Abstract
Chimpanzees remain fixed on a single strategy, even if a novel, more efficient, strategy is introduced. Previous studies reporting such findings have incorporated paradigms in which chimpanzees learn one behavioural method and then are shown a new one that the chimpanzees invariably do not adopt. This study provides the first evidence that chimpanzees show such conservatism even when the new method employs the identical required behaviour as the first, but for a different reward. Groups of chimpanzees could choose to exchange one of two types of inedible tokens, with each token type being associated with a different food reward: one type was rewarded with a highly preferred food (grape) and the other type was rewarded with a less preferred food (carrot). Individuals first observed a model chimpanzee from their social group trained to choose one of the two types of tokens. In one group, this token earned a carrot, while in the other, control, group the token earned a grape. In both groups, chimpanzees conformed to the trained model’s choice. This was especially striking for those gaining the pieces of carrot, the less favoured reward. This resulted in a population-level trend of food choices, even when counter to their original, individual, preferences. Moreover, the chimpanzees’ food preferences did not change over time, demonstrating that these results were not due to a simple shift in preferences. We discuss social factors apparent in the interactions and suggest that, despite seeming to be inefficient, in chimpanzees, conformity may benefit them, possibly by assisting with the maintenance of group relations.
Keywords: chimpanzee, conformity, conservatism, food preference, Pan troglodytes, social dynamics, social learning
Social learning, a process that has been demonstrated in a number species, can allow for the development of group-level behavioural traditions (Whiten et al. 1999; van Schaik et al. 2003; Bonnie et al. 2007; Dindo et al. 2009; Perry 2009). Theorists have suggested that the underlying mechanism for the maintenance of such group norms is the social learning strategy ‘follow-the-majority’ (Laland 2004; Rendell et al. 2011). Follow-the-majority predicts that, as the number of animals in a group performing a specific behaviour increases, so does the likelihood of a naïve individual adopting that same behaviour, thus driving the preservation of the tradition. Through empirical investigation, such a process, also termed ‘conformist transmission’ (Henrich & Boyd 1998), has been identified in a number of species, including fish (Day et al. 2001; Pike & Laland 2010), rats (Chou & Richerson 1992), nonhuman primates (Whiten et al. 2005; Perry 2009) and humans (Kameda & Nakanishi 2002).
Conformity is of particular interest because it may be a mechanism by which individuals identify the most successful strategies without having to spend time and energy on trial-and-error learning, and without inadvertently choosing strategies that may appear superficially beneficial but are not over the long term. Conformity exists even in species that presumably have the cognitive capacity to contrast alternative possibilities, such as humans, indicating its utility as a strategy for arriving at the best decision. Chimpanzees are a likely candidate for conformity given that they engage in complex behaviours for which the long-term outcome may not be immediately obvious (e.g. saving tools for use in the future, Mulcahy & Call 2006) and live in a social system that offers them many opportunities to observe others’ strategies.
In previous studies showing that animals conform to a group strategy, there was no apparent cost for them to do so, whereas, since Jean-Martin Charcot (Asch 1955), it has been shown that humans will go against their own preferences and opinions, often in situations in which their selected choice seems outlandish or is detrimental to a group’s success (e.g. Kelley & Shapiro 1954; reviewed in: Cialdini & Trost 1984; Bond & Smith 1996). We were interested in whether chimpanzees, which show a propensity to follow-the-majority (Whiten et al. 2005), would continue to do so if conforming meant going against their own individual food preferences. This has been shown in rats, which will override their dislike of a particular food after interacting with a conspecific that has eaten that same food (Galef & Whiskin 2008). Chimpanzees show a strong tendency, both in the wild and in captivity, for social learning (Whiten et al. 1999, 2009; Hopper et al. 2008), indicating that they pay close attention to the actions of others. Both their propensity for social learning and their need to bond and align with conspecifics suggest that chimpanzees, too, are a species likely to demonstrate conformity, even when it goes against their preferences (Coussi-Korbel & Fragaszy 1995).
Hrubesch et al. (2009) proposed ‘conservatism’ as an alternative mechanism to explain conformist transmission underlying social norms observed within groups of chimpanzees. They suggested that chimpanzees are unable to learn alternative behavioural strategies after having mastered a successful one, and so persist in using the originally seeded behaviour. Perhaps surprisingly, chimpanzees do indeed appear to be unable to learn a new strategy to solve an already-familiar task, even if this novel strategy is more efficient than the original method (Marshall-Pescini & Whiten 2008). The task employed by Marshall-Pescini & Whiten, however, incorporated a two-step technique for retrieving the food reward. The first, simpler, method enabled chimpanzees to gain honey, while the second, more complex, method, which built upon the first, resulted in the better reward of both honey and peanuts. The inability of chimpanzees in this study (and in that of Hrubesch et al. 2009) to transition to the second technique could either be explained by the second method being sufficiently difficult that learning it was not worth the better reward, or be due to an evolved conservatismin chimpanzees.
Many social learning studies with chimpanzees have used the two-action method (Dawson & Foss 1965), involving two physically distinct techniques to solve a single task. Although researchers have attempted to design apparatus for which the two methods are of equal complexity, there is often an inherent bias whereby one method is simpler or more open to individual discovery (see Hopper et al. 2007 for further discussion). We wished, therefore, to investigate whether this proposed conservatism would remain when the two methods required an identical physical action, and were only distinguished by the quality of food rewards obtained by the subjects. Would chimpanzees continue using the first method they encountered if another method that resulted in a more preferable food reward was observed, even when both methods were identical?
To test this, we employed an experimental exchange procedure that involved a subject returning one of two forms of inedible (and hence, otherwise valueless) token to an experimenter, who then gave the subject the designated food reward. Subjects could easily observe what other individuals exchanged and which rewards they received during these interactions (Brosnan et al. 2005). This exchange paradigm is quite simple, yet extremely flexible and intuitive. It has been successfully used to examine bartering behaviour (Hyatt & Hopkins 1998; Brosnan & de Waal 2004a, 2005; Brosnan & Beran 2009), inequity (Brosnan & de Waal 2003; Brosnan et al. 2005), the endowment effect (Brosnan et al. 2007), loss aversion (Chen & Santos 2006), social learning (Brosnan & de Waal 2004b) and symbolic communication (Savage-Rumbaugh et al. 1978).
In the present study, the two potential methods were created by two distinct forms of token available to the chimpanzees at all times, with each token type being associated with a different food reward. We predicted that because both methods required the same physical action, the chimpanzees would not evidence conservatism (Hrubesch et al. 2009) as neither option was more difficult to learn than the other. Thus, if a group-level norm arose, it could be attributed to conformity to a specific behaviour (Whiten et al. 2005). We hypothesized that chimpanzees would be attentive to the choice behaviour of their groupmates. Specifically, we predicted that chimpanzees would (1) return the modelled token regardless of the reward it gained them and (2) be unlikely to switch to returning the second token type, even if it were to gain them a better food reward, due to the propensity to conform. Finally, we investigated group dynamics during the targeted interactions to see whether and how social behaviour may have affected tendencies to conform to a group norm.
METHODS
Ethical Note
Twelve adult chimpanzees, housed in two social groups at the Michale E. Keeling Center for Comparative Medicine and Research of the University of Texas MD Anderson Cancer Center, Bastrop, TX, U.S.A. (KCCMR), were the subjects for this study. The chimpanzees were tested with their group, so that no animal was isolated from their cagemates. All testing took place in the chimpanzees’ large, enriched home enclosures (Primadomes®, diameter 10 m), but the chimpanzees had free access to their inside dens at all times (Fig. 1a), meaning that they chose whether or not to participate in the study. The chimpanzees were not deprived of food or water. Approval for this study was gained from the Institutional Animal Care and Use Committee (IACUC approval number: 07-92-03887) of the University of Texas. The KCCMR is fully accredited by American Association for the Accreditation of Laboratory Animal Care-International (AAALAC-I). We thank the animal care and enrichment staff for maintaining the health and wellbeing of the chimpanzees and making this research possible.
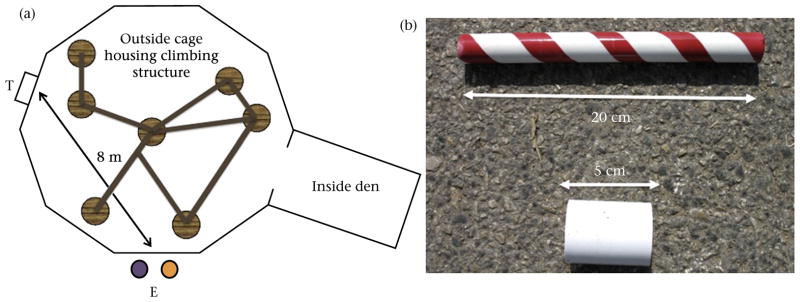
Figure 1.
Experimental set-up and tokens used for exchanging. (a) Footprint of the climbing frame within the chimpanzees’ cage and the experimental set-up. The experimenter (L.M.H.) stood at position E on the outside of the cage. To avoid priming the chimpanzees to select a particular token style, the experimenter delivered the tokens en masse (20 of each type, 40 in total) through a token delivery chute (location T) 8 m from where the chimpanzees had to exchange the tokens with the experimenter. The chimpanzees had to collect the tokens from location T and bring them to point E, walking either through or around the climbing structure in the cage, to exchange them with the experimenter. Two food reward forms (high-value reward (HR): grapes; medium-value reward (MR): carrot pieces) were constantly visible to the chimpanzees in two buckets at position E. (b) The candy-cane (CC) token (top) measured 20 × 2 cm; the short-squat (SS) token (bottom) measured 5 × 4 cm.
Subjects
Testing of the 12 chimpanzees took place in the large, outside portion of the enclosure. No subjects from either group were blocked from participation. The group that was exposed to a model trained to trade for the medium-value reward (carrots), the ‘MR group’, contained five individuals (3 males) with an age range of 18–20 years (average age: 18.6 years), while the group whose model was trained to trade for the high-value reward (grapes), the ‘HR group’, contained seven individuals (2 males) with an age range of 15–29 years (average age: 20.3 years).
Following Whiten et al. (2005, see also Biro et al. 2003), a dominant female from each group was selected to be the model. Female JO (age 20 years) was chosen to be the model for the MR group and female CH (age 29 years) was the model for the HR group. Neither JO nor CH had ever acted as models in previous social learning studies, and indeed, no individual from either group had ever been used in any previous social learning studies.
Food Preference Tests
We wished to identify two distinct foods items for the rewards; one rated highly by the chimpanzees and one that was less preferred but still eaten readily when no other food was available (Brosnan et al. 2010). Following our previous work with the chimpanzees (Brosnan et al. 2010), grapes were selected as the high-value reward (HR) and pieces of carrot as the medium-value reward (MR). Prior to conducting the experiment, we performed dichotomous food preference tests with each chimpanzee (sensu Brosnan & de Waal 2004b) to confirm that individuals in both groups preferred grapes over carrots. The proportion of grape choices made by each chimpanzee was recorded during two sessions of 10 forced-choice trials, conducted on different days prior to the test. To be considered ‘preferred’, subjects had to choose grapes over carrots on 8 of 10 trials in both sessions. (Note that all chimpanzees did so; however, had the chimpanzees not shown a preference for grapes, we would have selected different food stuffs and repeated these trials until we had identified a high-value reward selected 80% of the time over the medium-value reward.) A further food preference test was performed after the open diffusion experiment, to determine whether the chimpanzees’ experience affected their food preferences (for the individual food preferences of each chimpanzee see Supplementary Material, Appendix S1).
Token Preference Tests
To verify that chimpanzees did not have an innate preference for one of the two token types used in the open diffusion test (Fig. 1b), dichotomous choice tests were conducted with 12 control chimpanzees (housed in two groups) that were naïve to PVC tokens and the exchange paradigm. These token preference tests were not performed with the 12 experimental subjects (from the MR and HR groups) until after the open diffusion tests, as prior exposure to the two test token types might have affected their behaviour later in the open diffusion test. For instance, allowing them to form a preference based on some factor unrelated to the token’s associated food item. We wished to ensure that any preferences recorded during the open diffusion tests were due solely to social exposure and could not be explained by previous individual exposure to the tokens in a pretest preference test. The majority (10/12) of the naïve, control, chimpanzees were indifferent to the two token types, indicating that there was no innate preference for one token form or the other (individual preferences are reported in Supplementary Material, Appendix S2).
Procedure
To prepare the chimpanzees in the MR and HR groups for the open diffusion test phase, all were trained in the exchange paradigm in the 2 weeks prior to the open diffusion test. Wooden tokens selected for the training were distinctly different in form, colour and material from the PVC tokens used in the test phase. Training the chimpanzees to exchange tokens was run in a group setting by L.M.H., with all members of the respective groups learning together. We used a shaping procedure, and it took between one and five 20min training sessions for individuals to learn the procedure. The chimpanzees were rewarded with food (including apple pieces, orange pieces and pineapple), but neither grapes nor carrots (rewards used during the open diffusion test) were used in training.
Out of sight of their group, the models from each group were trained to exchange only one of the two token forms, the candy-cane (CC) token (Fig. 1). JO, the model from the MR group, was given one medium-value reward (MR, a grape-sized piece of carrot) for each successful exchange, while CH, the model for the HR group (control group), was given one high-value reward (HR, a grape). During training, exchange of the short-squat (SS) token resulted in no reward (they received rewards for the SS token during the open diffusion sessions, as did all chimpanzees). To ensure that they would initially trade the CC token in front of their group, this training lasted until the models selected the CC, for which they were rewarded, but ignored the SS token, on 10 consecutive choices. For both models, this training required two 15 min sessions conducted on two consecutive days.
Each group participated in 10 1-hour open diffusion test periods (Whiten et al. 2005). For each of these, the experimenter placed 20 CC and 20 SS tokens in the chimpanzees’ cage via a food dispenser (Fig. 1). The experimenter then moved to the far side of the cage (8m from the food dispenser, Fig. 1) and waited for the chimpanzees to bring the tokens to her and exchange them for the food rewards. This was done to ensure that the experimenter did not hand the tokens directly to the chimpanzees, and thus potentially inadvertently bias their token selection as a result of stimulus enhancement (Whiten et al. 2009). In both groups, the model exchanged the CC token prior to any other chimpanzee exchanging a token. Once chimpanzees exchanged 10 of either token form, the experimenter returned the tokens to the cage via the food dispenser to guarantee that the chimpanzees always had access to multiple tokens of both forms.
The chimpanzees could exchange either token form at any time and were rewarded according to their group’s pattern. Chimpanzees in the HR group were given one HR for each CC token they gave to the experimenter and one MR for each SS token. Conversely, chimpanzees in the MR group received one MR for each CC token exchanged and one HR for each SS token. Although all of the chimpanzees had been trained to generally exchange tokens for food rewards, prior to the first test session, no chimpanzees, other than the models, had ever seen the CC or SS tokens.
All sessions were recorded using a Canon ZR950 digital camcorder for detailed analysis. Running auditory commentary was provided by the experimenter (L.M.H.).
RESULTS
Token Exchanges
During the open diffusion sessions, all chimpanzees observed their cagemates exchange tokens and all chimpanzees exchanged tokens with the experimenter, showing that they all understood the principle of exchanging tokens for food. Throughout the 10 h of the open diffusion test, chimpanzees showed a strong interest in the actions of their groupmates and were classed as ‘observing’ if they were within 1 m, and oriented towards, another chimpanzee exchanging a token with the experimenter. Chimpanzees in the MR group observed an average of 208.0 token exchanges, with both token forms, by their groupmates, while chimpanzees in the HR group observed an average of 454.7 exchanges.
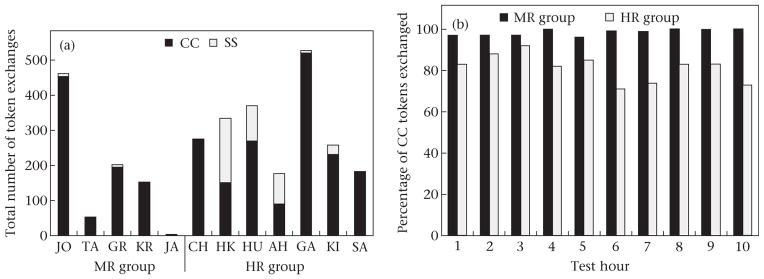
Individual chimpanzees in the HR group exchanged more tokens per individual (mean ± SD = 303.3 ± 121.3) than did those in the MR group (174.6 ± 178.3; t1.5 =10.0, P = 0.01; Fig. 2 shows individual chimpanzees’ data and data by hourly test period).
Figure 2.
(a) Number of candy-cane (CC) and short-squat (SS) tokens exchanged by individual chimpanzees in the medium-value reward (MR) group and the high-value reward (HR) group, in order of acquisition. (b) Percentage of CC tokens exchanged by all chimpanzees in both groups in each test hour.
Conformity to Seeded Method
All chimpanzees in the MR group conformed to the same strategy as the model, exchanging CC tokens for the MR. Over the 10 h, 404 of the 412 total token exchanges made by chimpanzees, excluding the model JO, utilized the CC token (98%; two-tailed binomial test: P < 0.001; Fig. 3). It is important to note that four of the five chimpanzees in this group made some exchanges with SS tokens one or more times during the 10 h open diffusion test, gaining the HR, but all reverted to predominantly exchanging CC tokens (only 2% of all exchanges were with SS tokens, and no individual exchanged more than 2.9% SS tokens). Chimpanzees in the HR group, who also observed the model exchange the CC tokens, but for the HR, also predominantly exchanged the CC tokens (1445 of 1848, token exchanges, or 78%; P < 0.001).
Figure 3.
Percentage of candy-cane (CC:■) and short-squat (SS:
 ) token exchanges by all chimpanzees in the high-value reward (HR) and medium-value reward (MR) groups.
) token exchanges by all chimpanzees in the high-value reward (HR) and medium-value reward (MR) groups.
When the model’s exchange responses were included in the analysis, the level of exchanging the CC tokens remained at the same high rate (MR group: 98% CC choices, 857/873; two-tailed binomial test: P = 0.0001; HR group: 81% CC choices, 1719/2123; P = 0.001). Furthermore, neither group had a change in the proportion of CC token exchanges across the 10 test periods (McNemar test: hour 1 versus hour 10: MR group: 113/117 versus 63/63; P > 0.05; HR group: 88/107 versus 155/210; P > 0.05).
Food and Token Preference Tests
Chimpanzees’ food preferences did not vary between the two groups. Prior to testing, both groups showed a comparably strong preference for grapes compared to carrot pieces (two-tailed Mann–Whitney U test: U = 0.81, N1 =7, N2 = 5, P > 0.05). Furthermore, food preferences remained the same before and after the open diffusion test for both groups. Before testing, chimpanzees in the MR group chose grapes 95% of the time, and after the open diffusion test they chose them 96% of the time. Chimpanzees in the HR group selected grapes 90% of the time before and 87% of the time after testing and there was also no difference in the preference for grapes shown by the two groups after the open diffusion test (two-tailed Mann–Whitney U test: U = 1.3 N1 =7, N2 = 5, P > 0.05; see Supplementary Material, Appendix S1 for individual data).
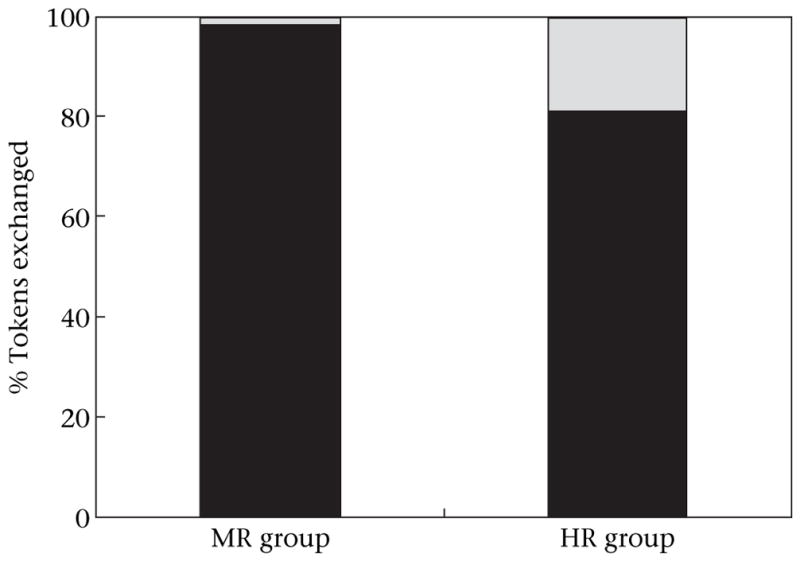
It was also possible that the chimpanzees’ propensity to conform during the open diffusion test might have affected their token preferences. In fact, after the experiment, eight of the 12 experimental chimpanzees showed a significant preference for the CC token, and the remaining four also showed a strong preference for CC tokens (three showed an 80% preference and one a 70% preference). We also compared their preferences to the 12 naïve chimpanzees tested prior to the open diffusion test (see Methods). Compared to these naïve chimpanzees, the experimental chimpanzees chose the CC tokens significantly more overall (two-tailed Mann–Whitney U test: U = 3.18, N1 =12, N2 = 12, P < 0.001), suggesting that the experience during the open diffusion test caused them to change their preferences in favour of CC tokens. Interestingly, the token’s value did not affect the subjects’ final token preferences; following testing, the chimpanzees from both groups equally preferred the CC token (two-tailed Mann–Whitney U test: U = 0.81, N1 =7, N2 = 5, P > 0.05).
Social Dynamics within Groups
Detailed analysis of the video footage revealed that chimpanzees would not only retrieve tokens from the food dispenser, but some also stole tokens from other chimpanzees (then exchanged them with the experimenter). We investigated the effect of this stealing behaviour on the exchanging strategies of all group members. For the purposes of this analysis, we defined ‘stealing’ as one chimpanzee taking a token from the possession (that is, from the hand of) of a second chimpanzee, who resisted relinquishing the token, often expressed through fear facial expressions and vocalizations. In both groups, only CC, and never SS, tokens were stolen.
Given the unlimited nature of the tokens, token stealing was a remarkably common behaviour. Over the 10 h, there were 139 incidents of stealing in the HR group (average rate of tokens stolen = 20 per chimpanzee), while in the MR group, there were 50 incidents of stealing, proportionally half the rate seen in the HR group (average rate of tokens stolen = 10 per chimpanzee). Token stealing may also explain the difference in conformity seen between the two groups. Although this difference was not significant (two-tailed Mann–Whitney U test: U = 0.89, N1 =7, N2 = 5, P > 0.05), chimpanzees of the HR group showed a reduced level of conformity compared to the chimpanzees of the MR group, despite getting the higher-value grape for each exchange. This trend is explained by the responses of three subordinate individuals in the HR group (two females, one male) who individually exchanged CC tokens at levels of 73%, 51% and 45% across the 10 h. The other four group members exchanged CC tokens at high levels (collectively above 97%), comparable to those seen in the MR group. Video analysis revealed that these three chimpanzees often had CC tokens stolen from them by more dominant group members, but at no point did they steal tokens from others (Table 1).
Table 1.
Number of times a chimpanzee stole a candy-cane (CC) token from another individual (‘Steals token’) or had a CC token stolen (‘Token stolen’), and the percentage of interactions in which a chimpanzee had a CC token stolen compared to when they stole from another (‘% Stolen’). Note that short-squat (SS) tokens were never stolen
| Group | Chimpanzee (sex, age in years) | Steals token | Token stolen | % Stolen |
|---|---|---|---|---|
| MR | JO (F, 20) | 18 | 20 | 52.6 |
| GR (F, 18) | 25 | 3 | 10.7 | |
| KR (M, 18)* | 0 | 21 | 100 | |
| TA (M, 18) | 6 | 5 | 45.5 | |
| JA (M, 19) | 1 | 1 | 50 | |
| HR | CH (F, 29) | 80 | 0 | 0 |
| SA (F, 29) | 12 | 8 | 40 | |
| AH (F, 15)† | 0 | 4 | 100 | |
| KI (F, 12) | 46 | 5 | 9.9 | |
| HU (F, 12)† | 0 | 59 | 100 | |
| GA (M, 18)* | 1 | 53 | 98.1 | |
| HK (M, 27)† | 0 | 10 | 100 |
MR: medium-value reward; HR: high-value reward.
Chimpanzees that avoided having their tokens stolen by exchanging when dominant chimpanzees were absent.
Chimpanzees that deviated from the group norm and exchanged SS tokens regularly.
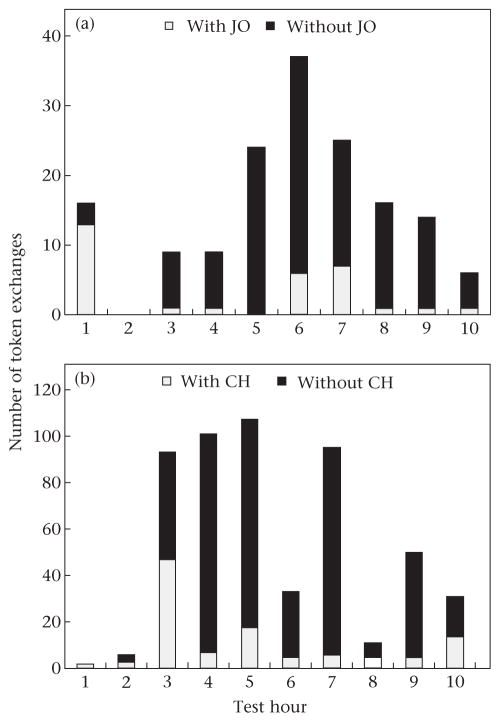
Switching token preferences was not the only strategy chimpanzees used to maximize their gains in the face of dominant animals controlling access to CC tokens. Two chimpanzees (one from each group) that also had their tokens stolen at very high rates continued to exchange CC tokens (both chimpanzees exchanged CC tokens 99% of the time; Table 1). However, they developed a strategy of avoidance. These two chimpanzees exchanged CC tokens at times when the individuals that stole their tokens were not present (i.e. further than 5m from the experimenter, see Fig. 4). In the MR group, the model JO stole CC tokens from male KR more than did any other chimpanzee (67% of his stolen tokens were taken by her), yet KR made 80% of his token exchanges when JO was not present. Similarly, in the HR group, male GA had his CC tokens stolen predominantly by model CH (85% of his stolen tokens were taken by her), and GA made 79% of his token exchanges when CH was not present. Interestingly, this strategy appeared to be a learned response by both KR and GA, as their pattern of avoidance increased over the 10 h. In hour 1, 19% of KR’s exchanges were performed when JO was not present, increasing to 83% by hour 10. Similarly, none of GA’s exchanges in hour 1 were when CH was absent, but by hour 10, 55% of his exchanges were performed in her absence.
Figure 4.
Avoidance of dominant individuals when exchanging candy-cane (CC) tokens to reduce the risk of having the tokens stolen. (a) Number of CC token exchanges made by KR when JO was present (<5 m from the experimenter) and not present (>5 m from the experimenter). (b) Number of CC token exchanges made by GA when CH was present (<5 m from the experimenter) and not present (>5 m from the experimenter).
DISCUSSION
In both groups, chimpanzees showed conformity to a group-level behavioural strategy of exchanging CC, but not SS, tokens with the experimenter. This occurred even when such a choice went against the chimpanzees’ food preferences, and cannot be explained by an innate preference for one token type or a change in food preferences. Strikingly, in the MR group, this remained true even after chimpanzees discovered that SS tokens gave them highly prized grapes, both through the observation of groupmates exchanging SS tokens (each chimpanzee saw an average of 5.6 SS token exchanges) and exchanging SS tokens themselves. The chimpanzees did not switch to the more profitable strategy (no more than 2% of the exchanges made were with the SS tokens), but instead they continued with the introduced method of exchanging CC tokens for the less preferred carrots.
These findings are consistent with previous studies suggesting that chimpanzees are unable to transition to a novel behaviour if they already are proficient at a productive strategy (Marshall-Pescini & Whiten 2008; Hrubesch et al. 2009), but they differ in two important ways. Firstly, the chimpanzees in the current study learned an application of an already-known behaviour, rather than having to learn two novel behaviours in succession. The chimpanzees had been pretrained on the exchange paradigm and so did not need to learn this technique from observing their group members; the chimpanzees learned only the models’ token choices. Thus, these results cannot be explained by hypothesizing that they did not, or could not, learn the alternative strategy (that is, there was no alternative method, just an alternative choice). Second, the chimpanzees in the MR group stuck to the introduced method, despite the fact that it caused them to act against their previously expressed (and maintained) food preferences.
Hrubesch et al. (2009) proposed that the conservatism of chimpanzees is what creates within-group behavioural homogeneity; despite other options being available, chimpanzees are unable to adopt them. In contrast Whiten and colleagues (Whiten et al. 2005, 2007; Bonnie et al. 2007) proposed that chimpanzees are conforming to a group norm; choosing the option selected by their groupmates in order to be like them (see also Meltzoff 2007). We propose that such conformity, rather than conservatism, is a more likely explanation for the behaviour of the chimpanzees in the MR group that continued to exchange the less profitable CC tokens (Whiten & van Schaik 2007).
Conformity appears to be the more parsimonious explanation for the results we present here. It is unlikely that the homogeneity of the chimpanzees’ token exchange pattern in the MR group can be explained by their inability to adopt the alternative strategy because four of the five chimpanzees exchanged the alternative SS tokens. Note, too, that six of the seven chimpanzees in the HR group also discovered the alternative strategy, and two chimpanzees (one female, AH and one male, HK) actually exchanged more SS than CC tokens (Fig. 2). Finally, we have direct evidence that chimpanzees can change their strategy when, and if, the need arises. The three chimpanzees that had their tokens stolen reverted to exchanging SS tokens, despite maintaining their preference for CC tokens (as evidenced by the preference tests following the conclusion of the study).
It has been argued that chimpanzees’ social learning is based on replicating the goals of their peers (Tennie et al. 2010), but this study reveals that chimpanzees attend to the details of conspecifics’ behaviour (i.e. the specific token exchanged). Moreover, these results cannot be explained by associative learning, as the majority of chimpanzees had experience with both token/reward pairs at some point during the experiment, yet continued to exchange following the group norm. Although it has been shown previously that chimpanzees create group-level traditions when there is no cost to themselves (Whiten et al. 2005), it has been argued that ‘…social learning in chimpanzees is a fairly fragile phenomenon, which can be fairly easily overridden by individual preferences’ (Tennie et al. 2009, page 2408). Our results call for this to be re-evaluated. Rather, chimpanzees’ motivations do appear to include conformity, and individual preferences may be, at least in some cases, the weaker of the two motivations.
Why should chimpanzees conform when it appears to be an inefficient strategy? This can be addressed on several levels. First, as discussed in the Introduction, conformity itself might be an evolutionary selected strategy due to the fact that, on average, it leads to the greatest benefit at the least relative cost. Of course, evolution works on averages, so this will not lead to the ‘best’ outcome for every individual. Nevertheless, conformity may give the chimpanzees the opportunity to acquire information and shape preferences in situations in which it may be difficult or costly to gather the requisite personal information (Henrich & Boyd 1998; Laland 2004; Pike & Laland 2010). Thus it may be a better strategy to rely on observations of one’s groupmates, even when they do not seem to make sense. Note, that by conforming, chimpanzees were merely copying preferences which, although different from their personal preferences, did not represent a hazardous or lethal alternative to their personal choice. Indeed, humans, too, engage in such seemingly irrational conformist behaviour, for instance, agreeing with one’s social group even in situations in which the response is clearly inaccurate (e.g. Asch 1952; Kelley & Shapiro 1954) and Cialdini & Trost (1984) emphasized that although in the Western world conformity may be considered to have a negative connotation, it may actually be adaptive and ‘virtuous’, allowing for group cohesion and interdependence.
If the conclusion of conformity is accepted, however, it is the more interesting because this strongly adopted behaviour was fuelled by just one female in each group. Unlike most tests of conformity, our two chimpanzee groups observed a minority ‘innovation’ (the trained behaviour of the model). We suggest that the chimpanzees adopted their model’s technique because this ‘minority’ (the female model)was consistent in its behaviour (token choice), as has been shown for humans who, too, adopt a consistent minority’s behaviour (Moscovici et al. 1969, reviewed in Maass & Clark 1984). It is also probably extremely important that the seeded method was introduced into each group by an individual who was not only consistent in their choice of token type, but also a dominant female. Previous research suggests that individuals are more likely to copy, and learn from, dominant individuals (Nicol & Pope 1999; Swaney et al. 2001; Biro et al. 2003; Horner et al. 2010).
In addition to the important characteristics of the model, which drove the conformity seen in this study, as with humans, who conform to ‘be like others’ and to strengthen social bonds (Meltzoff 2007), we propose that chimpanzees conform to aid social cohesion and the maintenance of group dynamics (Deutsch & Gerard 1955). Deutsch & Gerard (1955) proposed that humans may conform either because of ‘informational influence’, in which individuals are concerned with making the ‘correct’ choice, or because of ‘normative influence’, in which individuals seek to maintain social harmony through the approval of others. As the chimpanzees had much prior experience with both foodstuffs used in this experiment (grapes and carrots), we do not think that they required information from their cagemates to determine which food to select (informational influence), but rather, that their behaviour was shaped by normative influence. For primates, a comparative theory is described by de Waal’s (2001) ‘bonding- and identification- based observational learning’ model (BIOL).
Specifically, de Waal (2001) argued that BIOL is learning that is based on a desire to be like one’s peers, rather than on the rewards garnered from the behaviour. BIOL has also been proposed to explain why female capuchins are motivated to copy their groupmates (Perry 2009) and seems a likely explanation for the responses of the chimpanzees in the present study. In both groups tested, chimpanzees showed a strong tendency to copy their group, even when this meant selecting a nonpreferred food item. Thus, when considering social learning models (Laland 2004), it will be important to keep in mind not only material rewards to individuals, but intrinsic ones as well. Furthermore, Moscovici and colleagues (e.g. Moscovici et al. 1969) have suggested that the social factors that dictate, and influence, conformity differ depending on whether there is a minority- or majority-rule (reviewed in Cialdini & Trost 1984). It is possible that these same, changing, influences may well have affected the conformist behaviour of the chimpanzees in our study over the 10 h of testing, as more and more individuals adopted the seeded behaviour, but further research is required to specifically investigate this.
The detailed analysis of the social dynamics within groups provides interesting insight into what was occurring during the open diffusion sessions. Two strategies for exchanging tokens with the experimenter arose among those individuals that routinely had their CC tokens stolen. First, some of these chimpanzees preferentially exchanged CC tokens when dominant individuals were not around, while others adopted the alternative strategy of exchanging SS tokens. Ultimately, stealing was more common in the HR group than in the MR group.
We propose two potential factors to explain why stealing was more pronounced in the HR group. Firstly, de Waal’s (2001) BIOL model may provide an understanding of the apparent variance of the group dynamics. The HR group was formed in October 2008, only 18months prior to this experiment, while the composition of the MR group had been stable for at least 15 years. The more newly formed HR group may have been bonded less strongly than the MR group and so, following the BIOL model, their desire to conform may have been weaker than that of the HR group. Note, however, that comparable rates of conformity were found in both groups, making the apparent conformity in the newly formed MR group even more impressive. Therefore, the length of time since each group was formed cannot be the sole explanation for the increased rates of stealing in the HR group. Our second proposed explanation is that, in the HR group, chimpanzees garnered the better reward when using the seeded method (CC tokens), which in turn increased the demand for these tokens (especially compared to the seeded method in the MR group, which only garnered carrots). This increased desire for exchanging CC tokens in the HR group may also explain why there was a higher rate of exchange (per individual) compared to the MR group.
It would seem that those chimpanzees that had tokens regularly stolen and that adopted the alternative strategy (exchanging SS tokens), did so in order to gain some reward; for them, getting an MR was better than getting no reward at all. Furthermore, the alternative exchange strategy also allowed them to minimize the risk of attack by dominant individuals. This is reminiscent of meerkats, which, when faced with a novel task, do not show a preference to follow, and learn from, experienced dominants. This potentially reflects the interplay between the benefits (more reliable information) and costs (risk of attack) arising from observing dominants (Thornton & Malapert 2009). Note, too, that the chimpanzees in the HR group nevertheless showed a strong preference for CC tokens in the post-test token preference assessment, further supporting our suggestion that these three chimpanzees only used alternative strategies because of social pressure limiting their access to CC tokens, not because of a difference in the strength of the token preference. This highlights the interplay between the desire to conform and the social dynamics within an established community.
Supplementary Material
Acknowledgments
We are grateful to Michael Beran for comments on earlier drafts of this manuscript and to Tyrel McAdams for assisting with the manufacture of the tokens. This research was funded by a National Science Foundation CAREER award (SES 0847351 to S.F.B.). The chimpanzees are supported by a National Institutes of Health U42 (RR-15090) grant. We also wish to show our appreciation for all the staff at the UT MD Anderson facility for their support and help.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.anbehav.2011.03.002.
References
- Asch SE. Social Psychology. Englewood Cliffs, New Jersey: Prentice Hall; 1952. [Google Scholar]
- Asch SE. Opinions and social pressure. Scientific American. 1955;193:31–35. [Google Scholar]
- Biro D, Inoue-Nakamura N, Tonooka R, Yamakoshi G, Sousa C, Matsuzawa T. Cultural innovation and transmission of tool use in wild chimpanzees: evidence from field experiments. Animal Cognition. 2003;6:213–223. doi: 10.1007/s10071-003-0183-x. [DOI] [PubMed] [Google Scholar]
- Bond R, Smith PB. Culture and conformity: a meta-analysis of studies using Acsh’s (1952b, 1956) line judgment task. Psychological Bulletin. 1996;119:111–137. [Google Scholar]
- Bonnie KE, Horner V, Whiten A, de Waal FBM. Spread of arbitrary conventions among chimpanzees: a controlled experiment. Proceedings of the Royal Society B. 2007;274:367–372. doi: 10.1098/rspb.2006.3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosnan SF, Beran MJ. Trading behavior between conspecifics in chimpanzees, Pan troglodytes. Journal of Comparative Psychology. 2009;123:181–194. doi: 10.1037/a0015092. [DOI] [PubMed] [Google Scholar]
- Brosnan SF, de Waal FBM. Monkeys reject unequal pay. Nature. 2003;425:297–299. doi: 10.1038/nature01963. [DOI] [PubMed] [Google Scholar]
- Brosnan SF, de Waal FBM. A concept of value during experimental exchange in brown capuchin monkeys. Folia Primatologica. 2004a;75:317–330. doi: 10.1159/000080209. [DOI] [PubMed] [Google Scholar]
- Brosnan SF, de Waal FBM. Socially learned preferences for differentially rewarded tokens in the brown capuchin monkeys (Cebus apella) Journal of Comparative Psychology. 2004b;118:133–139. doi: 10.1037/0735-7036.118.2.133. [DOI] [PubMed] [Google Scholar]
- Brosnan SF, de Waal FBM. A simple response to barter in chimpanzees (Pan troglodytes) Primates. 2005;46:173–182. doi: 10.1007/s10329-005-0125-0. [DOI] [PubMed] [Google Scholar]
- Brosnan SF, Schiff HC, de Waal FBM. Chimpanzees’ (Pan troglodytes) reactions to inequity during experimental exchange. Proceedings of the Royal Society B. 2005;1560:253–258. [Google Scholar]
- Brosnan SF, Jones OD, Lambeth SP, Mareno MC, Richardson AS, Schapiro SJ. Endowment effects in chimpanzees. Current Biology. 2007;17:1–4. doi: 10.1016/j.cub.2007.08.059. [DOI] [PubMed] [Google Scholar]
- Brosnan SF, Talbot C, Ahlgren M, Lambeth SP, Schapiro SJ. Mechanisms underlying responses to inequitable outcomes in chimpanzees, Pan troglodytes. Animal Behaviour. 2010;79:1229–1237. doi: 10.1016/j.anbehav.2010.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MK, Santos LR. Some thoughts on the adaptive function of inequity aversion: an alternative to Brosnan’s social hypothesis. Social Justice Research. 2006;19:201–207. [Google Scholar]
- Cialdini RB, Trost MR. Social influence: social norms, conformity, and compliance. In: Gilbert DT, Fiske ST, Lindzey G, editors. The Handbook of Social Psychology. 4. Oxford: Oxford University Press; 1984. pp. 151–192. [Google Scholar]
- Chou LS, Richerson PJ. Multiple models in social transmission of food selection by Norway rats, Rattus norvegicus. Animal Behaviour. 1992;44:337–343. [Google Scholar]
- Coussi-Korbel S, Fragaszy DM. On the relation between social dynamics and social learning. Animal Behaviour. 1995;50:1441–1453. [Google Scholar]
- Dawson BV, Foss BM. Observational learning in budgerigars. Animal Behaviour. 1965;13:470–474. doi: 10.1016/0003-3472(65)90108-9. [DOI] [PubMed] [Google Scholar]
- Day R, MacDonald T, Brown C, Laland KN, Reader SM. Interactions between shoal size and conformity in guppy social foraging. Animal Behaviour. 2001;62:917–925. [Google Scholar]
- Deutsch M, Gerard HB. A study of normative and informational social influences upon individual judgment. Journal of Abnormal and Social Psychology. 1955;51:629–636. doi: 10.1037/h0046408. [DOI] [PubMed] [Google Scholar]
- Dindo M, Whiten A, de Waal FBM. In-group conformity sustains different foraging traditions in capuchin monkeys (Cebus apella) PLoS ONE. 2009;4:e7858. doi: 10.1371/journal.pone.0007858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galef BG, Whiskin EE. ‘Conformity’ in Norway rats? Animal Behaviour. 2008;75:2035–2039. [Google Scholar]
- Henrich J, Boyd R. The evolution of conformist transmission and the emergence of between-group differences. Evolution and Human Behavior. 1998;19:215–231. [Google Scholar]
- Hopper LM, Spiteri A, Lambeth SP, Schapiro SJ, Horner V, Whiten A. Experimental studies of traditions and underlying transmission processes in chimpanzees. Animal Behaviour. 2007;73:1021–1032. [Google Scholar]
- Hopper LM, Lambeth SP, Schapiro SJ, Whiten A. Observational learning in chimpanzees and children studied through ‘ghost’ conditions. Proceedings of the Royal Society B. 2008;275:835–840. doi: 10.1098/rspb.2007.1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner V, Proctor D, Bonnie KE, Whiten A, de Waal FBM. Prestige affects cultural learning in chimpanzees. PLoS ONE. 2010;5:e10625. doi: 10.1371/journal.pone.0010625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrubesch C, Preuschoft S, van Schaik CP. Skill mastery inhibits adoption of observed alternative solutions among chimpanzees (Pan troglodytes) Animal Cognition. 2009;12:209–216. doi: 10.1007/s10071-008-0183-y. [DOI] [PubMed] [Google Scholar]
- Hyatt CW, Hopkins WD. Interspecies object exchange: bartering in apes? Behavioural Processes. 1998;42:177–187. doi: 10.1016/s0376-6357(97)00075-2. [DOI] [PubMed] [Google Scholar]
- Kameda T, Nakanishi D. Cost-benefit analysis of social/cultural learning in a nonstationary uncertain environment: an evolutionary simulation and an experiment with human subjects. Evolution and Human Behavior. 2002;23:373–393. [Google Scholar]
- Kelley HH, Shapiro MM. An experiment on conformity to group norms where conformity is detrimental to group achievement. American Sociological Review. 1954;19:667–677. [Google Scholar]
- Laland KN. Social learning strategies. Learning & Behavior. 2004;32:4–14. doi: 10.3758/bf03196002. [DOI] [PubMed] [Google Scholar]
- Maass A, Clark RD. Hidden impact of minorities: fifteen years of minority influence research. Psychological Bulletin. 1984;95:428–450. [Google Scholar]
- Marshall-Pescini S, Whiten A. Chimpanzees (Pan troglodytes) and the question of cumulative culture: an experimental approach. Animal Cognition. 2008;11:449–456. doi: 10.1007/s10071-007-0135-y. [DOI] [PubMed] [Google Scholar]
- Meltzoff AN. ‘Like me’: a foundation for social cognition. Developmental Science. 2007;10:126–134. doi: 10.1111/j.1467-7687.2007.00574.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscovici S, Lage E, Naffrechoux M. Influence of a consistent minority on the responses of a majority in a color perception task. Sociometry. 1969;32:365–380. [PubMed] [Google Scholar]
- Mulcahy NJ, Call J. Apes save tools for future use. Science. 2006;312:1038–1040. doi: 10.1126/science.1125456. [DOI] [PubMed] [Google Scholar]
- Nicol CJ, Pope SJ. The effects of demonstrator social status and prior foraging success on social learning in laying hens. Animal Behaviour. 1999;57:163–171. doi: 10.1006/anbe.1998.0920. [DOI] [PubMed] [Google Scholar]
- Perry S. Conformism in the food processing techniques of white-faced capuchin monkeys (Cebus capucinus) Animal Cognition. 2009;12:705–716. doi: 10.1007/s10071-009-0230-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike TW, Laland KN. Conformist learning in nine-spined sticklebacks’ foraging decisions. Biology Letters. 2010;6:525–528. doi: 10.1098/rsbl.2009.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendell L, Fogarty L, Hoppitt WJE, Morgan TJH, Webster MM, Laland KN. Cognitive culture: theoretical and empirical insights into social learning strategies. Trends in Cognitive Sciences. 2011;15:68–76. doi: 10.1016/j.tics.2010.12.002. [DOI] [PubMed] [Google Scholar]
- Savage-Rumbaugh ES, Rumbaugh DM, Boysen S. Symbolic communication between two chimpanzees (Pan troglodytes) Science. 1978;201:641–644. doi: 10.1126/science.675251. [DOI] [PubMed] [Google Scholar]
- van Schaik CP, Ancrenaz M, Borgen G, Galdikas B, Knott CD, Singleton I, Suzuki A, Utami SS, Merrill M. Orangutan cultures and the evolution of material culture. Science. 2003;299:102–105. doi: 10.1126/science.1078004. [DOI] [PubMed] [Google Scholar]
- Swaney W, Kendal JR, Capon H, Brown C, Laland KN. Familiarity facilitates social learning of foraging behaviour in the guppy. Animal Behaviour. 2001;62:591–598. [Google Scholar]
- Tennie C, Call J, Tomasello M. Ratcheting up the ratchet: on the evolution of cumulative culture. Philosophical Transactions of the Royal Society B. 2009;364:2405–2415. doi: 10.1098/rstb.2009.0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennie C, Call J, Tomasello M. Evidence for emulation in chimpanzees in social settings using the floating peanut task. PLoS ONE. 2010;5:e10544. doi: 10.1371/journal.pone.0010544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton A, Malapert A. The rise and fall of an arbitrary tradition: an experiment with wild meerkats. Proceedings of the Royal Society B. 2009;276:1269–1276. doi: 10.1098/rspb.2008.1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Waal FBM. The Ape and the Sushi-Master: Cultural Reflections of a Primatologist. Cambridge, Massachusetts: Harvard University Press; 2001. [Google Scholar]
- Whiten A, van Schaik CP. The evolution of animal ‘cultures’ and social intelligence. Philosophical Transactions of the Royal Society B. 2007;362:603–620. doi: 10.1098/rstb.2006.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiten A, Goodall J, McGrew WC, Nishida T, Reynolds V, Sugiyama Y, Tutin CEG, Wrangham RW, Boesch C. Cultures in chimpanzees. Nature. 1999;399:682–685. doi: 10.1038/21415. [DOI] [PubMed] [Google Scholar]
- Whiten A, Horner V, de Waal FBM. Conformity to cultural norms of tool use in chimpanzees. Nature. 2005;437:737–740. doi: 10.1038/nature04047. [DOI] [PubMed] [Google Scholar]
- Whiten A, Spiteri A, Horner V, Bonnie KE, Lambeth SP, Schaprio SJ, de Waal FBM. Transmission of multiple experimentally-seeded traditions within and between groups of chimpanzees. Current Biology. 2007;17:1038–1043. doi: 10.1016/j.cub.2007.05.031. [DOI] [PubMed] [Google Scholar]
- Whiten A, McGuigan N, Marshall-Pescini S, Hopper LM. Emulation, imitation, over-imitation and the scope of culture for child and chimpanzee. Philosophical Transactions of the Royal Society B. 2009;364:2417–2428. doi: 10.1098/rstb.2009.0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.