ABSTRACT
Cervical cancer is a high-incidence female cancer most commonly caused by human papilloma virus (HPV) infection of the genital mucosa. Immunotherapy targeting HPV-derived tumor antigens (TAs) has been widely studied in animal models and in patients. Because the female genital tract is a portal for the entry of HPV and a highly compartmentalized system, the development of topical vaginal immunotherapy in an orthotopic cancer model would provide an ideal therapeutic. Thus, we examined whether flagellin, a potent mucosal immunomodulator, could be used as an adjuvant for a topical therapeutic vaccine for female genital cancer. Intravaginal (IVAG) co-administration of the E6/E7 peptides with flagellin resulted in tumor suppression and long-term survival of tumor-bearing mice. In contrast to IVAG vaccination, intranasal (IN) or subcutaneous (SC) immunization did not induce significant tumor suppression in the same model. The vaginal adjuvant effect of the flagellin was completely abolished in Toll-like receptor-5 (TLR5) knock-out mice. IVAG immunization with the E6/E7 peptides plus flagellin induced the accumulation of CD4+ and CD8+ cells and the expression of T cell activation-related genes in the draining genital lymph nodes (gLNs). The co-administered flagellin elicited antigen-specific IFNγ production in the gLNs and spleen. The intravaginally administered flagellin was found in association with CD11c+ cells in the gLNs. Moreover, after immunization with a flagellin and the E6/E7 peptides, the TLR5 expression in gLN cells was significantly upregulated. These results suggest that flagellin serves as a potent vaginal adjuvant for a therapeutic peptide cancer vaccine through the activation of TLR5 signaling.
KEYWORDS: Flagellin, human papilloma virus, peptide, therapeutic vaccine, vaginal adjuvant
Abbreviations
- Ag
antigen
- ANOVA
analysis of variance
- APC
antigen presenting cell
- CIN
cervical intraepithelial neoplasia
- ELISPOT
enzyme-linked immunospot
- gLN
genital lymph node
- HPV
human papillomavirus
- IFNγ
interferon gamma
- IN
intranasal
- IP
intraperitoneal
- IVAG
Intravaginal
- KO
knockout
- N9
nonxynol-9
- PBS
phosphate buffered saline
- PRR
pattern recognition receptor
- qRT-PCR
quantitative reverse transcription polymerase chain reaction
- SC
subcutaneous
- TA
tumor antigen
- TLR
Toll-like receptor
- WT
wild type
Introduction
Cervical cancer, the fourth leading cause of death among female cancers, is most commonly caused by high-risk human papillomavirus (HPV) infection.1,2 Following HPV infection, a series of phenotypic changes gradually develop from precancerous lesions called cervical intraepithelial neoplasia (CIN), graded 1–3 (CIN I-III) to invasive cervical cancer.3 Immunotherapy targeting the E6 and E7 (E6/E7) oncogenes of high-risk HPV type 16 (HPV-16) has been developed for the treatment of CIN and cancers.4,5
The female genital tract is a highly compartmentalized mucosal system that serves as an entry point for HPV.6,7 The tumor microenvironment is generally immunosuppressive, preventing an immune response to TAs.6-11 Therefore, for the successful clinical application of immunotherapy in cervical cancer or high-grade CIN, a topical immunization that can overcome immune tolerance and induce site-specific, local antitumor immune responses in the genital system is necessary.6,7,12-14 Ligands for pattern-recognition receptors (PRRs) have been actively evaluated as adjuvants for a wide spectrum of vaccines and immunotherapeutics.15-17 We previously reported that a bacterial flagellin, which is an agonist of TLR5,18,19 is an excellent adjuvant for various vaccines.17,20-24 It has been reported that not all TLRs are expressed in the female genital tract and that TLR5/TLR12 and TLR1/TLR2/TLR5/TLR13 are expressed in the uterus and vagina, respectively.25 The expression of TLR5 in the genital tissue in particular is much higher than that of other TLRs, regardless of the estrous cycle.25 As a therapeutic cancer vaccine regimen, whole tumor cell- or protein/peptide-antigens (Ags) have been suggested. Among these, the Ag-associated peptide vaccine can bypass Ag-processing and directly bind to MHC molecules on Ag-presenting cells (APCs) to interact with cognate T cells.26,27 We have previously shown that flagellin enhanced tumor-specific CD8+ T cell immune responses in a therapeutic cancer vaccine model.24 Given that the co-administration of flagellin with TA induced antitumor effects in a SC tumor implantation model, we hypothesized that flagellin could be used as a vaginal adjuvant for a peptide-based therapeutic anticancer vaccine in an orthotopic genital cancer model. It has been reported that IVAG administration of TLR3, 7, or 9 agonists enhances Ag-specific immune responses elicited by systemic immunization.13,28 In the present study, we examined whether flagellin, which is a potent mucosal immunomodulator, could be used as an adjuvant for a topical therapeutic cancer vaccine in a genital cancer model.
To trace in vivo tumor growth using non-invasive imaging technology, we implanted TC-1 cells expressing luciferase (TC-1-luc cells) in the female genitalia of mice, as previously described.29 Diestrus mice were co-administered topically with the E6/E7 peptides with or without flagellin. We showed that IVAG immunization with the E6/E7 peptides in combination with Vv-FlaB, a Vibrio vulnificus flagellin, suppressed tumor growth, leading to significantly longer survival of the tumor-bearing mice. In contrast to the IVAG vaccination, IN or SC administration did not induce significant tumor suppression in our orthotopic cancer model. Here, we propose that flagellin is a promising topical vaginal adjuvant for the enhancement of TA-specific antitumor immune responses in genital cancer or high-grade CIN (CIN II/III).
Results
IVAG immunization, and not IN or SC immunization, with the E6/E7 peptides plus flagellin suppresses tumor growth and promotes the long-term survival of tumor-bearing mice in a genital cancer model
To evaluate the efficacy of the flagellin-adjuvanted therapeutic cancer vaccine, we established an orthotopic genital cancer model by IVAG implantation of 1×105 TC-1-luc cells in C57BL/6 mice, as previously described.12,30 The tumor-bearing mice underwent IVAG, IN or SC immunization with phosphate-buffered saline (PBS) or the E6/E7 peptides plus Vv-FlaB (P+F) at days 3, 8 and 13 after TC-1-luc cell implantation, and tumor growth was monitored by bioluminescence detection (Fig. 1A). To enhance the transepithelial uptake of the E6/E7 peptides and Vv-FlaB, the TC-1-luc cell-implanted diestrus mice were treated with N9, as previously described.13,31 As shown in Fig. 1B and C, only the tumor-bearing mice with the IVAG immunization showed tumor suppression and long-term survival. In contrast to IVAG vaccination, IN or SC vaccination with P+F did not manifest any antitumor effect compared with the PBS vaccination in this genital cancer model (p > 0.05). The E6/E7-specific IFNγ spots were detected only in the IVAG group (Fig. S1). This result suggests that flagellin can be a promising topical vaginal adjuvant for inducing strong antitumor immune responses in genital tissues. In this context, IVAG immunization was selected for further mechanistic investigation.
Figure 1.
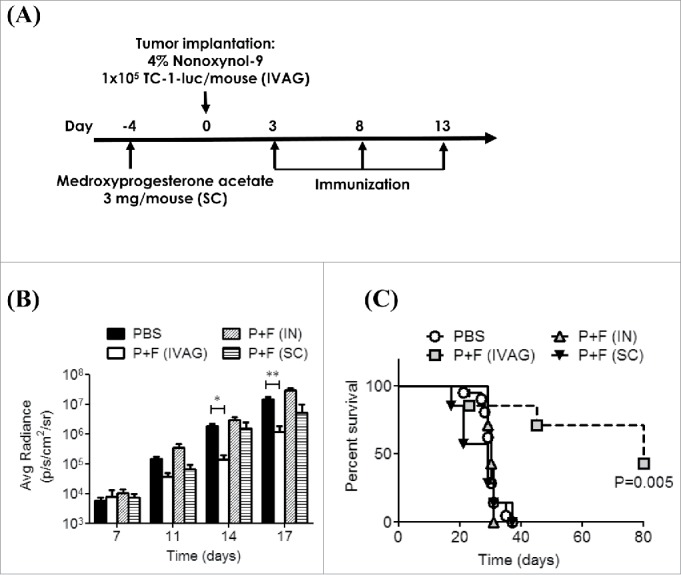
IVAG immunization, but not intranasal (IN) or SC immunization, with the E6/E7 peptides and flagellin suppresses tumor growth, leading to long-term survival in a genital cancer model. (A) Groups of C57BL/6 mice were pretreated with 3mg/mouse medroxyprogesterone (day -4) and N9 (day 0) and then the 1×105 TC-1-luc cells were intravaginally administered to the mice to induce tumor formation. The mice then underwent IVAG, IN or SC immunization with a mixture of 4 μg Vv-FlaB and 50 μg each E6 and E7 on days 3, 8 and 13, as described in the Materials and Methods section. Bioluminescence imaging was performed on day 7 or day 13. The imaging signals were collected using a cooled charge-coupled camera device, and signal intensities were assessed quantitatively in the tumor regions by measuring the maximum photons per second per centimeter squared per steradian (p/s/cm2/sr), followed by plotting as a function of time after the TC-1-luc challenge (B). The long-term survival of the tumor-bearing mice is shown in (C). (***indicates p < 0.001).
The antitumor effect induced by the co-administration of the E6/E7 peptides plus flagellin is abolished in TLR5 knockout mice
To determine whether the antitumor effect was mediated by the modulation of an Ag-specific immunity and whether the adjuvant effect was TLR5-dependent, wild-type (WT) or TLR5 knockout (KO) C57BL/6 mice were immunized with PBS (PBS), Vv-FlaB (F), the E6/E7 peptides (P) or the E6/E7 peptides plus Vv-FlaB (P+F). The in vivo tumor growth and the survival of the immunized mice were then assessed. The bioluminescence signal increased to 2.21×107 ± 4.96×106 p/s/cm2/sr in the PBS-treated control group (PBS) at 19 d after tumor cell implantation and that in the E6/E7 peptide-treated group (P) was 7.30×106 ± 3.68×106 p/s/cm2/sr in the WT mice (Fig. 2A). Notably, the co-administration group (P+F) showed significant inhibition of tumor growth (2.07×105 ± 1.67×105 p/s/cm2/sr) compared with the p group (p < 0.001) (Fig. 2A). Additionally, the Vv-FlaB-treated mice (F) showed tumor volumes (1.34×107 ± 6.49×106 p/s/cm2/sr) that were comparable to those of the PBS-treated control group (p > 0.05) (Fig. 2A). These results indicate that Vv-FlaB itself does not suppress TC-1 cell growth in vivo and potentiates the E6/E7-specific host immune response to suppress tumor growth in the present orthotopic therapeutic cancer vaccine model.
Figure 2.
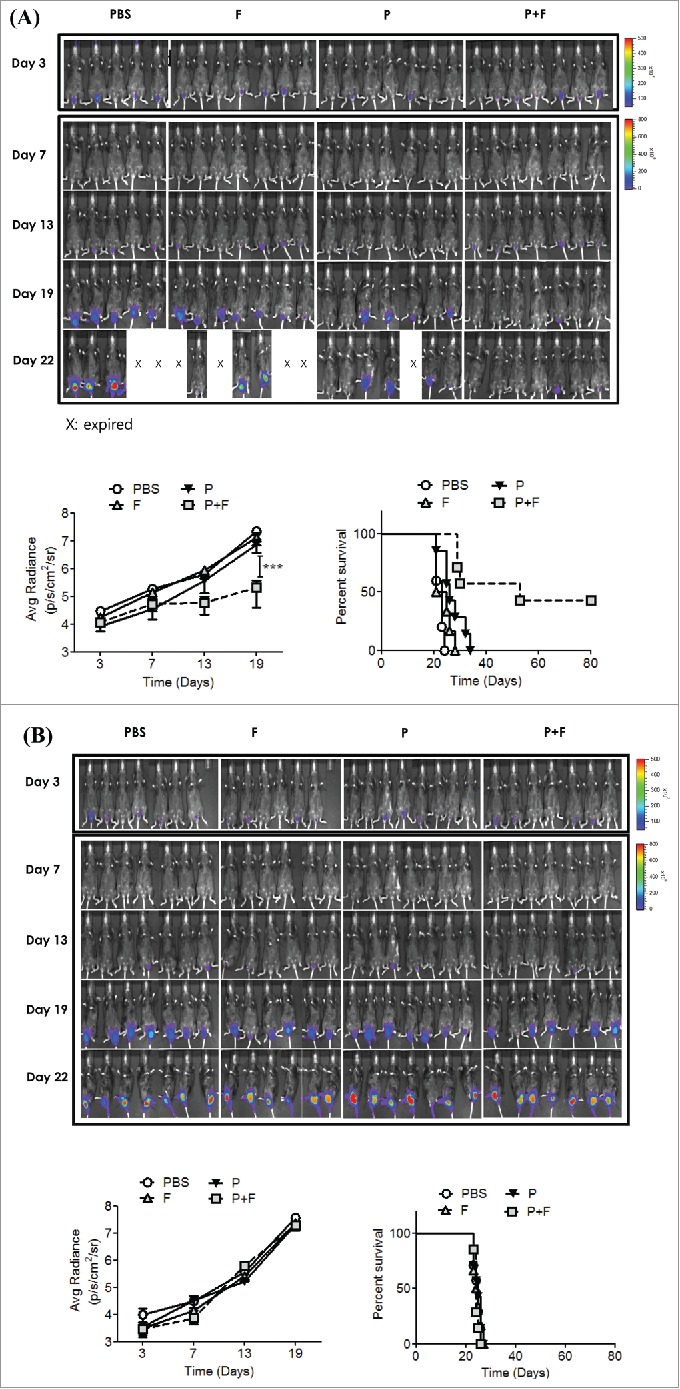
The antitumor effect induced by co-administration of the E6/E7 peptides with flagellin is TLR5 dependent. Groups of WT (A) and TLR5 KO (B) C57BL/6 mice were pretreated with 3mg/mouse medroxyprogesterone and N9 as described in the Materials and Methods section and then the 1×105 TC-1-luc cells were intravaginally administered to the mice to induce tumor formation. The mice then underwent IVAG immunization with PBS (PBS), 4 μg Vv-FlaB (F), 50 μg each E6 and E7 (P), or a mixture of 4 μg Vv-FlaB with 50 μg each E6 and E7 (P+F) on days 3, 8 and 13, as described in the Materials and Methods section. Bioluminescence imaging was performed four times in WT and TL-5 KO C57BL/6 mice, the imaging signals were collected using a cooled charge-coupled camera device, and signal intensities were assessed quantitatively in the tumor regions by measuring the maximum photons per second per centimeter squared per steradian (p/s/cm2/sr), followed by plotting as a function of time after the TC-1-luc challenge. The difference in tumor growth between the P+F and the p experimental groups was statistically significant (***indicates p < 0.001). Groups of vaccinated mice (n = 5–7) were monitored for survival. Representative results from one of three independent experiments are shown. The survival curve was constructed according to the Kaplan–Meier method, and the statistical significance was determined by the log-rank test. The difference in survival between the P+F and the p experimental groups was statistically significant (* indicates p < 0.05 on day 19).
We also evaluated the long-term survival of the vaccinated WT mice (Fig. 2A). Eighty days after the TC-1-luc cell challenge in WT mice, the cumulative survival rate in the P+F group (n = 7) was 43%. The survival rates in other groups (PBS, F and P) were all 0%. The P+F group survival was significantly prolonged compared to that of the p group (p < 0.05) (Fig. 2A). To determine whether the antitumor effect induced by IVAG E6/E7 plus flagellin was mainly mediated by host TLR5 signaling, we assessed the tumor growth and survival of the tumor-bearing TLR5 KO mice. As shown in Figure 2B, the E6/E7 peptides plus the flagellin-mediated antitumor effect was almost completely abolished in the TLR5 KO mice. Although the kinetics of tumor growth were delayed until day 13 in the TLR5 KO mice (p < 0.05, ANOVA), the end-point tumor burden in the KO mice at day 19 was comparable to that observed in the WT mice. Moreover, all of the groups of KO mice (PBS, P, F or P+F) died within 27 d (Fig. 2B). These results clearly indicate that the antitumor effect induced by IVAG E6/E7 co-administered with flagellin was dominated by the TLR5 signaling in the host.
IVAG immunization with the E6/E7 peptides plus flagellin induces E6/E7-specific IFNγ production in the gLNs
To determine whether IVAG vaccination with flagellin and the E6/E7 peptides could induce antitumor immune responses in the genital and systemic immune systems, we measured the number of E6/E7-specific, IFNγ-producing cells among gLN cells and splenic mononuclear cells (SPL) by the ELISPOT assay. To facilitate the transepithelial uptake of the E6/E7 peptides plus flagellin, diestrus-synchronized mice were administered N9 during the first IVAG vaccination (Fig. 3A). As shown in Figure 3B, the number of E6/E7-specific IFNγ spots in the P+F group was significantly higher than that in the p group (p < 0.01). Among the SPL, a significant number of E6/E7-specific IFNγ spots were detected only in the P+F group (P+F: 19.00 ± 4.79) (Fig. 3C). The E6/E7-specific IFNγ spots were not detected in the P+F group of TLR5 KO mice. This result shows that the IVAG P+F vaccination induced effective Ag-specific antitumor immune responses in both local and systemic compartments in a TLR5-dependent manner.
Figure 3.
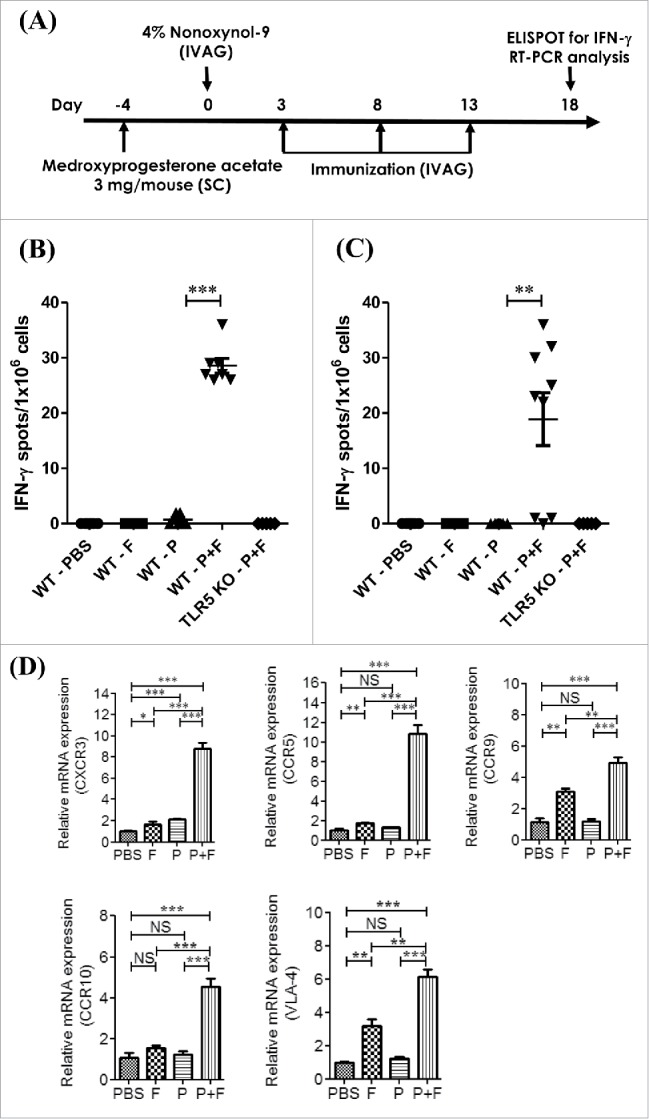
IVAG immunization with the E6/E7 peptides and flagellin induces E6/E7-specific IFNγ production in the gLNs. Groups of C57BL/6 mice (WT) and TLR5 KO mice were pretreated with 3mg/mouse medroxyprogesterone and N9 as described in the Materials and Methods section. The mice were intravaginally immunized with PBS (PBS), 4 μg Vv-FlaB (F), 50 μg each E6 and E7 (P) or 4 μg Vv-FlaB along with 50 μg each E6 and E7 (P+F) 3 times at 5-d intervals. The mice were euthanized 3 d after the last vaccination (A). The genital iliac lymph nodes (B) and splenocytes (C) were then prepared to evaluate E6/E7-specific IFNγ production. The immune cells were stimulated in vitro with the E6/E7 peptides (1 μg/mL each) for 3 d The number of E6/E7 peptide-specific IFNγ-producing cells generated in vaccinated mice was determined by group by ELISPOT assay. The genital iliac lymph nodes were also prepared to evaluate the mRNA expression of immune-related genes. Total RNA was isolated from the gLNs of C57BL/6 mice and mRNA expression levels were measured by qRT-PCR for the indicated genes (D). The bar charts show representative data, consisting of the mean ± SEM from three individual experiments. * indicates p < 0.05, ** indicates p < 0.01 and *** indicates p < 0.001.
IVAG immunization with the E6/E7 peptides plus flagellin induces immune cell activation in the gLNs
To evaluate the immune modulation in the gLNs induced by IVAG immunization with P+F, we determined the mRNA expression levels of genes related to T cell activation.28,32,33 The group of mice that was intravaginally immunized with the E6/E7 peptides plus flagellin (P+F) displayed significantly increased expression levels of the CXCR3, CCR5, CCR9, CCR10 and VLA-4 genes compared with the PBS group (p < 0.001; Fig. 3D). In contrast, the group immunized with the E6/E7 peptides alone (P group) did not exhibit enhanced CCR5, CCR9, CCR10 or VLA-4 expression but did show an increase in CXCR3. Although the E6/E7 peptides alone (P group) enhanced CXCR3 expression (p < 0.001), the gene expression was higher (p < 0.001) in the P+F group compared with the p group. In addition, IVAG administration of flagellin alone induced significantly higher expressions of the CXCR3, CCR5, CCR9, and VLA-4 genes. However, the expression levels of the CXCR3, CCR5, CCR9, CCR10 and VLA-4 genes in the P+F group were significantly higher than those in the F group (p < 0.01 or p < 0.005, respectively). This result indicates that TA or flagellin alone does not activate a sufficient immune response in draining LNs to induce tumor suppression and that co-administered flagellin along with TAs induces significant immunomodulation to induce local antitumor immune responses.
To determine the changes in the T cell population in the gLNs, we measured CD4+ and CD8+ cells in the gLNs by flow cytometry. The percentage of the cell population expressing CD8+ (p < 0.001) or CD4+ (p < 0.05) significantly increased in the P+F group compared with the p group. In contrast, the group that received flagellin alone (F) showed no increase in the CD4+ or CD8+ cell population compared with the PBS group (Fig. 4). These results suggest that only the IVAG co-administration of TAs and flagellin can induce efficacious T cell immune responses in the draining LNs.
Figure 4.
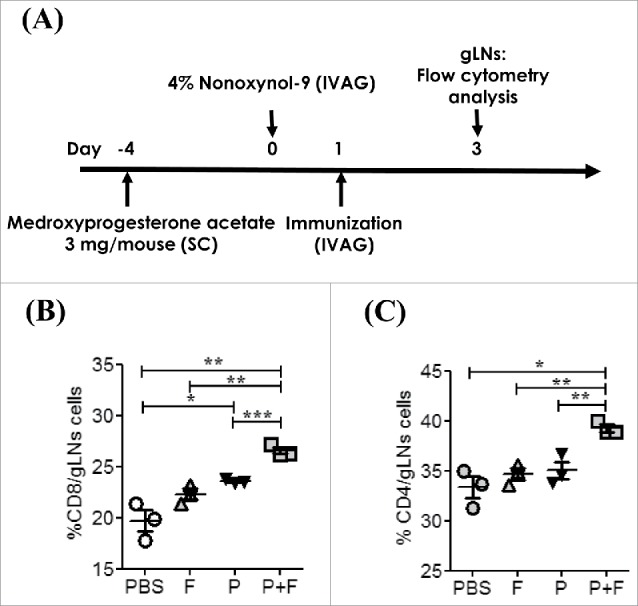
IVAG immunization with the E6/E7 peptides and flagellin induces immune cell activation in gLNs. (A) Groups of C57BL/6 mice were pretreated with 3mg/mouse medroxyprogesterone and N9 as described in the Materials and Methods section. The mice were intravaginally immunized with PBS (PBS) or 4 μg Vv-FlaB along with 50 μg each E6 and E7 (P+F) Three days after administration, the CD8+ (B) and CD4+ (C) cell populations in gLNs were measured by flow cytometry.
Intravaginally administered flagellin and peptide employ CD11c+ cells in the vagina and gLNs for their effects
To better understand the possible mechanisms underlying the effects of IVAG-administered flagellin in vivo, Vv-FlaB conjugated with FNR-675 (Vv-FlaB-FNR 675) was administered into N9-treated diestrus mice, and the interaction of Vv-FlaB-FNR 675 with the specific cell population was assessed by flow cytometry and confocal microscopy. Within one hour of Vv-FlaB-FNR 675 administration, we observed a clustering of CD11c+ cells in the luminal side epithelium of the vaginal canal that tended to co-localize with the administered Vv-FlaB-FNR 675 (Fig. S2–S4). Interestingly no such clustering of the CD11c+ cell population was observed in the PBS-administered group (Figs. S2–S5). Four to five hours after immunization, intravaginally administered Vv-FlaB-FNR675 was detected on CD11c+ cells (Fig. 5A and B). To locate the whereabouts of Vv-FlaB+CDllc+ cells in the gLN, we stained the cells in successive sections with CD11c and CD3 antibodies at the 4th h after administration. We observed that Vv-FlaB+CDllc+ cells located in the T cell areas of gLNs (Fig. 5C). By the 4th h post-flagellin plus E6/E7 peptide administration, CD11c+ cell numbers increased in the gLNs (Fig. 6). Taken together, these results show that intravaginally administered flagellin directly interacts with immune cells (mostly antigen-presenting cells) in situ in the vagina as well as in draining gLNs and, consequently, modulates the antigen-specific immune response.
Figure 5.
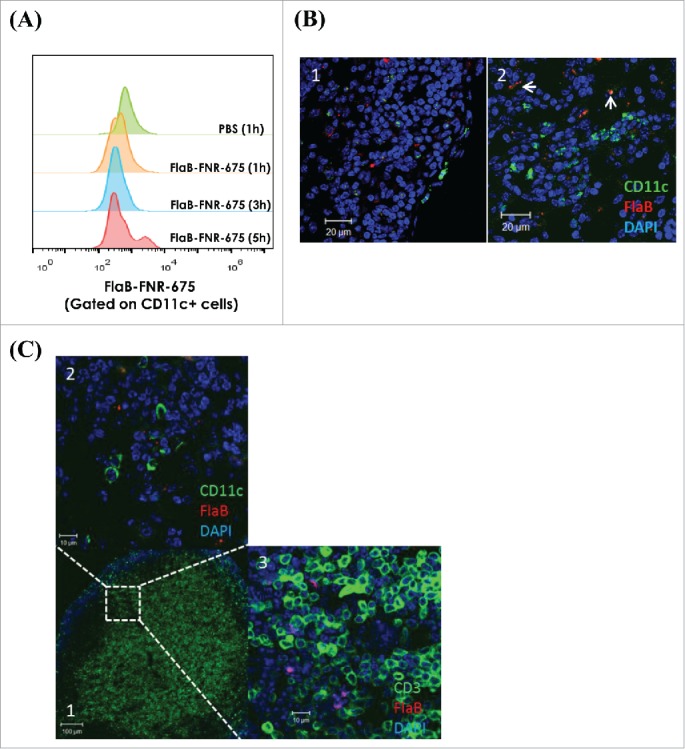
Intravaginally administered flagellin interacts with CD11c+ cells in the gLNs. Groups of C57BL/6 mice were pretreated with 3mg/mouse medroxyprogesterone and N9 as described in the Materials and Methods section. The mice were intravaginally administered 14 μg Vv-FlaB-FNR 675. One, three and five h after administration, the gLNs-cells were prepared to determine the localization of the Vv-FlaB-FNR 675 in the gLNs by flow cytometry (A). The genital iliac lymph nodes were also prepared for confocal microscopic observation 4 h after administration (B, C).
Figure 6.
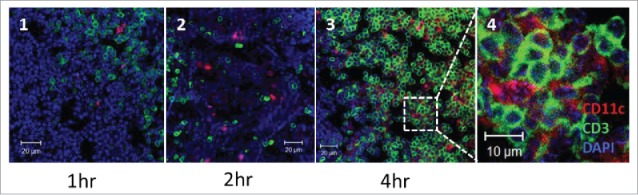
Intravaginally administered flagellin interacts with CD11c+ cells in the gLNs. Groups of N9-treated diestrus C57BL/6 mice as described in the Materials and Methods section were intravaginally administered with 4 μg Vv-FlaB along with 50 μg each E6 and E7. The mice were euthanized one, 2 and 4 h after administration (panel 1 at 1hr; panel 2 at 2 hr; panels 3 and 4 at 4 hr) and the genital iliac lymph nodes were prepared to observe CD11c+ and CD3+ cells in the gLNs by confocal microscopy.
IVAG immunization with the E6/E7 peptides plus flagellin induces TLR5 expression in the gLNs and the vagina
To decipher the reason for the notable immune responses in the P+F group post-vaccination, we measured the TLR5 gene expression levels in gLNs as well as in the vagina. The TLR5 expression was significantly enhanced upon flagellin plus E6/E7 peptides immunization in gLNs (Fig. 7A), which was confined to CD11c+ cells (Fig. 7B; Fig. 7C-2, white arrow). Non-CD11c+ cells also expressed TLR5 (Fig. 7C-2, red arrow). In the vaginal tissue, we also observed a marked increase in the TLR5 expression (Fig. S3A), especially in CD11c+ cells (Fig. S3B-S2 white arrow). The TLR5 expression on non-CD11c+ cells was observed in PBS-administered vaginal mucosa (Fig. S3B-S1, red arrow). This result suggests that IVAG vaccination with the E6/E7 peptides with flagellin upregulates TLR5-signaling cells in draining LNs, which presumably further augmented the adjuvant effect of flagellin.
Figure 7.
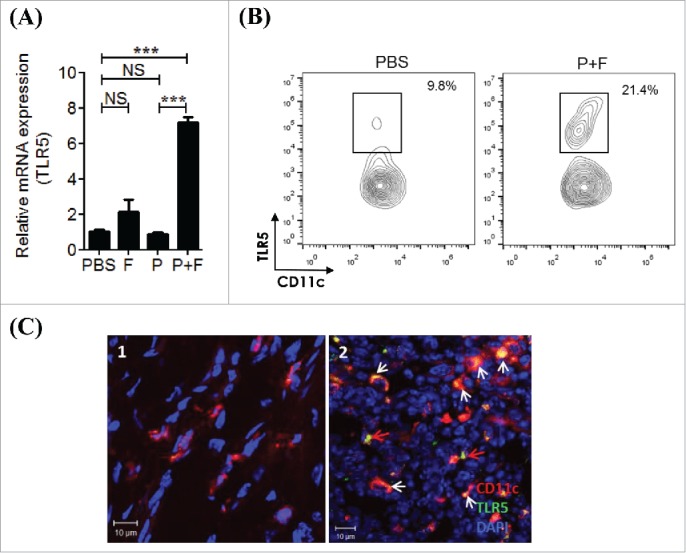
IVAG immunization with the E6/E7 peptides and flagellin induces TLR5 expression in the gLNs. (A) Groups of C57BL/6 mice were pretreated with 3mg/mouse medroxyprogesterone and N9 as described in the Materials and Methods section. The mice were intravaginally immunized with PBS (PBS), 4 μg Vv-FlaB (F), 50 μg each E6 and E7 (P) or 4 μg Vv-FlaB along with 50 μg each E6 and E7 (P+F) 3 times at 5-d intervals. The mice were euthanized 3 d after the last vaccination. The genital iliac lymph nodes were then prepared to determine the mRNA expression of immune-related genes. Total RNA was isolated from the gLNs and mRNA expression levels were measured by qRT-PCR for the indicated genes. (B) Groups of C57BL/6 mice were intravaginally immunized with PBS (PBS) or 4 μg Vv-FlaB along with 50 μg each E6 and E7 (P+F). Three days after immunization, single-cell suspensions of gLN cells were prepared and TLR5 expression on the CD11c+ cells in the gLN was measured by flow cytometry. (C) Groups of C57BL/6 mice were intravaginally immunized with PBS (PBS) or 4 μg Vv-FlaB along with 50 μg each E6 and E7 (P+F). Four hours after immunization, TLR5 expression was determined by confocal microscopy. *** indicates p < 0.001.
Discussion
We demonstrated that IVAG immunization with peptide-based TA in combination with flagellin induces orthotopic tumor suppression and long-term survival of tumor-bearing mice in a TLR5-dependent manner. The flagellin-mediated vaginal adjuvant effect was not observed for IN or SC immunization in the same orthotopic cancer model. Intravaginally administered soluble peptides and flagellin directly interacted with CD11c+ cells in the gLNs, leading to an increase in CD4+ and CD8+ cell accumulation in the gLNs. Furthermore, IVAG co-administration of the E6/E7 peptides with flagellin was sufficient to enhance T cell activation-related gene expression and E6/E7-specific IFNγ production in the gLNs.
The female genital tract is an entry point for HPV, the major etiologic agent of CIN and cervical cancer. A topical cancer vaccine for CIN or cervical cancer that induce site-specific and Ag-specific immune responses could be a useful approach in clinical applications. It is well-known that the anatomical site determines the microenvironments of implanted tumor cells and exerts a strong influence on the response to tumor immunotherapy.10,34 In the present study, we evaluated whether flagellin can be a useful vaginal adjuvant for therapeutic cervical cancer vaccines using an orthotopic genital cancer model, with the goal of developing a topical cancer vaccine system. We found that IVAG co-administration of flagellin with the E6/E7 peptides strongly induced site-specific immune responses against TAs. As shown in Figure 2, the strong vaginal adjuvant effect of flagellin was completely abolished in TLR5 KO mice. However, in TLR5 KO mice, the in vivo growth of implanted TC-1-luc cells was somewhat delayed by the P+F immunization (Fig. 2B; p < 0.05, ANOVA), suggesting the involvement of other PRR systems that can recognize flagellin, such as the NLRC4 inflammasome pathway.35 However, the tumor burden in the KO mice at day 19 was comparable to that observed in WT mice. Additionally, the P+F (E6/E7 peptides with flagellin) group of KO mice died within 27 d, whereas in WT mice, the survival rate at day 80 was 43%. These results clearly indicate that the TLR5 signaling pathway played a dominant role in the tumor suppression induced by the IVAG E6/E7 peptides plus flagellin vaccination. The usefulness of flagellin as a topical adjuvant for therapeutic vaccines for CIN and cervical cancer should be noted by the preferential expression of TLR5 in vaginal tissue.25 and the further induction of TLR5 in local tissue and draining LNs after topical treatment (Fig. 7).
In the present study, we used an orthotopic genital cancer model based on TC-1-luc cell implantation in N9-treated diestrus synchronized mice, as previously described.12,13 Compared with the SC implantation model, tumor progression was far more robust in the orthotopic model. For example, when 1×105 TC-1-luc cells or 5×105 TC-1 cells were implanted in the cervical genital tissues or the dorsal area, survival rates were comparable in the orthotopic and SC groups (Fig. 1 and Fig. 2).24 Because the in vivo tumor growths of TC-1 and TC-1-luc cells were not significantly different (data not shown), these results suggest that the genital tissue provides more a favorable microenvironment for the growth of TC-1 tumor cells. Despite more rapid tumorigenesis in the orthotopic genital cancer model, the IVAG vaccination with E6/E7 peptides plus flagellin potently suppressed tumor growth in genital tissue, leading to long-term survival of the tumor-bearing mice in this model (Figs. 1 and 2). Surprisingly, IN or SC immunization did not show in vivo tumor suppression in the same orthotopic genital cancer model (Fig. 1). In a previous report, we showed that IN immunization using flagellin and E6/E7 peptides in naïve mice induced E6/E7 peptide-specific IFNγ production in draining cervical lymph nodes (cLNs).23 In this study, we determined the E6/E7 peptide-specific IFNγ production only in the draining gLNs (not in cervical LNs) since we wanted to observe local immune responses that would more closely reflect antitumor activities in the genital cancer. Notably, IN immunization using E6/E7 peptides and flagellin was not sufficient to induce significant antitumor responses against the genital cancer (Fig. 1), while being able to induce Ag-specific IFNγ production in draining cLNs. Generally, intranasally immunized antigens induce paralleled secretory antibody responses both in the airway and in genital tracts.20,22 However, in the case of antitumor cellular immune responses, IN immunization may not necessarily induce resonant reactions in the genital tissue. These results suggest that flagellin is a strong vaginal (topical) adjuvant for the induction of site-specific antitumor immune responses. Peptide-based anticancer vaccines have been proposed and pursued by many groups because of their advantages in terms of safety, ease of production and stability.24,36-39 We also traced the movement of E6-FITC conjugates post-IVAG administration. The intravaginally administered E6-FITC could be detected in gLNs by FACS analysis. E6-FITC started to appear in the gLNs within the 1st h of its administration and remained co-localized with CD11c+ DC for extended periods (data not shown). Our data indicate that flagellin acts with minimal antigenic peptides (E6/E7, 8–9 amino acids) to activate a potent antitumor immune response. IVAG vaccination with a therapeutic peptide cancer vaccine in an orthotopic genital tumor would provide pharmacokinetic advantages, circumventing limitations in systemic administration by directly accessing target sites,10 and also by providing a dose-sparing effect.
It is believed that there are no organized mucosal-associated inductive sites in the genital tubes of naïve mice.40 Our data showed that administered soluble flagellin interacted with CD11c+ cells in vaginal mucosa and the gLNs (Fig. 5 and Fig. S2) Given that CXCR3, CCR5, CCR9, CCR10 and VLA-4 were highly expressed on effector T lymphocytes,33,41-43 our results clearly indicate that IVAG immunization with the E6/E7 peptides and flagellin could induce site-specific cellular immune responses. Although the flagellin-treated mice showed elevated expressions of T cell activation-related genes (CXCR3, CCR5, CCR9, and VLA-4), only the P+F group displayed Ag-specific IFNγ production. This result indicates that co-administration of the E6/E7 peptides and flagellin synergistically potentiated antitumor immune responses that should have been basally primed by the tumor by itself. Stimulation of the basally primed local immune system by only the peptide antigen was not sufficient to induce a therapeutic-level immune response. The advantage of the P+F vaccination would be additional stimulation of innate immune cells interacting with Ag-specific T cells and self-amplification of TLR5 signaling by further inducing TLR5 expression in local tissues. Under our in vivo experimental condition, the enhanced TLR5 expression could be due to either upregulation of the TLR5 or recruitment of TLR5-expressing cells to the gLNs. In our previous study of IN vaccination, we observed that flagellin treatment increased the number of TLR5+ CD11c+ DCs in the cLNs. Interestingly, each TLR5-positive cell showed a significantly higher expression level.20 To address the underlying mechanism, further study will be needed.
Our data suggest that administration of a flagellin-adjuvanted peptide vaccine as a topical therapeutic vaccine could induce regression of established genital cancer. However, systemic or IN vaccination of flagellin and peptides did not show tumor suppression. Though we did not test a systemic vaccination followed by IVAG administration of adjuvants as previously reported,13,28 we clearly showed that co-administration of flagellin and peptides effectively induces T cell immune responses in the draining lymph nodes, leading to tumor regression in responding lesions. Because a significant amount of flagellin was detected in the gLNs within 4 to 5 h of IVAG administration (Fig. 5), topical vaccination represents a promising approach for a therapeutic vaccine for CIN and cervical cancer.
For IVAG administration, the mice were pretreated with Sayana (medroxyprogesterone) and N9 as previously described.13,31 It is believed that this pretreatment enhances the transepithelial uptake of peptides and flagellin and accelerates immune responses in the gLNs. Generally, the barrier is disturbed in genital tumor-bearing mice. Therefore, it is probable that vaccine uptake and subsequent access to the draining lymph nodes are facilitated in tumor-bearing mice. Therefore, IVAG co-administration of the appropriate Ag with the vaginal adjuvant flagellin can elicit optimal site-specific immune responses, inducing tumor suppression in the genital compartment. Pettini et al showed that vaginal immunization can induce Ag-loaded dendritic cells within the draining lymph nodes and can recruit T cells.40 Cuburu et al also reported that IVAG immunization induces tissue-resident CD8+ T cell responses.31 This report supports the conclusion that IVAG vaccination is sufficient to induce immune responses in the genital compartment. Furthermore, tertiary lymphoid structures (TLSs) with the capacity to expand protective immune responses have been widely noted in chronic inflammatory settings (paper submitted).44 It has also been reported that a therapeutic vaccination targeting HPV-16 induces T cell responses that localize in mucosal lesions in humans.45
Taken together, these results suggest that IVAG administration is a promising alternative route for vaccination for CIN or cervical cancer and that flagellin, a TLR5 ligand, could be an optimal vaginal adjuvant candidate. To achieve the maximal immunotherapeutic efficacy of the flagellin-adjuvanted peptide vaccine, combination therapy with checkpoint targeting mAbs such as anti-CTLA-4 and/or anti-PD-1,46 could be applicable. Because the development of cervical cancer from high-grade precancerous lesions (CIN II/CIN III) into invasive cancer occurs over a long period of time, HPV-specific immunotherapy in the CIN II or CIN III period could present the opportunity to prevent invasive tumor development. In the present study, we propose consideration of the therapeutic application of flagellin as a vaginal adjuvant for peptide vaccines in immunotherapy for HPV-induced high-grade CIN (CIN II/III) or severe cervical cancer.
Materials and methods
Cell line and mice
The TC-1-luc cell line, TC-1 transfected with a luciferase gene, was used to trace orthotopic tumor growth.29 Six- to seven-week-old female C57BL/6 mice were purchased from the Korea Research Institute of Bioscience and Biotechnology (KRIBB, Daejeon, Korea). TLR5−/− knockout (KO) mice were bred in our animal facility as described previously.24 All of the animal experimental procedures were conducted in accordance with the guidelines of the Animal Care and Use Committee of Chonnam National University.
Peptides
The E6 (amino acids 50–57: YDFAFRDL) and E7 (amino acids 49–57: RAHYNIVTF) peptides from HPV-16 were synthesized by AnyGen (Gwangju, Korea) with a purity of > 95% as previously described.24
Preparation of flagellin
Recombinant Vibrio vulnificus FlaB (Vv-FlaB) was prepared as previously described.20,22 The LPS levels in the protein preparations adhered to the FDA guidelines (less than 0.15 EU/30 g per mouse). The TLR5 stimulating activity of the recombinant Vv-FlaB protein was determined as previously described.22
Orthotopic genital cancer model and determination of tumor growth
Groups of 6- to 7-week-old female mice were treated with 3 mg medroxyprogesterone acetate (Sayana®, Pfizer, RL1305) by SC injection. Four days later, the diestrus-synchronized mice were intravaginally administered 4% N9 (Sigma, I3021) under anesthesia (intraperitoneal (IP) injection of 100 μL of PBS containing 2 mg ketamine and 0.2 mg xylazine), as previously described.13,31 After 6 h, the cervico-vaginal areas of the N9-treated mice were washed with PBS. To establish an orthotopic genital tumor model, 1×105 TC-1-luc cells were intravaginally inoculated into the mice. Genital tumor growth was monitored by bioluminescence 15 min after IP injection of D-luciferin (Caliper Life Science, 122796) with the IVIS 100 (Caliper Life Science, USA), as previously described.47,48 The imaging signals were collected using a cooled charge-coupled camera device and the signal intensities were assessed quantitatively in the tumor regions by measuring the maximum photons per second per centimeter squared per steradian (p/s/cm2/sr).
Immunization
For cancer immunotherapy in the orthotopic genital model, the mice underwent IVAG administration of 25 μL PBS (PBS), 4 μg Vv-FlaB (F), 50 μg each E6/E7 (P) or 50 μg E6/E7 plus 4 μg Vv-FlaB (P+F) at 3, 8 and 13 d after tumor challenge under anesthesia (IP injection of 100 μL PBS containing 2 mg ketamine and 0.2 mg xylazine) (Fig. 1A). For the SC and IN vaccinations, 200 μL or 20 μL PBS alone or PBS containing 50 μg each E6/E7 along with 4 μg Vv-FlaB (P+F) was administered under anesthesia (IP injection of 100 μL PBS containing 2 mg ketamine and 0.2 mg xylazine) at the in appropriate time interval as described above.
E6/E7 peptides-specific IFNγ production
The N9-treated diestrus-synchronized mice underwent IVAG administration of 25 μL PBS (PBS), 4 μg Vv-FlaB (F), 50 μg each E6/E7 (P) or 50 μg E6/E7 plus 4 μg Vv-FlaB (P+F) three times at 5-d intervals. Three days after the last administration, single-cell suspensions of SPL and genital iliac lymph nodes were prepared. ELISPOT plates were pre-coated with capture antibody (anti-IFNγ, BD Biosciences, 51–2525). After 24 h, the plates were washed and blocked for 2 h at room temperature. SPL or gLN cells were added to a BD ELISPOT plate and then stimulated with 1 μg/mL each of the E6 and E7 peptides. After incubation for 3 d at 37 °C in 5% CO2, Ag-specific IFNγ-producing cells were identified by direct visualization of the spots produced by the addition of AEC reagents (BD Biosciences, 55–1951). The spots were measured using a CTL-ImmunoSpot Analyzer and analyzed with ImmunoSpot Professional Software version 5.0 (Cellular Technology, Shaker Heights, OH, USA).
Flow cytometry
The N9-treated diestrus-synchronized mice underwent IVAG administration of 25 μL PBS (PBS), 4 μg Vv-FlaB (F), 50 μg each E6/E7 (P) or 50 μg E6/E7 plus 4 μg Vv-FlaB (P+F). Three days after administration, single-cell suspensions of gLN cells were prepared. The isolated gLN cells were treated with Fc-Block (2.4G2, BD Bioscience, 55–3142) and then stained with the appropriate combinations of the following antibodies: FITC-anti-CD8+ (53_6.7, eBioscience, 55–3030); APC-anti-CD4+ (GK1.5, eBioscience, 17–0041); APC-anti-CD11c (N418, eBioscience, 17–0114); FITC-anti-CD11c (HL3, eBioscience, 55–7400); PE-anti-CD80 (16–10A1, BD Bioscience); PE-anti-86 (GC-1, BD Bioscience; PE-anti-MHC II (M5/114.15.2, BD Bioscience); and PE-anti-TLR5 (85B152.5, IMGENEX). Flow cytometry data were acquired using a BD Accuri C6 cytometer (BD Biosciences) and analyzed with FlowJo software (Tree Star, Ashland, OR).
Preparation of Vv-FlaB-FNR-675 and in vivo tracing
Vv-FlaB (24 µM in 1 mL 1× PBS) was mixed with FNR675-NHS ester (96 µM) (BioActs, Korea) at 4°C and maintained overnight in the dark with stirring. The labeled Vv-FlaB-FNR675 was then separated from the unconjugated dye using a centrifugal filter (30 kDa cutoff) (Amicon Ultra® -4, UFC801024), followed by washing (5×) in 1×PBS. Next, the amount of FNR675 conjugated to the Vv-FlaB was determined from the calibration curve of the FNR675-NHS ester using a UV-Vis spectrophotometer (UV-2700, Shimadzu, Japan). Percentage of FNR-675 conjugated to Vv-FlaB was determined by comparing the initial amount of FNR-675-NHS ester added, with the amount of FNR-675-NHS in the Vv-FlaB (determined from the calibration curve of the FNR-675-NHS). The percentage of FNR-675 conjugated to Vv-FlaB was calculated as 85%. The N9-treated diestrus-synchronized mice then underwent IVAG administration of 20 μL PBS or 14 μg Vv-FlaB-FNR675. gLN cells were prepared at the appropriate time points and stained with FITC-anti-CD11c (HL3, eBioscience, 55–7400,) as described above. Flow cytometry data were acquired using a BD Accuri C6 cytometer (BD Biosciences) and analyzed with FlowJo software (Tree Star, Ashland, OR).
Immunostaining and confocal imaging
Whole genital tissues and gLNs were removed at appropriate time points after immunization, embedded in OCT compound (Sakura Finetek, 4583) and snap frozen in liquid nitrogen. Sections of 5–7 µm were cut and stained with goat-anti-CD11c, Rabbit-anti-CD3, or biotin-anti-TLR5 antibodies. The sections were fixed in ice-cold acetone for 5 min, followed by rehydration in PBS and blocking in PBS containing 1% BSA and 0.05% Tween20 for 1 h. Between each staining step, sections were washed three times with PBS for 5 min.20 The sections were counterstained with DAPI and then mounted with ProLong Gold antifade reagent (Life Technologies, P36935). Confocal images were obtained with an LSM510 confocal microscope.
Quantitative RT-PCR
The N9-treated diestrus-synchronized mice underwent IVAG administration of 25 μL PBS (PBS), 4 μg Vv-FlaB (F), 50 μg each E6 and E7 (P) or 50 μg each E6 and E7 plus 4 μg Vv-FlaB (P+F) three times at 5-d intervals. Three days after the last administration, total RNA was isolated from the genital iliac lymph nodes using TRIzol reagent (Invitrogen, 15596–026) and an RNeasy kit (Qiagen, 74104). The gene expression levels of CXCR3, CCR5, CCR9, CCR10, VLA-4 and TLR5 in gLNs were then analyzed by qRT-PCR analysis. The total RNA was reverse transcribed using SuperScript® II Reverse Transcriptase (Invitrogen, 11904–018) and an oligo-dT primer. Quantitative PCR was performed using a Rotor-Gene™ 6000 (Corbett Life Science, Mortlake, Sydney, Australia) with the SensiMix SYBR® Green mixture (Quantace, QT615). Forward and reverse primer pairs were designed as shown in Table 1. Hypoxanthine-guanine phosphoribosyltransferase (HPRT) expression was used as an internal reference in all PCR experiments. The relative mRNA quantities in the samples were also calculated, as described elsewhere.49,50
Table 1.
Primers used in RT-PCR analysis.
| Gene | Primers | Sequences |
|---|---|---|
| CXCR3 | CXCR3-F | 5' – AACCTTCCTGCCAGCCCTCT – 3' |
| CXCR3-R | 5' – CGAAAACCCACTGGACAGCA – 3' | |
| CCR5 | CCR5-F | 5' – AGGCCATGCAGGCAACAG – 3' |
| CCR5-R | 5' – TCTCTCCAACAAAGGCATAGATGA – 3' | |
| CCR9 | CCR9-F | 5' – TCTCAGTTCCCCTACAACTCCATT– 3' |
| CCR9-R | 5' – CAGTTGGAGATGAACATGGCATA – 3' | |
| CCR10 | CCR10-F | 5' – TGCTCCTACTGAGACCCA – 3' |
| CCR1-R | 5' – CCCTGGGATTGTTTCTTT – 3' | |
| VLA-4 | VLA-4-F | 5' – AATGCCTCAGTGGTCAATCC – 3' |
| VLA-4-R | 5' – CTACCCAGCTGGAGCTGTTC – 3' | |
| TLR5 | TLR5-F | 5' – CAGTCCTGGAGCCTGTGTTGT– 3' |
| TLR5-R | 5' – ACCCGGCAAGCATTGTTCT – 3' | |
| HPRT | HPRT-F | 5' – AGCCTAAGATGAGCGCAAGT – 3' |
| HPRT-R | 5' – TTACTAGGCAGATGGCCACA – 3' |
Statistical analysis
The results are expressed as the mean ± SEM unless otherwise noted. Student's t-test or ANOVA was used to compare tumor volumes between the two groups. The difference in survival rates between the experimental groups was evaluated by Kaplan–Meier analysis. p values <0 .05 were considered statistically significant. All experiments were repeated more than three times, and the results from representative experiments are shown.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
Anti-TLR5 mAbs used in confocal analysis were kindly provided by Prof. Miyake of Osaka University, Japan.
Funding
This study was supported by an NRF grant from the MSIP [NRF-2013R1A2A2A01005011], Republic of Korea and a grant of the Korea Health Technology R&D Project through the KHIDI, funded by the MHW, Republic of Korea [HI14C0187].
Supplemental Material
Supplemental Material may be downloaded here: publisher's website
References
- 1.Conesa-Zamora P. Immune responses against virus and tumor in cervical carcinogenesis: treatment strategies for avoiding the HPV-induced immune escape. Gynecol Oncol 2013; 131:480-8; PMID:23994536; http://dx.doi.org/ 10.1016/j.ygyno.2013.08.025 [DOI] [PubMed] [Google Scholar]
- 2.Kumamoto Y, Iwasaki A. Unique features of antiviral immune system of the vaginal mucosa. Curr Opin Immunol 2012; 24:411-6; PMID:22673876; http://dx.doi.org/ 10.1016/j.coi.2012.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steenbergen RD, Snijders PJ, Heideman DA, Meijer CJ. Clinical implications of (epi)genetic changes in HPV-induced cervical precancerous lesions. Nat Rev Cancer 2014; 14:395-405; PMID:24854082; http://dx.doi.org/ 10.1038/nrc3728 [DOI] [PubMed] [Google Scholar]
- 4.Morrow MP, Yan J, Sardesai NY. Human papillomavirus therapeutic vaccines: targeting viral antigens as immunotherapy for precancerous disease and cancer. Expert Rev Vaccines 2013; 12:271-83; PMID:23496667; http://dx.doi.org/ 10.1586/erv.13.23 [DOI] [PubMed] [Google Scholar]
- 5.Tewari KS, Monk BJ. New Strategies in Cervical Cancer: From Angiogenesis Blockade to Immunotherapy. Clin Cancer Res. 2014; 20:5349-58; PMID:25104084; http://dx.doi.org/ 10.1158/1078-0432.CCR-14-1099 [DOI] [PubMed] [Google Scholar]
- 6.Czerkinsky C, Holmgren J. Topical immunization strategies. Mucosal Immunol 2010; 3:545-55; PMID:20861833; http://dx.doi.org/ 10.1038/mi.2010.55 [DOI] [PubMed] [Google Scholar]
- 7.Anjuere F, Bekri S, Bihl F, Braud VM, Cuburu N, Czerkinsky C, Hervouet C, Luci C. B cell and T cell immunity in the female genital tract: potential of distinct mucosal routes of vaccination and role of tissue-associated dendritic cells and natural killer cells. Clin Microbiol Infect 2012; 18 Suppl 5:117-22; PMID:22882377; http://dx.doi.org/21436444 10.1111/j.1469-0691.2012.03995.x [DOI] [PubMed] [Google Scholar]
- 8.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science 2011; 331:1565-70; PMID:21436444; http://dx.doi.org/ 10.1126/science.1203486 [DOI] [PubMed] [Google Scholar]
- 9.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144:646-74; PMID:21376230; http://dx.doi.org/ 10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- 10.Marabelle A, Kohrt H, Caux C, Levy R. Intratumoral immunization: a new paradigm for cancer therapy. Clin Cancer Res 2014; 20:1747-56; PMID:24691639; http://dx.doi.org/ 10.1158/1078-0432.CCR-13-2116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fridman WH, Remark R, Goc J, Giraldo NA, Becht E, Hammond SA, Damotte D, Dieu-Nosjean MC, Sautès-Fridman C. The immune microenvironment: a major player in human cancers. Int Arch Allergy Immunol 2014; 164:13-26; PMID:24852691; http://dx.doi.org/ 10.1159/000362332 [DOI] [PubMed] [Google Scholar]
- 12.Decrausaz L, Goncalves AR, Domingos-Pereira S, Pythoud C, Stehle JC, Schiller J, Jichlinski P, Nardelli-Haefliger D. A novel mucosal orthotopic murine model of human papillomavirus-associated genital cancers. Int J Cancer 2011; 128:2105-13; PMID:20635385; http://dx.doi.org/ 10.1002/ijc.25561 [DOI] [PubMed] [Google Scholar]
- 13.Soong RS, Song L, Trieu J, Knoff J, He L, Tsai YC, Huh W, Chang YN, Cheng WF, Roden RB et al.. Toll like receptor agonist imiquimod facilitates antigen-specific CD8+ T cell accumulation in the genital tract leading to tumor control through interferon-gamma. Clin Cancer Res 2014; 20:5456-67; PMID:24893628; http://dx.doi.org/ 10.1158/1078-0432.CCR-14-0344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paolini F, Massa S, Manni I, Franconi R, Venuti A. Immunotherapy in new pre-clinical models of HPV-associated oral cancers. Hum Vaccin Immunother 2013; 9:534-43; PMID:23296123; http://dx.doi.org/ 10.4161/hv.23232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hedayat M, Takeda K, Rezaei N. Prophylactic and therapeutic implications of toll-like receptor ligands. Med Res Rev 2012; 32:294-325; PMID:22383179; http://dx.doi.org/ 10.1002/med.20214 [DOI] [PubMed] [Google Scholar]
- 16.Akira S. Innate immunity and adjuvants. Philos Trans R Soc Lond B Biol Sci 2011; 366:2748-55; PMID:21893536; http://dx.doi.org/ 10.1098/rstb.2011.0106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rhee JH, Lee SE, Kim SY. Mucosal vaccine adjuvants update. Clin Exp Vaccine Res 2012; 1:50-63; PMID:23596577; http://dx.doi.org/ 10.7774/cevr.2012.1.1.50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hayashi F, Smith KD, Ozinsky A, Hawn TR, Yi EC, Goodlett DR, Eng JK, Akira S, Underhill DM, Aderem A. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 2001; 410:1099-103; PMID:11323673; http://dx.doi.org/ 10.1038/35074106 [DOI] [PubMed] [Google Scholar]
- 19.Smith KD, Andersen-Nissen E, Hayashi F, Strobe K, Bergman MA, Barrett SL, Cookson BT, Aderem A. Toll-like receptor 5 recognizes a conserved site on flagellin required for protofilament formation and bacterial motility. Nat Immunol 2003; 4:1247-53; PMID:14625549; http://dx.doi.org/ 10.1038/ni1011 [DOI] [PubMed] [Google Scholar]
- 20.Lee SE, Kim SY, Jeong BC, Kim YR, Bae SJ, Ahn OS, Lee JJ, Song HC, Kim JM, Choy HE et al.. A bacterial flagellin, Vibrio vulnificus FlaB, has a strong mucosal adjuvant activity to induce protective immunity. Infect Immun 2006; 74:694-702; PMID:16369026; http://dx.doi.org/ 10.1128/IAI.74.1.694-702.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nguyen CT, Kim SY, Kim MS, Lee SE, Rhee JH. Intranasal immunization with recombinant PspA fused with a flagellin enhances cross-protective immunity against Streptococcus pneumoniae infection in mice. Vaccine 2011; 29:5731-9; PMID:21696869; http://dx.doi.org/ 10.1016/j.vaccine.2011.05.095 [DOI] [PubMed] [Google Scholar]
- 22.Hong SH, Byun YH, Nguyen CT, Kim SY, Seong BL, Park S, Woo GJ, Yoon Y, Koh JT, Fujihashi K et al.. Intranasal administration of a flagellin-adjuvanted inactivated influenza vaccine enhances mucosal immune responses to protect mice against lethal infection. Vaccine 2012; 30:466-74; PMID:22051136; http://dx.doi.org/ 10.1016/j.vaccine.2011.10.058 [DOI] [PubMed] [Google Scholar]
- 23.Nguyen CT, Hong SH, Ung TT, Verma V, Kim SY, Rhee JH, Lee SE. Intranasal immunization with a flagellin-adjuvanted peptide anticancer vaccine prevents tumor development by enhancing specific cytotoxic T lymphocyte response in a mouse model. Clin Exp Vaccine Res 2013; 2:128-34; PMID:23858404; http://dx.doi.org/ 10.7774/cevr.2013.2.2.128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nguyen CT, Hong SH, Sin JI, Vu HV, Jeong K, Cho KO, Uematsu S, Akira S, Lee SE, Rhee JH. Flagellin enhances tumor-specific CD8(+) T cell immune responses through TLR5 stimulation in a therapeutic cancer vaccine model. Vaccine 2013; 31:3879-87; PMID:23831323; http://dx.doi.org/ 10.1016/j.vaccine.2013.06.054 [DOI] [PubMed] [Google Scholar]
- 25.Hickey DK, Fahey JV, Wira CR. Mouse estrous cycle regulation of vaginal versus uterine cytokines, chemokines, α-/β-defensins and TLRs. Innate Immunity 2013; 19:121-31; PMID:22855555; http://dx.doi.org/ 10.1177/1753425912454026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Makkouk A, Weiner GJ. Cancer immunotherapy and breaking immune tolerance: new approaches to an old challenge. Cancer Res 2014; 75(1):5-10; PMID:25524899; http://dx.doi.org/ 10.1158/0008-5472.CAN-14-2538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Slingluff CL, Jr. The present and future of peptide vaccines for cancer: single or multiple, long or short, alone or in combination. Cancer J 2011; 17:343-50; PMID:21952285; http://dx.doi.org/ 10.1097/PPO.0b013e318233e5b2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Domingos-Pereira S, Decrausaz L, Derre L, Bobst M, Romero P, Schiller JT, Jichlinski P, Nardelli-Haefliger D. Intravaginal TLR agonists increase local vaccine-specific CD8 T cells and human papillomavirus-associated genital-tumor regression in mice. Mucosal Immunol 2013; 6:393-404; PMID:22968420; http://dx.doi.org/ 10.1038/mi.2012.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim D, Hung CF, Wu TC. Monitoring the trafficking of adoptively transferred antigen- specific CD8-positive T cells in vivo, using noninvasive luminescence imaging. Human Gene Therapy 2007; 18:575-88; PMID:17576157; http://dx.doi.org/ 10.1089/hum.2007.038 [DOI] [PubMed] [Google Scholar]
- 30.Lin KY, Guarnieri FG, Staveley-O'Carroll KF, Levitsky HI, August JT, Pardoll DM, Wu TC. Treatment of established tumors with a novel vaccine that enhances major histocompatibility class II presentation of tumor antigen. Cancer Res 1996; 56:21-6; PMID:8548765 [PubMed] [Google Scholar]
- 31.Cuburu N, Graham BS, Buck CB, Kines RC, Pang YY, Day PM, Lowy DR, Schiller JT. Intravaginal immunization with HPV vectors induces tissue-resident CD8+ T cell responses. J Clin Invest 2012; 122:4606-20; PMID:23143305; http://dx.doi.org/21995573 10.1172/JCI63287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hailemichael Y, Overwijk WW. Cancer vaccines: Trafficking of tumor-specific T cells to tumor after therapeutic vaccination. Int J Biochem Cell Biol 2014; 53:46-50; PMID:24796845; http://dx.doi.org/21995573 10.1016/j.biocel.2014.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nolz JC, Starbeck-Miller GR, Harty JT. Naive, effector and memory CD8 T-cell trafficking: parallels and distinctions. Immunotherapy 2011; 3:1223-33; PMID:21995573; http://dx.doi.org/ 10.2217/imt.11.100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Devaud C, Westwood JA, John LB, Flynn JK, Paquet-Fifield S, Duong CP, Yong CS, Pegram HJ, Stacker SA, Achen MG et al.. Tissues in different anatomical sites can sculpt and vary the tumor microenvironment to affect responses to therapy. Mol Ther 2014; 22:18-27; PMID:24048441; http://dx.doi.org/ 10.1038/mt.2013.219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao Y, Yang J, Shi J, Gong YN, Lu Q, Xu H, Liu L, Shao F. The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus. Nature 2011; 477:596-600; PMID:21918512; http://dx.doi.org/ 10.1038/nature10510 [DOI] [PubMed] [Google Scholar]
- 36.Bijker MS, van den Eeden SJ, Franken KL, Melief CJ, Offringa R, van der Burg SH. CD8+ CTL priming by exact peptide epitopes in incomplete Freund's adjuvant induces a vanishing CTL response, whereas long peptides induce sustained CTL reactivity. J Immunol 2007; 179:5033-40; PMID:17911588; http://dx.doi.org/ 10.4049/jimmunol.179.8.5033 [DOI] [PubMed] [Google Scholar]
- 37.Melief CJ. Treatment of established lesions caused by high-risk human papilloma virus using a synthetic vaccine. J Immunotherapy 2012; 35:215-6; PMID:22421938; http://dx.doi.org/24349003 10.1097/CJI.0b013e318248f17f [DOI] [PubMed] [Google Scholar]
- 38.Arens R, van Hall T, van der Burg SH, Ossendorp F, Melief CJ. Prospects of combinatorial synthetic peptide vaccine-based immunotherapy against cancer. Seminars Immunol 2013; 25:182-90; PMID:23706598; http://dx.doi.org/24349003 10.1016/j.smim.2013.04.008 [DOI] [PubMed] [Google Scholar]
- 39.Comber JD, Philip R. MHC class I antigen presentation and implications for developing a new generation of therapeutic vaccines. Ther Adv Vaccines 2014; 2:77-89; PMID:24790732; http://dx.doi.org/24349003 10.1177/2051013614525375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pettini E, Prota G, Ciabattini A, Boianelli A, Fiorino F, Pozzi G, Vicino A, Medaglini D. Vaginal immunization to elicit primary T-cell activation and dissemination. PloS One 2013; 8:e80545; PMID:24349003; http://dx.doi.org/ 10.1371/journal.pone.0080545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Oliveira CE, Oda JM, Losi Guembarovski R, de Oliveira KB, Ariza CB, Neto JS, Banin Hirata BK, Watanabe MA. CC chemokine receptor 5: the interface of host immunity and cancer. Dis Markers 2014; 2014:126954; PMID:24591756; http://dx.doi.org/ 10.1155/2014/126954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Billottet C, Quemener C, Bikfalvi A. CXCR3, a double-edged sword in tumor progression and angiogenesis. Biochimica Et Biophysica Acta 2013; 1836:287-95; PMID:23994549; http://dx.doi.org/ 10.1016/j.bbcan.2013.08.002 [DOI] [PubMed] [Google Scholar]
- 43.Groom JR, Luster AD. CXCR3 in T cell function. Exp Cell Res 2011; 317:620-31; PMID:21376175; http://dx.doi.org/ 10.1016/j.yexcr.2010.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Neyt K, Perros F, GeurtsvanKessel CH, Hammad H, Lambrecht BN. Tertiary lymphoid organs in infection and autoimmunity. Trends Immunol 2012; 33:297-305; PMID:22622061; http://dx.doi.org/ 10.1016/j.it.2012.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maldonado L, Teague JE, Morrow MP, Jotova I, Wu TC, Wang C et al.. Intramuscular therapeutic vaccination targeting HPV16 induces T cell responses that localize in mucosal lesions. Sci Translational Med 2014; 6:221ra13; PMID:24477000; http://dx.doi.org/23724867 10.1126/scitranslmed.3007323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon RA, Reed K et al.. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med 2013; 369:122-33; PMID:23724867; http://dx.doi.org/ 10.1056/NEJMoa1302369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim DK, Jeong JH, Lee JM, Kim KS, Park SH, Kim YD, Koh M, Shin M, Jung YS, Kim HS et al.. Inverse agonist of estrogen-related receptor gamma controls Salmonella typhimurium infection by modulating host iron homeostasis. Nature Med 2014; 20:419-24; PMID:24658075; http://dx.doi.org/ 10.1038/nm.3483 [DOI] [PubMed] [Google Scholar]
- 48.Min JJ, Nguyen VH, Kim HJ, Hong Y, Choy HE. Quantitative bioluminescence imaging of tumor-targeting bacteria in living animals. Nature Protocols 2008; 3:629-36; PMID:18388945; http://dx.doi.org/ 10.1038/nprot.2008.32 [DOI] [PubMed] [Google Scholar]
- 49.Lee SE, Li X, Kim JC, Lee J, Gonzalez-Navajas JM, Hong SH, Park IK, Rhee JH, Raz E. Type I interferons maintain Foxp3 expression and T-regulatory cell functions under inflammatory conditions in mice. Gastroenterology 2012; 143:145-54; PMID:22475534; http://dx.doi.org/19945418 10.1053/j.gastro.2012.03.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Del Campo J, Lindqvist M, Cuello M, Backstrom M, Cabrerra O, Persson J, Perez O, Harandi AM. Intranasal immunization with a proteoliposome-derived cochleate containing recombinant gD protein confers protective immunity against genital herpes in mice. Vaccine 2010; 28:1193-200; PMID:19945418; http://dx.doi.org/ 10.1016/j.vaccine.2009.11.035 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


