ABSTRACT
Monoclonal antibodies (mAb) are central to the treatment of several types of malignancy. However, these reagents are subject to particular types of resistance. Several resistance mechanisms are regulated by the inhibitory FcγRIIB. We recently developed mAbs to block FcγRIIB and provided in vivo proof-of-concept for their ability to overcome FcγRIIB-mediated resistance.
Keywords: CD32B, FcgRIIB, immunotherapy, mAb resistance, monoclonal antibody, therapyrituximab
Introduction
It has long been appreciated that the inhibitory Fc gamma receptor (FcγR) IIB, expressed by numerous cells of the immune system, negatively regulates both innate and adaptive immunity through engagement of immune complexes (IC).1 Similarly, the knowledge that FcγRIIB negatively regulates mAb-mediated immunotherapy has been known for over a decade. As such, FcγRIIB-deficient mice are able to clear tumors more effectively than WT mice when treated with therapeutic mAbs, indicating that FcγRIIB expression on effector cells (i.e., macrophages and monocytes) leads to suppression of their phagocytic and cytotoxic potential in vivo.2 Moreover, FcγRIIB regulates the antigen-presenting potential of dendritic cells (DC) and FcγRIIB−ve DCs have an improved capacity to activate naive T cells.3 In addition, it has recently been demonstrated that high expression of FcγRIIB on target tumor cells may also be detrimental to targeted mAb therapy.4-8 Accordingly, on malignant B cells, such as chronic lymphocytic leukemia (CLL) and mantle cell lymphoma (MCL), the Fc portion of the direct targeting mAb (e.g., rituximab or alemtuzumab), is engaged by FcγRIIB and accelerates mAb internalization from the tumor surface.4,5,7 As a result of internalization, the Fc-dependent effector-functions of antibody-dependent cellular cytotoxicity (ADCC) and phagocytosis (ADCP) are severely hampered,4,6,7 reducing therapeutic efficacy in mouse models.9
Recently, we tested this hypothesis in humans by performing a retrospective study using tissues from follicular lymphoma (FL) patients treated with rituximab monotherapy and demonstrated that FL patients who expressed medium/high levels of FcγRIIB responded less effectively to rituximab compared to patients with negative/low FcγRIIB levels.8 These clinical observations add weight to the recent experimental discoveries highlighting the potential therapeutic importance of targeting FcγRIIB in lymphoid malignancies.4,5,7,8
To exploit these observations, we used a human phage-display library n-CoDeR®, to identify a panel of highly specific, fully human, FcγRIIB mAbs. Subsequent in vitro characterization enabled us to select antagonistic mAbs, capable of blocking IC binding and ligating FcγRIIB without activating it.10 We tested them in a variety of relevant in vitro assays, helping us to identify a lead clinical candidate clone 6G11 (BI-1206). Further in vitro and in vivo assays alone and in combination with clinically relevant therapeutic mAbs (e.g., rituximab) demonstrated its ability to augment immunotherapy. Of note, we confirmed previous observations that human FcγRIIB does not rapidly internalize from the surface of malignant B cells once ligated, making it a promising target for mAb therapy. Importantly, 6G11 was demonstrated to be safe in human FcγRIIB transgenic (Tg) mice and failed to induce any cytokine storm in vitro. Moreover, when combined with rituximab, 6G11 significantly enhanced the depletion of circulatory B cells in a novel human CD20 x FcγRIIB Tg mouse.10 Furthermore, using a primary CLL patient-derived xenograft (PDX) mouse model, we demonstrated the beneficial effects of combining FcγRIIB mAb with other clinically approved mAbs, including rituximab, obinituzumab and alemtuzumab.10 Encouragingly, when using CLL samples in the PDX model that were previously defined as refractory to mAb treatment, rituximab alone failed to significantly deplete xenografted CLL cells, whereas 6G11 and rituximab combination therapy strongly enhanced their depletion.10
In conclusion, our data indicate that human FcγRIIB may offer a safe and promising target for potentiating the activity of therapeutic mAbs currently administered in the clinic (For a schematic of potential mechanism of action see Figure 1). As a result of these pre-clinical data, we aim to test the safety and efficacy of 6G11 in a first-in-human clinical trial in combination with rituximab in non-Hodgkin's lymphoma patients. Additionally, we believe that blocking FcγRIIB on effector cells with an antagonistic mAb will lower their activation threshold, enhancing their cytotoxic potential, as well as potentially augmenting DC activation and cross-priming,3 Figure 1 perhaps suggesting a broader application for antagonistic FcγRIIB mAbs in the clinic, for example, FcγRIIB-ve malignancies.
Figure 1.
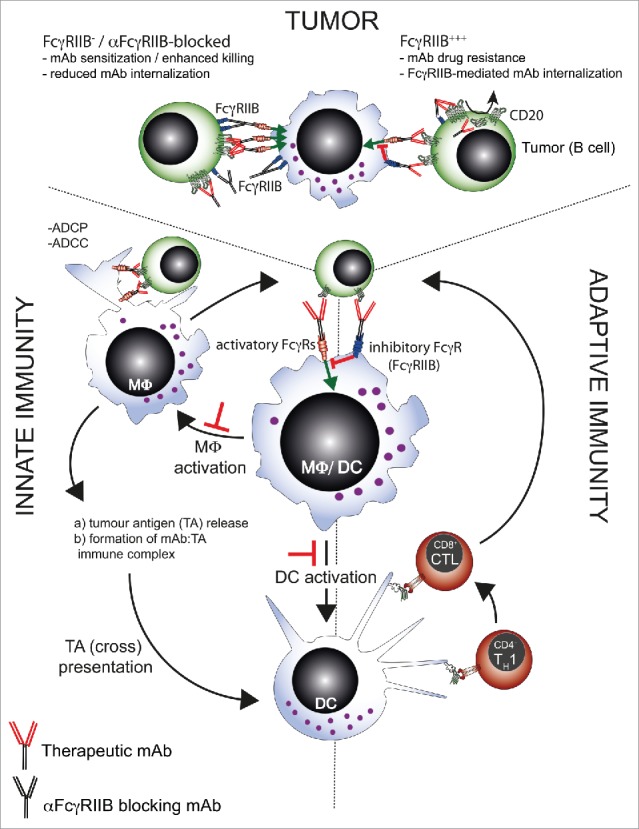
Schematic diagram demonstrating how FcγRIIB can regulate innate and adaptive immunity to influence immunotherapy. FcγRIIB can regulate mAb-mediated immunotherapy at multiple points indicated by the red ┴ symbol. Within the tumor, FcγRIIB can accelerate the internalization of direct targeting mAbs such as rituximab, leading to drug resistance. FcγRIIB can also transmit negative signals to the innate immune cells such as macrophages and dendritic cells, reducing tumor destruction, antigen release and uptake, presentation and activation of adaptive immunity. Targeting of FcγRIIB using anti-FcγRIIB mAbs may therefore potentially intersect at several points o boost mAb-mediated immunotherapy.
Disclosure of potential conflicts of interest
A.R. has received institutional support from BioInvent. M.S.C. acts as a consultant to BioInvent and has received institutional support from BioInvent, Roche and GSK for grants and patents. B.F. is a full-time employee of BioInvent International.
Acknowledgments
We are grateful to all patients and volunteers who generously donated their biological specimens for research, and the clinicians and scientists who collected and processed the clinical samples, respectively. We would like to thank colleagues from the Cancer Sciences Unit, University of Southampton and BioInvent International who provided advice and technical support.
References
- 1.Nimmerjahn F, Ravetch JV. Fcgamma receptors as regulators of immune responses. Nat Rev 2008; 8:34-47; PMID:18064051; http://dx.doi: 10.1038/nri2206 [DOI] [PubMed] [Google Scholar]
- 2.Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nat Med 2000; 6:443-6; PMID:10742152; http://dx.doi.org/ 10.1038/74704 [DOI] [PubMed] [Google Scholar]
- 3.van Montfoort N. 't Hoen PA, Mangsbo SM, Camps MG, Boross P, Melief CJ, Ossendorp F, Verbeek JS. Fcgamma receptor IIb strongly regulates Fcgamma receptor-facilitated T cell activation by dendritic cells. J Immunol 2012; 189:92-101; PMID:22649202; http://dx.doi.org/ 10.4049/jimmunol.1103703 [DOI] [PubMed] [Google Scholar]
- 4.Lim SH, Vaughan AT, Ashton-Key M, Williams EL, Dixon SV, Chan HT, Beers SA, French RR, Cox KL, Davies AJ et al.. Fc gamma receptor IIb on target B cells promotes rituximab internalization and reduces clinical efficacy. Blood 2011; 118:2530-40; PMID:21768293; http://dx.doi.org/ 10.1182/blood-2011-01-330357 [DOI] [PubMed] [Google Scholar]
- 5.Vaughan AT, Iriyama C, Beers SA, Chan CH, Lim SH, Williams EL, Shah V, Roghanian A, Frendéus B, Glennie MJ et al.. Inhibitory FcgammaRIIb (CD32b) becomes activated by therapeutic mAb in both cis and trans and drives internalization according to antibody specificity. Blood 2014; 123:669-77; PMID:24227819; http://dx.doi.org/ 10.1182/blood-2013-04-490821 [DOI] [PubMed] [Google Scholar]
- 6.Cohen-Solal JF, Cassard L, Fournier EM, Loncar SM, Fridman WH, Sautès-Fridman C. Metastatic melanomas express inhibitory low affinity fc gamma receptor and escape humoral immunity. Dermatology Research and Practice 2010; 2010:657406; PMID:20672001; http://dx.doi.org/ 10.1155/2010/657406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pallasch CP, Leskov I, Braun CJ, Vorholt D, Drake A, Soto-Feliciano YM, Bent EH, Schwamb J, Iliopoulou B, Kutsch N et al.. Sensitizing protective tumor microenvironments to antibody-mediated therapy. Cell 2014; 156:590-602; PMID:24485462; http://dx.doi.org/ 10.1016/j.cell.2013.12.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee CS, Ashton-Key M, Cogliatti S, Rondeau S, Schmitz SF, Ghielmini M, Cragg MS, Johnson P. Expression of the inhibitory Fc gamma receptor IIB (FCGR2B, CD32B) on follicular lymphoma cells lowers the response rate to rituximab monotherapy (SAKK 35/98). Br J Haematol 2015; 168:145-8; PMID:25142001; http://dx.doi.org/ 10.1111/bjh.13071 [DOI] [PubMed] [Google Scholar]
- 9.Tipton TR, Roghanian A, Oldham RJ, Carter MJ, Cox KL, Mockridge CI, French RR, Dahal LN, Duriez PJ, Hargreaves PG et al.. Antigenic modulation limits the effector cell mechanisms employed by type I anti-CD20 monoclonal antibodies. Blood 2015; 125:1901-9; PMID:25631769; http://dx.doi.org/ 10.1182/blood-2014-07-588376 [DOI] [PubMed] [Google Scholar]
- 10.Roghanian A, Teige I, Mårtensson L, Cox KL, Kovacek M, Ljungars A, Mattson J, Sundberg A, Vaughan AT, Shah V et al.. Antagonistic human FcgammaRIIB (CD32B) antibodies have anti-tumor activity and overcome resistance to antibody therapy In Vivo. Cancer Cell 2015; 27:473-88; PMID:25873171; http://dx.doi.org/ 10.1016/j.ccell.2015.03.005 [DOI] [PubMed] [Google Scholar]


