ABSTRACT
Treating cancer patients with oncolytic viruses that activate the immune system to fight cancer is an increasingly appealing option. Potency of the approach seems promising while safety has been consistent. Biological correlative data indicates that combining oncolytic immunostimulatory viruses with other existing treatments is tempting and many trials are ongoing.
Observations of human tumors reacting to viral infections have been made for more than a century. Genetic engineering and improved understanding of cancer biology in general and immunology in particular are leading to a new era of cancer therapy. Traditional treatments are being accompanied at an accelerating speed with drugs interacting with the immune system. For scientists, the rational design of oncolytic viruses has been possible for two decades. Initially, oncolysis (rupture of the cell due to virus replication) was regarded as the main mechanism of action but during the last 10 y it has been discovered that the patient´s immune system is a key participant in the response (Fig. 1), leading to many innovative virus modifications to stimulate the immune system further. In April 2015, these developments resulted in an FDA vote of 22-1 in favor of the approval of an oncolytic herpes virus for treatment of melanoma. Talimogene laherparepvec (T-VEC) led to 11% durable complete response rate,1 surpassing even the exciting data provided by checkpoint inhibiting antibodies. Moreover, time to treatment failure was increased from 2.9 to 8.2 mo (P < 0.001).1
Figure 1.
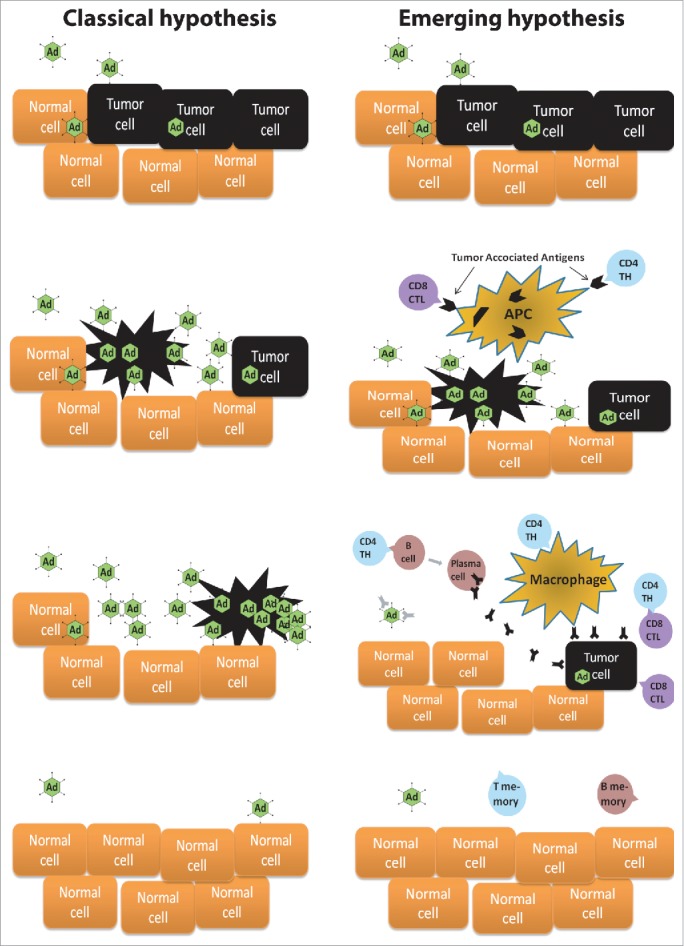
Classical hypothesis of the function of oncolytic virus in patients. Oncolytic viruses reach the tumor by direct injection or blood stream. They infect the tumor cells and start replicating. Oncolysis of a tumor cell cause millions of new virions to be released. New virions spread to neighboring tumor cells and to distant metastasis via the blood stream. Eventually, all tumor has been destructed. Emerging hypothesis of the function of immunostimulatory oncolytic virus in patients. Oncolytic viruses reach the tumor by direct injection or blood stream. They infect the tumor cells and start replicating. Oncolysis of the tumor cells cause inflammation that activates antigen-presenting cells (APC) to phagocytize lysed tumor cell remnants. The APCs present tumor associated antigens to cytotoxic T lymphocytes (CTL) and T helper (TH) cells. All tumor cells are not lysed by the Adenoviruses as the replication and spread is inhibited by the immune system and other mechanisms. APC-stimulated TH cells activate B cells, macrophages and CTLs. Activated B cells produce antibodies against the tumor while macrophages and CTLs have a direct antitumor activity. During this complex process, multiple immunomodulatory-cytokines, -co-receptors and -cells are also utterly important (not shown for clarity). After tumor destruction T-memory and B-memory cells are generated as a sign of antitumor immunity.
Our group at the University of Helsinki has developed oncolytic adenoviruses that have been made safer by genetic modifications while different arming devices have been added to stimulate the immune system. Our adenoviruses are based on serotype 5, serotype 3 or a chimeric 5/3 virus (the fiber knob that is important in the attachment to cells is from serotype 3). Many of the more promising viruses were used to treat cancer patients in an individualized therapy scheme, the Advanced Therapy Access Program: (ATAP), PETCT = Positron emission tomography–computed tomography, between 2007 and 2012.2-5 In general, the adverse events were tolerable and less severe than those related to common chemotherapy.
While these treatments (821 treatments in 290 patients) were able to help many patients, and perhaps the reported data has some interest to the medical community as well, it is key to realize that since patient benefit instead of scientific meticulousness was the goal, ATAP cohorts resemble an N = 1 setting more than a rigorous phase-2 clinical trial setting. Nevertheless, it can be summarized that all of the 10 used viruses were able to cause oncolysis and immune response while case examples of efficacy were repeatedly seen in patients.2-6 Viruses were given safely intravenously and intratumorally. Induction of neutralizing antibodies was seen regularly after treatments, but this is of unknown clinical relevance as there were examples of patient benefit in all classes of patients: those who had antibodies at baseline or not, and those whose antibodies were induced or not.2-6 Based on quantitation of viral genomes in blood, oncolytic replication seems to taper off after several days or weeks in some cases, and repeated doses result in smaller replication peaks. However, there were frequent examples of patients where replication seemed to persist for months, but this did not correlate with efficacy or lack thereof. Neutralization can be circumvented by switching the serotype; again with lack of clear clinical significance.7 The 50-y old notion of neutralizing antibodies being the key determinant of oncolytic virus efficacy did not correlate with the clinical ATAP experience. An explanation for this finding could be that anti-viral antibodies help in induction of danger signaling at the tumor, breaking immunosuppressive tolerance, thus contributing to antitumor immunity.2-5 Moreover, viruses may be able to hide from antibodies by using cells as stealth vehicles, or the mere quantity of virions produced by large tumors may simply overwhelm the opsonizing capacity of humoral immunity. Over and above antibodies, there might be also other relevant differences between different adenoviral serotypes.2,8
Many patients seemed to show signs of antitumor efficacy or surprisingly long survival.5 The best results seemed to associate with patients treated with GM-CSF or CD40L armed viruses4 or viruses based on serotype 32. With these viruses ca. 2/3 of the patients showed a decrease in tumor markers and/or stable disease or better in imaging. For most patients, the results seemed to be temporary lasting some months while a smaller proportion stabilized for years.2-5,7 Of note, many patients progressed while off therapy encouraging prolonged continuation of treatment.
Given the promising safety and efficacy data from ATAP, it was important that funding could be raised for a university spin-out company which completed a clinical phase I trial with one of the GM-CSF armed viruses. Oncos Therapeutics was recently successful in merging with Targovax, another immunotherapy company, which seems to ensure continuation of clinical testing (www.oncos.com/www.targovax.com).
Traditionally, cancer therapy responses have been evaluated mainly by CT imaging developed for the apoptotic response resulting from chemotherapy. However, with immunological therapies inflammatory tumor swelling is common and responses may emerge only months later. PETCT or MRI may have some advantages but they are not perfect.9,10 Clearly, however, immunological treatments require different endpoints from chemotherapy.
Changes in the quantity of tumor reactive T cells were generally seen in peripheral blood after treatments but blood data is confused by the overlapping phenomena of T-cell induction and trafficking to target tissues. Moreover, activity may be more important than cell number.2,3 More interestingly, data from patient tumor biopsies suggests variation in the immunological status of tumors, characterized by differences in cellular infiltrates.3 Preliminary results seem to suggest that tumors with a low amount of immune cells seem to react more clearly to treatment.3 We hypothesized that tumors with a high amount of immune cells indicates that the tumor is already recognized by the immune system but activation of effector cells is blocked by tumor mediated immunosuppression, facilitating combination treatment with checkpoint inhibitors.3
In summary, while signs of efficacy are regularly seen and safety has been consistent, oncolytic immunotherapy is rarely curative as a single agent. Therefore, combinations with traditional therapies seem rational. Co-emergence of PD1 and virus resistance immediately suggests interesting combinations now being tested in patients. Finally, understanding the immunological status of tumors could be important in optimizing treatments on a patient level. The excellent safety and multifaceted activity of oncolytic viruses pave the way for a multitude of tantalizing trial set-ups.
Disclosure of potential conflicts of interest
O.H. and A.H. are shareholders in Oncos Therapeutics Ltd and TILT Biotherapeutics Ltd. A.H. is an employee of TILT Biotherapeutics Ltd.
Funding
National Graduate School of Clinical Investigation, University of Helsinki, Sigrid Juselius Foundation, Biocentrum Helsinki, Biocenter Finland, Finnish Cancer Organizations. A.H. is Jane and Aatos Erkko Professor of Oncology at the University of Helsinki.
References
- 1.Andtbacka RH, Kaufman HL, Collichio F, Amatruda T, Senzer N, Chesney J, Delman KA, Spitler LE, Puzanov I, Agarwala SS et al.. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J Clin Oncol. 2015 Sep 1; 33(25):2780-8; PMID:26014293; http://dx.doi.org/ 10.1200/JCO.2014.58.3377 and DOI: [DOI] [PubMed] [Google Scholar]
- 2.Hemminki O, Diaconu I, Cerullo V, Pesonen SK, Kanerva A, Joensuu T, Kairemo K, Laasonen L, Partanen K, Kangasniemi L et al.. Ad3-hTERT-E1A, a fully Serotype 3 oncolytic adenovirus, in patients with chemotherapy refractory cancer. Mol Ther. 2012. Sep; 20(9):1821-30; PMID:22871667; http://dx.doi.org/25714011 10.1038/mt.2012.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hemminki O, Parviainen S, Juhila J, Turkki R, Linder N, Lundin J, Kankainen M, Ristimaki A, Koski A, Liikanen I et al.. Immunological data from cancer patients treated with Ad5/3-E2F-Delta24-GMCSF suggests utility for tumor immunotherapy. Oncotarget 2015; 6:4467-81; PMID:25714011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liikanen I, Koski A, Merisalo-Soikkeli M, Hemminki O, Oksanen M, Kairemo K, Joensuu T, Kanerva A, Hemminki A. Serum HMGB1 is a predictive and prognostic biomarker for oncolytic immunotherapy. Oncoimmunology 2015; 4:e989771; PMID:25949903; http://dx.doi.org/ 10.4161/2162402X.2014.989771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kanerva A, Koski A, Liikanen I, Oksanen M, Joensuu T, Hemminki O, Palmgren J, Hemminki K, Hemminki A. Case-control estimation of the impact of oncolytic adenovirus on the survival of patients with refractory solid tumors. Mol Ther 2015; 23:321-9; PMID:25381801; http://dx.doi.org/ 10.1038/mt.2014.218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nokisalmi P, Pesonen S, Escutenaire S, Sarkioja M, Raki M, Cerullo V, Laasonen L, Alemany R, Rojas J, Cascallo M et al.. Oncolytic adenovirus ICOVIR-7 in patients with advanced and refractory solid tumors. Clin Cancer Res 2010; 16:3035-43; PMID:20501623; http://dx.doi.org/ 10.1158/1078-0432.CCR-09-3167 [DOI] [PubMed] [Google Scholar]
- 7.Kanerva A, Nokisalmi P, Diaconu I, Koski A, Cerullo V, Liikanen I, Tahtinen S, Oksanen M, Heiskanen R, Pesonen S et al.. Antiviral and antitumor T-cell immunity in patients treated with GM-CSF-coding oncolytic adenovirus. Clin Cancer Res 2013; 19:2734-44; PMID:23493351; http://dx.doi.org/ 10.1158/1078-0432.CCR-12-2546 [DOI] [PubMed] [Google Scholar]
- 8.Lu ZZ, Wang H, Zhang Y, Cao H, Li Z, Fender P, Lieber A. Penton-dodecahedral particles trigger opening of intercellular junctions and facilitate viral spread during adenovirus serotype 3 infection of epithelial cells. PLoS pathogens 2013; 9:e1003718; PMID:24204268; http://dx.doi.org/ 10.1371/journal.ppat.1003718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koski A, Ahtinen H, Liljenback H, Roivainen A, Koskela A, Oksanen M, Partanen K, Laasonen L, Kairemo K, Joensuu T et al.. [(18)F]-fluorodeoxyglucose positron emission tomography and computed tomography in response evaluation of oncolytic adenovirus treatments of patients with advanced cancer. Hum Gene Ther 2013; 24:1029-41; PMID:24099555; http://dx.doi.org/ 10.1089/hum.2013.123 [DOI] [PubMed] [Google Scholar]
- 10.Hemminki O, Immonen R, Narvainen J, Kipar A, Paasonen J, Jokivarsi KT, Yli-Ollila H, Soininen P, Partanen K, Joensuu T et al.. In vivo magnetic resonance imaging and spectroscopy identifies oncolytic adenovirus responders. Inter J Cancer J Inter du Cancer 2014; 134:2878-90; PMID:24248808;http://dx.doi.org/ 10.1002/ijc.28615 [DOI] [PubMed] [Google Scholar]


