Abstract
Renal artery stenosis (RAS) most commonly due to atherosclerosis, with fibromuscular dysplasia (FMD) the most frequent among other less common etiologies. A high index of suspicion based upon clinical features is essential for diagnosis. Revascularization strategies are currently a topic of discussion and debate. When revascularization is deemed appropriate atherosclerotic RAS is most often treated with stent placement while patients with fibromuscular dysplasia are usually treated with balloon angioplasty. Ongoing randomized trials should help to better define the optimal management of RAS.
Keywords: Renal artery stenosis, stent, Peripheral vascular disease
Introduction
Renal artery stenosis (RAS) is somewhat unusual as a vascular disorder although renal artery ischemia can present as one of several overlapping clinical syndromes. Although several disease states can cause renal artery stenosis (Table 1), the vast majority of patients are affected by atherosclerotic lesions with fibromuscular dysplasia a distant second. Atherosclerosis typically occurs in older individuals, may present with hypertension or renal insufficiency, and has an equal prevalence in men and women. In contrast fibromuscular dysplasia is more often seen in the young, in women, and is usually associated with hypertension without renal insufficiency.(1)
Table 1.
Causes of main renal artery stenosis.
| Fibromuscular dysplasia |
|---|
| Atherosclerosis |
| Traumatic thrombosis or avulsion: usually due to blunt trauma |
| Non-traumatic thrombosis: hypercoagulable states |
| Thromboembolism |
| Renal or aortic dissection |
| Renal artery aneurysm |
| Congenital |
| William’s syndrome |
| Takayasu’s arteritis |
Diagnosis
With an understanding of the etiologic possibilities, consideration of the diagnosis of RAS should incorporate an understanding of the likely etiology (Table 2). One example would be a young (< 35 year old) woman who presents with resistant hypertension and normal renal function. It is reasonable to proceed with a non-invasive screening study such as Duplex ultrasound to rule out renovascular disease. A second example would be an elderly gentleman with known atherosclerotic coronary artery disease who has difficult to control hypertension and mild chronic kidney disease. He should be evaluated for atherosclerotic renal artery stenosis with a non-invasive screening test.
Table 2.
Increase risk of renal artery stenosis
|
Screening for Atherosclerotic Renal Artery Stenosis
A limited literature addresses the clinical factors that are predictive of finding atherosclerotic RAS and that may be useful in guiding appropriate screening. One of the early efforts, by Krijnen and coworkers in 1998 described clinical characteristics that were predictive of a RAS with imaging.(2) Importantly, in a follow-up publication in 2005 the results from the earlier work were validated. In brief, the authors demonstrated that older age, smoking history, and an elevated serum creatinine were significant predictors of atherosclerotic RAS in both the development and validation samples.(3)
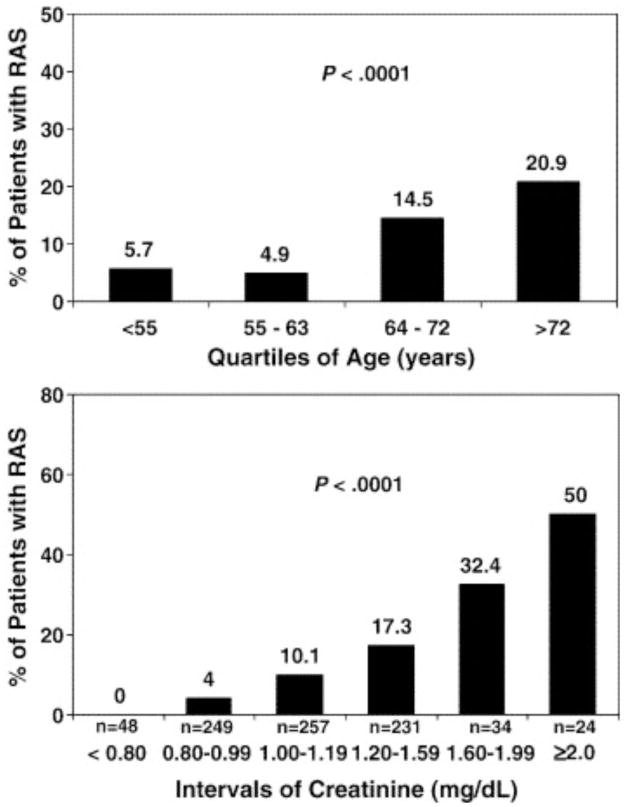
Several other investigators have evaluated the utility of abdominal angiography at the time of cardiac catheterization, specifically focusing on the “risk factors” that are predictive of finding angiographically significant RAS. Cohen et. al. demonstrated that elevated serum creatinine, advanced age, peripheral vascular disease, multiplicity of cardiovascular medications, hypertension, female gender and multivessel coronary artery disease were important (Figure 1).(4) Others have described factors including multivessel coronary disease, diabetes, carotid disease, abdominal bruit, pulmonary edema, and advanced age as factors.(5) Several have created risk scores to predict which patients are likely to benefit from screening.(4,5)
Figure 1.
Relationship of age and creatinine with the prevalence of significant RAS.
From Cohen et al. Am Heart J 2005;150:1204.
A number of expert panels have evaluated the topic of screening for renal artery stenosis. The first was part of the American Heart Association/American College of Cardiology (AHA/ACC) guidelines committee on peripheral vascular disease (PVD), the second was a committee assembled by the AHA to evaluate screening at the time of cardiac catheterization.(6,7) The guidelines for screening are summarized in Table 3.
Table 3.
Summary of current guidelines for screening patients with atherosclerotic RAS adapted from Hirsch et al: ACC/AHA 2006 guidelines for the management of patients with peripheral arterial disease.
| Class I | Class IIa | Class IIb |
|---|---|---|
| Onset of hypertension before the age of 30 years. (Level of Evidence: B) | Unexplained renal failure, including individuals starting renal replacement therapy(Level of Evidence: B) | Multivessel coronary artery disease and no clinical clues or PAD at the time of arteriography. (Level of Evidence: B) |
| Severe hypertension after the age of 55 years. (Level of Evidence: B) | Unexplained congestive heart failure or refractory angina (see Section 3.5.2.4 of the full-text guidelines). (Level of Evidence: C) | |
|
||
| New azotemia or worsening renal function after the administration of an ACE inhibitor or an angiotensin receptor blocking agent. (Level of Evidence: B) | ||
| Atrophic kidney or a discrepancy in size between the 2 kidneys of greater than 1.5 cm. (Level of Evidence: B) | ||
| Sudden, unexplained pulmonary edema (especially in azotemic patients). (Level of Evidence: B) |
Diagnostic methods
Invasive angiography has been considered the “gold standard” for the diagnosis and evaluation of RAS. Currently the most commonly employed methodology is intra-arterial digital subtraction angiography (DSA). (8) Carbon dioxide can be utilized for intra-arterial angiography (9), but image quality is reduced and this may create greater uncertainty about lesion severity unless combined with judicious use of iodinated contrast (10).
Duplex ultrasonography is an excellent screening test for RAS since it is non-toxic, involves no exposure to ionizing radiation and, in capable hands, is reliable (11). The major limitation to this method is its dependence on technician skill for acquisition of adequate images. In duplex ultrasound, peak systolic and end-diastolic velocities of the renal artery as well as the ratio of velocities in the renal artery to the aorta are obtained. Sensitivities of 92.5% to 98% and specificities of 96% to 98% have been reported.(12,13) A number of factors may limit image quality and thus the diagnostic accuracy of the test including obesity, bowel gas, and recent food intake.
Magnetic resonance angiography (MRA) and computerized tomographic angiography (CTA) are both non-invasive imaging methods which can visualize RAS (Figure 2). A meta-analysis by Vasbinder suggests that Duplex is inferior to MRA and CTA, however, the safety of Duplex makes it ideal as an index strategy for evaluation of patients with suspected RAS. (14). They conclude that CTA and gadolinium-enhanced three-dimensional MRA were more reliable noninvasive tests. A later publication by the same authors compared DSA with CTA and MRA and found both techniques lacked sensitivity and specificity.(15) Other limitations of tomographic imaging include the risk of contrast nephropathy with CTA and nephrogenic sclerosing fibrosis when gadolinium is utilized with MRA in patients with renal insufficiency. Among patients with significant renal insufficiency, non–contrast MRA techniques such as balanced steady state free precession may be useful.(16)
Figure 2.
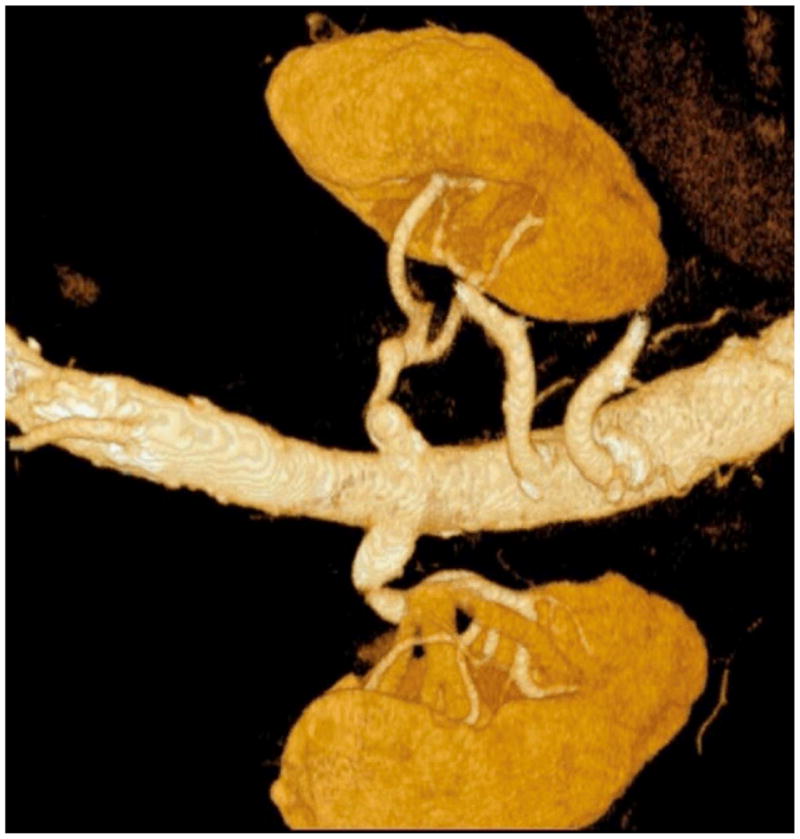
Screening renal computerized tomographic angiography.
An alternative strategy is to perform abdominal aortography when invasive angiography performed for other indications, such as peripheral arterial disease, or coronary disease in patients with a high likelihood of RAS. The obvious advantage is the ability to gain high quality diagnostic information during the already scheduled procedure. Limitations include increased contrast as the result of the aortic and renal imaging and the risk of atheroembolization if selective renal angiography is performed. For these reasons multidisciplinary guidelines were published in 2006.(7)
Another non-invasive test for RAS is captopril renography(17). Renography is dependent on comparative imaging of the right and left kidneys. Because the incidence of bilater RAS is approximately 30%, it makes a poor screening test. Subsequent meta-analysis has shown that captopril testing performs poorly compared to receiver operator curves of other non-invasive techniques. (14) Captopril renography may be useful when trying to determine the physiologic significance of a known intermediate stenosis.
Treatment
The treatment of RAS is performed for 1) blood pressure control, treatment of heart failure and/or pulmonary edema, and prevention of nephropathy. In addition renal artery revascularization is indicated to facilitate management of unstable angina, refractory to medical therapy. There is now general agreement that a ‘cure’ of hypertension with revascularization is uncommon (<10%) in patients with atherosclerotic RAS and is more frequently seen after revascularization in patients with FMD (approximately 50%).(1) Importantly, across a variety of observational studies there appears to be a consistent and sustained blood pressure lowering effect of revascularization.(18,19) In several of the early randomized studies of balloon angioplasty compared to medical therapy, balloon angioplasty was more effective than medical therapy alone in reducing hypertension.(1) In two recent studies of renal stenting in patients with uncertain indications for treatment or mild to moderate RAS, the patients in the medical therapy arm appear to have a similar lowering of their blood pressure (20,21).
Considerable controversy exists regarding the use of revascularization of atherosclerotic RAS to treat or prevent the development of ischemic nephropathy. This controversy stems from an apparent disparity between the effectiveness of stenting observed in clinical practice and practice-based registries versus the lack of effect seen in randomized studies. Several observational studies from the late 1990’s suggested that stent revascularization in patients with ischemic nephropathy and significant stenoses resulted in a slower rate of progression of nephropathy.(22,23) This effect has been described as stabilization. In a minority of patients, an actual improvement in renal function is seen with either stenting or surgical revascularization. (24) However, some patients experience a decline in kidney function that offset improvements seen in other patients. In contrast, there are now several randomized studies that have been conducted in patients with mild to moderate RAS or patients with uncertain indications for revascularization to determine the effect(s) of stenting on renal function. In general the following can be gleaned from the two most recent randomized trials of renal intervention.(20,21) The severity of the stenosis treated in these two trials were mild to modest and its not surprising that revascularization was not helpful. Core laboratories were not utilized for measurement of key study factors or outcomes such as lesion severity or renal function which were left to on-site reporting which is known to be highly variable and inconsistent. The skill level of the operators was uncertain and complication rates reported far exceeded those reported in the literature from skilled centers.
As a consequence, there still remains significant debate about revascularization in general, and more specifically our ability to discriminate between patients likely to benefit and those less likely to benefit from renal stenting.(25) One conclusion that can be made at this time, based upon the aggregate evidence, is that if stenting has effects that are superior to medical therapy for blood pressure control or renal function, the effects are likely modest and may not be present at all for patients with milder expressions of the disease. Furthermore, complications of a revascularization procedure have the potential to undermine the potential benefits.
Medical therapy
There has been considerable evolution in medical therapy for patients with ischemic renal disease. In the mid 1980’s there were observations demonstrating the potential for angiotensin converting enzyme (ACE) inhibitors to induce acute hemodynamically-mediated renal failure in patients with RAS (26). As a consequence all ACE inhibitors and angiotensin receptor blockers (ARBs) now carry a “black box” warning. Recent observational data, however, suggest that ACE inhibitors are associated with better outcomes. Specifically, data from Hackam et al suggests that treatment of RAS with ACE/ARB is associated with lower cardiovascular event rates (10 vs 13%) and need for dialysis (1.5 vs. 2.5%) although this comes at the cost of an increased risk of hospitalization for acute renal failure (1.2 vs. 0.6%).(27) These agents may reduce the adverse consequences of activation of the renin angiotensin system, controlling blood pressure and limiting downstream effects. However, as is the case with all observational data, a significant limitation may be selection bias; such that patients with better renal function and/or less severe disease are treated with these agents resulting in an apparent improvement of outcome.
In parallel, there is a growing consensus that other agents used to control the atherosclerotic process are important for the care of patients with atherosclerotic RAS. Observational data suggests that statins decrease death, limit lesion progression and promote restenosis-free survival.(28–31) There is general agreement that platelet inhibitors are important for the prevention of future cardiovascular events. However there is no data on antiplatelet agents effectiveness in patients with RAS.
Lifestyle Interventions
In addition to medical therapy, patients with atherosclerotic RAS need to be counseled regarding appropriate diet and lifestyle interventions. Specifically the therapeutic lifestyle changes recommended by the National Cholesterol Treatment Panel should be discussed with RAS patients (32). Furthermore all patients with atherosclerotic RAS and hypertension may benefit from the dietary recommendations, which include increased intake of fruits and vegetables, as well as dietary calcium through low fat dairy products.(33)
Revascularization
For patients with FMD the treatment of choice is balloon angioplasty.(34) A recent meta-regression suggests that early reports of hypertension cure with angioplasty may have overstated the benefit and that a reasonable estimate of the likelihood of cure is approximately 50%, with younger patients more likely to achieve this outcome.(1) In a minority of FMD cases there will be concomitant aneurysms of the renal artery. Some are small and can be followed, others that are larger or that compress on the kidney may require treatment. Such treatment may include exclusion with stent grafts, coil occlusion or even surgical reconstruction.(35)
In patients with atherosclerotic RAS stenting has proven superior to balloon angioplasty in randomized trials and is the recommended treatment (Figure 3).(1, 36) Surgical bypass or reconstruction is an alternative, but at least one randomized trial found no benefit over angioplasty (37). More importantly several series, including a large recent review of the Medicare experience suggests high rates of adverse outcomes with surgery, including peri-operative mortality of approximately 10% in this “real world” cohort.(38,39,40).
Figure 3.
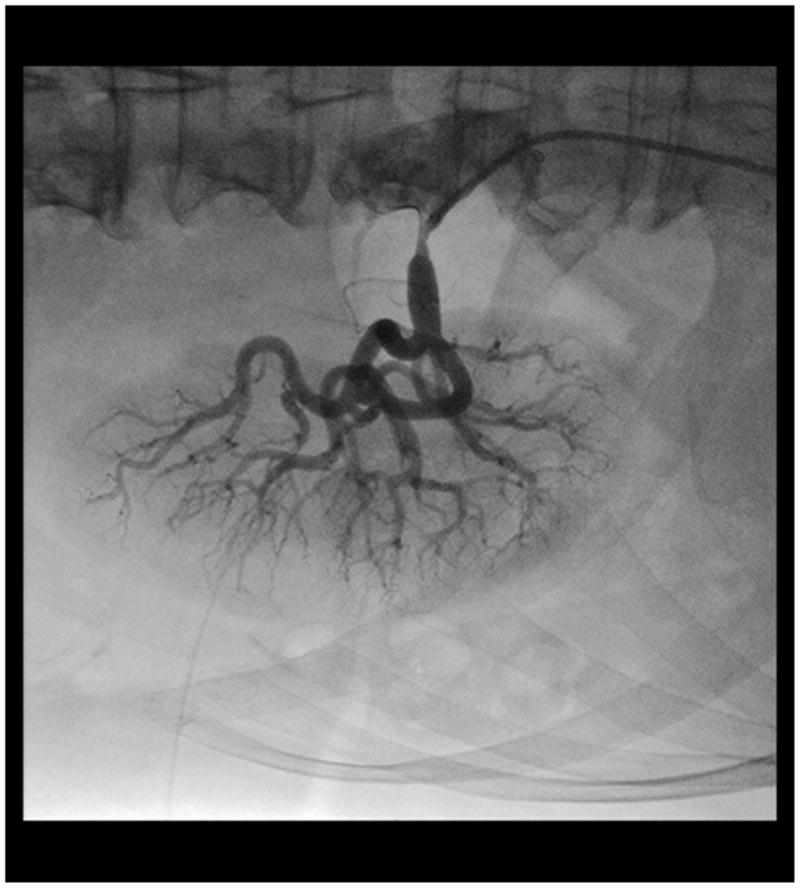
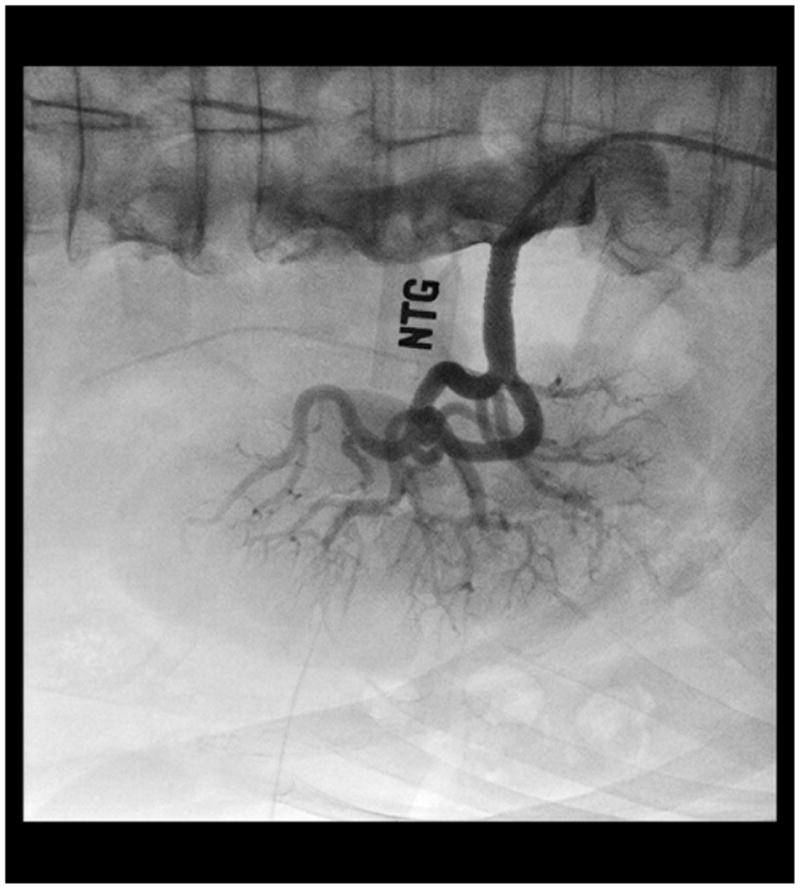
Successful renal artery stent placement.
When stenting is performed there are a number of technical factors that should be considered as part of the procedure. Previously, Feldman and colleagues described the “no touch” technique for engaging a catheter into the renal artery (41). This technique is recommended to reduce the risk of atheroembolism. No embolic protection device is approved by FDA for use in the renal artery. Data regarding the use of embolic protection systems suggest this strategy is safe and may improve renal function outcomes.(42–45) However, a small randomized trial demonstrated a benefit of embolic protection only in patients also assigned to abciximab (a platelet glycoprotein IIbIIIa inhibitor).(46) Several stents are approved by FDA for use in the renal artery for the indication of failed balloon angioplasty (47), regardless of the fact that primary stenting is the standard of care for atherosclerotic RAS worldwide.
Guidelines for treatment
The guidelines for treating RAS were published as part of the PAD guidelines in 2006 through a joint AHA/ACC committee.(6) An outline of the recommendations is provided in Table 4. There are some limitations though to this work: 1) the recommendations are largely based on expert opinion or observational studies, and 2) they predate the reporting of several randomized studies. With these limitations in mind a few conclusions are still worthy of consideration. The guidelines generally recommend treatment for complicated hypertension (drug resistant, accelerated, malignant) and for kidney disease in a setting that may imply a causal relationship between the stenosis and loss of renal function (bilateral stenosis and progressive kidney disease as an example.) Finally the guidelines also acknowledge the potential relationship(s) between RAS and either CHF or unstable angina as a rationale for treatment.
Table 4.
Summary of current guidelines for revascularization in patients with atherosclerotic RAS adapted from Hirsch et al: ACC/AHA 2006 guidelines for the management of patients with peripheral arterial disease.
| Class I | Class IIa | Class IIb |
|---|---|---|
| 1. Hemodynamically significant RAS and unexplained CHF or sudden unexplained pulmonary edema (Evidence level B) | 1. Hemodynamically significant RAS and accelerated HTN, resistant HTN, HTN with unexplained unilateral small kidney and HTN with medication intolerance (Evidence level B) | 1. Asymptomatic bilateral or solitary viable kidney with hemodynamically significant RAS (Evidence level C) |
| 2. Bilateral RAS or RAS of solitary functioning kidney and progressive CKD (Evidence level B) | 2. Unilateral hemodynamically significant RAS in a viable kidney (Evidence level C) | |
| 3. Hemodynamically significant RAS and unstable angina (Evidence level B) | 3. Unilateral RAS and CKD (Evidence level C) |
Conclusion
RAS is a common problem that affects the young (FMD) and old (atherosclerosis), and rarely is caused by other conditions. Treatment of FMD with balloon angioplasty and anti-hypertensive medications is well accepted. Treatment of atherosclerotic RAS remains somewhat controversial, largely due to the apparent disparity in results between observational cohorts suggesting a treatment advantage and randomized trials that have failed to detect such differences in treatment outcome. It is hoped that the reporting of ongoing studies may provide clarity in this area. (48)
Abbreviations
- RAS
Renal artery stenosis
- FMD
Fibromuscuclar Dysplasia
- AHA
American Heart Association
- ACC
American College of Cardiology
- PVD
Peripheral Vascular Disease
- DSA
Digital Subtraction Angiography
- CTA
computed Tomography Angiography
- MRA
Magnetic Resonance Angiography
- ACE
Angiotensin Converting Enzyme
- ARB
Angiotensin Receptor Blockers
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Trinquart L, Mounier-Vehier C, Sapoval M, Gagnon N, Plouin PF. Efficacy of Revascularization For Renal Artery Stenosis Caused by Fibromuscular Dysplasia, A Systematic Review and Meta-Analysis. Hypertension. 2010;56:525–532. doi: 10.1161/HYPERTENSIONAHA.110.152918. [DOI] [PubMed] [Google Scholar]
- 2.Krijnen P, van Jaarsveld BC, Steyerberg EW, Man in ‘t Veld AJ, Schalekamp MA, Habbema JD. A clinical prediction rule for renal artery stenosis. Ann Intern Med. 1998 Nov 1;129(9):705–11. doi: 10.7326/0003-4819-129-9-199811010-00005. [DOI] [PubMed] [Google Scholar]
- 3.Krijnen P, Steyerberg EW, Postma CT, Flobbe K, de Leeuw PW, Hunink MG. Validation of a prediction rule for renal artery stenosis. J Hypertens. 2005 Aug;23(8):1583–8. doi: 10.1097/01.hjh.0000174395.65267.e1. [DOI] [PubMed] [Google Scholar]
- 4.Cohen MG, Pascua JA, Garcia-Ben M, Rojas-Matas CA, Gabay JM, Berrocal DH, Tan WA, Stouffer GA, Montoya M, Fernandez AD, Halac ME, Grinfeld LR. A simple prediction rule for significant renal artery stenosis in patients undergoing cardiac catheterization. Am Heart J. 2005 Dec;150(6):1204–11. doi: 10.1016/j.ahj.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 5.Rigatelli G, Rigatelli G. Assessing the appropriateness and increasing the yield of renal angiography in patients undergoing coronary angiography: a scoring system. Int J Cardiovasc Imaging. 2006 Apr;22(2):135–9. doi: 10.1007/s10554-005-9000-8. [DOI] [PubMed] [Google Scholar]
- 6.Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, Hiratzka LF, Murphy WR, Olin JW, Puschett JB, Rosenfield KA, Sacks D, Stanley JC, Taylor LM, Jr, White CJ, White J, White RA, Antman EM, Smith SC, Jr, Adams CD, Anderson JL, Faxon DP, Fuster V, Gibbons RJ, Hunt SA, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic) Circulation. 2006 Mar 21;113(11):e463–654. doi: 10.1161/CIRCULATIONAHA.106.174526. [DOI] [PubMed] [Google Scholar]
- 7.White CJ, Jaff MR, Haskal ZJ, Jones DJ, Olin JW, Rocha-Singh KJ, Rosenfield KA, Rundback JH, Linas SL. Indications for renal arteriography at the time of coronary arteriography: a science advisory from the American Heart Association Committee on Diagnostic and Interventional Cardiac Catheterization, Council on Clinical Cardiology, and the Councils on Cardiovascular Radiology and Intervention and on Kidney in Cardiovascular Disease.; American Heart Association Committee on Diagnostic and Interventional Cardiac Catheterization, Council on Clinical Cardiology; American Heart Association Council on Cardiovascular Radiology and Intervention; American Heart Association Council on Kidney in Cardiovascular Disease. Circulation. 2006 Oct 24;114(17):1892–5. doi: 10.1161/CIRCULATIONAHA.106.178777. [DOI] [PubMed] [Google Scholar]
- 8.Kim D, Porter DH, Brown R, Crivello MS, Silva P, Leeming BW. Renal artery imaging: a prospective comparison of intra-arterial digital subtraction angiography with conventional angiography. Angiology. 1991;42:345–357. doi: 10.1177/000331979104200501. [DOI] [PubMed] [Google Scholar]
- 9.Hawkins IF, Jr, Wilcox CS, Kerns SR, Sabatelli FW. CO2 digital angiography: a safer contrast agent for renal vascular imaging? Am J Kidney Dis. 1994;24:685–694. doi: 10.1016/s0272-6386(12)80232-0. [DOI] [PubMed] [Google Scholar]
- 10.Liss P, Eklöf H, Hellberg O, Hägg A, Boström-Ardin A, Löfberg AM, Olsson U, Orndahl P, Nilsson H, Hansell P, Eriksson LG, Bergqvist D, Nyman R. Renal effects of CO2 and iodinated contrast media in patients undergoing renovascular intervention: a prospective, randomized study. J Vasc Interv Radiol. 2005 Jan;16(1):57–65. doi: 10.1097/01.RVI.0000144807.81633.79. [DOI] [PubMed] [Google Scholar]
- 11.Zierler RE. Is duplex scanning the best screening test for renal artery stenosis? Semin Vasc Surg. 2001 Sep;14(3):177–85. doi: 10.1053/svas.2001.25488. [DOI] [PubMed] [Google Scholar]
- 12.Olin JW, Piedmonte MR, Young JR, DeAnna S, Grubb M, Childs MB. The utility of duplex ultrasound scanning of the renal arteries for diagnosing significant renal artery stenosis. Ann Intern Med. 1995;122:833–888. doi: 10.7326/0003-4819-122-11-199506010-00004. [DOI] [PubMed] [Google Scholar]
- 13.Riehl J, Schmitt H, Bongartz D, Bergmann D, Sieberth HG. Renal artery stenosis: evaluation with colour duplex ultrasonography. Nephrol Dial Transplant. 1997;12:1608–1614. doi: 10.1093/ndt/12.8.1608. [DOI] [PubMed] [Google Scholar]
- 14.Vasbinder GB, Nelemans PJ, Kessels AG, Kroon AA, de Leeuw PW, van Engelshoven JM. Diagnostic tests for renal artery stenosis in patients suspected of having renovascular hypertension: a meta-analysis. Ann Intern Med. 2001;135:401–411. doi: 10.7326/0003-4819-135-6-200109180-00009. [DOI] [PubMed] [Google Scholar]
- 15.Vasbinder GB, Nelemans PJ, Kessels AG, Kroon AA, Maki JH, Leiner T, Beek FJ, Korst MB, Flobbe K, de Haan MW, van Zwam WH, Postma CT, Hunink MG, de Leeuw PW, van Engelshoven JM. Accuracy of computed tomographic angiography and magnetic resonance angiography for diagnosing renal artery stenosis. Ann Intern Med. 2004;141:674–682. doi: 10.7326/0003-4819-141-9-200411020-00007. [DOI] [PubMed] [Google Scholar]
- 16.Roditi G, Maki JH, Oliveira G, Michaely HJ. Renovascular imaging in the NSF era. J Magn Reson Imaging. 2009;30:1323–1334. doi: 10.1002/jmri.21977. [DOI] [PubMed] [Google Scholar]
- 17.Kaplan-Pavlovcic S, Nadja C. Captopril renography and duplex Doppler sonography in the diagnosis of renovascular hypertension. Nephrol Dial Transplant. 1998;13:313–317. doi: 10.1093/oxfordjournals.ndt.a027824. [DOI] [PubMed] [Google Scholar]
- 18.Burket MA, Cooper CJ, Kennedy DJ, Brewster PS, Ansel GM, Moore JA, Venkatesan J, Henrich WL. Renal artery angioplasty and stent placement: predictors of a favorable outcome. Am Heart J. 2000;139:64–71. doi: 10.1016/s0002-8703(00)90310-7. [DOI] [PubMed] [Google Scholar]
- 19.Balk E, Raman GMD, Chung M, Ip S, Tatsioni A, Alonso A, Chew P, Gilbert SJ, Lau J. Effectiveness of Management Strategies for Renal Artery Stenosis: A Systematic Review. Ann Intern Med. 2006;145:901–912. doi: 10.7326/0003-4819-145-12-200612190-00143. [DOI] [PubMed] [Google Scholar]
- 20.The Astral Investigators. Revascularization versus Medical Therapy for Renal-Artery Stenosis. N Engl J Med. 2009;361:1953–62. doi: 10.1056/NEJMoa0905368. [DOI] [PubMed] [Google Scholar]
- 21.Bax L, Woittiez AJ, Kouwenberg HJ, Mali WP, Buskens E, Beek FJ, Braam B, Huysmans FT, Schultze Kool LJ, Rutten MJ, Doorenbos CJ, Aarts JC, Rabelink TJ, Plouin PF, Raynaud A, van Montfrans GA, Reekers JA, van den Meiracker AH, Pattynama PM, van de Ven PJ, Vroegindeweij D, Kroon AA, de Haan MW, Postma CT, Beutler JJ. Stent Placement in Patients With Atherosclerotic Renal Artery Stenosis and Impaired Renal Function. Ann Intern Med. 2009;150:840–848. doi: 10.7326/0003-4819-150-12-200906160-00119. [DOI] [PubMed] [Google Scholar]
- 22.Harden PN, MacLeod MJ, Rodger RSC, Baxter GM, Connell JMC, Dominiczak AF, Junor BJR, Briggs JD, Moss JG. Effect of renal-artery stenting on progression of renovascular renal failure. Lancet. 1997;349:1133–1136. doi: 10.1016/s0140-6736(96)10093-3. [DOI] [PubMed] [Google Scholar]
- 23.Watson PS, Hadjipetrou P, Cox SV, Piemonte TC, Eisenhauer AC. Effect of renal artery stenting on renal function and size in patiens with atherosclerotic renovascular disease. Circulation. 2000;102:1671–1677. doi: 10.1161/01.cir.102.14.1671. [DOI] [PubMed] [Google Scholar]
- 24.Hansen KJ, Cherr GS, Dean RH. Dialysis-free survival after surgical repair of ischemic nephropathy. Cardiovasc Surg. 2002;10:400–404. doi: 10.1016/s0967-2109(02)00040-6. [DOI] [PubMed] [Google Scholar]
- 25.White CJ. Kiss my astral: one seriously flawed study of renal stenting after another. Catheter Cardiovasc Interv. 2010 Feb 1;75(2):305–7. doi: 10.1002/ccd.22416. [DOI] [PubMed] [Google Scholar]
- 26.Hricik DE, Browning PJ, Kopelman R, Goorno WE, Madias NE, Dzau VJ. Captopril-induced functional renal insufficiency in patients with bilateral renal-artery stenoses or renal-artery stenosis in a solitary kidney. N Engl J Med. 1983;308:373–6. doi: 10.1056/NEJM198302173080706. [DOI] [PubMed] [Google Scholar]
- 27.Hackam DG, Duong-Hua ML, Mamdani M, Li P, Tobe SW, Spence JD, Garg AX. Angiotensin inhibition in renovascular disease: a population-based cohort study. Am Heart J. 2008 Sep;156(3):549–55. doi: 10.1016/j.ahj.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 28.Silva VS, Martin LC, Franco RJ, Carvalho FC, Bregagnollo EA, Castro JH, Gavras I, Gavras H. Pleiotropic Effects of Statins May Improve Outcomes in Atherosclerotic Renovascular Disease. Am J Hypertens. 2008;21:1163–1168. doi: 10.1038/ajh.2008.249. [DOI] [PubMed] [Google Scholar]
- 29.Bates MC, Campbell JE, Stone PA, Jaff MR, Broce M, Lavigne PS. Factors affecting long-term survival following renal artery stenting. Catheter Cardiovasc Interv. 2007 Jun 1;69(7):1037–43. doi: 10.1002/ccd.21121. [DOI] [PubMed] [Google Scholar]
- 30.Corriere MA, Edwards MS, Pearce JD, Andrews JS, Geary RL, Hansen KJ. Restenosis after renal artery angioplasty and stenting: incidence and risk factors. J Vasc Surg. 2009 Oct;50(4):813–819. doi: 10.1016/j.jvs.2009.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheung CM, Patel A, Shaheen N, Cain S, Eddington H, Hegarty J, Middleton RJ, Cowie A, Mamtora H, Kalra PA. The effects of statins on the progression of atherosclerotic renovascular disease. Nephron Clin Pract. 2007;107(2):c35–42. doi: 10.1159/000107552. [DOI] [PubMed] [Google Scholar]
- 32.Executive summary of the third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation and treatment of high blood cholesterol in adults (adult treatment panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 33.Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, Bray GA, Vogt TM, Cutler JA, Windhauser MM, Lin PH, Karanja N. A clinical trial on the effects of dietary patterns on blood pressure. N Engl J Med. 1997;336:1117–1124. doi: 10.1056/NEJM199704173361601. [DOI] [PubMed] [Google Scholar]
- 34.Slovut DP, Olin JW. Fibromuscular dysplasia. N Engl J Med. 2004;350:1862–1871. doi: 10.1056/NEJMra032393. [DOI] [PubMed] [Google Scholar]
- 35.Olin JW, Pierce M. Contemporary management of fibromuscular dysplasia. Curr Opin Cardiol. 2008 Nov;23(6):527–36. doi: 10.1097/HCO.0b013e328313119a. [DOI] [PubMed] [Google Scholar]
- 36.van de Ven PJ, Kaatee R, Beutler JJ, Beek FJ, Woittiez AJ, Buskens E, Koomans HA, Mali WP. Arterial stenting and balloon angioplasty in ostial atherosclerotic renovascular disease: a randomised trial. Lancet. 1999 Jan 23;353(9149):282–6. doi: 10.1016/S0140-6736(98)04432-8. [DOI] [PubMed] [Google Scholar]
- 37.Weibull H, Bergqvist D, Bergentz SE, Jonsson K, Hulthén L, Manhem P. Percutaneous transluminal renal angioplasty versus surgical reconstruction of atherosclerotic renal artery stenosis: a prospective randomized study. J Vasc Surg. 1993 Nov;18(5):841–50. doi: 10.1067/mva.1993.45062. [DOI] [PubMed] [Google Scholar]
- 38.Modrall JG, Rosero EB, Smith ST, Arko FR, 3rd, Valentine RJ, Clagett GP, Timaran CH. Operative mortality for renal artery bypass in the United States: Results from the National Inpatient Sample. J Vasc Surg. 2008 Aug;48(2):317–322. doi: 10.1016/j.jvs.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 39.Modrall JG, Rosero EB, Smith ST, Arko FR, 3rd, Valentine RJ, Clagett GP, Timaran CH. Effect of hospital volume on in-hospital mortality for renal artery bypass. Vasc Endovascular Surg. 2009 Aug-Sep;43(4):339–45. doi: 10.1177/1538574409335919. [DOI] [PubMed] [Google Scholar]
- 40.Cherr GS, Hansen KJ, Craven TE, Edwards MS, Ligush J, Jr, Levy PJ, Freedman BI, Dean RH. Surgical management of atherosclerotic renovascular disease. J Vasc Surg. 2002 Feb;35(2):236–45. doi: 10.1067/mva.2002.120376. [DOI] [PubMed] [Google Scholar]
- 41.Feldman RL, Wargovich TJ, Bittl JA. No-touch technique for reducing aortic wall trauma during renal artery stenting. Catheter Cardiovasc Interv. 1999 Feb;46(2):245–8. doi: 10.1002/(SICI)1522-726X(199902)46:2<245::AID-CCD27>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 42.Holden A, Hill A. Renal angioplasty and stenting with distal protection of the main renal artery in ischemic nephropathy: early experience. J Vasc Surg. 2003;38:962–968. doi: 10.1016/s0741-5214(03)00606-2. [DOI] [PubMed] [Google Scholar]
- 43.Edwards MS, Craven BL, Stafford J, Craven TE, Sauve KJ, Ayerdi J, Geary RL, Hansen KJ. Distal embolic protection during renal artery angioplasty and stenting. J Vasc Surg. 2006;44:128–135. doi: 10.1016/j.jvs.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 44.Henry M, Klonaris C, Henry I, Tzetanov K, Le Borgne E, Foliguet B, Hugel M. Protected renal stenting with the Percusurge Guardwire device; a pilot study. J Endovasc Ther. 2001;8:227–237. doi: 10.1177/152660280100800301. [DOI] [PubMed] [Google Scholar]
- 45.Thatipelli MR, Misra S, Sanikommu SR, Schainfeld RM, Sharma SK, Soukas PA. Embolic protection device use in renal artery stent placement. J Vasc Interv Radiol. 2009 May;20(5):580–6. doi: 10.1016/j.jvir.2009.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cooper CJ, Haller ST, Colyer W, Steffes M, Burket MW, Thomas WJ, Safian R, Reddy B, Brewster P, Ankenbrandt MA, Virmani R, Dippel E, Rocha-Singh K, Murphy TP, Kennedy DJ, Shapiro JI, D’Agostino RD, Pencina MJ, Khuder S. Embolic protection and platelet inhibition during renal artery stenting. Circulation. 2008 May 27;117(21):2752–60. doi: 10.1161/CIRCULATIONAHA.107.730259. [DOI] [PubMed] [Google Scholar]
- 47.Rocha-Singh K, Jaff MR, Rosenfield K. Evaluation of the safety and effectiveness of renal artery stenting after unsuccessful balloon angioplasty the ASPIRE-2 study. J Am Coll Cardiol. 2005;46:776–783. doi: 10.1016/j.jacc.2004.11.073. [DOI] [PubMed] [Google Scholar]
- 48.Cooper CJ, Murphy TP, Matsumoto A, Steffes M, Cohen DJ, Jaff M, Kuntz R, Jamerson K, Reid D, Rosenfield K, Rundback J, D’Agostino R, Henrich W, Dworkin L. Stent revascularization for the prevention of cardiovascular and renal events among patients with renal artery stenosis and systolic hypertension: rationale and design of the CORAL trial. Am Heart J. 2006 Jul;152(1):59–66. doi: 10.1016/j.ahj.2005.09.011. [DOI] [PubMed] [Google Scholar]