ABSTRACT
Defects in natural killer (NK) cell functions are necessary for tumor immune escape, but their underlying regulatory mechanisms in human cancers remain largely unknown. Here we showed, in detailed studies of NK cells from 235 untreated patients with gastric cancer (GC), the NK cell density in GC tissues could predict improved survival of patients. However, NK cells were significantly decreased in number with advanced-stage GC. A multivariate Cox analysis revealed that the intratumoral NK cell density was an independent prognostic factor for overall survival and disease-free survival in the GC patients. Most of the intratumoral NK cells exhibited a normal phenotype and secreted normal levels of cytokines, but the expression of Ki67 was decreased compared with NK cells from nontumoral regions. Moreover, the levels of intratumoral NK cells were negatively correlated with the intratumoral expression of cyclooxygenase-2. Furthermore, we found that PGE2 derived from GC cells suppressed NK cell proliferation and increased apoptosis in vitro. These data reveal that tumor-derived PGE2 is critical for inducing NK cell dysfunction in GC and demonstrate that an extensive infiltration of NK cells predicts a good prognosis in patients with GC. Our findings suggest that immunosuppressive barriers erected by tumors greatly hamper the antitumor activity of human NK cells, thereby favoring tumor outgrowth and progression.
KEYWORDS: Cyclooxygenase-2, gastric cancer, natural killer cell, prostaglandin E2
Introduction
Innate immune surveillance plays a critical role in the control of cancer progression.1,2 NK cells, defined as effector lymphocytes of innate immunity, are cytolytic and cytokine-producing lymphocytes that can directly kill transformed or microbe-infected cells.3 NK cells also participate in shaping adaptive immune responses.4,5 Substantial clinical and experimental evidence demonstrates that the dysfunction of NK cells often leads to advanced disease progression in several types of human solid tumors.6,7 However, very little is known regarding the nature, regulation, and functions of NK cells in human GC.
Although NK cells are considered promising effector cells in the adoptive immunotherapy of cancer,8 NK cell-based immunotherapy has yielded limited clinical benefit.9 This reflects the poor capacity of adoptively transferred NK cells to home to tumor sites. Additionally, tumor cells under selective pressure from immune surveillance can undergo a process referred to as immune editing and thereby develop a phenotype capable of manipulating immune cells through the secretion of chemokines and cytokines;10 tumor cells thus become resistant to this first-line of defense.
The overexpression of cyclooxygenase-2 (COX-2) and an abundance of its enzymatic product, prostaglandin E2 (PGE2), play key roles in promoting inflammation and tumor progression.11,12 Multiple mechanisms involving COX-2 overexpression have been proposed to explain the tumor-promoting activity of the COX-2/PGE2 pathway, including the promotion of tumor cell proliferation, recruitment of myeloid-derived suppressor cells, induction of angiogenesis, and modification of immune cell responses to tumors.13
GC is the fifth most common malignancy worldwide,14 and the overexpression of COX-2 has been shown to enhance tumor progression.15 PGE2 has direct inhibitory effects on lymphocyte functions. To date, limited information is available on the effect of GC cells on NK cell function. Our study demonstrates, that NK cell infiltration is decreased in intratumoral regions and that this decrease is associated with a decreased survival in GC patients. The production of PGE2 by GC cells may play a primary role in suppressing NK cell proliferation and inducing apoptosis.
Results
Accumulation of functional NK cells in GC tissues suppresses disease progression and predicts improved survival
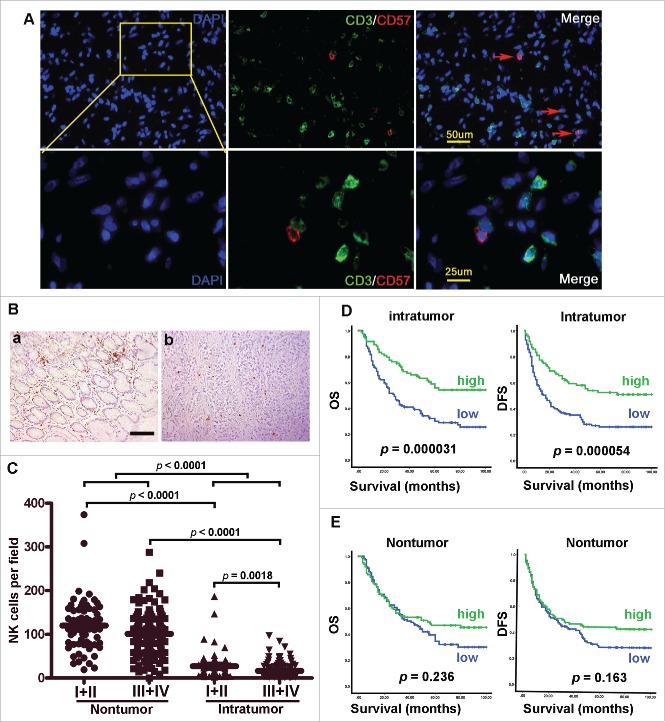
To clarify the role of NK cells in human GC immunopathology, we first investigated NK cell infiltration in the human GC intratumoral tissues and paired normal gastric tissues. CD57 antigen, namely human NK-1 (natural killer-1), is a carbohydrate. It has been demonstrated that CD57 is a specific marker for NK cells in hepatocellular carcinoma,6 we thus assessed the specificity of the enumeration of NK cells by double staining with anti-CD3 and anti-CD57. Immunofluorescent staining revealed that CD57 is a specific marker for NK cells in GC tissues (Fig. 1A). Therefore, we investigated the enumeration of NK cells by immunohistochemical staining of CD57 in 212 paraffin-embedded GC tissues. As shown in Fig. 1B and C, NK cells were predominant in normal gastric mucosa, in contrast to the intratumoral region (p < 0.0001). A decreased infiltration of NK cells into the intratumoral region occurred mainly in patients with advanced stage GC [stages I–II (n = 46) versus stages III–IV (n = 166); p < 0.0001; Fig. 1C]. Based on the above observations, we predicted that the presence of NK cells in GC tissues would have a favorable effect on patient survival. We then divided the GC patients into two groups according to the median value of the NK-1+ cell density in the intratumoral region. Remarkably, there was a striking positive association between the NK-1+ cell density in the intratumoral region and both overall survival (OS) and disease-free survival (DFS) (p < 0.0001 for both; Fig. 1D). By contrast, the number of NK-1+ cells in the nontumoral tissue was unrelated to the prognosis as assessed by either OS or DFS (Fig. 1E). The NK-1+ cell density in the intratumoral region was also associated with lymph node metastasis, distant metastasis and American Joint Committee on Cancer stage (AJCC, 7th edition. Table 1). Univariate and multivariate analyses (Tables 2 and 3) revealed that the number of NK-1+ cells in the intratumoral region was an independent prognostic factor for both OS and DFS.
Figure 1.
Accumulation of functional NK cells in GC tissues suppresses disease progression and predicts improved survival. (A) Assessment of the specificity of NK cells by double staining with anti-CD3 and anti-CD57 in GC tissues (n = 10). (B, C) Paraffin-embedded samples were stained with anti-CD57 Ab, the distribution of NK cells in remote nontumoral tissue and paired intratumoral tissues of GC patients. (D, E) Cumulative OS and DFS curves of the patients. Patients were divided into two groups according to the median density of CD57 cells in the nontumoral tissue (median: 112) and intratumoral tissue (median: 21). The cumulative OS and DFS time were calculated using the Kaplan-Meier method and analyzed by the log-rank test.
Table 1.
Association of intratumoral CD57 cells with clinicopathological characteristics.
| Total | Intratumoral CD57 cells |
|||
|---|---|---|---|---|
| Variable | (N = 212) | Low (cases) | High (cases) | P-value |
| Total study | 212 | 125 | 87 | |
| Gender | 0.321 | |||
| Male | 148 | 84 | 64 | |
| Female | 64 | 41 | 23 | |
| Age(years) | 0.074 | |||
| <55 | 91 | 60 | 31 | |
| ≥55 | 121 | 65 | 56 | |
| Tumor size(cm) | 0.315 | |||
| ≤4 | 118 | 66 | 52 | |
| >4 | 94 | 59 | 35 | |
| Tumor location | 0.300 | |||
| Cardia of stomach | 98 | 57 | 41 | |
| Body of stomach | 31 | 15 | 16 | |
| Antrum of stomach | 51 | 30 | 21 | |
| Whole | 32 | 23 | 9 | |
| Differentiation status | 0.223 | |||
| Low | 125 | 78 | 47 | |
| Well and Moderate | 87 | 47 | 40 | |
| CEA | 0.278 | |||
| Elevated | 52 | 34 | 18 | |
| Normal | 160 | 91 | 69 | |
| CA199 | 0.453 | |||
| Elevated | 62 | 39 | 23 | |
| Normal | 150 | 86 | 64 | |
| Depth of invasion | 0.163 | |||
| T1 + T2 | 46 | 23 | 23 | |
| T3 + T4 | 166 | 102 | 64 | |
| Lymph node metastasis | 0.003 | |||
| pN0 | 62 | 27 | 35 | |
| pN1 + pN2 + pN3 | 150 | 98 | 52 | |
| Distant metastasis | 0.003 | |||
| M0 | 175 | 95 | 80 | |
| M1 | 37 | 30 | 7 | |
| AJCC stage | 0.001 | |||
| I | 25 | 8 | 17 | |
| II | 45 | 24 | 21 | |
| III | 103 | 61 | 42 | |
| IV | 39 | 32 | 7 | |
| Intratumoral COX-2 expression | 0.000 | |||
| Low | 104 | 39 | 65 | |
| High | 108 | 86 | 22 | |
CEA: carcino-embryonic antigen; CA199: carbohydrate antigen 199;
COX-2: Cyclooxygenase-2.
Table 2.
Univariate analysis of factors associated with overall survival (OS) and disease-free survival (DFS) for gastric cancer.
| OS | DFS | |||||
|---|---|---|---|---|---|---|
| Univariate | Univariate | |||||
| Variable |
HR |
95%CI |
P |
HR |
95%CI |
P |
| Age(years) (≥55 vs. <55) | 1.188 | 0.835–1.691 | 0.339 | 1.311 | 0.932–1.846 | 0.120 |
| Gender (female vs. male) | 0.962 | 0.654–1.414 | 0.843 | 0.965 | 0.664–1.404 | 0.854 |
| Tumor size (>4 cm vs. ≤4 cm) | 1.314 | 0.924–1.869 | 0.129 | 1.386 | 0.985–1.952 | 0.061 |
| Tumor location(higher vs. lower) | 1.250 | 0.877–1.783 | 0.217 | 1.351 | 0.956–1.910 | 0.088 |
| Differentiation status(low vs. well+moderate) | 1.815 | 1.243–2.651 | 0.002 | 2.048 | 1.414–2.967 | 0.000 |
| CEA(ng/mL) (elevated vs. normal) | 1.128 | 0.751–1.694 | 0.561 | 1.148 | 0.780–1.689 | 0.485 |
| CA199(U/mL) (elevated vs. normal) | 1.493 | 1.026–2.172 | 0.036 | 1.600 | 1.115–2.295 | 0.011 |
| Depth of invasion(T3+4 vs. T1+2) | 2.692 | 1.590–4.559 | 0.000 | 2.915 | 1.748–4.861 | 0.000 |
| Lymph node metastasis(pN1+pN2+pN3 vs. pN0) | 4.755 | 2.765–8.178 | 0.000 | 4.885 | 2.925–8.157 | 0.000 |
| Stage(III+IV vs. I+II) | 4.899 | 2.960–8.106 | 0.000 | 5.242 | 3.241–8.479 | 0.000 |
| Intratumoral COX2 express (high vs. low) | 1.676 | 1.172–2.397 | 0.005 | 1.507 | 1.068–2.217 | 0.020 |
| Nontumoral CD57 cells (high vs. low) | 0.809 | 0.566–1.156 | 0.244 | 0.786 | 0.557–1.109 | 0.170 |
| Intratumoral CD57 cells (high vs. low) | 0.453 | 0.307–0.666 | 0.000 | 0.479 | 0.330–0.693 | 0.000 |
CEA: carcino-embryonic antigen; CA199: carbohydrate antigen 199; COX-2: Cyclooxygenase-2.
Table 3.
Multivariate analysis of factors associated with overall survival (OS) and disease-free survival (DFS) for gastric cancer.
| OS |
DFS |
|||||
|---|---|---|---|---|---|---|
| Variable | HR | 95%CI | P | HR | 95%CI | P |
| Intratumoral CD57 cells ( high vs. low) | 0.528 | 0.358–0.779 | 0.001 | 0.556 | 0.383–0.807 | 0.002 |
| Stage(III+IV vs. I+II) | 4.522 | 2.727–7.499 | 0.000 | 4.909 | 3.029–7.955 | 0.000 |
NK cell percentage, phenotype, functions and proliferation in GC patients
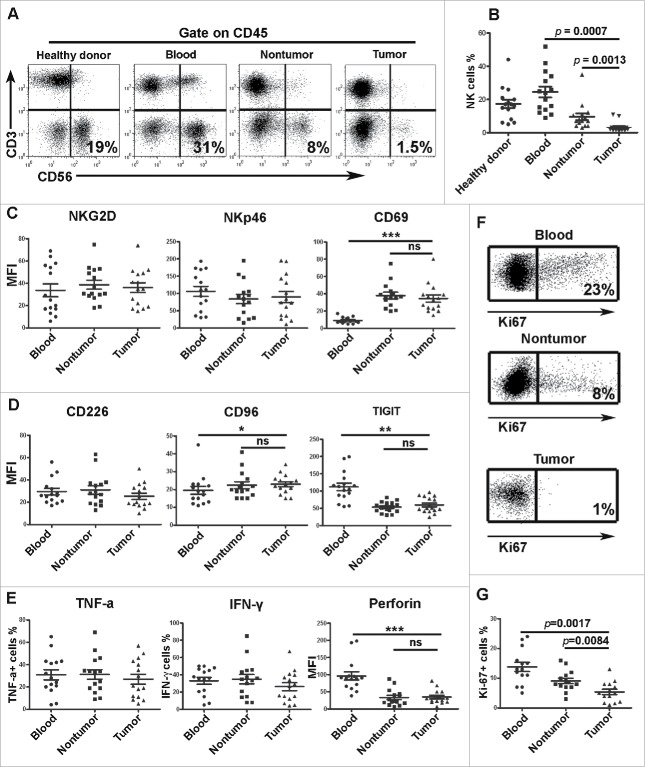
To investigate the percentage of NK cells in the lymphocyte population in vivo, we examined the percentage of NK cells among the CD45+ lymphocytes of GC patients. As indicated in Fig. 2A and B, the ratios of total (CD3− CD56+) NK cells were significantly lower in the intratumoral tissues. The above result is consistent with the results from the immunohistochemical analysis, which revealed that the number of NK cells was remarkably reduced in the intratumoral tissues of GC patients.
Figure 2.
NK cell percentage, phenotype, functions and proliferation in GC patients. For the measurement of intracellular cytokine production, mononuclear cells were isolated from blood, nontumoral tissues and intratumoral tissues. Per 2 × 106 mononuclear cells were cultured in 500 uL of complete medium with 2 uL Leukocyte Activation Cocktail (BD Bioscience) in 48 well plates in a 37°C humidified CO2 incubator for 4 h. Following activation, cells were stained with surface markers, fixed and permeabilized with IntraPre Reagent (Beckman Coulter), and finally stained with anti-TNF-α, anti-IFN-γ, anti-perforin and anti-granzyme B. (A, B) Representative image and statistical data of the percentages of total NK cells in lymphocytes as determined by flow cytometry. (C, D) Statistical results of the surface receptors expressed on the NK cells. (E) Statistical results of the assay for IFNγ, TNF-α and granzyme B secreted by the NK cells. (F, G) Representative image and statistical data of the percentage of Ki-67+ cells among the total NK cells. Results are expressed as the means ± SEM; * p < 0.05; ** p < 0.01; *** p < 0.001.
NK cells can function as both cytotoxic lymphocytes and inflammatory cells; the responsiveness of NK cells is fine-tuned through signals received via inhibitory and activating receptors expressed on the surface of these cells.16 We subsequently examined the phenotype of NK cells, and as depicted in Figure 2C, the mean fluorescence intensities (MFIs) of NKG2D, NKp46 and CD69 were not altered. CD96, CD226 (DNAM-1) and TIGIT, which belong to the family of receptors that interact with nectin and nectin-like proteins, were also unaltered (Fig. 2D). NK cells are major producers of cytokines such as interferon-gamma (IFNγ) and tumor necrosis factor-α (TNF-α), and the killing of target cells by NK cells is based mostly on the activity of perforin and granzymes A/B contained in NK cell granules. The NK cells from both intratumoral and nontumoral tissues did not reveal any significant difference in the secretion of TNF-α, IFN-γ, perforin (Fig. 2E) or granzyme B (data not shown).
NK cells derived from peripheral blood and intratumoral tissues show no difference in cytotoxic activity
Based on the above observations, we predicted that cytotoxic activity of NK cells in GC tissues would show no difference with NK cells in peripheral blood. To test this assumption, we assessed the lytic potential of NK cells derived from peripherial blood and intratumoral tissues toward AGS cells. Consistent with our hypothesis, the cytotoxic activity of NK cells derived from peripheral blood and intratumoral tissues against AGS cells did not reveal any significant difference at different effector-to-target (E/T) ratios (Fig. S1).
The above results indicate that although the phenotype, cytokine production and cytotoxicity of NK cells were not altered in GC tissues, the number of NK cells in intratumoral tissues was markedly decreased as evidenced by the immunohistochemical and flow cytometric results. Additionally, we also measured the expression of Ki-67 in NK cells. As shown in Fig. 2F and G, the expression of Ki-67 was significantly down-regulated in the NK cells from intratumoral tissues. This result suggested that NK cell proliferation may be suppressed in intratumoral tissue.
The density of NK cells and COX-2 expression are negatively associated in GC patients
COX-2 levels are normally low but are induced in inflammation and cancer. Therefore, we next examined a potential association between COX-2 expression and NK cells in GC patients using immunohistochemical staining of serial sections of GC tissues. As depicted in Fig. 3A, we found that 108 samples (51%) had moderate or strong COX-2 expression (i.e., were COX-2-positive), whereas 104 samples (49%) had weak or absent COX-2 expression (i.e., were COX-2-negative). The density of NK cells was negatively correlated with the expression of COX-2 in the same areas of the GC tissues (Fig. 3A and B, p = 0.007). Our results also revealed that the expression of COX-2 was a prognostic factor for both OS and DFS (Fig. 3C and D). The COX-2 expression in the intratumoral region was also associated with depth of invasion, lymph node metastasis and AJCC stage (Table S1).Taken together, the above results demonstrate a negative association between the density of NK cells and COX-2 expression in GC patients. PGE2 is a key mediator of immunopathology, and its production is tightly controlled by COX-2 expression,17,18 suggesting that NK cell density in vivo may be modulated by GC cells via PGE2.
Figure 3.
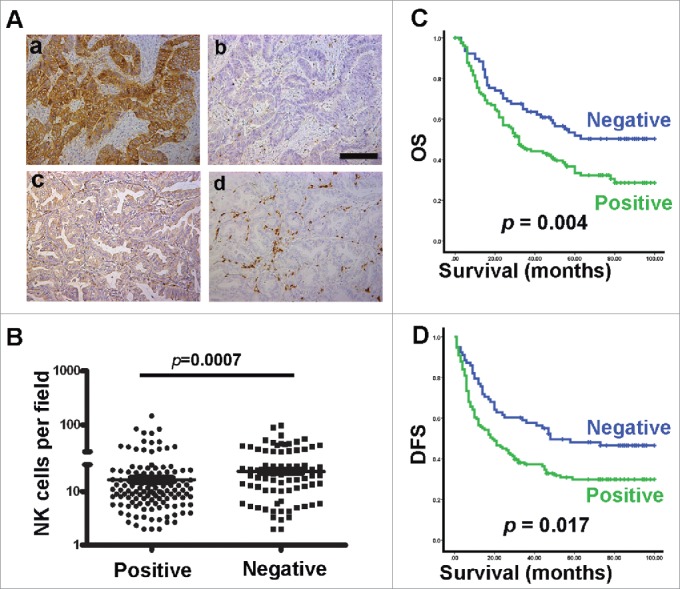
The density of NK cells and COX-2 expression are negatively associated in GC patient samples. (A) Serial sections of paraffin-embedded GC samples stained with anti-CD57 or anti-COX-2 Ab. Different levels of COX-2 expression can be observed in the intratumoral regions: a, strong or positive; c, none, slight or negative. Serial sections stained with anti-CD57 Ab: b, low density; d, high density. (B) Statistical analysis of the number of NK cells infiltrated into the intratumoral region with different levels of COX-2 expression. (C, D) Cumulative OS and DFS curves of the patients positive or negative for COX-2 expression.
Soluble factors derived from GC cells are involved in modulating the number of NK cells
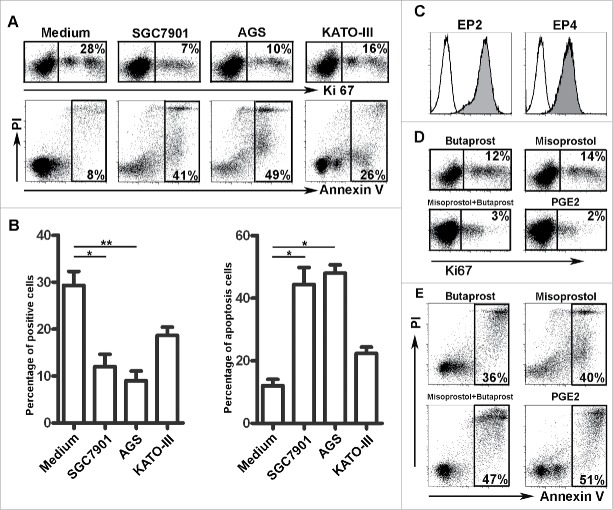
To determine whether GC cells could affect the proliferation of NK cells and induce NK cell apoptosis, purified human NK cells were cultured in medium with 30% tumor culture supernatants (TSNs) derived from three GC cell lines (i.e., SGC-7901, AGS, or KATO-III) for 60 h. The expression of Ki-67 by these NK cells was decreased compared with those cultured in complete medium alone (Fig. 4A and B). Furthermore, an analysis of the survival of these NK cells revealed that approximately 50% of the NK cells were positive for Annexin V, implying the cells were undergoing apoptosis (Fig. 4A and B). A previous report suggested that PGE2 acts via the prostaglandin receptors EP2 and EP4 to impair the function of immune cells.19,20 We next analyzed the expression of the PGE2 receptors EP2 and EP4 on NK cells. As shown in Fig. 4C, the NK cells constitutively expressed high levels of the EP2 and EP4 proteins. The addition of the EP2 agonist butaprost and the EP4 agonist misoprostol markedly inhibited NK cell proliferation; therefore, the actions of butaprost and misoprostol were synergistic. The addition of PGE2 strongly suppressed the proliferation of NK cells and induced NK cells apoptosis (Fig. 4D and E).
Figure 4.
Soluble factors derived from GC cells are involved in modulating the number of NK cells. (A) Representative data showing that TSNs derived from SGC-7901, AGS and KATO-III suppressed NK cell proliferation and induced NK cell apoptosis. (B) Statistical results of the GC cell lines that suppressed NK cell proliferation and induced NK cell apoptosis (n = 3). (C) Representative data of EP2 and EP4 receptor expression in NK cells. (D) Representative data of butaprost, misoprostol and PGE2 suppression of Ki-67 expression in NK cells (n = 3). (E) Representative data of butaprost, misoprostol and PGE2-induced NK cell apoptosis (n = 3).
TTCS suppresses NK cell proliferation and induces apoptosis
To further address our hypothesis, we cultured NK cells with 30% tumor tissue culture supernatants (TTCS) or nontumor tissue culture supernatants (NTCS). The proliferation of the NK cells was remarkably suppressed by TTCS (Fig. 5A and B, n = 3). We also observed that TTCS induced the apoptosis of NK cells significantly more than NTCS (Fig. 5C and D, n = 3). These functional data further confirmed that soluble factors derived from GC tissues were involved in modulating the number of NK cells.
Figure 5.
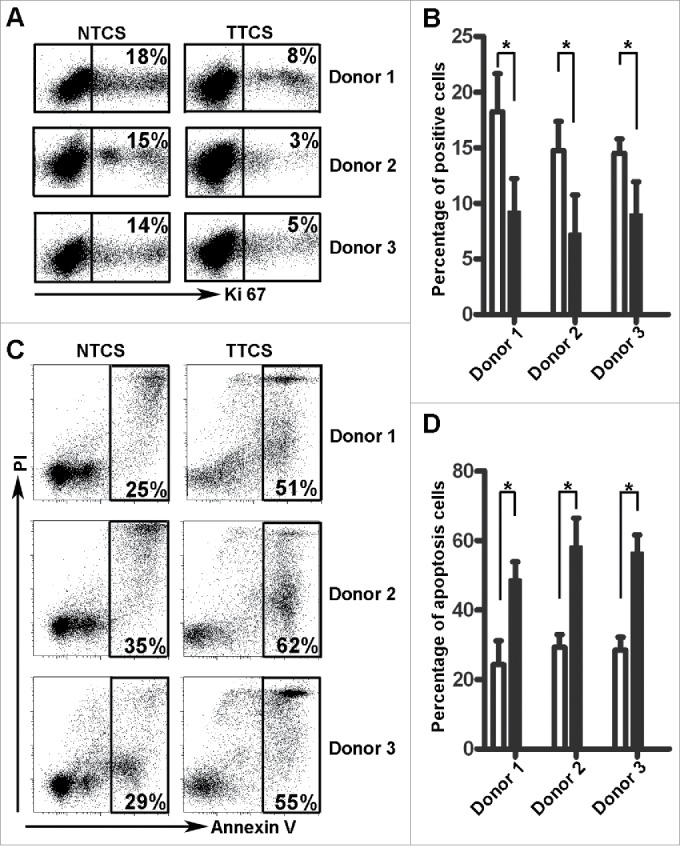
TTCS suppressed NK cell proliferation and induced apoptosis. (A, B) Representative image and statistical results of TTCS- and NTCS-induced suppression of NK cell proliferation. (C, D) Representative image and statistical results of TTCS- and NTCS-induced NK cell apoptosis.
Blocking PGE2 activities in GC cells restores NK cell function
Based on the above observations, we predicted that the PGE2 derived from TSNs would modulate NK cell function. To test this prediction, we used the specific inhibitor NS398 to block PGE2, and found that the dysfunction of NK cells was effectively attenuated, as indicated by the up-regulation of Ki-67 (Fig. 6A) and decrease in the level of NK cell apoptosis (Fig. 6B). To confirm our findings, we further analyzed the concentration of PGE2 in the TSNs. In support of our hypothesis, higher levels of PGE2 were found in the TSNs from SGC-7901 and AGS cells than in the TSN from KATO-III cells (Fig. 6C), and the concentration of PGE2 in TTCS was higher than that in NTCS (n = 5) (Fig. 6D). These data, together with the association between the density of NK cells and the expression of COX-2 in the same GC tissue area (Fig. 3), indicate that PGE2 derived from GC tissue plays a predominant role in modulating the functions of NK cells in intratumoral tissues.
Figure 6.
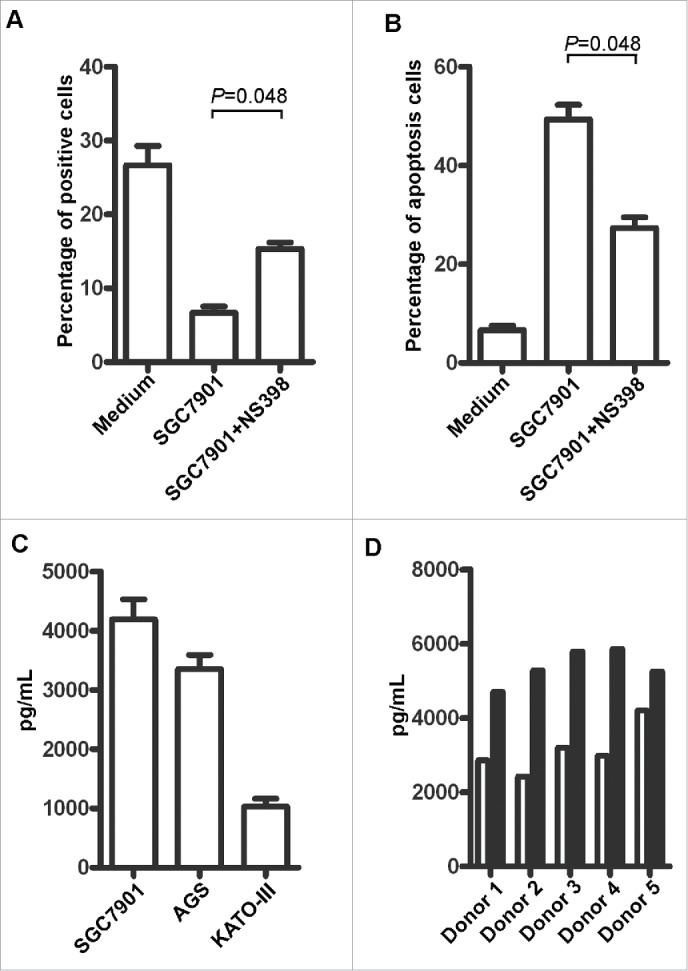
Role of PGE2 in the immunoregulatory activity of NK cells. (A) Statistical results for the restoration of Ki-67 expression and (B) the level of apoptosis with TSNs derived from SGC-7901 treated with NS398. (C) The concentration of PGE2 in TSNs as determined with an ELISA. (D) The concentration of PGE2 in TTCS and NTCS derived from five patients; white:NTCS; black:TTCS.
Discussion
NK cell dysfunction has been recognized as an important mechanism of tumor immune escape in both mice and cancer patients.6,21 The present study demonstrated that a high infiltration of functional NK cells in GC intratumoral regions predicts an improved survival; NK cells were significantly decreased in advanced stage GC, and the level of NK cells was negatively correlated with the expression of COX-2 in intratumoral tissue. We also found that soluble tumor-derived factors, including PGE2, dampen NK cell proliferation and induce apoptosis. These observations are in accord with the growing evidence that tumor cells have a fine-tuned mechanism to foster immune privilege and escape from innate immunity.2,22
It is generally accepted that immune surveillance plays a key role in the control of tumor development.23,24 NK cells are considered promising effector cells in the adoptive immunotherapy of cancer, and infiltration by these cells is associated with a better prognosis in many solid tumors.6,25,26 It has been reported that the NK cell phenotype and cytotoxic activity are altered in solid tumors.6,27 Our present study provided evidence that the NK cell phenotype and cytotoxic activity are at least partly normal compared with the NK cells from nontumoral tissue or blood. Several of our observations support this notion. First, the number of NK cells in the intratumoral region was an independent prognostic factor. Second, NK-cell functions are regulated by a series of surface receptors, but the activating and inhibitory receptors we studied were not altered. Third, the percentages of TNF-α- and IFNγ-positive NK cells in the intratumoral tissues were similar to those in the nontumoral tissues. Finally, the lytic potential of NK cells derived from peripherial blood and intratumoral tissues toward tumor cell lines showed no difference in vitro. These results suggest that the survival status of GC patients depends partly on the number of infiltrating NK cells and that these NK cells in the intratumoral tissues can function normally, once activated, to produce cytokines to stimulate themselves or other types of immune cells.
COX-2 expression is elevated in many cancers and correlates with a poor prognosis.17 A recent study suggests that Aspirin and COX-2 inhibitor use may be associated with improved outcomes in stage III colon cancer patients.13 It is evident that the phenotype and functional profile of effector lymphocytes in cancer are dramatically impacted by PGE2. The results of three sets of experiments provide evidence that the PGE2 derived from GC tissues is essential for NK cell dysfunction. First, immunohistochemical staining for CD57 indicated that the expression of COX-2 in tumor tissue negatively correlated with the number of NK cells. Second, blocking the PGE2 present in TSN restored NK cell proliferation and decreased the level of NK cell apoptosis. Third, TTCS and NTCS differed significantly in the NK cell dysfunction these supernatants induced. The research of other investigators also supports the notion that PGE2 production by cancer cells may promote local immunosuppression.17,27,28
The current knowledge of the effect of PGE2 on tumor immunity strongly supports our findings.18 Our research had limitations: first, the level of NK cell apoptosis was not examined in vivo. Second, we did not use carboxyfluorescein diacetate succinimidyl ester (CFSE)-labeled NK cells to examine the impaired capacity for proliferation induced in the dysfunctional NK cells, in addition to measuring Ki-67 production. Third, tumor-derived PGE2 plays a pivotal role in inducing recruitment of myeloid suppressor cells in cancer,28-30 and further studies are needed to elucidate the molecular basis of PGE2-regulated accumulation of myeloid suppressor cells and their functional consequence on NK cell activity in GC. Unfortunately, we were unable to perform this type of experiment in the current study.
In summary, the results of this study suggest that NK cells in intratumoral tissue contribute to immunosurveillance with a normal phenotype and cytotoxic function. At the same time, the ability of these NK cells to proliferate has been damaged by GC cells, accounting for the paradox that intratumoral NK cells can be activated and cytotoxic but are unable to control GC development once a tumor arises. These findings provide important new insight into the mechanisms by which PGE2 derived from cancer cells may perform a suppressive role by regulating NK cell functions. In view of this immunosuppressive effect, new strategies might be developed to prevent the inhibition of potentially efficient antitumor effector cells.
Materials and methods
Patients and specimens
Control blood samples were obtained from 15 healthy blood donors at the Guangzhou Blood Center, and tumor samples were obtained from 235 patients with pathologically confirmed GC at the Nanfang Hospital of Southern Medical University. None of the patients received anticancer therapy before samples were obtained. Individuals with an autoimmune disease, HIV or syphilis were excluded. Blood and paired intratumoral and nontumoral (at least 3 cm from the tumor site) tissues from patients (Group 1) who received therapy in 2014–2015 were processed for the isolation of peripheral and fresh tissue-infiltrating lymphocytes (n = 18, 15 patients for detection of NK cells percentage, surface markers and cytokines; three patients for examination of NK cells cytotoxic assays) or for the preparation of tumor and nontumor tissue-conditioned medium (n = 5). Another 212 patients (Group 2) who had undergone curative resection between 2005 and 2007 and had complete follow-up data were identified. Tissues from this group of patients were used for immunohistochemistry and for the analysis of OS and DFS. Clinical stages were classified according to the guidelines of the AJCC (7th edition). The clinical characteristics of all the patients are summarized in Table S2. All the samples were coded for anonymity in accordance with local ethical guidelines (as stipulated by the Declaration of Helsinki). Written informed consent was obtained from the patients, and the protocol was approved by the Review Board of Southern Medical University.
Tumor cell lines and preparation of tumor cell line culture supernatants
The human GC cell line, SGC7901, was purchased from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). The KATO III and AGS cell lines were obtained from the American Type Culture Collection (Manassas, VA, USA). All these cell types were tested for mycoplasma contamination using a single-step polymerase chain reaction (PCR) method31 and maintained in complete medium composed of Dulbecco's modified Eagle's medium (DMEM, Life Technologies) supplemented with 10% heat-inactivated fetal bovine serum (FBS; GIBCO, Australia). TSNs were prepared by plating 5 × 106 tumor cells in 10 mL of complete medium in 100-mm dishes, incubating the dishes for 24 h, and then changing the medium to complete medium with or without 5 μM NS398 (Cayman Chemicals). After 48 h, the supernatants were harvested, centrifuged and stored in aliquots at −80°C.
Tissue culture and preparation of tumor tissue and nontumor tissue culture supernatants
Gastric tissues were obtained from five independent cases of pathologically confirmed GC by gastroscopy. The tumor tissues were selected from minimally necrotic regions of the tumor mass. TTCS or NTCS were prepared as reported.32,33 Briefly, the tissues were cut into small pieces and then digested with collagenase type IV (1 mg/mL; Sigma, USA) and hyaluronidase (125 units/mL; Sigma, USA) at 37°C with agitation for 1 h in DMEM with 10% heat-inactivated FBS. The dissociated tissues were centrifuged, resuspended in DMEM with 10% FBS and cultured using one 25-cm2 flask per 1 g of tissue. After a 48-h culture period, the supernatants were harvested, centrifuged and stored.
Isolation of lymphocytes from peripheral blood and tissues
Peripheral lymphocytes were isolated by Ficoll density gradient centrifugation. Tumor- and nontumor-infiltrating lymphocytes were obtained from paired fresh tissue samples as described.33 Briefly, the human GC tissue was cut into small pieces and pre-incubated twice in RPMI 1640 (Life Technologies) containing 10% FBS, 1 mM DTT (Sigma, USA), 10 mM EDTA, 50 μg/mL gentamycin, 100 U/mL penicillin and 0.1 mg/mL streptomycin (PAA Laboratories, Germany) for 15 min. Next, the tissue was cut into fine pieces and digested in RPMI 1640 in the presence of 1 mg/mL type IV collagenase and 0.5 mg/mL DNAse (both from Sigma, USA) for 1 h. After digestion, the cell suspension was filtered, and the mononuclear cells were isolated by Ficoll (GE, USA) density gradient centrifugation. The lymphocytes were washed and resuspended in medium supplemented with 1% FBS for fluorescent-activated cell sorter (FACS) analysis.
Immunohistochemistry and Immunofluorescence
Paraffin-embedded and formalin-fixed samples were cut into 4-μm sections, which were then processed for immunohistochemistry as described.34 Following incubation with antibodies (Abs) directed against human CD57 (Abcam, Cambridge, MA; catalog number: ab187274) and COX-2 (Abcam, Cambridge, MA; catalog number: ab15191), the sections were stained in an Envision System (Dako Cytomation). At low power (×200), the tissue sections were screened using an inverted research microscope (Leica DM IRB, Germany), and the five most representative fields were selected. The extent of COX-2 staining was graded, and the number of nucleated NK-1+ NK cells per field were counted in each area. This analysis was performed by two independent observers who were blinded to the clinical outcome. For immunofluorescence analysis, tissues were stained with Abs against CD3 (Abcam, Cambridge, MA; catalog number: ab5690) and CD57, followed by Alexa Fluor 555–conjugated anti–mouse IgG and Alexa Fluor 488–conjugated anti–rabbit IgG (Molecular Probes, Carlsbad, CA). Nuclei were stained with 40-6-diamidino-2-phenylindole (DAPI). Images were viewed and assessed using a fluorescence microscope (Leica DMI 4000B, Germany) and analyzed by Leica Application suite software (version 4.0).
Isolation and culture of NK cells
Peripheral blood mononuclear cells (PBMCs) were isolated from the buffy coats of blood from healthy donors by Ficoll density gradient centrifugation as described previously.22 The NK cell isolation kit was from Miltenyi Biotec GmbH (Bergisch Gladbach, Germany). The cells were cultured in RPMI 1640 containing 20% heat-inactivated FBS and 100 U/mL IL-2 (R&D Systems) in the presence of 30% TSN or complete medium supplemented with 10 μM human PGE2 (Sigma-Aldrich), 10 μM butaprost (an EP2 agonist), and 20 μM misoprostol (an EP4 agonist; Cayman Chemical) for 60 h.
Flow cytometry
Leukocytes were stained for surface markers, fixed, permeabilized with IntraPreReagent (Beckman Coulter, Fullerton, CA), and further stained with Abs directed against intracellular markers. The data were acquired with a Gallios Flow Cytometer (Beckman Coulter, Brea, CA). To measure intracellular cytokine production, the cells were stimulated with Leukocyte Activation Cocktail (BD, Bioscience) at 37°C for 4 h before staining as described previously.22 The fluorochrome-conjugated monoclonal Abs are listed in Table S3. EP2 receptor (Item Number: 101750) and EP4 receptor (Item Number: 10479) were both from Cayman chemical, USA. Apoptosis of NK cells was quantified using an Annexin V apoptosis detection kit according to the manufacturer's instructions (R & D Systems; Abingdon, UK).
Cytotoxicity assays
AGS cells (2 × 104) were seeded in 48 wells and cultured in complete medium for 6 h. Mononuclear cells were purified from blood and intratumoral tissues; and the NK cell isolation kit was from EasySep™ Human CD56 Positive Selection Kit (Stem cell technologies, Canada). The lytic potential of NK cells derived from blood or intratumoral tissues were mixed with AGS cells at 5:1 and 10:1 effector-to-target (E/T) ratios respectively. Cells were cocultured in RPMI 1640 containing 20% heat-inactivated FBS and 100 U/mL IL-2 for 3 h, and then were washed three times to remove NK cells. Apoptosis of AGS cells was detected by morphological examination and was further confirmed by terminal deoxynucleotidyl transferase-mediated nick-end labeling (TUNEL) staining. For morphological examination, cells were stained with DAPI and those with condensed or fragmented nuclei were considered to be apoptotic cells. TUNEL staining was conducted using the In Situ Cell Death Detection kit, POD (Roche Diagnostics, Pleasanton, CA), according to the manufacturer's protocol.
ELISA
The concentrations of PGE2 (Enzo Life Sciences) in the TSNs, TTCS and NTCS were determined using ELISA kits according to the manufacturer's instructions.
Statistical analysis
The results are expressed as the mean ± SEM. The statistical significance of differences between groups was determined using Student's t test or a one-way ANOVA. The cumulative survival time was calculated using the Kaplan–Meier method, and survival was measured in months from the resection to either recurrence or the last review. The log-rank test was applied to compare the groups. Univariate and multivariate analyses of the prognostic factors for OS and DFS were performed using the Cox proportional hazards model. SPSS statistical software (version 19.0, IBM) and GraphPad Prism Version 5.0a (GraphPad) were used for all the statistical analyses. All the data were analyzed using two-tailed tests unless otherwise specified, and p < 0.05 was considered statistically significant.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
Grants support: This work was supported by: The State Key Projects on Infection Diseases of China (the 12th Five-Year Plan Period) 2012ZX10002017-005, 2012ZX10002016-011, 2012ZX10002010-001-007. National Natural Science Foundation of China, 81372243, 81370575, 81370555. Natural Science Foundation of Guangdong Province, S20120011190. Specialized Research Fund for the Doctoral Program of Higher Education, 20120171110082. Science and Technology Planning Project of Guangzhou, 201400000001-3. Public welfare in Health Industry, National Health and Family Planning Commission of China (201402015, 201502039). Chinese Post-doctoral foundation (2014M552216) and Key Clinical Specialty Discipline Construction Program.
Supplemental Material
Supplemental Material may be downloaded here: publisher's website
References
- 1.Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ. Natural innate and adaptive immunity to cancer. Ann Rev Immunol 2011; 29:235-71; PMID:21219185; http://dx.doi.org/ 10.1146/annurev-immunol-031210-101324 [DOI] [PubMed] [Google Scholar]
- 2.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell 2010; 140:883-99; PMID:20303878; http://dx.doi.org/ 10.1016/j.cell.2010.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parham P. Immunology. NK cells lose their inhibition. Science 2004; 305:786-7; PMID:15297654; http://dx.doi.org/ 10.1126/science.1102025 [DOI] [PubMed] [Google Scholar]
- 4.Vivier E, Raulet DH, Moretta A, Caligiuri MA, Zitvogel L, Lanier LL, Yokoyama WM, Ugolini S. Innate or adaptive immunity? The example of natural killer cells. Science 2011; 331:44-9; PMID:21212348; http://dx.doi.org/ 10.1126/science.1198687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Narni-Mancinelli E, Jaeger BN, Bernat C, Fenis A, Kung S, De Gassart A, Mahmood S, Gut M, Heath SC, Estellé J et al.. Tuning of natural killer cell reactivity by NKp46 and Helios calibrates T cell responses. Science 2012; 335:344-8; PMID:22267813; http://dx.doi.org/ 10.1126/science.1215621 [DOI] [PubMed] [Google Scholar]
- 6.Wu Y, Kuang DM, Pan WD, Wan YL, Lao XM, Wang D, Li XF, Zheng L. Monocyte/macrophage-elicited natural killer cell dysfunction in hepatocellular carcinoma is mediated by CD48/2B4 interactions. Hepatology 2013; 57:1107-16; PMID:23225218; http://dx.doi.org/ 10.1002/hep.26192 [DOI] [PubMed] [Google Scholar]
- 7.Vivier E, Ugolini S, Blaise D, Chabannon C, Brossay L. Targeting natural killer cells and natural killer T cells in cancer. Nat Rev Immunol 2012; 12:239-52; PMID:22437937; http://dx.doi.org/ 10.1038/nri3174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keener AB. Natural killers: Cataloging immune cells for immunotherapy. Nat Med 2015; 21:207-8; PMID:25742450; http://dx.doi.org/ 10.1038/nm0315-207 [DOI] [PubMed] [Google Scholar]
- 9.Parkhurst MR, Riley JP, Dudley ME, Rosenberg SA. Adoptive transfer of autologous natural killer cells leads to high levels of circulating natural killer cells but does not mediate tumor regression. Clin Cancer Res 2011; 17:6287-97; PMID:21844012; http://dx.doi.org/ 10.1158/1078-0432.CCR-11-1347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mantovani A, Romero P, Palucka AK, Marincola FM. Tumour immunity: effector response to tumour and role of the microenvironment. Lancet 2008; 371:771-83; PMID:18275997; http://dx.doi.org/ 10.1016/S0140-6736(08)60241-X [DOI] [PubMed] [Google Scholar]
- 11.Grosch S, Maier TJ, Schiffmann S, Geisslinger G. Cyclooxygenase-2 (COX-2)-independent anticarcinogenic effects of selective COX-2 inhibitors. J Natl Cancer Inst 2006; 98:736-47; PMID:16757698; http://dx.doi.org/ 10.1093/jnci/djj206 [DOI] [PubMed] [Google Scholar]
- 12.Chen JH, Perry CJ, Tsui YC, Staron MM, Parish IA, Dominguez CX, Rosenberg DW, Kaech SM. Prostaglandin E2 and programmed cell death 1 signaling coordinately impair CTL function and survival during chronic viral infection. Nat Med 2015; 21:327-34; PMID:25799228; http://dx.doi.org/ 10.1038/nm.3831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ng K, Meyerhardt JA, Chan AT, Sato K, Chan JA, Niedzwiecki D, Saltz LB, Mayer RJ, Benson AB 3rd, Schaefer PL, et al.. Aspirin and COX-2 inhibitor use in patients with stage III colon cancer. J Natl Cancer Inst 2015; 107:345; PMID:25432409; http://dx.doi.org/ 10.1093/jnci/dju345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McLean MH, El-Omar EM. Genetics of gastric cancer. Nat Rev Gastroenterol Hepatol 2014; 11:664-74; PMID:25134511; http://dx.doi.org/ 10.1038/nrgastro.2014.143 [DOI] [PubMed] [Google Scholar]
- 15.Sahin M, Sahin E, Gumuslu S. Cyclooxygenase-2 in cancer and angiogenesis. Angiology 2009; 60:242-53; PMID:18505747; http://dx.doi.org/ 10.1177/0003319708318378 [DOI] [PubMed] [Google Scholar]
- 16.Martinet L, Smyth MJ. Balancing natural killer cell activation through paired receptors. Nat Rev Immunol 2015;15:243-54; PMID:25743219; http://dx.doi.org/ 10.1038/nri3799 [DOI] [PubMed] [Google Scholar]
- 17.Xu L, Stevens J, Hilton MB, Seaman S, Conrads TP, Veenstra TD, Logsdon D, Morris H, Swing DA, Patel NL et al.. COX-2 inhibition potentiates antiangiogenic cancer therapy and prevents metastasis in preclinical models. Sci Translat Med 2014; 6:242ra84; http://dx.doi.org/ 10.1126/scitranslmed.3008455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalinski P. Regulation of immune responses by prostaglandin E2. J Immunol 2012; 188:21-8; PMID:22187483; http://dx.doi.org/22002714 10.4049/jimmunol.1101029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reader J, Holt D, Fulton A. Prostaglandin E2 EP receptors as therapeutic targets in breast cancer. Cancer Metastasis Rev 2011; 30:449-63; PMID:22002714; http://dx.doi.org/ 10.1007/s10555-011-9303-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kundu N, Ma X, Holt D, Goloubeva O, Ostrand-Rosenberg S, Fulton AM. Antagonism of the prostaglandin E receptor EP4 inhibits metastasis and enhances NK function. Breast Cancer Res Treat 2009; 117:235-42; PMID:18792778; http://dx.doi.org/ 10.1007/s10549-008-0180-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smyth MJ, Hayakawa Y, Takeda K, Yagita H. New aspects of natural-killer-cell surveillance and therapy of cancer. Nat Rev Cancer 2002; 2:850-61; PMID:12415255; http://dx.doi.org/ 10.1038/nrc928 [DOI] [PubMed] [Google Scholar]
- 22.Li T, Yang Y, Hua X, Wang G, Liu W, Jia C, Tai Y, Zhang Q, Chen G. Hepatocellular carcinoma-associated fibroblasts trigger NK cell dysfunction via PGE2 and IDO. Cancer Lett 2012; 318:154-61; PMID:22182446; http://dx.doi.org/ 10.1016/j.canlet.2011.12.020 [DOI] [PubMed] [Google Scholar]
- 23.Salgado R, Denkert C, Demaria S, Sirtaine N, Klauschen F, Pruneri G, Wienert S, Van den Eynden G, Baehner FL, Penault-Llorca F et al.. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol 2015; 26:259-71; PMID:25214542; http://dx.doi.org/ 10.1093/annonc/mdu450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer-related inflammation and treatment effectiveness. Lancet Oncol 2014; 15:e493-e503; PMID:25281468; http://dx.doi.org/ 10.1016/S1470-2045(14)70263-3 [DOI] [PubMed] [Google Scholar]
- 25.Villegas FR, Coca S, Villarrubia VG, Jimenez R, Chillon MJ, Jareno J, Zuil M, Callol L. Prognostic significance of tumor infiltrating natural killer cells subset CD57 in patients with squamous cell lung cancer. Lung cancer 2002; 35:23-8; PMID:11750709; http://dx.doi.org/ 10.1016/S0169-5002(01)00292-6 [DOI] [PubMed] [Google Scholar]
- 26.Senovilla L, Vacchelli E, Galon J, Adjemian S, Eggermont A, Fridman WH, Sautès-Fridman C, Ma Y, Tartour E, Zitvogel L et al.. Trial watch: Prognostic and predictive value of the immune infiltrate in cancer. Oncoimmunology 2012; 1:1323-43; PMID:23243596; http://dx.doi.org/ 10.4161/onci.22009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pietra G, Manzini C, Rivara S, Vitale M, Cantoni C, Petretto A, Balsamo M, Conte R, Benelli R, Minghelli S et al.. Melanoma cells inhibit natural killer cell function by modulating the expression of activating receptors and cytolytic activity. Cancer Res 2012; 72:1407-15; PMID:22258454; http://dx.doi.org/ 10.1158/0008-5472.CAN-11-2544 [DOI] [PubMed] [Google Scholar]
- 28.Mao Y, Sarhan D, Steven A, Seliger B, Kiessling R, Lundqvist A. Inhibition of tumor-derived prostaglandin-e2 blocks the induction of myeloid-derived suppressor cells and recovers natural killer cell activity. Clin Cancer Res 2014; 20:4096-106; PMID:24907113; http://dx.doi.org/ 10.1158/1078-0432.CCR-14-0635 [DOI] [PubMed] [Google Scholar]
- 29.Serafini P. Editorial: PGE2-producing MDSC: a role in tumor progression? J leukoc Biol 2010; 88:827-9; PMID:21041513; http://dx.doi.org/ 10.1189/jlb.0510303 [DOI] [PubMed] [Google Scholar]
- 30.Eruslanov E, Daurkin I, Ortiz J, Vieweg J, Kusmartsev S. Pivotal Advance: Tumor-mediated induction of myeloid-derived suppressor cells and M2-polarized macrophages by altering intracellular PGE(2) catabolism in myeloid cells. J leukoc Biol 2010; 88:839-48; PMID:20587738; http://dx.doi.org/ 10.1189/jlb.1209821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Uphoff CC, Drexler HG. Detection of mycoplasma in leukemia-lymphoma cell lines using polymerase chain reaction. Leukemia 2002; 16:289-93; PMID:11840297; http://dx.doi.org/ 10.1038/sj.leu.2402365 [DOI] [PubMed] [Google Scholar]
- 32.Zhuang Y, Peng LS, Zhao YL, Shi Y, Mao XH, Chen W, Pang KC, Liu XF, Liu T, Zhang JY et al.. CD8(+) T cells that produce interleukin-17 regulate myeloid-derived suppressor cells and are associated with survival time of patients with gastric cancer. Gastroenterology 2012; 143:951–62.e8; PMID:22710190; http://dx.doi.org/ 10.1053/j.gastro.2012.06.010 [DOI] [PubMed] [Google Scholar]
- 33.Glatzer T, Killig M, Meisig J, Ommert I, Luetke-Eversloh M, Babic M, Paclik D, Blüthgen N, Seidl R, Seifarth C et al.. RORgammat(+) innate lymphoid cells acquire a proinflammatory program upon engagement of the activating receptor NKp44. Immunity 2013; 38:1223-35; PMID:23791642; http://dx.doi.org/ 10.1016/j.immuni.2013.05.013 [DOI] [PubMed] [Google Scholar]
- 34.Wu Y, Zhao Q, Peng C, Sun L, Li XF, Kuang DM. Neutrophils promote motility of cancer cells via a hyaluronan-mediated TLR4/PI3K activation loop. J Pathol 2011; 225:438-47; PMID:21826665; http://dx.doi.org/ 10.1002/path.2947 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.