ABSTRACT
While major improvements have been made in targeting primary tumor growth, metastasis and combating cancer spread remain an enigma. We recently identified a systemic inflammatory cascade involving IL17-producing γδ T cells and neutrophils that advance breast cancer metastasis. These data provide insights into how immune cells promote cancer spread.
When deleterious events happen on the planet Earth whether natural or human-made, they often have far-reaching consequences beyond the local site of injury. Take the 2012 Fukushima power plant nuclear meltdown in Japan, and the 2010 eruption of Iceland's Eyjafjallajökull volcano for example. Low levels of radioactive cesium from Fukushima almost traveled to the California coast, around 8600 km away. The ash from Iceland, which disrupted European and trans-Atlantic flights for weeks, floated across the European continent and even as far as Russia. Like these events, localized tumors within an organism reach far beyond their boundaries to affect distant organs and evoke systemic responses. Confined tumors induce these long-distance changes that consequently create the right conditions for cultivation of tumor growth and metastasis.1
Recently, our lab unraveled a mechanism in the K14cre;Cdh1F/F;Trp53F/F (KEP) mouse model by which mammary tumors systemically reshape the host immune system to support metastasis.2 We showed that IL17-producing γδ T cells, activated by mammary tumor-derived IL1β, drive systemic expansion and polarization of neutrophils. The far-reaching accumulation of neutrophils and their reeducation establishes an organism-wide immunosuppressive environment that blocks the antitumor activity of CD8+ T cells and increases the efficiency of mammary cancer metastasis (Fig. 1).2
Figure 1.
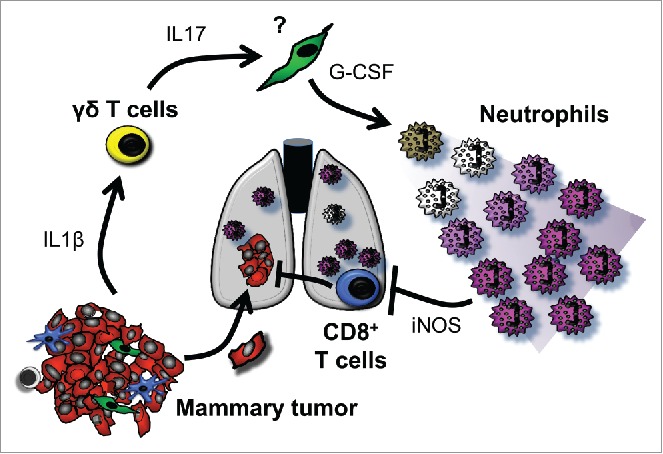
Mammary tumor-activated γδ T cells educate immunosuppressive neutrophils to advance metastasis. IL1β released into the circulation by mammary tumors activates γδ T cells to produce IL17. An unknown cell type responds to IL17 by upregulating G-CSF, causing neutrophil expansion and polarization toward an immunosuppressive phenotype. Through an iNOS-dependent mechanism, neutrophils dampen CD8+ T cell killer functions so that disseminated cancer cells are protected from attack.
Neutrophils suppress T cell activation by upregulation of iNOS,2 placing them under the myeloid-derived suppressor cell (MDSC) umbrella. These neutrophils express CD11b, Ly6G and Ly6C, but lack expression of F4/80 and CCR2, which distinguishes them from monocytes, macrophages and dendritic cells. However, neutrophils from tumor-bearing KEP mice contain both mature and immature populations, based on nuclear morphological analysis and expression of the hematopoietic stem cell marker, cKIT, by a fraction of the population. As expected, the depletion of these neutrophils unleashes the functions of CD8+ T cells and decreases metastasis formation. Importantly, the depletion of CD8+ T cells alone does not increase metastasis.2 These data indicate that CD8+ T cells are completely indolent in the KEP model until the neutrophil-generated immunosuppressive barrier is broken.
We found that IL17-induced G-CSF elicits the suppressive functions of neutrophils.2 TGFβ has also been proposed to regulate neutrophil polarization;3 although, in the KEP model, TGFβ does not seem to be as important as G-CSF. TGFβ levels are equivalent in tumor-bearing KEP;Rag1–/– mice and KEP mice, while IL17 levels, G-CSF levels and iNOS expression in neutrophils from KEP;Rag1–/– mice is reduced. As a result, TGFβ is more likely acting on γδ T cells or another population rather than neutrophils in our model.
Many of our findings are strikingly similar to other observations made about the pre-metastatic niche.4,5 First, we found that neutrophils are most important during the early steps of the metastatic cascade.2 Second, neutrophils in our model share several of the phenotypic characteristics with other cells implicated in the pre-metastatic niche, such as expression of VEGFR1 and cKIT.4,5 Third, in addition to iNOS, neutrophils in KEP mice upregulate expression of Bv8/Prok2, S100a8 and S100a9—three genes known to participate in establishment of the pre-metastatic niche.4,5 However, there is one major difference between our data and that of others: neutrophils are not restricted to metastasis-specific organs, like lungs or lymph nodes, they accumulate in every organ throughout the whole mouse. This almost rules out the possibility that neutrophils drive organ-specific tropism toward the lung and draining lymph node. At the same time, these observations raise the question of whether a pre-metastatic niche exists in the KEP model. Is there another mechanism at play that actively directs mammary cancer cells to pre-determined locations? Are other (immune) cells responsible? These questions remain answered.
Upstream of neutrophil expansion and polarization, γδ T cells produce IL17 to increase systemic G-CSF levels. All three components—γδ T cells, IL17 and G-CSF—are required for neutrophil expansion, since their depletion or neutralization prevents this cascade. The depletion or genetic knockout of γδ T cells in our spontaneous metastasis model also decreases multi-organ metastasis in an analogous fashion to neutrophil depletion. However, there is still one missing piece of the puzzle: What cell type is activated by IL17 to upregulate G-CSF and where does this occur? Previous studies have shown that tumor-associated fibroblasts produce G-CSF in response to IL17.6 It would be interesting to assess whether the same is true in the KEP model.
Together, our mechanistic data in mouse models and the clinical data of others indicate that γδ T cells and neutrophils join forces to expedite breast cancer metastasis.7-9 So how do we identify patients that may be most susceptible to this inflammatory cascade? As higher neutrophil-to-lymphocyte ratios are associated with worsened metastasis-specific survival,7 γδ T cell and neutrophil therapies may be effective in these patients. Determining which mutational signatures in primary tumors are correlated with systemic inflammation may also be beneficial for future stratification. How should γδ T cells and neutrophils be targeted in breast cancer patients without causing major toxicities or increased infections? One strategy may be to reverse the polarization of these cells as a means to downregulate their immunosuppressive and pro-metastatic abilities, without losing other anti-infectious properties. Bisphosphonates are one such way to alter γδ T cell behavior,10 and IL17 stands out as an ideal upstream candidate to modify neutrophil phenotype. One IL17 inhibitor was recently approved for use in patients with psoriasis. Our data provide several potential avenues to target systemic inflammation and combat metastasis—the primary cause of cancer-related deaths.
References
- 1.McAllister SS, Weinberg RA. The tumour-induced systemic environment as a critical regulator of cancer progression and metastasis. Nat Cell Biol 2014; 16:717-27; PMID:25082194; http://dx.doi.org/ 10.1038/ncb3015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coffelt SB, Kersten K, Doornebal CW, Weiden J, Vrijland K, Hau CS, Verstegen NJ, Ciampricotti M, Hawinkels LJ, Jonkers J et al.. IL-17-producing gammadelta T cells and neutrophils conspire to promote breast cancer metastasis. Nature 2015; 522:345-8; PMID:25822788; http://dx.doi.org/ 10.1038/nature14282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, Worthen GS, Albelda SM. Polarization of tumor-associated neutrophil phenotype by TGF-beta: "N1" versus "N2" TAN. Cancer Cell 2009; 16:183-94; PMID:19732719; http://dx.doi.org/ 10.1016/j.ccr.2009.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kowanetz M, Wu X, Lee J, Tan M, Hagenbeek T, Qu X, Yu L, Ross J, Korsisaari N, Cao T et al.. Granulocyte-colony stimulating factor promotes lung metastasis through mobilization of Ly6G+Ly6C+ granulocytes. Proc Natl Acad Sci USA 2010; 107:21248-55; PMID:21081700; http://dx.doi.org/ 10.1073/pnas.1015855107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hiratsuka S, Watanabe A, Aburatani H, Maru Y. Tumour-mediated upregulation of chemoattractants and recruitment of myeloid cells predetermines lung metastasis. Nat Cell Biol 2006; 8:1369-75; PMID:17128264; http://dx.doi.org/ 10.1038/ncb1507 [DOI] [PubMed] [Google Scholar]
- 6.Chung AS, Wu X, Zhuang G, Ngu H, Kasman I, Zhang J, Vernes JM, Jiang Z, Meng YG, Peale FV et al.. An interleukin-17-mediated paracrine network promotes tumor resistance to anti-angiogenic therapy. Nat Med 2013; 19:1114-23; PMID:23913124; http://dx.doi.org/ 10.1038/nm.3291 [DOI] [PubMed] [Google Scholar]
- 7.Noh H, Eomm M, Han A. Usefulness of pretreatment neutrophil to lymphocyte ratio in predicting disease-specific survival in breast cancer patients. J Breast Cancer 2013; 16:55-9; PMID:23593082; http://dx.doi.org/ 10.4048/jbc.2013.16.1.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ma C, Zhang Q, Ye J, Wang F, Zhang Y, Wevers E, Schwartz T, Hunborg P, Varvares MA, Hoft DF et al.. Tumor-infiltrating gammadelta T lymphocytes predict clinical outcome in human breast cancer. J Immunol 2012; 189:5029-36.; PMID:23034170; http://dx.doi.org/ 10.4049/jimmunol.1201892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen WC, Lai YH, Chen HY, Guo HR, Su IJ, Chen HH. Interleukin-17-producing cell infiltration in the breast cancer tumour microenvironment is a poor prognostic factor. Histopathol 2013; 63:225-33; PMID:23738752; http://dx.doi.org/ 10.1111/his.12156 [DOI] [PubMed] [Google Scholar]
- 10.Santini D, Martini F, Fratto ME, Galluzzo S, Vincenzi B, Agrati C, Turchi F, Piacentini P, Rocci L, Manavalan JS et al.. In vivo effects of zoledronic acid on peripheral gammadelta T lymphocytes in early breast cancer patients. Cancer Immunol, Immunother 2009; 58:31-8; PMID:18458903; http://dx.doi.org/ 10.1007/s00262-008-0521-6 [DOI] [PMC free article] [PubMed] [Google Scholar]


