Abstract
Cognitive regulation of emotions is a fundamental prerequisite for intact social functioning which impacts on both well being and psychopathology. The neural underpinnings of this process have been studied intensively in recent years, without, however, a general consensus. We here quantitatively summarize the published literature on cognitive emotion regulation using activation likelihood estimation in fMRI and PET (23 studies/479 subjects). In addition, we assessed the particular functional contribution of identified regions and their interactions using quantitative functional inference and meta-analytic connectivity modeling, respectively. In doing so, we developed a model for the core brain network involved in emotion regulation of emotional reactivity. According to this, the superior temporal gyrus, angular gyrus and (pre) supplementary motor area should be involved in execution of regulation initiated by frontal areas. The dorsolateral prefrontal cortex may be related to regulation of cognitive processes such as attention, while the ventrolateral prefrontal cortex may not necessarily reflect the regulatory process per se, but signals salience and therefore the need to regulate. We also identified a cluster in the anterior middle cingulate cortex as a region, which is anatomically and functionally in an ideal position to influence behavior and subcortical structures related to affect generation. Hence this area may play a central, integrative role in emotion regulation. By focusing on regions commonly active across multiple studies, this proposed model should provide important a priori information for the assessment of dysregulated emotion regulation in psychiatric disorders.
Keywords: Emotion regulation, ALE, DLPFC, VLPFC, aMCC, SMA, STG, Angular gyrus, MACM
Introduction
Humans are social individuals. Social interaction often demands regulation of individual behavior and responses. Thus, we are often (consciously and unconsciously) engaged in emotionally arousing situations that require us to regulate our emotions. Emotions are generally seen as responses generated by the human organism which are essential for adaptation to various challenges and needs (e.g. Levenson, 1994). Nevertheless, we are not simply prey to our emotional tides, but individual assessments of the situation (personal), predilections and general mind sets may shape how we emotionally react to a certain situation (Lazarus, 1991). One of the most influential and widely accepted concepts on how emotions are generated and regulated is often termed appraisal theory, which proposes that our emotional response is mediated by a physiological reaction triggered by internal or external stimuli and lastly shaped by appraisal (e.g. Arnold, 1960; Gross, 1998; Lazarus, 1966; Schachter and Singer, 1962) Emotional experience may thus be separated into different components, which can be modulated by the process of “emotion regulation” (Gross, 2007). Importantly, dysregulation of this process has been argued to lie at the heart of various psychiatric diseases (Gross and Muñoz, 1995). Theoretically, regulation of an emotion can occur during different stages of the generation process (Gross, 1998). Broadly, the stages can be separated into antecedent and response focused emotion regulation strategies. Antecedent focused emotion regulation strategies deal with regulating an emotion before it is fully expressed and thus aim at regulating the emotional experience, while response focused emotion regulation mainly targets the regulation of an emotional response to an already generated emotion (compare Gross, 1998, 2007). Furthermore, two important dimensions of emotion regulation are distinctions between automatic and conscious regulation as well as between functional and dysfunctional regulation strategies. A great variety of emotion regulation strategies have been investigated in the literature. In this paper, we concentrated on antecedent focused, conscious and functional regulation strategies applied in fMRI and PET studies.
In the past decade, an increasing number of neuroimaging studies have focused on neural correlates of emotion regulation (Beauregard et al., 2001; Domes et al., 2010; Ochsner et al., 2002, 2004; Schulze et al., 2011; Urry et al., 2006; van Reekum et al., 2007; Wager et al., 2008). Most studies used (negatively or positively) valenced visual stimuli and compared activation during an “attend” condition to activation during a “regulate” condition (based on Jackson et al., 2000). Wager et al. (2008) explicitly focused on cortical–subcortical interactions to elucidate the regulatory processes underlying successful emotion regulation. The authors applied a Mediation Effect Parametric Mapping (MEPM) approach, which basically reflects a structure equation model to investigate putative neural mediators of successful regulation. They focused on the right ventrolateral prefrontal cortex (VLPFC) which was correlated to regulation success and found that this correlation was mediated by nucleus accumbens and amygdala activity. While nucleus accumbens activity was positively associated with regulation success, amygdala activity showed a negative correlation instead. Additionally, the authors observed middle cingulate cortex (MCC) and pre-supplementary motor area (pre-SMA) activity to be related to regulation success. They conceded that their model must not be interpreted in terms of causality and that it might not fully reflect the complexity of interrelated regulatory processes in the frontal cortices during regulation of an emotion. Nevertheless, this work nicely relates behavioral measures of regulation success to functionality of frontal brain networks and demonstrates that a widespread network of brain areas is engaged in emotion regulation.
In neuroimaging studies on emotion regulation, “reappraisal” has been studied most often and describes the attempt to attribute a new meaning to the affective reaction generated by an arousing stimulus. Reappraisal is most commonly achieved by changing the initial interpretation of an emotional stimulus (e.g. a crying man seen on a picture is not sad, but sheds tears of joy for the return of a loved one; Ochsner et al., 2002). Reappraisal is an antecedent focused, conscious and functional regulation strategy and thus studies applying reappraisal in neuroimaging form a mayor part of this meta-analysis. Previous reviews suggest a key role in these processes for the insula, anterior cingulate and prefrontal regions (e.g., Ochsner and Gross, 2005; Quirk and Beer 2006). These reviews, though informative, are qualitative in nature, drawing inference from the results of relatively few studies. A recent meta-analysis found the ventromedial prefrontal cortex to be critically involved in the regulation of negative affect in different domains (Diekhof et al., 2011). Kalisch (2009) conducted the first quantitative meta-analysis of reappraisal studies and found initial support for the contribution of the above mentioned network. Nevertheless, the relative contribution of the different parts of this network remains largely unclear and leaves scope for different quantitative techniques that examine evidence from a broader spectrum of studies to elucidate which areas of the brain consistently contribute to (conscious and functional) emotion regulation and what functions these areas may serve.
Various tools to perform meta-analysis of brain imaging data were introduced in the last years and opened a way to the quantitative integration of findings across different studies. Activation likelihood estimation (ALE) was first implemented by Turkeltaub et al. (2002) and later integrated in the BrainMap’s framework. The BrainMap project aims at creating tools for data-mining and meta-analysis of the rapidly growing literature on brain mapping (Laird et al., 2009a, 2011). ALE estimates the probability that at least one activation focus from a collection of experiment truly lies at a specific voxels’ location by use of Gaussian assumptions of spatial uncertainty. Multilevel kernel density analysis (MKDA; Wager et al., 2007) is a second prominent approach, which relies on the proportion of reported foci within a certain radius of a voxel. Although a number of differences exist, ALE and MKDA seem to lead to qualitatively similar results (for details see Salimi-Khorshidi et al., 2009).
BrainMap and ALE not only allow retrospective meta-analysis, but can also be used to validate new paradigms or analyze co-occurring networks via MACM (Laird et al., 2009a) and, in this context for formal reverse inference on associated functions via functional decoding (Cieslik et al., 2012; Poldrack, 2011; Rottschy et al., 2012).
The aim of this study is thus (1) to quantitatively summarize the existing neuroimaging literature on cognitive, conscious emotion regulation, (2) to broaden the knowledge on functional connectivity patterns of brain areas reliably engaged in emotion regulation by mapping regions consistently interacting with these using meta-analytic connectivity modeling and (3) to analyze relevant mental processes by using the behavioral domain meta-data of the BrainMap database. Thus, our study should provide new insight into the relative contribution of different parts of the network of brain areas engaged in emotion regulation. This novel methodological approach of quantitative characterization relies on the data of a meta-analysis, providing a stringent, data-driven approach to the robust characterization of brain networks related to emotion regulation.
By this means we hope to contribute to the understanding of functional differentiation of frontal areas involved in the regulation of emotions and also to generate a neural model of conscious, cognitive emotion regulation.
Material and methods
Data for meta-analysis
BrainMap provides a database of systematically classified neuroimaging studies with whole brain coverage. The BrainMap Project is developed at the Research Imaging Institute of the University of Texas Health Science Center San Antonio with the aim to share neuroimaging data and enable meta-analysis of studies on different human brain functions (for details Laird et al., 2009a). At the time of submission, BrainMap consisted of 2336 papers and 45,188 subjects in the functional database. In the context of BrainMap the term “study” usually refers to a publication, which might report several “experiments” denoting the individual contrasts.
Our first step was to search the BrainMap database for studies related to emotion regulation by using the keywords “emotion regulation” and “affect regulation”. These two terms refer to studies in which an emotion is elicited (by a valenced visual stimulus) and in which the participants were asked to regulate their emotional response in at least one experimental condition. The results of our BrainMap search were filtered and experiments that compared an emotion regulation condition (REG) with a low-level baseline (e.g. fixation cross; only 3 experiments) or emotion perception condition were included whereas studies that compared one condition with another regulation condition or did not report results on a comparison with low level baseline or with emotion perception (but, e.g., only comparisons between regulation strategies or between-group differences) were not considered. We then added further studies to our results by searching PubMed and Google Scholar manually based upon the keywords “emotion regulation” “fmri” and “affect regulation” “fmri”. Additional relevant studies were identified by tracing the references in the already acquired papers and review articles. Inclusion criteria for studies were (cf. Chase et al., 2011) full brain coverage, at least one experiment with no pharmacological treatment or any kind of mental or neurological disorder (usually the control groups) and whole brain group analysis in a standard reference space (MNI, Talairach/Tournoux). In addition, only studies which used a strategy to regulate emotions attributably to the antecedent focused phase and aimed to directly influence the appraisal of an emotion by conscious, cognitive means were included (Gross, 2007). Hence, experiments were excluded, if predefined ROIs were investigated and the complete study was dismissed if no whole-brain group analysis was conducted. Studies reported in Talairach space were converted to MNI using the implemented algorithm in GingerALE (Laird et al., 2009a, 2010; Lancaster et al., 2007). This search and the employed inclusion/exclusion criteria led to 23 studies from peer-reviewed journals with 47 experiments, 479 subjects and 505 foci (see Table 1 for details).
Table 1.
All studies entered into the meta analysis are listed, including number of subjects, gender ratio, stimulus material and contrast.
| Authors | Number of subjects |
Gender ratio (f/m) |
Stimulus material | Contrasts | Mean age (SD) |
Analysis software |
|---|---|---|---|---|---|---|
| Campbell-Sills et al. (2011) | 26 | 22/4 | IAPS, negative | Reduce > baseline | 19.15 (1.83) | AFNI |
| Delgado et al. (2008) | 12 | 6/6 | Fear conditioning paradigm with instruction |
Decrease > attend | 23.29 (3.31) | BrainVoyager |
| Domes et al. (2010) | 33 | 17/16 | IAPS, negative | Decrease > maintain | m: 25.2 (1.9) | SPM5 |
| Increase > maintain | f: 24.6 (1.6) | |||||
| Eippert et al. (2007) | 24 | 24/0 | IAPS, neutral and negative (fear) |
Decrease > view Increase > view |
23.3 (range 18–28) |
SPM2 |
| Goldin et al. (2008) | 17 | 17/0 | Disgust-inducing and neutral film clips |
Reappraise > watch negative (early, middle, late) Suppress > watch negative (early, middle, late) |
22.7 (3.5) | AFNI |
| Harenski et Hamann (2006) | 10 | 10/0 | IAPS and popular media, moral vs non-moral, social unpleasant scenes |
Decrease moral > odd-even baseline Decrease non-moral > odd-even baseline Decrease moral > watch moral Decrease non-moral > watch non-moral |
(Range 18–29) | SPM99 |
| Hayes et al. (2010) | 25 | 11/14 | IAPS and in-house pictures, negative |
Reappraise > view Suppress > view |
21.6 (2.5) | FSL |
| Kanske et al. (2011) | 30 | 17/13 | IAPS, neg, pos, neutral, | Reappraise > view emotional (=neg and pos) Reappraise/distract > view |
21.8 (2.1) | SPM5 |
| Kim and Hamann (2007) | 10 | 10/0 | IAPS, negative and positive | Decrease positive > watch positive Decrease negative > watch negative Increase negative > watch negative Increase positive > watch positive |
20.7 (range 18–29) |
SPM99 |
| Kober et al. (2010) | 21 | 9/12 | Pictures of food and cigarettes | Down-regulation > baseline | 26.8 (8.94) | SPM5 |
| Koenigsberg et al. (2009) | 16 | 9/7 | IAPS, negative interpersonal scenes |
Distance > look | 31.8 (7.7) | SPM2 |
| Lang et al. (2012) | 15 | 15/0 | Standardized negative and neutral scripts from the Affective Norms for English Text (ANET) |
Enhance > maintain (early) Enhance > maintain (late) Distance > maintain (early) Distance > maintain (late) |
24.73 (5.64) | SPM8 |
| Mak et al. (2009) | 24 | 12/12 | IAPS and popular media, negative and positive |
Female: reduce negative > view negative Male: reduce negative > view negative Female: reduce positive > view positive male: reduce positive > view positive |
f: 24 (1.78) m: 24 (1.68) |
SPM2 |
| McRae et al. (2008) | 23 | n.a. | IAPS, negative | Decrease > look | f: 20.6 m: 20.36 (range 18–22) |
SPM2 |
| McRae et al. (2010) | 18 | 18/0 | IAPS and in-house set, negative |
Decrease > look masked by distraction > look | 24.4 (3.5) | SPM2 |
| Modinos et al. (2010) | 18 | 7/11 | IAPS, negative | Reappraisal > attend | 21.1 (2.8) | SPM5 |
| Ochsner et al. (2002) | 15 | 15/0 | IAPS, negative | Reappraise > attend/view | 21.9 (range 18–30) |
SPM99 |
| Ochsner et al. (2004) | 23 | 23/0 | IAPS, negative | Increase > look Reappraise > look |
20.6 | SPM99 |
| Phan et al. (2005) | 14 | 8/6 | IAPS, negative | Reappraise > baseline Reappraise > maintain |
27.6 (4.4) | SPM99 |
| Schulze et al. (2011) | 15 | 15/0 | IAPS and similar pictures, negative |
Decrease > maintain Increase > maintain |
24.53 (2.85) | SPM5 |
| Wager et al. (2008) | 30 | 18/12 | IAPS, negative | Reappraise > look | Median 22.3 | SPM2 |
| Walter et al. (2009) | 18 | 18/0 | IAPS, negative and neutral |
Decrease negative > no-regulation neutral Decrease > no-regulation Decrease negative > no-regulation negative Decrease neutral > no-regulation neutral |
24(3) | SPM2 |
| Winecoff et al. (2011) | 42 | n.a. | IAPS, negative and positive | Decrease positive > experience positive Decrease negative > experience negative |
Older: 69 (3.9) younger: 23.1 (4) |
FSL |
ALE methodology
The meta-analysis was conducted using the revised version (Eickhoff et al., 2009) of the activation likelihood estimation algorithm (Laird et al., 2005; Turkeltaub et al., 2002). The algorithm aims at identifying significantly overlapping clusters of activation between studies. ALE treats activation foci from single studies as 3D Gaussian probability distributions to compensate for spatial uncertainty. The width of these distributions was statistically determined based on empirical data for between subject and between template variability (Eickhoff et al., 2009). Additionally, studies were weighted according to sample size, holding the view that large sample sizes more likely reflect a true localization. This is implemented in terms of a widening Gaussian distribution with lower and a smaller Gaussian distribution (and thus a stronger impact on ALE scores) with larger sample sizes (Eickhoff et al., 2009).
Modeled activation maps (MA maps) for each study were generated by combining the probabilities of all activation foci for each voxel (Turkeltaub et al., 2012). The union of these MA maps was calculated to determine a voxel-wise ALE score, which quantifies the convergence between experiments. These ALE scores were then compared to an ALE null distribution (Eickhoff et al., 2012) in which the same number of activation foci was randomly relocated and restricted by a gray matter probability map (Evans et al., 1994). Spatial associations between experiments were treated as random while the distribution of foci within an experiment was treated as fixed. Thereby random effects inference focuses on significant convergence of foci between studies than convergence within one study. The ALE scores from the actual meta-analysis were then tested against the ALE scores obtained under this null-distribution yielding a p-value based on the proportion of equal or higher random values. The resulting non-parametric p-values were then thresholded at a family-wise error (FWE) corrected threshold of p = 0.05 (Eickhoff et al., 2012).
Meta-analytic connectivity modeling (MACM)
We aimed to analyze co-activation patterns of regions engaged in emotion regulation (ER) and conducted MACM analyses on the regions from the ALE meta-analysis to functionally segregate their putative contribution to emotion regulation. Therefore we created six different volumes of interest (VOIs) reflecting the six significant clusters obtained from the meta-analysis. MACM analyses were thus performed for the inferior frontal gyrus (IFG), the dorsolateral prefrontal gyrus (DLPFC) and the anterior middle cingulate gyrus (aMCC), the (pre-) supplementary motor area (SMA), the superior temporal gyrus (STG) and the angular gyrus (AG). Co-activation analysis was performed using the BrainMap database (Laird et al., 2009a, 2011) (www.brainmap.org). The VOI in the SMA/pre-SMA resulted in 686 experiments (9422 subjects, 10,284 foci), in the aMCC in 339 experiments (4505 subjects, 5326 foci), in the IFG in 751 experiments (10,333 subjects, 10,314 foci), in the DLPFC in 154 experiments (2325 subjects, 2430 foci), in the STG in 198 experiments (2859 subjects, 2895 foci) and in the AG in 207 experiments (2813 subjects, 2657 foci).
MACM is based on assessing the brain-wise co-activation patterns of a seed region across a large number of data based neuroimaging results (Eickhoff et al., 2011; Laird et al., 2009b). In practice this entailed an ALE meta-analysis over all foci reported in the experiments retrieved by the search per seed region. In other words, experiments were obtained by filtering the BrainMap database for experiments that activated the particular seed and significant co-activation was delineated by computing an ALE analysis over these to identify regions of significant convergence. Importantly, the experiments underlying the difference in co-activation pattern of the six ER meta-analysis derived clusters may then be described behaviorally, linking them to functional properties.
Functional decoding
The functional characterization of the emotion regulation derived clusters was based on the ‘Behavioral Domain (BD)’ and ‘Paradigm Class (PC)’ meta-data categories available for each neuroimaging experiment included in the BrainMap database. Behavioral domains include the main categories cognition, action, perception, emotion, and interoception, as well as their related sub-categories. Paradigm classes categorize the specific task employed (Turner and Laird, 2011; see http://brainmap.org/scribe/ for the complete BrainMap taxonomy).
As a first step, we determined the individual functional profile of the six emotion regulation derived clusters by using forward and reverse inference approaches. Forward inference is the probability of observing activity in a brain region given knowledge of the psychological process, whereas reverse inference is the probability of a psychological process being present given knowledge of activation in a particular brain region. In the forward inference approach, a cluster’s functional profile was determined by identifying taxonomic labels, for which the probability of finding activation in the respective cluster was significantly higher than the overall chance (across the entire database) of finding activation in that particular cluster. Significance was established using a binomial test (p < .05, corrected for multiple comparisons using Bonferroni’s method; Nickl-Jockschat et al., 2012; Reetz et al., 2012; Rottschy et al., 2012). That is, we tested whether the conditional probability of activation given a particular label [P(Activation|Task)] was higher than the baseline probability of activating the region in question per se [P(Activation)]. In the reverse inference approach, a cluster’s functional profile was determined by identifying the most likely behavioral domains and paradigm classes given activation in a particular cluster. This likelihood P(Task|Activation) can be derived from P(Activation| Task) as well as P(Task) and P(Activation) using Bayes rule. Significance (at p < .05, corrected for multiple comparisons using Bonferroni’s method) was then assessed by means of a chi-square test.
For the anatomical localization the meta-analytic data was referenced to probabilistic cytoarchitectonic maps provided in the SPM Anatomy Toolbox (Eickhoff et al., 2005, 2006, 2007).
Results
Meta-analysis
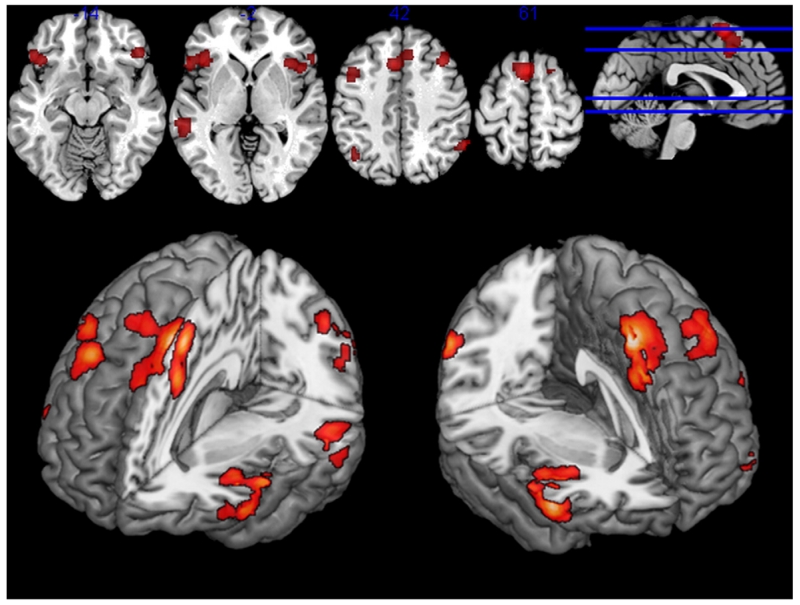
The ALE-meta-analysis across all included studies for emotion regulation (ER) revealed eight clusters of significant activation compared to low-level baseline or emotion perception (compare Table 2 and Fig. 1). ER reliably led to activation in bilateral inferior frontal gyrus (IFG) (right: p. triangularis and orbitalis; BA 44/45; peak MNI: 50/30/−8; 678 voxels, left: p. triangularis, opercularis and orbitalis, BA 44/45; peak MNI: −42/22/−6; 781 voxels), extending into bilateral anterior insula, which we will refer to as VLPFC VOI. One large cluster (1104 voxels) encompassing bilateral supplementary motor area (SMA, BA 6) and pre-SMA (peak MNI: −2/14/58), extending into anterior middle cingulate cortex (aMCC). Furthermore two clusters in the bilateral precentral gyrus (right: 114 voxels, peak MNI: 48/8/48; left: 372 voxels; peak MNI: −44/10/46), extending into bilateral middle frontal gyrus, which we will refer to as DLPFC VOI. Another activation focus was located in left middle temporal cortex (163 voxels; peak MNI: 38/22/44). Additional foci were found in bilateral angular gyri (left: IPC PFm, PGa and PGp; 136 voxels; peak MNI: −42/−60/44 right: PFm and PGa; 109 voxels; peak MNI: 60/−54/40).
Table 2.
Areas of activations resulting from the meta-analysis of emotions regulation, including peak voxel MNI-coordinates, cluster sizes and peak voxel t-values.
| Brain region | Hemisphere | MNI- coordinates |
Cluster size |
Peak voxel T-Value |
|---|---|---|---|---|
| Supplementary motor area/pre- supplementary motor area |
R + L | −2, 14, 58 | 1104 | 7.53 |
| Inferior frontal gyrus | L | −42, 22, −6 | 781 | 6.13 |
| Inferior frontal gyrus | R | 50, 30, −8 | 678 | 6.79 |
| Precentral gyrus | L | 44, 10, 46 | 372 | 5.80 |
| Middle temporal cortex | L | 38, 22, 44 | 163 | 6.01 |
| Angular gyrus | L | −42, −60, 44 | 136 | 4.27 |
| Precentral gyrus | R | 48, 8, 48 | 114 | 5.07 |
| Angular gyrus | R | 60, − 54, 40 | 109 | 6.27 |
Fig 1.
Displayed are significant results from the meta-analysis of emotion regulation (cFWE corrected > .05).
MACM analysis
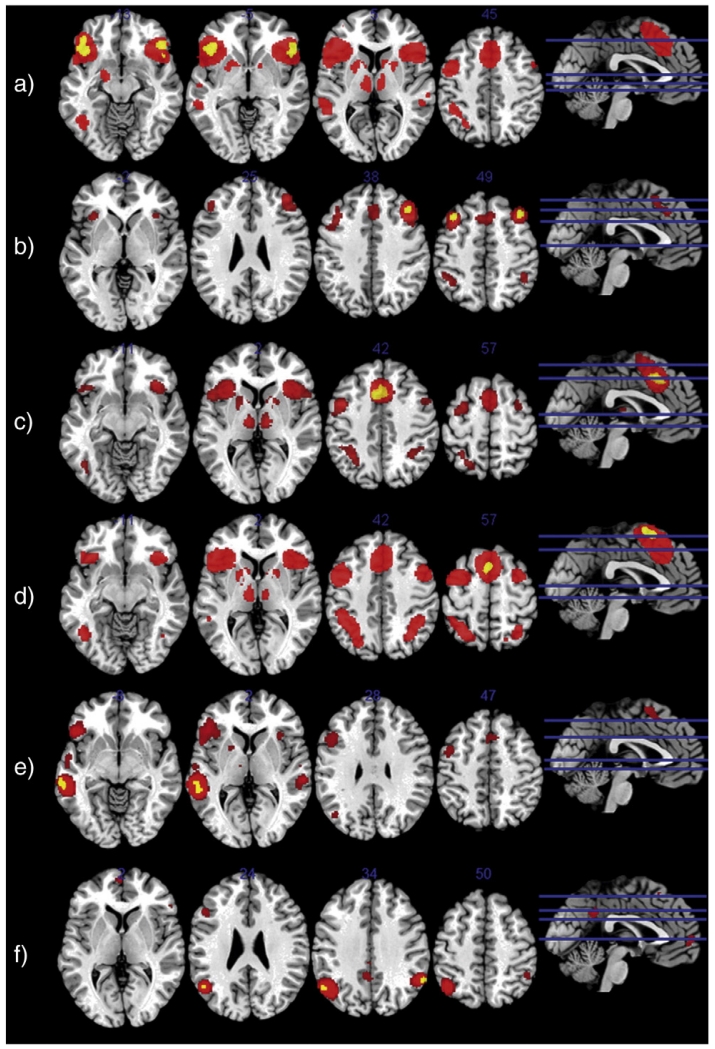
The IFG VOI from the ER meta-analysis showed convergent co-activation for bilateral insula, bilateral middle frontal (BA 45) and bilateral precentral gyrus (BA 44,6), bilateral aMCC and SMA, bilateral superior parietal lobe, bilateral thalamus, bilateral middle temporal gyrus, bilateral putamen, pallidum and caudate nucleus, left fusiform gyrus, left amygdala and left middle frontal gyrus (see Fig. 2a).
Fig. 2.
Depicted are connectivity maps corrected for multiple comparisons (FWE > 0.05) for the IFG (a), the DLPFC (b), the aMCC (c), the (pre-)SMA (d), the STG (e) and the AG (f). Coactivation patterns are labeled red and seed VOI are in yellow.
MACM analysis for the DLPFC VOI from the ER meta-analysis revealed convergent co-activation in aMCC, bilateral angular gyrus, bilateral anterior insula and left middle frontal gyrus (see Fig. 2b).
Co-activation maps for the MCC included bilateral insula, bilateral middle frontal (BA 45) and bilateral precentral gyrus (BA 44,6), bilateral SMA, bilateral superior parietal lobe, bilateral thalamus, bilateral fusiform gyrus, bilateral putamen and pallidum (see Fig. 2c).
Co-activation maps for the SMA were significant for bilateral insula, bilateral middle frontal (BA 45) and bilateral precentral gyrus (BA 44,6), bilateral aMCC, bilateral superior parietal lobe, bilateral thalamus, bilateral fusiform gyrus, bilateral putamen, pallidum and caudate nucleus, bilateral middle frontal gyrus and bilateral inferior temporal gyrus (see Fig. 2d).
For the angular gyrus significant co-activation was observed in bilateral intraparietal cortex and superior parietal lobule, left posterior and middle cingulate gyrus, left IFG (p. triangularis) and left SMA.
Significant co-activation for the superior temporal gyrus was observed in the left IFG (p. triangularis, opercularis and orbitalis) extending into the left precentral gyrus, the left inferior, middle and superior temporal gyrus and temporal pole (TE 1.0, 1.1, 1.2, 3, 4 and OP 4), bilateral SMA, right middle and superior temporal gyrus (TE 3), left intraparietal cortex, right insula, left putamen, left fusiform gyrus, left superior medial gyrus, and left prefrontal thalamus.
Functional characterization
Functional characterization according to the BrainMap meta-data was performed for all ER derived VOIs.
The VOI in the aMCC was associated to BDs related to language, working and explicit memory, as well as action inhibition. PCs most often involved were anti-saccades, word generation, Stroop, go/no-go and n-back tasks.
The SMA was associated to BDs on language, working and explicit memory. PCs recruiting this area were word generation, phonological discrimination, flanker tasks, mental rotation, n-back, Sternberg, Deception, Stroop and arithmetic tasks.
The IFG was strongly associated with language, social cognition, emotion, and action inhibition. PCs were associated with the IFG during deception tasks, word generation, reading, episodic recall, different monitor and discrimination tasks, and PCs related to nutrition and memory recall.
Our DLPFC VOI was significantly recruited in BDs action inhibition, working memory, reasoning, social cognition and cognition in general. Over-represented PCs were n-back, go/no-go and delay discounting tasks, film viewing and Stroop.
The STG VOI from the MA was associated with BDs language (different subcategories), social cognition, perception: audition and PCs on theory of mind, covert reading, semantic monitoring and discrimination, passive listening and phonological discrimination.
The AG VOI was associated with BDs of social cognition and PCs on chewing/swallowing, imagined objects or scenes, and deception tasks.
Discussion
The present study uses a meta-analytic approach to summarize the results of various studies on cognitive emotion regulation in fMRI and PET and found significant convergent activations in several areas previously proposed to play an important role in the regulation of emotional states (McRae et al., 2010; Ochsner and Gross, 2005; Schulze et al., 2011). The results from our meta-analysis point towards the consistent involvement in emotion regulation tasks of frontal areas, in particular the anterior middle cingulate cortex, superior temporal gyrus, angular gyrus and pre-SMA/SMA. Interestingly, all areas in our meta-analysis have also previously been shown to be related to regulation success (Ochsner et al., 2002; Wager et al., 2008). Our results are consistent with recent meta-analyses on the same topic (Buhle et al., 2013; Kalisch, 2009), additionally help clarify the functional contribution of different brain areas engaged in emotion regulation by quantitative means and led to formulation of a neural model of cognitive emotion regulation based on these results.
The DLPFC has often been shown to be involved in working memory and response selection (D’Esposito et al., 2000; Miller and Cohen, 2001), also its role in reward processing has been documented (Haber and Knutson, 2010). It has been proposed to be a central regulatory brain area in emotion processing, in which it regulates an emotion generated by amygdala, insula and VLPFC (Phillips et al., 2003) and has also been proposed to play an important role in emotion regulation (Ochsner and Gross, 2005). The DLPFC VOI in our data shows a widespread co-activation pattern with aMCC, bilateral angular gyrus, bilateral inferior insula and left middle frontal gyrus. The DLPFC VOI is functionally characterized by an overrepresentation in purely cognitive tasks such as working memory, reasoning, social cognition and cognition in general, while it is not strongly associated with emotion processing. The functional characterization demonstrates that while our DLPFC VOI is the result of a meta-analysis of emotion regulation, this area serves a more general role in cognitive control regardless of emotional or non-emotional content. In turn, this notion supports the initial assumption by Ochsner and colleagues that areas involved in “cold” cognition such as attention and memory, might also be involved in the regulation of “hot” emotions (Ochsner and Gross, 2005; Ochsner et al., 2002) and thereby underscores the interaction between emotion and cognition.
Anatomically, the DLPFC is in the position to regulate a very broad range of behavioral reactions, ranging from different motor behaviors (Cieslik et al., 2012), approach and avoidance via its connections to the ventral striatum (Haber, 2009a; Haber and Knutson, 2010). It lacks a direct anatomical connection to the amygdala (Ray and Zald, 2012), therefore it might exert a more indirect control over areas of affect generation by its projections to the pre-SMA and aMCC (Ray and Zald, 2012), which are both involved in emotion regulation and have an association to regulation success (Wager et al., 2008). The exact location of DLPFC activation might be of importance, when trying to determine its role in a specific task, as recent work has shown that the DLPFC can at least be subdivided into an anterior and posterior network with distinct connectivity patterns and functional characteristics (Cieslik et al., 2012).
As already introduced, the VLPFC (in conjunction with the anterior insula) plays a major role in generating and appraising emotion and affect (Ochsner and Gross, 2005; Phillips et al., 2008). Our VLPFC VOI (which extends into the anterior insula) shows a co-activation pattern in IFG, DLPFC, (pre-)SMA, aMCC, ventral striatal areas, amygdala, fusiform gyrus, and middle temporal gyrus (partially covering STG). These areas are involved in emotion processing (Lindquist et al., 2011; Phan et al., 2002; Wager et al., 2003), which is also reflected by our functional characterization. Besides language processes the VLPFC is associated to different kinds of emotion processing, social cognition and also action inhibition in the functional characterization. Anatomically, the VLPFC possesses direct efferent anatomical connections to the amygdala, while afferent projections from the amygdala most likely reach the VLPFC via the anterior insula (Ray and Zald, 2012). It is also anatomically connected to the STG (Ongür et al., 2003), which processes higher order multi-modal integration and also regulates amygdala activity (Müller et al., 2012). The medial prefrontal stream and the orbital prefrontal stream are the major white matter tracts providing interconnectivity between different prefrontal areas. Both of these neural pathways include the VLPFC (Ongür and Price, 2000; Ray and Zald, 2012). Therefore, it might be in an optimal position to integrate computations from various prefrontal areas. It may also relay information between subcortical areas and other prefrontal regions lacking direct connections to these brain areas, such as the frontal pole and dorsolateral prefrontal cortices (Ongür and Price, 2000; Ongür et al., 2003; Ray and Zald, 2012), rendering it in an ideal position to regulate activity in different brain networks. In summary, the VLPFC VOI may play a central part in emotion processing, which is supported by previous meta-analysis and our functional characterization, additionally it seems to play a role in regulatory processes as it is associated to action inhibition, which seems highly plausible given its anatomical position.
The aMCC VOI is functionally associated to memory tasks, language processing and action inhibition. It is not overrepresented in emotion processing in general, which supports the notion that the dorsal ACC may more strongly be associated with cognitive control and therefore reflects the “cognitive division” of the ACC (Bush et al., 2000). The aMCC plays a crucial role in intentional motor control (Hoffstaedter et al., 2012). This is in line with involvement of the aMCC VOI in action inhibition and memory tasks. Nevertheless, several authors see the aMCC as a key area for emotion related behavior. It is strongly involved in monitoring reward response behavior (Haber, 2009b) and punishment avoidance (Vogt, 2009). Thus, the aMCC has been termed a limbic motor control cortex and is involved in regulation of motor output systems for reward approach and punishment avoidance, and also in cognitive processes including selections, which do not require movement and employ the MCC to this end (Vogt, 2009). This notion may integrate our results from the functional characterization with literature on affective involvement of the aMCC as action inhibition, memory tasks and language processing can be seen as components of the functionality of a limbic motor control center described by Vogt (2009). Anatomically, the aMCC receives projections from the parietal cortex and interfaces skeletomotor systems via projections to the spinal cord, striatum, (pre)SMA and other motor cortices, thus the aMCC is in a unique position to control, monitor and reorganize computations in motor structures to produce behavior in response to approach and avoidance reactions (Vogt, 2009), which additionally supports the notion of limbic motor control attributed to the aMCC.
The (pre)SMA VOI is functionally associated with language processing and memory tasks. Because all studies of our meta-analysis focus on reconceptualization (changing the appraisal) we would argue that the (pre)SMA VOI in the meta-analysis reflects the execution of this reconceptualization by reformulating mental representations relying on language and memory processing, possibly also in the sense of embodied cognition (Barsalou, 2008; Niedenthal, 2007; Smith and Semin, 2007). The localization of our (pre)SMA VOI corresponds to the localization of both the anterior and posterior cluster of the SMA identified by functional characterization (Eickhoff et al., 2011), which were associated to cognitive and executive aspects of motor behavior, respectively. The recruitment of both clusters in emotion regulation may reflect crucial involvement of both cognitive and executive aspects and indicate that different subregions of the SMA cluster might be engaged in different stages of emotion regulation.
The AG VOI is associated with social cognition and paradigms in which scenes or objects have to be imagined, as well as deception tasks and chewing or swallowing. The angular gyrus is in general recognized as an associative cortex for semantic processes (Seghier et al., 2011), emotional stimuli (Kohn et al., 2011), episodic memory (Mazoyer et al., 2001; Anticevic et al., 2010; Yang et al., 2010), mental arithmetic (Grabner et al., 2009) and self-relevant internal cognitive processes (Andrews-Hanna et al., 2010). These rather different tasks all share the generation of an internal representation or image crucial for the respective performance, thus the AG’s association with social cognition may be explained by generation of imagined or remembered situations in social cognitive tasks. Anatomically, the AG has projections to the aMCC (Vogt, 2009) and DLPFC (Haber, 2009b), which is in line with the functional characteristics as the aMCC as limbic motor control center and the DLPFC as cognitive control center are related to mental imagery and thus AG functionality.
Our STG VOI is functionally characterized by language processing, which reflects its role as a posterior language and higher order visual processing area (Kandel et al., 2000). It computes higher order multimodal integrative processes and also affects amygdala activity (Müller et al., 2012), which may reflect its association to social cognition. In summary, the STG may play a role in verbalization of social scenes or mental imagery and additionally is in a position to modulate affective arousal via effective connectivity to the amygdala.
Intriguingly, the localization of STG, angular gyrus, (pre)SMA and DLPFC activation overlaps with activations found in a study about planning of everyday tool use (Johnson-Frey et al., 2005). The authors assumed activations in this network to be associated with interaction of semantic and motor representations underlying planning procedures of automatized tool use. In the sense of embodied emotion (Halberstadt et al., 2009; Niedenthal, 2007) thinking about an emotion is already related with bodily simulation of this emotion (Damasio, 1996; Niedenthal, 2007; Niedenthal et al., 2009). Such a bodily simulation of an emotional state can be seen as an analogy to planning procedures in tool use. In both processes a complex set of semantic and motor representations is engaged leading to an automatized motor procedure or an automatized affect regulation procedure. Therefore, the STG, angular gyrus and (pre)SMA might reflect a simulation of somatosensory, motor and possibly language processing with the intent to reach an emotional brain state. To this end, these regions might represent the “executive arm” of “embodied emotion regulation”. The aMCC may function as a mediator of regulation execution. It receives projections from the DLPFC and VLPFC (Ongür et al., 2003; Ray and Zald, 2012) and thus may integrate computations related to the regulatory process, which in turn propagate to the VLPFC (for re-appraisal) and to executive regulatory areas, such as (pre)SMA, STG and angular gyrus, which influence activation in subcortical regions.
Process of emotion regulation
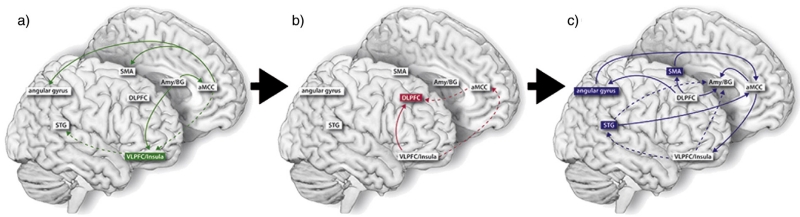
We propose a heuristic working model of conscious, cognitive emotion regulation, which aims to integrate the modal model of emotion regulation (Gross, 1998, 2007) and appraisalist theories of emotion with our findings of different functional characterizations of the areas involved in emotion regulation. This 3-stage model for the regulation of emotion proposes as an initial step the appraisal of an affective stimulus and the arousal generated by this stimulus (A, affective evaluation). Consecutive steps would be “detecting” the need for emotion regulation and the initiation of regulatory processing (B, initiation of regulation), followed by the regulation of affective arousal by generation of a new (regulated) emotional state (C, execution of regulation). The decision which affective state should be regulated can be triggered by various processes such as response tendencies, social norms and personality traits (see Fig. 3 for details and for discussion see Gross, 2007).
Fig. 3.
This heuristic model of neural processing of emotion regulation relates to the modal model of emotion (Gross, 1998). Affective arousal is relayed via amygdala and basal ganglia to the VLPFC and the anterior insula, as well as SMA, angular gyrus and STG (a). The VLPFC initiates the appraisal and signals the need to regulate the emotion to the DLPFC (b). The DLFPC processes the regulation itself and gives a feedforward signal (via the aMCC or directly) to angular gyrus, SMA, STG, amygdala and basal ganglia, which in turn participate in the generation of a (regulated) emotional state (c).
In general, subcortical brain structures, such as the amygdala and the ventral striatum are thought to play a major role in generation of an emotion (Lindquist et al., 2011; Phan et al., 2002; Wager et al., 2003). It can therefore be assumed that these brain areas will be involved in signaling affective arousal or excitation (Wager et al., 2008). We assume that subcortical areas project affective arousal to the VLPFC (via the anterior insula and the aMCC). Although the literature of emotion regulation has focused on the VLPFC as a core regulatory center (Ochsner and Gross, 2005; Phillips et al., 2008; Wager et al., 2008), we would argue that it might more strongly reflect the appraisal phase and the initiation of emotion regulation than the regulation itself as its functional involvement in emotion processing and social cognition as well as its anatomical connectivity indicates that it may represent a core hub of emotion perception and evaluation (A). Additionally, there is evidence that the VLPFC is a detector of salience rather signals when to inhibit and what to feel, than reflects the inhibitory process itself (Hampshire et al., 2010). Indeed, a recent meta-analytic connectivity study of the insula additionally underscored the importance of the anterior insula in detection of saliency (Cauda et al., 2012). In addition, some authors have stressed the relevance of the anterior insula in sustained tasks (Dosenbach et al., 2006, 2007) which also may relate to its “task-active” properties (Nelson et al., 2010).
In the next step (B), the VLPFC may signal the need for regulation via the aMCC and direct anatomical connections to the DLPFC (Ongür and Price, 2000; Ray and Zald, 2012). The anatomical connectivity of the DLPFC and our functional characterization underscore its involvement in “cold” regulatory processes, which may process information from the VLPFC and relay processed information to a brain network involved in (emoto-) motor control. Evidence for such a processing mode of the DLPFC comes from cytoarchitectonic features of the DLFPC. Barbas (2000) argued that the differential laminar patterns of the prefrontal cortex enable a distinction of functionality. She assumes that eulaminate, lateral prefrontal cortices (mainly Brodmann Area 46 and 8, which strongly overlap to the portion of the DLPFC observed in our meta-analysis) have a laminar connectivity pattern that implies a feed-forward function, while the laminar connectivity pattern of the orbitofrontal cortex and VLPFC implies a stronger involvement in feed-back functionality.
By simulating motor, somatosensory and language processes via imagination of scenes and congruent verbalization, pre-motor areas, angular gyrus and STG may initiate a reenacting of an emotional scene (Johnson-Frey et al., 2005; Niedenthal, 2007), which represents the execution of regulation (C). This may in turn influence activity in the ventral striatum and amygdala either directly or via the aMCC.
The VLPFC will in a final stage most probably be involved in a reappraisal of affective activity from subcortical regions. This dual role of the VLPFC in emotion regulation may be reflected in positive amygdala-VLPFC associations which negatively predict regulation success and positive VS-VLPFC associations which positively predict regulation success (Wager et al., 2008). The authors also point towards the dual role of the VLPFC in generation and regulation of emotions.
Nevertheless, our data does not support strong conclusion on the pathway of emotion regulation, but offers some interesting insights, which might guide future research as a heuristic working model.
Our line of argument implicitly assumes regulation of emotional reactivity to be mediated by an influence of (cortical) networks on subcortical affect generation structures. This view has been termed mediation hypothesis (Wager et al., 2008). Another perspective is the direct pathway hypothesis which assumes that regulation of affect is related to a change of appraisal in prefrontal areas and only slightly affects subcortical, affective regions (Wager et al., 2008). Our study is not designed to test these alternative hypotheses, but one can infer from our analyses and the literature, that a highly plausible candidate structure in the direct pathway model would be the VLPFC as it detects salience, is a core structure for appraisal processes (Hampshire et al., 2010; Wager et al., 2008) and would also be in a good position to initiate higher order cognitive processes or complex behaviors through its involvement in the medial prefrontal network (Ongür and Price, 2000).
A potential limitation of our results stems from the fact, that five studies included in our meta-analysis did not report information on the mental health status of their participants and the methods used for assessment of mental health. Rather they only stated that subjects were “normal”. Nevertheless, comparing activation patterns of the sample with and without these studies did not change the general pattern. Hence, we would argue that influence if present at all, is rather negligible.
Our data does not provide information on the causal relationship within this network. Nevertheless, our meta-analysis might provide a reliable map of anatomical regions for testing causal implications with suited models (such as DCM; Friston et al., 2003).
Results from our meta-analysis, MACM and functional decoding may aid in investigating psychiatric disease. The functional decoding and our model of emotion regulation allow the assessment of the potential nature dysfunctions in emotion regulation and their neural circuits in psychiatric disorders. Erk et al. (2010) found an association of DLPFC and IPC to amygdala decrease in healthy controls, which was identified as a measure of regulation success. In depressed patients the DLPFC did not show such an association, which according to our model may relate to global cognitive deficits in the patient population, rather than deficits in emotion regulation per se. Similarly, the finding of reduced ACC activation in Borderline patients (Koenigsberg et al., 2009) would according to our model be explainable in terms of disconnection of important relay centers connecting salience detection, assessment/judgments and execution. We would argue, that the current findings on brain areas robustly engaged in emotion regulation may provide important a priori information for the hypothesis-driven investigation of emotion-regulatory circuits in clinical populations.
Conclusion
We here identified core regions of the brain network involved in regulation of emotional reactivity and provide information on the relative contribution of these brain areas to the stages of the regulatory process. The DLPFC might be related to higher order “cold” regulatory processes; based on its anatomical connections it is not in an ideal position to directly regulate emotions and therefore might have a more indirect, initiatory influence. Contrary to models of emotion regulation, the VLPFC may not necessarily reflect the regulatory process per se, but signals salience and therefore the need to regulate. We identified a cluster in the aMCC as a region which is anatomically and functionally in an ideal position to influence behavior and subcortical structures related to affect generation and therefore this area may play a central, integratory role in the regulation of emotional reactivity. Additionally, we propose putative roles of non-frontal brain areas in emotion regulation.
Based on our results we propose a 3-stage neural network model of emotion regulation, consisting of emotion evaluation, initiation of regulation and execution of regulation. Emotion evaluation is associated with the VLPFC as core hub, initiation of regulation with the DLPFC. The STG, angular gyrus and (pre)SMA are involved in execution of regulation initiated by the DLPFC, while the aMCC may serve as a mediator for integrating these steps.
Acknowledgments
This work was supported by the Faculty of Medicine, RWTH Aachen University (START program 138/09) and by the German Research Foundation (DFG, IRTG 1328, International Research Training Group). UH is supported by a grant from the IZKF Aachen (Interdisciplinary Center for Clinical Research within the faculty of Medicine at the RWTH Aachen University, N4-4). This work was also supported by the National Institute of Mental Health (R01-MH074457; PTF, ARL, SBE) and the Helmholtz Alliance on Systems Biology (Human Brain Model; SBE).
References
- Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. Functional-anatomic fractionation of the brain’s default network. Neuron. 2010;65:550–562. doi: 10.1016/j.neuron.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A, Repovs G, Shulman GL, Barch DM. When less is more: TPJ and default network deactivation during encoding predicts working memory performance. Neuroimage. 2010;49:2638–2648. doi: 10.1016/j.neuroimage.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold M. Emotion and Personality. Columbia University Press; New York, NY, US: 1960. [Google Scholar]
- Barbas H. Proceedings of the Human Cerebral Cortex: From Gene to Structure and Function Connections Underlying the Synthesis of Cognition, Memory, and Emotion in Primate Prefrontal Cortices. 2000;52:319–330. doi: 10.1016/s0361-9230(99)00245-2. [DOI] [PubMed] [Google Scholar]
- Barsalou LW. Grounded cognition. Ann. Rev. Psychol. 2008;59:617–645. doi: 10.1146/annurev.psych.59.103006.093639. [DOI] [PubMed] [Google Scholar]
- Beauregard M, Levesque J, Bourgouin P. Neural correlates of conscious self-regulation of emotion. J. Neurosci. 2001;21:RC165. doi: 10.1523/JNEUROSCI.21-18-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhle JT, Silvers JA, Wager TD, Lopez R, Onyemekwu C, Kober H, Weber J, Ochsner KN. Cognitive reappraisal of emotion: a meta-analysis of human neuroimaging studies. Cereb. Cortex. 2013 doi: 10.1093/cercor/bht154. bht154–. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner M. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn. Sci. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Campbell-Sills L, Simmons AN, Lovero KL, Rochlin A, Paulus MP, Stein MB. Functioning of neural systems supporting emotion regulation in anxiety-prone individuals. Neuroimage. 2011;54:689–696. doi: 10.1016/j.neuroimage.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauda F, Costa T, Torta DME, Sacco K, D’Agata F, Duca S, Geminiani G, Fox PT, Vercelli A. Meta-analytic clustering of the insular cortex: characterizing the meta-analytic connectivity of the insula when involved in active tasks. Neuroimage. 2012;62:343–355. doi: 10.1016/j.neuroimage.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase HW, Eickhoff SB, Laird AR, Hogarth L. The neural basis of drug stimulus processing and craving: an activation likelihood estimation meta-analysis. Biol. Psychiatry. 2011;70:785–793. doi: 10.1016/j.biopsych.2011.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cieslik EC, Zilles K, Caspers S, Roski C, Kellermann TS, Jakobs O, Langner R, Laird AR, Fox PT, Eickhoff SB. Is there “one” DLPFC in cognitive action control? Evidence for heterogeneity from co-activation-based parcellation. Cereb. Cortex. 2012;22:2677–2689. doi: 10.1093/cercor/bhs256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Esposito M, Postle BR, Rypma B. Prefrontal cortical contributions to working memory: evidence from event-related fMRI studies. Exp. Brain Res. 2000;133:3–11. doi: 10.1007/s002210000395. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Nearing KI, Ledoux JE, Phelps E. Neural circuitry underlying the regulation of conditioned fear and its relation to extinction. Neuron. 2008;59:829–838. doi: 10.1016/j.neuron.2008.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasio AR. The somatic marker hypothesis and the possible functions of the prefrontal cortex. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1996;351:1413–1420. doi: 10.1098/rstb.1996.0125. [DOI] [PubMed] [Google Scholar]
- Diekhof EK, Geier K, Falkai P, Gruber O. Fear is only as deep as the mind allows: a coordinate-based meta-analysis of neuroimaging studies on the regulation of negative affect. Neuroimage. 2011;58:275–285. doi: 10.1016/j.neuroimage.2011.05.073. [DOI] [PubMed] [Google Scholar]
- Domes G, Schulze L, Böttger M, Grossmann A, Hauenstein K, Wirtz PH, Heinrichs M, Herpertz SC. The Neural Correlates Of Sex Differences In Emotional Reactivity And Emotion Regulation. Hum. Brain Mapp. 2010;31:758–769. doi: 10.1002/hbm.20903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NUF, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC, Burgund ED, Grimes AL, Schlaggar BL, Petersen SE. A core system for the implementation of task sets. Neuron. 2006;50:799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NUF, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RAT, Fox MD, Snyder AZ, Vincent JL, Raichle ME, Schlaggar BL, Petersen SE. Distinct brain networks for adaptive and stable task control in humans. Proc. Natl. Acad. Sci. U. S. A. 2007;104:11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 2005;25:1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Heim S, Zilles K, Amunts K. Testing anatomically specified hypotheses in functional imaging using cytoarchitectonic maps. Neuroimage. 2006;32:570–582. doi: 10.1016/j.neuroimage.2006.04.204. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Paus T, Caspers S, Grosbras M-H, Evans AC, Zilles K, Amunts K. Assignment of functional activations to probabilistic cytoarchitectonic areas revisited. Neuroimage. 2007;36:511–521. doi: 10.1016/j.neuroimage.2007.03.060. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Hum. Brain Mapp. 2009;30:2907–2926. doi: 10.1002/hbm.20718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Bzdok D, Laird AR, Roski C, Caspers S, Zilles K, Fox PT. Co-activation patterns distinguish cortical modules, their connectivity and functional differentiation. Neuroimage. 2011;57:938–949. doi: 10.1016/j.neuroimage.2011.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Bzdok D, Laird AR, Kurth F, Fox PT. Activation likelihood estimation meta-analysis revisited. Neuroimage. 2012;59:2349–2361. doi: 10.1016/j.neuroimage.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eippert F, et al. Regulation of emotional responses elicited by threat-related stimuli. Hum. Brain Mapp. 2007;28:409–423. doi: 10.1002/hbm.20291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erk S, Mikschl A, Stier S, Ciaramidaro A, Gapp V, Weber B, Walter H. Acute and sustained effects of cognitive emotion regulation in major depression. J. Neurosci. 2010;30:15726–15734. doi: 10.1523/JNEUROSCI.1856-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans A, Kamber M, Collins D. An MRI-based probabilistic atlas of neuroanatomy. NATO ASI Series A Life. 1994 [Google Scholar]
- Friston KJ, Harrison L, Penny W. Dynamic causal modelling. Neuroimage. 2003;19:1273–1302. doi: 10.1016/s1053-8119(03)00202-7. [DOI] [PubMed] [Google Scholar]
- Goldin PR, McRae K, Ramel W, Gross JJ. The neural bases of emotion regulation: reappraisal and suppression of negative emotion. Biol. Psychiatry. 2008;63:577–586. doi: 10.1016/j.biopsych.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabner RH, Ansari D, Koschutnig K, Reishofer G, Ebner F, Neuper C. To retrieve or to calculate? Left angular gyrus mediates the retrieval of arithmetic facts during problem solving. Neuropsychologia. 2009;47:604–608. doi: 10.1016/j.neuropsychologia.2008.10.013. [DOI] [PubMed] [Google Scholar]
- Gross JJ. The emerging field of emotion regulation: an integrative review. Rev. Gen. Psychol. 1998;2:271. [Google Scholar]
- Gross JJ. Emotion regulation: conceptual foundations. Handbook of Emotion Regulation. 2007;3:3–24. [Google Scholar]
- Gross JJ, Muñoz RF. Emotion regulation and mental health. Clin. Psychol. Sci. Pract. 1995;2:151–164. [Google Scholar]
- Haber SN. Connectivity of Primate Reward Centers. In: Neuroscience E-CLRSBT-E of, editor. Academic Press; Oxford: 2009a. pp. 91–98. [Google Scholar]
- Haber SN. Chapter 1 — anatomy and connectivity of the reward circuit. In: Dreher J-C, Tremblay L, editors. H of R and DM. Academic Press; New York: 2009b. pp. 1–27. [Google Scholar]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt J, Winkielmann P, Niedenthal P, Dalle J. Emotional conception how embodied emotion concepts guide perception and facial action. Psychol. Sci. 2009;20:1254–1261. doi: 10.1111/j.1467-9280.2009.02432.x. [DOI] [PubMed] [Google Scholar]
- Hampshire A, Chamberlain SR, Monti MM, Duncan J, Owen AM. The role of the right inferior frontal gyrus: inhibition and attentional control. Neuroimage. 2010;50:1313–1319. doi: 10.1016/j.neuroimage.2009.12.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harenski CL, Hamann S. Neural correlates of regulating negative emotions related to moral violations. Neuroimage. 2006;30:313–324. doi: 10.1016/j.neuroimage.2005.09.034. [DOI] [PubMed] [Google Scholar]
- Hayes JP, et al. Staying cool when things get hot: emotion regulation modulates neural mechanisms of memory encoding. Front. Hum. Neurosci. 2010;4:230. doi: 10.3389/fnhum.2010.00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffstaedter F, Grefkes C, Zilles K, Eickhoff SB. The “what” and “when” of self-initiated movements. Cereb. Cortex. 2012:1–11. doi: 10.1093/cercor/bhr391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson DC, Malmstadt JR, Larson CL, Davidson RJ. Suppression and enhancement of emotional responses to unpleasant pictures. Psychophysiology. 2000;37:515–522. [PubMed] [Google Scholar]
- Johnson-Frey S, Newman-Norlund R, Grafton S. A distributed left hemisphere network active during planning of everyday tool use skills. Cereb. Cortex. 2005;15:681–695. doi: 10.1093/cercor/bhh169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalisch R. The functional neuroanatomy of reappraisal: time matters. Neurosci. Biobehav. Rev. 2009;33:1215–1226. doi: 10.1016/j.neubiorev.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Kandel E, Schwartz J, Jessell T. Principles of Neural Science. 4th Mcgraw-Hill Professional; 2000. [Google Scholar]
- Kanske P, Heissler J, Schönfelder S, Bongers A, Wessa M. How to regulate emotion? Neural networks for reappraisal and distraction. Cereb. Cortex. 2011;21:1379–1388. doi: 10.1093/cercor/bhq216. [DOI] [PubMed] [Google Scholar]
- Kober H, et al. Prefrontal-striatal pathway underlies cognitive regulation of craving. Proc. Natl. Acad. Sci. U. S. A. 2010;107:14811–14816. doi: 10.1073/pnas.1007779107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Hamann S. Neural correlates of positive and negative emotion regulation. J. Cogn. Neurosci. 2007;19:776–798. doi: 10.1162/jocn.2007.19.5.776. [DOI] [PubMed] [Google Scholar]
- Koenigsberg HW, Fan J, Ochsner KN, Liu X, Guise KG, Pizzarello S, Dorantes C, Guerreri S, Tecuta L, Goodman M, New A, Siever LJ. Neural correlates of the use of psychological distancing to regulate responses to negative social cues: a study of patients with borderline personality disorder. Biol. Psychiatry. 2009;66:854–863. doi: 10.1016/j.biopsych.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn N, Kellermann T, Gur RC, Schneider F, Habel U. Gender differences in the neural correlates of humor processing: implications for different processing modes. Neuropsychologia. 2011;49:888–897. doi: 10.1016/j.neuropsychologia.2011.02.010. [DOI] [PubMed] [Google Scholar]
- Laird AR, Fox PM, Price CJ, Glahn DC, Uecker AM, Lancaster JL, Turkeltaub PE, Kochunov P, Fox PT. ALE meta-analysis: controlling the false discovery rate and performing statistical contrasts. Hum. Brain Mapp. 2005;25:155–164. doi: 10.1002/hbm.20136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, Eickhoff SB, Kurth F, Fox PM, Uecker AM, Turner JA, Robinson JL, Lancaster JL, Fox PT. ALE meta-analysis workflows via the brainmap database: progress towards a probabilistic functional brain atlas. Front. Neuroinformatics. 2009a;3:23. doi: 10.3389/neuro.11.023.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, Eickhoff SB, Li K, Robin DA, Glahn DC, Fox PT. Investigating the functional heterogeneity of the default mode network using coordinate-based meta-analytic modeling. J. Neurosci. 2009b;29:14496–14505. doi: 10.1523/JNEUROSCI.4004-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, Robinson JL, McMillan KM, Tordesillas-Gutiérrez D, Moran ST, Gonzales SM, Ray KL, Franklin C, Glahn DC, Fox PT, Lancaster JL. Comparison of the disparity between Talairach and MNI coordinates in functional neuroimaging data: validation of the Lancaster transform. Neuroimage. 2010;51:677–683. doi: 10.1016/j.neuroimage.2010.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, Eickhoff SB, Fox PM, Uecker AM, Ray KL, Saenz JJ, McKay DR, Bzdok D, Laird RW, Robinson JL, Turner JA, Turkeltaub PE, Lancaster JL, Fox PT. The BrainMap strategy for standardization, sharing, and meta-analysis of neuroimaging data. BMC Res. Notes. 2011;4:349. doi: 10.1186/1756-0500-4-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Tordesillas-Gutiérrez D, Martinez M, Salinas F, Evans A, Zilles K, Mazziotta JC, Fox PT. Bias between MNI and Talairach coordinates analyzed using the ICBM-152 brain template. Hum. Brain Mapp. 2007;28:1194–1205. doi: 10.1002/hbm.20345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang S, et al. Cognitive reappraisal in trauma-exposed women with borderline personality disorder. Neuroimage. 2012;59:1727–1734. doi: 10.1016/j.neuroimage.2011.08.061. [DOI] [PubMed] [Google Scholar]
- Lazarus RS. Psychological Stress and the Coping Process. McGraw-Hill; New York, NY, US: 1966. [Google Scholar]
- Lazarus RS. Emotion and Adaptation. Oxford University Press; New York and Oxford: 1991. [Google Scholar]
- Levenson RW. Human emotions: a functional view. In: Ekman P, Davidson RJ, editors. The Nature of Emotion: Fundamental Questions. Oxford University Press; New York: 1994. pp. 123–126. [Google Scholar]
- Lindquist K, Wager T, Kober H, Bliss-Moreau E, Barrett L. The brain basis of emotion: a meta-analytic review. Behav. Brain Sci. 2011:1–121. doi: 10.1017/S0140525X11000446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak AKY, Hu Z, Zhang JXX, Xiao Z, Lee TMC. Sex-related differences in neural activity during emotion regulation. Neuropsychologia. 2009;47:2900–2908. doi: 10.1016/j.neuropsychologia.2009.06.017. [DOI] [PubMed] [Google Scholar]
- Mazoyer B, Zago L, Mellet E, Bricogne S, Etard O, Houdeé O, Crivello F, Joliot M, Petit L, Tzourio-Mazoyer N. Cortical networks for working memory and executive functions sustain the conscious resting state in man. Brain Res. Bull. 2001;54:287–298. doi: 10.1016/s0361-9230(00)00437-8. [DOI] [PubMed] [Google Scholar]
- McRae K, Ochsner KN, Mauss IB, Gabrieli JJD, Gross JJ. Gender differences in emotion regulation: an fMRI study of cognitive reappraisal. 2008 doi: 10.1177/1368430207088035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae K, Hughes B, Chopra S, Gabrieli JDE, Gross JJ, Ochsner KN. The neural bases of distraction and reappraisal. J. Cogn. Neurosci. 2010;22:248–262. doi: 10.1162/jocn.2009.21243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu. Rev. Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Modinos G, Ormel J, Aleman A. Individual differences in dispositional mindfulness and brain activity involved in reappraisal of emotion. Soc. Cogn. Affect Neurosci. 2010;5:369–377. doi: 10.1093/scan/nsq006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller VI, Cieslik EC, Turetsky BI, Eickhoff SB. Crossmodal interactions in audiovisual emotion processing. Neuroimage. 2012;60:553–561. doi: 10.1016/j.neuroimage.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson SM, Dosenbach NUF, Cohen AL, Wheeler ME, Schlaggar BL, Petersen SE. Role of the anterior insula in task-level control and focal attention. Brain Struct. Funct. 2010;214:669–680. doi: 10.1007/s00429-010-0260-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickl-Jockschat T, Habel U, Michel TM, Manning J, Laird AR, Fox PT, Schneider F, Eickhoff SB. Brain structure anomalies in autism spectrum disorder—a meta-analysis of VBM studies using anatomic likelihood estimation. Hum. Brain Mapp. 2012;33:1470–1489. doi: 10.1002/hbm.21299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedenthal PM. Embodying emotion. Science. 2007;316:1002–1005. doi: 10.1126/science.1136930. [DOI] [PubMed] [Google Scholar]
- Niedenthal PM, Winkielman P, Mondillon L, Vermeulen N. Embodiment of emotion concepts. J. Pers. Soc. Psychol. 2009;96:1120–1136. doi: 10.1037/a0015574. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn. Sci. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JDE. Rethinking feelings: an FMRI study of the cognitive regulation of emotion. J. Cogn. Neurosci. 2002;14:1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Knierim K, Ludlow DH, Hanelin J, Ramachandran T, Glover G, Mackey SC. Reflecting upon feelings: an fMRI study of neural systems supporting the attribution of emotion to self and other. J. Cogn. Neurosci. 2004;16:1746–1772. doi: 10.1162/0898929042947829. [DOI] [PubMed] [Google Scholar]
- Ongür D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb. Cortex. 2000;10:206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- Ongür D, Ferry AT, Price JL. Architectonic subdivision of the human orbital and medial prefrontal cortex. J. Comp. Neurol. 2003;460:425–449. doi: 10.1002/cne.10609. [DOI] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage. 2002;16:331–348. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Phan KL, et al. Neural substrates for voluntary suppression of negative affect: a functional magnetic resonance imaging study. Biol. Psychiatry. 2005;57:210–219. doi: 10.1016/j.biopsych.2004.10.030. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception I: the neural basis of normal emotion perception. Biol. Psychiatry. 2003;54:504–514. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Ladouceur CD, Drevets WC. A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Mol. Psychiatry. 2008;13 doi: 10.1038/mp.2008.65. 829,833–829,857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack RA. Inferring mental states from neuroimaging data: from reverse inference to large-scale decoding. Neuron. 2011;72:692–697. doi: 10.1016/j.neuron.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Beer JS. Prefrontal involvement in the regulation of emotion: convergence of rat and human studies. Curr. Opin. Neurobiol. 2006;16:723–727. doi: 10.1016/j.conb.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Ray RD, Zald DH. Anatomical insights into the interaction of emotion and cognition in the prefrontal cortex. Neurosci. Biobehav. Rev. 2012;36:479–501. doi: 10.1016/j.neubiorev.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reetz K, Dogan I, Rolfs A, Binkofski F, Schulz JB, Laird AR, Fox PT, Eickhoff SB. Investigating function and connectivity of morphometric findings-exemplified on cerebellar atrophy in spinocerebellar ataxia 17 (SCA17) Neuroimage. 2012;62:1354–1366. doi: 10.1016/j.neuroimage.2012.05.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottschy C, Caspers S, Roski C, Reetz K, Dogan I, Schulz JB, Zilles K, Laird AR, Fox PT, Eickhoff SB. Differentiated parietal connectivity of frontal regions for “what” and “where” memory. Brain Struct. Funct. 2012:1–17. doi: 10.1007/s00429-012-0476-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salimi-Khorshidi G, Smith SM, Keltner JR, Wager TD, Nichols TE. Meta-analysis of neuroimaging data: a comparison of image-based and coordinate-based pooling of studies. Neuroimage. 2009;45:810–823. doi: 10.1016/j.neuroimage.2008.12.039. [DOI] [PubMed] [Google Scholar]
- Schachter S, Singer J. Cognitive, social, and physiological determinants of emotional state. Psychol. Rev. 1962;69:379–399. doi: 10.1037/h0046234. [DOI] [PubMed] [Google Scholar]
- Schulze L, Domes G, Krüger A, Berger C, Fleischer M, Prehn K, Schmahl C, Grossmann A, Hauenstein K, Herpertz SC. Neuronal correlates of cognitive reappraisal in borderline patients with affective instability. Biol. Psychiatry. 2011;69:564–573. doi: 10.1016/j.biopsych.2010.10.025. [DOI] [PubMed] [Google Scholar]
- Seghier ML, Fagan E, Price CJ. UKPMC funders group author manuscript functional subdivisions in the left angular gyrus where the semantic system meets and diverges from the default network. J. Neurosci. 2011;30:16809–16817. doi: 10.1523/JNEUROSCI.3377-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ER, Semin GR. Situated social cognition. Curr. Dir. Psychol. Sci. 2007;16:132–135. [Google Scholar]
- Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA. Meta-analysis of the functional neuroanatomy of single-word reading: method and validation. Neuroimage. 2002;16:765–780. doi: 10.1006/nimg.2002.1131. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, et al. Minimizing within-experiment and within-group effects in Activation Likelihood Estimation meta-analyses. Hum. Brain Mapp. 2012;33:1–13. doi: 10.1002/hbm.21186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JA, Laird AR. The cognitive paradigm ontology: design and application. Neuroinformatics. 2011;10:57–66. doi: 10.1007/s12021-011-9126-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urry HL, van Reekum CM, Johnstone T, Kalin NH, Thurow ME, Schaefer HS, Jackson CA, Frye CJ, Greischar LL, Alexander AL, Davidson RJ. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. J. Neurosci. 2006;26:4415–4425. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Reekum CM, Johnstone T, Urry HL, Thurow ME, Schaefer HS, Alexander AL, Davidson RJ. Gaze fixations predict brain activation during the voluntary regulation of picture-induced negative affect. Neuroimage. 2007;36:1041–1055. doi: 10.1016/j.neuroimage.2007.03.052. [DOI] [PubMed] [Google Scholar]
- Vogt BA. Regions and subregions of the cingulate gyrus. In: Vogt BA, editor. Cingulate Neurobiology and Disease. Oxford University Press; Oxford, England: 2009. pp. 3–30. [Google Scholar]
- Wager TD, Phan KL, Liberzon I, Taylor SF. Valence, gender, and lateralization of functional brain anatomy in emotion: a meta-analysis of findings from neuroimaging. Neuroimage. 2003;19:513–531. doi: 10.1016/s1053-8119(03)00078-8. [DOI] [PubMed] [Google Scholar]
- Wager TD, Lindquist M, Kaplan L. Meta-analysis of functional neuroimaging data: current and future directions. Soc. Cogn. Affect. Neurosci. 2007;2:150–158. doi: 10.1093/scan/nsm015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN. Prefrontal– subcortical pathways mediating successful emotion regulation. Neuron. 2008;59:1037–1050. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter H, et al. The temporal dynamics of voluntary emotion regulation. PLoS One. 2009;4:e6726. doi: 10.1371/journal.pone.0006726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winecoff A, Labar KS, Madden DJ, Cabeza R, Huettel S. Cognitive and neural contributors to emotion regulation in aging. Soc. Cogn. Affect Neurosci. 2011;6:165–176. doi: 10.1093/scan/nsq030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Weng X, Zang Y, Xu M, Xu X. Sustained activity within the default mode network during an implicit memory task. Cortex. 2010;46:354–366. doi: 10.1016/j.cortex.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]