ABSTRACT
Microsatellite instability (MSI-H) is caused by DNA mismatch repair deficiency and occurs in 15% of colorectal cancers. MSI-H cancers generate highly immunogenic frameshift peptide (FSP) antigens, which elicit pronounced local immune responses. A subset of MSI-H colorectal cancers develops in frame of Lynch syndrome, which represents an ideal human model for studying the concept of immunoediting. Immunoediting describes how continuous anti-tumoral immune surveillance of the host eventually leads to the selection of tumor cells that escape immune cell recognition and destruction. Between 30 and 40% of Lynch syndrome-associated colorectal cancers display loss of HLA class I antigen expression as a result of Beta2-microglobulin (B2M) mutations. Whether B2M mutations result from immunoediting has been unknown. To address this question, we related B2M mutation status of Lynch syndrome-associated colorectal cancer specimens (n = 30) to CD3-positive, CD8-positive and FOXP3-positive T cell infiltration in both tumor and normal mucosa. No significant correlation between B2M mutations and immune cell infiltration was observed in tumor tissue. However, FOXP3-positive T cell infiltration was significantly lower in normal mucosa adjacent to B2M-mutant (mt) compared to B2M-wild type (wt) tumors (mean: 0.98% FOXP3-positive area/region of interest (ROI) in B2M-wt vs. 0.52% FOXP3-positive area/ROI in B2M-mt, p = 0.023). Our results suggest that in the absence of immune-suppressive regulatory T cells (Treg), the outgrowth of less immunogenic B2M-mt tumor cells is favored. This finding supports the immunoediting concept in human solid cancer development and indicates a critical role of the immune milieu in normal colonic mucosa for the course of disease.
KEYWORDS: Beta2-microglobulin, colorectal cancer, hereditary cancer, immunoediting, Lynch syndrome, microsatellite instability, regulatory T cells
Abbreviations
- B2M
beta2-microglobulin
- cMS
coding microsatellite
- FSP
frameshift peptide
- IHC
immunohistochemistry
- MMR
mismatch repair
- MSI-H
high level microsatellite instability
- mt
mutant
- ROI
region of interest
- Treg
regulatory T cells
- wt
wild type
Introduction
The protective role of the immune system in cancer development has received considerable attention in recent years. There is obvious evidence that the host's immune response to cancer is related to patients' survival.1-3 More recently, immunotherapy aiming to activate therapeutic antitumor immunity by immune checkpoint blockade has been implemented in clinical practice with remarkable success.4
The hypothesis that the phenotype of tumors is influenced by the host's immune system is described by the concept of immunoediting.5,6 According to the immunoediting hypothesis, emerging tumor cells are initially recognized and eliminated by both the innate and adaptive immune system (elimination phase). However, few tumor cell clones may persist but are kept in a dormancy state which is mostly mediated by mechanisms of the adaptive immune system (equilibrium phase). This prevents further tumor cell outgrowth but also places tumor cells under a constant immune selection pressure that shapes their genotype and phenotype and eventually leads to the outgrowth of tumor cells that have acquired the ability to escape the host's immune cell attack (escape phase). These tumor cells can then progress to clinically overt cancers. The immunoediting concept has been supported in various mouse models6 with recent mouse studies even suggesting specific rejection antigens that appear to be responsible for the recognition and ultimately elimination of tumor cells by the immune system.7 In addition, preliminary evidence for immunoediting has also emerged in patients undergoing immunotherapy.5
However, to the best of our knowledge, conclusive evidence, that immunoediting plays a role in the natural history of human tumor development, is lacking. To examine this question, we focused on Lynch syndrome. Lynch syndrome is a particularly well-suited model of a close interaction between tumor and host immune system in humans.8-10 It is a hereditary cancer syndrome caused by germline mutations of DNA mismatch repair (MMR) genes, most frequently MLH1 or MSH2.11 Affected individuals are predisposed to the development of colorectal cancers with an estimated lifetime risk of about 40 to 80%.12,13 The pathogenesis of Lynch syndrome-associated cancers is driven by the inactivation of the MMR system, which results in the accumulation of numerous mutations at short repetitive DNA sequences (microsatellites), a phenotype termed high-level MSI-H. MMR deficiency-induced mutations affecting microsatellites located in gene-encoding regions then lead to gene inactivation and the generation of de-novo FSP sequences.11 These FSPs have been shown to elicit strong cytotoxic T cell responses14-16 and may therefore share properties of true rejection antigens. The immunogenicity of MSI-H induced FSPs is generally considered as a major contributor to the pronounced local immune responses regularly observed in Lynch syndrome-associated colorectal cancers.8 However, despite the dense presence of immune cells, Lynch syndrome-associated colorectal cancers grow out to clinically manifest tumors which suggests that immunosuppressive or immunoevasive mechanisms are required for tumor outgrowth.8 One major mechanism that may contribute to immune evasion of Lynch syndrome-associated cancers is loss of HLA class I-mediated antigen presentation due to mutations of the B2M gene,17,18 which impairs recognition of tumor cells by cytotoxic CD8-positive T cells.
However, only about 30–40% of all MSI-H colorectal cancers present with a B2M mutation.17,19 If these B2M mutations do in fact reflect the results of immune evasion, the question then arises why they occur only in a distinct subset of MSI-H colorectal cancers and why the remaining cancers can still grow out even though they continue to present highly immunogenic antigens to the host's immune system. This either argues in favor of alternative evasion mechanisms, which have been suggested in a subset of these lesions20-22 or in favor of a less stringent immune selection in patients with B2M-wt cancers. To examine whether B2M-wt cancers did in fact develop in a less stringent immune selection milieu, we analyzed the local infiltration with FOXP3-positive T cells, which represent major modulators and suppressors of adaptive immune responses in colorectal cancer,23-25 and correlated the results with B2M mutation status of the respective tumors. Similarly, CD3- and CD8-positive T cell counts as well as lymph follicle counts were quantified and related to B2M mutation status.
Results
Determination of B2M mutation status
All Lynch syndrome-associated colorectal cancer samples (n = 30) were stained by immunohistochemistry (IHC) with a B2M-specific antibody to determine the absence or presence of functional B2M protein. Nine carcinomas (30%) were B2M-negative whereas 21 carcinomas (70%) were B2M-positive. Staining results were unambiguous, showing either complete loss of B2M expression or strong, homogenous B2M expression throughout the tumor. Representative staining results are displayed in Fig. 1.
Figure 1.
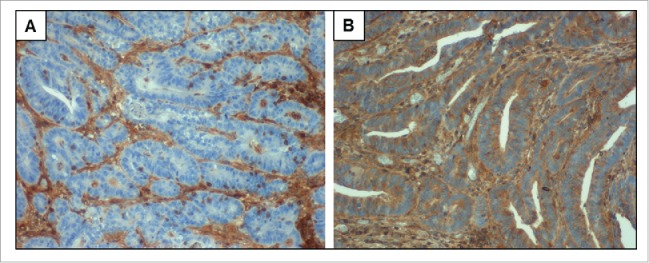
B2M expression in Lynch syndrome-associated colorectal cancers. (A) Carcinoma cells with complete loss of B2M expression. Expression of B2M is maintained in tumor-infiltrating lymphocytes and other stromal cells (brown staining). (B) Strong and homogenous B2M expression in carcinoma cells as well as in TILs and other stromal cells. Nuclei were counterstained with Hemalaun (blue). Pictures were taken at 40x objective magnification.
For all B2M-negative carcinomas, mutation analysis of the B2M gene was performed. Mutations of the B2M gene were identified in all 9 B2M-negative samples. Two distinct mutations were identified in 5 samples, corresponding to a total of 14 mutations. Mutations were mainly frameshift mutations located in the 4 most frequently affected cMS of the gene: Five mutations (35.7%) were located in the (CT)4 repeat in exon 1. Among the 3 cMS present in exon 2, 2 mutations (14.3%) were observed in the A5 repeat and 3 mutations (21.4%) occurred in the C5 repeat. No mutations were found in the second A5 repeat. Moreover, we observed 4 mutations not affecting these cMS (2 point mutations and 2 deletions), one of which (c.252_253delCT) had not been described previously in the literature. Table 1 gives an overview of the B2M mutations found in the B2M-negative Lynch syndrome-associated colorectal cancers. None of these mutations were found in normal tissue from the corresponding patients. All described mutations are predicted to cause a premature stop codon further downstream. This suggests mutation-induced functional inactivation of the B2M protein and provides a molecular explanation for loss of B2M expression in all analyzed B2M-negative tumor specimens.
Table 1.
Description of B2M mutations in Lynch syndrome-associated colorectal cancer samples.
| Sample | Description on nucleotide level | Description on amino acid level |
|---|---|---|
| Sample 1 | c.68–2A>G | p.Arg23_Lys26delinsHisfs*30; see § |
| Sample 2 | c.45_48delTTCT c.276delC | p.Ser16Alafs*27 p.Thr93Leufs*10 |
| Sample 3 | c.43_44delCT c.204delA | p.Leu15Phefs*41 p.Val68Trpfs*34 |
| Sample 4 | c.43_44delCT c.204delA | p.Leu15Phefs*41 p.Val68Trpfs*34 |
| Sample 5 | c.43_44delCT c.252_253delCT | p.Leu15Phefs*41 p.Leu85Valfs*4 |
| Sample 6 | c.101delC c.276delC | p.Pro34Glnfs*10 p.Thr93Leufs*10 |
| Sample 7 | c.276.delC | p.Thr93Leufs*10 |
| Sample 8 | c.43_44delCT | p.Leu15Phefs*41 |
| Sample 9 | c.68–2A>G | p.Arg23_Lys26delinsHisfs*30; see § |
§ The A>G substitution is located in the dinucleotide AG splice acceptor site of intron 1. This causes a splice acceptor defect immediately 5′ of exon 2 and results in the use of a cryptic splice site located within exon 2. In consequence, 11 bps of exon 2 that are located 5′ of this new cryptic splice site are deleted, causing a translational frameshift and a premature stop codon after 29 missense codons.44
A summary of clinicopathological characteristics is displayed in Table 2a and 2b. No significant correlation between B2M mutation status and any of the assessed parameters could be observed.
Table 2a.
Characteristics of Lynch syndrome-associated colorectal cancer patients.
| Total | B2M-wt | B2M-mt | p value | |
|---|---|---|---|---|
| Number of patients | n = 24 | n = 17 | n = 7 | |
| Age (median; range) | 44; 27–63 | 42; 27–53 | 45; 38–63 | 0.59 |
| Gender | 1.00 | |||
| Male | 19 (79.2) | 13 (76.5) | 6 (85.7) | |
| Female | 5 (21.8) | 4 (23.5) | 1 (14.3) | |
| Germline mutation | 0.55 | |||
| MLH1 | 16 (66.6) | 10 (58.8) | 6 (85.7) | |
| MSH2 | 7 (29.2) | 6 (35.3) | 1 (14.3) | |
| MSH6 | 1 (4.2) | 1 (5.9) | 0 (0.0) |
Numbers in brackets indicate percentage values if not indicated otherwise. P values for gender and germline mutations were calculated using Fisher´s exact test and Mann–Whitney U test was used for age.
Table 2b.
Characteristics of Lynch syndrome-associated colorectal cancer samples.
| Total | B2M-wt | B2M-mt | p value | |
|---|---|---|---|---|
| Number of tumor samples | n=30 | n=21 | n=9 | |
| Location in colon | 0.39 | |||
| proximal | 21 (70.0) | 16 (76.2) | 5 (55.6) | |
| distal | 9 (30.0) | 5 (23.8) | 4 (44.4) | |
| Primary tumor | 0.12 | |||
| T1 | 2 (6.7) | 2 (9.5) | 0 (0.0) | |
| T2 | 7 (23.3) | 7 (33.3) | 0 (0.0) | |
| T3 | 15 (50.0) | 8 (38.1) | 7 (77.8) | |
| T4 | 6 (20.0) | 4 (19.1) | 2 (22.2) | |
| Lymph nodes status | 0.11 | |||
| N0 | 21 (70.0) | 17 (81.0) | 4 (44.4) | |
| N1 | 5 (16.7) | 2 (9.5) | 3 (33.3) | |
| N2 | 4 (13.3) | 2 (9.5) | 2 (22.2) | |
| Distant metastasis | 1.00 | |||
| M0 | 27 (93.1) | 19 (90.5) | 8 (100.0) | |
| M1 | 2 (6.9) | 2 (9.5) | 0 (0.0) | |
| Mx | 1 | 0 | 1 | |
| UICC stage | 0.06 | |||
| UICC I | 8 (27.6) | 8 (38.1) | 0 (0.0) | |
| UICC II | 12 (41.4) | 8 (38.1) | 4 (50.0) | |
| UICC III | 7 (24.1) | 3 (14.3) | 4 (50.0) | |
| UICC IV | 2 (6.9) | 2 (9.5) | 0 (0.0) | |
| na | 1 | 0 | 1 |
Numbers in brackets indicate percentage values. All p values were calculated using Fisher´s exact test. na = not analyzable.
Correlation of B2M mutation status and immune infiltration data
Having established the B2M mutation status for all Lynch syndrome-associated colorectal cancers, these results were then correlated to FOXP3-positive T cell infiltration in the tumor.
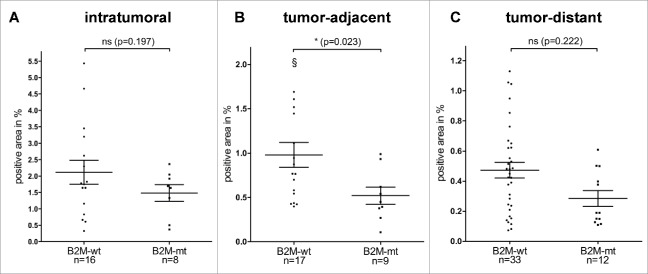
No statistically significant correlation between B2M mutation status and intratumoral FOXP3-positive T cell infiltration was observed (mean: 2.12% FOXP3-positive area/ROI in B2M-wt vs. 1.48% FOXP3-positive area/ROI in B2M-mt, p = 0.197, Fig. 2A).
Figure 2.
FOXP3-positive T cell infiltration depending on B2M mutation status. (A) Intratumoral FOXP3-positive T cell infiltration of B2M-wt and B2M-mt Lynch syndrome-associated colorectal cancers. FOXP3-positive T cell infiltration (B) in tumor-adjacent mucosa and (C) in tumor-distant mucosa of B2M-wt and B2M-mt Lynch syndrome-associated colorectal cancers. A significantly lower infiltration with FOXP3-positive T cells was observed in tumor-adjacent mucosa of B2M-mt tumors compared to B2M-wt tumors. Data are shown as scatter dot plots with horizontal lines indicating mean values and SEM. § represents an outlier: 2.44%. ns = not significant.
The effect of immune cells on tumor outgrowth likely plays an important role at very early stages of tumor development, when neoplastic cells are still closely surrounded by normal mucosa. As a next step, we therefore analyzed FOXP3-positive T cell counts in tumor-adjacent normal mucosa as this was felt to best represent the immune milieu during the early stages of tumor development.
In tumor-adjacent mucosa, B2M-mt Lynch syndrome-associated colorectal cancers presented with a significantly lower FOXP3-positive T cell infiltrate than B2M-wt Lynch syndrome associated colorectal cancers (mean: 0.98% FOXP3-positive area/ROI in B2M-wt vs. 0.52% FOXP3-positive area/ROI in B2M-mt, p = 0.023, Fig. 2B). To examine whether this correlation was restricted to FOXP3-positive T cell counts or also detectable for other T cell subtypes, we examined CD3-positive and CD8-positive T cell infiltration. However, no significant differences were observed for CD3-positive and CD8-positive T cell infiltration (Fig. S1). Moreover, no difference in the number of lymph follicles was observed (Fig. S1).
To confirm the correlation between B2M mutation status and FOXP3-positive T cell infiltration in tumor-adjacent mucosa, we repeated our analysis in a second, independent cohort consisting of 12 Lynch syndrome-associated colorectal cancers (B2M-wt: n = 9, B2M-mt: n = 3; see Tables S1 and S2 for description of B2M mutations and clinicopathological characteristics). We were able to affirm the lower number of FOXP3-positive T cells in the tumor-adjacent mucosa of B2M-mt tumors compared to B2M-wt cancers (mean: 0.67% FOXP3-positive area/ROI in B2M-wt vs. 0.32% FOXP3-positive area/ROI in B2M-mt) although statistical significance was not reached due to the lower number of samples available (p = 0.262, see Fig. S2A).
Having established a significant correlation between B2M mutations in Lynch syndrome-associated CRCs and FOXP3-positive T cell infiltration in tumor-adjacent mucosa, the analysis was extended to tumor-distant normal mucosa to see whether similar effects could also be observed there. However, no significant difference in FOXP3-positive T cell infiltration between B2M-mt Lynch syndrome-associated colorectal cancers and B2M-wt Lynch syndrome-associated colorectal cancers was found in tumor-distant normal mucosa (mean: 0.47% FOXP3-positive area/ROI in B2M-wt vs. 0.29% FOXP3-positive area/ROI in B2M-mt, p = 0.222, Fig. 2C). The lack of a significant correlation between B2M mutation status and FOXP3-positive T cell infiltration in tumor-distant mucosa was confirmed in the validation cohort (mean: 0.17% FOXP3-positive area/ROI in B2M-wt vs. 0.18% FOXP3-positive area/ROI in B2M-mt, p = 1.000, see Fig. S2B). Similarly, no differences were observed for CD3-positive and CD8-positive T cell infiltration as well as for lymph follicle count in tumor-distant mucosa (Fig. S3).
Discussion
We observed a statistically significant relationship between the B2M mutation status of Lynch syndrome-associated colorectal cancers and the density of FOXP3-positive T cells in the normal colonic mucosa: In patients with B2M-mt tumors, a significantly lower density of FOXP3-positive T cells in the normal colonic mucosa directly adjacent to the tumor was observed. Conversely, patients with B2M-wt tumors showed a significantly higher infiltration with FOXP3-positive T cells in the tumor-adjacent mucosa; a finding, which was confirmed in a second, independent sample collection.
This demonstrates for the first time that the immune cell infiltration of the colonic mucosa is related to the phenotype of tumors developing in the respective mucosal region. Specifically we provide evidence that high numbers of FOXP3-positive T cells in the colonic mucosa are associated with the occurrence of tumors that are B2M-wt and thus still capable of antigen presentation via HLA class I. B2M mutations are known to completely abrogate HLA class I-mediated antigen presentation, a key mechanism of tumor cell recognition and elimination by cells of the adaptive immune system.18 On the other hand, FOXP3-positive Treg cells have been implicated as crucial mediators in suppressing antigen-specific immune responses in colorectal tumors.23-25 Our observation may therefore imply that B2M-wt MSI-H colorectal cancers are more likely to grow out in the presence of high numbers of FOXP3-positive Treg cells. In an environment of sparse infiltration with FOXP3-positive Treg cells, which may reflect a more active local immune environment and thus a stronger immunoselective pressure, emerging tumor cell clones may be eliminated more effectively. This may consequently favor the outgrowth of poorly immunogenic, B2M-mt MSI-H colorectal cancer cells that no longer present FSP-derived neoantigens to the immune system via HLA class I antigens (Fig. 3). Hence, the significant correlation between the occurrence of B2M mutations and mucosal Treg cell infiltration supports the concept that MSI-H colorectal cancers in Lynch syndrome develop through a process of immune selection. This lends further support to the validity of the immunoediting hypothesis in humans and especially in colorectal cancer development. It is in line with the existence of an elimination and equilibrium phase lasting for many years before escape variants develop and finally grow out to manifest cancers.5,6 We have previously shown that FSP-specific T cells are commonly observed in the peripheral blood drawn from MSI-H colorectal cancer patients.15,25 Interestingly, FSP-specific T cells were also detected in the peripheral blood drawn from Lynch syndrome mutation carriers without any history of cancer,15 whereas they were virtually absent in MSS cancer patients and healthy controls. These observations further support the concept that FSP-specific T cells may contribute to immunoediting of MSI-H colorectal cancers and potentially to the outgrowth of B2M-mutant cancers in Lynch syndrome.
Figure 3.
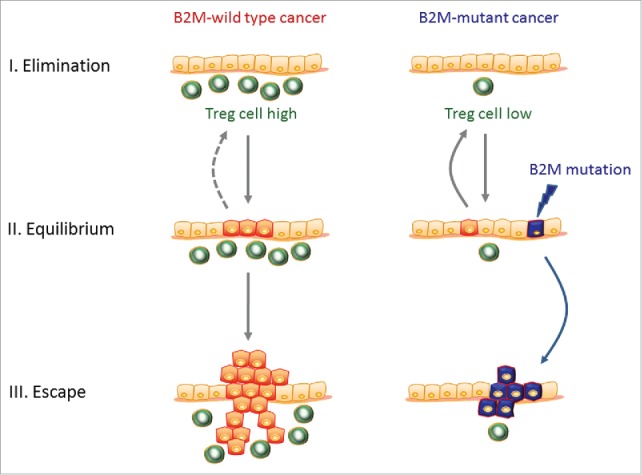
Influence of mucosal Treg cell levels on phenotype of developing colorectal cancers. Left panel: High levels of immunosuppressive Treg cells within the normal colonic mucosa (green cells) may reflect a less active local immune milieu, which potentially applies a less stringent immune selection pressure and is less capable of eliminating emerging tumor cells (red cells). Consequently, this may also permit the outgrowth of more immunogenic, i.e., B2M-wt, cancers. Right panel: In contrast, low levels of immunosuppressive Treg cells within the normal colonic mucosa may be indicative of a more active local immune milieu and a stronger immunoselective pressure. This may enable a more effective elimination of emerging cancer cell clones and only allow the outgrowth of poorly immunogenic, i.e., B2M-mt, cancers (blue cells). This sequence corresponds to the three phase immunoediting concept consisting of an elimination and equilibrium phase followed by an escape phase in which clinically overt cancers manifest.
This new link between the presence and number of FOXP3-positive cells and the occurrence of B2M mutations in cancer underlines the importance of tumor - immune cell interactions and the inter-relation of molecular tumor classifiers and immune responses of the host.26 It is particularly interesting in view of emerging immune checkpoint blockade therapies that have shown very promising results in clinical trials, particularly in highly mutated tumor types such as melanoma.27,28 One of the most promising checkpoint modulators is ipilimumab, an antibody directed against human CTLA4, which has also been shown to specifically target FOXP3-positive Treg cells.29-31 Although CTLA4 blockade failed to show a significant efficacy in colorectal cancer so far,32 its use specifically in the highly mutant subgroup of MSI-H colorectal cancers has been proposed recently.33,34
Our observation suggests that a therapeutic reduction of FOXP3-positive T cell counts, which may be induced by CTLA4 blockade, might not only support tumor cell elimination directly, but also, if elimination fails, lead to the induction of B2M mutations in persisting MSI-H cancer cells. Interestingly, MSI-H colorectal cancers with B2M mutations are associated with an excellent prognosis and the absence of disease relapses or distant organ metastases.17,35,36 Consequently, Treg cell depletion may improve survival not only by facilitating tumor cell killing, but also by shaping the tumor in a way that favors the formation of a less aggressive, locally restricted phenotype.
The association between B2M mutation status of the tumor and FOXP3-positive Treg cell infiltration was restricted to tumor-adjacent normal mucosa, but not observed in tumor-distant normal mucosa, i.e., normal mucosa located at the surgical resection margins. This is compatible with the concept that the process of immune selection during colorectal carcinogenesis is rather a locally restricted than a systemic phenomenon.
Interestingly, B2M mutation status was not associated with intratumoral FOXP3-positive Treg cell counts. This may reflect the fact that immune cell infiltration in the tumor, in contrast to non-tumorous mucosa, is influenced by a variety of factors that are not only related to the host's systemic and local immune status, but also related to secondary effects mediated by tumor cells, such as tumor cytokine secretion.36-39
Our study has several limitations: Due to the observational design, we cannot functionally prove that FOXP3-positive T cells have a direct impact on tumor cell phenotype through immune selection or other mechanisms. An alternative explanation for the association of high FOXP3-positive cell counts in the vicinity of B2M-wt tumors may be provided by the observation that the activation of CD8-positive T cells, which is expected to be restricted to B2M-wt tumors, can contribute to the recruitment of FOXP3-positive cells to these tumors and the tumor environment,40 although intratumoral FOXP3-positive cell densities did not differ significantly between B2M-wt and B2M-mutant tumors in our study. Moreover, we cannot prove that FOXP3-positive T cells are in fact suppressor or Treg cells, although evidence from the literature strongly suggests that the vast majority of FOXP3-positive cells in the colon are of the regulatory or suppressor phenotype.41 Finally, the number of patients included in this study is limited to 24 Lynch syndrome mutations carriers (plus another 10 patients in the validation cohort), and we were only able to assess immune cell infiltration in specimens retrieved at the time point of surgery, i.e., when a tumor had already formed. Therefore, we cannot exclude potential immunological effects triggered by the tumor. Accordingly, larger and prospective studies are required to further evaluate our findings. Moreover, it will be interesting to study whether similar associations between B2M mutations and immune cell infiltration of tumor-adjacent mucosa are restricted to Lynch syndrome or whether they can also be observed in sporadic MSI-H colorectal cancers.
In summary, our study provides further evidence for the validity of the immunoediting concept in human MSI-H colorectal cancer development. Beyond the perspective of MSI-H cancers and Lynch syndrome, our results suggest that the immune milieu in normal colonic mucosa may play a critical role as a host factor in determining the individual risk of solid cancer development and the course of the disease. Moreover, our data indicate that therapeutic Treg cell depletion may improve patient outcome, by favoring tumor cell elimination as well as by allowing persistence and outgrowth only of less aggressive, i.e., B2M-mt, tumor cells.
Materials and methods
Patients
Tumor and normal tissue material from Lynch syndrome patients were collected at the Department of Applied Tumor Biology, University Hospital Heidelberg, as a center of the German HNPCC Consortium. Informed consent was obtained from all patients. All patients underwent surgery at the Department of Surgery at Heidelberg University Hospital between 2000 and 2014. None of the patients had received neoadjuvant treatment prior to surgery. The study was approved by the local Ethics Committee. Three different types of tissue samples were included in this study: (1) Lynch syndrome-associated colorectal cancer samples, (2) tumor-adjacent mucosa samples (i.e., from histologically normal-appearing, non-malignant colonic mucosa located immediately next to the respective tumor) and (3) tumor-distant mucosa samples (i.e., from histologically normal colonic mucosa located at the resection margins). In total, 24 Lynch syndrome patients were included in this study. From these patients, 30 tumor specimens as well as 30 tumor-adjacent mucosa samples and 46 tumor-distant mucosa samples were obtained for analysis. The difference between the number of Lynch syndrome patients and the number of tumor specimens/tumor-adjacent mucosa samples is due to the fact that some Lynch syndrome patients presented with two or more synchronous colorectal cancers. In these cases, tumor samples and tumor-adjacent normal mucosa samples from all individual colorectal cancers were included separately in this study. The increase in sample size for tumor-distant mucosa samples compared to the tumor-adjacent normal mucosa sample collection is due to the fact that for some patients two different FFPE specimens containing tumor-distant normal mucosa were available (usually from the two surgical resection margins). Concerning the specimens analyzed for intratumoral Treg infiltration, the same 30 tumor samples from 24 patients as outlined before were assessed. However, 6 tumor samples were excluded from analysis because of signet ring cell or 100% mucinous differentiation which is why only 24 tumor samples could be considered for statistical analysis. These 24 tumor samples were obtained from 21 different patients with three patients presenting with two synchronous tumors. Also, four tumor-adjacent mucosa samples and one tumor-distant mucosa sample had to be excluded because of poor sample quality.
As an independent validation cohort, 10 additional Lynch syndrome patients were assessed. From these patients, 12 tumor specimens corresponding to 12 tumor-adjacent mucosa samples and 19 tumor-distant mucosa samples were obtained for analysis of FOXP3-positive T cell infiltration in tumor-adjacent and tumor-distant normal mucosa. Intratumoral FOXP3-positive T cell infiltration was not assessed in this cohort.
Molecular tumor analysis
DNA was isolated from normal colonic mucosa and tumor tissue after manual microdissection of HE-stained tissue sections using the DNeasy Blood&Tissue Kit (Qiagen, Cat.No. 69581). For all samples that stained B2M-negative, exon-wise sequencing analysis of the B2M gene was performed as described previously.17
Immunohistochemistry
Immunohistochemical staining of tissue sections was performed as described previously.20,41 The following monoclonal antibodies were used: CD3: clone PS1, Acris Antibodies, Cat.No.DM112-05; CD8: clone 4B11, Novocastra, Product Code: NCL−CD8+-4B11; FOXP3: clone 236A/E7, eBioscience, Cat.No. 14-4777-82; B2M: clone L368.42
Quantification of T cells and lymph follicles
T cell infiltration (CD3, CD8, FOXP3) was assessed using computer-based image analysis software. Full scans of all IHC sections were obtained using a Hamamatsu NanoZoomer 2.0 HT scan system (Hamamatsu Tissue Imaging and Analysis Center, University of Heidelberg). All slides were scanned at 40x magnification (resolution of 230 nm/pixel). Special imaging software (TissuemorphDP, Visiopharm) was used for further analysis of the scans. Three regions of interest (ROI), each 2 mm2 for tumor tissue and 1 mm2 for normal mucosa, were defined within the tissue contained in each section. In a few cases, only two ROIs could be assessed due to limited amount of tissue available on the section. A quantification algorithm from TissuemorphDP software was applied to the ROIs that classified cells according to the strength of their DAB staining as positive or negative. Results were expressed as the percentage of the total ROI that stained positively. The results were then averaged for all three ROIs of a section.
Because FOXP3, in contrast to CD3 and CD8, is expressed in the nucleus, FOXP3-positive areas were adjusted to CD3-positive and CD8-positive areas to allow a direct comparison. This was achieved by using a conversion factor (1.5874) that was empirically determined by analyzing the area covered by 40 CD3-positive and 40 FOXP3-positive cells from 3 patients and subsequently averaging these results.
Lymph follicles situated in the mucosa or submucosa were counted manually on CD3-stained slides using a microscope (40x objective magnification). Total mucosa length on each slide was determined on CD3-scans using a measurement tool from TissuemorphDP software in order to normalize the number of lymph follicles to mucosa length.
Statistical evaluation
Fisher's exact test was applied for all pairwise comparisons regarding clinicopathological characteristics except for age for which the Mann–Whitney U test was used. To assess the effect of immune infiltration on B2M mutation status logistic regression models were applied. To account for repeated measurements within patients, this was done using generalized linear mixed models (GLMM). For numerical optimization Powell's BOBYQA algorithm was used.43 Likelihood ratio tests were used for comparing nested regression models. Results of tests with p values lower than 0.05 were considered to be statistically significant.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We greatly acknowledge the technical assistance of Petra Höfler and Beate Kuchenbuch.
Funding
This work was supported by a grant of the Deutsche Forschungsgemeinschaft (DFG, grant number DFG-KFO 227).
Supplemental Material
Supplemental Material may be downloaded here: publisher's website
References
- 1.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P et al.. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 2006; 313:1960-4; PMID:17008531; http://dx.doi.org/ 10.1126/science.1129139 [DOI] [PubMed] [Google Scholar]
- 2.Pages F, Galon J, Dieu-Nosjean MC, Tartour E, Sautes-Fridman C, Fridman WH. Immune infiltration in human tumors: a prognostic factor that should not be ignored. Oncogene 2010; 29:1093-102; PMID:19946335; http://dx.doi.org/ 10.1038/onc.2009.416 [DOI] [PubMed] [Google Scholar]
- 3.Pages F, Kirilovsky A, Mlecnik B, Asslaber M, Tosolini M, Bindea G, Lagorce C, Wind P, Marliot F, Bruneval P et al.. In situ cytotoxic and memory T cells predict outcome in patients with early-stage colorectal cancer. J Clin Oncol 2009; 27:5944-51; PMID:19858404; http://dx.doi.org/ 10.1200/JCO.2008.19.6147 [DOI] [PubMed] [Google Scholar]
- 4.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012; 12:252-64; PMID:22437870; http://dx.doi.org/ 10.1038/nrc3239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mittal D, Gubin MM, Schreiber RD, Smyth MJ. New insights into cancer immunoediting and its three component phases-elimination, equilibrium and escape. Curr Opin Immunol 2014; 27C:16-25; PMID:24531241; http://dx.doi.org/ 10.1016/j.coi.2014.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science 2011; 331:1565-70; PMID:21436444; http://dx.doi.org/ 10.1126/science.1203486 [DOI] [PubMed] [Google Scholar]
- 7.Matsushita H, Vesely MD, Koboldt DC, Rickert CG, Uppaluri R, Magrini VJ, Arthur CD, White JM, Chen YS, Shea LK et al.. Cancer exome analysis reveals a T-cell-dependent mechanism of cancer immunoediting. Nature 2012; 482:400-4; PMID:22318521; http://dx.doi.org/ 10.1038/nature10755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kloor M, Michel S, von Knebel Doeberitz M. Immune evasion of microsatellite unstable colorectal cancers. Int J Cancer 2010; 127:1001-10; PMID:20198617; http://dx.doi.org/ 10.1002/ijc.25283 [DOI] [PubMed] [Google Scholar]
- 9.Drescher KM, Sharma P, Lynch HT. Current hypotheses on how microsatellite instability leads to enhanced survival of Lynch Syndrome patients. Clin Dev Immunol 2010; 2010:170432; PMID:20631828; http://dx.doi.org/ 10.1155/2010/170432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bernal M, Garcia-Alcalde F, Concha A, Cano C, Blanco A, Garrido F, Ruiz-Cabello F. Genome-wide differential genetic profiling characterizes colorectal cancers with genetic instability and specific routes to HLA class I loss and immune escape. Cancer Immunol Immunother 2012; 61:803-16; PMID:22072317; http://dx.doi.org/ 10.1007/s00262-011-1147-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boland CR, Goel A. Microsatellite instability in colorectal cancer. Gastroenterology 2010; 138:2073-87.e3; PMID:20420947; http://dx.doi.org/ 10.1053/j.gastro.2009.12.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hampel H, Stephens JA, Pukkala E, Sankila R, Aaltonen LA, Mecklin JP, de la Chapelle A. Cancer risk in hereditary nonpolyposis colorectal cancer syndrome: later age of onset. Gastroenterology 2005; 129:415-21; PMID:16083698; http://dx.doi.org/ 10.1016/j.gastro.2005.05.011 [DOI] [PubMed] [Google Scholar]
- 13.Jasperson KW, Tuohy TM, Neklason DW, Burt RW. Hereditary and familial colon cancer. Gastroenterology 2010; 138:2044-58; PMID:20420945; http://dx.doi.org/ 10.1053/j.gastro.2010.01.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Linnebacher M, Gebert J, Rudy W, Woerner S, Yuan YP, Bork P, von Knebel Doeberitz M. Frameshift peptide-derived T-cell epitopes: a source of novel tumor-specific antigens. Int J Cancer 2001; 93:6-11; PMID:11391614; http://dx.doi.org/ 10.1002/ijc.1298 [DOI] [PubMed] [Google Scholar]
- 15.Schwitalle Y, Kloor M, Eiermann S, Linnebacher M, Kienle P, Knaebel HP, Tariverdian M, Benner A, von Knebel Doeberitz M. Immune response against frameshift-induced neopeptides in HNPCC patients and healthy HNPCC mutation carriers. Gastroenterology 2008; 134:988-97; PMID:18395080; http://dx.doi.org/ 10.1053/j.gastro.2008.01.015 [DOI] [PubMed] [Google Scholar]
- 16.Saeterdal I, Gjertsen MK, Straten P, Eriksen JA, Gaudernack G. A TGF betaRII frameshift-mutation-derived CTL epitope recognised by HLA-A2-restricted CD8+ T cells. Cancer Immunol Immunother 2001; 50:469-76; PMID:11761441; http://dx.doi.org/ 10.1007/s002620100222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kloor M, Michel S, Buckowitz B, Ruschoff J, Buttner R, Holinski-Feder E, Dippold W, Wagner R, Tariverdian M, Benner A et al.. Beta2-microglobulin mutations in microsatellite unstable colorectal tumors. Int J Cancer 2007; 121:454-8; PMID:17373663; http://dx.doi.org/ 10.1002/ijc.22691 [DOI] [PubMed] [Google Scholar]
- 18.Bernal M, Ruiz-Cabello F, Concha A, Paschen A, Garrido F. Implication of the beta2-microglobulin gene in the generation of tumor escape phenotypes. Cancer Immunol Immunother 2012; 61:1359-71; PMID:22833104; http://dx.doi.org/ 10.1007/s00262-012-1321-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dierssen JW, de Miranda NF, Ferrone S, van Puijenbroek M, Cornelisse CJ, Fleuren GJ, van Wezel T, Morreau H. HNPCC versus sporadic microsatellite-unstable colon cancers follow different routes toward loss of HLA class I expression. BMC Cancer 2007; 7:33; PMID:17316446; http://dx.doi.org/ 10.1186/1471-2407-7-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kloor M, Becker C, Benner A, Woerner SM, Gebert J, Ferrone S, von Knebel Doeberitz M. Immunoselective pressure and human leukocyte antigen class I antigen machinery defects in microsatellite unstable colorectal cancers. Cancer Res 2005; 65:6418-24; PMID:16024646; http://dx.doi.org/ 10.1158/0008-5472.CAN-05-0044 [DOI] [PubMed] [Google Scholar]
- 21.Surmann EM, Voigt AY, Michel S, Bauer K, Reuschenbach M, Ferrone S, von Knebel Doeberitz M, Kloor M. Association of high CD4-positive T cell infiltration with mutations in HLA class II-regulatory genes in microsatellite-unstable colorectal cancer. Cancer Immunol Immunother 2015; 64:357-366; PMID:25445815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Michel S, Linnebacher M, Alcaniz J, Voss M, Wagner R, Dippold W, Becker C, von Knebel Doeberitz M, Ferrone S, Kloor M. Lack of HLA class II antigen expression in microsatellite unstable colorectal carcinomas is caused by mutations in HLA class II regulatory genes. Int J Cancer 2010; 127:889-98; PMID:20013806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bonertz A, Weitz J, Pietsch DH, Rahbari NN, Schlude C, Ge Y, Juenger S, Vlodavsky I, Khazaie K, Jaeger D et al.. Antigen-specific Tregs control T cell responses against a limited repertoire of tumor antigens in patients with colorectal carcinoma. J Clin Invest 2009; 119:3311-21; PMID:19809157; http://dx.doi.org/ 10.1172/JCI39608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clarke SL, Betts GJ, Plant A, Wright KL, El-Shanawany TM, Harrop R, Torkington J, Rees BI, Williams GT, Gallimore AM et al.. CD4+CD25+FOXP3+ regulatory T cells suppress anti-tumor immune responses in patients with colorectal cancer. PLoS One 2006; 1:e129; PMID:17205133; http://dx.doi.org/ 10.1371/journal.pone.0000129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bauer K, Nelius N, Reuschenbach M, Koch M, Weitz J, Steinert G, Kopitz J, Beckhove P, Tariverdian M, von Knebel Doeberitz M et al.. T cell responses against microsatellite instability-induced frameshift peptides and influence of regulatory T cells in colorectal cancer. Cancer Immunol Immunother 2013; 62:27-37; PMID:22729559; http://dx.doi.org/ 10.1007/s00262-012-1303-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ogino S, Galon J, Fuchs CS, Dranoff G. Cancer immunology–analysis of host and tumor factors for personalized medicine. Nat Rev Clin Oncol 2011; 8:711-9; PMID:21826083; http://dx.doi.org/ 10.1038/nrclinonc.2011.122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC et al.. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363:711-23; PMID:20525992; http://dx.doi.org/ 10.1056/NEJMoa1003466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robert C, Thomas L, Bondarenko I, O'Day S, Weber J, Garbe C, Lebbe C, Baurain JF, Testori A, Grob JJ et al.. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med 2011; 364:2517-26; PMID:21639810; http://dx.doi.org/ 10.1056/NEJMoa1104621 [DOI] [PubMed] [Google Scholar]
- 29.Yano H, Thakur A, Tomaszewski EN, Choi M, Deol A, Lum LG. Ipilimumab augments antitumor activity of bispecific antibody-armed T cells. J Transl Med 2014; 12:191; PMID:25008236; http://dx.doi.org/ 10.1186/1479-5876-12-191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nancey S, Boschetti G, Cotte E, Ruel K, Almeras T, Chauvenet M, Stroeymeyt K, Moussata D, Kaiserlian D, Flourie B. Blockade of cytotoxic T-lymphocyte antigen-4 by ipilimumab is associated with a profound long-lasting depletion of Foxp3+ regulatory T cells: a mechanistic explanation for ipilimumab-induced severe enterocolitis? Inflamm Bowel Dis 2012; 18:E1598-600; PMID:22069060; http://dx.doi.org/ 10.1002/ibd.21927 [DOI] [PubMed] [Google Scholar]
- 31.Zhou J, Bashey A, Zhong R, Corringham S, Messer K, Pu M, Ma W, Chut T, Soiffer R, Mitrovich RC et al.. CTLA-4 blockade following relapse of malignancy after allogeneic stem cell transplantation is associated with T cell activation but not with increased levels of T regulatory cells. Biol Blood Marrow Transplant 2011; 17:682-92; PMID:20713164; http://dx.doi.org/ 10.1016/j.bbmt.2010.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chung KY, Gore I, Fong L, Venook A, Beck SB, Dorazio P, Criscitiello PJ, Healey DI, Huang B, Gomez-Navarro J et al.. Phase II study of the anti-cytotoxic T-lymphocyte-associated antigen 4 monoclonal antibody, tremelimumab, in patients with refractory metastatic colorectal cancer. J Clin Oncol 2010; 28:3485-90; PMID:20498386; http://dx.doi.org/ 10.1200/JCO.2010.28.3994 [DOI] [PubMed] [Google Scholar]
- 33.Xiao Y, Freeman GJ. The microsatellite instable subset of colorectal cancer is a particularly good candidate for checkpoint blockade immunotherapy. Cancer Discov 2015; 5:16-8; PMID:25583798; http://dx.doi.org/ 10.1158/2159-8290.CD-14-1397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Llosa NJ, Cruise M, Tam A, Wicks EC, Hechenbleikner EM, Taube JM, Blosser RL, Fan H, Wang H, Luber BS et al.. The vigorous immune microenvironment of microsatellite instable colon cancer is balanced by multiple counter-inhibitory checkpoints. Cancer Discov 2015; 5:43-51; PMID:25358689; http://dx.doi.org/ 10.1158/2159-8290.CD-14-0863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tikidzhieva A, Benner A, Michel S, Formentini A, Link KH, Dippold W, von Knebel Doeberitz M, Kornmann M, Kloor M. Microsatellite instability and Beta2-Microglobulin mutations as prognostic markers in colon cancer: results of the FOGT-4 trial. Br J Cancer 2012; 106:1239-45; PMID:22353804; http://dx.doi.org/ 10.1038/bjc.2012.53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koelzer VH, Baker K, Kassahn D, Baumhoer D, Zlobec I. Prognostic impact of beta-2-microglobulin expression in colorectal cancers stratified by mismatch repair status. J Clin Pathol 2012; 65:996-1002; PMID:22859396; http://dx.doi.org/ 10.1136/jclinpath-2012-200742 [DOI] [PubMed] [Google Scholar]
- 37.Simpson JA, Al-Attar A, Watson NF, Scholefield JH, Ilyas M, Durrant LG. Intratumoral T cell infiltration, MHC class I and STAT1 as biomarkers of good prognosis in colorectal cancer. Gut 2010; 59:926-33; PMID:20581241; http://dx.doi.org/ 10.1136/gut.2009.194472 [DOI] [PubMed] [Google Scholar]
- 38.Walsh MD, Dent OF, Young JP, Wright CM, Barker MA, Leggett BA, Bokey L, Chapuis PH, Jass JR, Macdonald GA. HLA-DR expression is associated with better prognosis in sporadic Australian clinicopathological Stage C colorectal cancers. Int J Cancer 2009; 125:1231-7; PMID:19462453; http://dx.doi.org/ 10.1002/ijc.24484 [DOI] [PubMed] [Google Scholar]
- 39.Baker K, Chong G, Foulkes WD, Jass JR. Transforming growth factor-beta pathway disruption and infiltration of colorectal cancers by intraepithelial lymphocytes. Histopathology 2006; 49:371-80; PMID:16978200; http://dx.doi.org/ 10.1111/j.1365-2559.2006.02520.x [DOI] [PubMed] [Google Scholar]
- 40.Spranger S, Spaapen RM, Zha Y, Williams J, Meng Y, Ha TT, Gajewski TF. Up-regulation of PD-L1, IDO, and T(regs) in the melanoma tumor microenvironment is driven by CD8(+) T cells. Sci Transl Med 2013; 5:200ra116; PMID:23986400; http://dx.doi.org/ 10.1126/scitranslmed.3006504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Michel S, Benner A, Tariverdian M, Wentzensen N, Hoefler P, Pommerencke T, Grabe N, von Knebel Doeberitz M, Kloor M. High density of FOXP3-positive T cells infiltrating colorectal cancers with microsatellite instability. Br J Cancer 2008; 99:1867-73; PMID:18985040; http://dx.doi.org/ 10.1038/sj.bjc.6604756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lampson LA, Fisher CA, Whelan JP. Striking paucity of HLA-A, B, C and beta 2-microglobulin on human neuroblastoma cell lines. J Immunol 1983; 130:2471-8; PMID:6187860 [PubMed] [Google Scholar]
- 43.Powell MJD. The BOBYQA algorithm for bound constrained optimization without derivatives. Cambridge, England: Department of Applied Mathematics and Theoretical Physics, Centre for Mathematical Sciences, University of Cambridge; 2009. [Google Scholar]
- 44.Hicklin DJ, Wang Z, Arienti F, Rivoltini L, Parmiani G, Ferrone S. beta2-Microglobulin mutations, HLA class I antigen loss, and tumor progression in melanoma. J Clin Invest 1998; 101:2720-9; PMID:9637706; http://dx.doi.org/ 10.1172/JCI498 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.