ABSTRACT
Estrogen receptors have recently been demonstrated at the cell surface. Unlike nuclear receptors, they are able to trigger rapid responses inside the cells. In this study, we evaluated the presence and the possible role of autoantibodies specific to estrogen receptor (anti-ER Abs) in the peripheral blood of breast cancer patients. Anti-ERα Abs were detectable in 22/48 (46%) patients' sera and their levels positively correlated with the percentage of Ki-67-positive breast cancer cells. Anti-ERα Abs purified from breast cancer patients' sera were able: (i) to recognize ERα epitopes expressed at the cell surface of ER-positive breast cancer cells, (ii) to trigger rapid extracellular signal-regulated kinase (ERK) phosphorylation, and (iii) to induce cell proliferation. Our results suggest that anti-ERα Abs can act as estrogen agonists playing a pathogenetic role as breast cancer-promoting factors. These autoantibodies could also be considered as possible peripheral blood biomarkers indicative of the breast cancer growth potential.
KEYWORDS: Autoantibodies, breast cancer, biomarkers, estrogen receptor, proliferation
The growth and proliferation of breast tissue are normally responsive to estrogen (17β estradiol, E2), a steroid hormone that binds to and activates the estrogen receptor (ER)α and ERβ. ERs function as nuclear transcription factors that regulate the expression of genes coordinating survival and proliferation.1 In many neoplastic breast cells the ER signaling network contributes to controlling the relative rates of cell proliferation and programmed cell death, with pro-survival and proliferation signals overwhelming pro-death and quiescence signals.2 More than 70% of breast tumors express ERα (ER+) at diagnosis and many of these tumors are initially responsive to hormonal therapy.3 The selective estrogen receptor modulator (SERM) tamoxifen, which binds to and neutralizes ERα, is the most commonly used treatment for patients with ER+ breast cancer.4 However, the application of this therapy appears to be still limited by the occurrence of drug resistance.5 A better comprehension of the ER signaling network could provide novel and useful insights into the pathogenesis and response to therapy of breast cancer. In this regard, the discovery of membrane-associated ER (mER) has greatly expanded our understanding of estrogen action.6-8 Upon ligand binding, mER rapidly activates different signaling pathways (e.g., protein kinase cascades) influencing downstream transcription factors. In ER+ MCF-7 breast cancer cells, the activation of mERα by membrane-impermeant estrogen dendrimer conjugates has been shown to stimulate nongenomic responses,9 also contributing to the regulation of breast cancer cell number.10 In this context, the identification of autoantibodies reacting with ERs and their possible pathogenic role in cancer have made this scenario even more complex, opening a new path for the mechanistic research in the exploring estrogen-related receptor activity.11-14
Anti-ER autoantibodies (anti-ER Abs) were originally described in sera from patients with autoimmune disorders.15-17 More recently, our research group demonstrated the presence of anti-ERα Abs (but not of anti-ERβ Abs) in sera from patients with two paradigmatic autoimmune diseases, characterized by a female-to-male ratio ranging from 3:1 to 14:1, i.e., systemic lupus erythematosus and systemic sclerosis,13,14 in whose pathogenesis E2 has been supposed to play a key role. These antibodies behave as true ERα agonists activating extracellular signal-regulated kinase (ERK) signaling and significantly correlate with disease activity and severity. At the present time, little is known about the role of anti-ER Abs in breast cancer. Pioneer studies showed that anti-ER Abs were able to displace estradiol from its complex with ER and to induce an estrogenic response in MCF-7 cells, producing an ER downregulation.11,12 Aim of this study was to evaluate the presence and levels of anti-ER Abs in sera from patients with breast cancer and their correlation with clinical features. The specific binding and the effect of anti-ER Abs as estrogen agonists on human breast cancer cells were also investigated.
Results and discussion
Anti-ERα autoantibodies are detectable in sera from patients with breast cancer
Cancer patients develop an immune humoral response against tumor-associated antigens.17 Autoantibody responses have been mainly associated to cancers with an elevated inflammatory component such as breast cancer. In this study, we evaluated the presence of anti-ER Abs in sera from 48 patients with newly diagnosed early breast cancer before any pharmacological treatment, by an immunoenzymatic assay previously described.13,14 Our analysis demonstrated the presence of anti-ERα Abs in 22/48 (46%) sera from breast cancer patients, independently of their age and of their median body mass index (BMI) (Table 1). No positivity was detected for anti-ERβ Abs in these sera. Sera from healthy donors were all negative for both anti-ERα and anti-ERβ Abs. The initial events that lead to the production of anti-ERα Abs are still unknown, but the chronic inflammation during the breast cancer development could play a role. The presence of serum anti-ERα Abs was independent of ER, progesterone receptor (PgR) and human epidermal growth factor receptor 2 (HER2) breast cancer expression (Table 1). The occurrence of anti-ERα Abs in both ER positive (17/38) and ER negative (5/10) patients let us to hypothesize that these autoantibodies could develop in early stage of carcinogenesis, before the loss of ER expression in breast cancer cells. Whether the autoantibody itself could play a role in this loss of expression, thus contributing to the onset of antiestrogen therapy-resistant tumor cells, needs further investigation. In addition to ER, PgR and HER2 expression, classical clinico-pathological features of breast cancer indicating patient prognosis include tumor stage and grading. Accordingly, we investigated the possible correlation of these parameters with anti-ERα Abs to assess their potential prognostic value. Dividing the patients on the basis of the stage of disease, we found that 6 out of the 13 patients in stage I, 13/24 in stage II and 3/11 in stage III were positive for serum anti-ERα Abs. The analysis of tumor grading indicated that 10 out of the 22 patients classified as G2 and 12/26 patients classified as G3 were positive for serum anti-ERα Abs. No differences were also found between anti-ERα Abs levels and stage or grading of the tumors (Fig. 1A and B). Notably, on the other hand, a significant positive correlation between serum anti-ERα Abs levels and the percentage of Ki-67-positive cells in breast cancer tissue samples was found (Fig. 1C). Uncontrolled proliferation is a hallmark of malignancy and in breast cancer the immunohistochemical assessment of Ki-67 protein, a nuclear marker expressed during all the active phases of the cell cycle but absent in resting cells, has become the most widely used method for measuring the proliferative status of tumor cells.18 Our findings suggest a role for anti-ERα Abs in cancer cell proliferation and future studies are needed to better elucidate the interplay between Ki-67 antigen and anti-ERα Abs considering the role for Ki-67 as an independent prognostic parameter for disease-free survival and overall survival in breast cancer patients.19
Table 1.
Clinico-pathological features and anti-ERα Abs in patients with breast cancer. Anti ERα Abs are reported as OD490 value, in bold are represented the value > of cut-off (0.45) measured as reported in Methods.
| N. | Age (Years) | BMI | HER2 | ER | PgR | Stage | Grading | Ki-67 | anti-ERα Abs |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 54 | 22.1 | − | + | + | II | G2 | 10% | 0.50 |
| 2 | 55 | 27 | − | − | − | I | G2 | 65% | 0.43 |
| 3 | 36 | 19.2 | − | + | − | I | G3 | 25% | 0.46 |
| 4 | 59 | 21.1 | − | − | − | I | G3 | 25% | 0.67 |
| 5 | 70 | 30.1 | − | + | + | IIIA | G3 | n/a | 0.19 |
| 6 | 69 | 26 | − | − | − | I | G3 | 70% | 0.70 |
| 7 | 50 | 23.8 | + | − | − | I | G2 | 35% | 0.78 |
| 8 | 52 | 30.5 | + | + | + | II | G2 | 10% | 0.54 |
| 9 | 60 | 27.3 | − | + | + | IIIA | G2 | <5% | 0.36 |
| 10 | 49 | 30.8 | − | + | + | II | G3 | 5% | 0.35 |
| 11 | 34 | 20.4 | + | + | + | I | G3 | 20% | 0.22 |
| 12 | 53 | 25.4 | − | + | + | IIB | G2 | 15% | 0.52 |
| 13 | 54 | 38.2 | − | + | + | IIA | G2 | 1% | 0.40 |
| 14 | 56 | 26.7 | − | − | − | I | G3 | 20% | 0.41 |
| 15 | 64 | 21.8 | − | − | − | I | G3 | 15% | 0.00 |
| 16 | 73 | 20.4 | − | − | − | I | G3 | 50% | 0.68 |
| 17 | 49 | 21.2 | − | + | + | II | G3 | 50% | 0.60 |
| 18 | 72 | 28.8 | − | + | + | IIIA | G3 | 20% | 0.25 |
| 19 | 59 | 24.7 | − | − | − | IIIA | G3 | 30% | 0.70 |
| 20 | 49 | 27.6 | + | + | + | IIA | G3 | 15% | 0.66 |
| 21 | 53 | 29.6 | − | + | + | IA | G2 | 20% | 0.48 |
| 22 | 56 | 22.2 | − | + | + | II | G3 | 20% | 0.51 |
| 23 | 65 | 29.4 | + | + | + | II | G3 | 20% | 0.35 |
| 24 | 42 | 25.3 | − | + | + | IIIA | G2 | 10% | 0.20 |
| 25 | 64 | 24 | − | + | + | II | G3 | 20% | 0.57 |
| 26 | 62 | 18.9 | − | + | + | IIB | G2 | n/a | 0.29 |
| 27 | 54 | 27.8 | − | + | + | II | G2 | 5% | 0.00 |
| 28 | 43 | 19.8 | − | + | + | IIIA | G2 | 10% | 0.39 |
| 29 | 49 | 24 | − | + | + | II | G2 | 25% | 0.52 |
| 30 | 39 | 20.7 | − | + | + | IIIA | G2 | 5% | 0.34 |
| 31 | 63 | 20.6 | + | − | − | I | G3 | 20% | 0.25 |
| 32 | 45 | 24.3 | + | + | + | II | G2 | 30% | 0.61 |
| 33 | 48 | 20.2 | − | + | + | IIIA | G3 | 30% | 0.36 |
| 34 | 49 | 27.2 | − | + | + | II | G3 | 25% | 0.12 |
| 35 | 46 | 29.4 | − | + | + | I | G2 | 25% | 0.43 |
| 36 | 50 | 19.8 | − | + | + | IIB | G2 | 40% | 0.55 |
| 37 | 53 | 26.2 | − | + | + | IIB | G3 | 5% | 0.16 |
| 38 | 46 | 25.1 | − | + | + | IIB | G3 | 10% | 0.63 |
| 39 | 45 | 28 | − | + | + | IIB | G2 | 15% | 0.48 |
| 40 | 37 | 25 | + | + | + | IIIC | G2 | 12% | 0.58 |
| 41 | 49 | 29.4 | + | + | + | II | G3 | 30% | 0.74 |
| 42 | 56 | 24.2 | − | + | + | II | G2 | 25% | 0.33 |
| 43 | 55 | 27.6 | − | − | − | IIB | G3 | 35% | 0.39 |
| 44 | 45 | 21.3 | − | + | − | IIA | G3 | 25% | 0.33 |
| 45 | 49 | 18.4 | + | + | + | IB | G2 | 15% | 0.30 |
| 46 | 71 | 24.9 | − | + | + | IIIC | G3 | 10% | 0.54 |
| 47 | 40 | 23.5 | − | + | + | IIIA | G2 | 40% | 0.31 |
| 48 | 67 | 22.8 | − | + | + | II | G3 | 25% | 0.24 |
Figure 1.
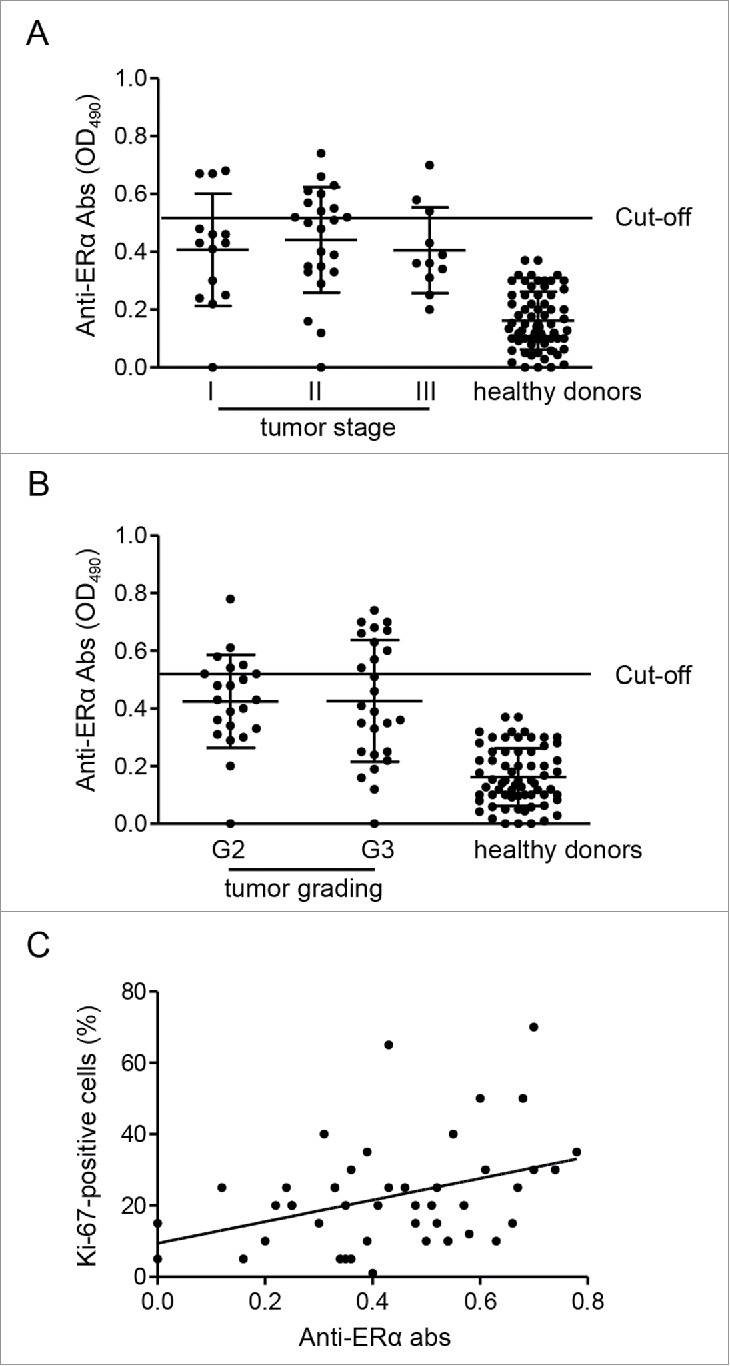
Association of anti-ERα Abs levels with tumor characteristics. Means ± SD of anti-ERα Abs levels (OD490) in patients divided on the basis of tumor stage (A) and grading (B). (C) Correlation and linear regression analysis of the percentage of tumor cells that are positive for Ki-67 protein and serum anti-ERα Abs levels (Spearman's rho, r = 0.35; P = 0.015).
Summarizing, we found that (i) anti-ERα Abs are detectable in patients with breast cancer, and (ii) their levels were correlated with the proliferation index of breast cancer cells, but not with the classical, established prognostic markers. To note, since a significant anti-ERα Ab positivity was also found in patients with autoimmune diseases such as systemic lupus erythematosus or systemic sclerosis,13,14 the possibility that anti-ERα Abs could exert pathogenetic roles in determining the gender prevalence of these diseases in females cannot be ruled out.
Epitope mapping
To identify the epitopes recognized by anti-ERα Abs, we performed aPEPperMAP® Epitope Mapping (PEPperPRINT) of human affinity purified anti-ERα Abs against ERα. The sequence of ERα was translated into 15 aa peptides with a peptide-peptide overlap of 14 aa. The resulting microarrays contained 444 different peptides printed in duplicate (888 peptide spots). A clear epitope-like spot pattern formed by a row of neighbored peptides with the consensus motif YTFLSSTLKSLEE was shown (Fig. 2A, underlined). To note, the peptide is located in ligand binding domain of the ERα (see ref.1) (Fig. 2A, bold).
Figure 2.
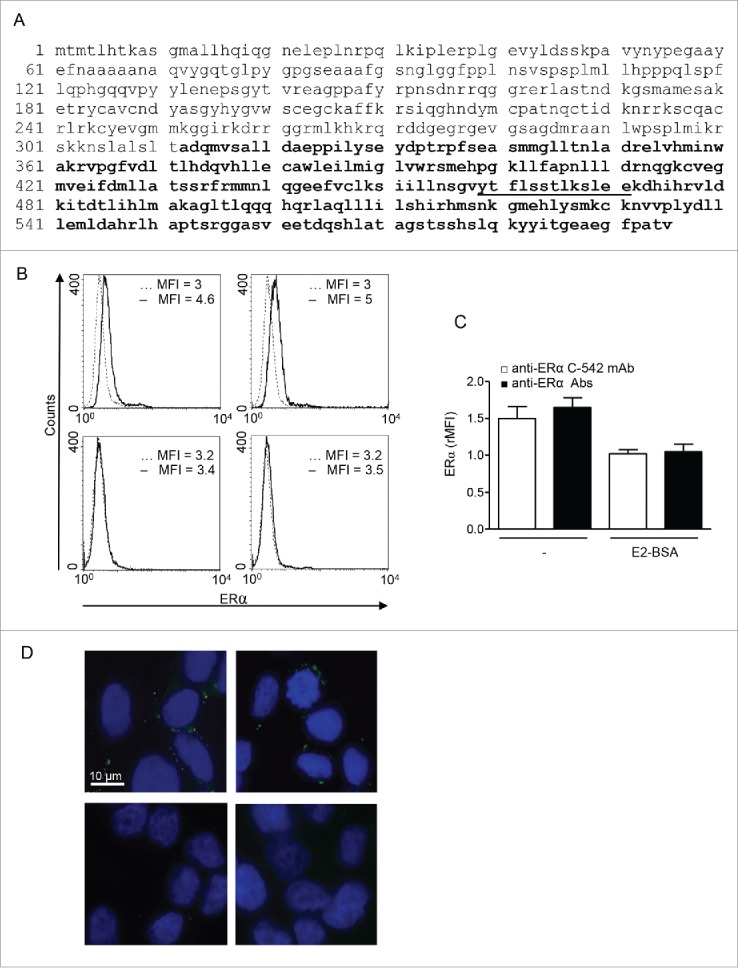
Anti-ERα Abs reacted to binding domain of mERα on MCF-7 breast cancer cells. (A) Amino acid sequence of ERα (UniProtKB/Swiss-Prot: P03372.2). aPEPperMAP® Epitope Mapping (PEPperPRINT) defined the main reactive epitope (underlined) into the ligand binding domain (bold). (B) Representative flow cytometric analysis of mERα in MCF-7 cells by anti-ERα C-542 mAb (left panels) and human purified anti-ERα Abs (right panels) in untreated- (upper panels) or E2-BSA-treated (bottom panels) cells. Isotype control staining is represented by the broken line and anti-ERα-labeled cells are represented by the solid line. MFI, mean fluorescence intensity. A representative experiment out of five is shown. (C) Values of mERα/isotype mean fluorescence intensity ratio are reported (rMFI). Data are expressed as mean ± SD of five independent experiments. (D) Immunofluorescence analysis of mERα in MCF-7 cells by anti-ERα C-542 mAb (left panels) and human purified anti-ERα Abs (right panels) in untreated- (upper panels) or E2-BSA-treated (bottom panels) cells.
Anti-ERα autoantibodies purified from patients' sera recognized ER expressed on the cell surface of ER positive MCF-7 human breast cancer cells
Flow cytometry (Fig. 2B and C) and immunofluorescence (Fig. 2D) analyses showed that the commercially available C-542 mAb specific to ERα (Fig. 2B and D, upper left panels) and anti-ERα Abs purified from breast cancer patients' sera (Fig. 2B and D, upper right panels) were able to recognize ERα epitopes expressed at the cell surface of ER+ MCF-7 breast cancer cells. ER negative MDA-MB-231 cells did not reveal any positivity for mERα staining by human purified anti-ERα Abs (not shown). Preincubation of MCF-7 cells with membrane non-permeant E2 conjugated with bovine serum albumin (E2-BSA), which is not able to enter the cell, significantly decreased mERα staining, confirming antibody specificity for surface ER (Fig. 2B and D, bottom panels).
Anti-ERα autoantibodies are effective in stimulating nongenomic estrogen action
We monitored the time course of ERK phosphorylation by anti-ERα Abs purified from patients' sera in MCF-7 cells (Fig. 3A). ERK phosphorylation occurred rapidly upon anti-ERα Abs treatment. Maximal phosphorylation was observed at 5 min, with a decline thereafter, returning to the control levels at 30 min. Control human antibodies (IVIG) did not exert any effect on ERK phosphorylation (Fig. S1). Moreover, we found that the ER negative MDA-MB-231 cells did not show any enhancement of ERK phosphorylation after treatment with anti-ERα Abs (Fig. S2). To investigate if anti-ERα Abs could also activate the PI3K/Akt/mTOR pathway, recently suggested to represent a target in ER positive breast cancer,20 we evaluated the level of Akt phosphorylation after autoantibody treatment. However, Akt phosphorylation showed only a slight not significant tendency to increase after 15–30 min (Fig. 3B). We also analyzed whether anti-ERα Abs modulate total expression levels of ERK and Akt. The anti-ERα Ab treatment did not affect the total expression levels of ERK and Akt both at 5 and 30 min (Fig. 3A and B) and after 24 h (data not shown). Hence, anti-ERα Abs seem to be able to act as mERα agonists inducing the rapid activation of ERK. To note, 24 h treatment with anti-ERα Abs induced a 1.7-fold increase of cell proliferation (Fig. 3C), without modifying apoptosis levels (annexin V-positive cells were below 12% in untreated as well as in treated cells, data not shown). In conclusion, our data suggest that anti-ERα Abs might contribute to the pathogenesis of breast cancer. At the best of our knowledge, this is the first proof-of-concept study with breast cancer patients in which a simple and non-invasive analysis, i.e., in the peripheral blood, is hypothesized to provide useful information as concern tumor progression. However, a larger study with more extended clinical endpoints, such as relapse, survival and response to therapy, will be mandatory in order to validate the possible use of anti-ERα Abs as prognostic and/or predictive serum biomarkers. More specifically, the hypothesis that anti-ERα Abs, binding to ERα, might have reflections on treatment outcome in patients undergoing antiestrogenic therapy, e.g., with tamoxifen, cannot be ruled out.
Figure 3.
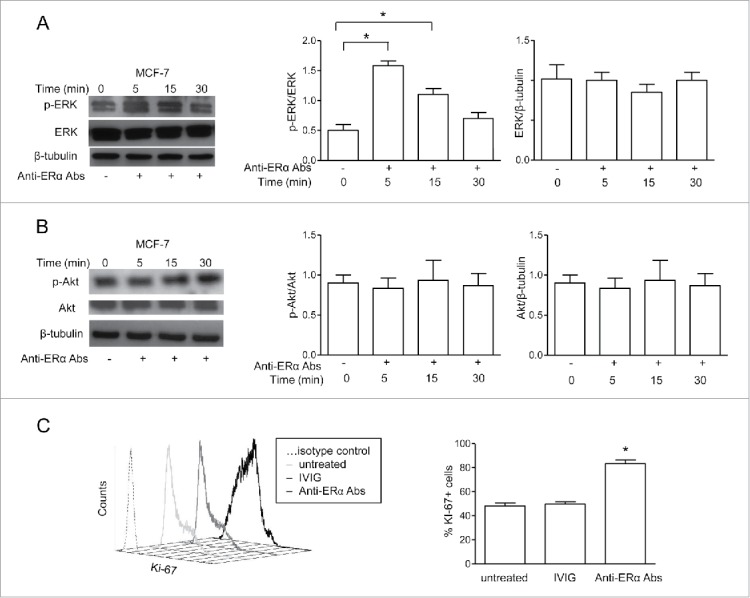
Anti-ERα Abs induced ERK phosphorylation and proliferation in MCF-7 breast cancer cells. (A) Western blot analysis of phosphorylated ERK (p-ERK) and total ERK in ER+ MCF-7 cells treated with anti-ERα Abs (50 μg/ml) for 5, 15, and 30 min. Blots are representative of those from five experiments. Densitometric analyses of p-ERK and total ERK levels are also shown. (B) Western blot analysis of phosphorylated Akt (p-Akt) and total Akt in ER+ MCF-7 cells treated as described above. Blots are representative of those from five experiments. Densitometric analyses of p-Akt and total Akt levels are also shown. (C) Representative flow cytometry analysis of Ki-67 expression levels in ER+ MCF-7 cells after 24 h treatment with anti-ERα Abs or IVIG (both at 50 μg/mL). Mean ± SD from five independent experiments are also reported. * P < 0.05.
Patients and methods
Patients
Forty-eight pre or postmenopausal patients with newly diagnosed early breast cancer [median age: 53 years, range: 34-73; BMI: 24.95, range: 18.4-38.2] were enrolled at Regina Elena National Cancer Institute of Rome and assessed after 30–40 days from surgery (total or partial mastectomy) of primary tumor before the start of chemotherapy. Exclusion criteria included patients with ipsilateral breast and/or axillary recurrences and/or a new contralateral breast cancer diagnosed after previous primary tumor. Clinico-pathological characteristics of enrolled patients were summarized in Table 1. The control group consisted of 75 age- and sex-matched healthy donors. The study was reviewed and approved by the Local Ethical Committee at the Regina Elena National Cancer Institute, and a written informed consent was obtained from all patients. All clinical investigations have been conducted according to the principles expressed in the Declaration of Helsinki.
The immunohistochemical assessment of ER and PgR as well as HER2 and Ki-67 status was performed in formalin-fixed paraffin-embedded tissues. ER and PgR expression was analyzed using the mAbs 6F11 and 1A6 (Novocastra, Menarini, Florence, Italy), respectively, whereas HER2 immunostaining was performed using the polyclonal antibody A0485 (Dako, Milan, Italy). Ki-67 was tested using the mAb MIB1 (Dako). Immunoreactions were revealed by a streptavidin-biotin enhanced immunoperoxidase technique (Super Sensitive MultiLink, Menarini) in an automated autostainer. Diaminobenzidine was used as chromogenic substrate. Tumors were graded using the Bloom and Richardson scoring system, as moderately (G2, 46%) and poorly differentiated (G3, 54%) carcinomas. Staging was performed by following the Union Internationale Contre le Cancer tumor-node-metastasis system criteria.21
Enzyme-linked immunosorbent assay (ELISA)
ELISA was developed essentially as previously described.13 Briefly, polystyrene plates (Maxisorp, Nunc, Roskilde, Denmark) were coated with the antigen (2 μg/well ERα and ERβ, Sigma, St Louis, MO) in 0.05 M NaHCO3 buffer, pH 9.5, and incubated overnight at 4 °C. Plates were blocked with 100 μl/well of 3% milk, for 1 h at 37 °C. Human sera were diluted in PBS-Tween and 1% milk (1:100 for total IgG), 100 μl per well. Peroxidase-conjugated goat anti-human IgG (BioRad, Richmond, CA) were diluted in PBS-Tween containing 1% milk (1:3,000) and incubated for 1 h at room temperature. O-phenylenediamine dihydrochloride (Sigma) was used as a substrate and the optical density was measured at 490 nm (OD490). Mean + 3 standard deviations of the OD reading of the healthy donors was considered as the cut-off level for positive reactions. All assays were performed in quadruplicate. Data were presented as the mean OD corrected for background (wells without coated antigen).
Purification of specific autoantibodies from patients' sera
Antibody purification was performed as previously described.13 Recombinant ERα (50 μg, Sigma) was spotted onto a nitrocellulose filter and incubated with sera from 10 patients that had OD > 0.6 by ELISA. The antibodies were eluted with 100 mM glycine, pH 2.5, immediately neutralized with 1 M Tris HCl, pH 8, and dialyzed against PBS. Endotoxin contamination of antibodies was determined by the quantitative chromogenic Limulus amebocyte cell lysate assay (QCL-1000; BioWhittaker, Walkersville, MD). Antibodies from a preparation of intravenous immunoglobulin (IVIG) precipitated by saturated ammonium sulfate solution were used as control.
PEPperMAP® epitope mapping
The epitope mapping of the purified human anti-ERα was done against ERα amino acid sequence translated into 15 aa peptides with peptide-peptide overlap of 14 aa. The peptide microarray with the protein-derived peptides was incubated with the antibody sample at concentrations of 1 μg/mL, 4 μg/mL, 10 μg/mL, and 50 μg/mL in incubation buffer. The incubation was followed by staining with a secondary goat anti-human IgG DyLight680 antibody and read-out with a LI-COR Odyssey Imaging System. Quantification of spot intensities and peptide annotation were done with PepSlide® Analyzer.
Cell culture and treatment
MCF-7 (ATCC® HTB-22™) and MDA-MB-231 (ATCC® HTB-26™) were obtained from the American Type Culture Collection (ATCC). Cells were cultured in RPMI 1640 medium without phenol red (Gibco BRL, Grand Island, NY) supplemented with 10% charcoal stripped fetal bovine serum (EuroClone, Pero, Milan, Italy), 2 mM glutamine (Sigma), and 50 µg/mL gentamycin (Sigma) at 37 °C in a humidified 5% CO2 atmosphere and passaged for fewer than 6 months after receipt.
For ERK activation cells were treated with 50 µg/mL of human anti-ERα Abs or IVIG. We selected this antibody concentration on the basis of a dose response curve constructed by incubating MCF-7 cells with serial dilutions of anti-ERα Abs (5-50 µg/mL) for 5 min.
Flow cytometry
Human anti-ERα Abs purified from patient's sera, anti-ERα mAb C-542 (Abcam, Cambridge, UK) (1 µg per 1 × 106 cells), and the appropriate FITC-conjugated secondary antibodies were used for staining mERα on breast cancer cell lines. Equal amounts of IVIG or mouse IgG isotype control were used as negative controls. At 4 °C, pre-incubation with 1 µM E2-BSA was also used to verify the specificity of staining. Proliferation was evaluated by measuring the Ki-67 nuclear Ag expression using the PE-mouse anti-human Ki-67 Set according to the manufacturer's protocol (BD Biosciences, San Jose, CA). Apoptosis was quantified using a FITC-conjugated annexin V and propidium iodide apoptosis detection kit according to the manufacturer's protocol (Marine Biological Laboratory, Woods Hole, MA). Acquisition was performed on FACSCalibur flow cytometer (BD Biosciences) and 50,000 events per sample were run. Data were analyzed using the Cell Quest Pro software (BD Biosciences).
Immunofluorescence assay
Cells were fixed with 4% formaldehyde in PBS for 30 min at 4 °C. After blocking in PBS 2% BSA, containing 5% glycerol and 0.2% Tween-20 cells were incubated for 1 h at 4 °C with purified human anti-ERα Abs or with the commercial mAb C-542 (Abcam) in PBS containing 1% BSA. FITC- anti-human or anti-mouse antisera were then added and incubated at 4 °C for 30 min. Fluorescence was analyzed with an Olympus U RFL microscope (Olympus, Hamburg, Germany).
SDS-PAGE and western blot
Breast cancer cells were lysed in RIPA buffer (100 mM Tris–HCL pH 8, 150 mM NaCl, 1% Triton X-100, 1 mM MgCl, 100 mM sodium orthovanadate) in the presence of complete protease-inhibitor mixture (Sigma). Protein content was determined by the Bradford assay (BioRad). The level of phosphorylated ERK (p-ERK), total ERK, p-Akt, and total Akt were analyzed by Western blot as previously described.13 Cell lysate (25 µg) was loaded in SDS-PAGE and Western blot was performed using rabbit anti-p-ERK-1/2 mAb (137F5), rabbit anti-p-Akt mAb (D25E6), 1:1,000 dilution, (Cell Signaling Technology, Beverly, MA), mouse anti-ERK-1/2 mAb (sc-135900) or rabbit anti-Akt polyclonal Ab (sc-5298), 1:500 dilution, (Santa Cruz Biotechnology, CA). To ensure the presence of equal amounts of protein, the membranes were reprobed with a mouse anti-β-tubulin mAb (N357, Amersham, Gent, Belgium). Quantification of protein expression was performed by densitometry analysis of the autoradiograms (GS-700 Imaging Densitometer, BioRad).
Statistical analyses
The two-tailed Mann–Whitney U test, the Spearman's rank correlation analysis, and χ2 test were performed. Statistical significance was set at P < 0.05.
Disclosure of potential conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgment
We are grateful to Prof. Lev Berstein for his support and critical revision of the manuscript.
Funding
The study was partially supported by the Ministry of Health (Ricerca Finalizzata 2009), AIRC and Fondazione Veronesi 2012.
Supplemental Material
Supplemental Material may be downloaded here: publisher's website
References
- 1.Ascenzi P, Bocedi A, Marino M. Structure-function relationship of estrogen receptor alpha and beta: impact on human health. Mol Aspects Med 2006; 27:299-402; PMID:16914190; http://dx.doi.org/ 10.1016/j.mam.2006.07.001 [DOI] [PubMed] [Google Scholar]
- 2.Tyson JJ, Baumann WT, Chen C, Verdugo A, Tavassoly I, Wang Y, Weiner LM, Clarke R. Dynamic modelling of oestrogen signalling and cell fate in breast cancer cells. Nat Rev Cancer 2012; 11:523-32; PMID:21677677; http://dx.doi.org/ 10.1038/nrc3081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics. CA Cancer J Clin 2009; 59:225-49; PMID:19474385; http://dx.doi.org/ 10.3322/caac.20006 [DOI] [PubMed] [Google Scholar]
- 4.Early Breast Cancer Trialists' Collaborative Group (EBCTCG), Davies C, Godwin J, Gray R, Clarke M, Cutter D, Darby S, McGale P, Pan HC, Taylor C et al.. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet 2011; 378:771-84; PMID:21802721; http://dx.doi.org/ 10.1016/S0140-6736(11)60993-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ali S, Coombes RC. Endocrine-responsive breast cancer and strategies for combating resistance. Nat Rev Cancer. 2002; 2:101-12; PMID:12635173; http://dx.doi.org/ 10.1038/nrc721 [DOI] [PubMed] [Google Scholar]
- 6.Pietras RJ, Marquez-Garban DC. Membrane-associated estrogen receptor signaling pathways in human cancers. Clin Cancer Res 2007; 13:4672-6; PMID:17699844; http://dx.doi.org/ 10.1158/1078-0432.CCR-07-1373 [DOI] [PubMed] [Google Scholar]
- 7.Levin ER. Plasma membrane estrogen receptors. Trends Endocrinol Metab 2009; 20:477-82; PMID:19783454; http://dx.doi.org/ 10.1016/j.tem.2009.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mendelsohn ME, Karas RH. Rapid progress for non-nuclear estrogen receptor signaling. J Clin Investig 2010; 120:2277-9; PMID:20577045; http://dx.doi.org/ 10.1172/JCI43756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harrington WR, Kim SH, Funk CC, Madak-Erdogan Z, Schiff R, Katzenellenbogen JA, Katzenellenbogen BS. Estrogen dendrimer conjugates that preferentially activate extranuclear, nongenomic versus genomic pathways of estrogen action. Mol Endocrinol 2006; 20:491-502; PMID:16306086; http://dx.doi.org/ 10.1210/me.2005-0186 [DOI] [PubMed] [Google Scholar]
- 10.Zivadinovic D, Gametchu B, Watson CS. Membrane estrogen receptor-alpha levels in MCF-7 breast cancer cells predict cAMP and proliferation responses. Breast Cancer Res 2005; 7: R101-12; PMID:15642158; http://dx.doi.org/ 10.1186/bcr958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borkowski A, Gyling M, Muquardt C, Body JJ, Leclercq G. Estrogen-like activity of a subpopulation of natural antiestrogen receptor autoantibodies in man. Endocrinology 1991; 128:3283-92; PMID:2036991; http://dx.doi.org/ 10.1210/endo-128-6-3283 [DOI] [PubMed] [Google Scholar]
- 12.Tassignon J, Haeseleer F, Borkowski A. Natural antiestrogen receptor autoantibodies in man with estrogenic activity in mammary carcinoma cell culture: study of their mechanism of action; evidence for involvement of estrogen-like epitopes. J Clin Endocrinol Metab 1997; 82:3464-70; PMID:9329387; http://dx.doi.org/ 10.1210/jcem.82.10.4313 [DOI] [PubMed] [Google Scholar]
- 13.Colasanti T, Maselli A, Conti F, Sanchez M, Alessandri C, Barbati C, Vacirca D, Tinari A, Chiarotti F, Giovannetti A et al.. Autoantibodies to estrogen receptor alpha interfere with T lymphocyte homeostasis and are associated with disease activity in systemic lupus erythematosus. Arthritis Rheum 2012; 64:778-87; PMID:21968947; http://dx.doi.org/ 10.1002/art.33400 [DOI] [PubMed] [Google Scholar]
- 14.Giovannetti A, Maselli A, Colasanti T, Rosato E, Salsano F, Pisarri S, Mezzaroma I, Malorni W, Ortona E, Pierdominici M. Autoantibodies to estrogen receptor alpha in systemic sclerosis (SSc) as pathogenetic determinants and markers of progression. PLoS One 2013; 8: e74332; PMID:24058548; http://dx.doi.org/ 10.1371/journal.pone.0074332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feldman M. Steroid receptor antibodies in autoimmune disorders. Biochem Biophys Res Commun 1987; 145:1342-8; PMID:3606605; http://dx.doi.org/ 10.1016/0006-291X(87)91585-3 [DOI] [PubMed] [Google Scholar]
- 16.Counihan KA, Vertosick FT, Kelly RH. Anti-estrogen antibodies in systemic lupus erythematosus: a quantitative evaluation of serum levels. Immunol Investig 1991; 20:317-31; PMID:1874561; http://dx.doi.org/ 10.3109/08820139109026233 [DOI] [PubMed] [Google Scholar]
- 17.Ortona E, Pierdominici M, Berstein L. Autoantibodies to estrogen receptors and their involvement in autoimmune diseases and cancer. J Steroid Biochem Mol Biol 2014; 144:260-7; PMID:25038321; http://dx.doi.org/ 10.1016/j.jsbmb.2014.07.004 [DOI] [PubMed] [Google Scholar]
- 18.Dowsett M, Nielsen TO, A'Hern R, Bartlett J, Coombes RC, Cuzick J, Ellis M, Henry NL, Hugh JC, Lively T et al.. International Ki-67 in breast cancer working group. Assessment of Ki67 in breast cancer: recommendations from the International Ki67 in breast cancer working group. J Natl Cancer Inst. 2011; 103:1656-64; PMID:21960707; http://dx.doi.org/ 10.1093/jnci/djr393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Inwald EC, Klinkhammer-Schalke M, Hofstädter F, Zeman F, Koller M, Gerstenhauer M, Ortmann O. Ki-67 is a prognostic parameter in breast cancer patients: results of a large population-based cohort of a cancer registry. Breast Cancer Res Treat. 2013; 139:539-52; PMID:23674192; http://dx.doi.org/ 10.1007/s10549-013-2560-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ciruelos Gil EM. Targeting the PI3K/AKT/mTOR pathway in estrogen receptor-positive breast cancer. Cancer Treat Rev. 2014; 40:862-71; PMID:24774538; http://dx.doi.org/ 10.1016/j.ctrv.2014.03.004 [DOI] [PubMed] [Google Scholar]
- 21.Sobin LH, Wittekind C (Eds.). TNM classification of malignant tumors (6th ed.), John Wiley & Sons, Inc, New York; 2002. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


