ABSTRACT
We recently completed a phase 1 clinical trial demonstrating the safety of a mammaglobin-A DNA vaccine in patients with metastatic breast cancer. We are currently enrolling patients with early stage breast cancer in a phase 1b clinical trial. The mammaglobin-A DNA vaccine will be administered concurrently with neoadjuvant endocrine therapy, providing a unique opportunity to examine the impact of vaccination in the tumor microenvironment.
Keywords: Cancer vaccine, breast cancer, immunotherapy, mammaglobin-A, DNA vaccine, T cells
Cancer vaccines have the potential to induce antitumor immunity, without the side effects of more traditional treatment modalities. Breast cancer is an attractive target for cancer vaccine therapy given the strong evidence of interactions between the immune system and breast cancer.1 For example, the presence of specific immune subsets within the breast cancer tumor microenvironment (TME) has been associated with response to conventional therapies and survival.1-3 In addition, there is increasing evidence that breast cancer vaccine therapy targeting HER2, carcinoembryonic antigen, and mucin-1 can impact clinical outcomes.4,5
Mammaglobin-A (MAM-A) is a 93 amino acid secretoglobin protein that exhibits several characteristics of an ideal antigen for breast cancer vaccine therapy. First, MAM-A is highly expressed in breast cancers but is absent or expressed at very low levels in normal tissues. High MAM-A expression is observed in 40–80% of breast cancers, and in breast cancers of all intrinsic subtypes.6 Second, MAM-A is highly immunogenic. In vitro, MAM-A expressing cells can be used to generate MAM-A-specific CD8+ and CD4+ T cells that are capable of specific recognition and lysis of MAM-A expressing breast cancers.7,8. Of note, MAM-A-specific CD8 T cells have been detected in patients with breast cancer but are absent in patients without disease.7 We have often questioned why the endogenous immune response to MAM-A cannot eliminate developing breast cancers. We hypothesize that MAM-A-specific T cells may be unable to eliminate breast cancers for a variety of reasons: (1) insufficient number of MAM-A-specific T cells, (2) insufficient infiltration of MAM-A-specific T cells into the TME, and (3) downregulation of MAM-A-specific T cells at the TME due to the presence of immunoregulatory elements such as regulatory T cells (Tregs), myeloid-derived suppressor cells (MDSCs) and expression of PD-1/PD-L1 (Fig. 1).
Figure 1.
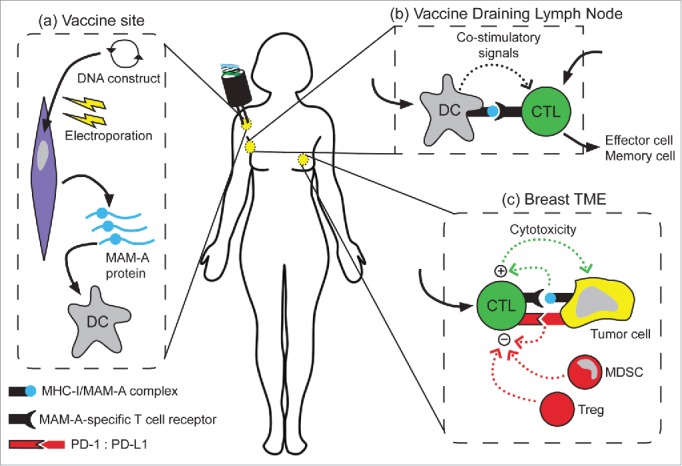
Schematic depiction of the proposed mechanism of action of the MAM-A DNA vaccine. (A) DNA vaccination by electroporation results in expression of mammaglobin-A. Dendritic cells (DC) in the periphery ultimately acquire the recombinant mammaglobin-A protein. (B) DC then transit to the vaccine draining lymph node and prime mammaglobin-A-specific T cells. We have demonstrated that the MAM-A DNA vaccine can successfully induce a MAM-A-specific CD8+ T cell response (CTL). (C) Activated effector cells then migrate to the tumor microenvironment (TME) where they can recognize MHC-I/MAM-A complexes on tumor cells. Immunoregulatory mechanisms such as regulatory T cells (Treg), myeloid-derived suppressive cells (MDSC), and PD-1-PD-L1 interactions may modulate antitumor activity. In our current phase 1B clinical trial, we will examine the functional capacity of CD8+ T cells in the TME, and any potential factors that may modulate their cytotoxic activity.
Previously, we have demonstrated the ability to induce MAM-A specific CD8 T cells in HLA-A2 transgenic mice by DNA vaccination.9 To test the safety and efficacy of this vaccine strategy in breast cancer patients, we recently vaccinated 15 patients with MAM-A+ breast cancers in a phase 1 clinical trial. Subjects were vaccinated on days 1, 29, and 57, and PBMC was collected at various time points up to a year post-vaccination. There were no grade 3 or 4 toxicities reported. Of the 15 patients, four developed flu-like symptoms, one developed vaccine-site tenderness, one developed a rash, and one developed a shingles episode treated with Valtrex.
Immune monitoring studies demonstrated increased numbers of MAM-A-specific T cells in the peripheral blood. In preliminary studies of the first seven patients enrolled, we found an increase in ICOShiCD4+ T cells and a decrease in Foxp3+CD4+ T cells at 6 months post-vaccination in the peripheral blood. These ICOShiCD4+ T cells exhibited a cytotoxic, Th1 phenotype: after vaccination, they expressed higher levels of T-bet and IFN-γ but decreased levels of IL-10.8 In the eight vaccinated patients who expressed HLA-A*0201, we demonstrated an increase in MAM-A-specific CD8 T cells by tetramer analysis as well as IFN-γ enzyme-linked ImmunoSpot (ELISPOT) against the full-length MAM-A protein. Cytotoxic activity of these CD8 T cells was assessed against HLA-A2+, MAM-A+ breast cancer cell lines and was found to be dependent on the target cell expression of HLA-A2 and MAM-A as well as the CD8 T cell expression of TNF-α and IFN-γ. Moreover, on preliminary analysis comparing patients who received vaccination against patients who were not vaccinated because they did not express the required HLA type, vaccination with the MAM-A DNA vaccine was associated with an improved progression-free survival at 6 months (53% vs 33%).10
The efficacy of MAM-A-specific CD8 T cells is not just dependent on expansion of this population, it also requires modulation of the regulatory networks present in the TME. Prior work by our group and others has demonstrated the presence of these regulatory networks in breast cancer patients. Decreased levels of CD68 tumor-associated macrophages and CD4 T lymphocytes with high levels of CD8 T lymphocytes by immunohistochemistry correlated with longer recurrence-free survival.1 Moreover, patients with PD-L1+ breast cancer or PD-L1+ TILs had decreased overall survival across various breast cancer subtypes.2,3 Questions remain about the interaction between vaccination and these regulatory networks present in the breast cancer TME.
We plan to further investigate the safety of the MAM-A DNA vaccine, as well as its ability to induce MAM-A-specific CD8 T cells and impact the breast cancer TME in our phase 1b clinical trial (NCT02204098). Patients with newly diagnosed T2-T4c Nx M0 ER+Her2- breast cancer undergoing neoadjuvant endocrine therapy are eligible for enrollment. This study will provide additional data about the safety and immunogenicity of the MAM-A DNA vaccine, but may also provide important insights into the function of MAM-A-specific T cells in the TME. These analyses may provide important insights into how to optimize the efficacy of the MAM-A DNA vaccine.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.DeNardo DG, Brennan DJ, Rexhepaj E, Ruffell B, Shiao SL, Madden SF, Gallagher WM, Wadhwani N, Keil SD, Junaid SA et al.. Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov 2011; 1(1):54-67; PMID:22039576; http://dx.doi.org/ 10.1158/2159-8274.CD-10-0028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Muenst S, Schaerli AR, Gao F, Däster S, Trella E, Droeser RA, Muraro MG, Zajac P, Zanetti R, Gillanders WE et al.. Expression of programmed death ligand 1 (PD-L1) is associated with poor prognosis in human breast cancer. Breast Cancer Res Treat 2014; 146(1):15-24; PMID:24842267; http://dx.doi.org/ 10.1007/s10549-014-2988-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muenst S, Soysal SD, Gao F, Obermann EC, Oertli D, Gillanders WE. The presence of programmed death 1 (PD-1)-positive tumor-infiltrating lymphocytes is associated with poor prognosis in human breast cancer. Breast Cancer Res Treat 2013; 139(3):667-76; PMID:23756627; http://dx.doi.org/ 10.1007/s10549-013-2581-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Madan RA, Bilusic M, Heery C, Schlom J, Gulley JL. Clinical evaluation of TRICOM vector therapeutic cancer vaccines. Semin Oncol 2012; 39(3):296-304; PMID:22595052; http://dx.doi.org/ 10.1053/j.seminoncol.2012.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mittendorf EA, Alatrash G, Xiao H, Clifton GT, Murray JL, Peoples GE. Breast cancer vaccines: ongoing National Cancer Institute-registered clinical trials. Expert Rev Vaccines 2011; 10(6):755-74; PMID:21692698; http://dx.doi.org/ 10.1586/erv.11.59 [DOI] [PubMed] [Google Scholar]
- 6.Fleming TP, Watson MA. Mammaglobin, a breast-specific gene, and its utility as a marker for breast cancer. Ann N Y Acad Sci 2000; 923:78-89; PMID:11193781; http://dx.doi.org/ 10.1111/j.1749-6632.2000.tb05521.x [DOI] [PubMed] [Google Scholar]
- 7.Jaramillo A, Majumder K, Manna PP, Fleming TP, Doherty G, Dipersio JF, Mohanakumar T. Identification of HLA-A3-restricted CD8+ T cell epitopes derived from mammaglobin-A, a tumor-associated antigen of human breast cancer. Int J Cancer 2002; 102(5):499-506; PMID:12432553; http://dx.doi.org/ 10.1002/ijc.10736 [DOI] [PubMed] [Google Scholar]
- 8.Tiriveedhi V, Fleming TP, Goedegebuure PS, Naughton M, Ma C, Lockhart C, Gao F, Gillanders WE, Mohanakumar T. Mammaglobin-A cDNA vaccination of breast cancer patients induces antigen-specific cytotoxic CD4+ICOShi T cells. Breast Cancer Res Treat 2013; 138(1):109-18; PMID:22678162; http://dx.doi.org/ 10.1007/s10549-012-2110-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bharat A, Benshoff N, Fleming TP, Dietz JR, Gillanders WE, Mohanakumar T. Characterization of the role of CD8+T cells in breast cancer immunity following mammaglobin-A DNA vaccination using HLA-class-I tetramers. Breast Cancer Res Treat 2008; 110(3):453-63; PMID:17874294; http://dx.doi.org/ 10.1007/s10549-007-9741-2 [DOI] [PubMed] [Google Scholar]
- 10.Tiriveedhi V, Tucker N, Herndon J, Li L, Sturmoski M, Ellis M, Ma C, Naughton M, Lockhart AC, Gao F et al.. Safety and preliminary evidence of biologic efficacy of a mammaglobin-a DNA vaccine in patients with stable metastatic breast cancer. Clin Cancer Res 2014; 20(23):5964-75; PMID:25451106; http://dx.doi.org/ 10.1158/1078-0432.CCR-14-0059 [DOI] [PMC free article] [PubMed] [Google Scholar]


