ABSTRACT
Accumulating evidence suggests that B cells play important roles in inhibiting the immune response in autoimmune disorders and human tumors as well as murine tumor models. In an effort to explore the role of B cells in human breast cancer etiology, we examined the presence of CD19+ B lymphocytes in 134 cases of invasive breast carcinoma (IBCa) and 31 breast fibroadenoma, and assessed its relationship with PD-L1 (programmed death-ligand 1) expression in breast cancer. We found that the density of CD19+ B lymphocytes was higher in IBCa compared with fibroadenoma, and significantly associated with increasing tumor grade, negative estrogen status. Similar findings were observed for the expression of IL-10 in IBCa. Meanwhile, CD19+ B lymphocytes were shown to be highly coincident with PD-L1 and IL-10 in IBCa. We further demonstrated that CD19+ B cells can differentiate into CD19+CD24+CD38+ B cells when co-cultured with PD-L1hi MDA-MB231 cells. In addition, the percentage of CD19+CD24+CD38+ B cells was higher in breast tissue and peripheral blood cells of IBCa patients than that of benign tumor and health individuals. And CD19+CD24+CD38+ B cells were found to be IL-10 secreting B cells. Finally, we showed that CD19+ B cells from IBCa patients but not healthy individuals induced formation of CD4+CD25+Foxp3+ T cells when co-cultured with T cells from IBCa patients and healthy subjects (80.4% and 30.8% respectively). The induction of CD4+CD25+Foxp3+ T cells by CD19+ B cells was further shown to be mediated by PD-L1. Together, these results are suggestive of a role for CD19+ B lymphocytes in immune suppression and tumor evasion via PD-L1 in breast cancer.
KEYWORDS: Breast cancer, B lymphocytes, CD19, IL-10, PD-L1, regulatory B cell, regulatory T cell
Abbreviations
- APCs
antigen-presenting cells
- Bregs
regulatory B cells
- DAB
diaminobenzydine
- ER
estrogen-receptor
- HE staining
hematoxylin/eosin staining
- HER-2
human epidermal growth factor receptor 2
- IBCa
invasive carcinoma of breast
- IHC
immunohistochemical staining
- PBMC
peripheral blood mononuclear cell
- PD-1
programmed death 1
- PD-L1
programmed death-ligand 1
- PR
progesterone-receptor
- siRNA
small interfering RNAs
- Th
CD4+ helper T
- Th0
T helper type-0
- TIL
tumor-infiltrating lymphocytes
- TNM stage
tumor node and metastasis stage
- Tregs
regulatory T cells
- B10
IL-10–competent B cells
- DAPI
4′,6-diamidino-2-phenylindol
- IF
Immunofluorescence
Introduction
B lymphocytes are a type of lymphocytes that present antigen, secrete immunoglobulin and produce cytokines.1-3 As antigen presenting cells (APCs), B lymphocytes transfer activation signal to T cells and positively regulate T-cell activation in tumor immunity response.4-6 Hansen et al. reported the tumor-infiltrating B lymphocytes can induce medullary breast cancer apoptosis through antibody production.6 However, several recent studies using murine tumor models showed that B lymphocytes negatively regulate tumor immune response.7 These studies showed that growth of cancer cells could be limited without metastasis in B-cell-deficient mice.8 In addition, a subset of B cells with negative regulatory function were identified as IL-10–competent B cells (or B10) which produces IL-10 upon stimulation.9-11 Recently, Blair et al. reported that CD19+CD38+CD24+ B lymphocytes in both humans and mice were linked with protection from autoimmune diseases.10 Further studies showed that blockade of IL-10 and IL-10 receptor of CD19+CD38+CD24+ B cells, the subset B cells lost suppressive effect. The IL-10 expressing subset B cells with regulatory functions, independently of secreted antibodies, are regarded as regulatory B cells (Bregs).12 Further investigations suggested the Bregs might inhibit immune response via upregulating CD4+CD25+Foxp3+ T cells.8 CD4+CD25+Foxp3+ T cell, as negative regulator of immune response, was associated with inhibition of immune response against cancer, tumor immune escape and metastasis. It has been shown that significantly more B lymphocytes were expressed in breast cancer than in benign lesions.13 However, the exact role of tumor-infiltrating B lymphocytes in immune responses of breast invasive carcinoma remains largely unknown and needs further investigation.
PD-L1 (B7-H1/CD274) has been identified as ligand for PD-1. Interactions of PD-L1 and PD-1 can inhibit T-cell activity, control the induction and maintenance of peripheral T-cell tolerance during normal immune responses.14,15 These interactions also play an important role in tumor immune evasion.16-18 And numbers of studies have suggested that PD-L1 acts as a negative regulator of immune responses.19-21
B lymphocytes play a central role in the immune system, presenting antigen, secreting immunoglobulin, producing cytokines and activating T cells.1-3 However, the characteristics and molecular mechanisms of CD19+CD38+CD24+ B cells are still poorly defined so far, therefore further studies are warranted. In the present study, we examined the presence and density of tumor-infiltrating CD19+ B cells, CD19+CD38+CD24+ B cells and CD4+CD25+Foxp3+ T cells in IBCa, and assessed the relationship among CD19+ B cells, CD4+CD25+Foxp3+ T cells and PD-L1 expression in IBCa tissue. Meanwhile, we examined the relationship between IL-10 and CD19+ or CD19+CD38+CD24+ B cells to further define the phenotype and function of CD19+CD38+CD24+ B cells in IBCa.
Results
Expression of CD19, IL-10 and PD-L1 in fibroadenoma and IBCa
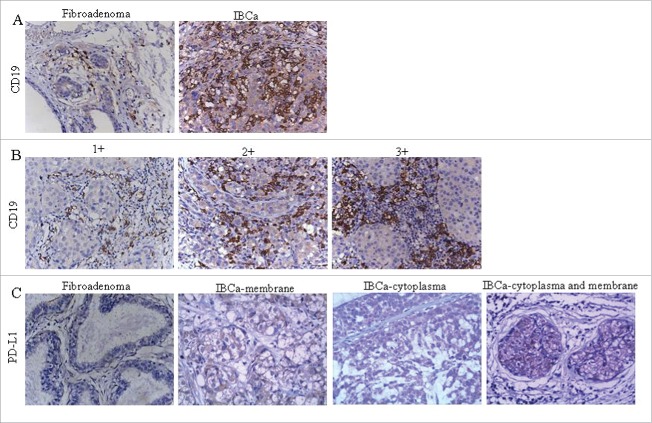
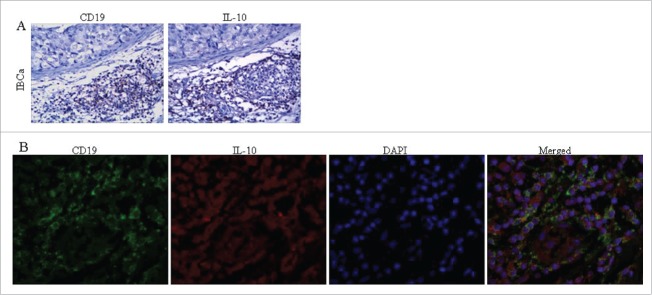
Immunohistochemistry staining analysis showed that CD19 was primarily located in the cell membrane of B lymphocytes (Fig. 1A and 1B). PD-L1 was shown to be expressed predominantly in both the cell membrane and cytoplasm, with some detected only on the membrane or in the cytoplasm (Fig. 1C) and was observed in the breast epithelial cells, cancer cells and tumor-infiltrating lymphocytes (TILs) in fibroadenoma and IBCa. As expected, IL-10 was detected only in the cytoplasm of TILs (Fig. 2A).
Figure 1.
Immunochistochemical staining of CD19 and PD-L1 in IBCa and fibroadenoma tissue of breast. Sections of fibroadenoma tissues and IBCa were stained with anti-CD19, anti-PD-1 and anti-PD-L1 antibodies, as described in “Materials and Methods”, shown in Fig. 1 are representative microphotographs (× 400). (A) The density of CD19+ B lymphocytes is significantly higher in IBCa tissue than that in fibroadenomas of breast. (B) The density of CD19+ B lymphocytes in grade 1, 2 and 3 IBCa. C: PD-L1 is strongly expressed in the cell membrane, cytoplasm or both the IBCa tissues than that in fibroadenomas of the breast. Isotype-matched mouse IgG1 mAb was used as control.
Figure 2.
The colocalization of CD19 and IL-10 expression in IBCa tissues. The colocalization of CD19 and IL-10 expression in IBCa were analyzed by immunohistochemistry and immunoflurescence staining as described in “Materials and Methods”, shown in Fig. 2 are representative microphotographs (× 400). (A) The immunohistochemistry staining of serial sections showed the location of CD19 expression as well as IL-10 in TILs of IBCa tissue. (B) Confocal Laser-Scanning Microscopy was applied to detect CD19 and IL-10 in IBCa specimens. Membrane of B cells was visualized by CD19 staining (Green), cytoplasm are stained with IL-10 (Red), and nuclei are counterstained with DAPI (Blue). Merged figure exhibit a colocalization of CD19 and IL-10 in the B cells.
Immunophenotyping demonstrated that CD19+ B TILs were predominantly noted in the stroma surrounding and separating the tumor masses or at the tumor's edge. Semi-quantitative scoring of cell density revealed that CD19+ B lymphocytes were present in 8 of the 31 fibroadenomas (25.8%) and 93 of the 134 IBCa (69.4%) (Table 1). A total of 38.1% (51 out of 134) of IBCa displayed strong positive (3+) for CD19+ B cells, while none of the fibroadenoma had this level of CD19 B+ cells (p < 0.0001, Table 1). The density of CD19+ B cells with 1+ cell membrane staining was comparable in fibroadenomas and IBCa (16.1% and 18.6% respectively). Further analysis of the relationships between the frequency of CD19+ B cells in IBCa and the histopathological characteristics of IBCa demonstrated that CD19+ B cells were positively associated with histological grade 3, lymph node metastasis, TNM stage T4, ER negative status and PR negative status (all are indicators of poor prognosis) (Table 2). Particularly, the density of CD19+ B cell was significantly associated with histological grade 3 (p < 0.0001) and ER negative status (p = 0.047, Table 2). In addition, a statistically significant association between the frequency of CD19+ B cells and a small tumor size was also noticed (p = 0.021) (Table 2).
Table 1.
Comparisons of the frequency of tumor-infiltrating B cells in breast fibroadenoma and IBCa tissue.
| CD19+ B cells |
||||||
|---|---|---|---|---|---|---|
| All case | – | 1+ | 2+ | 3+ | p | |
| Fibroadenoma | 31 | 23(74.2%) | 5(16.1%) | 3(9.7%) | 0(0.0%) | <0.0001 |
| IBCa | 134 | 41(30.6%) | 25(18.6%) | 17(12.7%) | 51(38.1%) | |
Table 2.
Relationship between the tumor-infiltrating CD19+ B cell density and the clinicopathological parameters of IBCa patients.
| CD19+ B cell |
||||||
|---|---|---|---|---|---|---|
| Variables | All cases | – | 1+ | 2+ | 3+ | p |
| IBCa | 134 | 41(30.6%) | 25(18.7%) | 17(12.7%) | 51(38.1%) | |
| Grade | ||||||
| G1 | 52 | 22(42.3%) | 10(19.2%) | 7(13.5%) | 13(25.0%) | <0.0001 |
| G2 | 52 | 16(30.8%) | 14(26.9%) | 5(9.6%) | 17(32.7%) | |
| G3 | 30 | 3(10.0%) | 1(3.3%) | 5(16.7%) | 21(70.0%) | |
| LN metastasis | ||||||
| No | 81 | 23(28.4%) | 17(21.0%) | 12(14.8%) | 29(35.8%) | 0.984 |
| Yes | 53 | 18(34.0%) | 8(15.1%) | 5(9.4%) | 22(41.5%) | |
| TNM | ||||||
| T1 | 40 | 11(27.5%) | 4(10.0%) | 7(17.5%) | 18(45.0%) | 0.438 |
| T2 | 51 | 14(27.5%) | 15(29.4%) | 4(7.8%) | 18(35.3%) | |
| T3 | 31 | 12(38.7%) | 6(19.4%) | 4(12.9%) | 9(29.0%) | |
| T4 | 12 | 4(33.3%) | 0(0.0%) | 2(16.7%) | 6(50.0%) | |
| ER | ||||||
| Positive | 86 | 28(32.6%) | 19(22.1%) | 14(16.3%) | 25(29.1%) | 0.047 |
| Negative | 48 | 13(27.1%) | 6(12.5%) | 3(6.3%) | 26(54.2%) | |
| PR | ||||||
| Positive | 82 | 26(31.7%) | 18(22.0%) | 14(17.1%) | 24(29.3%) | 0.103 |
| Negative | 52 | 15(28.8%) | 7(13.5%) | 3(5.8%) | 27(51.9%) | |
| HER2 | ||||||
| Positive | 21 | 4(19.0%) | 3(14.3%) | 2(9.5%) | 12(57.1%) | 0.069 |
| Negative | 113 | 37(32.7%) | 22(19.5%) | 15(13.3%) | 39(34.5%) | |
| Age | ||||||
| <49 y | 61 | 17(27.9%) | 10(16.4%) | 10(16.4%) | 24(39.3%) | 0.455 |
| ≥49 y | 73 | 24(32.9%) | 15(20.5%) | 7(9.6%) | 27(37.0%) | |
| Tumor size | ||||||
| <3cm | 74 | 15(20.3%) | 16(21.6%) | 11(14.9%) | 32(43.2%) | 0.021 |
| ≥3cm | 60 | 26(43.3%) | 9(15.0%) | 6(10.0%) | 19(31.7%) | |
As mentioned above, the expression of PD-L1 was detected in membrane and/or cytoplasm (Fig.1C and Table 3), a pattern that has been previously reported. 21 The percentage of positive PD-L1 staining was lower in fibroadenomas (54.8%), significantly increased in IBCa (83.6%) (P = 0.001, Table 3). The expression of PD-L1 in IBCa was significantly associated with TNM staging with 75.0%, 82.3%, 93.5% and 91.7% in T1, T2, T3 and T3 respectively (P = 0.030, Table 4). A marginal significance was observed between PD-L1 expression in breast cancer cells and in IBCa tumor grade with the highest positivity seen in G2 tumors (47/52 or 90.4%) (P = 0.063, Table 4).
Table 3.
PD-L1 expression in breast fibroadenoma and IBCa tissue.
| PD-L1 expression |
||||||
|---|---|---|---|---|---|---|
| All case | – | membrane + | cytoplasm + | both + | p | |
| Fibroadenoma | 31 | 9(29.0%) | 3(9.7%) | 2(6.5%) | 17(54.8%) | 0.001 |
| IBCa | 134 | 15(11.2%) | 4(3.0%) | 3(2.2%) | 112(83.6%) | |
Table 4.
Relationship between PD-L1 expression in IBCa tissue with histopathological features of IBCa.
| PD-L1 expresion |
||||||
|---|---|---|---|---|---|---|
| Variables | All cases | − | Membrane + | Cytoplasm + | Both+ | p |
| IBCa | 134 | 15(11.2%) | 4(3.0%) | 3(2.2%) | 112(83.6%) | |
| Grade | ||||||
| G1 | 52 | 9 (17.3%) | 2 (3.8%) | 1 (1.9%) | 40 (76.9%)* | 0.063 |
| G2 | 52 | 2 (3.8%) | 1 (1.9%) | 2 (3.8%) | 47 (90.4%) | |
| G3 | 30 | 4 (13.3%) | 1(3.3%) | 0 (0.0%) | 25 (83.3%) | |
| LN metastasis | ||||||
| No | 81 | 10 (12.3%) | 3 (3.7%) | 3 (3.7%) | 65 (80.2%) | 0.359 |
| Yes | 53 | 5 (9.4%) | 1 (1.9%) | 0 (0.0%) | 47 (88.7%) | |
| TNM | ||||||
| T1 | 40 | 7 (17.5%) | 3 (7.5%) | 0 (0.0%) | 30 (75.0%) | 0.030 |
| T2 | 51 | 6 (11.8%) | 1 (2.0%) | 2 (3.9%) | 42 (82.3%) | |
| T3 | 31 | 1 (3.2%) | 0 (0.0%) | 1 (3.2%) | 29 (93.5%) | |
| T4 | 12 | 1 (8.3%) | 0 (0.0%) | 0 (0.0%) | 11 (91.7%) | |
| ER | ||||||
| Positive | 86 | 10 (11.6%) | 1 (1.2%) | 2 (2.3%) | 73 (84.9%) | 0.724 |
| Negative | 48 | 5 (10.4%) | 3 (6.3%) | 1 (2.1%) | 39 (81.3%) | |
| PR | ||||||
| Positive | 82 | 9 (11.0%) | 2 (2.4%) | 1 (1.2%) | 70 (85.4%) | 0.685 |
| Negative | 52 | 6 (11.5%) | 2 (3.8%) | 2 (3.8%) | 42 (80.8%) | |
| HER2 | ||||||
| Positive | 21 | 0 (0.0%) | 1 (4.8%) | 0 (0.0%) | 20 (95.2%) | 0.104 |
| Negative | 113 | 15 (13.3%) | 3 (2.7%) | 3 (2.7%) | 92 (81.4%) | |
| Age | ||||||
| <49 y | 61 | 6 (9.8%) | 2 (3.3%) | 1 (1.6%) | 52 (85.2%) | 0.663 |
| ≥49 y | 73 | 9 (12.3%) | 2 (2.7%) | 2 (2.7%) | 60 (82.2%) | |
| Tumor size | ||||||
| <3cm | 74 | 10 (13.5%) | 4 (5.4%) | 1 (1.4%) | 59 (79.7%) | 0.157 |
| ≥3cm | 60 | 5 (8.3%) | 0 (0.0%) | 2 (3.3%) | 53 (88.3%) | |
The percentage of positive IL-10 staining was lower in tumor grade G1 and G2 (40.4% and 42.3%, respectively), significantly increased in grade G3 IBCa (66.7%) (P =0.048, Table 5). In addition, the expression level of IL-10 was higher in ER, PR negative and HER2 positive case (Table 5).
Table 5.
Relationship between IL-10 expression in IBCa tissue with histopathological features of IBCa.
| IL-10 expression |
||||
|---|---|---|---|---|
| Variables | All cases | – | + | p |
| IBCa | 134 | 71 (53.0%) | 63 (47.0%) | |
| Grade | ||||
| G1 | 52 | 31 (59.6%) | 21(40.4%) | 0.048 |
| G2 | 52 | 30(57.7%) | 22 (42.3%) | z=−2.114 |
| G3 | 30 | 10 (33.3%) | 20 (66.7%) | |
| LN metastasis | ||||
| No | 81 | 40 (49.4%) | 41(50.6%) | 0.302 |
| Yes | 53 | 31 (58.5%) | 22 (41.5%) | |
| TNM | ||||
| T1 | 40 | 23 (57.5%) | 17(42.5%) | 0.346 |
| T2 | 51 | 22 (43.1%) | 29 (56.9%) | z=0.380 |
| T3 | 31 | 19 (61.3%) | 12 (38.7%) | |
| T4 | 12 | 7 (58.3%) | 5 (41.7%) | |
| ER | ||||
| Positive | 86 | 52 (60.5%) | 34 (39.5%) | 0.020 |
| Negative | 48 | 19 (39.6%) | 29 (60.4%) | |
| PR | ||||
| Positive | 82 | 49 (59.8%) | 33 (40.2%) | 0.049 |
| Negative | 52 | 22 (42.3%) | 30 (57.7%) | |
| HER2 | ||||
| Positive | 21 | 7 (33.3%) | 14 (66.7%) | 0.049 |
| Negative | 113 | 64 (56.6%) | 49 (43.4%) | |
| Age | ||||
| <49 y | 61 | 34 (55.7%) | 27 (44.3%) | 0.559 |
| ≥49 y | 73 | 37 (50.7%) | 36 (49.3%) | |
| Tumor size | ||||
| <3 cm | 74 | 39 (52.7%) | 35 (47.3%) | 0.942 |
| ≥3 cm | 60 | 32 (53.3%) | 28 (46.7%) | |
Relationship between CD19+ B lymphocytes and PD-L1 expression, CD19+ B lymphocytes and IL-10 expression in IBCa
The McNemar's test was applied to examine the relationship between CD19+ B cells and PD-L1 expression in IBCa. A high coincidence of tumor-infiltrating CD19+ B lymphocytes and PD-L1 expression in IBCa was observed (p = 0.001, κ = 0.056, Table 6). Seventy-eight out of one hundred twenty-seven (61.4%) IBCa cases with positive CD19+ B lymphocytes also displayed positive staining for PD-L1 in cancer cells, while only 9/127 (7.1%) CD19+ B lymphocytes positive cases showed negative staining for PD-L1 (Table 6).
Table 6.
Correlation of tumor-infiltrating CD19+ B cells with PD-L1 expression in cell membrane and cytoplasm of IBCa tissue.
| PD-L1 expression |
|||
|---|---|---|---|
| + | – | p and κ | |
| CD19+ n | 78 | 9 | p = 0.001 |
| CD19– n | 34 | 6 | κ=0.056 |
As shown in Table 7, a high coincidence of tumor-infiltrating CD19+ B lymphocytes and IL-10 expression was observed (p = 0.001, κ = 0.227). Fifty-two out of sixty-three (82.5%) IBCa cases with positive staining for IL-10 in TILs also displayed positive CD19+ B lymphocytes, while only 11/63 (17.5%) IL-10 positive cases showed negative staining for CD19+ B lymphocytes (Table 7), together suggesting that majority of the CD19+ B lymphocytes in IBCa tissue are Bregs as IL-10 is considered a surrogate marker for Bregs.22 Additionally, multivariate logistic regression analyses revealed that CD19+ B lymphocytes were higher in poor differentiation of IBCa (P = 0.002, Table S2), and significantly associated with IL-10 expression level (P = 0.011, Table S2). The results of immunohistochemistry staining of serial IBCa tissue sections showed that the locations of CD19 and IL-10 expression were similar (Fig. 2A) which was further confirmed by immunofluorescence (IF) staining showing that IL-10 was expressed in the cytoplasm of CD19+ B cells (Fig. 2B).
Table 7.
Correlation of tumor-infiltrating CD19+ B cells with IL-10 expression in IBCa tissue.
| IL-10 expression |
|||
|---|---|---|---|
| + | – | p and κ | |
| CD19+ n | 52 | 41 | p = 0.001 |
| CD19– n | 11 | 28 | κ = 0.227 |
PD-L1 stimulated the differentiation of CD19+ B cells into CD19+CD24+CD38+ B cell subtype
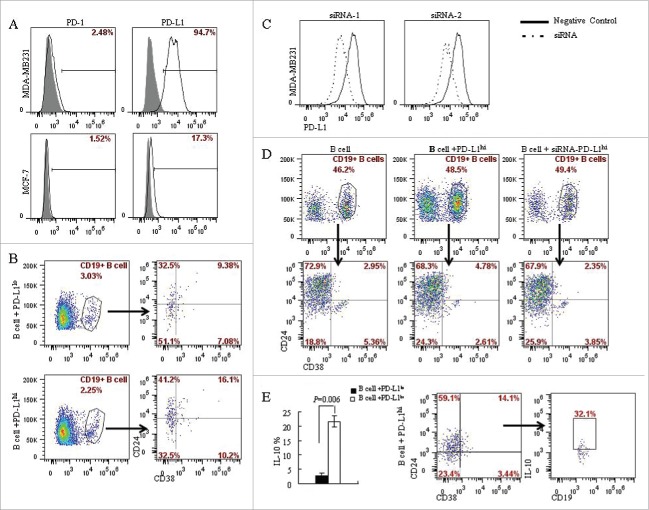
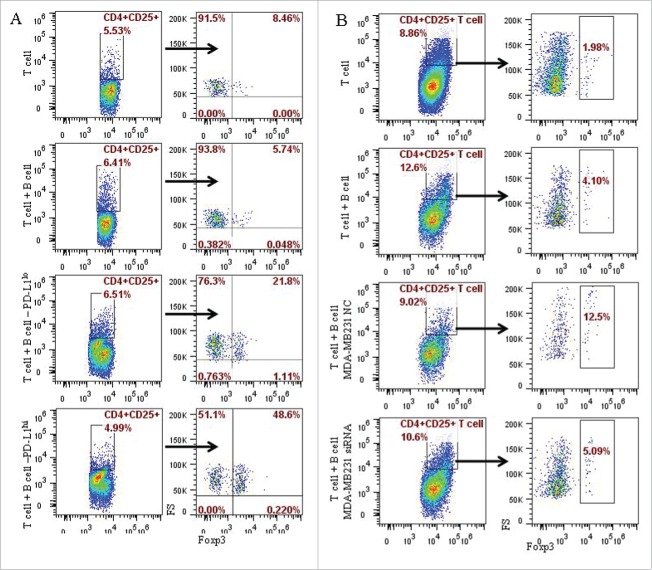
Lymphocyte/tumor co-culture experiments were used to investigate the interaction between PD-L1 expressed in the tumor cells and CD19+ B cells in the tumor microenvironment. Activated CD19+ B cells via anti-CD40 antibody were co-cultured with PD-L1 expressing and deficient breast cancer cells, MDA-MB231 and MCF-7 respectively (Fig. 3A). CD19+ B cells were isolated by CD19+ positive selection kit from peripheral blood of healthy individuals, and activated by anti-CD40 antibody (5C11). The percentage of CD19+CD24+CD38+ B cells was found to be higher following co-culture with PD-L1hi MDA-MB231 cells than that co-cultured with PD-L1lo MCF-7 cells (16.1% vs. 9.38%; P = 0.030) (Fig. 3B). To confirm whether the induction of CD19+CD24+CD38+ B cells was due to PD-L1, we knocked down PD-L1 in MDA-MB231 cells by using PD-L1 specific siRNA (Fig. 3C). The MDA-MB231 cells with PD-L1 knocked down were then co-cultured with activated CD19+ B cells. The percentages of CD19+CD24+CD38+ B cells reduced by half (4.78% vs. 2.35%; P = 0.0478) when following co-culture of activated CD19+ B cells and MDA-MB231 with PD-L1 knocked down when compared with that without PD-L1 knockdown (Fig. 3C and 3D). These results demonstrated that the differentiation of CD19+ B cells into CD19+CD24+CD38+ phenotype was mediated by PD-L1. To identify the phenotype of CD19+CD24+CD38+ B cells, CD19+ B cells in the co-culture system were stimulated with PMA plus ionomycin, stained with CD24, CD38 and IL-10 and analyzed by flow cytometry. A significantly higher level of IL-10 (21.65±1.91%) in the PD-L1hi groups compared to that in the PD-L1lo cells (2.83 ± 0.81%; P = 0.006) (Fig. 3E). In addition, 32.1% of CD19+CD24+CD38+ B lymphocytes were found to be IL-10 secreting B cells (Fig. 3E). This result indicated that like B10 cells, the CD19+CD24+CD38+ B cells derived from CD19+ B cells upon stimulation with PD-L1 also carry immunosuppressive function.
Figure 3.
PD-L1 mediates the differentiation of CD19+ B cells into CD19+CD24+CD38+ B cell subtype. (A) The expression of PD-L1 and PD-1 on breast cancer cell lines was analyzed by flow cytometry. MDA-MB231 expresses high level of PD-L1 (94.7%) compared with MCF-7 (17.3%); Both MCF-7 and MDA-MB231 lacks PD-1 expression. (B) CD19+ B lymphocytes/ breast cancer cells co-culture systems were established to investigate the interaction between PD-L1 expression in breast cancer cells and CD19+ B cells. 16.1% of CD19+CD38+CD24+ B cells were detected in PD-L1hi MDA-MB231, while only 9.38% of CD19+CD38+CD24+ B cells were detected in PD-L1lo MCF-7. (C) To confirmed the relationship between PD-L1 and CD19+ B cells, PD-L1siRNA were used to knockdown the expression of PD-L1. (D) Co-culture of CD19+ B cells and PD-L1hi MDA-MB231 with or without siRNA. The percentage of CD19+CD38+CD24+ B cells were reduced when CD19+ B cells were co-cultured with MDA-MB231 cells with PD-L1 knocked down. (E) A high level of IL10 was detected in PD-L1hi co-culture compared to that in PD-L1lo co-culture system (P < 0.05). And CD19+CD24+CD38+ B cells produced IL-10 following activation by PMA and ionomycin.
Phenotype and function of CD19+ B cells from IBCa patients
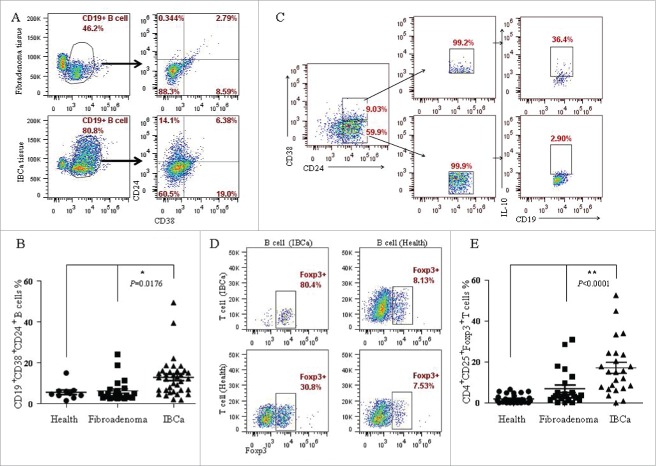
CD19+CD24+CD38+ B cells are regarded as Bregs which have been shown to have immunosuppressive function.10 To identify the phenotypic changes of B cells in patients, mononuclear cells isolated from resected specimens of breast tumor patients were stained with multicolor-labeled antibodies (CD19, CD24 and CD38) and analyzed by flow cytometry. The peripheral blood mononuclear cells (PBMC) were also analyzed in the same cell population. Among the CD19+ B cells, a population with clear CD24+CD38+ staining was identified, and a variable amount of CD19+CD24+CD38+ B cells were also detected in IBCa tissues and patients' peripheral blood. The expression of the B cell markers in PBMC from IBCa was indistinguishable from that of fibroadenoma and health individuals (p = 0.0176) (Fig. 4B). Whereas the percentage of CD19+CD24+CD38+ B cells in IBCa tissues was 6.38%, higher than that in fibroadenoma tissues (2.79%) (P = 0.0281) (Fig. 4A). In order to define the phenotypic characteristics of CD19+CD24+CD38+ B cells, freshly purified B cells from peripheral blood samples of IBCa patients were assessed by gating them on IL-10 high-expressing B cells. Phenotypic analysis revealed that the level of IL-10 was significantly higher in CD19+CD24+CD38+ B cells (36.4%) compared to that in CD19+CD24+CD38− B cells (2.90%) (p = 0.0001, Fig. 4C). Thus, IL-10high B cells were also CD24+CD38+, whereas IL-10low/negative B cells were CD38−. These observations indicated that CD19+CD24+CD38+ B cells exhibit regulatory capacity in the patients with breast cancer.
Figure 4.
Phenotype and function of CD19+ B cells from IBCa patients. To investigate the phenotype and function of CD19+ B cells in patients and healthy individuals, CD19+ B cells were isolated from peripheral blood or breast tumor tissues, incubated with CD19-ECD, CD38-APC and CD24-PE-cy7, and analyzed by flow cytometry. CD4+ T cells in patients and healthy individuals were purified and incubated with CD4-ECD, CD25-PE-cy7, fixed and stained by Foxp3-APC. B/T lymphocytes co-culture systems were set up to examine the interaction between CD19+ B cells and CD4+ T cells. (A) Higher percentage of CD19+CD38+CD24+ B cells were detected in fresh IBCa tissues that in breast fibroadenoma tissue (6.38% vs. 2.79%). (B) Significantly higher level of CD19+CD38+CD24+ B cells was detected in the PBMC of IBCa patients compared with that in fibroadenoma or health individuals (p = 0.0176). (C) Elevated expression of IL-10 (36.4%) was detected in CD19+CD24+CD38+ B cells activated by LPS, PMA and ionomycin compared to that in CD19+CD24+CD38− B cells (2.90%, P < 0.05). (D) A total of 80.4% of CD4+CD25+Foxp3+ T cells were found in the co-culture system, in which both CD19+ B cells and CD4+ T cells were originated from IBCa patients, versus 30.8% of CD4+CD25+Foxp3+ T cells in co-culture system of CD19+ B cells from patients and CD4+ T cells from health individuals. However, only 7.53% and 8.13% of CD4+CD25+Foxp3+ T cells were detected in the co-culture system of CD19+ B cells from health individuals with CD4+ T cells originated from health individual or IBCa patients, respectively. (E) Significantly higher level of CD4+CD25+Foxp3+ Treg cells were detected in the PBMC of IBCa patients compared with that in fibroadenoma patients or health individuals (P < 0.0001).
Meanwhile, we investigated the relationship between CD19+ B cells and CD4+CD25+Foxp3+ Tregs using B/T lymphocytes co-culture systems. High level of CD4+CD25+Foxp3+ T cells (80.4%) was found in the co-culture system, in which both CD19+ B cells and CD4+ T cells were originated from IBCa patients (Fig. 4D). A total of 30.8% CD4+CD25+Foxp3+ T cells was detected in the system of CD19+ B cells from IBCa patient co-cultured with CD4+ T cells from healthy individuals (Fig. 4D). In contrast, only 7.53% and 8.13% of the CD4+CD25+Foxp3+ T cells were detected in the co-culture systems of CD19+ B cells from health individuals and CD4+ T cells originated from health individual or IBCa patients, respectively (P = 0.0108 and P = 0.0277 respectively, Fig. 4D). The results indicated that CD19+ B cells from IBCa were different from that originated from healthy subject, the former induced high levels of CD4+CD25+Foxp3+ Treg in in vitro co-culture system.
CD4+CD25+Foxp3+ T cells, regarded as Tregs, play an important role in tumor immune suppression. In agreement with previous study,23 the present study also revealed a high level of CD4+CD25+Foxp3+ Treg cells in IBCa patients compared with that in fibroadenoma or health individuals (P <0.0001) (Fig. 4E).
PD-L1 contributed to the induction of the CD4+CD25+Foxp3+ Tregs by CD19+ B cells in in vitro system
As has been discussed above, CD19+CD24+CD38+ B cells can be induced by PD-L1 in breast cancer cells, and CD4+CD25+Foxp3+ Tregs can be promoted by CD19+ B cells originated from IBCa. We further investigated the interaction between CD19+ B cell and CD4+ T cell using co-culture experiments. CD19+ B cells were collected after co-culturing with PD-L1hi MD-MB231 or PD-L1lo MCF-7. CD4+ T cells were isolated using CD4+ positive selection kit from peripheral blood of healthy individuals, and activated as described in previous section. The activated CD4+ T cells and stimulated B cells were then co-cultured at a ratio of 1:1. As illustrated in Fig. 5A, 48.6% CD4+CD25+Foxp3+ T cells were produced in co-culture with B cells pre-cultured with PD-L1hi MD-MB231, higher than that pre-cultured with PD-L1lo MCF-7 (21.8%) and without pre-culture with breast cancer cells (5.74%) (P = 0.0021) (Fig. 5A). When PD-L1 expression in MDA-MB231 cells was knocked down by siRNA, the percentage of CD4+CD25+Foxp3+ T cells was reduced to 5.09% following B/T lymphocytes co-culture (P = 0.0459) (Fig. 5B). Together, these results are suggestive of a role for CD19+CD24+CD38+ in stimulating the formation of CD4+CD25+Foxp3+ Tregs.
Figure 5.
PD-L1 contributed to the induction of the CD4+CD25+Foxp3+ Tregs by CD19+ B cells in in vitro system. The potential role of PD-L1 in the B cells and T cells interaction were examined in co-culture experiments of CD4+ T cell with CD19+ B cells, or CD19+ B cells pre-cultured with MCF-7 or MD-MB231 cells, or MD-MB231 cells with or without PD-L1 knocked down. CD4+ T cells were isolated by CD4+ positive selection kit from peripheral blood of healthy individuals. Following activation with anti-human CD3 and anti-human CD28 antibodies, CD4+ T cells and B cells as described above were mixed together at a ratio of 1:1, and analyzed by flow cytometry following culture for the specified time as described in the Materials and Methods section. (A) A total of 48.6% CD4+CD25+ Foxp3+ T cells were produced in co-culture system in which B cells were pre-cultured (or stimulated) by PD-L1hi MD-MB231, 21.8% CD4+CD25+ Foxp3+ T cells in co-culture system of T cells with B cells stimulated by PD-L1lo MCF-7. (B) A total of 5.09% and 12.5% of CD4+CD25+ Foxp3+ T cells were detected in co-culture with B cells stimulated by MD-MB231 with or without PD-L1 depletion by PD-L1-siRNA or PD-L1-NC (negative control), respectively.
Discussion
B lymphocytes are a type of lymphocytes in the humoral immunity of adaptive immune system. Once a B lymphocyte encounters its cognate antigen and receives an additional signal from a T helper cell, it further differentiates into plasma B cells or memory B cells that secret antibodies and cytokines against antigens.1,2 On the other hand, as antigen-presenting cells (APCs), B lymphocytes can transfer activation signal to T cells.1,2 Therefore, B lymphocytes are recognized as a positive regulator of immune response.3,4 Recently, a contradictory role for B lymphocytes in autoimmune disorders has been reported showing that B cells can suppress the allergic and autoimmune response in EAE, SLE and ultraviolet irradiation animal models.5,6 Several studies reported that CD19+ B cells involve in the regulation of several immune-mediated pathologic processes in both mice and humans, including autoimmune diseases and infections.10 Further investigations emphasized a potential role for B cell in treating patients with various autoimmune disorders such as rheumatoid arthritis and systemic lupus erythematosus.10,24 Blair et al. reported that the CD19+CD24hiCD38hi B cells carry immunosuppressive capacity in healthy individuals but loss this function in SLE patients.10 The subset of B cells was further identified to be IL-10 producing cells (or B10 cells) upon stimulation by PMA and ionomycin.9-11,25 Olkhanud et al. confirmed that the special B cell subset promoted breast cancer metastasis by converting resting CD4+ T cells to T-regulatory cells in murine tumor model.26
In this study, we examined the density of CD19+ B lymphocytes in fibroadenomas and IBCa. CD19+ tumor-infiltrating B cells were detected predominantly in IBCa compared with that in fibroadenomas, indicating that CD19+ B cells might play an important role in breast tumor immune response. More importantly, the density and frequency of CD19+ B cells was higher in advanced histological grade (grade 3), lymph node metastasis, ER negative status, PR negative status and TNM stage T4 than that in other groups. In particular, the density of CD19+ B cells was significantly associated with histopathological parameters, including ER-negative status, poor differentiation. An increasing trend toward the density of CD19+ B cells was also observed with increasing tumor grade. The results suggested the density of CD19+ B lymphocytes in IBCa tissue might be indicative of poor prognosis in patients with this malignancy.
PD-L1, a ligand for PD-1, also known as negative regulatory molecule in immune responses, is broadly expressed on non-immune cells as well as T cells, B cells, and dendritic cells and upregulated following their activation.14-16 The association between PD-L1 expression and tumor aggressiveness, poor clinicopathologic features, as well as reduced survival has been recently reported in a group of human malignancies.17,18 Another study using murine tumor models reported that PD-L1 blockade using anti-PD-L1 monoclonal antibody enhanced antitumor immunity and inhibited tumor growth.19 In the present study, the expression of PD-L1 was higher in IBCa compared with fibroadenoma (P = 0.001), and associated with increasing tumor grade with marginal significance (P = 0.063). Similar result has been previously reported.17 In addition, a significant coincidental presence of CD19+ B lymphocytes and PD-L1 was observed on carcinoma cells. These results suggested that CD19+ B lymphocytes, which maintained a significant correlation with PD-L1 expression in poorly differentiated of IBCa, may play an important role in tumor immune response in IBCa.
Previous study has demonstrated that interactions of PD-L1 and PD-1 inhibit T-cell activation, maintain immune tolerance, and induce tumor immune evasion via a significant positive correlation between PD-L1 expression and Tregs infiltration.16,18 The relationship between PD-L1 expression in IBCa cells and CD19+ B cells in tumor microenvironment requires further investigation.
We utilized a CD19+ B cells /breast cancer cells interaction experiment to investigate a potential association between tumor cell PD-L1 expression and CD19+ B cells. The CD19+CD24+CD38+ B cells was found to be induced in the PD-L1hi MDA-MB-231 cells co-cultured with CD19+ B cells compared with that co-cultured with PD-L1lo MCF-7 cells. This was further confirmed by using siRNA technique to knock down PD-L1 in MDA-MB231 cells. The percentage of CD19+CD24+CD38+ B cells was found significantly reduced when CD19+ B cells was co-cultured with MDA-MB231 cells with PD-L1 knocked down. These observations suggested that PD-L1 in breast cancer cells promoted the differentiation of CD19+ B cells into CD19+CD24+CD38+ B subset in in vitro. Additionally, a variable amount of CD19+CD24+CD38+ B cells were detected in IBCa tissues and patients' peripheral blood, higher than that of fibroadenoma and health individuals. IL-10 is regarded as a surrogate marker for Bregs.9-11 Results from the present study showed that IL-10 expression was associated with poor differentiation, ER negative, PR negative and HER2 positive case. Importantly, co-location of IL-10 and CD19 was observed in IBCa by IHC and IF staining. In agreement with previous report,10 we demonstrated that high level of IL-10 could be detected in CD19+CD24hiCD38hi B cells which were derived from IBCa patients or CD19+ B cell /breast cancer cells co-culture system compared to that CD19+CD24hiCD38− B cells. The IL-10 producing CD19+CD24+CD38+ B cells, regarded as Bregs, have been reported to be linked with protection from autoimmune diseases in both humans and mice.10 The origin and role of CD19+CD24+CD38+ B cells in breast cancer is yet to be elucidated.
Tadmor et al. indicated that absence of B lymphocytes could reduce the number and function of T-regulatory cells.8 Olkhanud et al. confirmed that the special B cell subset promoted breast cancer metastasis by converting resting CD4+ T cells to T-regulatory cells in murine tumor model.26 To further define the characteristics and functions of CD19+ B cells in IBCa, we employed co-culture experiments of CD19+ B cells and CD4+ T cells to investigate the role of CD19+ B cells in immune response. We showed that CD19+ B cells from IBCa patients induced 80.4% CD4+CD25+Foxp3+ T cells in CD4+ T cells from IBCa patients compared to 30.8% from healthy individuals. However, CD19+ B cells from health individuals induced only 8.13% and 7.53% Tregs when co-cultured with CD4+ T cells from patients or health individuals, respectively. The results demonstrated that CD19+ B cells from IBCa patients were different from those from healthy individuals, and they had the potential to promote CD4+CD25+Foxp3+ T cells. We further showed that the CD4+CD25+Foxp3+ Tregs are among the highest in IBCa compared with fibroadenoma and healthy individuals. In addition, higher level of CD4+CD25+Foxp3+ Tregs could be promoted by CD19+ B cells pre-cultured with PD-L1hi breast cancer cells in vitro. Several studies reported that B regulatory cells suppressed immune response via promoting Tregs.8,26 However, the origin of the subset B cells remains unclear. Our results suggested that the CD19+CD24+CD38+ B cells were promoted in tumor microenvironment via PD-L1 in breast cancer cells. We also confirmed that CD4+CD25+Foxp3+ Tregs could be promoted by this special CD19+ B cells subset stimulated by PD-L1hi breast cancer cells.
In summary, we have shown that elevated CD19+ B cells in IBCa are significantly associated with poor prognostic factors including high tumor grade, ER-negative status, IL-10 and PD-L1 expression in IBCa. We also have demonstrated that the formation of the B cell subset CD19+CD24+CD38+ B cells from CD19+ B cells, as well as the induction of CD4+CD25+Foxp3+ Tregs are both mediated by PD-L1. Hence, it is logical to speculate that CD19+ B cells may have a crucial role in breast cancer immune escape via PD-L1 and are valuable in precisely formulating a prognosis. The underlying mechanisms warrant further investigation.
Materials and methods
Reagents
The following monoclonal antibodies and reagents were used in these studies: Mouse anti-human CD19 antibody (clone: DM1B, Gene Tex, Inc.); Rabbit anti-human IL-10 antibody (clone: ab34843, Abcam, USA); rabbit anti-human PD-L1 (NBP1-03220, Novus Biologicals, USA); CD4-ECD (Beckman Coulter. Inc. USA); CD19-ECD (Beckman Coulter Company, Marseille, France); PD-1-FITC (clone: MIH4) and PD-L1-PE (clone: 29E.2A3) were from Biolegend; CD38-APC (clone: HIT2), CD24-PE-cyanire7 (clone: eBioSN3), CD25-PE-cy7 (clone: BC96), IL-10-FITC (clone: BT-10) and anti-Foxp3-APC (clone: PCH101) were purchased from eBiosciences (San Jose, CA, USA); Anti-CD3e monoclonal antibody (clone: OKT3, Glucan Bio-tech., Changzhou, China); Lipopolysaccharide (Sigma, USA); PMA(Sigma, USA); calcium-ionomycin (Sigma, USA); Foxp3 Fixation /Permeabilization Solution (Biolegend, USA); Cell Fixation/Permeabilization Kits for Intracellular Cytokine Analysis (BD Biosciences, USA); CD4+ T cell and CD19+ B cell positive isolation kit (Miltenyi Biotec, Germany); ChemMate™ Envision/HRP technique (Gene Tech, Shanghai, China). Alexa Fluor 488 donkey anti-Mouse IgG (H+L) antibody (Invitrogen, USA); Cy™3-conjugated donkey anti-Rabbit IgG (H+L) antibody (Jackson ImmunoResearch Laboratories, Inc.).
The following monoclonal antibodies were prepared in our laboratory: anti-human CD28 antibodies (Clone: 18G8),27 agonist anti-human CD40 monoclonal antibody (Clone: 5C11).28
Patients
Tumor tissues
Tissue samples from 134 cases of IBCa and 31 cases of fibroadenoma from breast were analyzed in the present study. All cases were randomly selected from the archive of the Department of Pathology of the First and Second Affiliated Hospital of Soochow University, including resected biopsies or mastectomies. None of the patients with IBCa had received any kind of chemical or radiation therapy before surgery. The age of the IBCa patients ranged from 22 to 88 y, with a mean of 52.4 y. Fibroadenoma biopsies were taken from 31 symptom-free female subjects for which the median age was 28.4 (17–45) y old. All hematoxylin/eosin (HE)-stained sections were re-evaluated. The histopathological diagnosis for breast tumor was made according to cellular morphological changes and tissue architecture by using previously established criteria.29-31 Fifty-two out of one hundred thirty-four IBCa were graded as well-differentiated (38.8%), 52/134 were moderately-differentiated (38.8%) and the rest (30/134) were poorly-differentiated (22.3%). The TNM stage of invasive carcinomas was also assessed following the 2012 WHO classification of breast tumors. 29,30 Carcinoma specimens were classified as stage T1 (40/134, 29.9%), T2 (51/134, 38.1%), T3 (31/134, 23.1%) and T4 (12/134, 9.0%). In addition, local lymph node metastasis occurred in 53 cases of 134 cases (39.6%) (Table S1). A case was considered estrogen-receptor-positive (ER+) and progesterone-receptor-positive (PR+) if more than 15% of the tumor cells showed nuclear staining of ER or PR. HER2 expression was scored according to the guidelines of the National Comprehensive Cancer Network.30,32 Positivity of HER-2 was scored as “++” when strong and complete membrane staining was present in more than 30% of invasive tumor cells.30,32
Peripheral blood cells and fresh tissues samples
Peripheral blood cells were collected from IBCa and fibroadenoma patients as well as healthy individuals for flow cytometry analysis of cell surface markers. Age-matched patients with IBCa, fibroadenoma patients and female health individuals were included in this study as disease control. Fresh tissues were obtained from eight cases of IBCa and four cases of fibroadenoma during surgery. This study was approved by the ethics committee of the University of Soochow Hospitals National Health Service Trust. Patients and healthy volunteers were recruited after informing consent was obtained.
Breast cancer cell lines and culture
The human breast cancer lines, MB-M231 and MCF-7 were purchased from ATCC, and were cultured in RPMI 1,640 medium (Gibco BRL, Grand Island, NY, USA) containing 10% heat-inactivated fetal bovine serum (FBS) (Biological Industries, Israel), 1% penicillin/streptomycin (Biological Industries, Israel). All breast cancer cell lines used in these studies were assessed by Flow Cytometry analysis for constitutive cell surface PD-L1 or PD-1 expression.
Cell isolation from tumor tissues and peripheral blood
For isolating cells from tumor tissue, fresh tumor specimens were gently minced on a wire mesh screen to obtain a cell suspension. The cell suspension was centrifuged over Ficoll-Hypaque (Amersham Biosciences, Sweden) at 1,800 r/min for 25 min. After density gradient centrifugation, the mononuclear cells were collected and washed with PRMI 1,640 media containing 10% FBS and 1% penicillin /streptomycin. PBMC were also isolated with Ficoll–Hypaque density gradient centrifugation. The mononuclear cells were used for flow cytometry analysis immediately or CD4+ T cells and CD19+ B cells isolation.
CD4+ T cells were purified from the peripheral blood of healthy individuals or IBCa patients using a CD4+ T cell positive isolation kit, according to the manufacturer's instructions (Miltenyi Biotec, Germany). To establish Th0 cell conditions, 2×105 CD4+ T cells/well in 96-well plates were stimulated with anti-CD3e monoclonal antibody (1 μg/mL), and anti-CD28 monoclonal antibody (1 μg/mL) for 72 h. On day 3, activated CD4+ T cells were collected for co-culture experiments.
A CD19+ B cell isolation kit was used to purify CD19 positive B cells from the peripheral blood of healthy individuals or IBCa patients according to the manufacturer's instructions (Miltenyi Biotec, Germany). 2×105 purified CD19+ B cells were stimulated with lipopolysaccharide (1 μg/mL) or anti-CD40 monoclonal antibody (1 μg/mL, clone: 5C11) for 48h, and simulated with or without breast cancer cell for an additional 24 h. On day 3, B cells were collected and used for subsequent co-culture experiments with or without activated CD4+ T cells.
Immunohistochemical staining (IHC)
Tissue processing and IHC procedure
All the surgically resected specimens and biopsy samples were fixed with 10% neutral buffered formalin, embedded in paraffin, and serially sectioned at 4 μm. IHC was performed on selected slides using the ChemMateTM Envision/HRP technique.33 Briefly, the sections were deparaffinized and dehydrated; following block of endogenous peroxidase activity using H2O2, the sections were incubated with primary antibodies for CD19, IL-10 or PD-L1,19,34 and secondary antibody, and visualized with diaminobenzydine (DAB). Finally, slides were counterstained with hematoxylin. Negative controls were established by replacing the primary antibody with PBS or normal mouse IgG1 (BD PharMingen). Tonsil tissue and colorectal cancer tissue were used as positive controls for CD19, IL-10 and PD-L1, respectively.19
Evaluation of IHC slides
CD19 expression was seen in the cell membrane of TILs in breast tumor tissues. 13 IHC staining of PD-L1 appeared as fine granular in cell membrane or cytoplasm of tumor cells and non-tumorous breast tissue.17
The immunopositivity for CD19 were defined semiquantitatively according to the following criteria: 1+, minimal (<10% of cells); 2+, moderate (10–50%); and 3+, diffuse/marked/brisk (>50%).13 IHC for IL-10 or PD-L1 expression was evaluated according to the overall staining intensity and proportion (percentage of positively stained tissue).17 The intensity score was determined as 0 (no staining), 1 (weak staining), 2 (moderate staining) and 3 (strong staining). The proportion score was defined as 1 (<30% of cells) and 2 (>30% of cells). The intensity score and the proportion score were multiplied together for a total score with 0–1 as negative and 2–6 as positive. All slides were evaluated independently by two investigators without knowledge of the identity of the patient and clinical outcome.
Immunofluorescence staining (IF)
The CD19 and IL-10 colocalization studies were performed on human IBCa tissue samples. After deparaffinization, dehydration, blocking of endogenous peroxidase activity as described above, sections were incubated with a mouse anti-human CD19 antibody (Dako) and a rabbit anti-human IL-10 antibody (Abcam). A donkey anti-rabbit Cy3 antibody and an Alexa 488 goat anti-mouse antibody were used as secondary antibodies. Finally, sections were counterstained with 4′,6-diamidino-2-phenylindol (DAPI) and analyzed with Confocal Laser-Scanning Microscopy (LSM 710, Zeiss, Germany).
Flow cytometry analysis
Surface staining and flow cytometry
The isolated mononuclear cells were washed in PBS containing 2.5% FBS and incubated with the specific fluorochrome-conjugated antibodies including CD19-ECD, CD38-APC and CD24-PE-cy7 to identify B cells surface molecules for 30 min at 4°C. Labeled cells were re-suspended in 0.5 mL cell staining buffer, and analyzed with flow cytometry software (FlowJo version 7.6.2, USA). Isotype controls were done for each staining.
Intracellular staining and flow cytometry
The surface molecules of Tregs were also staining as described above using fluorochrome-conjugated antibodies CD4-ECD and CD25-PE-cy7. For intracellular staining, washed cells were fixed with Foxp3 Fixation /Permeabilization Solution and incubated with APC conjugated anti-Foxp3 anti-body in the dark for 30 min. Labeled cells were re-suspended in 0.5 mL cell staining buffer, and analyzed with flow cytometry.
Activated CD19+ B cells were stimulated with PMA and ionomycin for 2 h, and incubated with GolgiStop for additional 4 h. Then the CD19+ B cells were collected, washed, stained with surface mark CD19, CD24 and CD38 as described above. For intracellular staining, washed cells were fixed with Fixation /Permeabilization Solution and incubated with FITC conjugated anti-IL-10 anti-body in the dark for 30 min. Labeled cells were re-suspended in 0.5 mL cell staining buffer, and analyzed with flow cytometry.
Depletion of PD-L1 by synthetic small interfering RNAs
We synthesized small interfering RNAs (siRNA) specific to PD-L1 with the sequence of siRNA1 and siRNA2 to knockdown endogenous PD-L1 expression in MDA-MB231 (Table S3). Twenty-four hours after plating, cells were transfected with PD-L1 siRNA or control siRNA using Oligofectamine transfection Reagent (Dharmacon, THERMO) in accordance with the manufacturer's instructions. At 72 h after transfection, cells were harvested and subjected to flow cytometry analysis. Each transfection was done in triplicate and repeated three times.
Co-culture system of CD19+ B cells and CD4+ T cells, or CD19+ B cells and breast cancer cells
Two co-culture systems were established to study the interactions between subtype immune cells or immune cells and breast cancers cells. 2×105 CD19+ B cells stimulated with 5C11 from IBCa patients were collected and cultured with activated CD4+ T cells from IBCa patients or health individuals after at a ratio of 1:1, respectively. Similarly, activated CD19+ B cell from health individuals were cultured with activated CD4+ T cells from IBCa patients or health individuals. On day 3, CD4+ T cells were collected, washed for intracellular staining.
The CD19+ B cells/breast cancer cells co-culture experiments was employed to investigate a potential association between PD-L1 expression and CD19+ B cells. Breast cancer cell lines, either PD-L1hi MDA-MB231 or PD-L1lo MCF-7, were seeded at 2×104 cells per well in 96-well culture plates and co-cultured with activated CD19+ B cells from health individuals at ratio of 1:10. On the day 2, CD19+ B cells were collected for flow cytometry analysis. To investigate the potential role of PD-L1 in B cells differentiation, co-culture experiments of B cells and MDA-MB231 breast cancer cells with or without PD-l depletion were carried out. In addition, CD19+ B cells/CD4+ T cells co-culture was used to investigate the role of CD19+ B cells on differentiation of CD4+ T cells. In brief, B cells, collected following culture with breast cancer cell lines with or without PD-Ll depletion, were co-cultured with activated T cells to examine whether PD-L1 play a role in T cells differentiation in breast cancer via CD19+ B cells.
Statistical analysis
The χ2 test was used for analyzing the percentage of samples with positive staining. Spearman correlation test and trend test were used for the correlation analysis between positive rates and histopathological parameters, ER, PR, or HER2. ANOVA-test was used for analyzing the percentage of Tregs, Bregs in patients and healthy individuals. The McNemar's test was applied to examine the relationship between CD19+ B cells and PD-L1 expression. Multivariate logistic regression was used to assess the influence of clinical parameters, PD-L1 expression and IL-10 expression on CD19+ B lymphocytes. P values<0.05 were considered statistically significant. All analyses were done using SAS 9.2 (SAS Enterprise Guide 3.0, Cary, NC).
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported in part by the National Natural Science Foundation of China grant number 81372343; social development project of Suzhou grant number SS201246 and SYSD2012093; a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Supplemental Material
Supplemental Material may be downloaded here: publisher's website
References
- 1.Cooper MD, Alder MN. The evolution of adaptive immune systems. Cell (2006); 124:815-22; PMID:16497590; http://dx.doi.org/ 10.1016/j.cell.2006.02.001 [DOI] [PubMed] [Google Scholar]
- 2.Jager E, Jager D, Knuth A. Clinical cancer vaccine trials. Curr Opin Immunol (2002); 14:178-82; PMID:11869889; http://dx.doi.org/ 10.1016/S0952-7915(02)00318-7 [DOI] [PubMed] [Google Scholar]
- 3.Yanaba K, Bouaziz JD, Matsushita T, Magro CM, St Clair EW, Tedder TF. B-lymphocyte contributions to human autoimmune disease. Immunol Rev (2008); 223:284-99; PMID:18613843; http://dx.doi.org/ 10.1111/j.1600-065X.2008.00646.x [DOI] [PubMed] [Google Scholar]
- 4.Christensen JP, Kauffmann SO, Thomsen AR. Deficient CD4+ T cell priming and regression of CD8+ T cell functionality in virus-infected mice lacking a normal B cell compartment. J Immunol (2003); 171:4733-41; PMID:14568949; http://dx.doi.org/ 10.4049/jimmunol.171.9.4733 [DOI] [PubMed] [Google Scholar]
- 5.LeBien TW, Tedder TF. B lymphocytes: how they develop and function. Blood (2008); 112:1570-80; PMID:18725575; http://dx.doi.org/ 10.1182/blood-2008-02-078071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hansen MH, Nielsen H, Ditzel HJ. The tumor-infiltrating B cell response in medullary breast cancer is oligoclonal and directed against the autoantigen actin exposed on the surface of apoptotic cancer cells. Proc Natl Acad Sci U S A (2001); 98:12659-64; PMID:11606714; http://dx.doi.org/ 10.1073/pnas.171460798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inoue S, Leitner WW, Golding B, Scott D. Inhibitory effects of B cells on antitumor immunity. Cancer Res (2006); 66:7741-7; PMID:16885377; http://dx.doi.org/ 10.1158/0008-5472.CAN-05-3766 [DOI] [PubMed] [Google Scholar]
- 8.Tadmor T, Zhang Y, Cho HM, Podack ER, Rosenblatt JD. The absence of B lymphocytes reduces the number and function of T-regulatory cells and enhances the anti-tumor response in a murine tumor model. Cancer Immunol Immunother (2011); 60:609-19; PMID:21253724; http://dx.doi.org/ 10.1007/s00262-011-0972-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iwata Y, Matsushita T, Horikawa M, Dilillo DJ, Yanaba K, Venturi GM, Szabolcs PM, Bernstein SH, Magro CM, Williams AD et al.. Characterization of a rare IL-10-competent B-cell subset in humans that parallels mouse regulatory B10 cells. Blood (2011); 117:530-41; PMID:20962324; http://dx.doi.org/ 10.1182/blood-2010-07-294249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blair PA, Noreña LY, Flores-Borja F, Rawlings DJ, Isenberg DA, Ehrenstein MR, Mauri C. CD19(+)CD24(hi)CD38(hi) B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic lupus erythematosus patients. Immunity (2010); 32:129-40; PMID:20079667; http://dx.doi.org/ 10.1016/j.immuni.2009.11.009 [DOI] [PubMed] [Google Scholar]
- 11.Das A, Ellis G, Pallant C, Lopes AR, Khanna P, Peppa D, Chen A, Blair P, Dusheiko G, Gill U et al.. IL-10-producing regulatory B cells in the pathogenesis of chronic hepatitis B virus infection. J Immunol (2012); 189:3925-35; PMID:22972930; http://dx.doi.org/24449569 10.4049/jimmunol.1103139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mamula MJ. Editorial: B cells: not just making immunoglobulin anymore. Arthritis Rheumatol (2014); 66:2-5; PMID:24449569; http://dx.doi.org/ 10.1002/art.38208 [DOI] [PubMed] [Google Scholar]
- 13.Helal TE, Ibrahim EA, Alloub AI. Immunohistochemical analysis of tumor-infiltrating lymphocytes in breast carcinoma: relation to prognostic variables. Indian J Pathol Microbiol (2013); 56:89-93; PMID:24056641; http://dx.doi.org/ 10.4103/0377-4929.118676 [DOI] [PubMed] [Google Scholar]
- 14.Dorfman DM, Brown JA, Shahsafaei A, Freeman GJ. Programmed death-1 (PD-1) is a marker of germinal center-associated T cells and angioimmunoblastic T-cell lymphoma. Am J Surg Pathol (2006); 30:802-10; PMID:16819321; http://dx.doi.org/ 10.1097/01.pas.0000209855.28282.ce [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ren X, Akiyoshi K, Vandenbark AA, Hurn PD, Offner H. Programmed death-1 pathway limits central nervous system inflammation and neurologic deficits in murine experimental stroke. Stroke (2011); 42:2578-83; PMID:21737801; http://dx.doi.org/ 10.1161/STROKEAHA.111.613182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park JJ, Omiya R, Matsumura Y, Sakoda Y, Kuramasu A, Augustine MM, Yao S, Tsushima F, Narazaki H, Anand S et al.. B7-H1/CD80 interaction is required for the induction and maintenance of peripheral T-cell tolerance. Blood (2010); 116:1291-8; PMID:20472828; http://dx.doi.org/ 10.1182/blood-2010-01-265975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghebeh H, Mohammed S, Al-Omair A, Qattan A, Lehe C, Al-Qudaihi G, Elkum N, Alshabanah M, Bin Amer S, Tulbah A et al.. The B7-H1 (PD-L1) T lymphocyte-inhibitory molecule is expressed in breast cancer patients with infiltrating ductal carcinoma: correlation with important high-risk prognostic factors. Neoplasia (2006); 8:190-8; PMID:16611412; http://dx.doi.org/ 10.1593/neo.05733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hua D, Sun J, Mao Y, Chen LJ, Wu YY, Zhang XG. B7-H1 expression is associated with expansion of regulatory T cells in colorectal carcinoma. World J Gastroenterol (2012); 18:971-8; PMID:22408358; http://dx.doi.org/ 10.3748/wjg.v18.i9.971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen Y, Wang Q, Shi B, Xu P, Hu Z, Bai L, Zhang X. Development of a sandwich ELISA for evaluating soluble PD-L1 (CD274) in human sera of different ages as well as supernatants of PD-L1+ cell lines. Cytokine (2011); 56:231-8; PMID:21733718; http://dx.doi.org/ 10.1016/j.cyto.2011.06.004 [DOI] [PubMed] [Google Scholar]
- 20.Flies DB, Sandler BJ, Sznol M, Chen L. Blockade of the B7-H1/PD-1 pathway for cancer immunotherapy. Yale J Biol Med (2011); 84:409-21; PMID:22180678 [PMC free article] [PubMed] [Google Scholar]
- 21.Gao Q, Wang XY, Qiu SJ, Yamato I, Sho M, Nakajima Y, Zhou J, Li BZ, Shi YH, Xiao YS et al.. Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clin Cancer Res (2009); 15:971-9; PMID:19188168; http://dx.doi.org/ 10.1158/1078-0432.CCR-08-1608 [DOI] [PubMed] [Google Scholar]
- 22.Mauri C, Blair PA. Editorial: regulatory B cells: are we really ready to manipulate them for the benefit of patients with autoimmune diseases? Arthritis Rheumatol (2014); 66:1982-3; PMID:24729488; http://dx.doi.org/ 10.1002/art.38667 [DOI] [PubMed] [Google Scholar]
- 23.Ghebeh H, Barhoush E, Tulbah A, Elkum N, Al-Tweigeri T, Dermime S. FOXP3+ Tregs and B7-H1+/PD-1+ T lymphocytes co-infiltrate the tumor tissues of high-risk breast cancer patients: Implication for immunotherapy. BMC Cancer (2008); 8:57; PMID:18294387; http://dx.doi.org/ 10.1186/1471-2407-8-57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Picchianti Diamanti A, Rosado MM, Scarsella M, Germano V, Giorda E, Cascioli S, Laganà B, D'Amelio R, Carsetti R. Abatacept (cytotoxic T lymphocyte antigen 4-immunoglobulin) improves B cell function and regulatory T cell inhibitory capacity in rheumatoid arthritis patients non-responding to anti-tumour necrosis factor-alpha agents. Clin Exp Immunol (2014); 177:630-40; PMID:24773026; http://dx.doi.org/ 10.1111/cei.12367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DiLillo DJ, Weinberg JB, Yoshizaki A, Horikawa M, Bryant JM, Iwata Y, Matsushita T, Matta KM, Chen Y, Venturi GM et al.. Chronic lymphocytic leukemia and regulatory B cells share IL-10 competence and immunosuppressive function. Leukemia (2013); 27:170-82; PMID:22713648; http://dx.doi.org/ 10.1038/leu.2012.165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olkhanud PB, Damdinsuren B, Bodogai M, Gress RE, Sen R, Wejksza K, Malchinkhuu E, Wersto RP, Biragyn A. Tumor-evoked regulatory B cells promote breast cancer metastasis by converting resting CD4(+) T cells to T-regulatory cells. Cancer Res (2011); 71:3505-15; PMID:21444674; http://dx.doi.org/ 10.1158/0008-5472.CAN-10-4316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qiu YH, Zhang X, Ji Y, Ju S, Wang T. Preparation of functional monoclonal antibody against human CD28 and analysis of its biological feature. Chin J Cell Mol Immunol (2001); 17:368-70 [Google Scholar]
- 28.Zhou ZH, Wang JF, Wang YD, Qiu YH, Pan JZ, Xie W, Jiang LY, Klein B, Zhang XG. An agonist anti-human CD40 monoclonal antibody that induces dendritic cell formation and maturation and inhibits proliferation of a myeloma cell line. Hybridoma (1999); 18:471-8; PMID:10626675 [DOI] [PubMed] [Google Scholar]
- 29.Khokher S, Qureshi MU, Chaudhry NA. Comparison of WHO and RECIST criteria for evaluation of clinical response to chemotherapy in patients with advanced breast cancer. Asian Pac J Cancer Prev (2012); 13:3213-8; PMID:22994736; http://dx.doi.org/ 10.7314/APJCP.2012.13.7.3213 [DOI] [PubMed] [Google Scholar]
- 30.Adegboyega TO, Landercasper J, Linebarger JH, Johnson JM, Andersen JJ, Dietrich LL, Driscoll CD, Raghavendra M, Madadi AR, Al-Hamadani M et al.. Institutional review of compliance with NCCN guidelines for breast cancer: lessons learned from real-time multidimensional synoptic reporting. J Natl Compr Canc Netw (2015); 13:177-83; PMID:25691610 [DOI] [PubMed] [Google Scholar]
- 31.Zagouri F, Liakou P, Bartsch R, Peccatori FA, Tsigginou A, Dimitrakakis C, Zografos GC, Dimopoulos MA, Azim HA Jr. Discrepancies between ESMO and NCCN breast cancer guidelines: An appraisal. Breast, (2015); 24(4):513-23; PMID:25818651; http://dx.doi.org/ 10.1016/j.breast.2015.02.031 [DOI] [PubMed] [Google Scholar]
- 32.Theriault RL, Carlson RW, Allred C, Anderson BO, Burstein HJ, Edge SB, Farrar WB, Forero A, Giordano SH, Goldstein LJ et al.. Breast cancer, version 3.2013: featured updates to the NCCN guidelines. J Natl Compr Canc Netw (2013); 11:753-60; quiz 761; PMID:23847214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xie F, Wang Q, Chen Y, Gu Y, Mao H, Zeng W, Zhang X. Costimulatory molecule OX40/OX40L expression in ductal carcinoma in situ and invasive ductal carcinoma of breast: an immunohistochemistry-based pilot study. Pathol Res Pract (2010); 206:735-9; PMID:20634005; http://dx.doi.org/ 10.1016/j.prp.2010.05.016 [DOI] [PubMed] [Google Scholar]
- 34.Zhou Y, Chen YJ, Bai LX, Zhu GC, Wang XF, Zhang XG. Preparation and characterization of three novel monoclonal antibodies against human PD-L1. Chin J Cell Mol Immunol (2011); 27:1208-11; PMID:22078450 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.