Summary
Coumarin derivative RKS262 belongs to a new class of potential anti-tumor agents. RKS262 was identified by structural optimization of Nifurtimox which is currently undergoing phase II clinical trials to treat high-risk neuroblastoma. In a NCI60 cell-line assay RKS262 exhibited significant cytotoxicity in ovarian cancer cells and a variety of other cell lines exceeding effects of commercial drugs such as cisplatin, 5-FU, cyclophosphamide or sapacitabine. Various leukemia cell-lines were most sensitive (GI50:~10 nM) while several non-small cell lung cancer cell lines and few cell lines from other tissues were relatively resistant (GI50> *1µM) to RKS262 treatment. The mechanism of cytotoxicity was examined using ovarian cancer cell-line OVCAR-3 as a model. RKS262 treatment resulted in a reduced mitochondria-transmembrane-depolarization potential. RKS262 effects included up-regulation of apoptotic markers and were not correlated with activation of proapoptotic MAP-Kinases (p38, SAP/JNK). RKS262 exerted strong inhibitory effects on oncogene ras, down-regulated DNA-pk KU-80 subunit expression and caused activation of Akt. A signature effect of RKS262 is the regulation of the mitochondrial Bcl2-family pathway. Pro-apoptotic factors Bid, Bad and Bok were up-regulated while expression of pro-survival factors Bcl-xl and Mcl-1 was inhibited. Moreover, at sub-cytotoxic doses RKS262 delayed OVCAR-3 cell-cycle progression through G2 phase and up-regulated p27 while cyclin-D1 and Cdk-6 were down-regulated, indicating that RKS262 is a specific cyclin/CDK inhibitor. In summary, RKS262 has been identified as a molecule belonging to a new class of potential chemotherapeutic agents affecting the viability of multiple cancer cell-lines and causing selective adverse effects on the viability of ovarian cancer cells.
Keywords: Coumarin derivative RKS262, Ovarian cancer, Cytotoxicity, Cell-cycle regulation, Bcl2 class proteins, MAPK, Akt signaling
Introduction
Ovarian cancer is the eighth most common cause of death caused by cancer in women with worldwide more than 190,000 newly diagnosed cases each year. Incidence rates vary considerably, with the highest found in the United States and Northern Europe and the lowest in Africa and Asia [1]. The majority of patients with ovarian cancer present late with advanced disease (FIGO stage III/IV) and despite multimodality treatment with surgical debulking followed by platinum-taxane combination chemotherapy, median survival is only 3 years. While re-treatment with a platinum-based drug is possible for some women the response rate to current second line chemotherapy is 15–30% due to the rise of resistance to these drugs requiring the development of new drugs to treat this cancer.
Nifurtimox [Fig. 1A; compound 1], an agent originally employed to treat Chagas’ disease, causes cytotoxic and antitumor effects in neuroblastoma cell lines [2, 3], has shown promise in a phase 1 trial [4] and is currently being evaluated in a phase 2 trial for the treatment of childhood neuroblastoma. Nifurtimox also exhibited cytotoxicity against cells lines derived from ovarian cancer even though with a lower efficacy [unpublished data]. In order to identify an analog of Nifurtimox with higher potency we carried out a structural optimization. A variety of analogs were synthesized and their cytotoxicity evaluated against a platinum-resistant ovarian cancer cell line (OVCAR-3) as a model system. RKS262 [Fig. 1A; compound 5], a coumarine derivative that includes the 1-aminotetrahydrothiazine ring of Nifurtimox, revealed the most potent activity among Nifurtimox analogs tested and was selected as ‘lead’ molecule for our study.
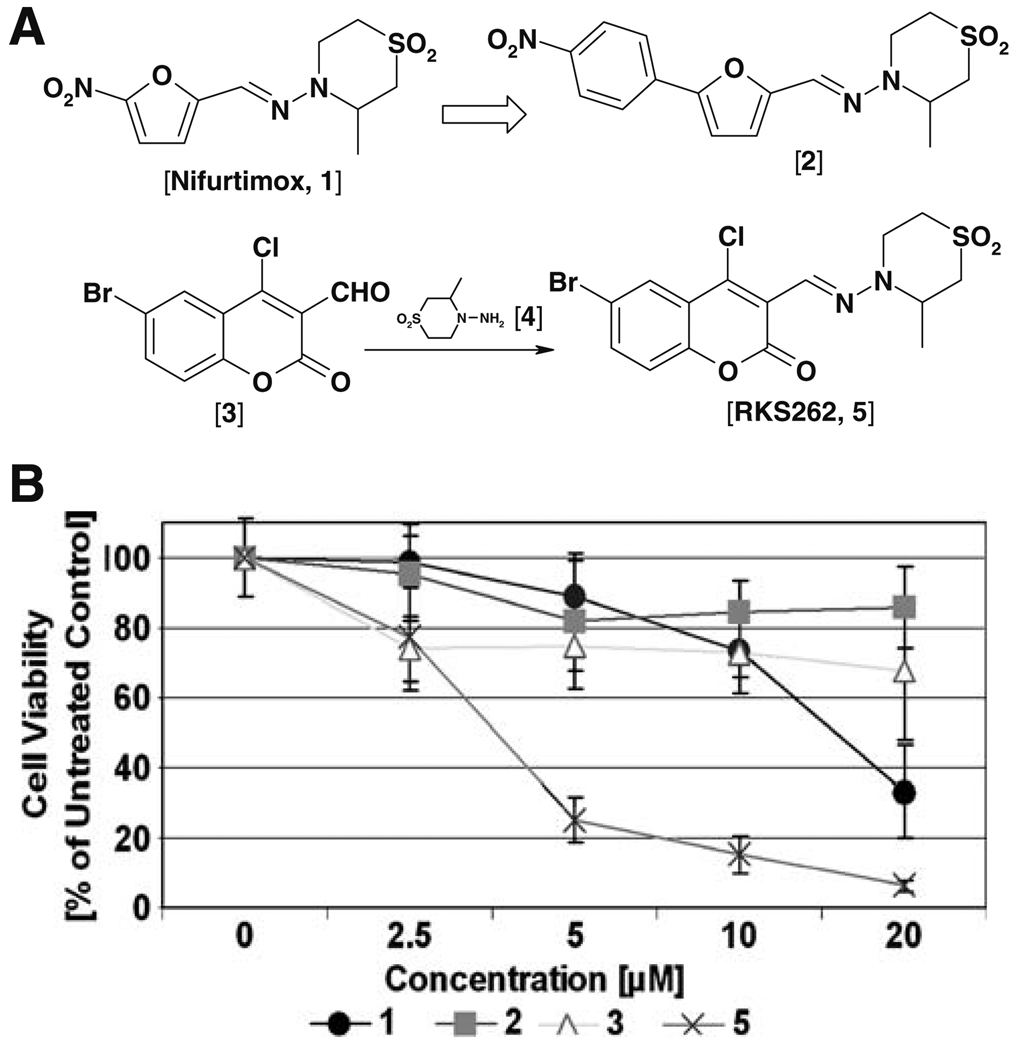
Fig. 1.
Design of coumarin derivative RKS262 and cytotoxic effects in platinum resistant OVCAR-3 ovarian cancer cells. A RKS262, related to Nifurtimox [1] and Nifurtimox derivative [2] was synthesized in multiple steps; the synthesis of a key intermediate, the 1-aminotetrahydrothiazane ring [4] was carried out by developing a more efficient, less toxic and safer methodology (details to the experimental protocol are described in the Supplementary Materials, Online Resource 1). The condensation of commercially available coumarin aldehyde [3] with [4] in anhydrous EtOH at 60–70°C afforded RKS262 [5] as a yellow colored solid, which was characterized by NMR and Mass. Purity was ascertained by HPLC. B Comparative analysis of the cytotoxic effect of RKS262 [5], its precursor coumarin aldehyde [3], Nifurtimox [1] and its close structural analog [2] in OVCAR-3 cells (human ovarian epithelial adenocarcinoma cell line). Cells were treated with compounds in various concentrations (0–20 µM) for 48 h. An MTS viability assay was carried out as described (Materials and methods). Data are expressed as the mean of triplicate determinations (X±SD) of a representative experiment in % cell viability of samples with untreated cells [100%]
The antitumor potential of RKS262 was examined in multiple assays. To evaluate the tumor type selectivity of this compound a NCI60 cell line growth/viability assay featuring cell-lines derived from refractory tumors of 12 different tissues (http://dtp.nci.nih.gov/screening.html) was utilized. Next, the mechanism of cytotoxicity was analyzed in OVCAR-3 cells which, apart from platinum-resistance, feature various mutations present in a variety of ovarian and other solid tumors [5]. In addition, the antitumor potential of RKS262 to treat ovarian cancer cells was investigated by measuring anti-proliferative effects in conjunction with the expression profile of pro-apoptotic and pro-survival Bcl2 family proteins. Furthermore we studied the effects of sub-cytotoxic concentrations of RKS262 on cell-cycle progression and the expression of regulatory proteins such as p27, cyclins and CDKs in OVCAR-3 cells.
RKS262 revealed potent cytotoxicity in multiple chemoresistant cancer cell types including ovarian cancer. In OVCAR-3 cells induction of apoptosis through RKS262 treatment is not directly correlated with activation of proapoptotic MAP-Kinases (p38, SAP/JNK) but with inhibitory effects on oncogene ras, down-regulation of DNA-pk KU-80 subunit expression and activation of AKT. RKS262 up-regulated pro-apoptotic Bcl2 family members such as Bad, Bid and Bok and down-regulated prosurvival Bcl2 family proteins Mcl-1 and Bcl-xl. Moreover, at sub-cytotoxic concentrations, RKS262 delayed progression of OVCAR-3 cells through G2 phase and upregulated p27 while cyclins-D1, cyclin-D3 and Cdk-6 were down-regulated.
Materials and methods
Synthesis of RKS262 and analogs
RKS262 was synthesized in multiple steps [Fig. 1] and details of the synthesis and experimental protocol are described in the supplementary material (Online Resource 1). The synthesis of a key intermediate, the tetrahydrothiazane ring, could not be achieved by the procedure described in the literature [6]. We developed a new route to synthesize this intermediate in multi-gram quantities with high yield. The treatment of sulfone diol with tertiary butyl carbazate in refluxing sodium hydroxide solution overnight afforded the tetrahydrothiazane ring. Removal of the tert-Boc protecting group on the primary nitrogen achieved the cyclization of the sulfone diol to its corresponding amine. This improved methodology provides a strategy to avoid the use of carcinogenic and highly explosive hydrazine reagents used previously [6]. Finally, the condensation of commercially available coumarin aldehyde [Fig. 1; compound 3] with the newly synthesized amine afforded RKS262 [compound 5] as a yellow colored solid, which was characterized by NMR and Mass Spectroscopy. Purity was ascertained by HPLC. Stock solutions of RKS262 in 10 mM DMSO were stored at −20°C.
Cell lines (human)
OVCAR-3 (ovarian epithelial adenocarcinoma) cells were purchased from ATCC (Manassas, VA) and grown in T75/T150 cell culture flasks (Corning, New York, NY) in DMEM medium (Gibco, Rockville, MD) supplemented with 20% fetal bovine serum (Atlanta Biologicals, Lawrenceville, GA) and 100 U/ml penicillin, and 100 µg/ml streptomycin at 37°C, 5% CO2, in a humidified incubator. For all assays cells were allowed to attach overnight after seeding and treated in complete medium.
Cell viability assay
Viability of cells was determined by the 96® Aqueous-One-Solution Assay (Promega, Madison, WI) following the manufacturer’s recommendations. This colorimetric assay is based on the ability of mitochondria to reduce a substrate (MTS) into a soluble formazan product with an absorbance at 490 nm (ELISA plate reader; (Thermo Labsystems, Waltham, MA) directly proportional to the number of living cells (Promega, Madison, WI). Cells (5×103) were plated into 96 well flat bottom plates (Corning, Inc., Corning, NY) and treated with RKS262 as indicated (Results section). Following incubation for 46 h the MTS reagent was added for an additional 2 h and absorbance measured at 490 nm. Experiments were performed in triplicates; data are expressed as the mean of the triplicate determinations (X±SD) of a representative experiment in % of absorbance of samples with untreated cells [100%]. The description of NCI60 cell lines assay can be found at http://dtp.nci.nih.gov and a protocol is provided in the Supplementary Material (Online Resource 1).
Mitochondrial transmembrane-potential
OVCAR-3 cells (1 × 106) were seeded in a 100 mm2 petridish and treated with 5 µM of RKS262 for 3 h or 24 h. Following treatment cells were washed with PBS, resuspended in fresh medium (5 × 105 cells/ml) and incubated with 15 nM DiOC18(3) (Invitrogen Corporation) for 30 min at 37°C. The cells were washed twice with DPBS, resuspended in an equal volume of DPBS analyzed by flow cytometry (excitation at 488 nm, emission at 520 nm). Data was acquired on a BD FACSort flow cytometer using CellQuest software (BD Immunocytometry-Systems, San Jose, CA) and analyzed (ModFit LT software, Verity Software House, Inc., Topsham, ME). Ten thousand cells were analyzed for each sample.
Cell-cycle analysis (by FACS)
Cell-cycle analysis was carried out by flow cytometry. OVCAR-3 cells were seeded into 100 mm2 tissue culture dishes (1 × 106 cells/dish) (Corning Inc., Corning, NY), treated with RKS262 (1 µM or 3 µM) for 24 h or 48 h and the assay carried out as described previously [7]. Appropriate gating was used to select the single cell population. The same gate was used on all samples, ensuring that the measurements were made on a standardized cell population. Experiments were performed in duplicate.
Western blot analysis
OVCAR-3 cells were seeded into 100 mm2 tissue culture dishes (1 × 106 cells/dish) and treated with RKS262 (5 µM) for various time intervals as indicated (Results section). Preparation of cell lysates, PAGE and immunoblotting was carried out as described elsewhere [7]. Primary antibodies were all purchased from (Cell Signaling Technology, Beverly, MA). The bands were visualized using horseradish peroxidase-conjugated secondary antibodies (Amersham-Pharmacia Biotech, Piscataway, NJ), followed by enhanced chemiluminescence (Upstate, Waltham, MA) and documented autoradiography (F-Bx810 Film, Phoenix, Hayward, CA). As a size standard prestained Precision Plus Protein Kaleidoscope (Biorad, Hercules, CA) marker was used. Experiments were performed in duplicate.
Data analysis
Mean and standard deviation (SD) were calculated. Mean differences were determined by Student’s t-test or determined by one-way ANOVA. Software used for these analyses was STATA 9.0 (StataCorp, College Station, TX).
Results
Development of a new route to synthesize RKS262 and analogs
We modified and improved the synthesis of 1-aminotetrahydrothiazane [4], the key intermediate in the synthesis of Nifurtimox, because the method previously described [6] could not be reproduced in our hands despite repeated attempts. In addition the previously described method [6] required the use of hydrazine, a known carcinogen and a highly explosive agent. We instead used tert-butyl carbazate to accomplish the ring cyclization and achieved tert-butyl Boc deprotection of the amine group in situ in one step to generate a 1-amino tetrahydrothiazane ring [coumpound 4] in multi-gram quantities in good yield (60–70%, un-optimized). Coupling of the ring with 6-bromo,4-choloro coumarin-3-aldehyde [compound 3] generated RKS262 in good yield (60%, un-optimized). Other analogs such as Nifurtimox [compound 1] and nitrophenyl analog [coumpound 2] were prepared under similar conditions (Fig. 1A).
Viability of various cancer cell types upon treatment with RKS262
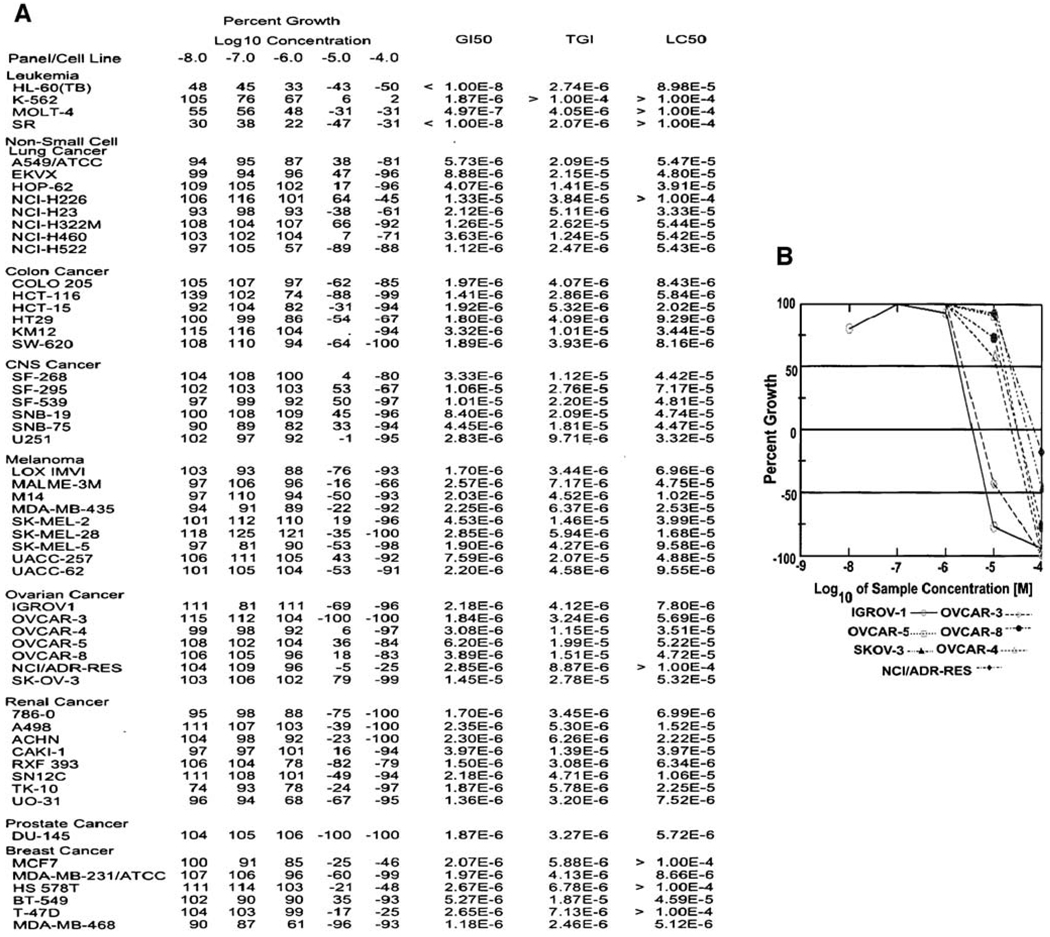
In an initial approach to analyze the effects of RKS262 on ovarian cancer cells, we performed a cytotoxicity assay (Fig. 1B) comparing Nifurtimox [compound 1] and analog [compound 2] to RK262 [compound 5] and precursors i.e. coumarin aldehyde [compound 3]. RKS262 proved to be highly and dose-dependently cytotoxic to OVCAR-3 (75% cytotoxicity at 5 µM) cells. For Nifurtimox, similar toxicity required a concentration of 20 µM while the other derivatives displayed minimal toxicity even at 20 µM. To evaluate potential tumor-type selectivity, RKS262 was screened in a NCI60 cell-line growth/viability assay (Fig. 2A). RKS262 treatment resulted in cytotoxicity to most of NCI60 cell-lines tested. Specifically, leukemia cell lines (e.g. HL-60/MOLT-4/SR) were most sensitive (GI50:10 nM) whereas several non-small cell lung cancer (incl. NCI322M/NCI-H226) as well as some cell lines from other tissues were relatively resistant (breast/BT549, CNS/SNB19, melanoma/UACC257, renal/786-0, ovarian/SKOV-3) to RKS262 treatment (GI50 >1 µM). The comparison of key parameters such as GI50, TGI and LC50 (Fig. 2A) which define growth inhibition (50%), total growth inhibition and lethal concentration (50%), respectively, indicate that RKS262 is superior to various clinically used anti-cancer drugs (e.g. cisplatin, 5-FU, cyclophosphamide, sapacitabine) (http://dtp.nci.nih.gov/screening.html). In the same assay the dose-dependent effect of various concentrations of RKS262 (100 nM–100 µM) on a panel of ovarian cancer cell lines was also compared (Fig. 2B). The growth of IGROV-1 and OVCAR-3 were highly affected, OVCAR-8, OVCAR-4 and OVCAR-5 responded strongly to treatment, whereas SKOV-3 revealed less reduction in growth upon RKS262 treatment. The NCI60 cell-line screen and our cytotoxicity assays clearly suggest RKS262 to be highly damaging to cell lines derived from a variety of tumor types, including ovarian cancer. Similarly cell-line selectivity, yet not tumor-type specificity, has been observed in other classes of drugs screened in NCI60 cell-lines [7, 8].
Fig. 2.
Effect of RKS262 [NSC-D-746733-1] on cell growth in a NCI60 cancer screen. A Cells were treated in 96-well plates with RKS262 (10 nM–10 µM) or vehicle and cell growth of the TCA fixed treated and untreated cells was assessed after 48 h with sulphorhodamine-B (SRB) solution and absorbance read at 515 nM. (For details see Supplementary Material, Online Resource 1). B Dose-dependent effect of RKS262 on ovarian cancer cell lines in the NCI60 screen
Cell-cycle analysis of OVCAR-3 cells after RKS262 treatment
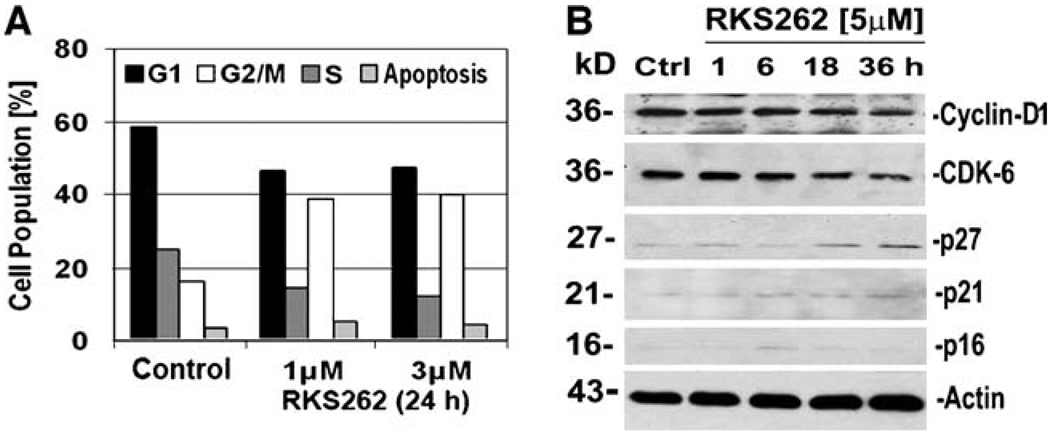
Cytotoxic agents at subcytotoxic concentrations often display cell-cycle regulatory effects. For example, isothiocyanate 7Me-IEITC arrested progression of SKOV-3 ovarian cancer cells in G2/M phase and its close structural analog NB7M in SKOV-3 caused G1 phase arrest [7]. Using FACS we analyzed the effect of subcytotoxic (1 µM) and IC50 (~3 µM) concentration of RKS262 (24 h treatment) in OVCAR-3 cells. Our analysis revealed a significant increase in the G2/M-phase cell population followed by reduction of cells in G1-phase as compared to untreated control (Fig. 3A). This indicated that proliferation of OVCAR-3 cells was adversely affected, while a block of cell-cycle progression in G2/M-phase in this non-synchronized culture appeared within 24 h of treatment. We performed western blot analysis of the lysates of OVCAR-3 cells to investigate the expression of cell-cycle regulatory factors upon drug treatment (Fig. 3B). Western blotting revealed that RKS262 caused cyclin D1 down-regulation and a p21-independent inhibition of CDK-6. In contrast p27 which previously has been suggested as a target for cancer therapeutics [9] was up-regulated by RKS262 treatment. Immunoblotting further revealed that RKS262 treatment of OVCAR-3 did not alter the expression of members of the Cip/Kip family of cyclin-dependent kinase (CDK) inhibitors, p21 and p16 (Fig. 3B).
Fig. 3.
Effect of RKS262 on cell-cycle progression and regulators in OVCAR-3 cells. A OVCAR-3 cells were treated with RKS262 (1or 3 µM) or vehicle for 24 h. Cell-cycle analysis of treated and untreated cells by FACS was carried out as described (Materials and Methods). Data are presented as the relative fluorescence intensity of cell subpopulations in a bar chart. B Expression of Cyclin-D1, CDK-6, p16, p21 and p27 in RKS262 and vehicle-treated OVCAR-3 cells was analyzed by Western blotting (Materials and Methods) of cell lysates using appropriate primary and secondary antibodies. As an internal standard for equal loading the blots were probed with an anti-β-actin antibody. Experiments were performed independently in duplicates showing identical outcomes
RKS262 disrupted the mitochondrial membrane potential of OVCAR-3 and caused morphological hallmarks of apoptosis
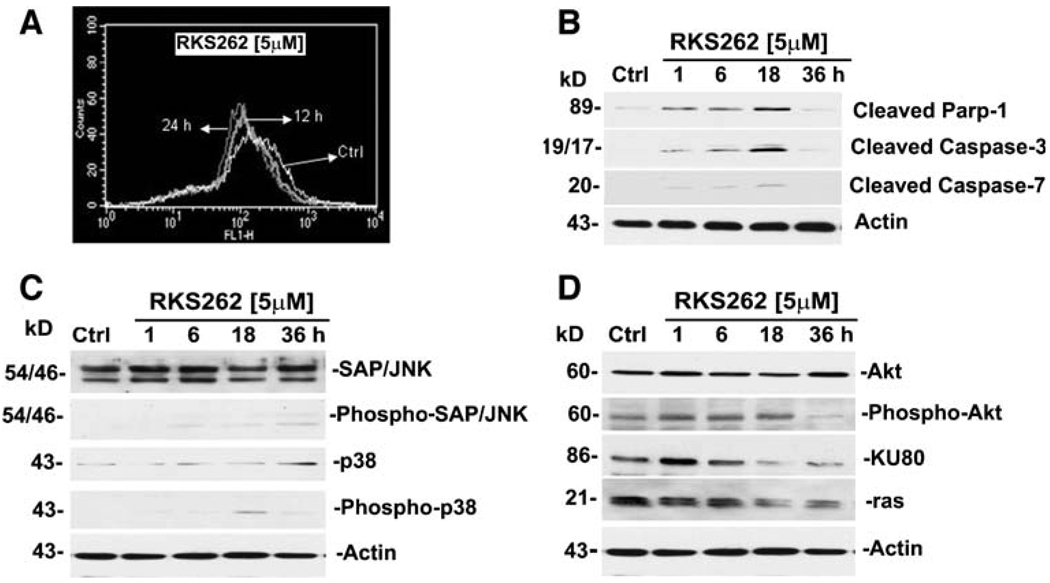
To understand the mechanism of cytotoxic response of OVCAR-3 cell to RKS262, we examined the mitochondrial transmembrane depolarization potential (ΔYm) cells by flow cytometry. RKS262 at a concentration of 5 µM caused a loss of ΔYm within 12 h of treatment (Fig. 4A). RKS262 treatment also led to similar reductions in the ΔYm of SMS-KCNR, a chemotherapy-resistant neuroblastoma cancer cell line (unpublished data). Loss of ΔYm due to chemical agents has been reported to be an indicator of onset of early and irreversible apoptotic events [10].
Fig. 4.
RKS262 causes apoptosis in OVCAR-3 platinum-resistant ovarian cancer cells. A Mitochondrial membrane depolarization analysis: OVCAR-3 cells were treated for 12 or 24 h with vehicle or 5 µM RKS262 and stained with DiOC18(3) as described (Materials and Methods). Fluorescence of the single-cell population was measured by flow cytometry and the transmembrane depolarization potential of the single-cell populations plotted. Ten thousand cells were analyzed in each sample. B Caspase activation following RKS262 treatment: OVCAR-3 cells were treated with vehicle or 5 µM RKS262 for 1, 6, 18 or 36 h. Analysis of the expression of proteins in the lysates of treated and untreated cells was carried out by Western blot analysis as described (Material and Methods). Primary antibodies against activated caspases-3, -7 and inactivated/cleaved PARP-1 were used. C Expression of pro-apoptotic MAPK in OVCAR-3: Cells were treated with vehicle or 5 µM RKS262 for 1, 6, 18 or 36 h. Analysis of the expression of proteins in the lysates of treated and untreated cells by Western blot analysis was carried out as described (Material and Methods) using primary antibodies against pro- and activated/phosphorylated (P-) SAP/JNK and p38. D Inactivation of survival signaling proteins in OVCAR-3: Cells were treated with vehicle or 5 µM of RKS262 for 1 6, 18 or 36 h. Analysis of the expression of proteins in the lysates of treated and untreated cells by PAGE/western blot analysis was carried out using primary antibodies against ras, KU80, pro- and activated/phosphorylated Akt proteins
Induction of apoptosis in OVCAR-3 ovarian cancer cells by RKS262
Cell death via apoptosis is executed by initiator caspases (such as caspases-2, -8, -9 and -10) which leads to activation of downstream effector caspases (such as caspases-3, -6 and -7) and, subsequently, of many intracellular proteins and morphological and biochemical changes associated with apoptosis [11, 12]. RKS262 treatment of OVCAR-3 cells (5 µM) resulted in the activation/cleavage of executioner caspase-3 and caspase-7 within 1 h (reaching maximal activation at 18 h) as shown by immunoblotting (Fig. 4B). The activation of proteolytic caspases-3 and -7 following RKS262 exposure to OVCAR-3 cells resulted in the cleavage of PARP-1 within 1 h of treatment (Fig. 4B). PARP, a 116 kD nuclear poly (ADP-ribose) polymerase, is involved in DNA repair, and cleavage of PARP facilitates cellular disassembly and serves as a marker of cells undergoing apoptosis [13].
RKS262 treatment suppression of pro-survival markers in OVCAR-3 ovarian cancer cells
To define key signaling responses of OVCAR-3 cells upon treatment with RKS262, we analyzed the expression and activation/phosphorylation of cellular markers involved in pro-apoptotic signaling. Immunoblotting of cell lysates revealed that RKS262 (at 5 µM) caused only a minor upregulation/activation of SAP/JNK and p38 MAPK (Fig. 3C). These two MAPKs are crucial factors in signaling cascades responding to inflammatory cytokines, stress, UV light, osmotic shock, cytotoxic drugs and diverse pro-apoptotic stimuli [14]. It appeared from our experiments that RKS262 induced cytotoxicity of OVCAR-3 cells was mainly independent of the MAPK studied.
In contrast, RKS262 (5 µM) exerted strong inhibitory effects on oncogene ras (Fig. 4D) within 1 h after treatment. Another protein of current interest in ovarian cancer treatment is DNA-pk (see Discussion section) essential for DNA repair. RKS262 treatment (5 µM) initially upregulated the KU-80 subunit expression and after 6 h down-regulated KU-80 to background levels within 18 h of treatment (Fig. 4D) suggesting that RKS262 has a significant effect on DNA-pk holozyme expression and function. Moreover, RKS262 treatment (5 µM) initially and within 1 h activated Akt but over time down-regulated phosphorylation of Akt to reach background levels at 36 h, which was counteracted by the cells through increased expression of Akt (Fig. 4D). Among various other signal transduction pathways, Akt is emerging as a major therapeutic oncogenic target in several cancers including ovarian cancer (see Discussion section).
RKS262 selectively regulated expression of pro-apoptotic Bcl2 family member proteins
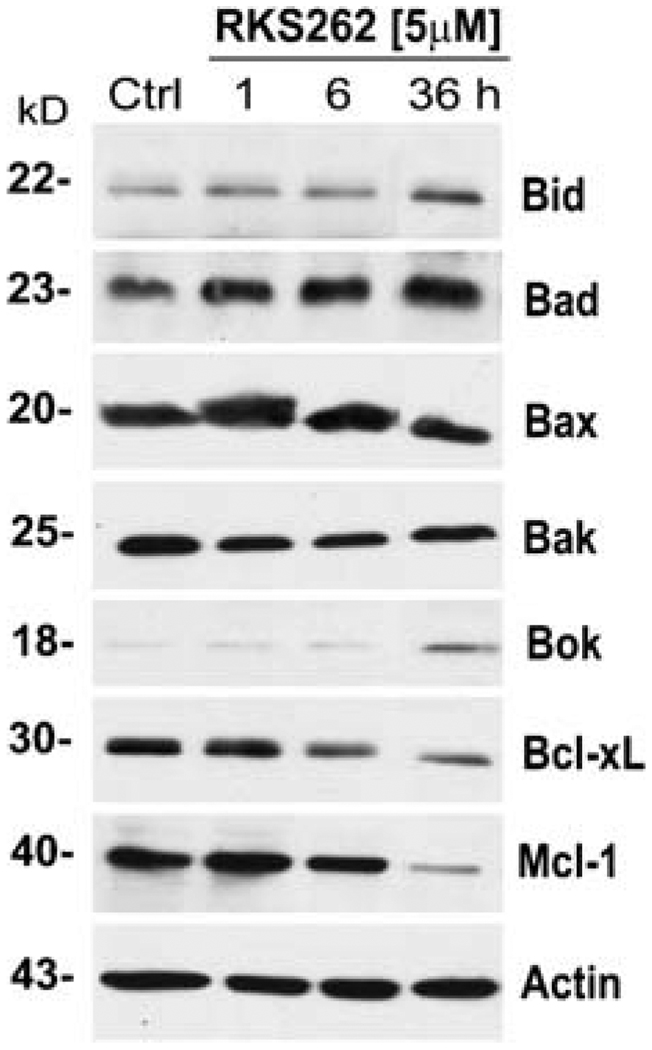
The expression of Bcl2 family members are a major determinant of cancer cell-survival. Relevant pro-apoptotic markers include Bid, Bad, Bax, Bak and Bok. Immunoblotting of OVCAR-3 cell lysates revealed that RKS262 (at 5 µM) caused a strong, rapid (within 1 h), and sustained (36 h) activation of Bid and Bad as well as an up-regulation of Bok after 36 h whereas levels of Bax and Bak remained unchanged (Fig. 5). Further analysis of the lysates proved that expression of anti-apoptotic Bcl2 family members Mcl-1 and Bcl-xL was strongly down-regulated by RKS262 treatment (Fig. 5).
Fig. 5.
Effect of RKS262 treatment on the expression profile of extended Bcl2 class proteins in OVCAR-3 cells. Expression of proapoptotic Bax, Bid, Bad, Bax, Bak and Boc and anti-apoptotic Bcl-xl and Mcl-1 in RKS262 (5 µM; for 1, 6, 18 and 36 h) and vehicle-treated OVCAR-3 cells were analyzed by Western blotting of lysates (Materials and Methods). As an internal standard for equal loading the blots were probed with an anti-β-actin antibody. Experiments were performed independently in duplicates showing identical outcomes
Discussion
Nifurtimox, an agent originally employed to treat Chagas’ disease displays antitumor effects in neuroblastoma in vitro and in vivo [2–4] and is currently being tested in a phase 2 treatment trial for childhood neuroblastoma. Nifurtimox forms a nitro-anion radical metabolite and may produce superoxide anions/hydrogen peroxide in cells [3]. These metabolites react with nucleic acids causing a significant breakage in deoxyribonucleic acid (DNA) [15]. As suggested for other chemotherapeutic drugs, Nifurtimox may be used in oxidation therapy by elevating H2O2 and superoxide radical in tumor cells above the survival/death threshold (see introduction) [16]. Production of reactive oxygen species results in apoptosis and/or necrosis and can be used for selective targeting of tumor cells which possess higher oxidative stress level and display alteration of antioxidant enzymes (catalase, SOD) as compared to normal cells. However, cytotoxicity screening in our lab revealed that cell-lines derived from a variety of tissues including ovarian cancer are relatively resistant to Nifurtimox treatment (unpublished data). The objective of the present study was to design a potent synthetic analog of Nifurtimox to potentially treat various human solid tumors including platinum-resistant ovarian cancers.
The cytotoxic potential of RKS262, a Nifurtimox derivative with specific modifications in the chemical structure including a coumarin scaffold was identified by using an ovarian cancer cell line as a model system. In RKS262 [Fig. 1A; compound 5] the tetrahydrothiazane ring was retained and linked to a 6-bromo, 4-chloro coumarin ring which replaced the nitrofuran ring present in Nifurtimox [compound 1]. Another Nifurtimox analog [compound 2] was synthesized by inserting a phenyl ring spacer between the nitro-functionality and the furan ring while the tetrahydrothiazane ring of Nifurtimox and the imine spacer were maintained. RKS262, now possessing the tetrahydrothiazane ring and a coumarin/benzopyranone scaffold with enhanced lipophilicity (bromide-functionality) revealed potent cytotoxicity in a viability assay and was chosen as the ‘lead’ structure for our studies. Viability experiments with OVCAR-3 cells settled some intriguing structure-activity relationship questions: (i) RKS262, multiple-fold more cytotoxic than Nifurtimox or analog [compound 2] (inactive at concentrations ≤ 20 µM), is structurally devoid of a nitro group which appears to not be essential for cytotoxicity; (ii) the phenyl spacer present only in inactive compound 2 appears to be detrimental to cytotoxicity; (iii) the tetrahydrothiazane ring present in Nifurtimox and RKS262 likely contributes to cytotoxicity; (iv) the imine spacer common to Nifurtimox, inactive compound 2 and RKS262 is not important for the activity of these compounds. In summary, we postulate that the superior activity of RKS262 is based on an additive cytotoxic effect of the synthetic lipophilic coumarin scaffold in conjunction with the 1-aminotetrahydrothiazane ring originally derived from Nifurtimox.
Screening the effects of RKS262 in a NCI60 cell-line growth/viability assay [17] revealed remarkable responses in 48 of 60 cell lines. This panel represents cell lines derived from tumors of 12 different tissues types for which current chemotherapy regimens are inadequate to provide cure or extended disease free survival periods. While most of the cell lines responded to a standard single dose of 10µM RKS262, we are intrigued by the differential activity with exceptional sensitivity of leukemia cells types such as HL-60 TB and SR (GI50<10 nM) but minimal activity against several non-small cell lung cancer cells (NCI-H322H, NCI-226, HOP-62, EKVX and A549). A comparison analysis (COMPARE program, http://www.dtp.nci.nih.gov) of known cytotoxic drugs indicated that RKS262 is more potent than a variety of clinically used drugs, such as cisplatin, oxaloplatin, seliciclib, 5-FU and cyclophosphamide in most of the NCI60 cell lines including various ovarian cancer cells and compares well with paclitaxel and doxorubicin [18]. The high degree of cytotoxic action by RKS262 could be explained by its unique/native stereoelectronic properties. It combines lipophilic properties (e.g. modified coumarin scaffold) with an aminotetrahydrothiazane moiety of primarily hydrophilic character which likely endows this drug with enhanced solubility, improved uptake and cellular retention [19].
This present study reveals that RKS262, in addition to its selective cytotoxic properties, acts as a potent inhibitor of OVCAR-3 ovarian cancer cell proliferation at sub-cytotoxic concentrations. RKS262 treatment inhibited cell-cycle progression through G2/M phase and this arrest was correlated with the cumulative effects of up-regulation of p27 and down-regulation of CDK-6 and cyclin-D1. In contrast, the expression of p21 and p16 proteins which are primarily linked to G1-phase regulation [20] remained unaffected by RKS262 treatment. It is noteworthy that tumor suppressor gene p27 has been suggested as a target for cancer therapeutics [9]. Similarly, cyclin-D1 and CDKs, specifically CDK-6 and CDK-7, are key regulators during G2/M cell-cycle progression in cancer cells [21, 22] and targeting such checkpoints has been suggested as an alternative approach to anti-cancer therapies [23, 24]. Regulators of the cell-cycle machinery are frequently altered in human cancer and apparently transformed cells can be more sensitive to the cyclin-dependent kinases (CDK) inhibition [25, 26]. Based on the specific effect of RKS262 on G2/M phase, p27-, cyclin-D1-and CDK-6 expression in OVCAR-3 cells we suggest future studies on subcytotoxic concentrations of RKS262 and related compounds to include a full examination of the expression profile of cell-cycle regulators in synchronized ovarian cancer cell lines as well as in in vivo models.
Recent approaches to understand the mechanism of anticancer drugs in general, and in ovarian cancer cells in particular, have focused regularly on the involvement of MAP-Kinases [27, 28]. Based on immunoblot experiments, we assessed that contrary to many cytotoxic agents, RKS262-induced cytotoxicity is not significantly correlated with the activation of key pro-apoptotic MAPK P38 and SAP/JNK. These, as well as other MAPKs, mediate signaling pathways in cancer and control cells responding to inflammatory cytokines, UV light, diverse pro-apoptotic stimuli as well as cytotoxic drugs [14, 29–31] such as with cisplatin in ovarian cancer cells [27]. To confirm that RKS262 effects do not include major changes in the expression, activation and function of such MAPK, future studies could include detailed kinetic and dose-dependent cellular responses to RKS262 during co-treatment with MAPK inhibitors or growth factors (e.g. EGF) which can modulate drug induced MAPK activities in ovarian cancer cells [32, 33].
In addition to a variety of undefined factors, protooncogenes, repair enzymes and signaling factors such as ras, DNA-pk, and Akt are over-expressed in ovarian and other cancers. These factors play multiple roles in resistance to therapies and inactivation of a broad panel of critical proapoptotic events [34–36].
Constitutive expression of ras enhances ovarian cancer cell migration via the ras-MEK-1 kinase pathway [35] and may be a target for cancer treatment. Down-regulation of ras by RKS262 in OVCAR-3 cells indicates that this compound possesses the capability to interfere with tumor progression. Similarly, it has been suggested that agents that can down-regulate peptide components Ku-70, Ku-80 or p350 of the DNA-pk holozyme may have potential in the treatment of ovarian or other cancers [36]. DNA-pk essential for DNA repair is composed of a large catalytic subunit (Mr 460,000) and heterodimeric autoantigen Ku 70 and Ku 80, is a key enzyme in determining the response to DNA-damaging agents [37, 38]. RKS262 treatment time dependently down-regulated KU-80 indicating that RKS262 has a significant effect on DNA-pk holozyme constitution and function. In addition, RKS262 treatment of OVCAR-3 cells resulted in a time-dependent down-regulation of Akt phosphorylation. Akt (a serine-threonine kinase) is emerging as a major therapeutic oncogenic target in several cancers including ovarian cancer where elevated signaling by the Akt pathway has been associated with poor prognosis and a more aggressive phenotype [36]. Therefore, RKS262 and other agents that directly target this pathway and its downstream molecules might be developed to mitigate the oncogenic effects of Akt.
A major determinant of cancer cell-survival is the expression of Bcl2 family members with wide ranging effects in ovarian and other cancer cells both in vitro and in vivo. It is known that the effective treatment of ovarian cancer is significantly hampered by drug resistance, which is directly correlated to the expression and function of Bcl-2 regulators [39].
We show here that RKS262 down-regulates the expression of pro-survival Bcl-xl and Mcl-1 proteins in platinum-resistant OVCAR-3 ovarian cancer cells within 6 h of treatment. These pro-survival proteins are crucial components of gynecological cancer cells where Bcl-xL over-expression is associated with a shorter disease-free interval in breast cancer patients [40] or over-expression of Mcl-1 correlates with an advanced clinical stage and poor survival of ovarian cancer patients [41]. RKS262 also up-regulates the expression of several pro-apoptotic Bcl2 class proteins such as Bid, Bad and Bok in OVCAR-3 cells. Expression of Bid is an adverse prognostic factor for radiotherapy outcome in carcinoma [42]. This protein plays a central role in apoptosis by connecting the intrinsic and extrinsic pathway, converging signals in mitochondria and acting agonistically in concert with other pro-apoptotic Bcl2 proteins [43]. The significance of the RKS262 effect on Bid, however, remains to be further investigated because the expression of Bax and Bak remained unaltered by RKS262 even though the proapoptotic activities of these two proteins are generally coupled to expression of Bid [44].
RKS262 also induces the expression of Bad in OVCAR-3 cells. Bad has been described as a crucial factor in chemotherapy-induced apoptosis of epithelial ovarian cancer cells and apparently Bad is capable of partly reversing paclitaxel chemoresistance in ovarian cancer through inhibition of Bcl-xL [45]. Bad like Bid possesses a BH3 domain which allows binding to other Bcl2 family members, thus triggering mitochondrial events associated with apoptosis. It can translocate between the cytosol and membrane-bound BCL-xL or Bcl2. Similarly Bok can induce apoptosis by promoting the release of pro-apoptotic mitochondrial factors to the cell cytosol. Bok expression is down-regulated through RKS262 and its activity has been shown to be inhibited by Mcl-1 but not Bcl-xl [46] (both of which are down-regulated by RKS262). These observations suggest a multilayer effect of RKS262 in the induction of Bcl2 protein class mediated apoptosis. While numerous antagonists of pro-survival Bcl2 class proteins are under investigation in clinical trials [47], similarly, the selective effect of drugs such as RKS262 on specific pro-apoptotic and pro-survival Bcl2 proteins deserves further investigation.
In conclusion, novel compound RKS262 displays characteristics of a therapeutic anti-cancer drug. In platinum-resistant OVCAR-3 ovarian cancer cells RKS262 regulates multiple aspects of cell viability such as cell-cycle regulation, mitochondrial integrity, induction of apoptosis and various oncogenic signaling events. The present report suggests that RKS262 is a potent growth-suppressing agent in vitro for cell lines derived from ovarian cancer. Future studies on RKS262 will include animal models to determine the compounds toxicity and potential to treat such tumors in vivo.
Supplementary Material
Acknowledgments
We thank NIH COBRE Grant 1-P20RR018728 for providing instrumentation support.
Abbreviations
- DMEM
Dulbecco’s Modified Eagle’s medium
- DMSO
dimethyl sulfoxide
- FACS
fluorescent activated cell sorting
- FBS
fetal bovine serum
- MAPK
mitogen activated protein kinases
- PBS
phosphate-buffered saline
- GI
growth inhibition
- TGI
total growth inhibition
- LC
lethal concentration
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s10637-009-9335-4) contains supplementary material, which is available to authorized users.
Contributor Information
Rakesh K. Singh, Molecular Therapeutics Laboratory, Program in Women’s Oncology, Department of Obstetrics and Gynecology, Women and Infants’ Hospital of RI, The Warren Alpert Medical School of Brown University, 101 Dudley Street, Providence, RI 02905, USA
Thilo S. Lange, Molecular Therapeutics Laboratory, Program in Women’s Oncology, Department of Obstetrics and Gynecology, Women and Infants’ Hospital of RI, The Warren Alpert Medical School of Brown University, 101 Dudley Street, Providence, RI 02905, USA Division of Biology and Medicine, Brown University, Providence, RI 02912, USA.
Kyu Kwang Kim, Molecular Therapeutics Laboratory, Program in Women’s Oncology, Department of Obstetrics and Gynecology, Women and Infants’ Hospital of RI, The Warren Alpert Medical School of Brown University, 101 Dudley Street, Providence, RI 02905, USA.
Laurent Brard, Email: lbrard@wihri.org, Laurent_Brard_MD@Brown.edu, Molecular Therapeutics Laboratory, Program in Women’s Oncology, Department of Obstetrics and Gynecology, Women and Infants’ Hospital of RI, The Warren Alpert Medical School of Brown University, 101 Dudley Street, Providence, RI 02905, USA.
References
- 1.Cancer Facts and Figures. 2008 http://www.cancer.org/downloads/STT/2008CAFFfinalsecured.pdf. [Google Scholar]
- 2.Saulnier-Sholler GL, Kalkunte S, Greenlaw C, McCarten K, Forman E. Antitumor activity of Nifurtimox observed in a patient with neuroblastoma. J Pediatr Hematol Oncol. 2006;28:693–695. doi: 10.1097/01.mph.0000212994.56812.f2. [DOI] [PubMed] [Google Scholar]
- 3.Saulnier-Sholler GL, Brard L, Straub JA, Dorf L, Illyene S, Kalkunte S, Bosenberg M, Ashikaga N, Nishi R. Nifurtimox induces apoptosis of neuroblastoma cells in vitro and in vivo. J Pediatr Hematol Oncol. 2009;31(3):187–193. doi: 10.1097/MPH.0b013e3181984d91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saulnier-Sholler G, Ferguson W, Brard L, Johnson G, Heath B, Bingham P, Taka A, Kamen B, Homans A. A phase I study of nifurtimox in patients with relapsed/refractory neuroblastoma. 44th American Society of Clinical Oncology Annual Meeting; May 30–June 3 2008; Chicago, IL. 2008. [Google Scholar]
- 5.Anderson NS, Bermudaze Y, Badgewell D, Chen R, Nicosia SV, Bast RC, Jr, Kruk PA. Urinary levels of Bcl2 are elevated in ovarian cancer patients. Gynecol Oncol. 2009;112:60–67. doi: 10.1016/j.ygyno.2008.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bock M, Haberkorn A, Herlinger H, Mayer KH, Petersen S. The structure activity relationship of 4-(5′-nitrofurfurylidene-amino)-tetrahydro-4H-1-, 1-dioxide against Trypanosoma cruzi. Arzneimittelforschung. 1972;22:1564–1569. [PubMed] [Google Scholar]
- 7.Singh RK, Lange TS, Kim KK, Singh AP, Vorsa N, Brard L. Isothiocyanate NB7M causes selective cytotoxicity, proapoptotic signaling and cell-cycle regression in ovarian cancer cells. Br J Cancer. 2008;99:1823–1831. doi: 10.1038/sj.bjc.6604778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Serova M, Galmarini CM, Ghoul A, Benhadji K, Green SR, Chiao J, Faivre S, Cvitkovic E, Tourneau C, Calvo F, Raymond E. Antiproliferative effects of sapacitabine (CYC682), a novel 20-deoxycytidine-derivative, in human cancer cells. Br J Cancer. 2007;97:628–636. doi: 10.1038/sj.bjc.6603896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blain S, Scher H, Cordon-Cardo C, Koff A. p27 as a target for cancer therapeutics. Cancer Cell. 2003;3:111–115. doi: 10.1016/s1535-6108(03)00026-6. [DOI] [PubMed] [Google Scholar]
- 10.Petit PX, Lecoeur H, Zorn E, Dauguet C, Mignotte B, Gougeon M. Alterations in mitochondrial structure and function are early events of dexamethasone-induced thymocyte apoptosis. J Cell Biol. 1995;130:157–167. doi: 10.1083/jcb.130.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thornberry NA, Lazebnik Y. Caspases: enemies within. Science. 1998;281:1312–1316. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- 12.Cohen GM. Caspases: the executioners of apoptosis. Biochem J. 1997;326:1–16. doi: 10.1042/bj3260001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Satoh MS, Lindahl T. Role of poly(ADP-ribose) formation in DNA repair. Nature. 1992;356:356–358. doi: 10.1038/356356a0. [DOI] [PubMed] [Google Scholar]
- 14.Pearson G, Robinson F, Beers GT, Xu BE, Karandikar M, Berman K, Cobb MH. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev. 2001;22:153–183. doi: 10.1210/edrv.22.2.0428. [DOI] [PubMed] [Google Scholar]
- 15.Diaz EG, Montelto DMM, Castro JA. Reactions of Nifurtimox with critical sulfhydryl-containing biomolecules: their potential toxicological relevance. J Appl Toxicol. 2004;24:189–195. doi: 10.1002/jat.970. [DOI] [PubMed] [Google Scholar]
- 16.Renschler MF. The emerging role of reactive oxygen species in cancer therapy. Eur J Cancer. 2004;40:1934–1940. doi: 10.1016/j.ejca.2004.02.031. [DOI] [PubMed] [Google Scholar]
- 17.Boyd MR, Paull KD. Some practical considerations and applications of the National Cancer Institute in vitro anticancer drug discovery screen. Drug Dev Res. 1995;34:91–109. [Google Scholar]
- 18.Shoemaker RH. The NCI60 human tumor cell line anticancer drug screen. Nat Rev Cancer. 2006;6:813–823. doi: 10.1038/nrc1951. [DOI] [PubMed] [Google Scholar]
- 19.Stasio BD, Frochot C, Dumas D, Even P, Zwier J, Müller A, Didelon J, Guillemin F, Viriot ML, Heyob MB. The 2-aminoglucosamide motif improves cellular uptake and photodynamic activity of tetraphenylporphyrin. Eur J of Med Chem. 2005;40:1111–1122. doi: 10.1016/j.ejmech.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 20.Sherr CJ. Cancer cell cycles. Science. 1996;274:1672–1677. doi: 10.1126/science.274.5293.1672. [DOI] [PubMed] [Google Scholar]
- 21.Sandor V, Senderowicz A, Mertins S, Sackett D, Sausville E, Blagosklonny MV, Bates SE. P21-dependent g(1)arrest with downregulation of cyclin D1 and upregulation of cyclin E by the histone deacetylase inhibitor FR901228. Br J Cancer. 2000;83:817–825. doi: 10.1054/bjoc.2000.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aquilina G, Cresenzi M, Bignami M. Mismatch repair, G2/M cell cycle arrest and lethality after DNA damage. Carcinogenesis. 1999;20:2317–2325. doi: 10.1093/carcin/20.12.2317. [DOI] [PubMed] [Google Scholar]
- 23.Shapiro GI, Harper JW. Anticancer drug targets: cell-cycle and checkpoint control. J Clin Invest. 1999;104:1645–1653. doi: 10.1172/JCI9054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mazumder S, DuPree EL, Almasan A. A dual role of cyclin E in cell proliferation and apoptosis may provide a target for cancer therapy. Curr Cancer Drug Targets. 2004;4:65–75. doi: 10.2174/1568009043481669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hartwell LH, Kastan MB. Cell-cycle control and cancer. Science. 1994;266:1821–1828. doi: 10.1126/science.7997877. [DOI] [PubMed] [Google Scholar]
- 26.Gladden AB, Diehl JA. Cell-cycle progression without cyclin E/CDK2: breaking down the walls of dogma. Cancer Cell. 2003;4:160–162. doi: 10.1016/s1535-6108(03)00217-4. [DOI] [PubMed] [Google Scholar]
- 27.Mansouri A, Ridgway LD, Korapati AL, Zhang Q, Tian L, Wang Y, Siddik ZH, Mills GB, Claret FX. Sustained activation of JNK-p38 MAP kinase pathways in response to cisplatin leads to Fas ligand induction and cell death in ovarian carcinoma cells. J Biol Chem. 2003;278:19245–19256. doi: 10.1074/jbc.M208134200. [DOI] [PubMed] [Google Scholar]
- 28.Lange TS, Stuckey AR, Robison K, Kim KK, Singh RK, Raker CA, Brard L. Effect of a Vitamin D(3) derivative (B3CD) with postulated anti-cancer activity in an ovarian cancer animal model. Invest New Drugs. 2009 Jul 7; doi: 10.1007/s10637-009-9284-y. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singh RK, Lange TS, Shaw S, Kim KK, Brard L. A novel Indole Ethyl Isothiocyanate (7Me-IEITC) with anti-proliferative and pro-apoptotic effects on platinum-resistant ovarian cancer cells. Gyn Onc. 2008;109:240–249. doi: 10.1016/j.ygyno.2008.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Birkenkamp KU, Dokter WH, Esselink MT, Jonk LJ, Kruijer W, Vellenga E. A dual function for p38 MAP kinase in hematopoietic cells: involvement in apoptosis and cell activation. Leukemia. 1999;13:1037–1045. doi: 10.1038/sj.leu.2401447. [DOI] [PubMed] [Google Scholar]
- 31.Ahmed-Choudhury J, Williams KT, Young LS, Adams DH, Afford SC. SCCD40 mediated human cholangiocyte apoptosis requires JAK2 dependent activation of STAT3 in addition to activation of JNK1/2 and ERK1/2. Cell Signal. 2006;18:456–468. doi: 10.1016/j.cellsig.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 32.Wang TH, Chan YH, Chen CW, Kung WH, Lee YS, Wang ST, Chang TC, Wang HS. Paclitaxel (Taxol) upregulates expression of functional interleukin-6 in human ovarian cancer cells through multiple signaling pathways. Oncogene. 2006;25:4857–4866. doi: 10.1038/sj.onc.1209498. [DOI] [PubMed] [Google Scholar]
- 33.Zhang CC, Shapiro DJ. Activation of the p38 Mitogen activated protein kinase pathway by estrogen or by 4-hydroxytamoxifen is coupled to estrogen receptor-induces apoptosis. J Biol Chem. 2000;275:479–486. doi: 10.1074/jbc.275.1.479. [DOI] [PubMed] [Google Scholar]
- 34.Bian D, Su S, Mahanivong C, Cheng RK, Han Q, Pan ZK, Sun P, Huang S. Lysophosphatidic acid stimulates ovarian cancer cell migration via a Ras-MEK Kinase-1 pathway. Cancer Res. 2004;64:4209–4217. doi: 10.1158/0008-5472.CAN-04-0060. [DOI] [PubMed] [Google Scholar]
- 35.Townsend DM, Shen H, Staros AL, Gate L, Tew KD. Efficacy of a Glutathione S-Transferase-activated prodrug in platinum-resistant ovarian cancer cells. Mol Canc Ther. 2002;1:1089–1095. [PMC free article] [PubMed] [Google Scholar]
- 36.Nicholson KM, Anderson NG. The protein kinase B/Akt signalling pathway in human malignancy. Cell Signal. 2002;14:381–395. doi: 10.1016/s0898-6568(01)00271-6. [DOI] [PubMed] [Google Scholar]
- 37.Weaver DT. What to do at an end: DNA double-strand-break repair. Trends Genet. 1995;11:388–392. doi: 10.1016/s0168-9525(00)89121-0. [DOI] [PubMed] [Google Scholar]
- 38.Jackson SP, Jeggo PA. DNA double-strand break repair and V(D)J recombination: involvement of DNA-PK. Trends Biochem Sci. 1995;20:412–415. doi: 10.1016/s0968-0004(00)89090-8. [DOI] [PubMed] [Google Scholar]
- 39.Kupryjanczyk J, Szymanska T, Madry R, Timorek A, Stelmachow J, Karpinska G, Rembiszewska A, Ziokowska I, Kraszewska E, Debniak J, Emerich J, Ulanska M, Płuzanska A, Jedryka M, Goluda M, Chudecka-Glaz A, Rzepka-Gorska I, Klimek M, Urbanski K, Breborowicz J, Zielinski J, Markowska J. Evaluation of clinical significance of TP53, Bcl-2, Bax and Mek1 expression in 229 ovarian carcinomas treated with platinum-based regimen. Br J Cancer. 2003;88:848–854. doi: 10.1038/sj.bjc.6600789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fiebig AA, Zhu W, Hollerbach C, Leber B, Andrews DW. Bcl-xL is qualitatively different from and ten times more effective than Bcl-2 when expressed in a breast cancer cell line. BMC Cancer. 2006;6:213. doi: 10.1186/1471-2407-6-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shigemasa K, Katoh O, Shiroyama Y, Mihara S, Mukai K, Nagai N, Ohama K. Increased MCL-1 expression is associated with poor prognosis in ovarian carcinomas. Jpn J Cancer Res. 2002;93:542–550. doi: 10.1111/j.1349-7006.2002.tb01289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Green MML, Hutchison GJ, Valentine HR, Fitzmaurice RJ, Davidson SE, Hunter RD, Dive C, West CML, Stratford IJ. Expression of the pro-apoptotic protein Bid is an adverse prognostic factor for radiotherapy outcome in carcinoma of the cervix. Br J of Cancer. 2005;92:449–458. doi: 10.1038/sj.bjc.6602344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang K, Yin XM, Chao DT, Milliman CL, Korsmeyer SJ. BID: a novel BH3 domain-only death agonist. Genes Dev. 1996;10:2859–2869. doi: 10.1101/gad.10.22.2859. [DOI] [PubMed] [Google Scholar]
- 44.Degli-Esposti M. The roles of Bid. Apoptosis. 2002;7:433–440. doi: 10.1023/a:1020035124855. [DOI] [PubMed] [Google Scholar]
- 45.Strobel T, Tai Y-T, Korsmeyer S, Cannistra SA. Oncogene. 1998;17:419–2427. doi: 10.1038/sj.onc.1202180. [DOI] [PubMed] [Google Scholar]
- 46.Rodriguez JM, Glozak MA, Ma Y, Cress WD. Bok, Bcl-2 related ovarian killer is cell cycle-regulated and sensitizes to stress-induced apoptosis. J Biol Chem. 2006;281:22729–22735. doi: 10.1074/jbc.M604705200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lessene G, Czabotar PE, Colman PM. Bcl-2 family antagonists for cancer therapy. Nat Rev Drug Discov. 2008;7:989–1000. doi: 10.1038/nrd2658. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.