Abstract
In a wide range of organisms, including humans, mothers can influence offspring via the care they provide. Comparatively little is known about the effects of fathering on offspring. Here, we test the hypothesis that fathers are capable of programming their offspring for the type of environment they are likely to encounter. Male threespine sticklebacks, Gasterosteus aculeatus, were either exposed to predation risk while fathering or not. Fathers altered their paternal behaviour when exposed to predation risk, and consequently produced adult offspring with phenotypes associated with strong predation pressure (smaller size, reduced body condition, reduced behavioural activity). Moreover, more attentive fathers produced offspring that showed stronger antipredator responses. These results are consistent with behaviourally mediated paternal programming: fathers can alter offspring phenotypes to match their future environment and influence offspring traits well into adulthood.
Keywords: behavioural development, fathering, Gasterosteus aculeatus, maternal effect, parental effect, paternal care, phenotypic plasticity, predation risk, transgenerational plasticity
In a wide range of organisms, including humans, mothers' experiences can affect offspring (Mousseau & Fox, 1998; Uller, 2008). For example, maternal exposure to predation risk alters offspring morphology (Agrawal, Laforsch, & Tollrian, 1999; Weisser, Braendle, & Minoretti, 1999), physiology (Sheriff, Krebs, & Boonstra, 2010) and behaviour (Storm & Lima, 2010). There is also an emerging literature showing that the way mothers behave towards their offspring can have a long-lasting influence on their offspring (Champagne, 2008).
Comparatively less is known about the significance of fathers' experiences and behaviour for offspring. At first glance it might appear that there is little opportunity for fathers' experiences to become embedded in offspring because there is rarely intimate contact between fathers and developing embryos. However, a growing number of studies is showing that fathers' experiences prior to fertilization can influence offspring via, for example, changes in sperm morphology or seminal fluid (beetles: Sirot, Lapointe, Shatters, & Bausher, 2006), the sperm epigenome (mammals: Curley, Mashoodh, & Champagne, 2011) and sperm microRNAs (rats: Rodgers, Morgan, Bronson, Rovello, & Bale, 2013). A relatively unexplored possibility is that fathers adjust their parenting in response to stressors, and adjustments in care have long-term consequences for offspring, as has been shown for mothers (McLeod, Sinal, & Perrot-Sinal, 2007).
Predation is a strong selective pressure that shapes many traits (Abrams & Rowe, 1996; Endler, 1995). In a predator-rich environment, antipredator defences are key for reproductive success and offspring survival, and predation risk often alters phenotypes in predictable ways. For example, prey in high-predation environments tend to be smaller, less active and have faster life history trajectories than prey from low-predation environments (guppies: Endler, 1995; tadpoles: Relyea, 2004; lizards: Vervust, Grbac, & Van Dame, 2007). If parents can respond to cues that future predation risk is likely to be high, and if they can prepare offspring for living in a predator-rich environment, then this transgenerational plasticity could be adaptive.
Here, we investigate the effects of paternal experience with predation risk on offspring morphological, behavioural and physiological traits in threespine sticklebacks, Gasterosteus aculeatus. Sticklebacks are teleost fish in which the father is the sole provider of parental care that is necessary for offspring survival. Therefore, there is no opportunity for differential allocation or compensation by the mother (Curley et al., 2011). During the approximately 5-day incubation period, male sticklebacks ‘fan’ the eggs with pectoral fins, providing oxygen and clearing carbon dioxide, and remove rotten eggs and debris (Wootton, 1984). Once the eggs hatch, fathers continue to defend their offspring and retrieve young fry that stray too far from the nest. Previous studies suggested that offspring learn appropriate antipredator behaviour from their father after hatching (Feuth-De Bruijn & Sevenster, 1983; Tulley & Huntingford, 1987).
We used a within-subjects breeding design to test the hypothesis that paternal exposure to predation risk during parenting influences offspring traits. Specifically, males experienced predation risk during one parenting episode (‘predator-exposed’) and were not exposed to predation risk during the other parenting episode (‘unexposed’). We evaluated the effect of paternal predator exposure on the morphology, behaviour and physiology of reproductively mature adult offspring. We further examined correlations between paternal behaviour and offspring behaviour to test the hypothesis that paternal effects on offspring are mediated by paternal behaviour.
METHODS
Study Population and Breeding
Adult threespine sticklebacks were collected from Putah Creek, a dammed, regulated freshwater stream in northern California, in April 2010. Sculpin (Cottus spp.), a fish predator known to prey on stickleback eggs, fry and adults (Moodie,1972; Pressley,1981) are present at this site. Fish were shipped to the University of Illinois at Urbana- Champaign, and males were introduced into separate 9.5-litre (36 × 21 × 18 cm) tanks with a refuge (plastic ‘plant’), an open plastic box (13 × 13 × 3 cm) filled with fine sand, and filamentous algae for nest building. Following nest completion, males were presented with a gravid female and allowed to spawn. Each male spawned with a unique female. After spawning, the female was removed. Fish were kept at 20 °C on a summer (16:8 h light:dark) photoperiod. Water was cleaned via a recirculating flow-through system that consisted of a series of particulate, biological and UV filters (Aquaneering, San Diego, CA, U.S.A.). Ten per cent of the water volume in the tanks was replaced each day. Fish were fed a mixed diet consisting of frozen bloodworm, brine shrimp and Mysis shrimp in excess each day.
Exposing Fathers to Predation Risk and Recording Paternal Behaviour
Males were randomly assigned to either the ‘unexposed’ or ‘predator-exposed’ treatment for their first clutch. On the third day after males spawned (when the embryos were 3 days old), males in the ‘predator-exposed’ treatment were chased with a 10 cm rubber model sculpin (Jewel Bait Company, Bakersfield, MO, U.S.A.) for 2 min to simulate a nest predation attempt. A predator of this size is a threat to the eggs and fry, but not to the adult males (Moodie, 1972; Pressley, 1981). Previous research has shown that adult sticklebacks show relevant antipredator behaviours when confronted with a realistically painted model (Grobis, Pearish, & Bell, 2013). It is unlikely that the embryos were exposed to visual cues of the model predator, as during the ‘predator-exposed’ treatment, the optic cups of the 3-day-old embryos were still developing (Swarup, 1958) and the eggs were covered by nesting material. For males in the ‘unexposed’ treatment, we removed the top of the tank and gently splashed the water when the eggs were 3 days old to simulate the water disturbance caused when the model predator entered the tank. Males were only exposed to the predator once.
After spawning, we observed paternal behaviour every day for 5 min between 1000 and 1300 hours Central Standard Time (CST) from 1 day after spawning through 5 days after the eggs hatched (when fry from this population naturally disperse in the wild). We measured the total time that the male was within one body length of the nest (total time at nest) and the total amount of time that the male spent fanning his eggs, a paternal behaviour that oxygenates the eggs (Wootton, 1984), is important for proper offspring development (von Hippel, 2000) and consistently varies among fathers (Stein & Bell, 2012). The simulated predation threat (or water splashing) occurred after the daily observation of paternal behaviour. There were subtle but detectable effects of predator exposure on paternal behaviour. For example, ‘predator-exposed’ fathers decreased total time fanning relative to control males for 2 days following exposure to the model predator, but afterwards resumed normal activity (Stein & Bell, 2012). More details on parental behaviour are presented in Stein and Bell (2012).
Five days after the eggs hatched, males were placed in new tanks and allowed to construct second nests and the entire process, including daily behavioural observations, was repeated for the second clutch. Males that had been in the ‘predator-exposed’ treatment in the first clutch were assigned to the ‘unexposed’ treatment for the second clutch and vice versa.
Ten males completed at least one clutch; of these, eight completed two clutches. Initial treatment did not affect whether males completed a second clutch (of those that completed two clutches, four were ‘unexposed’ and four were ‘predator-exposed’ in their first clutch), and parental behaviour in the first clutch did not predict whether a male completed a second clutch (Stein & Bell, 2012). We did not detect a difference in parental behaviour between the first and second clutches or an effect of the order in which a male experienced a model predator (i.e. a male's experience with parenting or with a predator did not influence his behaviour in his second clutch).
Offspring Morphology and Antipredator Behaviour
Once fry were approximately 1 cm in length (~1 month old), each full-sibling family was split across at least two tanks at a density of six fish per tank. Offspring were fed newly hatched Artemia nauplii shrimp in excess each day until they reached 3 cm in length, at which time they were fed the adult slurry of frozen food. Offspring were kept this way for 1 year; during this time they experienced a simulated winter (LD 8:16) photoperiod from November 2010 to March 2011.
At 1 year of age, when the offspring were reproductively mature, we measured their morphology, behaviour and cortisol response to predation risk. Specifically, we measured standard length and weight and scored colour (males only) using a ranking method (Boughman, 2007). Throat redness was measured as the sum of throat red area and red intensity scores (range 0–3 for each). Body brightness ranged from 0 to 5, with 5 being very bright. Throat hue and body brightness were measured on the side of the fish.
For behavioural testing of predator responses, fish were transferred individually to an observation tank in an opaque cylinder (10 cm in height, 10 cm diameter) plugged with a cork. The observation tank (53 × 33 × 24 cm) had a 5 × 2 grid drawn on the front, a gravel bottom and two plastic plants for refuge, one on each side of the tank. After a 15 min acclimation period, we removed the cork remotely and, after emerging from the cylinder, the fish acclimated to the observation tank for 1 h.
We recorded behaviour with a high-definition JVC Everio camcorder from behind a blind. Behaviour was recorded (see below for details) for 3 min without a stimulus to obtain a baseline level of behaviour (‘before’). After 3 min, we introduced a 15 cm clay sculpin (model predator) painted with natural markings to the tank to measure antipredator behaviour. A predator this size is a threat to adult sticklebacks (Moodie, 1972; Pressley, 1981). The model predator was attached with fishing wire to a rod that could be manipulated from behind the blind. We introduced the model to the right side of the tank and moved it in a clockwise direction around the tank for 1 min. We then placed the model on the gravel for 2 min, simulating the sit-and-wait predation style of sculpin, and recorded behaviour during the 3 min period that the predator was in the tank (‘during’). After 2 min, we removed the sculpin model and recorded behaviour for an additional 3 min to determine whether behaviour differed from baseline after offspring observed a predator (‘after’).We recorded the total number of squares moved (a measure of activity), total time freezing (an antipredator behaviour) and total number of jerky swims (a quick ‘burst’ of speed, a conspicuous behaviour) using JWatcher (http://www.jwatcher.ucla.edu/). The three observers recording behaviours were blind to offspring family and father treatment, and we did not detect an effect of observer on behaviour.
Behavioural observations were carried out between 1100 and 1700 hours CST during August–September 2011. Only one fish per rearing tank was tested each day; we recorded the behaviour of 12 fish per day. There were a total of 18 full-sibling families, with eight pairs of half-sibling families. Nine clutches were from predator-exposed fathers and nine clutches were from unexposed fathers. In total, there were 91 offspring from fathers in the predator-exposed treatment and 66 offspring from fathers in the unexposed treatment (total N = 157 offspring).
Measuring Plasma Cortisol in Offspring
We measured circulating plasma cortisol after the behavioural assay as a means of detecting offspring cortisol response to predation risk. Previous studies in threespine sticklebacks showed that circulating plasma cortisol peaks 15min after a predator encounter (Mommer & Bell, 2013). Therefore, 15 min following the introduction of the predator to the observation tank, we quickly netted the stickleback and immediately euthanized it with an overdose (>0.2 mM) of MS-222.We then removed the caudal peduncle just before the cloaca with sharp scissors. We collected blood from the caudal vein using 75 mm heparinized microhaematocrit tubes (Statspin, Westwood, MA, U.S.A.). Tubes were centrifuged on a microhaematocrit rotor (Statspin) to pellet circulating cells. Plasma supernatant was aspirated and kept at −20 °C until enzyme-linked immunosorbent assay (ELISA) could be performed. At this time we also removed the caudal fin and stored it in 70% ethanol for later determination of genetic sex using a male-specific genetic marker (Peichel et al., 2004).
To confirm that exposure to predation risk during the behavioural assays elicited a cortisol stress response, a subset of 20 offspring (N = 10 from predator-exposed fathers, N = 10 from unexposed fathers) were not put through the behaviour assay. Instead, these fish were randomly selected from their home tanks and immediately euthanized with an overdose of MS-222 (‘control’ treatment). We then measured standard length, weight, scored coloration, collected the caudal fin and extracted plasma as above. These control measurements also allowed us to determine whether offspring from predator-exposed fathers had higher ‘baseline’ levels of cortisol than offspring from unexposed fathers.
Plasma samples were thawed and their cortisol concentration measured in duplicate by competitive ELISA according to the manufacturer's protocol (Enzo Life Sciences, Plymouth Meeting, PA, U.S.A.). To extend the manufacturer's recommended standard curve for the ELISA, we included an eighth standard of cortisol (78 pg/ml) on each plate. Plasma samples across both levels of paternal treatment (predator-exposed and unexposed) and for behaviourally assayed and control offspring were represented on each of the four plates. The inter-assay coefficient of variation (CV) between all ELISA plates (N = 4 plates) was 6.65%; intra-assay CVs averaged 6.77 ± 6.20% (mean ± SD). Thirty-five individuals either gave very little blood or the plasma was lost from the capillary tube during isolation; therefore, the final sample size for cortisol analysis was 123 offspring exposed to the model predator and 19 control individuals.
Statistical Analysis
To determine whether paternal exposure to predation risk influences offspring, we compared offspring between paternal treatments (‘predator-exposed’ or ‘unexposed’ fathers). We constructed separate models to test for the effect of paternal treatment on offspring (1) body size, (2) body condition (calculated as the residuals of length by weight regression), (3) male nuptial coloration, (4) activity, (5) time freezing, (6) jerky swims and (7) circulating plasma cortisol concentration. All models included paternal treatment (predator-exposed, unexposed) and offspring sex as fixed effects, and father ID, clutch nested within father ID, offspring home tank nested within clutch and order in which the father saw a predator as random effects. We did not detect any differences between gravid and nongravid female offspring (results not shown).
Offspring activity (total squares moved) and time freezing were measured at three time points: before, during and after predator exposure. Therefore, for these variables, we also included stage (before, during and after predator exposure) as a fixed effect and individual ID as a random effect to account for multiple measurements of the same individual. To determine whether exposure to a model predator triggered a cortisol stress response in offspring, we used a t test comparing offspring that had or had not been exposed to the model predator.
We wished to control for the effect of offspring body size on behaviour and cortisol, but paternal treatment affected offspring length (see Results). Therefore, we regressed each behaviour and cortisol concentration on length and analysed the length-corrected residuals. Squares moved and total time freezing were ln-transformed (trait + 1 to account for zeroes) prior to regression to obtain normality; number of jerky swims was square-root (+1) transformed.
To test the hypothesis that paternal behaviour influenced offspring traits, we examined correlations between father behaviour (averaged within a clutch) and offspring behaviour (regressed on length and averaged within a clutch) using Pearson correlations. This analysis was performed at the clutch level rather than at the father level because a given male behaved differently during his two clutches, so each of a male's clutches experienced a different rearing environment. We used sequential Bonferroni correction to account for multiple correlation tests (Rice, Schork, & Rao, 2008).
All statistical analyses were conducted with R version 2.15.2 (R Foundation for Statistical Computing, Vienna, Austria). Linear mixed models (LMMs) were performed using the lmer function from the ‘lme4’ (Bates, Maechler, & Bolker, 2012) and ‘lmerTest’ (Kuznetsova, Brockhoff, & Christensen, 2013) packages. We used REML estimation and a diagonal covariance structure for our models, with Satterth waite approximation for degrees of freedom. We determined whether levels of fixed factors differed from one another using Tukey HSD test. We calculated effect sizes (Cohen's d) from means and standard deviations and interpret 0.5 as a large effect, 0.3 as a medium effect and 0.1 as a small effect (Cohen,1992).
Ethical Note
We took measures to maximize animal welfare by minimizing the duration of exposure to simulated predation risk and by providing refuges (plants and gravel) in the sticklebacks' tanks. Animals were killed via overdose of anaesthetic to minimize suffering. The experiments were approved by the Institutional Animal Care and Use Committee at the University of Illinois at Urbana-Champaign (Protocol number 09204, approved on 1 September 2009).
RESULTS
Paternal Effects on Offspring Morphological Traits
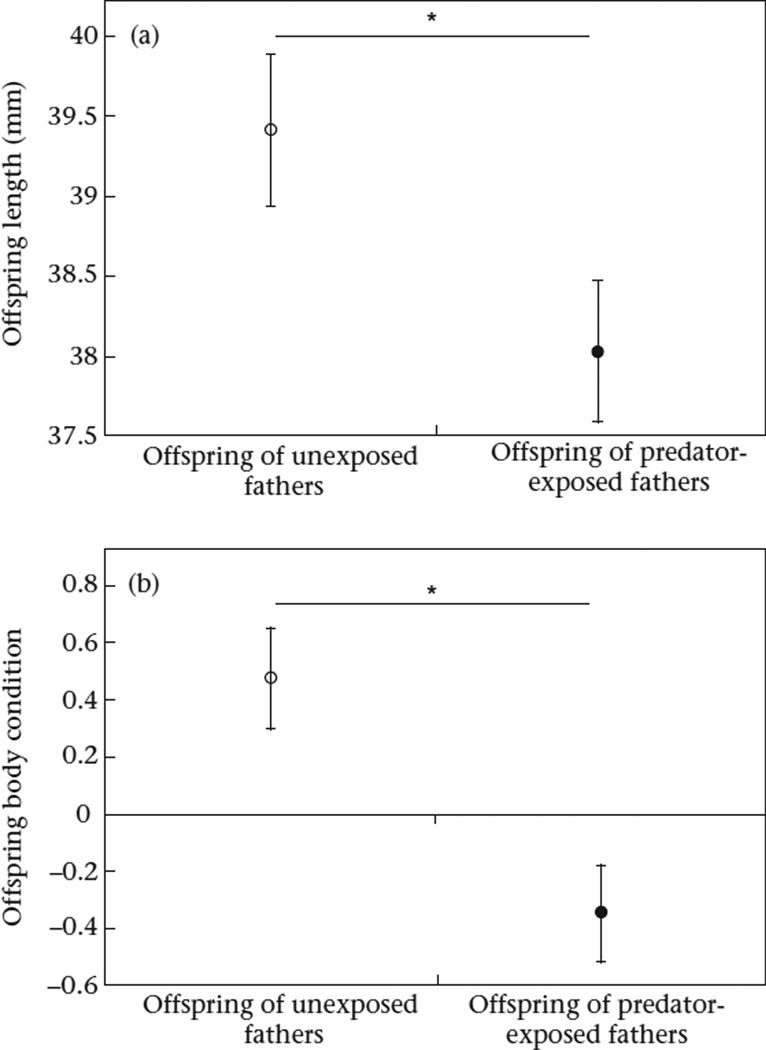
Whether or not a father experienced predation risk influenced the body size of his offspring (Cohen's d = 0.35; Table 1, Fig. 1a). When fathers were exposed to predation risk, they produced offspring that were significantly smaller (mean ± SE: 38.09 ± 4.20 mm) than when they were not exposed to predation risk (mean ± SE: 39.48 ± 3.84 mm). Paternal treatment also influenced body condition: when fathers were exposed to predation risk, they produced offspring in worse condition (mean ± SE: −0.35 ± 1.64) than when they were not exposed to predation risk (mean ± SE: 0.48 ± 1.46; Cohen's d = 0.54; Fig. 1b). Male offspring of fathers in the predator-exposed treatment had lower colour scores (mean ± SE: 2.49 ± 1.52) than male offspring of fathers in the unexposed treatment (mean ± SE: 3.12 ± 2.22; Cohen's d = 0.33). However, this effect was driven by the negative effect of paternal predator exposure on body size: when male offspring nuptial coloration was corrected for offspring length, there was no difference between paternal treatments (LMM: effect of paternal treatment: F1,41.15 = 0.12, P = 0.73).
Table 1.
Linear mixed model results for offspring morphological traits in threespine sticklebacks
| Factor | Length | Body condition | ||
|---|---|---|---|---|
| F (df) | P | F (df) | P | |
| Paternal treatment | 4.78 (1, 60.1) | 0.03 | 10.82 (1, 62.4) | 0.002 |
| Sex | 0.63 (1, 152.9) | 0.43 | 0.27 (1, 152.5) | 0.60 |
| Paternal treatment*sex | 0.02 (1, 152.2) | 0.89 | 1.86 (1, 152.1) | 0.18 |
Significant values are shown in bold.
Figure 1.
Effect of paternal treatment (unexposed versus predator-exposed) on (a) body size and (b) body condition of stickleback offspring. Means (uncorrected for length) ± SE are shown.
Paternal Effects on Offspring Antipredator Behaviour
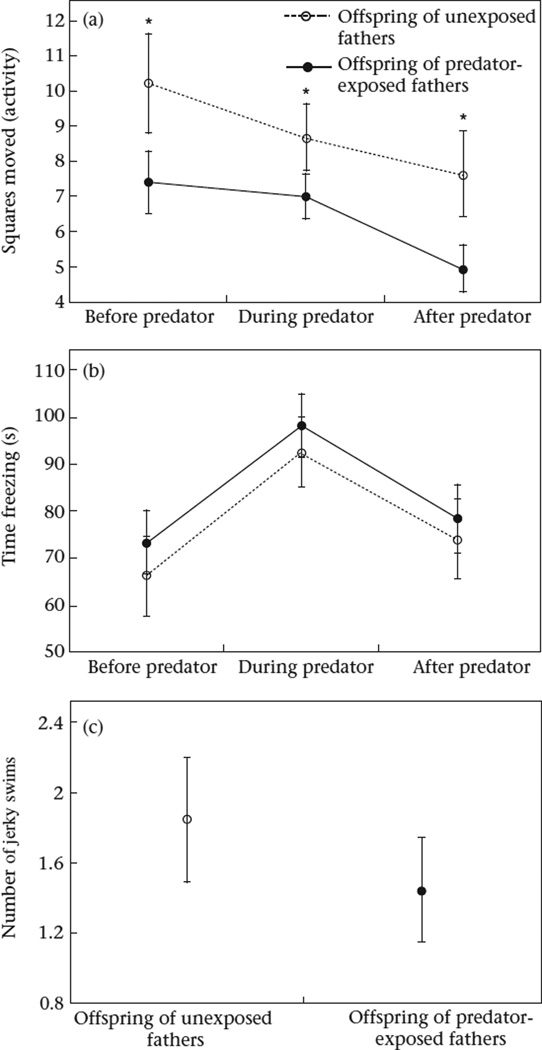
Offspring reacted to the model predator during the behavioural assay (Table 2, effect of stage). For example, when the predator was present, offspring reduced activity (mean ± SE: during: 7.7 ± 6.9 squares) compared to the period before the predator was introduced (mean ± SE: before: 8.6 ± 9.9 squares). Offspring maintained relatively low levels of activity after the predator was removed (mean ± SE: after: 6.09 ± 8.06 squares). Offspring increased the amount of time spent frozen when the predator was present (mean ± SE: before: 70.6 ± 67.3 s; during: 96.1 ± 62.3 s), and then returned to ‘before’ levels after the predator was removed (mean ± SE: after: 76.7 ± 69.8 s). Offspring only performed the jerky swimming behaviour when the predator was present. Altogether these behavioural data indicate that offspring reacted to the predator as if it were a threat.
Table 2.
Linear mixed model results for offspring antipredator behaviours in threespine sticklebacks
| Squares moved | Time freezing | Jerky swims | ||||
|---|---|---|---|---|---|---|
| F (df) | P | F (df) | P | F (df) | P | |
| Paternal treatment | 4.22 (1, 50.9) | 0.045 | 1.42 (1, 153) | 0.24 | 0.78 (1, 12.86) | 0.40 |
| Stage | 7.18 (2, 306) | 0.0009 | 13.62 (2, 306) | <0.0001 | — | |
| Sex | 0.35 (1, 152.7) | 0.55 | 0.35 (1, 153) | 0.56 | 0.74 (1, 152.8) | 0.39 |
| Paternal treatment*sex | 0.40 (1, 152.4) | 0.53 | 0.20 (1, 153) | 0.66 | 1.82 (1, 152.8) | 0.18 |
| Paternal treatment*stage | 0.78 (2, 306) | 0.46 | 0.63 (2, 306) | 0.54 | — | |
| Stage*sex | 1.08 (2, 306) | 0.34 | 0.67 (2, 306) | 0.51 | — | |
| Paternal treatment*stage*sex | 1.28 (2, 306) | 0.28 | 0.46 (2, 306) | 0.63 | — | |
LMMs were run on length-corrected residuals of behaviours.
Significant values are shown in bold.
Paternal predator exposure influenced offspring activity (Cohen's d: before: d = 0.28; during: d = 0.23; after: d = 0.32; Table 2, effect of paternal treatment). During all stages of the behavioural assay (before, during and after predator exposure), offspring of predator-exposed fathers were less active compared to offspring of unexposed fathers (Fig. 2a).We did not detect an effect of paternal predator exposure on freezing behaviour (mean ± SE: predator-exposed father: 83.6 ± 67.5 s; unexposed father: 77.7 ± 66.9 s; Fig. 2b) or jerky swimming (mean ± SE: predator-exposed father: 1.45 ± 2.87; unexposed father: 1.85 ± 2.87; Fig. 2c).
Figure 2.
Effect of paternal treatment on (a) activity (squares moved), (b) time spent freezing and (c) number of jerky swims performed by offspring of unexposed and predator-exposed stickleback fathers before, during and after exposure to a model predator. Means (uncorrected for length) ± SE are shown.
Paternal Effects on Offspring Cortisol Stress Response
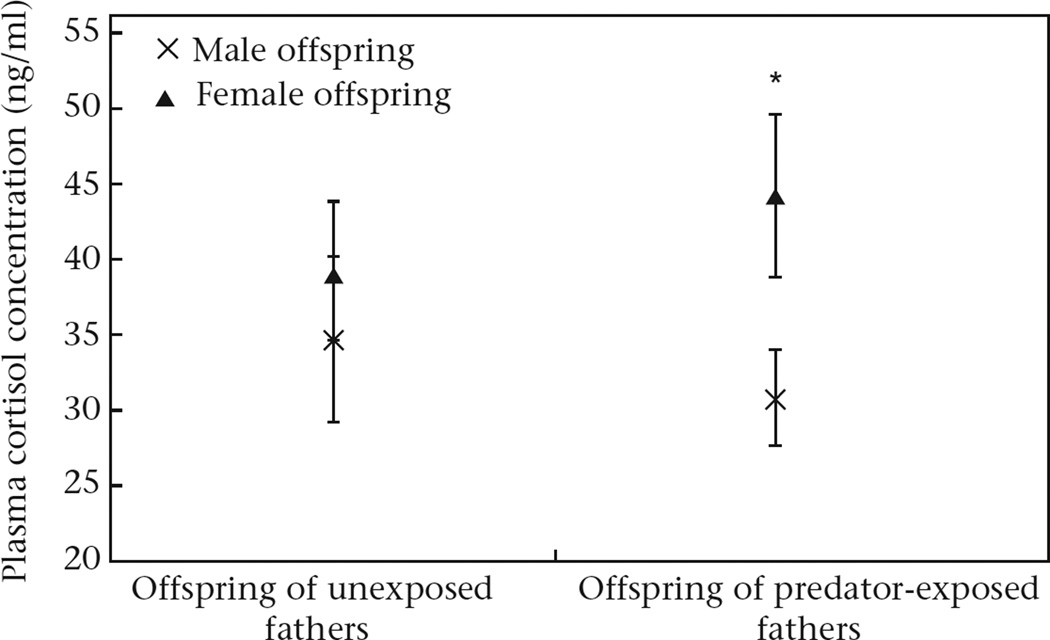
We did not find an effect of paternal treatment on circulating cortisol (LMM: F1,114.4, P = 0.69; Cohen's d = −0.06). However, the LMM suggested that the effect of paternal treatment on offspring cortisol response might depend on sex (LMM: effect of paternal treatment*sex: F1,114.3 = 2.77, P = 0.09). Therefore, we ran separate LMMS for the two paternal treatments, testing for the effects of sex as a fixed factor and father ID, clutch nested within father, home tank nested within clutch, order in which father saw a predator and ELISA plate as random factors. While there was no sex difference in offspring of unexposed fathers (LMM: effect of sex: F1,45.9 = 0.14, P = 0.70), female offspring had higher circulating cortisol than male offspring of predator-exposed fathers (LMM: effect of sex: F1,68.9 = 9.71, P = 0.003; Fig. 3).
Figure 3.
Circulating cortisol levels in stickleback offspring of unexposed fathers (N = 28 females, N = 20 males) and predator-exposed fathers (N = 45 females, N = 29 males) following exposure to a predator model. Means (uncorrected for length) ± SE are shown.
We also collected plasma from a baseline ‘control’ group of offspring that were removed directly from their home tanks and killed. These data verify that offspring reacted to the model predator as a stressor: control offspring had lower cortisol than offspring that were exposed to the predator during the behavioural assay (t test: t21.4 = 6.05, P < 0.0001).We did not detect a difference in levels of baseline circulating cortisol between paternal treatments (F1,15 = 0.065, P = 0.80) or sexes (F1,15 = 0.007, P = 0.93).
Relationships between Fathers' Behaviour and Offspring Traits
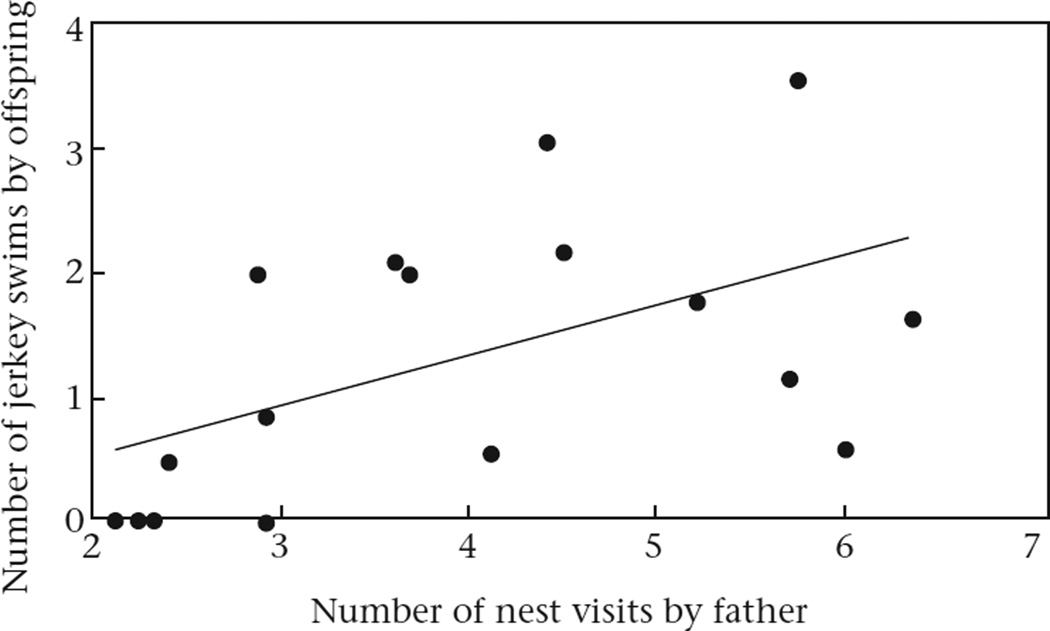
Previous analyses (Stein & Bell, 2012) revealed subtle, but detectable, effects of predator exposure on fathers' behaviour. In particular, when fathers were predator-exposed, they performed less parental behaviour than when they were unexposed. Therefore, we hypothesized that changes in fathers' behaviour might be driving some of the differences between offspring of predator-exposed versus unexposed fathers. Consistent with this hypothesis, fathers that made more visits to the nest produced offspring that performed more jerky swims (Pearson correlation: r14 = 0.57, P = 0.016, a result that passed the sequential Bonferroni test; Fig. 4). We did not find any significant correlations between fathers' behaviour, body size, body condition, nuptial colouration, cortisol, total squares moved during predator exposure or total time freezing during predator exposure.
Figure 4.
Relation between the number of nest visits by the father and the number of jerky swims performed by stickleback offspring in the presence of a predator. Regression line is shown for illustrative purposes only. Figure shows raw data (uncorrected for length).
DISCUSSION
Exposing fathers to predation risk caused them to produce offspring with phenotypes associated with living under high predation risk. In particular, offspring of predator-exposed fathers were smaller, in poorer condition and less active than offspring of unexposed fathers, and paternal exposure to predation risk induced a sex-specific difference in offspring cortisol stress response to predation. A growing literature suggests that mothering can influence offspring via nongenetic mechanisms (reviewed in Champagne, 2008), our results suggest that fathers are capable of something similar. Studies on fathering to date have focused on the effects of fathers' experience prior to parenting on offspring (Crean, Dwyer, & Marshall, 2013; Curley et al., 2011; Dias & Ressler, 2014; Rodgers et al., 2013; Sirot, Lapointe, Shatters, & Bausher, 2006) and have suggested that the magnitude of paternal effects can be similar to that of maternal effects (Head, Berry, Royle, & Moore, 2012). Our results show that a father's experience during parenting can influence offspring phenotype in much the same way as a mother's experience can. Our results also suggest that long-term differences between offspring of predator-exposed and unexposed fathers can be attributed to short-term adjustments in paternal behaviour in response to predation risk. Together, these results suggest that fathers from natural populations can transmit information about the current environment to their offspring, and that the way that fathers behave towards their offspring can shape their developmental trajectories into adulthood.
One of the advantages of the stickleback system is that there is an extensive literature on morphology and behaviour in the field and laboratory that provides a framework for interpreting some of the paternal effects observed in this study. For example, predation pressure on sticklebacks (and other species; see Endler, 1995; Relyea, 2004; Vervust et al., 2007) is associated with smaller body size at sexual maturity (Bell, Dingemanse, Hankison, Langenhof, & Rollins, 2011), lower body condition (Frommen et al., 2011), reduced male nuptial coloration (Candolin, 1998) and reduced activity (Lacasse & Aubin-Horth, 2012). In this study, offspring of predator-exposed fathers had traits associated with predator-rich conditions: they were smaller, in poorer body condition, had reduced nuptial coloration (males) and were less active than offspring of unexposed fathers. These findings suggest that the nongenetic paternal effects found here could reflect transgenerational plasticity for coping with a high-predation environment. Remarkably, our results also suggest that fathers are capable of flexibly ‘programming’ their offspring for the environment that they themselves experienced while parenting, or adaptive anticipatory paternal effects. Fathers made short-term adjustments to their parenting behaviour in response to predation risk, but a father's experience with predation risk during one breeding episode did not carry over to influence his behaviour or offspring in a subsequent breeding episode (Stein & Bell, 2012), consistent with paternal programming. Offspring of predator-exposed fathers were less active than offspring of unexposed fathers. Lower activity in the presence of predation risk can improve stickleback survival, presumably because it makes them less conspicuousness to predators (McGhee, Pintor, Suhr, & Bell, 2012). Offspring of predator-exposed fathers were less active at all stages of the behavioural assay (before, during and after the predator was present). Interestingly, we did not detect a predator-induced paternal effect on offspring freezing behaviour or jerky swimming, two behaviours that can be effective strategies to avoid capture (McGhee et al., 2012). The fact that paternal predator exposure influenced offspring activity generally rather than influencing behaviours involved in immediately avoiding predation suggests that fathers might provide their offspring with general skills for the environment they are likely to encounter rather than providing offspring with specific tools for avoiding capture. An alternative explanation for the patterns observed in this study is that perturbations while parenting caused males to reduce parenting behaviour, which caused males to produce offspring in worse condition, and poor condition caused offspring to be inactive. However, we did not detect a relationship between offspring condition and activity. Therefore, effects on behaviour are unlikely to have been driven by effects on condition. An obvious task for future studies on paternal programming is to determine whether offspring of predator-exposed fathers have higher fitness in the face of predation risk.
We did not find an overall difference in offspring cortisol response to predation risk based on paternal experience. However, we detected a trend suggesting a sex-specific paternal effect on cortisol concentration. Specifically, female offspring of predator-exposed fathers showed a higher cortisol response to predation risk than male offspring of predator-exposed fathers (similar to Zohar & Weinstock, 2011; for rats). Other studies have also found sex differences in hormonal and behavioural stress responses of offspring based on maternal experience with a stressor (reviewed in Brunton, 2013), but the proximate mechanisms that contribute to them and their ecological consequences are not well understood.
There are multiple mechanisms by which the paternal effects observed in this study might have occurred. We found that fathers that visited their nest more frequently in the presence of a model predator produced offspring that performed more antipredator behaviour (jerky swimming), which is consistent with the hypothesis that the paternal effects are behaviourally mediated. Fathers decreased fanning behaviour following predator exposure, which is also consistent with behavioural mediation (Stein & Bell, 2012). As fanning is an important predictor of reproductive success and provides oxygen to developing embryos (von Hippel, 2000), it is possible that a reduction in oxygenation might have affected offspring development. It is also possible that olfactory cues from the father might be involved. In sticklebacks, paternal odour is an important cue for imprinting (Kozak, Head, & Boughman, 2011). Spiggin, the protein produced by male kidneys and used to glue the nest together, may also contain cues for offspring about the fathers' state (Kozak et al., 2011) and is continually added to the nest throughout parenting. The paternal effects observed here might also have been hormonally mediated: fish release steroid hormones, including cortisol, into the water via the gills (Scott & Ellis, 2007), fathers are in close contact with their nests, and fish embryos can take up cortisol from their surroundings (McCormick, 1999). Therefore, it is possible that offspring were exposed to paternal hormones, potentially even paternal cortisol, which could have had organizational effects on the development of the hypothalamic–pituitary–interrenal (HPI axis), body size and behaviour.
That being said, we can rule out some alternative explanations for the differences between offspring of predator-exposed and unexposed fathers that were observed in this study. For example, fathers were exposed to predation risk after their eggs were already fertilized, so the paternal effects cannot be due to changes in sperm (Curley et al., 2011; Dias & Ressler, 2014) or seminal fluid (Rodgers et al., 2013; Simmons, 2011; Sirot et al., 2006). It is also unlikely that the effects of paternal treatment can be attributed to the direct exposure of offspring to visual cues of the predator (Darmaillacq, Lesimple, & Dickel, 2008). Fathers in the ‘predator-exposed’ treatment were exposed to predation risk by a model sculpin when their offspring were 3 days old (postfertilization, prehatching), when the optic cups of the embryo are still developing (Swarup, 1958) and when the eggs are covered by nesting material. In addition, because fathers were exposed to a rubber model predator rather than a live predator, there was no opportunity for olfactory cues from a predator to reach the eggs (Ferrari & Chivers, 2010; Nelson, Alemadi, & Wisenden, 2013).
While maternal effects on offspring have been examined in natural systems, paternal effects on offspring and their ecological and evolutionary implications have remained relatively understudied. Our results provide some of the first evidence for behavioural transmission of the paternal environment to offspring. Fathers made short-term adjustments in response to predation risk that influenced both the morphology and behaviour of their offspring, but these effects were reversible within fathers (i.e. a male's subsequent clutches were not influenced by his previous experience). In a system such as sticklebacks, where fathers breed more than once during the breeding season (Wootton, 1984), this suggests that fathers' experiences and behaviour from one clutch to another can produce offspring with potentially different fitness outcomes based on the environment in which they occur. Whether this is a true anticipatory parental effect, such that offspring show greater fitness when raised in the environment that their father experienced, remains to be explored.
Acknowledgments
We thank G. Kozak, M. Stager, members of the Bell laboratory and two anonymous referees for valuable feedback on this manuscript. We thank Rebecca Trapp, Jessica Mulcrone and Valerie Sefton for help with video coding. This project was supported by the University of Illinois Department of Animal Biology, National Science Foundation (NSF) Doctoral Dissertation Improvement Grant (IOS 1210696) and NSF Graduate Research Fellowship to L.R.S., and NSF IOS 1121980 and National Institutes of Health (NIH) R01 GM082937 to A.M.B.
References
- Abrams PA, Rowe L. The effects of predation on the age and size of maturity of prey. Evolution. 1996;50:1052–1061. doi: 10.1111/j.1558-5646.1996.tb02346.x. [DOI] [PubMed] [Google Scholar]
- Agrawal AA, Laforsch C, Tollrian R. Transgenerational induction of defences in animals and plants. Nature. 1999;401:60–63. [Google Scholar]
- Bates D, Maechler M, Bolker B. lme4: Linear mixed-effects models using S4 classes. 2012 (R package version 0.999999-0) http://CRAN.R-project.org/package=lme4. [Google Scholar]
- Bell AM, Dingemanse NJ, Hankison SJ, Langenhof MBW, Rollins K. Early exposure to nonlethal predation risk by size-selective predators increases somatic growth and decreases size at adulthood in threespined sticklebacks. Journal of Evolutionary Biology. 2011;24:943–953. doi: 10.1111/j.1420-9101.2011.02247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boughman JW. Condition-dependent expression of red colour differs between stickleback species. Journal of Evolutionary Biology. 2007;20:1577–1590. doi: 10.1111/j.1420-9101.2007.01324.x. [DOI] [PubMed] [Google Scholar]
- Brunton PJ. Effects of maternal exposure to social stress during pregnancy: consequences for mother and offspring. Reproduction. 2013;146:R175–R189. doi: 10.1530/REP-13-0258. [DOI] [PubMed] [Google Scholar]
- Candolin U. Reproduction under predation risk and the trade-off between current and future reproduction in the threespine stickleback. Proceedings of the Royal Society B: Biological Sciences. 1998;265:1171–1175. [Google Scholar]
- Champagne FA. Epigenetic mechanisms and the transgenerational effects of maternal care. Frontiers in Neuroendocrinology. 2008;29:386–397. doi: 10.1016/j.yfrne.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. A power primer. Psychological Bulletin. 1992;112:155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- Crean AJ, Dwyer JM, Marshall DJ. Adaptive paternal effects? Experimental evidence that the paternal environment affects offspring performance. Ecology. 2013;94:2575–2582. doi: 10.1890/13-0184.1. [DOI] [PubMed] [Google Scholar]
- Curley JP, Mashoodh R, Champagne FA. Epigenetics and the origins of paternal effects. Hormones and Behavior. 2011;59:306–314. doi: 10.1016/j.yhbeh.2010.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darmaillacq A, Lesimple C, Dickel L. Embryonic visual learning in the cuttlefish, Sepia officinalis. Animal Behaviour. 2008;76:131–134. [Google Scholar]
- Dias BG, Ressler KJ. Parental olfactory experience influences behaviour and neural structure in subsequent generations. Nature Neuroscience. 2014;17:89–96. doi: 10.1038/nn.3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endler JA. Multiple-trait coevolution and environmental gradients in guppies. Trends in Ecology & Evolution. 1995;10:22–29. doi: 10.1016/s0169-5347(00)88956-9. [DOI] [PubMed] [Google Scholar]
- Ferrari MCO, Chivers DP. The ghost of predation future: threat-sensitive and temporal assessment of risk by embryonic woodfrogs. Behavioral Ecology and Sociobiology. 2010;64:549–555. [Google Scholar]
- Feuth-De Bruijn E, Sevenster P. Parental reactions to young in sticklebacks (Gasterosteus aculeatus L) Behaviour. 1983;83:186–203. [Google Scholar]
- Frommen JG, Herder F, Engqvist L, Mehlis M, Bakker TCM, Schwarzer J, et al. Costly plastic morphological responses to predator specific odour cues in three-spined sticklebacks (Gasterosteus aculeatus) Evolutionary Ecology. 2011;25:641–656. [Google Scholar]
- Grobis MM, Pearish SP, Bell AM. Avoidance or escape? Discriminating between two hypotheses for the function of schooling in threespine sticklebacks. Animal Behaviour. 2013;85:187–194. doi: 10.1016/j.anbehav.2012.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head ML, Berry LK, Royle NJ, Moore AJ. Paternal care: direct and indirect genetic effects of fathers on offspring performance. Evolution. 2012;66:3570–3581. doi: 10.1111/j.1558-5646.2012.01699.x. [DOI] [PubMed] [Google Scholar]
- von Hippel FA. Vigorously courting male sticklebacks are poor fathers. Acta Ethologica. 2000;2:83–89. [Google Scholar]
- Kozak GM, Head ML, Boughman JW. Sexual imprinting on ecologically divergent traits leads to sexual isolation in sticklebacks. Proceedings of the Royal Society B: Biological Sciences. 2011;278:2604–2610. doi: 10.1098/rspb.2010.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznetsova A, Brockhoff PB, Christensen RHB. lmerTest: Test for random and fixed effects for linear mixed models (lmer objects of lme4 package) 2013 (R package version 1.2-0) http://CRAN.R-project.org/package=lmerTest. [Google Scholar]
- Lacasse J, Aubin-Horth N. A test of the coupling of predator defense morphology and behaviour variation in two threespine stickleback populations. Current Zoology. 2012;58:53–65. [Google Scholar]
- McCormick MI. Experimental test of the effect of maternal hormones on larval quality of a coral reef fish. Oecologia. 1999;118:412–422. doi: 10.1007/s004420050743. [DOI] [PubMed] [Google Scholar]
- McGhee KE, Pintor LM, Suhr EL, Bell AM. Maternal exposure to predation risk decreases offspring antipredator behaviour and survival in threespined stickleback. Functional Ecology. 2012;26:932–940. doi: 10.1111/j.1365-2435.2012.02008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod J, Sinal CJ, Perrot-Sinal TS. Evidence for non-genomic transmission of ecological information via maternal behaviour in female rats. Genes, Brain, and Behavior. 2007;6:19–29. doi: 10.1111/j.1601-183X.2006.00214.x. [DOI] [PubMed] [Google Scholar]
- Mommer BC, Bell AM. A test of maternal programming of offspring stress response to predation risk in threespine sticklebacks. Physiology & Behavior. 2013;122:222–227. doi: 10.1016/j.physbeh.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moodie GEE. Predation, natural-selection and adaptation in an unusual 3 spine stickleback. Heredity. 1972;28:155–167. [Google Scholar]
- Mousseau TA, Fox CW. The adaptive significance of maternal effects. Trends in Ecology & Evolution. 1998;13:403–407. doi: 10.1016/s0169-5347(98)01472-4. [DOI] [PubMed] [Google Scholar]
- Nelson AB, Alemadi SD, Wisenden BD. Learned recognition of novel predator odour by convict cichlid embryos. Behavioral Ecology and Sociobiology. 2013;67:1269–1273. [Google Scholar]
- Peichel CL, Ross JA, Matson CK, Dickson M, Grimwood J, Schmutz J, et al. The master sex-determination locus in threespine sticklebacks is on a nascent Y chromosome. Current Biology. 2004;14:1416–1424. doi: 10.1016/j.cub.2004.08.030. [DOI] [PubMed] [Google Scholar]
- Pressley PH. Parental effort and the evolution of nest-guarding tactics in the threespine stickleback, Gasterosteus aculeatus L. Evolution. 1981;35:282–295. doi: 10.1111/j.1558-5646.1981.tb04887.x. [DOI] [PubMed] [Google Scholar]
- Relyea RA. Fine-tuned phenotypes: tadpole plasticity under 16 combinations of predators and competitors. Ecology. 2004;85:172–179. [Google Scholar]
- Rice TK, Schork NJ, Rao DC. Methods for handling multiple testing. Advances in Genetics. 2008;60:293–308. doi: 10.1016/S0065-2660(07)00412-9. [DOI] [PubMed] [Google Scholar]
- Rodgers AB, Morgan CP, Bronson SL, Rovello S, Bale TL. Paternal stress exposure alters sperm microRNA content and reprograms offspring HPA stress axis regulation. Journal of Neuroscience. 2013;33:9003–9012. doi: 10.1523/JNEUROSCI.0914-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott AP, Ellis T. Measurement of fish steroids in water: a review. General and Comparative Endocrinology. 2007;153:392–400. doi: 10.1016/j.ygcen.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Sheriff MJ, Krebs CJ, Boonstra R. The ghosts of predators past: population cycles and the role of maternal programming under fluctuating predation risk. Ecology. 2010;91:2983–2994. doi: 10.1890/09-1108.1. [DOI] [PubMed] [Google Scholar]
- Simmons LW. Allocation of maternal- and ejaculate-derived proteins to reproduction in female crickets, Teleogryllus oceanicus. Journal of Evolutionary Biology. 2011;24:132–138. doi: 10.1111/j.1420-9101.2010.02158.x. [DOI] [PubMed] [Google Scholar]
- Sirot LK, Lapointe SL, Shatters R, Bausher M. Transfer and fate of seminal fluid molecules in the beetle, Diaprepes abbreviatus: implications for the reproductive biology of a pest species. Journal of Insect Physiology. 2006;52:300–308. doi: 10.1016/j.jinsphys.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Stein LR, Bell AM. Consistent individual differences in fathering in threespined stickleback Gasterosteus aculeatus. Current Zoology. 2012;58:45–52. doi: 10.1093/czoolo/58.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm JJ, Lima SL. Mothers forewarn offspring about predators: a transgenerational maternal effect on behaviour. American Naturalist. 2010;175:382–390. doi: 10.1086/650443. [DOI] [PubMed] [Google Scholar]
- Swarup H. Stages in the development of the stickleback Gasterosteus aculeatus (L) Journal of Embryology and Experimental Morphology. 1958;6:373–383. [PubMed] [Google Scholar]
- Tulley JJ, Huntingford FA. Paternal care and the development of adaptive variation in antipredator responses in sticklebacks. Animal Behaviour. 1987;35:1570–1572. [Google Scholar]
- Uller T. Developmental plasticity and the evolution of parental effects. Trends in Ecology & Evolution. 2008;I:432–438. doi: 10.1016/j.tree.2008.04.005. [DOI] [PubMed] [Google Scholar]
- Vervust B, Grbac I, Van Dame R. Differences in morphology, performance and behaviour between recently diverged populations of Podarcis sicula mirror differences in predation pressure. Oikos. 2007;116:1343–1352. [Google Scholar]
- Weisser WW, Braendle C, Minoretti N. Predator-induced morphological shift in the pea-aphid. Proceedings of the Royal Society B: Biological Sciences. 1999;266:1175–1181. [Google Scholar]
- Wootton RJ. A functional biology of sticklebacks. London, U.K.: Croom Helm; 1984. [Google Scholar]
- Zohar I, Weinstock M. Differential effect of prenatal stress on the expression of cortiocotrophin-releasing hormone and its receptors in the hypothalamus and amygdala in male and female rats. Journal of Neuroendocrinology. 2011;23:320–328. doi: 10.1111/j.1365-2826.2011.02117.x. [DOI] [PubMed] [Google Scholar]