ABSTRACT
Here, we introduce a double-stranded RNA mimic that is chemically synthesized to exclusively stimulate toll-like receptor 3 (TLR3). This function-defined TLR3 ligand, named ARNAX, acts as an adjuvant to induce antitumor CTL and NK without significant cytokinemia in mice, and thus superior to polyI:C for therapeutic vaccine immunotherapy against tumor.
Keywords: Antitumor CTL, Priming-phase adjuvant, no cytokine toxicity, tumor remission
Introduction
PolyI:C, a double-stranded RNA mimic, has been employed as an adjuvant for cancer immunotherapy. Together with tumor-associated antigen peptides (TAAs), it efficiently induces antigen-specific cytotoxic T-lymphocytes (CTL). Tumor shrinkage was observed in patients with various cancers treated with immunotherapy in clinical phase trials. However, due to toxic manifestations which were deemed intolerable, most of the clinical studies were stopped, although a significant therapeutic effect against cancer was observed in these trials.1 Life-threatening side-effects, represented by hypotonic shock, disseminating thrombosis and renal failure, are signs of cytokinemia secondary to cytokine toxicity.2
Accumulating evidence on innate immunity suggests that without adjuvant, cellular immunity does not respond to antigens, a state known as tolerance or anergy, and is exhausted shortly after stimulation.3,4 The concept of anergy is that dendritic cells (DC), which in the presence of adjuvant mature and promote differentiation of specific CTL against TAAs, are barely evoked because tumors possess TAAs but no adjuvants (such as PAMPs). Our previous studies on RNA adjuvants,5,6 revealed that polyI:C induces cross-presentation of exogenous antigens, upregulation of MHC class I molecules and co-stimulators, and increases serum levels of cytokines/chemokines. In some cases, tumor necroptosis is induced to contribute to the back-up of immune cell-mediated tumor cytotoxicity.7 PolyI:C exerts all these aspects of the adjuvant effect in tumor-bearing mice. PolyI:C is a ligand for MDA5 and TLR3, which signal activation of the MAVS and TICAM-1 pathways, respectively.6,7 The only weak point of polyI:C use is its induction of marked cytokinemia, which is rooted in the MDA5/MAVS pathway.3
Development of a TLR3-defined adjuvant
Our attempt to overcome the toxic manifestations caused by polyI:C was to make polyI:C non-toxic. We found that the cytokine-toxicity was mainly attributable to the MAVS pathway,3,8 while the antitumor NK/CTL induction was based on Batf3 transcriptional activity and the TLR3 pathway in polyI:C treatment. Production of IL-12p70 and direct DC maturation are indispensable to the polyI:C antitumor function, and this effect is independent of the MAVS signal.3 Hence, the problem would be solved if we could develop a compound that specifically activates TLR3.
To design aTLR3-specific ligand that sufficiently activates the TICAM-1 pathway but not the MAVS pathway, we monitored several markers.8 Activation of the IFN-β promoter, function of splenic DC from Mavs−/− and Ticam1−/− mice and NK cell IFNγ production were determined with >50 compounds consisting of DNA and dsRNA. These compounds were in vitro-transcribed RNA 5′-capped with phosphorothioated GpC not CpG DNA (sODN-dsRNA). sODN capping blocks RIG-I activation and facilitates trafficking of the dsRNA to endosomes.8 The leader–trailer sequence of measles virus was employed as the dsRNA, which will never coincidentally induce RNAi in human transcripts. The length of the RNA chain is 140, which is too short to activate MDA5, but induces TLR3 activation.8 The sequence of the dsRNA would be further improved by modification, but the minimal essential is to deliver the dsRNA to endosomes without degradation and thus to stimulate TLR3 (Fig. 1). Ultimately, one compound, named sODN with 140 dsRNA (sODN-140), was synthesized that fulfilled the above criteria.8
Figure 1.
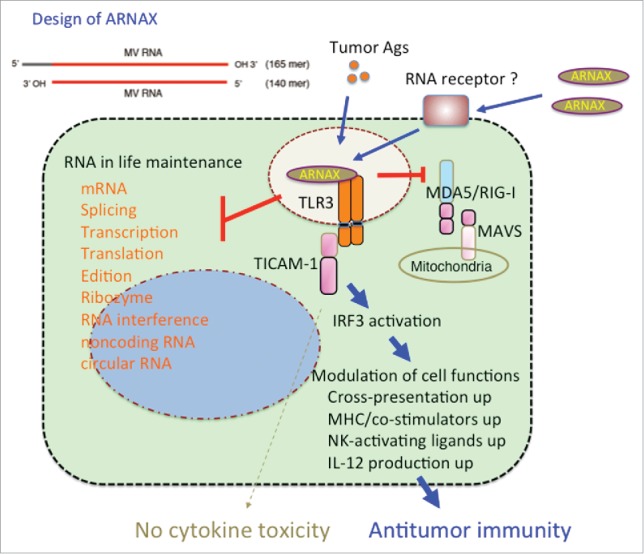
TLR3 targeting by ARNAX for cancer immunotherapy.The chemical structure of ARNAX is shown to the upper left. MV: measeles virus. RNA essentially plays functions for life support in the nucleus and cytoplasm (left), but ARNAX does not affect these fundamental RNA functions. On the other hand, ARNAX taken up into cells induces signal response capable of activating IRF3 in myeloid cells (right). Although type I IFN and inflammatory cytokines are produced via the TICAM-1 pathway in this context, the serum cytokine levels are not significantly increased by ARNAX, unlike polyI:C. ARNAX reserves the ability to mature DC followed by immune cells activation, resulting in tumor repression (right). Molecular mechanism for capturing RNA in the membrane and transferring it to endosomes is not clear yet. MDA5 and RIG-I are cytoplasmic RNA sensors which cause robust cytokine production. TICAM-1 and MAVS are adapter molecules.
Tumor growth retardation was determined using mouse implant models of the syngeneic system. EG7 (OVA), B16 (survivin) and C1498 (WT1) were found to be good systems in C57BL/6 mice, and we mainly used the EG7 (OVA)-implant system, since OVA is a multi-epitope antigen and CTL activation is represented by SL8-tetramer.8,9 OT-1 proliferation, tetramer assay and IFNγ production were employed to measure CTL production. Therapeutic response was monitored by tumor shrinkage, and parallelism was checked by monitoring cytokine levels in serum, NK cell and TAA-specific CTL activation.8 The inflammatory cytokines, IL-6, TNF-α and IL-10, and the instructive cytokine IL-12, were measured in sera 6 h after sub-cutaneous injection of cM362–140.8 Surprisingly, cM362–140 did not increase inflammatory cytokines, but sufficiently induced IL-12p70. Hence, we decided to chemically synthesize this molecule instead of using in vitro synthesis to enable future GMP regulation.
Five years after the plan was accepted, sODN-140 was synthesized using a DNA/RNA synthesizer. The synthetic device will be described separately. The results of sODN-140 were as expected.8 The yield of IL-12p40 was more than expected in Mavs−/− mice compared to wild-type mice. We next wanted to improve the method to increase the yield of this compound. Generation of a method of more efficient recovery is currently under consideration. We named the synthetic sODN-140 ‘ARNAX’.
We are currently testing whether ARNAX exhibits effective adjuvancy for TAA-dependent CTL proliferation in a non-inflammatory environment in human blood cells. Although ARNAX has not yet undergone phase I trials, we are aware that the mouse results are essentially reproducible in human blood cells. If clinical trials reproduce these effects, immunotherapy will be improved by abolishing the side effects of the DC-priming by using ARNAX.
Immunotherapy perspectives
Here, we emphasize the importance of a function-defined adjuvant to enable use of vaccine immunotherapy for cancer (Fig. 1). The adjuvant directs the DC-priming host immune system to respond to the appropriate target: generating mature DC for tumor eradication.9 We show in this report that a TLR3-specific adjuvant preferentially induces TAA-specific CTL as well as NK cell activation without provoking inflammation or systemic cytokinemia.8 Immunotherapy with antibodies against PD-L1 or PD-1 has brought cancer patients hope of a positive outcome: in patients with an inoperable solid tumor, more than 20% diminished without severe side-effects.10 The combination of PD-1/PD-L1 with TAA+ARNAX may be a promising therapeutic regimen, where TAA exerts cytotoxicity specifically on the tumor, while ARNAX induces DC to promote proliferation of CTL by breaking their tolerance and exhaustion (Matsumoto M, Seya T, in preparation). These functions of ARNAX increase tumor immunity in conjunction with release of the suppressive co-stimulatory function of PD-1. Such therapies will enable creation of a more extensive vaccine immunotherapy to facilitate eradication of cancer as in the vaccine therapies against infections.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Galluzzi L, Vacchelli E, Eggermont A, Fridman WH, Galon J, Sautès-Fridman C, Tartour E, Zitvogel L, Kroemer G. Trial watch: experimental toll-like receptor agonists for cancer therapy. Oncoimmunology 2012; 1:699-716; PMID:22934262; http://dx.doi.org/ 10.4161/onci.20696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aranda F, Vacchelli E, Obrist F, Eggermont A, Galon J, Sautès-Fridman C, Cremer I, Henrik Ter Meulen J, Zitvogel L, Kroemer G, Galluzzi L. Trial watch: toll-like receptor agonists in oncological indications. Oncoimmunology 2014; 3:e29179; PMID:25083332; http://dx.doi.org/ 10.4161/onci.29179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seya T, Azuma M, Matsumoto M. Targeting TLR3 with no RIG-I/MDA5 activation is effective in immunotherapy for cancer. Expert Opin Ther Targets 2013; 17:533-544; PMID:23414438; http://dx.doi.org/ 10.1517/14728222.2013.765407 [DOI] [PubMed] [Google Scholar]
- 4.Mescher MF, Curtsinger JM, Agarwal P, Casey KA, Gerner M, Hammerbeck CD, Popescu F, Xiao Z. Signals required for programming effector and memory development by CD8+ T cells. Immunol Rev 2006; 211: 81-92; PMID:16824119; http://dx.doi.org/ 10.1111/j.0105-2896.2006.00382.x [DOI] [PubMed] [Google Scholar]
- 5.Akazawa T, Ebihara T, Okuno M, Okuda Y, Shingai M, Tsujimura K, Takahashi T, Ikawa M, Okabe M, Inoue N, et al. Antitumor NK activation induced by the Toll-like receptor 3-TICAM-1 (TRIF) pathway in myeloid dendritic cells. Proc Natl Acad Sci U S A 2007; 104:252-257; PMID:17190817; http://dx.doi.org/ 10.1073/pnas.0605978104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Azuma M, Ebihara T, Oshiumi H, Matsumoto M, Seya T. Cross-priming for antitumor CTL induced by soluble Ag + polyI:C depends on the TICAM-1 pathway in mouse CD11c(+)/CD8α(+) dendritic cells. Oncoimmunology 2012; 1:581-592; PMID:22934250; http://dx.doi.org/ 10.4161/onci.19893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seya T, Shime H, Takaki H, Azuma M, Oshiumi H, Matsumoto M. TLR3/TICAM-1 signaling in tumor cell RIP3-dependent necroptosis. Oncoimmunology 2012; 1:917-23; PMID:23162759; http://dx.doi.org/ 10.4161/onci.21244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsumoto M, Tatematsu M, Nishikawa F, Azuma M, Shime H, Seya T. Defined TLR3-specific adjuvant that induces NK and cytotoxic T cell activation without significant cytokine production in vivo. Nat Commun 2015; 6:6280; PMID:25692975; http://dx.doi.org/ 10.1038/ncomms7280 [DOI] [PubMed] [Google Scholar]
- 9.Woo SR, Corrales L, Gajewski TF. Innate Immune Recognition of Cancer. Annu Rev Immunol 2015 Jan 22. [Epub ahead of print [DOI] [PubMed] [Google Scholar]
- 10.Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, Wolchok JD, Hersey P, Joseph RW, Weber JS, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med 2013; 369:134-144; PMID:23724846; http://dx.doi.org/ 10.1056/NEJMoa1305133 [DOI] [PMC free article] [PubMed] [Google Scholar]


