ABSTRACT
Breast cancer is a heterogeneous disease, characterized by several distinct biological subtypes, among which triple-negative breast cancer (TNBC) is one associated with a poor prognosis. Oncolytic virus replication is an immunogenic phenomenon, and viruses can be armed with immunostimulatory molecules to boost virus triggered antitumoral immune responses. Cyclophosphamide (CP) is a chemotherapy drug that is associated with cytotoxicity and immunosuppression at higher doses, whereas immunostimulatory and anti-angiogenic properties are observed at low continuous dosage. Therefore, the combination of oncolytic immuno-virotherapy with low-dose CP is an appealing approach.
We investigated the potency of oncolytic adenovirus Ad5/3-D24-GMCSF on a TNBC cell line and in vivo in an orthotopic xenograft mouse model, in combination with low-dose CP or its main active metabolite 4-hydroperoxycyclophosphamide (4-HP-CP). Furthermore, we summarized the breast cancer-specific human data on this virus from the Advanced Therapy Access Program (ATAP).
Low-dose CP increased the efficacy of Ad5/3-D24-GMCSF in vitro and in a TNBC mouse model. In ATAP, treatments appeared safe and well-tolerated. Thirteen out of 16 breast cancer patients treated were evaluable for possible benefits with modified RECIST 1.1 criteria: 1 patient had a minor response, 2 had stable disease (SD), and 10 had progressive disease (PD). One patient is alive at 1,771 d after treatment.
Ad5/3-D24-GMCSF in combination with low-dose CP showed promising efficacy in preclinical studies and possible antitumor activity in breast cancer patients refractory to other forms of therapy. This preliminary data supports continuing the clinical development of oncolytic adenoviruses for treatment of breast cancer, including TNBC.
KEYWORDS: Breast cancer, combination therapies, cyclophosphamide, GM-CSF, oncolytic adenovirus, oncolytic virotherapy, triple-negative breast cancer
Introduction
Breast cancer is the most frequently diagnosed cancer in women and the second cause of cancer death in women (after lung cancer), with an estimated 40,290 deaths expected in 2015. 1 It is a heterogeneous disease, characterized by several distinct biological subtypes associated with specific biological behavior and different clinical outcomes. 2 TNBC is defined as estrogen receptor (ER)-negative, progesterone receptor (PR)-negative and lacking overexpression of human epidermal growth factor receptor 2 (HER2). 2 TNBC accounts for 10–15% of all breast cancers subtypes and is associated with a worse than average prognosis, due to its aggressive clinical course and insensitivity to many of the treatment modalities available for hormone receptor positive and HER2 positive diseases, including HER2-directed therapy such as trastuzumab and endocrine therapies such as tamoxifen or aromatase inhibitors. 3 TNBC is typically treated with a combination of therapies such as surgery, radiation therapy, and chemotherapy. 4 The relatively aggressive clinical course, poor prognosis, and limited treatment choices highlight the need for research in this patient group. A classic but little explored concept in the treatment of cancer is the use of low-dose metronomic chemotherapy regimens, which aims to ablate the dividing endothelial cells in tumors (preventing angiogenesis) 5 and to alter the immunological tumor microenvironment, thus hindering tumor growth. 6,7 In a phase II study by Colleoni et al., addressing the effects of 50 mg of CP given once daily and 2.5 mg of methotrexate twice daily on patients with metastatic breast carcinoma, the authors concluded that low-dose chemotherapy is possible even in patients with metastatic disease refractory to conventional treatments, it can potentiate the activity of other anticancer drugs and has low toxicity. 8 Currently, several randomized phase III clinical trials that combine metronomic chemotherapy with other antitumor drugs are under way. 9
CP is one of the most common alkylating agents used in the treatment of breast cancer, particularly as an adjuvant agent. 10 In 2004 a study showed that the biological actions of CP are dose-dependent: at higher doses, it is associated with increased cytotoxicity and immunosuppression, while at low continuous dosage it shows immunostimulatory and anti-angiogenic properties. 11 Low-dose CP has been used as adjuvant therapy or in combination with other antitumor therapies. 12 In addition to its anti-angiogenic properties 13,14 and alteration of tumor microenvironment,7 low-dose CP can deplete regulatory T-cells, increasing the immunotherapeutic effect of concomitant treatments. 15 Low-dose CP has been shown to lead to a reduction of regulatory T-cells also in metastatic breast cancer patients. 16 Combination of immunotherapeutic modalities such as oncolytic viruses with regulatory T-cell depletion by low-dose CP has been found efficacious in several preclinical models as well as feasible in cancer patients. 17,18-20
In this study, we have combined the oncolytic adenovirus Ad5/3-D24-GMCSF, a 5/3-capsid chimeric oncolytic adenovirus coding for GM-CSF 18,20-23 with low-dose CP for the treatment of breast cancer, including TNBC.
Results
CP and 4-HP-CP increase efficacy of oncolytic adenovirus
We first investigated the cell killing effect of CP and 4-HP-CP as single agents on MDA-MB-436 TNBC cell line. Two concentrations of CP (0.0075 mg/mL and 0.05 mg/mL) and three of 4-HP-CP (0.001 mg/mL, 0.0025 mg/mL, and 0.005 mg/mL), able to kill 20–40% of cells in vitro (data not shown), were selected for further experiments. Combination of oncolytic adenovirus Ad5/3-D24-GMCSF and CP or 4-HP-CP was then tested in MDA-MB-436 TNBC cell line (Fig. 1). Five days post-infection, combination of 1 to 10 VP/cell of virus with 4-HP-CP results in statistically significant increased cell killing, compared to virus only or 4-HP-CP alone (p < 0.001, p < 0.01, p < 0.05). CP combined with 1 VP/cell of virus, resulted in increased cell killing compared to virus only (p < 0.01), but CP was more effective alone than when combined with low doses of virus, and improvement of efficacy was achieved only when combined with 100 and 1,000 VP/cell of virus (p < 0.01).
Figure 1.
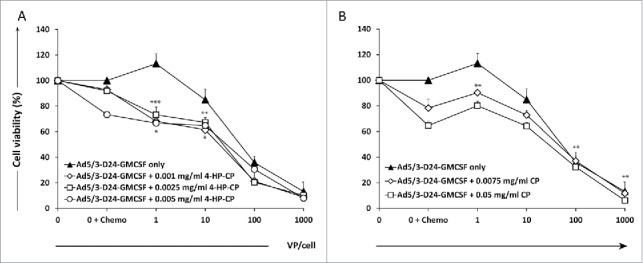
Combination of Ad5/3-D24-GMCSF with 4-hydroperoxycyclophosphamide (4-HP-CP) or cyclophosphamide (CP) increases cell killing of MDA-MB-436 TNBC cells in vitro. Viability of MDA-MB-436 TNBC cells after infection with (A) Ad5/3-D24-GMCSF + CP or (B) Ad5/3-D24-GMCSF + 4-HP-CP. Cell viability was measured 5 d after infection by MTS assay. Viability of mock-infected cells was set as 100%. Means with standard deviations of triplicates are shown. VP, viral particles; CP, cyclophosphamide; 4-HP-CP, 4-hydroperoxycyclophosphamide. (A) * p < 0.05 Virus + 4-HP-CP vs. 4-HP-CP alone; ** p < 0.01 Virus + 4-HP-CP vs. virus alone; *** p < 0.001 Virus + 4-HP-CP vs. virus alone. (B) ** p < 0.01 Virus (1 VP/cell) + CP vs. virus alone; Virus (100 VP/cell and 1,000 VP/cell respectively) vs. CP alone.
Low-dose CP enhances the antitumor efficacy of Ad5/3-D24-GMCSF in an orthotopic TNBC xenograft mouse model
We tested the combination of Ad5/3-D24-GMCSF with low-dose metronomic CP in a MDA-MB-436 TNBC xenograft model in nude mice. Antitumor efficacy was observed only in groups treated with a combination of oncolytic virus (Ad5/3-D24-GMCSF or Ad5/3-D24) and low-dose CP (Fig. 2, Fig. S1). Treatment with Ad5/3-D24 in combination with CP was the most effective (p = 0.018 vs. Ad5/3-D24 alone on day 33 post-infection, p = 0.024 vs. CP alone on day 60 post-infection,) (Fig. S1). Also Ad5/3-D24-GMCSF in combination with CP was significantly more effective than Ad5/3-D24-GMCSF alone (p = 0.002 on day 33 post-infection) and there was also a trend for better efficacy than CP alone (p = 0.144 on day 60 post-infection) (Fig. 2). There was no significant difference between combinations of CP to Ad5/3-D24-GMCSF and Ad5/3-D24, as expected, since human GM-CSF is not biologically active in mice. 24
Figure 2.
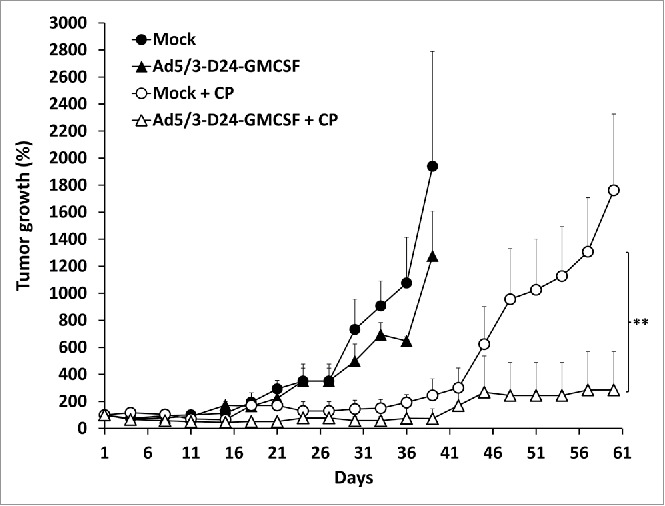
Combination of Ad5/3-D24-GMCSF with low-dose CP displays enhanced antitumor efficacy in a TNBC mouse model. Nude/NMRI mice were inoculated orthotopically into two different mammary fat pad sites with human TNBC cells (MDA-MB-436). When tumors reached the size of approximately 5 mm diameter, they were injected with 7 × 109 VP/tumor of Ad5/3-D24-GMCSF or saline on days 1, 4, 8, 15, 29, 43. Low-dose CP (20 mg/kg) was administered to indicated groups intraperitoneally. Tumor sizes are indicated as percentage respective to day 1, which was set as 100%. Data is presented as means + standard deviations. ** p < 0.01 Ad5/3-D24-GMCSF + CP vs. Ad5/3-D24-GMCSF alone on day 33 post-infection. Tumor sizes were compared between the treatment groups using Mann-Whitney U test with Bonferroni correction. n = 8 tumors/group; n = 10 tumors/mock groups.
Safety of Ad5/3-D24-GMCSF in breast cancer patients
A total of 33 Ad5/3-D24-GMCSF treatments were given to 16 patients with advanced breast cancer, progressing after previous therapies, in the context of an ATAP (Table S1). The most common adverse reactions were grade 1–2 constitutional symptoms (fever, fatigue, and rigors), nausea, transient anemia, and leukocytopenia (Table S2). Grade 3 and 4 adverse reactions were reported for three patients, but none were classified as a SAE (defined as serious adverse events possibly related to the treatment and leading to patient prolongation of hospitalization, malformation or death): patient R328 had grade 3 fever, thrombocytopenia, hyponatremia and aspartate aminotransferase (AST) increase; patient R317 had grade 3 glucose imbalance and grade 4 ketoacidosis, most likely related to her pre-existing diabetes. One patient (R172) had grade 3 pain a few hours after treatment, which was successfully relieved with pain medication and did not cause prolongation of hospitalization. As frequently observed,18,20-22,25 all 16 patients showed a transient decrease in lymphocyte numbers in the peripheral blood, possibly related to redistribution of lymphocytes. 22,26 No treatment related deaths occurred.
Neutralizing antibody titer and presence of Ad5/3-D24-GMCSF genomes in patient serum
At baseline, three out of five evaluable patients had low detectable neutralizing antibody titer against the Ad5/3 capsid (1–4), while one patient had medium titer (256), and one was not evaluable (NE) for baseline titer. After treatment, the titer increased or remained stable in all five patients (Table S3). Ten patients were evaluable for presence of viral genomes in serum. All eight for whom the baseline sample was available were negative for Ad5/3-D24-GMCSF before treatment. After treatment, virus titer increased in most patients and in one patient the titer was highest on day 21 (Table S3).
Possible signs of efficacy of Ad5/3-D24-GMCSF in breast cancer patients were observed
Fourteen out of sixteen patients were imaged with contrast-enhanced computed tomography (CT) and/or [18F]-fluorodeoxyglucose positron emission tomography (PET-CT),27 and treatment response was evaluated according to modified RECIST 1.1. and/or PET criteria.27,28 Serum breast cancer antigen 15–3 (Ca15–3) and carcinoembryonic antigen (CEA) levels were also followed up as possible indicators of treatment outcome in patients who had elevated marker levels at baseline (Table 1). Overall 3 out of 14 patients had some signs of possible treatment benefit. Patient R247 had a radiological MR according to RECIST1.1, with a 10% reduction of tumor diameter, and also partial metabolic response (PMR) (–38%) in PET-CT, after a serial treatment with Ad5/3-D24-GMCSF (imaging was done four weeks after the last virus injection). The same patient showed a partial response (PR) with Ca15–3 marker (–30%). Two patients, R73 and R203, had a SD in CT, and a MR in tumor marker measurements (29% and 18% reduction of Ca15–3 respectively). Patient R248 had stable metabolic disease (SMD) in PET-CT and PD in CT and Ca15–3 measurements due to a new metastasis. Patient R8, who was radiologically in complete response (CR) after two prior virus treatments with other oncolytic adenoviruses (Ad5-D24-GMCSF 29 and ICOVIR-7 30,31), received one treatment with Ad5/3-D24-GMCSF and remained in radiological CR when imaged 7.5 mo after Ad5/3-D24-GMCSF injection. However, the patient was considered NE according to modified RECIST 1.1, due to the lack of measurable lesions when treated with Ad5/3-D24-GMCSF (Table 1). As a response to Ad5/3-D24-GMCSF treatment, the same patient showed a 16% decrease in Ca15–3 marker level following Ad5/3-D24-GMCSF injection, suggesting possible therapeutic efficacy. Of note, TNBC patient R328 had a 22% reduction of injected lesions and 70% decrease of tumor marker CEA suggesting antitumor efficacy. However, formal radiological evaluation results in PMD, due to two new metastases. In summary, of the 13 patients evaluated for tumor response, one patient had a MR/PMR, two a SD, ten a PD/progressive metabolic disease (PMD) (Fig. 3(A), (B)). With regard to tumor markers, of the 13 patients measured for tumor markers levels, one patient had a CR (with CEA), one had a PR (with Ca15–3), five had a MR (three with Ca15–3 and two with CEA), one a SD (with Ca15–3), and five a PD (with Ca15–3). In patient R170, both Ca15–3 and CEA were measured, and discordant responses were obtained (129% increase of Ca15–3 and 10% reduction of CEA marker levels) (Fig. 3(C)).
Table 1.
Clinical responses and patient survival. Patients R184, R195, R317, R328 had triple negative breast cancer.
| Patient code | Number of treatments with Ad5/3-D24-GMCSF | Concomitant CP | Imaging result | Tumor markers | Survival (days)1 |
|---|---|---|---|---|---|
| R8 | 1 | Yes | NE2 | Ca15–3: MR (−16%) | 717 |
| R73 | 2 | Yes | SD3 | 896 | |
| Yes | SD (+2%)4,5 | Ca15–3: MR (−29%) | |||
| R161 | 1 | Yes | Ca15–3: PD (+34%) | 137 | |
| R170 | 1 | Yes | PD (+60%)4,6 | CEA: MR (−10%) Ca15–3: PD (+129%) | 107 |
| R172 | 1 | No | 112 | ||
| R184 | 3 | Yes | PD (+21%)4 | 227 | |
| R195 | 1 | No | PD (+33%)4,6 | 241 | |
| R196 | 1 | No | PD (+47%)4,6 | Ca15–3: PD | 162 |
| R203 | 1 | Yes | SD (+4%)4,6 | Ca15–3: MR (−18%) | 442 |
| R247 | 3 | Yes | PET-CT: PMR (−38%)4 CT: MR (−10%)4 | Ca15–3: PR (−30%) | 195 |
| R248 | 3 | Yes | PET-CT: SMD (+19%)4 CT: PD (+45% +new mets) | Ca15–3: PD (+56%) | 393 |
| R249 | 3 | Yes | PET-CT: PMD (+36%)4 | CEA: MR (−24%) | 497 |
| R255 | 3 | Yes | PET-CT: PMD (+56%)4 | Ca15–3: PD (+29%) | 17717 |
| R256 | 3 | Yes | PET-CT: PMD (+70%)4 CT: PD (new bone mets) | Ca15–3: SD (−5%) | 189 |
| R317 | 3 | Yes | PET-CT: PMD (+54%+new mets)4 | Ca15–3: PD (+74%) | 190 |
| R328 | 3 | Yes | PET-CT: PMD (–22% in injected lesions + two new mets)4 | CEA: CR (–70%) | 239 |
Numbers indicate the survival from their 1st Ad5/3-D24-GMCSF treatment
Patient not evaluable according to modified RECIST 1.1. Patient showed a complete response (CR) after two additional virus treatments prior to Ad5/3-D24-GMCSF and remained in CR when imaged 7.5 mo after Ad5/3-D24-GMCSF treatment.
Patient received one additional virus treatment prior to Ad5/3-D24-GMCSF. Imaging was performed 9.5 weeks after Ad5/3-D24-GMCSF treatment
Serial treatment, imaging was performed after the third treatment
Patient received one additional virus treatment prior to Ad5/3-D24-GMCSF and one after it. Imaging was performed after the third treatment, 6 weeks after Ad5/3-D24-GMCSF injection. New CT 15 weeks after Ad5/3-D24-GMCSF injection: PD (+1.4% + new liver metastasis + a few new lymph nodes in the peritoneum)
Patient received two additional virus treatments after Ad5/3-D24-GMCSF. Imaging was performed after the third treatment.
Patient alive at the end of follow-up (March 2015)
Abbreviations: NE, not evaluable; CP, cyclophosphamide; CT, contrast-enhanced computed tomography; PET-CT, [18F]-fluorodeoxyglucose positron emission tomography; PD, progressive disease; SD, stable disease; MR, minor response; PMD, progressive metabolic disease (in PET); SMD, stable metabolic disease; mets, metastases. Percentage change in tumor diameters and appearance of new lesions indicated for CT and percentage change in summed SUVmax, as well as appearance of new lesions for PET-CT.
Figure 3.
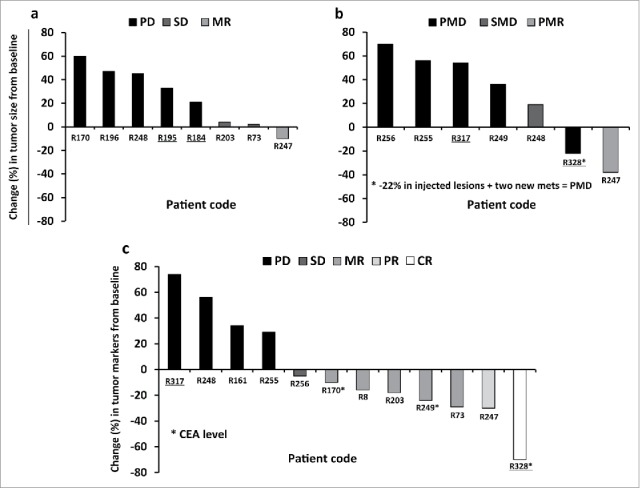
Waterfall plots showing radiological and tumor marker responses after Ad5/3-D24-GMCSF treatments in breast cancer patients. (A,B) Percent change in tumor burden relative to pre-treatment baseline is represented. (A) CT, and (B) PET-CT are shown. (C) Percent change in Ca15–3 or CEA levels (patients R170, R249 and R328) relative to pre-treatment baseline is represented. Patient R196 (PD) is not represented in the graph (percent change was not measured). For patient R170, “best response” is shown. TNBC patients are underlined. Mets, metastases. (A,B) n = 13. Patients R247 and R248 have been imaged with both CT and PET-CT; (C) n = 12.
At the end of the follow up (March 2015) one patient was still alive at 1,771 d post treatment: this patient had a PMD in PET-CT at three weeks after the last Ad5/3-D24-GMCSF treatment, and a PD in the Ca15–3 measurements. Median survival of all treated patients was 233 d after the first Ad5/3-D24-GMCSF treatment (Fig. S2).
Discussion
Oncolytic immunotherapy is an emerging treatment modality for advanced solid tumors refractory to current therapies. The efficacy and safety of oncolytic adenoviruses armed with immunostimulatory cytokines has been studied in preclinical studies, and patients have been treated. 20,29,32 However, the overall conclusion is in line with unarmed oncolytic viruses, suggesting that single agent efficacy would benefit from improvement. 20,29 Previous work has shown that oncolytic viruses can be combined with conventional anticancer treatments, such as chemotherapy, enhancing the efficacy of the viral treatment without significantly increasing side effects. 17,18,23,33-36 In this study we saw an additional cell killing in vitro, and enhanced tumor growth inhibition in TNBC xenografts when Ad5/3-D24-GMCSF was combined with low-dose CP. For the in vitro study, we used both CP, which is a prodrug, and its first active metabolite 4-HP-CP. Both drugs were more efficient in TNBC cell killing when combined with Ad5/3-D24-GMCSF. CP is a prodrug and thus not expected to be active in cells in vitro which lack cytochrome P450 enzymes. 37 There was nevertheless some activity in vitro, suggesting that pathways other than liver enzymes can also activate CP. 38 The cytolytic activity of a virus similar to Ad5/3-D24-GMCSF but without the GM-CSF transgene (Ad5/3-D24) has been previously studied also in other breast cancer cell lines,39 suggesting that the antitumor effect of Ad5/3-D24-GMCSF (keeping in mind that GM-CSF arming has no relevance in vitro) may not be limited to MDA-MB-436 TNBC cells. However, cell line-specific effects cannot be excluded, and further studies on other TNBC cell lines are needed.
Since our in vivo study was conducted in immunodeficient mice, which lack T-cells, the results suggest that low-dose CP had a significant impact on inhibition of tumor growth via a mechanism other than depletion of regulatory T-cells. 7,19 Overall, it can be concluded that Ad5/3-D24-GMCSF displays effective antitumour activity in a TNBC xenograft model with concomitant low-dose metronomic CP, suggesting that adding low-dose metronomic CP may have a useful multifactorial combination effect with the virus, in accordance with previous studies. 18,19 Ad5/3-D24 has been studied in mouse models,40,41 showing promising results in breast cancer studies. 39 In our in vivo study, the results with Ad5/3-D24 were similar to Ad5/3-D24-GMCSF as expected, since human GM-CSF is not active in mice. 24 To assess the possible immune mechanism triggered by the virus and the effects of low-dose CP on tumor vasculature and tumor immunological microenvironment, an immunocompetent animal model susceptible to human transgenes would be appealing. Syrian hamsters have been considered the “best available immunocompetent animal model” for the study of oncolytic adenoviruses, since these animals allow replication of human adenovirus and expression of replication-linked transgenes, such as human GM-CSF. 42,43 Unfortunately, there are currently no specific reagents available that would allow the study of tumor vasculature and immunological cells in Syrian hamsters,21 and thus, further work is required for development of hamster breast cancer model. In humans, previous studies have shown an increase in the total number of survivin-specific CD8+ T-lymphocytes in the blood of patients with advanced solid tumors treated with Ad5/3-D24-GMCSF, suggesting that the virus is able to stimulate a tumor-specific T-cell response. 20,22
In 16 breast cancer patients treated in ATAP, Ad5/3-D24-GMCSF was well-tolerated overall. Three out of 14 imaged patients had tumor shrinkage or disease stabilization after Ad5/3-D24-GMCSF treatments. One patient, who showed a CR after a previous virus treatment, still remained in radiological CR when imaged after Ad5/3-D24-GMCSF treatment. Suggesting that also treatment with Ad5/3-D24-GMCSF contributed to the long progression-free survival seen in this patient, there was decrease in tumor marker Ca15–3. Interestingly, all the patients who seemed to benefit from virus treatment received low-dose CP as concomitant therapy. Two patients have been imaged with both CT and PET-CT on the same day. A previous study suggests that PET using [(18)F]-fluorodeoxyglucose (FDG-PET), which measures tissue metabolism based on quantification of glucose uptake, may be more sensitive than CT for detecting early possible treatment benefit. 27 CT alone would miss many patients possibly benefiting from therapy, since in the case of immunologically active treatments such as oncolytic virus therapy, initial increase in tumor diameter may indicate mounting of an inflammatory response to the treatment (“pseudoprogression”) instead of progression. 44 Tumors may also remain stable for extended periods before eventual shrinkage. Thus, to avoid false interpretations as treatment failure, both CT and PET-CT responses were shown.
Many patients had post-treatment decreases in tumor markers measured from blood. Tumor markers can be sensitive indicators of tumor burden since they can be easily measured from blood, even at short intervals. However, they are considered to be less reliable than imaging in most tumor types but not all. 45,46 Median survival was 233 d and one patient has a long ongoing survival of 1,771 d from her virus treatment, which is unusual for patients with chemotherapy-refractory ductal carcinoma of the breast.
The ATAP patient series included 4/16 TNBC patients. In RECIST analysis, these patients showed PD after treatment. Interestingly one of these patients showed a reduction in the size of injected lesions, suggesting antitumor activity of the virus. However, there were two new metastases. Overall, clinical efficacy in TNBC patients was less dramatic than in the animal experiment, underlining the aggressive nature of this particular breast cancer subtype, and that preclinical studies are poorly predictive of human patient outcomes. Combination with chemotherapy or other immune therapies could be explored to increase efficacy. Also, treatment of earlier-stage patients could yield benefits. Major issues in individualized treatments such as ATAP, which are not a replacement for clinical trials,47,48 are heterogeneity of the treatment and patient populations. Clinical trials with this virus, also known as CGTG-102 and ONCOS-102, are currently ongoing, and could ultimately determine if the safety and efficacy obtained in preclinical experiments are retained in humans. 49
This work supports future clinical application of Ad5/3-D24-GMCSF in combination with metronomic low-dose CP, for the treatment of patients with currently incurable breast cancer, including TNBC. Given promising data obtained also in other indications,18,20,22 Ad5/3-D24-GMCSF may have wide applicability in immunotherapy of cancer.
Materials and methods
Adenoviruses
Ad5/3-D24-GMCSF, Ad5/3-D24, and Ad5/3luc1 have been described previously. 20,50,51 Ad5wt is the wild-type Ad5 strain Ad300, obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). Replication-deficient and replication-competent adenoviruses were purified on cesium chloride gradients according to standard protocols, after propagation on 293 or A549 cells respectively.
Cell lines
Human triple-negative breast adenocarcinoma cell line MDA-MB-436 (ATCC, Manassas, VA, USA) was cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum (FBS, PAA, Cölbe, Germany), 100 IU/mL penicillin and 100 μg/mL streptomycin (both Invitrogen). Cells were grown at humidified 37°C and 5% CO2.
Chemotherapeutic agents and preliminary tests
CP (Sendoxan, Baxter) was purchased from the Helsinki and Uusimaa Hospital District Pharmacy (HUS apteekki, Helsinki, Finland) and diluted in 0.9% sodium chloride (NaCl) solution (B. Braun, Melsungen, Germany). Four-hydroperoxycyclophosphamide (4-HP-CP) was purchased from Niomech – IIT GmbH (Bielefeld, Germany) and diluted in sterile water. MDA-MB-436 cells were seeded in 96-well plates at 10, 000 cells/well in 100 µL of growth medium supplemented with 5 % FBS. After 24 h, cells were treated with serial dilutions of CP and 4-HP-CP. Cell viability was measured 4 d later. Experiments were performed in triplicates. Absorbance was measured at 490 nm using Multiskan Ascent and Ascent Software v2.6 (Thermo Labsystems, Helsinki, Finland). Background absorbance was subtracted and viability of infected cells was calculated in relation to mock-infected cells, whose mean absorbance was defined as 100 % viability.
In vitro combination cytotoxicity assay
MDA-MB-436 TNBC cells were seeded in 96-well plates at 10,000 cells/well in 100 µL of DMEM supplemented with 5 % FBS and 1% penicillin/streptomycin. After 24 h, cells in triplicates were infected with tenfold dilutions (from 1,000 VP/cell to 1 VP/cell) of Ad5/3-D24-GMCSF alone or in combination with different doses of CP (0.0075, 0.05 mg/mL) or 4-HP-CP (0.001, 0.0025, 0.005 mg/mL) or with GM only as control. Infection was done in 100 μL of DMEM supplemented with 2% FBS, and 100 μL of DMEM supplemented with 10% FBS was added 24 h later. Five days post-infection, cell viability was measured using CellTiter 96 Aqueous One Solution Cell Proliferation Assay (Promega, Madison, WI). Absorbance was measured at 490 nm using Multiskan Ascent and Ascent Software v2.6 (Thermo Labsystems, Helsinki, Finland). Background absorbance was subtracted and viability of infected cells was calculated in relation to mock-infected cells, whose mean absorbance was defined as 100 % viability.
Animal experiments
Female nude/NMRI mice (3–4 weeks old) were obtained from Harlan Laboratories (Indianapolis, IN, USA). The animals were quarantined for at least one week. MDA-MB-436 TNBC cells (5 × 106 cells/tumor) were injected orthotopically into two upper most mammary fat pad sites. When tumors reached the size of approximately 5 mm diameter, mice were randomized into 9 groups (4 mice/group; 5 animals for mock groups), and viruses diluted in 0.9% NaCl solution (B. Braun, Melsungen, Germany) were injected intratumorally (i.t.) at 7 × 109 VP/tumor (2 tumors/mouse) on days 1, 4, 8, 15, 29, and 43. Concomitant low-dose CP (Sendoxan, Baxter, CP: 20 mg/kg 18,20) was injected intraperitoneally (i.p.) the day after the first virus injection and every 3 d thereafter. NaCl 0.9% and low-dose CP as single agent were used as mock treatments. Tumor growth was followed by measuring the width and the length of the tumors and approximating the volumes (volume = 0.5 × length × (width)2). The health status of the animals was followed daily during the experiment, and animals were euthanized according to local animal care rules and the humane end-point guidelines. Interim analyses were performed at the time point when animals in all groups treated with virus alone were still alive (Day 33 after first virus treatment), and final analyses were performed when animals from the remaining mock + CP group had to be sacrificed (Day 60 after first virus treatment). Animal experiments were approved by the Experimental Animal Committee of the University of Helsinki and the Provincial Government of Southern Finland.
Patients
A total of 16 patients with chemotherapy-refractory breast cancer (4/16 TNBC, 6/16 ER+ PR+ HER2-, 3/16 ER+ PR-HER2-, 1/16 ER+ PR- HER2+, 2/16 ER+ PR+ HER2 unknown) were treated with Ad5/3-D24-GMCSF in ATAP. 52 ATAP was regulated by Finnish Medicines Agency (FIMEA) as determined by the European Committee (EC) Regulation No 1394/2007 on advanced therapy medicinal products, amending Directive 2001/83/EC and Regulation (EC) No 726/2004 (Table S1). Analysis of patient samples for this retrospective study has been approved by Helsinki University Central Hospital (HUCH) operative ethics committee (HUS 62/13/03/02/2013). Some data from these patients has been published previously, focusing on different aspect of the treatments. 22,27 Here we report new information regarding clinical responses, viral genomes and neutralizing antibody titers in patient serum, focusing on breast cancer patients and Ad5/3-D24-GMCSF treatments.
Patient treatments
Patients received intratumoral (primary tumor and/or metastases), or a combination of intratumoral and intravenous injections of Ad5/3-D24-GMCSF as a single treatment or serial treatment consisting of three virus administrations within 10 weeks. In ATAP, the treatment was personalized for each patient according to the location and size of tumors. In absence of injectable lesions (patient R8), two-third of Ad5/3-D24-GMCSF dose was administered intraperitoneally and one-third was given intravenously. In the case of intrapleural or intraperitoneal disease, injections could be given correspondingly. Two patients had received other virus treatments prior to Ad5/3-D24-GMCSF (Ad5-D24-GMCSF,29 ICOVIR-7,30,31 or Ad5-D24-RGD-GMCSF 32). In the absence of contraindications, patients received concomitant low-dose CP either per oral (50 mg daily, starting one week prior to virus treatment), or by single intravenous bolus (1,000 mg),17 or as combination of these. One patient also received verapamil as concomitant therapy, to increase virus replication. 33 These “virus sensitizers” are not expected to yield antitumor efficacy on their own; instead they were used to increase the effects of the virus. 17,33 Patients were monitored for 24 h in the hospital and 4 weeks as outpatients. Adverse reactions were monitored for 28 d and recorded according to Common Terminology Criteria for Adverse Events (CTCAE) v3.0. Tumor size was assessed by contrast-enhanced CT (CT) scanning after a median of 4 weeks from the latest virus treatment, and maximum tumor diameters were calculated according to modified RECIST v1.1 criteria,27,28 including injected and uninjected lesions. In four cases, [18F]-fluorodeoxyglucose positron emission tomography with a low resolution CT scan (PET-CT) was used instead of contrast-enhanced CT for response evaluation. 27 In three patients, treatment response was evaluated with both CT and PET-CT. Response evaluation criteria are summarized in Supplementary Materials.
Neutralizing antibody titer and detection of viral genomes in patient serum
Neutralizing antibody titer measurements were done using Ad5/3luc1, as described previously 29 and in Supplementary Materials. DNA extraction and real-time PCR to determine viral titers from patient serum samples were performed using primers specific for adenovirus and GM-CSF sequences, as described previously. 20,29
Statistical analysis
Statistics were done with SPSS v18.0 (SPSS, Chicago, IL). Unpaired Student's t-test for independent samples was used to assess combination effect of CP and 4-HP-CP with virus in vitro. Mann-Whitney U test with Bonferroni correction was used to assess tumor volume for nude mice experiment. p-values of <0.05 were considered significant. Kaplan–Meier analysis was used to process survival data.
Disclosure of potential conflicts of interest
A.H. is shareholder in Targovax AS. A.H. is employee and shareholder in TILT Biotherapeutics Ltd. M.S. is employee of TILT Biotherapeutics Ltd.
Acknowledgments
The authors thank all current and previous technicians in the Cancer Gene Therapy Group, and the Docrates and Eira hospital personnel for help and support.
Funding
This study was supported by: Oncos Therapeutics Ltd, Doctoral Program in Biomedicine (DPBM), Doctoral Program in Clinical Research (KLTO), American Society of Clinical Oncology (ASCO) Foundation, HUCH Research Funds (EVO), Sigrid Juselius Foundation, Academy of Finland, Biocentrum Helsinki, Biocenter Finland, Cancer Organizations and University of Helsinki. A.H. is Jane and Aatos Erkko Professor of Oncology at the University of Helsinki.
Supplemental Material
Supplemental Material may be downloaded here: publisher's website
References
- 1.American Cancer Society : Cancer Facts and Figures 2015. Atlanta, Ga: American Cancer Society, 2015. http://www.cancer.org/research/cancerfactsstatistics/cancerfactsfigures2015/ [Google Scholar]
- 2.Sandhu R, Parker JS, Jones WD, Livasy CA, Coleman WB. Microarray-Based gene expression profiling for molecular classification of breast cancer and identification of new targets for therapy. Lab Medicine 2010; 41:364-72; http://dx.doi.org/ 10.1309/LMLIK0VIE3CJK0WD [DOI] [Google Scholar]
- 3. http://www.cancer.gov/types/breast/patient/breast-treatment-pdq
- 4.Kern P, Kalisch A, Kolberg HC, Kimmig R, Otterbach F, von Minckwitz G, Sikov WM, Pott D, Kurbacher C. Neoadjuvant, anthracycline-free chemotherapy with carboplatin and docetaxel in triple-negative, early-stage breast cancer: a multicentric analysis of feasibility and rates of pathologic complete response. Chemotherapy 2013; 59:387-94; PMID:24852315; http://dx.doi.org/ 10.1159/000362756 [DOI] [PubMed] [Google Scholar]
- 5.Browder T, Butterfield CE, Kraling BM, Shi B, Marshall B, O'Reilly MS, Folkman J. Antiangiogenic scheduling of chemotherapy improves efficacy against experimental drug-resistant cancer. Cancer Res 2000; 60:1878-86; PMID:10766175 [PubMed] [Google Scholar]
- 6.Pasquier E, Kavallaris M, Andre N. Metronomic chemotherapy: new rationale for new directions. Nat Rev Clin Oncol 2010; 7:455-65; PMID:20531380; http://dx.doi.org/ 10.1038/nrclinonc.2010.82 [DOI] [PubMed] [Google Scholar]
- 7.Loven D, Hasnis E, Bertolini F, Shaked Y. Low-dose metronomic chemotherapy: from past experience to new paradigms in the treatment of cancer. Drug Discov Today 2013; 18:193-201; PMID:22868084; http://dx.doi.org/ 10.1016/j.drudis.2012.07.015 [DOI] [PubMed] [Google Scholar]
- 8.Colleoni M, Rocca A, Sandri MT, Zorzino L, Masci G, Nole F, Peruzzotti G, Robertson C, Orlando L, Cinieri S et al.. Low-dose oral methotrexate and cyclophosphamide in metastatic breast cancer: antitumor activity and correlation with vascular endothelial growth factor levels. Ann Oncol 2002; 13:73-80; PMID:11863115; http://dx.doi.org/ 10.1093/annonc/mdf013 [DOI] [PubMed] [Google Scholar]
- 9.http://clinicaltrials.gov/.
- 10.Gor PP, Su HI, Gray RJ, Gimotty PA, Horn M, Aplenc R, Vaughan WP, Tallman MS, Rebbeck TR, DeMichele A. Cyclophosphamide-metabolizing enzyme polymorphisms and survival outcomes after adjuvant chemotherapy for node-positive breast cancer: a retrospective cohort study. Breast Cancer Res 2010; 12:R26; PMID:20459744; http://dx.doi.org/ 10.1186/bcr2570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nicolini A, Mancini P, Ferrari P, Anselmi L, Tartarelli G, Bonazzi V, Carpi A, Giardino R. Oral low-dose cyclophosphamide in metastatic hormone refractory prostate cancer (MHRPC). Biomed Pharmacother 2004; 58:447-50; PMID:15464874; http://dx.doi.org/ 10.1016/j.biopha.2004.08.006 [DOI] [PubMed] [Google Scholar]
- 12.Lien K, Georgsdottir S, Sivanathan L, Chan K, Emmenegger U. Low-dose metronomic chemotherapy: a systematic literature analysis. Eur J Cancer 2013; 49:3387-95; PMID:23880474; http://dx.doi.org/ 10.1016/j.ejca.2013.06.038 [DOI] [PubMed] [Google Scholar]
- 13.Man S, Bocci G, Francia G, Green SK, Jothy S, Hanahan D, Bohlen P, Hicklin DJ, Bergers G, Kerbel RS. Antitumor effects in mice of low-dose (metronomic) cyclophosphamide administered continuously through the drinking water. Cancer Res 2002; 62:2731-5; PMID:12019144 [PubMed] [Google Scholar]
- 14.Wang R, Qin S, Chen Y, Li Y, Chen C, Wang Z, Zheng R, Wu Q. Enhanced antitumor and anti-angiogenic effects of metronomic cyclophosphamide combined with Endostar in a xenograft model of human lung cancer. Oncol Rep 2012; 28:439-45; PMID:22641525; http://dx.doi.org/ 10.3892/or.2012.1828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghiringhelli F, Menard C, Puig PE, Ladoire S, Roux S, Martin F, Solary E, Le Cesne A, Zitvogel L, Chauffert B. Metronomic cyclophosphamide regimen selectively depletes CD4+CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol Immunother 2007; 56:641-8; PMID:16960692; http://dx.doi.org/ 10.1007/s00262-006-0225-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ge Y, Domschke C, Stoiber N, Schott S, Heil J, Rom J, Blumenstein M, Thum J, Sohn C, Schneeweiss A et al.. Metronomic cyclophosphamide treatment in metastasized breast cancer patients: immunological effects and clinical outcome. Cancer Immunol Immunother 2012; 61:353-62; PMID:21915801; http://dx.doi.org/ 10.1007/s00262-011-1106-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cerullo V, Diaconu I, Kangasniemi L, Rajecki M, Escutenaire S, Koski A, Romano V, Rouvinen N, Tuuminen T, Laasonen L et al.. Immunological effects of low-dose cyclophosphamide in cancer patients treated with oncolytic adenovirus. Mol Ther 2011; 19:1737-46; PMID:21673660; http://dx.doi.org/ 10.1038/mt.2011.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liikanen I, Ahtiainen L, Hirvinen ML, Bramante S, Cerullo V, Nokisalmi P, Hemminki O, Diaconu I, Pesonen S, Koski A et al.. Oncolytic adenovirus with temozolomide induces autophagy and antitumor immune responses in cancer patients. Mol Ther 2013; 21:1212-23; PMID:23546299; http://dx.doi.org/ 10.1038/mt.2013.51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bramante S, Kaufmann JK, Veckman V et al.. Treatment of melanoma with a serotype 5/3 Chimeric oncolytic adenovirus coding for GM-CSF: results in vitro, in rodents and in humans. Int J Cancer 2015 Oct 1; 137(7):1775-83; PMID:25821063; http://dx.doi.org/ 10.1002/ijc.29536 [DOI] [PubMed] [Google Scholar]
- 20.Koski A, Kangasniemi L, Escutenaire S, Pesonen S, Cerullo V, Diaconu I, Nokisalmi P, Raki M, Rajecki M, Guse K et al.. Treatment of cancer patients with a serotype 5/3 chimeric oncolytic adenovirus expressing GMCSF. Mol Ther 2010; 18:1874-84; PMID:20664527; http://dx.doi.org/ 10.1038/mt.2010.161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bramante S, Koski A, Kipar A, Diaconu I, Liikanen I, Hemminki O, Vassilev L, Parviainen S, Cerullo V, Pesonen SK et al.. Serotype chimeric oncolytic adenovirus coding for GM-CSF for treatment of sarcoma in rodents and humans. In J Cancer 2014; 135:720-30; PMID:24374597; http://dx.doi.org/23493351 10.1002/ijc.28696 [DOI] [PubMed] [Google Scholar]
- 22.Kanerva A, Nokisalmi P, Diaconu I, Koski A, Cerullo V, Liikanen I, Tahtinen S, Oksanen M, Heiskanen R, Pesonen S et al.. Antiviral and antitumor T-cell immunity in patients treated with GM-CSF-coding oncolytic adenovirus. Clin Cancer Res 2013; 19:2734-44; PMID:23493351; http://dx.doi.org/ 10.1158/1078-0432.CCR-12-2546 [DOI] [PubMed] [Google Scholar]
- 23.Siurala M, Bramante S, Vassilev L, Hirvinen M, Parviainen S, Tahtinen S, Guse K, Cerullo V, Kanerva A, Kipar A et al.. Oncolytic adenovirus and doxorubicin-based chemotherapy results in synergistic antitumor activity against soft-tissue sarcoma. Int J Cancer 2015; 136:945-54; PMID:24975392; http://dx.doi.org/ 10.1002/ijc.29048 [DOI] [PubMed] [Google Scholar]
- 24.Shanafelt AB, Johnson KE, Kastelein RA. Identification of critical amino acid residues in human and mouse granulocyte-macrophage colony-stimulating factor and their involvement in species specificity. J Biol Chem 1991; 266:13804-10; PMID:1856212 [PubMed] [Google Scholar]
- 25.Hemminki O, Diaconu I, Cerullo V, Pesonen SK, Kanerva A, Joensuu T, Kairemo K, Laasonen L, Partanen K, Kangasniemi L et al.. Ad3-hTERT-E1A, a fully serotype 3 oncolytic adenovirus, in patients with chemotherapy refractory cancer. Mol Ther 2012; 20:1821-30; PMID:22871667; http://dx.doi.org/ 10.1038/mt.2012.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hemminki O, Parviainen S, Juhila J, Turkki R, Linder N, Lundin J, Kankainen M, Ristimaki A, Koski A, Liikanen I et al.. Immunological data from cancer patients treated with Ad5/3 E2F Delta24 GMCSF suggests utility for tumor immunotherapy. Oncotarget 2015; 6:4467-81; PMID:25714011; http://dx.doi.org/ 10.18632/oncotarget.2901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koski A, Ahtinen H, Liljenback H, Roivainen A, Koskela A, Oksanen M, Partanen K, Laasonen L, Kairemo K, Joensuu T et al.. (18)F-fluorodeoxyglucose positron emission tomography and computed tomography in response evaluation of oncolytic adenovirus treatments of patients with advanced cancer. Hum Gene Ther 2013; 24:1029-41; PMID:24099555; http://dx.doi.org/ 10.1089/hum.2013.123 [DOI] [PubMed] [Google Scholar]
- 28.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M et al.. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45:228-47; PMID:19097774; http://dx.doi.org/ 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 29.Cerullo V, Pesonen S, Diaconu I, Escutenaire S, Arstila PT, Ugolini M, Nokisalmi P, Raki M, Laasonen L, Sarkioja M et al.. Oncolytic adenovirus coding for granulocyte macrophage colony-stimulating factor induces antitumoral immunity in cancer patients. Cancer Res 2010; 70:4297-309; PMID:20484030; http://dx.doi.org/ 10.1158/0008-5472.CAN-09-3567 [DOI] [PubMed] [Google Scholar]
- 30.Rojas JJ, Cascallo M, Guedan S, Gros A, Martinez-Quintanilla J, Hemminki A, Alemany R. A modified E2F-1 promoter improves the efficacy to toxicity ratio of oncolytic adenoviruses. Gene Ther 2009; 16:1441-51; PMID:19710704; http://dx.doi.org/ 10.1038/gt.2009.103 [DOI] [PubMed] [Google Scholar]
- 31.Nokisalmi P, Pesonen S, Escutenaire S, Sarkioja M, Raki M, Cerullo V, Laasonen L, Alemany R, Rojas J, Cascallo M et al.. Oncolytic adenovirus ICOVIR-7 in patients with advanced and refractory solid tumors. Clin Cancer Res 2010; 16:3035-43; PMID:20501623; http://dx.doi.org/ 10.1158/1078-0432.CCR-09-3167 [DOI] [PubMed] [Google Scholar]
- 32.Pesonen S, Diaconu I, Cerullo V, Escutenaire S, Raki M, Kangasniemi L, Nokisalmi P, Dotti G, Guse K, Laasonen L et al.. Integrin targeted oncolytic adenoviruses Ad5-D24-RGD and Ad5-RGD-D24-GMCSF for treatment of patients with advanced chemotherapy refractory solid tumors. Int J Cancer 2012; 130:1937-47; PMID:21630267; http://dx.doi.org/ 10.1002/ijc.26216 [DOI] [PubMed] [Google Scholar]
- 33.Koski A, Raki M, Nokisalmi P, Liikanen I, Kangasniemi L, Joensuu T, Kanerva A, Pesonen S, Alemany R, Hemminki A. Verapamil results in increased blood levels of oncolytic adenovirus in treatment of patients with advanced cancer. Mol Ther 2012; 20:221-9; PMID:22044933; http://dx.doi.org/ 10.1038/mt.2011.230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raki M, Kanerva A, Ristimaki A, Desmond RA, Chen DT, Ranki T, Sarkioja M, Kangasniemi L, Hemminki A. Combination of gemcitabine and Ad5/3-Delta24, a tropism modified conditionally replicating adenovirus, for the treatment of ovarian cancer. Gene Ther 2005; 12:1198-205; PMID:15800658; http://dx.doi.org/ 10.1038/sj.gt.3302517 [DOI] [PubMed] [Google Scholar]
- 35.Ottolino-Perry K, Diallo JS, Lichty BD, Bell JC, McCart JA. Intelligent design: combination therapy with oncolytic viruses. Mol Ther 2010; 18:251-63; PMID:20029399; http://dx.doi.org/ 10.1038/mt.2009.283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khuri FR, Nemunaitis J, Ganly I, Arseneau J, Tannock IF, Romel L, Gore M, Ironside J, MacDougall RH, Heise C et al.. A controlled trial of intratumoral ONYX-015, a selectively-replicating adenovirus, in combination with cisplatin and 5-fluorouracil in patients with recurrent head and neck cancer. Nat Med 2000; 6:879-85; PMID:10932224; http://dx.doi.org/ 10.1038/78638 [DOI] [PubMed] [Google Scholar]
- 37.Sladek NE. Therapeutic efficacy of cyclophosphamide as a function of inhibition of its metabolism. Cancer Res 1972; 32:1848-54; PMID:4641276 [PubMed] [Google Scholar]
- 38.Ginsberg AH, Monte WT, Johnson KP. Effect of cyclophosphamide in vitro and on vaccinia virus replication in tissue culture. J Virol 1977; 21:277-83; PMID:833925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ranki T, Sarkioja M, Hakkarainen T, von Smitten K, Kanerva A, Hemminki A. Systemic efficacy of oncolytic adenoviruses in imagable orthotopic models of hormone refractory metastatic breast cancer. Int J Cancer 2007; 121:165-74; PMID:17315187; http://dx.doi.org/ 10.1002/ijc.22627 [DOI] [PubMed] [Google Scholar]
- 40.Guse K, Ranki T, Ala-Opas M, Bono P, Sarkioja M, Rajecki M, Kanerva A, Hakkarainen T, Hemminki A. Treatment of metastatic renal cancer with capsid-modified oncolytic adenoviruses. Mol Cancer Ther 2007; 6:2728-36; PMID:17938266; http://dx.doi.org/ 10.1158/1535-7163.MCT-07-0176 [DOI] [PubMed] [Google Scholar]
- 41.Kanerva A, Raki M, Ranki T, Sarkioja M, Koponen J, Desmond RA, Helin A, Stenman UH, Isoniemi H, Hockerstedt K et al.. Chlorpromazine and apigenin reduce adenovirus replication and decrease replication associated toxicity. J Gene Med 2007; 9:3-9; PMID:17149790; http://dx.doi.org/ 10.1002/jgm.984 [DOI] [PubMed] [Google Scholar]
- 42.Cohen AM, Hines DK, Korach ES, Ratzkin BJ. In vivo activation of neutrophil function in hamsters by recombinant human granulocyte colony-stimulating factor. Infect Immun 1988; 56:2861-5; PMID:2459064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thomas MA, Spencer JF, La Regina MC, Dhar D, Tollefson AE, Toth K, Wold WS. Syrian hamster as a permissive immunocompetent animal model for the study of oncolytic adenovirus vectors. Cancer Res 2006; 66:1270-6; PMID:16452178; http://dx.doi.org/ 10.1158/0008-5472.CAN-05-3497 [DOI] [PubMed] [Google Scholar]
- 44.Wolchok JD, Hoos A, O'Day S, Weber JS, Hamid O, Lebbe C, Maio M, Binder M, Bohnsack O, Nichol G et al.. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res 2009; 15:7412-20; PMID:19934295; http://dx.doi.org/ 10.1158/1078-0432.CCR-09-1624 [DOI] [PubMed] [Google Scholar]
- 45.Bast RC Jr., Klug TL, St John E, Jenison E, Niloff JM, Lazarus H, Berkowitz RS, Leavitt T, Griffiths CT, Parker L et al.. A radioimmunoassay using a monoclonal antibody to monitor the course of epithelial ovarian cancer. New Engl J Med 1983; 309:883-7; PMID:6310399; http://dx.doi.org/ 10.1056/NEJM198310133091503 [DOI] [PubMed] [Google Scholar]
- 46.Wallace TJ, Torre T, Grob M, Yu J, Avital I, Brucher B, Stojadinovic A, Man YG. Current approaches, challenges and future directions for monitoring treatment response in prostate cancer. J Cancer 2014; 5:3-24; PMID:24396494; http://dx.doi.org/ 10.7150/jca.7709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hemminki A. Treatment of chemotherapy-refractory cancer in the advanced therapy access program. Mol Ther 2012; 20:1654-5; PMID:22945227; http://dx.doi.org/ 10.1038/mt.2012.170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hemminki A, Oksanen M, Merisalo-Soikkeli M. Oncolytic virotherapy trials–letter. Clin Cancer Res 2013; 19:4541-2; PMID:23946419; http://dx.doi.org/ 10.1158/1078-0432.CCR-13-1471 [DOI] [PubMed] [Google Scholar]
- 49. www.oncos.com.
- 50.Kanerva A, Zinn KR, Chaudhuri TR, Lam JT, Suzuki K, Uil TG, Hakkarainen T, Bauerschmitz GJ, Wang M, Liu B et al.. Enhanced therapeutic efficacy for ovarian cancer with a serotype 3 receptor-targeted oncolytic adenovirus. Mol Ther 2003; 8:449-58; PMID:12946318; http://dx.doi.org/ 10.1016/S1525-0016(03)00200-4 [DOI] [PubMed] [Google Scholar]
- 51.Kanerva A, Mikheeva GV, Krasnykh V, Coolidge CJ, Lam JT, Mahasreshti PJ, Barker SD, Straughn M, Barnes MN, Alvarez RD et al.. Targeting adenovirus to the serotype 3 receptor increases gene transfer efficiency to ovarian cancer cells. Clin Cancer Res 2002; 8:275-80; PMID:11801569 [PubMed] [Google Scholar]
- 52.Hemminki A. Oncolytic immunotherapy: where are we clinically? Scientifica 2014; 2014:862925; PMID:24551478; http://dx.doi.org/ 10.1155/2014/862925 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


