate efficient antigen processing by DCs.25
The primary goal of this study was to directly compare the in vivo immunogenicity of DCs pulsed with either FT-necrotic cells or X-ray irradiated FT-necrotic cells, in the context of HGG. Moreover we explored the contribution of protein carbonylation-based OAMPs in this setting. To address these questions, we utilized the well-established, immunocompetent, orthotopic GL261 mouse HGG model. This model has been abundantly used to evaluate the potency of anti-HGG immunotherapies.30
Results
Clinical evidence generated from DC vaccination trials in HGG patients hints toward improved efficacy of irradiated FT-necrotic lysate
Since the year 2,000, over 30 phase I/II studies of DC-based immunotherapy for HGG have been published in which over 500 patients were involved.31 To this end, we decided to do a literature-based meta-analysis to ascertain the methodologies of tumor lysate preparation used and the associated patient responses. We found that 19 trials reported the use of whole tumor lysate as an antigen source for loading DCs (Table 1). The method of preparing this lysate however randomly (i.e., without any specified reason or rationale) involved either FT-necrotic cells 16,32–40 or irradiated FT-necrotic cells.41–49 Retrospective analysis of primary GBM patients’ survival data with a Karnofsky performance score (KPS) of more than 70 revealed a trend toward prolonged overall survival in patients vaccinated with DCs fed with irradiated (IR) FT-necrotic GBM cells (FT+IR-DC vaccine, n = 27, median survival of 33.5 mo) as compared to patients treated with DCs fed with FT-necrotic GBM cells (FT-DC vaccine, n = 34, median survival of 22.5 mo, data not shown). These results have to be interpreted with due caution, as a more stringent and better powered meta-analysis is required to correctly compare the two treatment groups. Insufficient data were available for comparison of immunogenicity-related parameters.
Table 1.
Autologous tumor lysate-pulsed DC vaccination studies in HGG patients
| Author | Year | No. of patients (type of trial) | Grade III/IV | ND/R | Lysate preparation | Injection route | Treatment schedule | Immune response | Clinical response |
|---|---|---|---|---|---|---|---|---|---|
| Yamanaka et al. 32 | 2003 | 10 (phase I-II) | III /IV | R | FT | ID and/or IT | 1–10 vaccinations at 3-week intervals | Increase in NK cells in PBMCs (5/5); positive DTH reaction (3/6); increased IFNγ ELISPOT (2/5) | MR (2/6) |
| Wheeler et al. 33 | 2003 | 17 (phase I) | IV | ND/R | FT | NS | 3 vaccinations at 2- week intervals | NS | NS |
| 17 (phase II) | IV | ND/R | FT | 3 vaccinations at 2-week intervals; 4th vaccination 6 weeks later | |||||
| Wheeler et al. 34 | 2004 | 5 (phase I) and 12 (Phase II) | III/IV | ND/R | FT | NS | 3 vaccinations at 2-week intervals (phase I) and 4th vaccination 6 weeks later (phase II) | In GBM patients receiving post-vaccine chemotherapy, CD8+ TRECs predicted longer chemotherapy responses | Vaccinated patients receiving subsequent chemotherapy exhibited longer times to tumor recurrence after chemotherapy |
| Yu et al. 35 | 2004 | 14 (phase I-II) | III/IV | ND/R | FT | SC | 3 vaccinations at 2-week intervals; 4th vaccination 6 weeks later | Increased IFNγ ELISPOT (6/10); expansion of CD8+ antigen specific T cell clones (4/9); systemic T cell cytotoxicity against tumor (1/1) | OS: 133 weeks in DC-group and 30 weeks in control-group (recurrent GBM) |
| Rutkowski et al. 41 | 2004 | 12 (phase I) | IV | R | FT + IR | ID | 2–7 vaccinations; 2nd vaccine 2 weeks after 1st, then monthly vaccines | Positive DTH reaction (6/8) | PR (1/12); tumor-free survival 5 y after vaccination (2/12); PFS: 3 months; OS: 105 months |
| Yamanaka et al. 36 | 2005 | 24 (phase I-II) | III/IV | R | FT | ID or ID + IT | 1–10 vaccinations at 3-week intervals | Positive DTH reaction (8/17); positive IFNγ ELISOT (7/16); positive immune monitoring predicts good clinical outcome | PR (1/24); MR (3/24); SD (10/24); significantly increased MS |
| Wheeler et al. 37 | 2008 | 34 (phase II) | III/IV | ND/R | FT | SC | 3 vaccinations at 2-week intervals; 4th vaccination 6 weeks later | Increased IFNγ ELISPOT (17/34); DTH-test resulted in cutaneous GBM in 1 patient (DTH was subsequently discontinued) | Significant positive correlation between post-vaccine response magnitude and TTS; 2-year OS: 41 % in vaccine responders versus 7 % in non-responders; patients relapsing after vaccination showed increased chemosensitivity. |
| De Vleeschouwer et al. 42 | 2008 | 56 (phase I-II) | IV | R | FT + IR | ID | Cohort comparison | Positive DTH (9/21 at time of diagnosis and 9/17 after 2 vaccinations) | PFS: 3 months; OS: 9.6 months; 2-year OS: 14.8 %; total resection is a predictor for better PFS; younger age and total resection are predictors for better OS in univariable analysis; tendency toward improved PFS when faster DC vaccination schedule with tumor lysate boosting was applied |
| Ardon et al. 43 | 2010 | 8 (pilot) | IV | ND | FT + IR | ID | 4 weekly vaccines, 3 monthly vaccines, then 3-months intervals | Increased IFNγ ELISPOT (5/8), positive DTH reaction (3/6) | 6-months PFS: 75 %; OS: 24 months; PFS: 18 months |
| Ardon et al. 44 | 2010 | 33 children (phase I/II) | III/IV | R | FT + IR | ID | Depending on the cohort | NS | 6-months PFS: 42 %; PFS: 4.4 months; OS: 13.5 months |
| Fadul et al. 45 | 2011 | 10 (phase I/II) | IV | ND | IR + FT | IN | 3 vaccines at 2-week intervals | Increased IFNγ ELISPOT (4/10) | PFS: 9.5 months; OS: 28 months |
| Prins et al. 38 | 2011 | 23 (phase I) | IV | ND/R | FT | ID | 3 vaccines at 2–week intervals, booster vaccines every 3 months (in combination with Imiquimod/Poly-ICLC) | Mesenchymal tumors had a higher number of CD3+ and CD8+ tumor-infiltrating lymphocytes. | OS: 31.4 months and a 1-, 2- and 3-year survival rate of 91 %, 55 % and 47 %. Better survival in patients having a mesenchymal gene signature. |
| Elens et al. 46 | 2012 | 39 (phase I/II) | III | R | FT + IR | ID | Depending on the cohort | NS | Median PFS/OS were 3.4/20.5 mon (AA), 3.4/18.8 months (AOD) and 7.8/13.3 months (AOA) |
| Ardon et al. 47 | 2012 | 77 (phase I/II) | IV | ND | FT + IR | ID | 4 weekly vaccines, 3 monthly vaccines, then 3-months intervals | Immunological profiling could not predict clinical outcome. | 6-months PFS: 70.1 %; median OS: 18.3 months; OS depending on RPA classification |
| De Vleeschouwer et al. 48 | 2012 | 117 (phase II) | III/IV | R | FT + IR | ID | Changing per cohort | NS | OS: 6–48.4 months ( according to HGG-immuno RPA classification) |
| Fong et al. 39 | 2012 | 24 (2 phase I trials): 19 (DC-ATL) and 5 (DC-GAA) | IV | ND/R | FT | ID | 3 vaccinations at 2-week intervals | Decreased Treg frequency and decreased expression of CTLA-4 on T cells after DC vaccination was associated with better survival | OS: 33.8 months (DC-ATL), 14.5 months (DC-GAA); TTP: 13.9 months (DC-ATL), 9.6 months (DC-GAA) |
| Valle et al. 49 | 2012 | 5 (pilot) | IV | ND | FT + IR | NS | 1st vaccine one week before radiotherapy, 2nd 3 weeks after radiotherapy, 2 monthly vaccines and 4 every 2 months and later quarterly | PBMC proliferation (3/3); positive IFNγ ELISA (3/3); positive IFNγ ELISPOT (0/3) | PFS: 16.1 months; OS: 27 months |
| Lasky et al. 40 | 2013 | 7 (pilot, children) | III/IV | ND/R | FT | ID | 2-week intervals | Cytokine production upon in vitro stimulation (1/3) | MR: 1/3; CR: 2/3 |
| Prins et al. 16 | 2013 | 28 (phase I) | III/IV | ND/R | FT | ID | 3 vaccines at 2-week intervals, booster vaccines every 3 months | Significant correlation between decreased Treg ratio (post/pre-vaccination) and OS | PFS: 18.1 months; OS: 34.4 months |
AA, anaplastic astrocytoma; AOD, anaplastic oligodendroglioma; AOA, anaplastic oligoastrocytoma; ATL, autologous tumor lysate; CR, complete response; CTL, cytotoxic T lymphocyte; CTLA-4, cytotoxic T lymphocyte-associated protein 4; DC, dendritic cell; DTH, delayed-type hypersensitivity; ELISA, enzyme-linked immunosorbent assay; ELISPOT, enzyme-linked immunosorbent spot assay; FT, freeze-thaw; GAA, glioma-associated antigens; GBM, glioblastoma multiforme; IFNγ, interferon-γ; ID, intradermal; IN, intranodal; IR, irradiation; IT, intratumoral; MR, minor response; MS, median survival; ND, newly diagnosed; NK, natural killer; NS, not specified; OS, overall survival; PBMC, peripheral blood mononuclear cell; PFS, progression-free survival; PR, partial response; R, relapsed; RPA, recursive partitioning analysis; SC, subcutaneous, SD, stable disease; TREC, T cell receptor excision circle; Treg, regulatory T cell; TTP, time to progression; TTS, time to survival.
In conclusion, this literature survey showed that several clinical trials utilized FT-DC vaccine and FT+IR-DC vaccine ad libitum for anti-HGG immunotherapy. Preliminary survival analysis hints toward giving preference to the use of irradiated necrotic lysate for loading DCs; however the two treatment regimens were indiscernible at the level of immunoscoring parameters.
Irradiation of necrotic cells potentiates DC vaccine-induced overall survival in glioma-challenged mice
Since we were unable to reach a consensus on immunogenicity-related differences between the FT-DC vaccine and the FT+IR-DC vaccine based on above analysis, we decided to conduct preclinical experiments to directly compare the efficacy of these two DC vaccine ‘types’.
Using a prophylactic treatment strategy we observed a significant increase (p < 0.05) in the median survival of mice vaccinated with the FT+IR-DC vaccine (53.5 d) as compared to mice treated with the FT-DC vaccine (34 d) (Fig. 1A). Moreover, treatment with FT+IR-DC vaccine protected 5 of 14 animals (36%) from tumor development, while only 2 of 14 (14%) mice that received FT-DC vaccine were protected. Of note, in line with our previously published data, vaccination with FT-DC vaccine induced a significant improvement in median survival (p < 0.01) in comparison to untreated animals (34 versus 23 d, respectively).50
Figure 1.
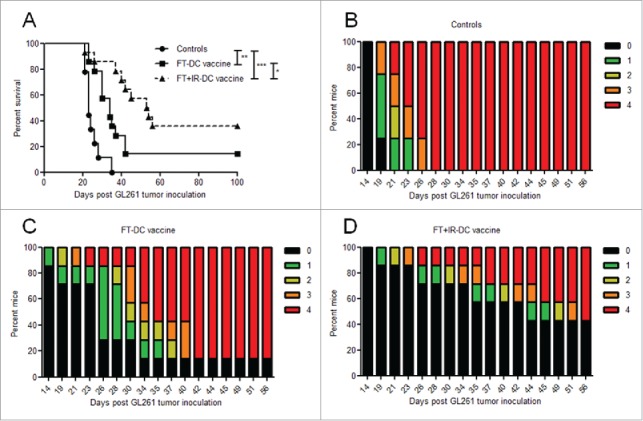
Irradiation of necrotic tumor lysate prolongs DC-vaccine induced survival of glioma-bearing mice. (A) Kaplan–Meier graph of two independent experiments depicting survival of mice immunized with the FT-DC vaccine (▪, n = 14), the FT+IR-DC vaccine (▴, dashed line, n = 14) and untreated control mice (•, n = 9). *, p < 0.05; **, p < 0.01; ***, p < 0.0001 (Log-rank test). (B-D) The tumor-induced neurological deficit of one representative experiment is displayed graphically over time by color-coding symptom severity for (B) control mice (n = 4), (C) FT-DC vaccine treated mice (n = 7) and (D) FT+IR-DC vaccine immunized mice (n = 7): grade 0 (black), healthy mice; grade 1 (green), slight unilateral paralysis; grade 2 (yellow), moderate unilateral paralysis and/or beginning hunchback; grade 3 (orange), severe unilateral or bilateral paralysis and/or pronounced hunchback; grade 4 (red), moribund mice.
Consistent with the survival data, graphical representation of the tumor-induced neurological deficit scores over time revealed a delay in the onset of clinically-relevant symptoms and a less pronounced clinical manifestation in mice treated with the FT+IR-DC vaccine compared to FT-DC vaccine immunized animals (Fig. 1B-D).
As irradiation is a known enhancer of tumor cell immunogenicity,51,52 we also wished to address if IR on its own or IR treatment prior to FT of the GL261 cells could impact the in vivo immunogenicity of the tumor cell preparations. Both treatment groups (IR-DC vaccine and IR+FT-DC vaccine, respectively) however failed to induce significant survival benefit as compared to untreated mice or FT+IR-DC vaccine treated mice (Fig. S1). Interestingly, to achieve a 90% cell killing rate the GL261 cells had to receive an 800 Gy X-ray dose, underlining the high radiotherapy resistance of this HGG cell line (data not shown). Of note, because of the requirement of 100 % tumor cell avitality for clinical translation of DC vaccines, the IR-DC vaccine cannot be considered as a clinically relevant alternative for the FT+IR-DC vaccine.
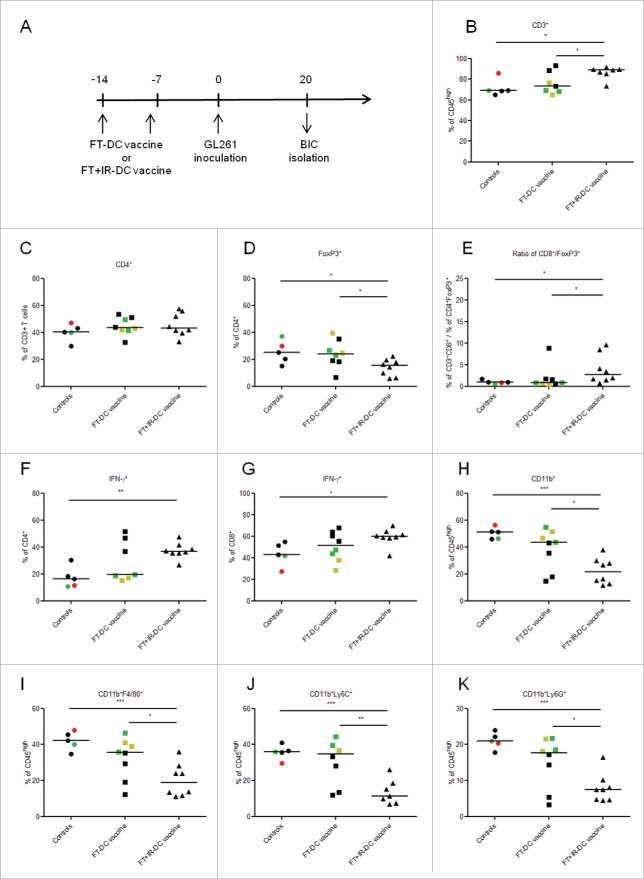
Increased potency of DCs pulsed with irradiated FT-necrotic cells is associated with a favorable shift in brain immune contexture
Next, we investigated whether the increased in vivo immunogenicity of the FT+IR-DC vaccine was associated with an immunostimulatory shift in the brain immune contexture. To this end, brain-infiltrating immune cells (BICs) were analyzed by flow cytometry, 20 d after intracranial GL261 tumor inoculation (Fig. 2A). Each graphical point (depicting one mouse) was given a color according to it neurological deficit score at the moment of sacrifice (Fig. 2B-K). Evaluating the adaptive immune compartment, a significantly increased infiltration of CD3+ T cells was observed in mice vaccinated with the FT+IR-DC vaccine as compared to both untreated and FT-DC vaccine treated animals (p < 0.05, Fig. 2B). We observed no significant differences in the percentages of total CD4+ brain-infiltrating T cells between the different groups (Fig. 2C). Moreover, FT+IR-DC vaccine injected mice showed a reduced infiltration of CD4+FoxP3+ Tregs and an increase in the clinically relevant ratio of CD8+ T cells to CD4+FoxP3+ Tregs (p < 0.05, Fig. 2D and E, respectively). As IFNγ production by T cells is considered crucial for the induction of Th1 polarization-based antitumor immunity,53 we evaluated the intracellular levels of this cytokine in the brain-infiltrating lymphocytes. Only in FT+IR-DC vaccine treated mice the frequency of IFNγ+CD4+ T cells i.e., Th1 cells and IFNγ+CD8+ T cells i.e., CTLs was significantly higher as compared to untreated control mice (p < 0.05 and p < 0.01 respectively, Fig. 2F and G, respectively). In addition, to the adaptive immune compartment, we also evaluated the brain infiltration of different myeloid cell populations in tumor-inoculated mice (Fig. 2H-K). Analysis of the percentages of brain-infiltrating myeloid cells (CD11b+) revealed a significantly reduced influx of these cells in mice treated with the FT+IR-DC vaccine compared to both untreated mice (p < 0.05) and FT-DC vaccine immunized mice (p < 0.01, Fig. 2H). A more detailed phenotypic characterization of the brain-infiltrating myeloid cells showed a significant decrease in the percentage of TAMs (CD11b+F4/80+, Fig. 2I), mononuclear MDSCs (CD11b+Ly6C+, Fig. 2J) and granulocytic MDSCs (CD11b+Ly6G+, Fig. 2K) in mice receiving the FT+IR-DC vaccine in comparison to both FT-DC vaccine injected (p < 0.05, p < 0.01 and p < 0.05, respectively) and untreated animals (p < 0.001 for all three populations). Although there was a decreased infiltration of these myeloid cell populations in FT-DC vaccine treated mice as compared to untreated mice, this did not reach statistical significance.
Figure 2.
Prophylactic vaccination with FT+IR-DC vaccines favorably impacts the brain immune contexture. (A) Timeline depicting the prophylactic treatment protocol. Mice received two weekly i.p. vaccinations with either the FT-DC vaccine (n = 8) or the FT+IR-DC vaccine (n = 8). One week after the last vaccination, mice were intracranially inoculated with GL261 cells. Mice not immunized with vaccines were used as controls (n = 5). The BICs were isolated on day 20 post GL261 tumor inoculation. (B-K) Flow cytometry of the mononuclear BICs. Each point (representing data from one mouse) is given a color according to its neurological deficit score: grade 0 (black), healthy mice; grade 1 (green), slight unilateral paralysis; grade 2 (yellow), moderate unilateral paralysis and/or beginning hunchback; grade 3 (orange), severe unilateral or bilateral paralysis and/or pronounced hunchback; grade 4 (red), moribund mice. (B) Percentages of CD3+ T cells (CD45high-gated), (C) CD4+ T cells (CD3+-gated), (D) FoxP3+ Tregs (CD4+-gated) and (E) the ratio of CD8+ T cells (CD3+-gated) over FoxP3+ Tregs (CD4+-gated). (F-G) Percentages of IFNγ+ cells in the (F) CD4+ and (G) CD8+ T cell populations, analyzed by intracellular IFNγ staining. (H) Percentages of CD11b+ myeloid cells, (I) CD11b+F4/80+ macrophages, (J) CD11b+Ly6C+ mononuclear MDSCs and (K) CD11b+Ly6G+ granulocytic MDSCs within the CD45high gate, thereby excluding CD45intCD11b+ microglia. *, p < 0.05; **, p < 0.01; ***, p < 0.001 (one-way ANOVA). Data are presented as medians.
Of note, mice experiencing neurological deficits (grade 1–4) were only present in the untreated and FT-DC vaccine treated groups. These mice in general showed the lowest infiltration of Th1 cells and CTLs, the highest infiltration of the different myeloid populations (especially in the FT-DC vaccine group) and the highest Treg levels (Fig. 2).
Together, these data suggest that prophylactic vaccination with FT+IR-DC vaccines leads to an increased accumulation of immunostimulatory, IFNγ producing CD4+/CD8+ T cells while simultaneously impeding the infiltration of immunosuppressive Tregs, TAMs and MDSCs.
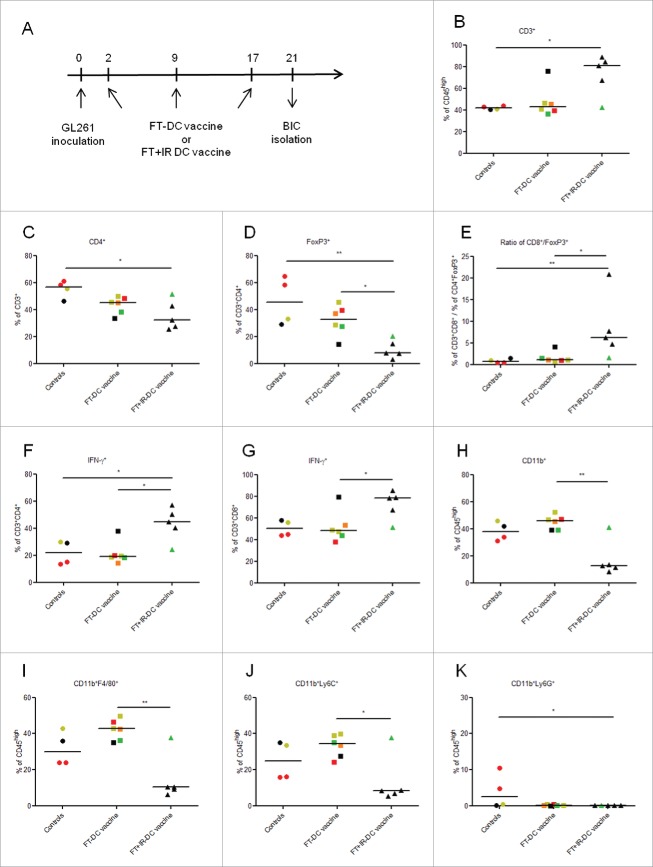
Curative treatment with DC vaccines fed with irradiated necrotic cells establishes a shift toward Th1 and CTL driven antitumor immunity
As prophylactic treatment does not really represent the clinical status quo, we next evaluated the in vivo immunogenicity of the two vaccine ‘types’ in a curative vaccination setup. Treated mice received three weekly DC vaccines and all mice were sacrificed four days after the last vaccination to analyze the BICs populations (Fig. 3A). This study revealed an increased infiltration of CD3+ T lymphocytes and a reduced infiltration of CD4+ T cells in FT+IR-DC vaccine injected mice as compared to control mice (p < 0.05 for both populations, Fig. 2B and C). However, this reduced infiltration of general CD4+ T cells might be explained by the strong significant reduction in Treg levels in these mice as compared to both untreated mice (p < 0.01) and FT-DC vaccine treated mice (p < 0.05, Fig. 3D). Moreover, vaccination with the FT+IR-DC vaccine resulted in a significant increase in the CD8+ T cell to Treg ratio (p < 0.05 versus FT-DC vaccine and p < 0.01 versus controls, Fig. 3E) and this was paralleled by an enhanced infiltration of Th1 cells and CTLs (Fig. 3F and G, respectively; p < 0.05 in comparison to FT-DC vaccine treated mice for both populations). With respect to the myeloid cell populations, we observed a significantly reduced infiltration of total CD11b+ myeloid cells (Fig. 3H), F4/80+ TAMs (Fig. 3I) and Ly6C+ MDSCs (Fig. 3J) in FT+IR-DC vaccine injected mice as compared to FT-DC vaccine immunized mice (p < 0.01, p < 0.01 and p < 0.05, respectively). Although Ly6G+ MDSCs were almost absent in the brains of both vaccinated groups, statistical analysis revealed a lower infiltration of these granulocytic MDSCs only in the FT+IR-DC vaccine treated mice in comparison to untreated animals (Fig. 3K, p < 0.05). Interestingly, also in this setting, mice injected with FT-DC vaccine showed no significant differences as compared to untreated mice for any of the studied immune cell populations.
Figure 3.
Curative vaccination with the FT+IR-DC vaccine induces a pro-inflammatory shift in brain immune contexture. (A) Timeline depicting the curative treatment protocol. Treated mice received three i.p. vaccinations with either FT-DC vaccines (n = 6) or FT+IR-DC vaccines (n = 5) on days 2, 9 and 17 post intracranial GL261 inoculation. Mice not immunized with vaccines were used as controls (n = 4). The BICs were isolated on day 21 post GL261 tumor inoculation. (B-K) Flow cytometry of the mononuclear BICs. Each point (representing data from one mouse) is given a color according to its neurological deficit score: grade 0 (black), healthy mice; grade 1 (green), slight unilateral paralysis; grade 2 (yellow), moderate unilateral paralysis and/or beginning hunchback; grade 3 (orange), severe unilateral or bilateral paralysis and/or pronounced hunchback; grade 4 (red), moribund mice. (B) Percentages of CD3+ T cells (CD45high-gated), (C) CD4+ T cells (CD3+-gated), (D) FoxP3+ Tregs (CD4+-gated) and (E) the ratio of CD8+ T cells (CD3+-gated) over FoxP3+ Tregs (CD4+-gated). (F-G) Percentages of IFNγ+ cells in the (F) CD4+ and (G) CD8+ T cell populations, analyzed by intracellular IFNγ staining. (H) Percentages of CD11b+ myeloid cells, (I) CD11b+F4/80+ macrophages, (J) CD11b+Ly6C+ mononuclear MDSCs and (K) CD11b+Ly6G+ granulocytic MDSCs within the CD45high gate, thereby excluding CD45intCD11b+ microglia. *, p < 0.05; **, p < 0.01 (one-way ANOVA). Data are presented as medians.
Of note, in accordance with the results obtained in the prophylactic setting, mice showing neurological deficits (predominantly present in the untreated and FT-DC vaccine group) in general showed the highest infiltration of Tregs and myeloid cell populations, while having a less evident infiltration of IFNγ producing effector T cells (Fig. 3).
In conclusion, these remarkable results indicate that the productive/pro-inflammatory immune interphase between the tumor and its immune contexture is maintained in the clinically relevant setting where vaccination with FT+IR-DC vaccine is applied in a pre-established tumor setting.
Pulsing of DCs with irradiated FT-necrotic cells does not impact DC maturation
Different tumor lysate preparations have been found to impact the phenotypic and functional maturation of DCs interacting with them.18,20 To this end, we examined whether the pulsing of DCs with FT+IR cells could directly modify the DC phenotype. As shown in Fig. 4A, we observed no differences in the expression levels of the costimulatory proteins CD80, CD86 and CD40 and the MHC class I and II molecules (H-2Kb and I-A/I-E, respectively) between DCs constituting the FT-DC vaccine and the FT+IR-DC vaccine.
Figure 4.
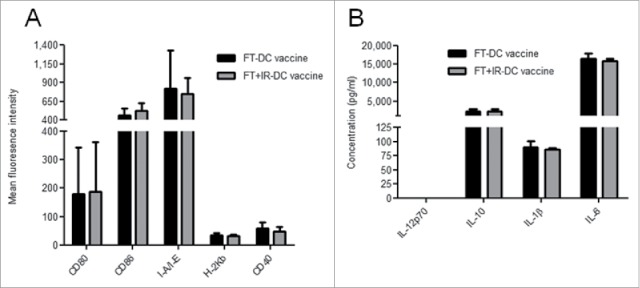
Loading of DCs with irradiated GL261 tumor lysate does not impact DC maturation. (A) Surface expression of CD80, CD86, I-A/I-E, H-2Kb and CD40 on the FT-DC vaccine and the FT+IR-DC vaccine was evaluated by means of flow cytometry. Data are presented as the mean fluorescent intensity ± SD of six independent experimental determinations. (B) The conditioned media of the FT-DC vaccines and the FT+IR DC vaccines were collected followed by analysis for concentrations of IL-12p70, IL-10, IL-1β and IL-6. Data are presented as mean ± SD of two independent experiments, each performed in triplicate.
In order to gain more insight into the functional status of the lysate-pulsed DCs, we evaluated the conditioned media of the FT-DC vaccine and the FT+IR-DC vaccine for the pattern of certain important cytokines like IL-10, IL-12p70, IL-6 and IL-1β (Fig. 4B). The exposure of DCs to both lysate preparations did not induce production of IL-12p70. Moreover, we did not detect any differences in the expression levels of IL-10, IL-1β and IL-6 between the two vaccines. Conditioned media of the GL261 lysates (in the absence of DCs) did not contain detectible levels of any of the cytokines (data not shown).
Together, these data suggest that irradiation of FT-necrotic cells to pulse DCs does not influence the phenotypic and functional maturation of DCs.
Oxidation-induced protein carbonylation in tumor lysates in vitro, correlates with DC vaccine's ability to induce tumor rejection in vivo
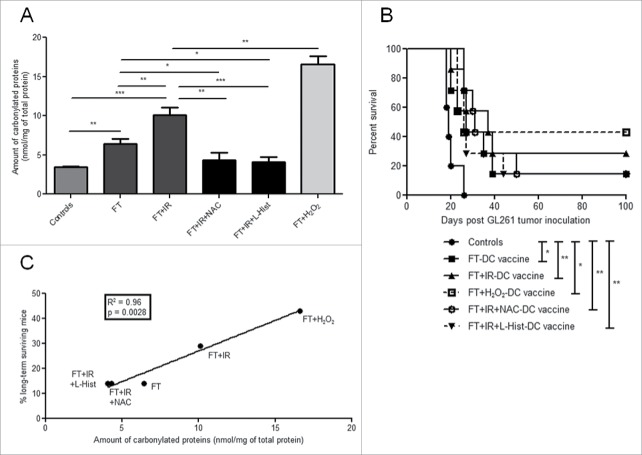
Given the well described ROS-inducing features of irradiation,22 and the increased immunogenicity observed with OAMPs accumulation,54 we wondered whether ROS-induced OAMPs (like protein carbonylation) might contribute to the increased in vivo immunogenicity of FT+IR GL261 cells.
To this end, we first decided to measure whether IR treatment can increase accumulation of protein carbonylation, an indicator of irreversible protein oxidation.55 Indeed, the level of protein carbonylation was significantly higher in FT+IR treated cells compared to FT treated cells (p < 0.01, Fig. 5A). Moreover, as positive control, we employed the bona fide oxidizing agent hydrogen peroxide (H2O2)56 and as expected, H2O2 induced even higher levels of protein carbonylation compared to FT+IR treated cells (p < 0.01, Fig. 5A). Next, we wondered whether the increase in the levels of carbonylated proteins was caused by ROS production induced by exposing the FT-necrotic cells to IR. To prove this, we decided to employ well-established anti-oxidants like N-acetylcysteine (NAC)57 and L-histidine (L-Hist)55,58 in combination with the FT+IR treatment. We observed that the presence of these ROS-scavengers in combination with IR treatment of FT-necrotic cells reduced the protein carbonyl content, back to the level of untreated GL261 cells (Fig. 5A). This result proved that, IR of FT-necrotic cells is associated with increased ROS-based biomolecule modifications.
Figure 5.
Oxidation-induced protein carbonylation in GL261 tumor lysates correlates with in vivo tumor rejection. The carbonyl content (calculated as nmols of carbonylated proteins per mg of total proteins) was evaluated in untreated GL261 cells (controls) and in the following lysate conditions: FT, FT+IR, FT+IR+NAC, FT+IR+L-Hist and FT+H2O2. Data are presented as mean values ± SD from one experiment performed in triplicate. *, p < 0.05; **, p < 0.01; ***, p < 0.001 (Student's t-test). (B) Kaplan–Meier graph of an experiment in which GL261 inoculated mice were either left untreated (controls, •, n = 5) or were immunized with FT-DC vaccines (▪, n = 7), FT+IR-DC vaccines (▴, n = 7), FT+IR+NAC-DC vaccines (○, n = 7), FT+IR+L-Hist-DC vaccines (▾, dashed line, n = 7) or FT+H2O2-DC vaccines (□, dashed line, n = 7). *, p < 0.05; **, p < 0.01 (Log-rank test). (C) Linear correlation between the amount of protein carbonylation in the different lysate preparations (as depicted in A) and the percentages of long-term surviving animals that were vaccinated with DCs pulsed with the respective lysate preparations (p = 0.0028, R2 = 0.96, linear regression analysis).
Next, we decided to employ our prophylactic DC vaccination model to address whether the increased OAMPs were mediating the in vivo immunogenicity of the FT+IR-DC vaccine (Fig. 5B). Reduction of the protein oxidative burden in the tumor lysates used to pulse the DCs was able to reduce (albeit not significant) the median survival of FT+IR+L-Hist-DC vaccine treated mice (26 d) and FT+IR+NAC-DC vaccine treated mice (31 d) as compared to mice vaccinated with the FT+IR-DC vaccine only (37 d).
Interestingly though, the percentage of mice that became long-term survivors was the same in the anti-oxidant treated groups as in the FT-DC vaccine treated group (14 %), while 29 % of mice became long-term survivors in the FT+IR-DC vaccine injected group. Mice vaccinated with the FT+H2O2-DC vaccine showed the highest percentage of tumor-rejecting mice (43 %). Intriguingly, the amount of protein carbonylation in the different lysate preparations showed a highly significant linear correlation with the percentage of long-term surviving mice (R2 = 0.96, p = 0.0028, Fig. 5C). These data clearly indicate that the levels of OAMPs, like carbonylated proteins, in lysates used to pulse DCs, are highly associated with (and may partly mediate) long-term immunity in this DC vaccination model for murine HGG.
Discussion
In this study, we show the superiority of DC vaccines pulsed with X-ray irradiated FT-necrotic glioma cells as compared to DC vaccines pulsed with FT-necrotic glioma cells only, in terms of improving the survival of HGG-bearing mice. Irradiation of necrotic glioma cells did not impact DC maturation but did, however, increase the amount of protein carbonylation in the lysate preparation. Most intriguingly, the protein carbonyl content in the tumor lysate preparations used to pulse the DC vaccines correlated significantly with the percentage of long-term surviving HGG-inoculated animals.
Meta-analysis of clinical data has indicated that subtle differences in the production process of DC vaccines, like DC maturation status, DC dose and use and non-use of adjuvants can impact clinical parameters in human melanoma, prostate cancer and renal cell cancer patients.59,60 Moreover, a meta-analysis of 173 vaccination trials in a wide range of cancers concluded that patients vaccinated with whole tumor antigen vaccines had a higher rate of clinical responses compared to those vaccinated with defined tumor antigens.61 Indeed, whole tumor vaccine strategies have the advantage of not relying on selected haplotypes such that immune responses are raised against a plethora of tumor antigens (including neo-antigens, if any), thereby avoiding the risk of immune escape variants.62 Our literature overview of clinical studies reporting the use of whole tumor lysate-pulsed DCs in HGG patients (both adults and children) revealed that there is no consensus yet in how FT-necrotic tumor lysates ought to be prepared (Table 1). However, based on our study, it can be hypothesized that FT-necrotic cells/lysates, if utilized for DC vaccines, should be preferably combined with high oxidation-inducing modalities.
Moreover, to the best of our knowledge, this is the first preclinical study evaluating the tumor immune contexture in a prophylactic as well as in a curative DC vaccination setup. This study revealed a reduced brain infiltration of Tregs, TAMs and MDSCs in GL261-inoculated mice previously immunized with the FT+IR-DC vaccine in both settings. All three of the abovementioned immunosuppressive leukocyte populations have been known to accumulate in the circulation and/or in the tumor microenvironment of HGG patients.63 Accumulation of CD4+FoxP3+ Tregs in the peripheral blood of GBM patients tends to correlate with impairment of T cell proliferation and the frequency of tumor-infiltrating Tregs has been shown to correlate with tumor grade in astrocytomas.10,64 Moreover, transient depletion of Tregs in the GL261 glioma model is capable of rescuing all tumor-challenged animals, however without the induction of immunological memory upon GL261 rechallenge.17 Similarly, in human GBM, increased percentages of circulating MDSC (CD15+CD33+HLA-DR−) have been reported.65 Nevertheless, TAMs are the predominant innate immune cells infiltrating gliomas, far outweighing the T cells, and their (TAMs) number has been shown to correlate with the tumor grade.66 Depletion of CD11b+ cells in GL261-bearing mice has been shown to slow down glioma progression, further indicating that the majority of tumor-infiltrating CD11b+ cells serve a tumor-promoting role in the context of HGG.67 Whether vaccination of GL261-inoculated mice with FT+IR-DC vaccine can also influence the functional status of Tregs, TAMs and MDSCs remains an open question. Additional ex vivo functional studies are required to address this question.
In addition, we observed an enriched population of CD3+ T cells and a stronger infiltration of Th1 cells and CTLs in mice receiving the FT+IR-DC vaccine. This finding suggests that this vaccination therapy can tackle the suppressive immune compartment, while simultaneously promoting the immune stimulating arm. This shift in immune balance toward a more antitumorigenic immune contexture is further demonstrated by the increased ratio of CD8+ T cells to Tregs in our FT+IR-DC vaccination set-up. Sayour and colleagues recently disclosed a positive correlation between the CD8+ to Treg ratio in primary GBM patients and survival time.68
Radiotherapy is a known inducer of immunogenic cell death (ICD) due to its combined action of generating ROS and inducing collateral endoplasmatic reticulum stress.51,52 This ICD-inducing feature of radiotherapy however does not apply in the setting where FT-necrosis is applied before X-ray irradiation (thereby making them ‘signaling incompetent’ from ICD's perspective). On the other hand, the ROS-inducing features of radiotherapy are still applicable in this situation. Accumulating research reveals that OAMPs like carbonylated proteins can act as danger signals.25-29 Irradiation of the necrotic GL261 cells upregulated the levels of protein carbonylation. Moreover, treatment of irradiated FT-cells with two different anti-oxidants completely abolished this effect. Of note, anti-oxidants based abrogation of irradiation-induced protein carbonylation (partially but not significantly) reduced overall median survival as well as the percentage of long-term surviving mice in our prophylactic vaccination model. The lack of significance between the different treatment conditions in this in vivo experiment (Fig. 5B) might be due to the small survival benefit observed with the FT+IR-DC vaccine (as compared to the FT-DC vaccine), leaving a very stiff window for intervention. DC vaccines pulsed with tumor lysate treated with the chemical oxidizer H2O2 showed the highest level of glioma protection. These data are in line with a recent study of Chiang and colleagues in the ID8 ovarian cancer model.18 They observed an increased in vivo efficacy of DCs pulsed with hypochlorous acid-oxidized tumor lysate as compared to DCs pulsed with FT-lysate. These findings suggest that the level of irreversible protein carbonylation in lysates — a convenient surrogate of the overall oxidative/OAMPs burden — used to pulse DC vaccines associates with (and possibly partially mediates) long-term antitumor immunity in this GL261 glioma model.
Interestingly, irradiation of the GL261 cells prior to freeze-thawing was shown to negatively affect the vaccine's in vivo immunogenicity. This could be explained by the fact that freeze-thawing is described to destroy carbonyl adducts.69 This is in accordance with our own observation of lower protein carbonyl content in the IR+FT treated cells in comparison to FT+IR treated cells (data not shown).
Various oxidatively damaged components caused by the irradiation, including lipid (per-)oxidation products, which have not been investigated here, may also ultimately contribute to the heightened protein carbonylation status of the lysates.70 This might be paralleled by formation of high-molecular weight aggregates of unfolded proteins (i.e., oxidation-induced aggregation), which can further enhance immunogenicity.71
All together, these data warrant the use of X-ray irradiated FT necrotic lysates, over the use of FT necrotic lysates alone, as an antigen source to pulse DCs in clinical DC vaccination studies. Moreover, these data foster the preclinical investigation of strong oxidizing or OAMPs-inducing compounds to further increase the potency of whole tumor antigen-pulsed DC vaccinations.72
Materials and methods
Mice and tumor cell line
Adult, female (8 to 10 weeks old) C57BL/6J mice were purchased from Harlan (Horst, The Netherlands). The mice were housed in conventional pathogen-free conditions. All animal experiments were approved by the bioethics committee of the KU Leuven that follows international guidelines.
Methylcholanthrene-induced murine C57BL/6J syngeneic GL261 glioma cells were kindly provided by Dr. Eyüpoglu (University of Erlangen, Germany). The cells were maintained in DMEM supplemented with 10% heat-inactivated FCS (Sigma-Aldrich, F7524), 2 mM L-glutamine, 100 U/mL penicillin and 100 mg/mL streptomycin (both from Lonza, BE17-605E and DE17-602E, respectively). Cell morphology was evaluated by microscopic examination multiple times per week.
Orthotopic glioma model and DC immunotherapy
The mice were intracranially injected with 5 × 105 GL261 tumor cells as previously described.17 Briefly, mice were anesthetized and fixed in a stereotactic frame (Kopf Instruments, Tujunga, CA), which allows for accurate injection at 2 mm lateral and 2 mm posterior from the bregma and at 3 mm below the dura mater. Stereotactic inoculation was performed under sterile conditions.
DC immunotherapy was applied in the prophylactic setting by intraperitoneal (i.p.) injection of 1 × 106 lysate-pulsed mature DCs on day 14 and 7 prior to GL261 tumor inoculation. The mice were defined as long-term survivors when their survival exceeded 3 times the median survival of the untreated control animals. In the curative treatment set-up, mice received 3 i.p. administered DC vaccines on days 2, 9 and 17 after intracranial GL261 tumor inoculation.
Preparation of GL261 lysates
GL261 cells were harvested, washed and suspended in phosphate-buffered saline (DPBS, Lonza) supplemented with 1% FCS. Seven different GL261 lysate formulations were prepared; GL261 cells subjected to 6 consecutive FT cycles (FT, 3 min in liquid nitrogen and 3 min on 56°C, respectively), GL261 cells subjected to 6 FT cycles and followed by 60 Gy X-ray irradiation (FT+IR), GL261 cells exposed to 60 Gy X-ray irradiation prior to 6 FT cycles (IR+FT), GL261 cells subjected to 800 Gy X-ray irradiation only (IR), GL261 cells exposed to the anti-oxidants NAC (5 mM, Sigma-Aldrich, A9165) or L-Hist (250 mM, Sigma-Aldrich, 53319) before, during and after the FT and irradiation treatment (FT+IR+NAC and FT+IR+L-Hist, respectively) and GL261 cells incubated with the strong oxidizer H2O2 for 24 h (750 μM, Sigma, H1009) after the FT cycles (FT+H2O2). Protein concentrations were determined via colorimetric assay (Bradford protein assay) according to the manufacturer's instructions (Bio-Rad).
Generation and characterization of DCs
DCs were derived from bone marrow progenitor cells as described.17 In short, bone-marrow progenitor cells were cultured for 7 d in the presence of GM-CSF (20 ng/mL, Peprotech, 312-03). Medium was refreshed on day 3 and day 5. On day 7 immature DCs were harvested using a cell scraper. For lysate loading, immature DCs were incubated with GL261 lysates at 2 mg protein per 10 × 106 DCs per mL culture medium at 37°C for 90 min. Immediately after loading, the DCs were transferred to culture flasks for 24 h in DC medium containing 20 ng/mL GM-CSF and 1 μg/mL Escherichia coli-derived lipopolysaccharide (LPS, Sigma-Aldrich, L6529–1MG) to induce maturation. Twenty-four hours later, the mature lysate-loaded DCs were harvested, counted and resuspended to 1 × 106 DCs in 100 μL PBS for i.p. injection.
Surface expression of DC maturations markers was assessed on lysate-pulsed mature DCs by flow cytometry using the following monoclonal antibodies (mAbs): FITC-conjugated anti-H-2Kb (Becton Dickinson (BD), 553569), PE-conjugated anti-I-A/I-E and anti-CD40 (BD, 557000 and 553791, respectively) and PE-conjugated anti-CD80 and anti-CD86 (eBioscience, 12-0862-83 and 12-0114-83, respectively). For each staining the appropriate isotypes were used. Analysis was performed using the Cellquest software on a FACSort cytometer (BD, Erembodegem, Belgium). The conditioned media derived from the lysate-loaded mature DCs were collected and checked for cytokine levels of IL-10 (88-7104-88), IL-12p70 (88-7121-88), IL-1β (88-7013-88) and IL-6 (88-7064-88) by means of ELISA kits from eBioscience, according to the manufacturer's instructions. Cytokine levels in GL261 lysates were also evaluated to rule out GL261 cell-derived cytokine production.
Isolation and characterization of brain-infiltrating immune cells
BICs were isolated from GL261-inoculated mice as previously described.17 Two different staining panels were used respectively for the prophylactic and curative setup. The staining protocol was however exactly the same for the two experiments. In the prophylactic setting surface staining was performed with anti-CD11b PE (eBioscience, 12-0112-83), anti-CD4+ PE and anti-CD8+ PE (BD, 553049 and 553033), anti-F4/80 FITC (AbD Serotec, MCA497DB), anti-Ly6G FITC (eBioscience, 11-5931-83), anti-CD3 FITC, anti-Ly6C FITC (BD, 553062 and 553104, respectively) and anti-CD45 PerCP-Cy5.5 mAbs (eBioscience, 45-0451-82). Intracellular FoxP3 was detected using a FoxP3-Alexa Fluor 488 staining kit (eBioscience, 73-5776) according to the manufacturer's protocols. For intracellular IFNγ staining, cells were stimulated for 4 h in vitro with 100 ng/mL phorbol myristate acetate, 1 μg/mL ionomycin and 0.7 μg/mL monensin (all from Sigma-Aldrich, P8139-1MG, I0634-1MG and M5273-500MG, respectively). After restimulation, surface staining for CD4+, CD8+ and CD45 was performed and cells were washed with a permeabilization buffer containing 0.5% saponin and 0.5% bovine serum albumin (BSA). Intracellular staining was performed with an anti-IFNγ FITC mAb (BD, 554411). Data acquisition was performed on a FACSort flow cytometer (BD) and the Cellquest software was used for data analysis.
In the curative setting surface staining was performed with anti-Ly6C Alexa Fluor® 647 (Bio-Rad, MCA2389A6457), anti-CD11b BV421, anti-Ly6G FITC, anti-CD8+ BV421 (all three from BD, 562605, 551460 and 563898, respectively), anti-CD45 Alexa Fluor® 700, anti-F4/80 PE, anti-CD3 FITC and anti-CD4+ PerCP-Cyanine5.5 mAbs (all four from eBioscience, 56-0451-82, 12-4801-80, 11-0031-82 and 45-0042-82, respectively). Intracellular FoxP3 was detected using an anti-FoxP3 PE staining set (eBioscience, 72-5775). For intracellular IFNγ staining, surface staining of stimulated cells was performed with anti-CD45 Alexa Fluor® 700, anti-CD3 PE, anti-CD4+ APC-eFluor® 780 (all three from eBioscience, 56-0451-82, 12-0031-82 and 47-0042-82, respectively) and anti-CD8+ BV421 mABs (BD), followed by intracellular staining performed with an anti-IFNγ PerCP-Cyanine5.5 mAb (BD, 560660). Data acquisition was performed on a LSRFortessa flow cytometer (BD Biosciences) and the FlowJo software was used for data analysis.
Protein carbonylation detection assay
The detection of protein carbonyl derivatives was performed as previously described.55 In brief, 50 μL of GL261 cell lysates or untreated GL261 cells, corresponding to a total protein concentration of 5 to 10 mg/mL were incubated with an equal volume of 200 μM fluorescein-5-thiosemicarbazide (FTC, Sigma-Aldrich, 46985-100MG-F) overnight at room temperature in the dark. Twenty-four hours later, proteins were precipitated and centrifuged after which the supernatant was discarded and consequently the protein precipitates were washed three times with acetone. The acetone supernatant was discarded and the precipitates were air-dried. These precipitates were then solubilized with 50 μL of guanidine hydrochloride (GuHCl, 6M) and diluted with 450 μL of NaH2PO4 (pH 7.0). Subsequently, the protein concentrations in these samples were measured via the bicinchoninic (BCA) protein assay kit from Thermo Scientific (23225). Fifty microliters of these samples were aliquoted in a black microtiter plate and fluorescence was measured with a FlexStation 3 microplate reader (Molecular Devices, Berkshire, United Kingdom, 0310-5627). A standard curve that was prepared using different concentrations of FTC allowed for the calculation of the nanomoles of FTC-reacted carbonyls. These values were then divided by the respective protein concentrations to derive the amount of protein carbonyls expressed as nmoL/mg protein.
Literature search of tumor lysate-pulsed DC vaccination trials in HGG patients
The studies listed in Table 1 were identified through an electronic search of the PubMed database, reference lists of published trials and relevant review articles. The search strategy included the following search terms: ‘glioma’ AND ‘dendritic cells’ AND ‘tumor lysate’. We only included studies that performed FT cycles to obtain tumor lysates and excluded case reports, articles not published in English and protocol studies.
Statistical analysis
Statistical analyses were performed using GraphPad Prism 5 software (San Diego, USA). Survival analysis was performed using the log-rank test. Student's t-test was performed for statistical analysis with significance level set at p < 0.05. Wherever applicable (with respect to comparison between multiple data-sets), a one-way ANOVA analysis was performed. Linear regression analysis was performed to investigate a possible association between protein carbonylation levels and the percentages of long-term surviving mice.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
L.V. is the recipient of a Strategic Basic Research grant from the Agency for Innovation by Science and Technology in Flanders (IWT Vlaanderen). A.D.G. and T.V are recipients of the FWO Postdoctoral Fellowship awarded by the Research Foundation-Flanders (FWO-Vlaanderen). S.W.V.G. is senior clinical investigator at the FWO-Vlaanderen.
Funding
This work was supported by the Olivia Hendrickx Research fund, the James E. Kearney Foundation, the Herman Memorial Research Fund, the Belgian Brain Tumor Support, individual donors and the FWO-grants G058412N (of P.A.) and GA01111N (of S.W.V.G.). The authors would also like to thank Dr. C.E. Fadul, Dr. C.J. Wheeler, Dr. R.M. Prins, Dr. R.D. Valle and Dr. H. Ardon for sharing their clinical data.
Supplemental Material
Supplemental Material may be downloaded here: publisher's website
References
- 1. Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 2007; 114:97-109; PMID:17618441; http://dx.doi.org/ 10.1007/s00401-007-0243-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005; 352:987-96; PMID:15758009; http://dx.doi.org/ 10.1056/NEJMoa043330 [DOI] [PubMed] [Google Scholar]
- 3. Koks CA, Garg AD, Ehrhardt M, Riva M, Vandenberk L, Boon L, De Vleeschouwer S, Agostinis P, Graf N, Van Gool SW. Newcastle disease virotherapy induces long-term survival and tumor-specific immune memory in orthotopic glioma through the induction of immunogenic cell death. Int J Cancer 2015; 136:E313-25; PMID:25208916; http://dx.doi.org/ 10.1002/ijc.29202 [DOI] [PubMed] [Google Scholar]
- 4. Galluzzi L, Vacchelli E, Bravo-San Pedro JM, Buque A, Senovilla L, Baracco EE, Bloy N, Castoldi F, Abastado JP, Agostinis P, et al. Classification of current anticancer immunotherapies. Oncotarget 2014; 5:12472-508; PMID:25537519; http://dx.doi.org/ 10.18632/oncotarget.2998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dudek AM, Martin S, Garg AD, Agostinis P. Immature, semi-mature, and fully mature dendritic cells: toward a DC-cancer cells interface that augments anticancer immunity. Front Immunol 2014; 4:438; PMID:24376443; http://dx.doi.org/ 10.3389/fimmu.2013.00438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cao JX, Zhang XY, Liu JL, Li D, Li JL, Liu YS, Wang M, Xu BL, Wang HB, Wang ZX. Clinical efficacy of tumor antigen-pulsed DC treatment for high-grade glioma patients: evidence from a meta-analysis. PLoS One 2014; 9:e107173; PMID:25215607; http://dx.doi.org/ 10.1371/journal.pone.0107173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Garg AD, Martin S, Golab J, Agostinis P. Danger signalling during cancer cell death: origins, plasticity and regulation. Cell Death Differ 2014; 21:26-38; PMID:23686135; http://dx.doi.org/ 10.1038/cdd.2013.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol 2002; 3:991-8; PMID:12407406; http://dx.doi.org/ 10.1038/ni1102-991 [DOI] [PubMed] [Google Scholar]
- 9. Palucka K, Banchereau J. Dendritic-cell-based therapeutic cancer vaccines. Immunity 2013; 39:38-48; PMID:23890062; http://dx.doi.org/ 10.1016/j.immuni.2013.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Heimberger AB, Abou-Ghazal M, Reina-Ortiz C, Yang DS, Sun W, Qiao W, Hiraoka N, Fuller GN. Incidence and prognostic impact of FoxP3+ regulatory T cells in human gliomas. Clin Cancer Res 2008; 14:5166-72; PMID:18698034; http://dx.doi.org/ 10.1158/1078-0432.CCR-08-0320 [DOI] [PubMed] [Google Scholar]
- 11. Crane CA, Ahn BJ, Han SJ, Parsa AT. Soluble factors secreted by glioblastoma cell lines facilitate recruitment, survival, and expansion of regulatory T cells: implications for immunotherapy. Neuro Oncol 2012; 14:584-95; PMID:22406925; http://dx.doi.org/ 10.1093/neuonc/nos014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Komohara Y, Ohnishi K, Kuratsu J, Takeya M. Possible involvement of the M2 anti-inflammatory macrophage phenotype in growth of human gliomas. J Pathol 2008; 216:15-24; PMID:18553315; http://dx.doi.org/ 10.1002/path.2370 [DOI] [PubMed] [Google Scholar]
- 13. Parney IF, Waldron JS, Parsa AT. Flow cytometry and in vitro analysis of human glioma-associated macrophages. Laboratory investigation. J Neurosurg 2009; 110:572-82; PMID:19199469; http://dx.doi.org/ 10.3171/2008.7.JNS08475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hussain SF, Yang D, Suki D, Aldape K, Grimm E, Heimberger AB. The role of human glioma-infiltrating microglia/macrophages in mediating antitumor immune responses. Neuro Oncol 2006; 8:261-79; PMID:16775224; http://dx.doi.org/ 10.1215/15228517-2006-008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kepp O, Senovilla L, Vitale I, Vacchelli E, Adjemian S, Agostinis P, Apetoh L, Aranda F, Barnaba V, Bloy N, et al. Consensus guidelines for the detection of immunogenic cell death. Oncoimmunology 2014; 3:e955691; PMID:25941621; http://dx.doi.org/23377664 10.4161/21624011.2014.955691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Prins RM, Wang X, Soto H, Young E, Lisiero DN, Fong B, Everson R, Yong WH, Lai A, Li G, et al. Comparison of glioma-associated antigen peptide-loaded versus autologous tumor lysate-loaded dendritic cell vaccination in malignant glioma patients. J Immunother 2013; 36:152-7; PMID:23377664; http://dx.doi.org/ 10.1097/CJI.0b013e3182811ae4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Maes W, Rosas GG, Verbinnen B, Boon L, De Vleeschouwer S, Ceuppens JL, Van Gool SW. DC vaccination with anti-CD25 treatment leads to long-term immunity against experimental glioma. Neuro Oncol 2009; 11:529-42; PMID:19336528; http://dx.doi.org/ 10.1215/15228517-2009-004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chiang CL, Kandalaft LE, Tanyi J, Hagemann AR, Motz GT, Svoronos N, Montone K, Mantia-Smaldone GM, Smith L, Nisenbaum HL, et al. A dendritic cell vaccine pulsed with autologous hypochlorous acid-oxidized ovarian cancer lysate primes effective broad antitumor immunity: from bench to bedside. Clin Cancer Res 2013; 19:4801-15; PMID:23838316; http://dx.doi.org/ 10.1158/1078-0432.CCR-13-1185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kim HS, Choo YS, Koo T, Bang S, Oh TY, Wen J, Song SY. Enhancement of antitumor immunity of dendritic cells pulsed with heat-treated tumor lysate in murine pancreatic cancer. Immunol Lett 2006; 103:142-8; PMID:16313973; http://dx.doi.org/ 10.1016/j.imlet.2005.10.021 [DOI] [PubMed] [Google Scholar]
- 20. Hatfield P, Merrick AE, West E, O'Donnell D, Selby P, Vile R, Melcher AA. Optimization of dendritic cell loading with tumor cell lysates for cancer immunotherapy. J Immunother 2008; 31:620-32; PMID:18600182; http://dx.doi.org/ 10.1097/CJI.0b013e31818213df [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Goldszmid RS, Idoyaga J, Bravo AI, Steinman R, Mordoh J, Wainstok R. Dendritic cells charged with apoptotic tumor cells induce long-lived protective CD4+ and CD8+ T cell immunity against B16 melanoma. J Immunol 2003; 171:5940-7; PMID:14634105; http://dx.doi.org/ 10.4049/jimmunol.171.11.5940 [DOI] [PubMed] [Google Scholar]
- 22. Obeid M, Panaretakis T, Joza N, Tufi R, Tesniere A, van Endert P, Zitvogel L, Kroemer G. Calreticulin exposure is required for the immunogenicity of gamma-irradiation and UVC light-induced apoptosis. Cell Death Differ 2007; 14:1848-50; PMID:17657249; http://dx.doi.org/ 10.1038/sj.cdd.4402201 [DOI] [PubMed] [Google Scholar]
- 23. Kaminski JM, Shinohara E, Summers JB, Niermann KJ, Morimoto A, Brousal J. The controversial abscopal effect. Cancer Treat Rev 2005; 31:159-72; PMID:15923088; http://dx.doi.org/ 10.1016/j.ctrv.2005.03.004 [DOI] [PubMed] [Google Scholar]
- 24. Kim EM, Yang HS, Kang SW, Ho JN, Lee SB, Um HD. Amplification of the gamma-irradiation-induced cell death pathway by reactive oxygen species in human U937 cells. Cell Signal 2008; 20:916-24; PMID:18262755; http://dx.doi.org/ 10.1016/j.cellsig.2008.01.002 [DOI] [PubMed] [Google Scholar]
- 25. Allison ME, Fearon DT. Enhanced immunogenicity of aldehyde-bearing antigens: a possible link between innate and adaptive immunity. Eur J Immunol 2000; 30:2881-7; PMID:11069070; http://dx.doi.org/ [DOI] [PubMed] [Google Scholar]
- 26. Carrasco-Marin E, Paz-Miguel JE, Lopez-Mato P, Alvarez-Dominguez C, Leyva-Cobian F. Oxidation of defined antigens allows protein unfolding and increases both proteolytic processing and exposes peptide epitopes which are recognized by specific T cells. Immunology 1998; 95:314-21; PMID:9824492; http://dx.doi.org/ 10.1046/j.1365-2567.1998.00618.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Moghaddam AE, Gartlan KH, Kong L, Sattentau QJ. Reactive carbonyls are a major Th2-inducing damage-associated molecular pattern generated by oxidative stress. J Immunol 2011; 187:1626-33; PMID:21742965; http://dx.doi.org/ 10.4049/jimmunol.1003906 [DOI] [PubMed] [Google Scholar]
- 28. Miller YI, Choi SH, Wiesner P, Fang L, Harkewicz R, Hartvigsen K, Boullier A, Gonen A, Diehl CJ, Que X, et al. Oxidation-specific epitopes are danger-associated molecular patterns recognized by pattern recognition receptors of innate immunity. Circ Res 2011; 108:235-48; PMID:21252151; http://dx.doi.org/ 10.1161/CIRCRESAHA.110.223875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. West XZ, Malinin NL, Merkulova AA, Tischenko M, Kerr BA, Borden EC, Podrez EA, Salomon RG, Byzova TV. Oxidative stress induces angiogenesis by activating TLR2 with novel endogenous ligands. Nature 2010; 467:972-6; PMID:20927103; http://dx.doi.org/ 10.1038/nature09421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Szatmari T, Lumniczky K, Desaknai S, Trajcevski S, Hidvegi EJ, Hamada H, Safrany G. Detailed characterization of the mouse glioma 261 tumor model for experimental glioblastoma therapy. Cancer Sci 2006; 97:546-53; PMID:16734735; http://dx.doi.org/ 10.1111/j.1349-7006.2006.00208.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Anguille S, Smits EL, Lion E, van Tendeloo VF, Berneman ZN. Clinical use of dendritic cells for cancer therapy. Lancet Oncol 2014; 15:e257-67; PMID:24872109; http://dx.doi.org/ 10.1016/S1470-2045(13)70585-0 [DOI] [PubMed] [Google Scholar]
- 32. Yamanaka R, Abe T, Yajima N, Tsuchiya N, Homma J, Kobayashi T, Narita M, Takahashi M, Tanaka R. Vaccination of recurrent glioma patients with tumour lysate-pulsed dendritic cells elicits immune responses: results of a clinical phase I/II trial. Br J Cancer 2003; 89:1172-9; PMID:14520441; http://dx.doi.org/ 10.1038/sj.bjc.6601268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wheeler CJ, Black KL, Liu G, Ying H, Yu JS, Zhang W, Lee PK. Thymic CD8+ T cell production strongly influences tumor antigen recognition and age-dependent glioma mortality. J Immunol 2003; 171:4927-33; PMID:14568975; http://dx.doi.org/ 10.4049/jimmunol.171.9.4927 [DOI] [PubMed] [Google Scholar]
- 34. Wheeler CJ, Das A, Liu G, Yu JS, Black KL. Clinical responsiveness of glioblastoma multiforme to chemotherapy after vaccination. Clin Cancer Res 2004; 10:5316-26; PMID:15328167; http://dx.doi.org/ 10.1158/1078-0432.CCR-04-0497 [DOI] [PubMed] [Google Scholar]
- 35. Yu JS, Liu G, Ying H, Yong WH, Black KL, Wheeler CJ. Vaccination with tumor lysate-pulsed dendritic cells elicits antigen-specific, cytotoxic T-cells in patients with malignant glioma. Cancer Res 2004; 64:4973-9; PMID:15256471; http://dx.doi.org/ 10.1158/0008-5472.CAN-03-3505 [DOI] [PubMed] [Google Scholar]
- 36. Yamanaka R, Homma J, Yajima N, Tsuchiya N, Sano M, Kobayashi T, Yoshida S, Abe T, Narita M, Takahashi M, et al. Clinical evaluation of dendritic cell vaccination for patients with recurrent glioma: results of a clinical phase I/II trial. Clin Cancer Res 2005; 11:4160-7; PMID:15930352; http://dx.doi.org/ 10.1158/1078-0432.CCR-05-0120 [DOI] [PubMed] [Google Scholar]
- 37. Wheeler CJ, Black KL, Liu G, Mazer M, Zhang XX, Pepkowitz S, Goldfinger D, Ng H, Irvin D, Yu JS. Vaccination elicits correlated immune and clinical responses in glioblastoma multiforme patients. Cancer Res 2008; 68:5955-64; PMID:18632651; http://dx.doi.org/ 10.1158/0008-5472.CAN-07-5973 [DOI] [PubMed] [Google Scholar]
- 38. Prins RM, Soto H, Konkankit V, Odesa SK, Eskin A, Yong WH, Nelson SF, Liau LM. Gene expression profile correlates with T-cell infiltration and relative survival in glioblastoma patients vaccinated with dendritic cell immunotherapy. Clin Cancer Res 2011; 17:1603-15; PMID:21135147; http://dx.doi.org/ 10.1158/1078-0432.CCR-10-2563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fong B, Jin R, Wang X, Safaee M, Lisiero DN, Yang I, Li G, Liau LM, Prins RM. Monitoring of regulatory T cell frequencies and expression of CTLA-4 on T cells, before and after DC vaccination, can predict survival in GBM patients. PLoS One 2012; 7:e32614; PMID:22485134; http://dx.doi.org/23645755 10.1371/journal.pone.0032614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lasky JL, 3rd, Panosyan EH, Plant A, Davidson T, Yong WH, Prins RM, Liau LM, Moore TB. Autologous tumor lysate-pulsed dendritic cell immunotherapy for pediatric patients with newly diagnosed or recurrent high-grade gliomas. Anticancer Res 2013; 33:2047-56; PMID:23645755 [PMC free article] [PubMed] [Google Scholar]
- 41. Rutkowski S, De Vleeschouwer S, Kaempgen E, Wolff JE, Kuhl J, Demaerel P, Warmuth-Metz M, Flamen P, Van Calenbergh F, Plets C, et al. Surgery and adjuvant dendritic cell-based tumour vaccination for patients with relapsed malignant glioma, a feasibility study. Br J Cancer 2004; 91:1656-62; PMID:15477864; http://dx.doi.org/ 10.1038/sj.bjc.6602195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. De Vleeschouwer S, Fieuws S, Rutkowski S, Van Calenbergh F, Van Loon J, Goffin J, Sciot R, Wilms G, Demaerel P, Warmuth-Metz M, et al. Postoperative adjuvant dendritic cell-based immunotherapy in patients with relapsed glioblastoma multiforme. Clin Cancer Res 2008; 14:3098-104; PMID:18483377; http://dx.doi.org/ 10.1158/1078-0432.CCR-07-4875 [DOI] [PubMed] [Google Scholar]
- 43. Ardon H, Van Gool S, Lopes IS, Maes W, Sciot R, Wilms G, Demaerel P, Bijttebier P, Claes L, Goffin J, et al. Integration of autologous dendritic cell-based immunotherapy in the primary treatment for patients with newly diagnosed glioblastoma multiforme: a pilot study. J Neurooncol 2010; 99:261-72; PMID:20146084; http://dx.doi.org/ 10.1007/s11060-010-0131-y [DOI] [PubMed] [Google Scholar]
- 44. Ardon H, De Vleeschouwer S, Van Calenbergh F, Claes L, Kramm CM, Rutkowski S, Wolff JE, Van Gool SW. Adjuvant dendritic cell-based tumour vaccination for children with malignant brain tumours. Pediatr Blood Cancer 2010; 54:519-25; PMID:19852061; http://dx.doi.org/ 10.1002/pbc.22319. [DOI] [PubMed] [Google Scholar]
- 45. Fadul CE, Fisher JL, Hampton TH, Lallana EC, Li Z, Gui J, Szczepiorkowski ZM, Tosteson TD, Rhodes CH, Wishart HA, et al. Immune response in patients with newly diagnosed glioblastoma multiforme treated with intranodal autologous tumor lysate-dendritic cell vaccination after radiation chemotherapy. J Immunother 2011; 34:382-9; PMID:21499132; http://dx.doi.org/ 10.1097/CJI.0b013e318215e300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Elens I, De Vleeschouwer S, Pauwels F, Van Gool S. Resection and immunotherapy for recurrent grade Iii Glioma. Neuro-Oncology 2012; 14:61; http://dx.doi.org/ 10.5402/2012/530179 [DOI] [Google Scholar]
- 47. Ardon H, Van Gool SW, Verschuere T, Maes W, Fieuws S, Sciot R, Wilms G, Demaerel P, Goffin J, Van Calenbergh F, et al. Integration of autologous dendritic cell-based immunotherapy in the standard of care treatment for patients with newly diagnosed glioblastoma: results of the HGG-2006 phase I/II trial. Cancer Immunol Immunother 2012; 61:2033-44; PMID:22527250; http://dx.doi.org/ 10.1007/s00262-012-1261-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. De Vleeschouwer S, Ardon H, Van Calenbergh F, Sciot R, Wilms G, van Loon J, Goffin J, Van Gool S. Stratification according to HGG-IMMUNO RPA model predicts outcome in a large group of patients with relapsed malignant glioma treated by adjuvant postoperative dendritic cell vaccination. Cancer Immunol Immunother 2012; 61:2105-12; PMID:22565485; http://dx.doi.org/ 10.1007/s00262-012-1271-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Valle RD, de Cerio AL, Inoges S, Tejada S, Pastor F, Villanueva H, Gallego J, Espinos J, Aristu J, Idoate MA, et al. Dendritic cell vaccination in glioblastoma after fluorescence-guided resection. World J Clin Oncol 2012; 3:142-9; PMID:23293753; http://dx.doi.org/ 10.5306/wjco.v3.i11.142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Verschuere T, Toelen J, Maes W, Poirier F, Boon L, Tousseyn T, Mathivet T, Gerhardt H, Mathieu V, Kiss R, et al. Glioma-derived galectin-1 regulates innate and adaptive antitumor immunity. Int J Cancer 2014; 134:873-84; PMID:23929302; http://dx.doi.org/ 10.1002/ijc.28426 [DOI] [PubMed] [Google Scholar]
- 51. Krysko DV, Garg AD, Kaczmarek A, Krysko O, Agostinis P, Vandenabeele P. Immunogenic cell death and DAMPs in cancer therapy. Nat Rev Cancer 2012; 12:860-75; PMID:23151605; http://dx.doi.org/ 10.1038/nrc3380 [DOI] [PubMed] [Google Scholar]
- 52. Dudek AM, Garg AD, Krysko DV, De Ruysscher D, Agostinis P. Inducers of immunogenic cancer cell death. Cytokine Growth Factor Rev 2013; 24:319-33; PMID:23391812; http://dx.doi.org/ 10.1016/j.cytogfr.2013.01.005 [DOI] [PubMed] [Google Scholar]
- 53. Lakshmi Narendra B, Eshvendar Reddy K, Shantikumar S, Ramakrishna S. Immune system: a double-edged sword in cancer. Inflamm Res: Off J Euro Histamine Res Soc [et al] 2013; 62:823-34; PMID:23868500; http://dx.doi.org/ 10.1007/s00011-013-0645-9 [DOI] [PubMed] [Google Scholar]
- 54. Garg AD, Krysko DV, Vandenabeele P, Agostinis P. DAMPs and PDT-mediated photo-oxidative stress: exploring the unknown. Photochem Photobiol Sci 2011; 10:670-80; PMID:21258717; http://dx.doi.org/ 10.1039/c0pp00294a [DOI] [PubMed] [Google Scholar]
- 55. Garg AD, Dudek AM, Ferreira GB, Verfaillie T, Vandenabeele P, Krysko DV, Mathieu C, Agostinis P. ROS-induced autophagy in cancer cells assists in evasion from determinants of immunogenic cell death. Autophagy 2013; 9:1292-307; PMID:23800749; http://dx.doi.org/ 10.4161/auto.25399 [DOI] [PubMed] [Google Scholar]
- 56. Verfaillie T, Rubio N, Garg AD, Bultynck G, Rizzuto R, Decuypere JP, Piette J, Linehan C, Gupta S, Samali A, et al. PERK is required at the ER-mitochondrial contact sites to convey apoptosis after ROS-based ER stress. Cell Death Differ 2012; 19:1880-91; PMID:22705852; http://dx.doi.org/ 10.1038/cdd.2012.74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Panaretakis T, Kepp O, Brockmeier U, Tesniere A, Bjorklund AC, Chapman DC, Durchschlag M, Joza N, Pierron G, van Endert P, et al. Mechanisms of pre-apoptotic calreticulin exposure in immunogenic cell death. EMBO J 2009; 28:578-90; PMID:19165151; http://dx.doi.org/ 10.1038/emboj.2009.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Garg AD, Krysko DV, Verfaillie T, Kaczmarek A, Ferreira GB, Marysael T, Rubio N, Firczuk M, Mathieu C, Roebroek AJ, et al. A novel pathway combining calreticulin exposure and ATP secretion in immunogenic cancer cell death. EMBO J 2012; 31:1062-79; PMID:22252128; http://dx.doi.org/ 10.1038/emboj.2011.497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Nakai N, Hartmann G, Kishimoto S, Katoh N. Dendritic cell vaccination in human melanoma: relationships between clinical effects and vaccine parameters. Pigment Cell Melanoma Res 2010; 23:607-19; PMID:20579307; http://dx.doi.org/ 10.1111/j.1755-148X.2010.00736.x [DOI] [PubMed] [Google Scholar]
- 60. Draube A, Klein-Gonzalez N, Mattheus S, Brillant C, Hellmich M, Engert A, von Bergwelt-Baildon M. Dendritic cell based tumor vaccination in prostate and renal cell cancer: a systematic review and meta-analysis. PLoS One 2011; 6:e18801; PMID:21533099; http://dx.doi.org/ 10.1371/journal.pone.0018801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Neller MA, Lopez JA, Schmidt CW. Antigens for cancer immunotherapy. Semin Immunol 2008; 20:286-95; PMID:18951039; http://dx.doi.org/ 10.1016/j.smim.2008.09.006 [DOI] [PubMed] [Google Scholar]
- 62. Sampson JH, Heimberger AB, Archer GE, Aldape KD, Friedman AH, Friedman HS, Gilbert MR, Herndon JE, 2nd, McLendon RE, Mitchell DA, et al. Immunologic escape after prolonged progression-free survival with epidermal growth factor receptor variant III peptide vaccination in patients with newly diagnosed glioblastoma. J Clin Oncol 2010; 28:4722-9; PMID:20921459; http://dx.doi.org/ 10.1200/JCO.2010.28.6963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ahn BJ, Pollack IF, Okada H. Immune-checkpoint blockade and active immunotherapy for glioma. Cancers (Basel) 2013; 5:1379-412; PMID:24202450; http://dx.doi.org/ 10.3390/cancers5041379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Fecci PE, Mitchell DA, Whitesides JF, Xie W, Friedman AH, Archer GE, Herndon JE, 2nd, Bigner DD, Dranoff G, Sampson JH. Increased regulatory T-cell fraction amidst a diminished CD4 compartment explains cellular immune defects in patients with malignant glioma. Cancer Res 2006; 66:3294-302; PMID:16540683; http://dx.doi.org/ 10.1158/0008-5472.CAN-05-3773 [DOI] [PubMed] [Google Scholar]
- 65. Raychaudhuri B, Rayman P, Ireland J, Ko J, Rini B, Borden EC, Garcia J, Vogelbaum MA, Finke J. Myeloid-derived suppressor cell accumulation and function in patients with newly diagnosed glioblastoma. Neuro Oncol 2011; 13:591-9; PMID:21636707; http://dx.doi.org/ 10.1093/neuonc/nor042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Nishie A, Ono M, Shono T, Fukushi J, Otsubo M, Onoue H, Ito Y, Inamura T, Ikezaki K, Fukui M, et al. Macrophage infiltration and heme oxygenase-1 expression correlate with angiogenesis in human gliomas. Clin Cancer Res 1999; 5:1107-13; PMID:10353745 [PubMed] [Google Scholar]
- 67. Zhai H, Heppner FL, Tsirka SE. Microglia/macrophages promote glioma progression. Glia 2011; 59:472-85; PMID:21264953; http://dx.doi.org/ 10.1002/glia.21117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Sayour EJ, McLendon P, McLendon R, De Leon G, Reynolds R, Kresak J, Sampson JH, Mitchell DA. Increased proportion of FoxP3+ regulatory T cells in tumor infiltrating lymphocytes is associated with tumor recurrence and reduced survival in patients with glioblastoma. Cancer Immunol Immunother 2015; 64:419-27; PMID:25555571; http://dx.doi.org/ 10.1007/s00262-014-1651-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Hawkins CL, Morgan PE, Davies MJ. Quantification of protein modification by oxidants. Free Radical Biol Med 2009; 46:965-88; PMID:19439229; http://dx.doi.org/ 10.1016/j.freeradbiomed.2009.01.007 [DOI] [PubMed] [Google Scholar]
- 70. Fritz KS, Petersen DR. Exploring the biology of lipid peroxidation-derived protein carbonylation. Chem Res Toxicol 2011; 24:1411-9; PMID:21812433; http://dx.doi.org/ 10.1021/tx200169n [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. van Beers MM, Sauerborn M, Gilli F, Brinks V, Schellekens H, Jiskoot W. Oxidized and aggregated recombinant human interferon beta is immunogenic in human interferon beta transgenic mice. Pharm Res 2011; 28:2393-402; PMID:21544687; http://dx.doi.org/ 10.1007/s11095-011-0451-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Garg AD, Agostinis P. ER stress, autophagy and immunogenic cell death in photodynamic therapy-induced anti-cancer immune responses. Photochem Photobiol Sci: Off J Euro Photochem Assoc Eur Soc Photobiol 2014; 13:474-87; PMID:24493131; http://dx.doi.org/ 10.1039/c3pp50333j [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.