Abstract
Natural adult aging is associated with many functional impairments of the human neuromuscular system. One of the more observable alterations is the loss of contractile muscle mass, termed sarcopenia. The loss of muscle mass occurs primarily due to a progressive loss of viable motor units, and accompanying atrophy of remaining muscle fibers. Not only does the loss of muscle mass contribute to impaired function in old age, but alterations in fiber type and myosin heavy chain isoform expression also contribute to weaker, slower, and less powerful contracting muscles. This review will focus on motor unit loss associated with natural adult aging, age-related fatigability, and the age-related differences in strength across contractile muscle actions.
Keywords: Aging, Eccentric, Force, Motor unit, Muscle atrophy, Power, Residual force enhancement, Sarcopenia, Velocity
1. Introduction
Skeletal muscle is a remarkable, highly organized tissue which regulates metabolic processes, is important in thermo-regulation and serving ultimately as a “molecular motor”. Muscles produce tension, and through tendons move bones to produce meaningful movement and locomotion. With natural human adult aging, there is a loss of muscle mass and alterations to the structural components of the neuromuscular system resulting in impaired contractile function and performance of the entire system. This brief review outlines age-related alterations to the structure and function of the human neuromuscular system, with a particular emphasis on age-related fatigability and dynamic muscle actions. The mechanisms and aetiology of sarcopenia are beyond the scope of this review, but there are excellent reviews on the topic.1–6
2. Aging and motor unit loss
Arguably, one of the more recognizable structural changes to the neuromuscular system associated with adult aging is the loss of contractile muscle mass. On average 10%–20% of skeletal muscle mass is lost by the 7th decade of life, and a further ~20% reduction occurs within the 8th decade; this decline may be accelerated into very old age unless interventions as described below occur.7 The loss of muscle mass occurs primarily through the loss of motor units (MUs)8–10 and atrophy of surviving muscle fibers.10 The MU is the basic functional contractile unit of the neuromuscular system consisting of an α-motoneuron (MN) housed in the spinal cord, a motor nerve and all the individual muscle fibers innervated by that nerve. MUs are consistently undergoing remodelling throughout the lifespan, and it is not until the process of denervation outpaces re-innervation in old age that a loss of MU integrity and the ensuing death of MUs occurs.11 The loss of MUs is suggested to occur in a “die-back” fashion11 with the loss of MU integrity, preceding its death, occurring at the site of the neuromuscular junction.5 The early loss of MUs in old age often goes undetected from a functional perspective due to successful remodelling (collateral re-innervation) with relative maintenance of muscle mass and strength.12,13 This process5,11 helps to maintain muscle mass such that, following the death of an MN in the spinal cord, or axonal degeneration, those muscle fibers orphaned from their parent MN, which are no longer part of a functional MU will atrophy and become non-functional, unless, they are re-innervated via a nerve sprout from an adjacent intact functional MN axon, resulting in a larger and often slower MU.14,15 In humans, the loss of muscle fibers seems to be equal among fast (Type II) and slow (Type I) muscle fiber types.10,16–18 Conversely, there is evidence in animal models to suggest that predominately slow type MUs are better protected from MU loss than fast type MUs.19,20
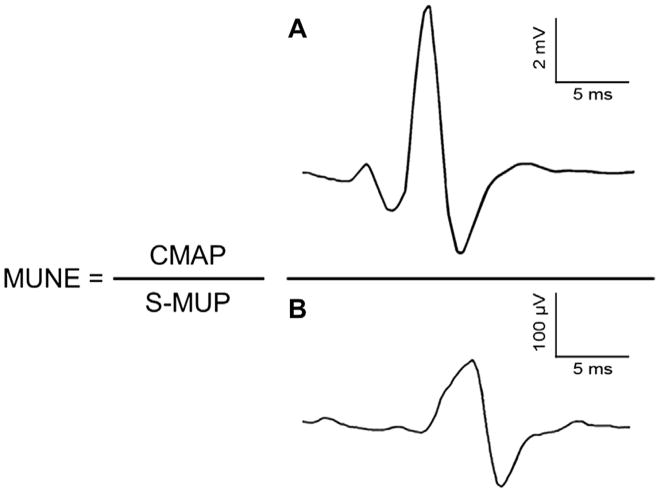
The number of MNs in old age can be estimated only via cadaveric studies. Tomlinson and Irving21 provided a thorough examination of MNs in the human throughout the lifespan. Using histochemical staining procedures on the lower lumbar segment of cadavers they estimated the numbers of MNs in previously healthy individuals, aged 13–95 years. Necessarily, these studies were cross-sectional in nature, but they found no evidence of MN loss in the first 7 decades, but beyond the 7th decade there was clear and increasing evidence of MN loss. The average MN loss from the 2nd to 10th decade was approximately 25%, although several people older than 60 years had counts 50% less than those specimens taken in early adulthood or middle age. These initial observations indicated that MN loss is not a linear process. Because MU death is suggested to occur in a die back fashion11 the loss of functioning MUs may precede the death of the MN at the spinal cord. The number of functional MUs in human muscle can also be estimated in vivo electrophysiologically using minimally invasive techniques (e.g., multiple point stimulation,14,22 incremental method,23,24 automated incremental method,25 a combination of electrical stimulation and voluntary contractions, spike triggered averaging,26,27 and decomposition-enhanced spike triggered averaging12,28–31). For a review of these techniques see Ref. 32. The basic tenets of all these electrophysiological techniques for MU number estimates (MUNEs) involves obtaining two size parameters: (1) the maximal electrical size of the entire MU pool, as reflected by the compound muscle action potential (CMAP; or M-wave) and (2) the electrical size of an individual MU, as reflected by the surface detected MU potential (S-MUP) (Fig. 1). Accordingly, if one assumes the CMAP represents the electrical size of all functional MUs, and the S-MUP is the electrical size of an individual functional MU, the number of functional MUs can be estimated easily by dividing the CMAP by the average S-MUP. A caveat to this metric is the derived MU number is not the “true” number of MUs present in a human limb muscle. However, this estimate is a reliable measure33–35 of the number of MUs and it is sensitive to clinical changes of the neuromuscular system,31,36,37 as well as age-related decrements in the number of functioning MUs.12 Considering that it is impossible to determine the actual number of MUs in living or deceased humans (i.e., even staining techniques21 are not without error in staining only α-MNs), estimates and comparison of MU loss have proven useful in understanding age-related alterations in the neuromuscular system.
Fig. 1.
Formula used in calculating a motor unit number estimate (MUNE). The compound muscle action potential (CMAP, sum of electrical contribution of all motor units) is divided by the average surface detected motor unit potential (S-MUP, sample of motor unit potentials representing the average electrical size of the constituent units) to derive a MUNE. Panel A is a representative CMAP and Panel B is a representative S-MUP.
For the most part, age-related losses of MUs are consistent across muscles of the upper and lower limbs, and the reported age-related loss of MUs ranges from 40% to 60% for most muscle groups. In the upper limb muscles, age-related reductions in MUNEs have been reported for the biceps brachii26,27,29 and small intrinsic hand muscles.14,22 For lower limb muscles, age-related declines in MUNEs have been reported for the extensor digitorum brevis,8,38 tibialis anterior,12,30 and soleus24 muscles. However, Dalton et al.28 reported a non-significant reduction in the number of MUs of the soleus by the 8th decade, whereas Vandervoort and McComas24 reported a 70% age-related loss of MUs in the soleus of men greater than 90 years of age. These two studies suggest that MU loss and remodelling may be delayed in a habitually active postural muscle, up to a critical age-related threshold. They also raise the possibility of a fiber type dependence of MU loss. Thus, not only the habitual activity level of the soleus, but also its predominantly slow type (>85%) muscle fiber composition39 may have helped mitigate MU loss into old age when compared with results reported for other limb muscles. These findings of fiber type-dependent MU loss are also supported by work on animals.19 Results in humans, however, are inconclusive on fiber type-dependent MU loss, possibly owing to the more heterogeneous fiber type distribution39 of human compared with animal muscle.
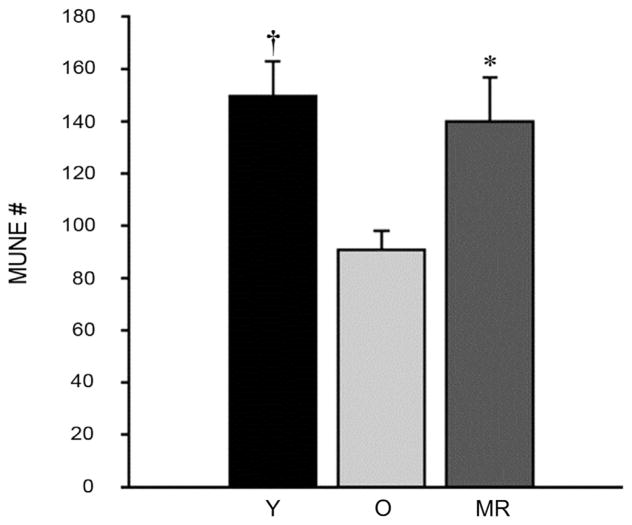
Recent work from our group on masters athletes indicate that high levels of life-long physical activity (PA) has the potential to mitigate the loss of functional MUs in the tibialis anterior into the 7th decade of life,30 thus maintaining excitable muscle mass (Fig. 2). To provide insight on whether habitual PA had a local preservation effect (on the exercised muscles), or a systemic preservation effect, muscles of the masters athletes not directly loaded during running were also tested.29 Unlike the tibialis anterior, estimates of the number of MUs in the biceps brachii of masters runners were decreased in old age compared with young. These findings indicate that chronic activation of the MN pool specific to the muscle action is required for delaying the typical age-related loss of MUs during healthy adult aging.29 The old adage, “use it or lose it” appears to hold true for the loss of MUs and muscle mass based on cross-sectional studies. However, it is not entirely known whether the number of functional MUs into very old age (>80 years) is maintained. Preliminary data from our work on very old world-class masters athletes40 suggest that MUNEs in male world-class masters athletes are higher than age-matched controls, but are lower than young controls. Interestingly, it appears that in this very old group of masters athletes denervation is not entirely outpacing re-innervation. More specifically, the surviving MUs in these athletes are larger (greater S-MUP size) suggesting the process of collateral re-innervation is still contributing to the maintenance of excitable muscle mass (as indirectly reflected by the larger size of the CMAP) in very old masters athletes, but it is still a losing game in that MU loss seems inevitable.
Fig. 2.
Motor unit number estimates (MUNE) in masters athletes and old men. MUNE did not differ between masters runners (MR, ~65 years) and young men (Y, ~25 years), but were higher in MR than old men (O, ~65 years), and less in O compared with Y. Values are presented as mean ± SE. *Significant difference between MR and O. †Significant difference between Y and O. Adapted with permission from Ref. 29.
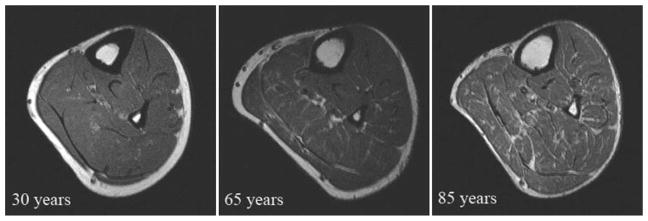
A comprehensive gerontological study of MUNEs to date was performed by McNeil et al.12 on the tibialis anterior muscle across three age groups: young (~25 years), old (~65 years) and very old (~80 years), using an age range from 23 to 89 years. They found no difference in strength between the young and old group, however the estimated number of MUs was reduced in the old. In the very old, there was a significant loss of strength, and a further reduction in the MUNEs compared with the young and old subjects, supporting that the functional significance of MU loss may not occur until after the 7th decade of life. At this point, the extent of MU loss cannot be adequately compensated for by collateral re-innervation, and the loss of MUs and muscle mass contributes to strength loss into very old age. The loss of MUs, and its influence on muscle mass, was quantified in a separate investigation using MR imaging13 (Fig. 3). Muscle mass of the tibialis anterior was maintained in the old group, but declined significantly in the very old subjects. In summary, the age-related loss of MUs is eventually immutable,41,42 nonlinear,12,21,24 not equal across muscles,28 and influenced by habitual PA.29,30 Notwithstanding, muscle tissue retains its plasticity into very old age and thus appropriate resistance training can reverse some of the atrophy of muscle fibers.7,43–47
Fig. 3.
Axial magnetic resonance images of the right leg from a young (30 years), old (65 years), and very old (85 years) man. Note the smaller amount of infiltration of non-contractile tissue in legs of those 65 years of age but it is not until the very old age (85 years) when a significant overall loss of muscle mass is evident including more infiltration. Adapted with permission from Ref. 13.
3. Cross-sectional area and muscle fiber composition
As mentioned above, the loss of MUs is one factor, and perhaps likely a key contributing factor48 to the reduction in whole muscle cross-sectional area (CSA) associated with adult aging. The second factor is the atrophy of those remaining muscle fibers. Lexell et al.48 reported an 18% reduction in whole muscle CSA and 25% fewer muscle fibers in older adults compared with young adults. The combined consequence of MU loss and muscle fiber atrophy ultimately leads to an upwards of a 40% decline in total muscle CSA into very old age.10 The suggested loss of muscle mass, as reflected by reductions in total CSA is on the magnitude of 0.5%–0.8% per year when investigated in cross-sectional studies of older adults.7 However, this may be an underestimation of the actual degree of muscle loss. A longitudinal study by Frontera et al.49 found greater CSA loss per year when tracked in the same subjects over a 12-year period (initial age ~65 years) compared to cross-sectional study designs. The loss of muscle mass was on the magnitude of 1.4% per year. Additionally, the infiltration of non-contractile tissue into the whole muscle and muscle compartments increases with age. Depending on the muscle group, the amount of non-contractile tissue in young is ~2%–5% and in old is ~8%–18% of the whole muscle compartment CSA.50,51 Thus, when estimating the age-related changes in muscle mass based solely on whole muscle CSA, or girth measures,52 if the non-contractile tissue that is dispersed throughout the muscle is not quantified and subtracted from the overall CSA, the actual loss of contractile tissue may be underestimated. Results from studies involving animal preparations are equivocal regarding fiber type loss.53 Previously, it was believed there was a preferential loss of Type II fibers with adult aging.54 However, this is not necessarily the case for humans where Types II and I muscle fibers are suggested to be affected equally by cell apoptosis,48 although there is a preferential atrophy (~26%) of Type II compared with Type I fibers.10,55 This combined effect results in a greater whole muscle Type I:II fiber area.
Investigations using histochemical techniques are useful in identifying the relative muscle fiber type composition. However, another factor, perhaps more functionally relevant, which contributes to the performance of a single muscle fiber is the myosin heavy chain (MHC) isoform expression. In humans there are three MHC isoform expressions, Type I, Type IIa, and Type IIx. Single muscle fibers expressing MHC isoforms IIa and IIx are associated with 3–9 times stronger, faster, and more powerful contractile properties, than those single fibers expressing MHC I isoforms.56–58 With adult aging, the reduction in proportional area of Type II to Type I muscle fibers results directly in a relative loss of MHC IIa and IIx isoform expression, which contributes to weaker, slower, and less powerful whole muscle contractile properties in older adults.6,59–61 Interestingly, in older adults, even when matched for MHC isoform expression61 and normalized to muscle fiber CSA to account for the reduction in myofibrillar content, there are nonetheless age-associated impairments in muscle contractile function, known as muscle quality.3,62 The loss of contractile function, even when normalized to muscle mass, indicates possible impairments in cross-bridge force generation kinetics. An age-associated loss of myosin content relative to muscle fiber CSA consequently results in a decreased number of actomyosin interactions.61 The loss of myosin content coupled with a lower percentage of strongly bound cross-bridges, as measured using electron paramagnetic resonance spectroscopy to determine the fraction of myosin heads in a strongly bound state (i.e., 32% in young compared to 22% in old) during contraction,63 contributes to impaired force production in old age. Additionally, decreased actin sliding speed as assessed with in vitro motility assays,61 independent of MHC expression,64,65 is associated with reductions in maximal shortening velocity in old age. These findings point to a slowing of the kinetic steps within cross-bridge cycling, or structural changes to the cross-bridge affecting the excursion per step cycle contributing to impaired muscle function. The former being relatively easy to measure, while the latter is virtually impossible to determine, at least with any accuracy. Despite the magnitude of indirect evidence on cross-bridge function, it is currently unknown in older adults which step(s) in the cross-bridge cycle is/are impaired. However, these impairments are reflected in reduced whole muscle contractile performance.
4. Muscle function
A reduction in myofibrillar content, a lower Type II:I fiber area, a shift in MHC isoform to a predominately slow type56–58 expression, and molecular changes at the site of actin-myosin interaction,58,59 all act in unison with declines at the neural level2 to contribute to the functional declines associated with aging. This includes reductions in muscle strength, shortening velocity, and ultimately power output. Natural adult aging results, on average, in a loss of strength of ~1%–1.5% per year after the 6th decade of life.49 When strength loss is investigated in the same population (46–78 years of age) and compared both cross-sectionally and longitudinally over a 10-year time span, there is an ~60% greater decline in longitudinal versus cross-sectional data,66 highlighting the importance of longitudinal studies in gerontological research. Another longitudinal investigation of ~70–80-year-olds indicated that strength loss may be muscle-dependent: greater for the ankle plantar flexors (25%–30% reduction) compared with the dorsiflexors (4%–10% reduction).67
Motor units of varying sizes and muscle fiber types contribute to muscle function and strength, but the estimated loss of MUs in old age may not directly result in immediate and observable impairments in whole muscle performance. As shown above, McNeil et al.12 found, despite a loss of MUs, that strength was maintained into the 7th decade. However into the 9th decade, there was a precipitous loss of MUs which led to a loss of muscle mass and associated strength deficits. Even though changes in various anatomic and physiologic factors located at the spinal and supraspinal levels have been reported with age,2 it does not seem likely that voluntary activation is a limiting factor in the ability of older adults to produce maximal isometric efforts.68,69 The loss of maximal voluntary strength in old age can be attributed to structural changes (see above) rather than an inability to activate fully the contractile muscle mass, at least in simple movements of individual joints. Voluntary activation is commonly defined as the level of voluntary drive to the muscles during an effort.68–70 The general consensus using a superimposed twitch interpolation technique is that well practiced and physically active young and older adults familiarized with a simple isometric task appear to be able to activate equally their muscles to usually more than 95% depending on the muscle group.68,69 However, the ability to produce this maximal effort during dynamic contractions is less clear and it is uncertain whether voluntary activation is maintained and similar between young and old for dynamic actions.69
5. Velocity-dependent power
At the whole muscle level, older individuals are ~15%–40% slower for voluntary angular contractile velocity at relative submaximal loads13,71–80 than young subjects. Additionally, in vivo human studies have consistently reported estimated maximum contraction velocities of various muscles to be ~30%–40% lower in healthy older adults than young,76–79 and the majority of studies at the single fiber level have reported that fibers from old are slower than young for tests of unloaded shortening velocity.81–85 The age-related loss of strength and shortening velocity lead to alterations in the force–velocity (F–V) relationship, shifting the curve to the left and downward.86 The leftward shift is owing to the decrease in contractile velocity at each relative load, and the loss of strength causes the downward shift. Both force production and velocity of shortening are necessary for power production, and if either of these two factors is impaired, power production is affected greatly.
Optimal power generation is based on the finely tuned relationship between force and shortening velocity, and expressed as the F–V relationship. As velocity increases, less force can be generated owing to fewer cross-bridge attachments, and a shift of the cross-bridge attachment distribution function to smaller “x” distances as defined by Huxley.87 Thus a combination of submaximal forces and velocities is required to achieve peak power.88,89 Because muscle power is the product of force and velocity both of which are independently influenced by aging, the age-related loss of power is greater than either force or velocity decrements alone.13 Power is reduced consistently across a range of isovelocity76,78,79,90–94 and isotonic-like actions,13,72–75,95,96 even when normalized to muscle mass.13 Not only is power reported to be impaired in older adults for cross-sectional studies, it is also reduced by 10%–35% in longitudinal studies.17,49,67,97,98 Indeed age-related declines in muscle power are greater and more rapid than isometric strength alone and it is this variable that can lead to marked impairments in physical function.99–101
6. Neuromuscular fatigue
Fatigue can be defined as any exercise induced reduction in the ability to generate force or power regardless of whether or not the task can be sustained.102 It is commonly accepted that fatigue can vary between populations103–106 and is dependent highly upon the task performed.107 Not surprisingly, reports on age-related fatigability are equivocal. Older adults can experience less, the same or more fatigue compared with younger subjects because the level of fatigue depends ultimately upon the task imposed and the criterion measure.72
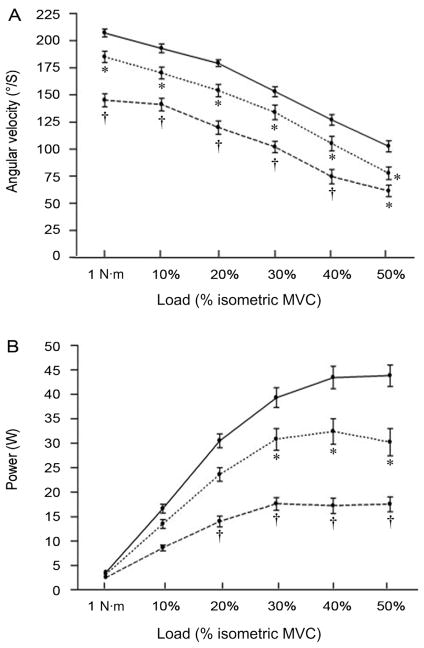
Older adults have slower, weaker, and less powerful contracting muscles compared to their younger counterparts13,86 (Fig. 4). However, the slower muscle contractile properties in old age may be advantageous in the sense that lower levels of activation are required to reach fused tetanus either during electrically stimulated108 or voluntary efforts.109 The age-related slowing of muscle contractile properties, and lower firing rates provide a strategy whereby older adults can reach a fused muscle contraction for a given lower firing rate than young, resulting in old experiencing less fatigue than young during tasks requiring no joint movement (i.e., isometric)106 or movement at slow constrained isovelocities.110 This apparent fatigue resistance is attributed to a lower ATP cost and more ATP generated through oxidative processes, via a greater Type I:II muscle composition in old age.111,112 Conversely, during tasks which require fast movement velocities, the slower contracting muscles of older adults lead to a greater fatigue response than young,113,114 especially when the fast movements are performed under velocity-dependent (i.e., isotonic like) conditions.13,72–75,96 As outlined above, there is a relationship between the intrinsic slowing of the older adults’ muscle contractile properties and lower MU firing rates leading to a more “energetically efficient” muscle when tested during an isometric task, although exceptions have been reported.115,116 During dynamic actions it is less clear whether old are more or less fatigable than young, which can be confounded by the task-dependent nature of fatigue. For dynamic contractions, fatigue can be assessed on a dynamometer using the isokinetic (isovelocity) mode. Isovelocity actions are characterized by sustaining a constant angular velocity during the participants’ maximal effort throughout a range of motion, while the criterion measure is torque production. However, if the participant cannot match the imposed angular velocity, perhaps because the speed is beyond the inherent ability or habitual capability of the neuromuscular system, as is the case for older adults, this contraction mode may not correctly reflect a full maximal voluntary torque effort.90 During these isovelocity tasks, despite shortening velocity being a key component strongly related to the inherent design properties of skeletal muscle,89 velocity is constrained artificially (i.e., preset) and changes in power are related directly and limited to alterations in torque production. Findings are equivocal regarding age-related fatigue during such isovelocity tasks, ultimately because the task is not stressing the components of the aged system which are prone to failure, but rather allowing for an “energetic advantage” of aged muscle on a relative basis to be less fatigable.
Fig. 4.
Comparison of angular velocity (A) and velocity-dependent power (B) at 1 nm and different submaximal loads normalized to a relative percentage of maximum voluntary contraction (MVC) in young (~25 years; solid line), old (~65 years; dotted line), and very old (~80 years; dashed line) men for the ankle dorsiflexors. Values are means ± SE. Old men were slower than young men at all loads but 30% maximal voluntary contraction (MVC) (*p < 0.05). Very old men were slower than young men at 50% MVC (*p < 0.05) and slower than both young and old men at all other loads (†p < 0.05). Old men generated less power than young men at 30%, 40%, and 50% MVC (*p < 0.05). Very old men generated less power than both young and old men at 20%, 30%, 40%, and 50% MVC (†p < 0.05). Adapted with permission from Ref. 13.
Moreover, older adults are unequivocally more fatigable than young for tasks in which the aged system is stressed with an isotonic load/resistance based on a relative percentage of their maximal force production capacity (i.e., MVC) and the shortening muscle action is performed maximally with shortening velocity as the criterion measure of fatigability.72–75,96 This paradigm of stressing the aged system is termed velocity-dependent117 allowing the muscle to function in conditions that more closely approximate real world conditions than isovelocity contractions. Maximal unconstrained shortening velocity is indeed slower in old76–79 compared with younger adults. Therefore, as efficiency is related to the speed of muscle shortening118 older adults, with slower contracting muscles may experience a greater perturbation in ATP homeostasis during fast velocity-dependent actions, consequently exacerbating their fatigue response and resulting in a greater reduction in shortening velocity and subsequent velocity-dependent power than young adults. Numerous studies from our laboratory and others72–75,96 support the notion that older adults are indeed more fatigable than young when their neuromuscular system is stressed with fast dynamic unconstrained velocity actions.
Age-related fatigability of the knee extensors was investigated during an isovelocity task relative to the subject’s individual torque-angular velocity relationship.113 To account for differences in angular velocity between young and old, subjects were fatigued at the angular velocity which corresponded to 50% of their maximum isokinetic torque across a range of angular velocities. The authors found no differences in fatigability between young and old. More comprehensively, Dalton et al.73 tested age-related fatigue in the knee extensors during three dynamic tasks: (1) slow isovelocity, (2) moderate speed isovelocity, and (3) fast velocity-dependent. The old were more fatigable than young during the fast velocity-dependent task compared with the isovelocity tasks, even when the fatiguing contractions were performed at a similar power output for both the isovelocity and velocity-dependent task. For the velocity-dependent task, upon task termination, the older men had a greater slowing in both twitch time to peak tension and half-relaxation time than the young. However for the moderate isovelocity task, there was only a slowing in half-relaxation time. Thus, the velocity-dependent task resulted in a greater slowing of whole muscle contractile properties in the older adults, thus contributing to their greater loss of power and fatigability during fast dynamic actions. Additionally a significant negative relationship between slowing of half-relaxation time and power existed for the fast velocity-dependent task, but not for the slow or moderate isovelocity tasks. These relationships could indicate that loaded shortening velocity, and therefore power, are influenced by similar fatigue processes as those affecting half-relaxation time, but remain undetected during isometric and slower isovelocity actions.119 It appears that, depending on the task performed, the contributing factors involved in power production may be dominated by a reduction in torque (i.e., slow isovelocity task); whereas for other tasks the key factors may relate to the reduction in shortening velocity. Therefore, when exploring age-related fatigability, factors relating to shortening velocity may be of greater importance than torque per se. Perhaps the isovelocity task employed by Callahan et al.113 was not “fast” enough to induce significant slowing of the contractile properties in old compared with young. It would be interesting to compare young and old across various percentages of their maximum shortening velocity to elucidate the upper limits of velocity which evoke the fatigue response found in all velocity-dependent age-related studies. Additionally, the actual criterion measure chosen to assess fatigue is a key factor when assessing age-related fatigability (Fig. 5). Following a series of repeated velocity-dependent fatiguing shortening actions, when fatigue was assessed using the typical method of isometric strength (i.e., MVC), there did not appear to be a difference in fatigue between young and old, however when tested with the criterion measure of angular velocity, older adults were more fatigable than their younger counterparts.72 Together, these studies highlight the task-dependent nature of age-related fatigue in addition to the importance of the criterion measure used to quantify fatigue in older adults.
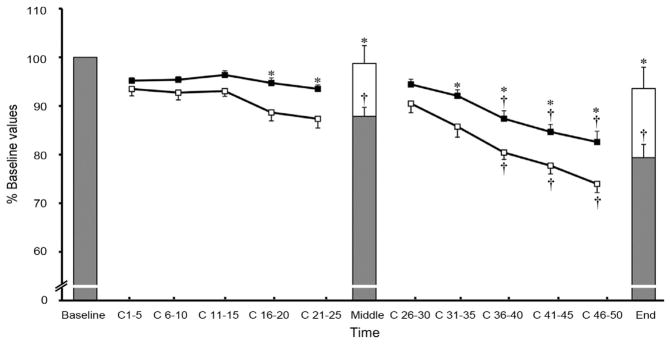
Fig. 5.
Fatigue response of peak power (squares) and isometric maximum voluntary contraction (MVC, bars). *Age-related difference between the old (open) and young (filled) men (p < 0.05). †Difference from C 1–20 for peak power and a difference from baseline for MVC (p < 0.01). C represents dynamic contraction number. Values are means ± SE. Duration between C 25 and C 26 was ~15 s and time between C 50 and End MVC was ~5 s. Adapted with permission from Ref. 73.
7. Contraction type
Not only do muscles pull and shorten, but when an external load overcomes the tension produced by the muscle, they lengthen actively. Lengthening muscle actions are a part of normal daily activity whether landing from a jump or walking down a flight of stairs. For a given resistance, these contractions are less energetically demanding, causing less metabolic perturbation and generally produce greater forces (1.5–2 times) than shortening or isometric contractions.120 Age-related reductions in muscle contractile capacity are disparate when considered in terms of contractile mode.7 The seminal observation of Vandervoort et al.121 suggested that older women had well-maintained knee extensor eccentric strength compared with young. This finding was later confirmed in old mice,122 older men16 and other human muscle groups.123,124 The relative maintenance of eccentric to isometric strength ratios in old age range from 2% to 48%, and is on average ~20% greater than isometric strength.45 The maintenance of eccentric strength with adult aging is an interesting phenomenon and useful in exploring age-related alterations to the human neuromuscular system whereby one contraction mode is relatively maintained whereas others (i.e., isometric and concentric) show large age-related impairments. Two factors are potentially responsible for the maintenance of eccentric strength of older adults: neural and mechanically mediated mechanisms.45 Neural factors likely include: reduced agonist and increased antagonist activation during concentric actions thus reducing the overall net force produced. However older adults, if well practiced and accustomed with the task have little problem fully activating their muscles, and antagonist co-activation is often not altered with aging, at least for isometric tasks.68,116,125,126 Thus, it is highly probable that the mechanisms behind the relative maintenance of eccentric strength in old age are likely related to the non-contractile and structural properties intrinsic to the muscle tissue. An increase in muscle passive stiffness82,127 in older adults may enhance resistance during lengthening actions16 and this elevated passive stiffness could potentially increase the effective “storage” of elastic energy to generate passive mechanical work and optimise force production. The greatest support of an intrinsic muscle property leading to maintained eccentric strength is the elevated force following a quick active stretch in single muscle fibers of older adults.128 Elevated force during lengthening was suggested to be related to an increased number of weakly bound cross-bridges, with the possibility of converting to a strongly bound state. As well, instantaneous stiffness82 was increased following a very quick shortening step (defined as the ratio between the change in force and sarcomere length change) in maximally activated single skinned muscle fibers from old relative to young. This provides evidence of altered elastic, or structural properties of the muscle, independent of cross-bridge cycling times being partly responsible for the maintenance of eccentric strength, although the precise contributions of the various potential mechanisms are unknown.
It was also an intriguing question to study how repeated eccentric loading affected the muscles of older adults. Despite the maintenance of eccentric strength in old age, neuromuscular function in older adults remains more impaired than young during and following repeated isovelocity114 or velocity-dependent75 lengthening contractions. Contrary to earlier reports from animal models,129–131 older adults do not appear to experience more muscle damage following repetitive lengthening contractions compared with their younger counterparts perhaps due to differing strain magnitudes and activation patterns.132 A major contributor to initial force loss following lengthening contractions is a reduction in the amount of Ca2+ released from the sarcoplasmic reticulum, and reduced myofibrillar Ca2+ sensitivity.133,134 Consequently, lengthening induced impairments, combined with impaired excitation contraction coupling in old age135 results in even greater impairments in neuromuscular function in older than younger adults.75
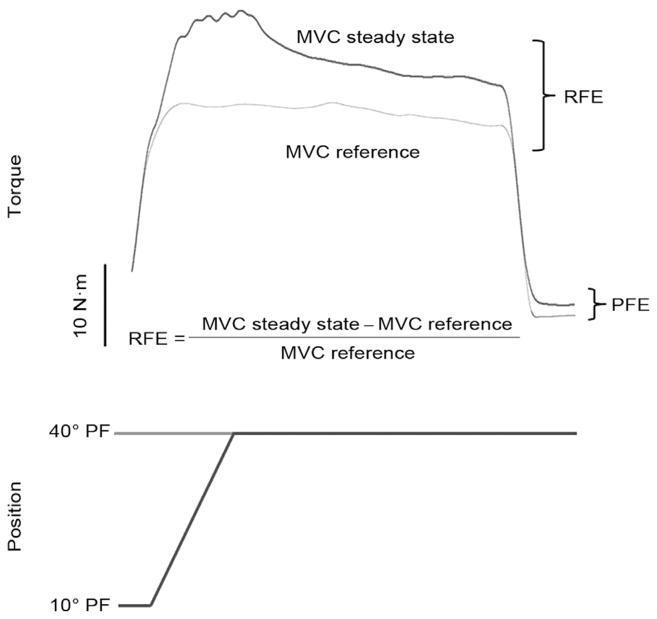
8. Residual force enhancement
Muscle force production is dependent highly upon prior contractile history.136 For example, isometric force of an activated skeletal muscle during a steady-state phase following stretch is greater than isometric force produced at the same muscle length prior to stretch.137–139 This intrinsic property of the skeletal muscle, unaccounted for by the cross-bridge and sliding filament theories of muscle contraction,140 is termed residual force enhancement (RFE). Residual force enhancement can last for up to 30 s following stretch,137 although for most investigations of human electrically stimulated141,142 and voluntary contractions, RFE dissipates quickly (10 s). Active and passive structural properties of muscle force generating and transmitting structures143 contribute to RFE. Active force enhancement is dependent upon cross-bridge kinetics and is observed along the entire force–length (F–L) relationship.143 Proposed mechanisms of active force enhancement are an increased proportion of strongly bound cross-bridges138 and an increase in the average force produced by each cross-bridge.142 Passive force enhancement (PFE) on the other hand is attributed less to cross-bridge interactions but more to length-dependent properties of muscle. Passive force enhancement occurs on the descending limb of the F–L relationship and is associated with the engagement of passive viscoelastic elements during and following stretch.143 It is suggested that PFE may be dependent upon the giant molecular spring titin which spans the sarcomere from Z-disc to M-line. Furthermore, titin may have the capacity to change its stiffness in a Ca2+ dependent manner as indicated in experiments showing elevated passive force upon full deactivation of the muscle.144–146 Most notably, RFE is greater when stretch is applied on the descending limb (long muscle length) versus the plateau or ascending limb (short muscle length) of the F–L relationship owing to the greater contribution of passive elements.147
Given that force enhancement (i.e., relative maintenance of eccentric strength) is greater in old age, it is not unreasonable to speculate that RFE may be greater in older compared with younger adults. In a recent investigation148 older adults had similar values for eccentric strength of the ankle dorsiflexors compared with young. Following stretch, the old group had 2.5 times greater residual force enhancement than the young. As well, upon relaxation the older adults experienced greater passive force enhancement than the young, thus, contributing more to the total residual force enhancement. Additionally, immediately following the active stretch, old and young followed the typical exponential decline in force,147 but more time was required for the old to reach an isometric steady-state force level compared to young148,149 (Fig. 6). These findings suggest that structural viscoelastic mechanisms may have a disproportionately greater contribution to force enhancement and RFE in old age. The elevated residual force enhancement in old age is likely related to mechanical factors rather than neural influences/limitations. Indeed muscle activity (as reflected by surface EMG) is often lower during lengthening contractions,150 however, as reported by Power et al.,148 central activation (agonist; root mean squared EMG amplitude) levels were similar for the reference isometric MVC and steady-state MVC following stretch in both young and old men. Antagonist co-activation could be ruled out as this parameter was not significantly different between young and old, a common finding for the ankle dorsiflexors. Finally, voluntary activation as assessed using the interpolated twitch technique70 was near maximal and was similar for young and old. Recently these findings were confirmed for the knee extensors in young and old adults.149 Thus, any confounding influence of age-related reductions in neuromuscular activation was avoided in the determination of RFE and the enhancement in older men likely can be attributed to age-related alterations within the muscle’s structure. Further studies of titin’s influence on aged muscle function will be particularly interesting in this regard.
Fig. 6.
Raw data depicting the determination of residual force enhancement (RFE) in an old adult (77 years). PFE, passive force enhancement; MVC, maximum voluntary isometric contraction. Adapted with permission from Ref. 137.
9. Conclusions and future directions
The age-related loss of MUs is an immutable process. Reductions in muscle strength and velocity lead to even greater declines in power than either variable alone. Furthermore, while the slowing of contractile properties may be advantageous during static or slow dynamic tasks, when the aged neuromuscular system is stressed with faster unconstrained velocity movements, older adults are indeed more fatigable than young. This modality may more closely represent day to day normal shortening contractile actions than other previous paradigms. Despite these apparently negative consequences of natural adult aging, older adults possess a unique ability to experience greater force enhancement and residual force enhancement during and following lengthening muscle actions. It is also rather auspicious that high levels of life-long activity have the potential to mitigate MU loss well into old age and maintain some levels of neuromuscular function. It is less clear at this time how fatigue may be affected by PA or fitness levels. Most studies of fatigue have explored these features in relatively healthy older adults and results may not apply to frail elderly populations.
Acknowledgments
This work is supported by an operating grant to Dr. Charles Rice from the Natural Sciences and Engineering Research Council of Canada (NSERC). Dr. Brian Dalton is currently a post-doctoral fellow at the University of British Columbia supported by the Canadian Institutes of Health Research (CIHR) and the Michael Smith Foundation for Health Research (MSFHR). Dr. Geoffrey Power is currently a postdoctoral fellow at the University of Calgary supported by Alberta Innovates Health Solutions (AIHS). The authors would like to thank Dr. Anthony Vandervoort for support and helpful comments on versions of this manuscript.
References
- 1.Manini TM, Hong SL, Clark BC. Aging and muscle: a neuron’s perspective. Curr Opin Clin Nutr Metab Care. 2013;16:21–6. doi: 10.1097/MCO.0b013e32835b5880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aagaard P, Suetta C, Caserotti P, Magnusson SP, Kjaer M. Role of the nervous system in sarcopenia and muscle atrophy with aging: strength training as a countermeasure. Scand J Med Sci Sports. 2010;20:49–64. doi: 10.1111/j.1600-0838.2009.01084.x. [DOI] [PubMed] [Google Scholar]
- 3.Doherty TJ. Invited review: aging and sarcopenia. J Appl Physiol. 2003;95:1717–27. doi: 10.1152/japplphysiol.00347.2003. [DOI] [PubMed] [Google Scholar]
- 4.Narici MV, Maffulli N. Sarcopenia: characteristics, mechanisms and functional significance. Br Med Bull. 2010;95:139–59. doi: 10.1093/bmb/ldq008. [DOI] [PubMed] [Google Scholar]
- 5.Deschenes MR. Motor unit and neuromuscular junction remodeling with aging. Curr Aging Sci. 2011;4:209–20. doi: 10.2174/1874609811104030209. [DOI] [PubMed] [Google Scholar]
- 6.Canepari M, Pellegrino MA, D’Antona G, Bottinelli R. Single muscle fiber properties in aging and disuse. Scand J Med Sci Sports. 2010;20:10–9. doi: 10.1111/j.1600-0838.2009.00965.x. [DOI] [PubMed] [Google Scholar]
- 7.Vandervoort AA. Aging of the human neuromuscular system. Muscle Nerve. 2002;25:17–25. doi: 10.1002/mus.1215. [DOI] [PubMed] [Google Scholar]
- 8.Campbell MJ, McComas AJ, Petito F. Physiological changes in ageing muscles. J Neurol Neurosurg Psychiatry. 1973;36:174–82. doi: 10.1136/jnnp.36.2.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lexell J. Human aging, muscle mass, and fiber type composition. J Gerontol A Biol Sci Med Sci. 1995;50:11–6. doi: 10.1093/gerona/50a.special_issue.11. [DOI] [PubMed] [Google Scholar]
- 10.Lexell J, Taylor CC, Sjostrom M. What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J Neurol Sci. 1988;84:275–94. doi: 10.1016/0022-510x(88)90132-3. [DOI] [PubMed] [Google Scholar]
- 11.Gordon T, Hegedus J, Tam SL. Adaptive and maladaptive motor axonal sprouting in aging and motoneuron disease. Neurol Res. 2004;26:174–85. doi: 10.1179/016164104225013806. [DOI] [PubMed] [Google Scholar]
- 12.McNeil CJ, Doherty TJ, Stashuk DW, Rice CL. Motor unit number estimates in the tibialis anterior muscle of young, old, and very old men. Muscle Nerve. 2005;31:461–7. doi: 10.1002/mus.20276. [DOI] [PubMed] [Google Scholar]
- 13.McNeil CJ, Vandervoort AA, Rice CL. Peripheral impairments cause a progressive age-related loss of strength and velocity-dependent power in the dorsiflexors. J Appl Physiol. 2007;102:1962–8. doi: 10.1152/japplphysiol.01166.2006. [DOI] [PubMed] [Google Scholar]
- 14.Doherty TJ, Brown WF. The estimated numbers and relative sizes of thenar motor units as selected by multiple point stimulation in young and older adults. Muscle Nerve. 1993;16:355–66. doi: 10.1002/mus.880160404. [DOI] [PubMed] [Google Scholar]
- 15.Doherty TJ, Brown WF. Age-related changes in the twitch contractile properties of human thenar motor units. J Appl Physiol. 1997;82:93–101. doi: 10.1152/jappl.1997.82.1.93. [DOI] [PubMed] [Google Scholar]
- 16.Hortobagyi T, Zheng D, Weidner M, Lambert NJ, Westbrook S, Houmard JA. The influence of aging on muscle strength and muscle fiber characteristics with special reference to eccentric strength. J Gerontol A Biol Sci Med Sci. 1995;50:B399–406. doi: 10.1093/gerona/50a.6.b399. [DOI] [PubMed] [Google Scholar]
- 17.Aniansson A, Hedberg M, Henning GB, Grimby G. Muscle morphology, enzymatic activity, and muscle strength in elderly men: a follow-up study. Muscle Nerve. 1986;9:585–91. doi: 10.1002/mus.880090702. [DOI] [PubMed] [Google Scholar]
- 18.Klitgaard H, Mantoni M, Schiaffino S, Ausoni S, Gorza L, Laurent-Winter C, et al. Function, morphology and protein expression of ageing skeletal muscle: a cross-sectional study of elderly men with different training backgrounds. Acta Physiol Scand. 1990;140:41–54. doi: 10.1111/j.1748-1716.1990.tb08974.x. [DOI] [PubMed] [Google Scholar]
- 19.Chopek JW, Gardiner PF. Life-long caloric restriction: effect on age-related changes in motoneuron numbers, sizes and apoptotic markers. Mech Ageing Dev. 2010;131:650–9. doi: 10.1016/j.mad.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 20.Valdez G, Tapia JC, Kang H, Clemenson GD, Jr, Gage FH, Lichtman JW, et al. Attenuation of age-related changes in mouse neuromuscular synapses by caloric restriction and exercise. Proc Natl Acad Sci USA. 2010;107:14863–8. doi: 10.1073/pnas.1002220107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tomlinson BE, Irving D. The numbers of limb motor neurons in the human lumbosacral cord throughout life. J Neurol Sci. 1977;34:213–9. doi: 10.1016/0022-510x(77)90069-7. [DOI] [PubMed] [Google Scholar]
- 22.Brown WF. A method for estimating the number of motor units in thenar muscles and the changes in motor unit count with ageing. J Neurol Neurosurg Psychiatry. 1972;35:845–52. doi: 10.1136/jnnp.35.6.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McComas AJ, Fawcett PR, Campbell MJ, Sica RE. Electrophysiological estimation of the number of motor units within a human muscle. J Neurol Neurosurg Psychiatry. 1971;34:121–31. doi: 10.1136/jnnp.34.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vandervoort AA, McComas AJ. Contractile changes in opposing muscles of the human ankle joint with aging. J Appl Physiol. 1986;61:361–7. doi: 10.1152/jappl.1986.61.1.361. [DOI] [PubMed] [Google Scholar]
- 25.Galea V. Changes in motor unit estimates with aging. J Clin Neurophysiol. 1996;13:253–60. doi: 10.1097/00004691-199605000-00010. [DOI] [PubMed] [Google Scholar]
- 26.Brown WF, Strong MJ, Snow R. Methods for estimating numbers of motor units in biceps-brachialis muscles and losses of motor units with aging. Muscle Nerve. 1988;11:423–32. doi: 10.1002/mus.880110503. [DOI] [PubMed] [Google Scholar]
- 27.Doherty TJ, Vandervoort AA, Taylor AW, Brown WF. Effects of motor unit losses on strength in older men and women. J Appl Physiol. 1993;74:868–74. doi: 10.1152/jappl.1993.74.2.868. [DOI] [PubMed] [Google Scholar]
- 28.Dalton BH, McNeil CJ, Doherty TJ, Rice CL. Age-related reductions in the estimated numbers of motor units are minimal in the human soleus. Muscle Nerve. 2008;38:1108–15. doi: 10.1002/mus.20984. [DOI] [PubMed] [Google Scholar]
- 29.Power GA, Dalton BH, Behm DG, Doherty TJ, Vandervoort AA, Rice CL. Motor unit survival in lifelong runners is muscle dependent. Med Sci Sports Exerc. 2012;44:1235–42. doi: 10.1249/MSS.0b013e318249953c. [DOI] [PubMed] [Google Scholar]
- 30.Power GA, Dalton BH, Behm DG, Vandervoort AA, Doherty TJ, Rice CL. Motor unit number estimates in masters runners: use it or lose it? Med Sci Sports Exerc. 2010;42:1644–50. doi: 10.1249/MSS.0b013e3181d6f9e9. [DOI] [PubMed] [Google Scholar]
- 31.Allen MD, Choi IH, Kimpinski K, Doherty TJ, Rice CL. Motor unit loss and weakness in association with diabetic neuropathy in humans. Muscle Nerve. 2013;48:298–300. doi: 10.1002/mus.23792. [DOI] [PubMed] [Google Scholar]
- 32.Bromberg MB. Updating motor unit number estimation (MUNE) Clin Neurophysiol. 2007;118:1–8. doi: 10.1016/j.clinph.2006.07.304. [DOI] [PubMed] [Google Scholar]
- 33.Boe SG, Dalton BH, Harwood B, Doherty TJ, Rice CL. Inter-rater reliability of motor unit number estimates and quantitative motor unit analysis in the tibialis anterior muscle. Clin Neurophysiol. 2009;120:947–52. doi: 10.1016/j.clinph.2009.02.168. [DOI] [PubMed] [Google Scholar]
- 34.Boe SG, Stashuk DW, Doherty TJ. Motor unit number estimation by decomposition-enhanced spike-triggered averaging: control data, test-retest reliability, and contractile level effects. Muscle Nerve. 2004;29:693–9. doi: 10.1002/mus.20031. [DOI] [PubMed] [Google Scholar]
- 35.Boe SG, Stashuk DW, Doherty TJ. Within-subject reliability of motor unit number estimates and quantitative motor unit analysis in a distal and proximal upper limb muscle. Clin Neurophysiol. 2006;117:596–603. doi: 10.1016/j.clinph.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 36.Boe SG, Rice CL, Doherty TJ. Estimating contraction level using root mean square amplitude in control subjects and patients with neuromuscular disorders. Arch Phys Med Rehabil. 2008;89:711–8. doi: 10.1016/j.apmr.2007.09.047. [DOI] [PubMed] [Google Scholar]
- 37.Boe SG, Stashuk DW, Doherty TJ. Motor unit number estimates and quantitative motor unit analysis in healthy subjects and patients with amyotrophic lateral sclerosis. Muscle Nerve. 2007;36:62–70. doi: 10.1002/mus.20784. [DOI] [PubMed] [Google Scholar]
- 38.Murga Oporto L, Menéndez-de León C, Bauzano Poley E, Núñez-Castaín MJ. Statistical (Poisson) motor unit number estimation. Methodological aspects and normal results in the extensor digitorum brevis muscle of healthy subjects. Rev Neurol. 2003;36:601–4. in Spanish. [PubMed] [Google Scholar]
- 39.Johnson MA, Polgar J, Weightman D, Appleton D. Data on the distribution of fibre types in thirty-six human muscles. An autopsy study. J Neurol Sci. 1973;18:111–29. doi: 10.1016/0022-510x(73)90023-3. [DOI] [PubMed] [Google Scholar]
- 40.Allen MD, Power GA, Filion M, Doherty TJ, Rice CL, Taivassalo T, et al. Motor unit number estimates in world-class masters athletes: is 80 the new 60? FASEB J. 2013;27:1150–1. [Google Scholar]
- 41.Kanda K, Hashizume K. Effects of long-term physical exercise on age-related changes of spinal motoneurons and peripheral nerves in rats. Neurosci Res. 1998;31:69–75. doi: 10.1016/s0168-0102(98)00026-1. [DOI] [PubMed] [Google Scholar]
- 42.Kanda K, Hashizume K, Miwa T, Miwa Y. Overloading a muscle does not alter the rate of motoneuronal loss in aged rats. Neurobiol Aging. 1996;17:613–7. doi: 10.1016/0197-4580(96)00004-8. [DOI] [PubMed] [Google Scholar]
- 43.Hunter GR, McCarthy JP, Bamman MM. Effects of resistance training on older adults. Sports Med. 2004;34:329–48. doi: 10.2165/00007256-200434050-00005. [DOI] [PubMed] [Google Scholar]
- 44.Porter MM. The effects of strength training on sarcopenia. Can J Appl Physiol. 2001;26:123–41. doi: 10.1139/h01-009. [DOI] [PubMed] [Google Scholar]
- 45.Roig M, Macintyre DL, Eng JJ, Narici MV, Maganaris CN, Reid WD. Preservation of eccentric strength in older adults: evidence, mechanisms and implications for training and rehabilitation. Exp Gerontol. 2010;45:400–9. doi: 10.1016/j.exger.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Trappe S, Williamson D, Godard M, Porter D, Rowden G, Costill D. Effect of resistance training on single muscle fiber contractile function in older men. J Appl Physiol. 2000;89:143–52. doi: 10.1152/jappl.2000.89.1.143. [DOI] [PubMed] [Google Scholar]
- 47.Morse CI, Thom JM, Mian OS, Muirhead A, Birch KM, Narici MV. Muscle strength, volume and activation following 12-month resistance training in 70-year-old males. Eur J Appl Physiol. 2005;95:197–204. doi: 10.1007/s00421-005-1342-3. [DOI] [PubMed] [Google Scholar]
- 48.Lexell J, Henriksson-Larsen K, Winblad B, Sjostrom M. Distribution of different fiber types in human skeletal muscles: effects of aging studied in whole muscle cross sections. Muscle Nerve. 1983;6:588–95. doi: 10.1002/mus.880060809. [DOI] [PubMed] [Google Scholar]
- 49.Frontera WR, Hughes VA, Fielding RA, Fiatarone MA, Evans WJ, Roubenoff R. Aging of skeletal muscle: a 12-yr longitudinal study. J Appl Physiol. 2000;88:1321–6. doi: 10.1152/jappl.2000.88.4.1321. [DOI] [PubMed] [Google Scholar]
- 50.Rice CL, Cunningham DA, Paterson DH, Lefcoe MS. Arm and leg composition determined by computed tomography in young and elderly men. Clin Physiol. 1989;9:207–20. doi: 10.1111/j.1475-097x.1989.tb00973.x. [DOI] [PubMed] [Google Scholar]
- 51.Overend TJ, Cunningham DA, Paterson DH, Lefcoe MS. Thigh composition in young and elderly men determined by computed tomography. Clin Physiol. 1992;12:629–40. doi: 10.1111/j.1475-097x.1992.tb00366.x. [DOI] [PubMed] [Google Scholar]
- 52.Rice CL, Cunningham DA, Paterson DH, Lefcoe MS. A comparison of anthropometry with computed tomography in limbs of young and aged men. J Gerontol. 1990;45:M175–9. doi: 10.1093/geronj/45.5.m175. [DOI] [PubMed] [Google Scholar]
- 53.Kanda K, Hashizume K. Changes in properties of the medial gastrocnemius motor units in aging rats. J Neurophysiol. 1989;61:737–46. doi: 10.1152/jn.1989.61.4.737. [DOI] [PubMed] [Google Scholar]
- 54.Larsson L, Sjodin B, Karlsson J. Histochemical and biochemical changes in human skeletal muscle with age in sedentary males, age 22–65 years. Acta Physiol Scand. 1978;103:31–9. doi: 10.1111/j.1748-1716.1978.tb06187.x. [DOI] [PubMed] [Google Scholar]
- 55.Klein CS, Marsh GD, Petrella RJ, Rice CL. Muscle fiber number in the biceps brachii muscle of young and old men. Muscle Nerve. 2003;28:62–8. doi: 10.1002/mus.10386. [DOI] [PubMed] [Google Scholar]
- 56.Bottinelli R, Schiaffino S, Reggiani C. Force-velocity relations and myosin heavy chain isoform compositions of skinned fibres from rat skeletal muscle. J Physiol. 1991;437:655–72. doi: 10.1113/jphysiol.1991.sp018617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bottinelli R, Betto R, Schiaffino S, Reggiani C. Maximum shortening velocity and coexistence of myosin heavy chain isoforms in single skinned fast fibres of rat skeletal muscle. J Muscle Res Cell Motil. 1994;15:413–9. doi: 10.1007/BF00122115. [DOI] [PubMed] [Google Scholar]
- 58.Bottinelli R, Betto R, Schiaffino S, Reggiani C. Unloaded shortening velocity and myosin heavy chain and alkali light chain isoform composition in rat skeletal muscle fibres. J Physiol. 1994;478(Pt 2):341–9. doi: 10.1113/jphysiol.1994.sp020254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.D’Antona G, Pellegrino MA, Carlizzi CN, Bottinelli R. Deterioration of contractile properties of muscle fibres in elderly subjects is modulated by the level of physical activity. Eur J Appl Physiol. 2007;100:603–11. doi: 10.1007/s00421-007-0402-2. [DOI] [PubMed] [Google Scholar]
- 60.Canepari M, Rossi R, Pellegrino MA, Orrell RW, Cobbold M, Harridge S, et al. Effects of resistance training on myosin function studied by the in vitro motility assay in young and older men. J Appl Physiol. 2005;98:2390–5. doi: 10.1152/japplphysiol.01103.2004. [DOI] [PubMed] [Google Scholar]
- 61.D’Antona G, Pellegrino MA, Adami R, Rossi R, Carlizzi CN, Canepari M, et al. The effect of ageing and immobilization on structure and function of human skeletal muscle fibres. J Physiol. 2003;552:499–511. doi: 10.1113/jphysiol.2003.046276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Russ DW, Gregg-Cornell K, Conaway MJ, Clark BC. Evolving concepts on the age-related changes in “muscle quality”. J Cachexia Sarcopenia Muscle. 2012;3:95–109. doi: 10.1007/s13539-011-0054-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lowe DA, Surek JT, Thomas DD, Thompson LV. Electron paramagnetic resonance reveals age-related myosin structural changes in rat skeletal muscle fibers. Am J Physiol Cell Physiol. 2001;280:540–7. doi: 10.1152/ajpcell.2001.280.3.C540. [DOI] [PubMed] [Google Scholar]
- 64.Hook P, Li X, Sleep J, Hughes S, Larsson L. The effect of age on in vitro motility speed of slow myosin extracted from single rat soleus fibres. Acta Physiol Scand. 1999;167:325–6. doi: 10.1046/j.1365-201x.1999.00619.x. [DOI] [PubMed] [Google Scholar]
- 65.Hook P, Sriramoju V, Larsson L. Effects of aging on actin sliding speed on myosin from single skeletal muscle cells of mice, rats, and humans. Am J Physiol Cell Physiol. 2001;280:782–8. doi: 10.1152/ajpcell.2001.280.4.C782. [DOI] [PubMed] [Google Scholar]
- 66.Hughes VA, Frontera WR, Wood M, Evans WJ, Dallal GE, Roubenoff R, et al. Longitudinal muscle strength changes in older adults: influence of muscle mass, physical activity, and health. J Gerontol A Biol Sci Med Sci. 2001;56:209–17. doi: 10.1093/gerona/56.5.b209. [DOI] [PubMed] [Google Scholar]
- 67.Winegard KJ, Hicks AL, Sale DG, Vandervoort AA. A 12-year follow-up study of ankle muscle function in older adults. J Gerontol A Biol Sci Med Sci. 1996;51:202–7. doi: 10.1093/gerona/51a.3.b202. [DOI] [PubMed] [Google Scholar]
- 68.Jakobi JM, Rice CL. Voluntary muscle activation varies with age and muscle group. J Appl Physiol. 2002;93:457–62. doi: 10.1152/japplphysiol.00012.2002. [DOI] [PubMed] [Google Scholar]
- 69.Klass M, Baudry S, Duchateau J. Voluntary activation during maximal contraction with advancing age: a brief review. Eur J Appl Physiol. 2007;100:543–51. doi: 10.1007/s00421-006-0205-x. [DOI] [PubMed] [Google Scholar]
- 70.Gandevia SC. Spinal and supraspinal factors in human muscle fatigue. Physiol Rev. 2001;81:1725–89. doi: 10.1152/physrev.2001.81.4.1725. [DOI] [PubMed] [Google Scholar]
- 71.Aoki H, Demura S. Age differences in hand grip power in the elderly. Arch Gerontol Geriatr. 2011;52:176–9. doi: 10.1016/j.archger.2010.10.025. [DOI] [PubMed] [Google Scholar]
- 72.Dalton BH, Power GA, Vandervoort AA, Rice CL. Power loss is greater in old men than young men during fast plantar flexion contractions. J Appl Physiol. 2010;109:1441–7. doi: 10.1152/japplphysiol.00335.2010. [DOI] [PubMed] [Google Scholar]
- 73.Dalton BH, Power GA, Vandervoort AA, Rice CL. The age-related slowing of voluntary shortening velocity exacerbates power loss during repeated fast knee extensions. Exp Gerontol. 2012;47:85–92. doi: 10.1016/j.exger.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 74.McNeil CJ, Rice CL. Fatigability is increased with age during velocity-dependent contractions of the dorsiflexors. J Gerontol A Biol Sci Med Sci. 2007;62:624–9. doi: 10.1093/gerona/62.6.624. [DOI] [PubMed] [Google Scholar]
- 75.Power GA, Dalton BH, Rice CL, Vandervoort AA. Power loss is greater following lengthening contractions in old versus young women. Age. 2012;34:737–50. doi: 10.1007/s11357-011-9263-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Valour D, Ochala J, Ballay Y, Pousson M. The influence of ageing on the force-velocity-power characteristics of human elbow flexor muscles. Exp Gerontol. 2003;38:387–95. doi: 10.1016/s0531-5565(02)00265-6. [DOI] [PubMed] [Google Scholar]
- 77.Labarque V, BOTE, Van Leemputte M. Resistance training alters torque-velocity relation of elbow flexors in elderly men. Med Sci Sports Exerc. 2002;34:851–6. doi: 10.1097/00005768-200205000-00019. [DOI] [PubMed] [Google Scholar]
- 78.Thom JM, Morse CI, Birch KM, Narici MV. Influence of muscle architecture on the torque and power-velocity characteristics of young and elderly men. Eur J Appl Physiol. 2007;100:613–9. doi: 10.1007/s00421-007-0481-0. [DOI] [PubMed] [Google Scholar]
- 79.Thom JM, Morse CI, Birch KM, Narici MV. Triceps surae muscle power, volume, and quality in older versus younger healthy men. J Gerontol A Biol Sci Med Sci. 2005;60:1111–7. doi: 10.1093/gerona/60.9.1111. [DOI] [PubMed] [Google Scholar]
- 80.Pojednic RM, Clark DJ, Patten C, Reid K, Phillips EM, Fielding RA. The specific contributions of force and velocity to muscle power in older adults. Exp Gerontol. 2012;47:608–13. doi: 10.1016/j.exger.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Korhonen MT, Cristea A, Alen M, Hakkinen K, Sipila S, Mero A, et al. Aging, muscle fiber type, and contractile function in sprint-trained athletes. J Appl Physiol. 2006;101:906–17. doi: 10.1152/japplphysiol.00299.2006. [DOI] [PubMed] [Google Scholar]
- 82.Ochala J, Frontera WR, Dorer DJ, Van Hoecke J, Krivickas LS. Single skeletal muscle fiber elastic and contractile characteristics in young and older men. J Gerontol A Biol Sci Med Sci. 2007;62:375–81. doi: 10.1093/gerona/62.4.375. [DOI] [PubMed] [Google Scholar]
- 83.Frontera WR, Hughes VA, Krivickas LS, Roubenoff R. Contractile properties of aging skeletal muscle. Int J Sport Nutr Exerc Metab. 2001;11:16–20. doi: 10.1123/ijsnem.11.s1.s16. [DOI] [PubMed] [Google Scholar]
- 84.Yu F, Hedstrom M, Cristea A, Dalen N, Larsson L. Effects of ageing and gender on contractile properties in human skeletal muscle and single fibres. Acta Physiol (Oxf) 2007;190:229–41. doi: 10.1111/j.1748-1716.2007.01699.x. [DOI] [PubMed] [Google Scholar]
- 85.Krivickas LS, Suh D, Wilkins J, Hughes VA, Roubenoff R, Frontera WR. Age- and gender-related differences in maximum shortening velocity of skeletal muscle fibers. Am J Phys Med Rehabil. 2001;80:447–55. doi: 10.1097/00002060-200106000-00012. [DOI] [PubMed] [Google Scholar]
- 86.Raj IS, Bird SR, Shield AJ. Aging and the force-velocity relationship of muscles. Exp Gerontol. 2010;45:81–90. doi: 10.1016/j.exger.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 87.Huxley AF. Muscle structure and theories of contraction. Prog Biophys Biophys Chem. 1957;7:255–318. [PubMed] [Google Scholar]
- 88.Abbott BC, Wilkie DR. The relation between velocity of shortening and the tension-length curve of skeletal muscle. J Physiol. 1953;120:214–23. doi: 10.1113/jphysiol.1953.sp004886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lieber RL, Ward SR. Skeletal muscle design to meet functional demands. Philos Trans R Soc Lond B Biol Sci. 2010;366:1466–76. doi: 10.1098/rstb.2010.0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lanza IR, Towse TF, Caldwell GE, Wigmore DM, Kent-Braun JA. Effects of age on human muscle torque, velocity, and power in two muscle groups. J Appl Physiol. 2003;95:2361–9. doi: 10.1152/japplphysiol.00724.2002. [DOI] [PubMed] [Google Scholar]
- 91.Lindle RS, Metter EJ, Lynch NA, Fleg JL, Fozard JL, Tobin J, et al. Age and gender comparisons of muscle strength in 654 women and men aged 20–93 yr. J Appl Physiol. 1997;83:1581–7. doi: 10.1152/jappl.1997.83.5.1581. [DOI] [PubMed] [Google Scholar]
- 92.Harries UJ, Bassey EJ. Torque–velocity relationships for the knee extensors in women in their 3rd and 7th decades. Eur J Appl Physiol Occup Physiol. 1990;60:187–90. doi: 10.1007/BF00839157. [DOI] [PubMed] [Google Scholar]
- 93.Gajdosik RL, Vander Linden DW, Williams AK. Concentric isokinetic torque characteristics of the calf muscles of active women aged 20 to 84 years. J Orthop Sports Phys Ther. 1999;29:181–90. doi: 10.2519/jospt.1999.29.3.181. [DOI] [PubMed] [Google Scholar]
- 94.Ochala J, Lambertz D, Pousson M, Goubel F, Hoecke JV. Changes in mechanical properties of human plantar flexor muscles in ageing. Exp Gerontol. 2004;39:349–58. doi: 10.1016/j.exger.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 95.Petrella JK, Kim JS, Tuggle SC, Bamman MM. Contributions of force and velocity to improved power with progressive resistance training in young and older adults. Eur J Appl Physiol. 2007;99:343–51. doi: 10.1007/s00421-006-0353-z. [DOI] [PubMed] [Google Scholar]
- 96.Petrella JK, Kim JS, Tuggle SC, Hall SR, Bamman MM. Age differences in knee extension power, contractile velocity, and fatigability. J Appl Physiol. 2005;98:211–20. doi: 10.1152/japplphysiol.00294.2004. [DOI] [PubMed] [Google Scholar]
- 97.Grimby G. Muscle performance and structure in the elderly as studied cross-sectionally and longitudinally. J Gerontol A Biol Sci Med Sci. 1995;50:17–22. doi: 10.1093/gerona/50a.special_issue.17. [DOI] [PubMed] [Google Scholar]
- 98.Clark DJ, Pojednic RM, Reid KF, Patten C, Pasha EP, Phillips EM, et al. Longitudinal decline of neuromuscular activation and power in healthy older adults. J Gerontol A Biol Sci Med Sci. 2013;68:1419–25. doi: 10.1093/gerona/glt036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bean JF, Kiely DK, Herman S, Leveille SG, Mizer K, Frontera WR, et al. The relationship between leg power and physical performance in mobility-limited older people. J Am Geriatr Soc. 2002;50:461–7. doi: 10.1046/j.1532-5415.2002.50111.x. [DOI] [PubMed] [Google Scholar]
- 100.Bean JF, Kiely DK, LaRose S, Goldstein R, Frontera WR, Leveille SG. Are changes in leg power responsible for clinically meaningful improvements in mobility in older adults? J Am Geriatr Soc. 2010;58:2363–8. doi: 10.1111/j.1532-5415.2010.03155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kent-Braun JA, Callahan DM, Fay JL, Foulis SA, Buonaccorsi JP. Muscle weakness, fatigue, and torque variability: effects of age and mobility status. Muscle Nerve. 2013 doi: 10.1002/mus.23903. http://dx.doi.org/10.1002/mus.23903[Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 102.Bigland-Ritchie B, Woods JJ. Changes in muscle contractile properties and neural control during human muscular fatigue. Muscle Nerve. 1984;7:691–9. doi: 10.1002/mus.880070902. [DOI] [PubMed] [Google Scholar]
- 103.Hicks AL, Kent-Braun J, Ditor DS. Sex differences in human skeletal muscle fatigue. Exerc Sport Sci Rev. 2001;29:109–12. doi: 10.1097/00003677-200107000-00004. [DOI] [PubMed] [Google Scholar]
- 104.Hunter SK. Sex differences and mechanisms of task-specific muscle fatigue. Exerc Sport Sci Rev. 2009;37:113–22. doi: 10.1097/JES.0b013e3181aa63e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hunter SK, Critchlow A, Enoka RM. Influence of aging on sex differences in muscle fatigability. J Appl Physiol. 2004;97:1723–32. doi: 10.1152/japplphysiol.00460.2004. [DOI] [PubMed] [Google Scholar]
- 106.Kent-Braun JA. Skeletal muscle fatigue in old age: whose advantage? Exerc Sport Sci Rev. 2009;37:3–9. doi: 10.1097/JES.0b013e318190ea2e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Enoka RM, Duchateau J. Muscle fatigue: what, why and how it influences muscle function. J Physiol. 2008;586:11–23. doi: 10.1113/jphysiol.2007.139477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Allman BL, Rice CL. An age-related shift in the force-frequency relationship affects quadriceps fatigability in old adults. J Appl Physiol. 2004;96:1026–32. doi: 10.1152/japplphysiol.00991.2003. [DOI] [PubMed] [Google Scholar]
- 109.Connelly DM, Rice CL, Roos MR, Vandervoort AA. Motor unit firing rates and contractile properties in tibialis anterior of young and old men. J Appl Physiol. 1999;87:843–52. doi: 10.1152/jappl.1999.87.2.843. [DOI] [PubMed] [Google Scholar]
- 110.Lanza IR, Russ DW, Kent-Braun JA. Age-related enhancement of fatigue resistance is evident in men during both isometric and dynamic tasks. J Appl Physiol. 2004;97:967–75. doi: 10.1152/japplphysiol.01351.2003. [DOI] [PubMed] [Google Scholar]
- 111.Tevald MA, Foulis SA, Lanza IR, Kent-Braun JA. Lower energy cost of skeletal muscle contractions in older humans. Am J Physiol Regul Integr Comp Physiol. 2010;298:729–39. doi: 10.1152/ajpregu.00713.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Russ DW, Lanza IR. The impact of old age on skeletal muscle energetics: supply and demand. Curr Aging Sci. 2011;4:234–47. doi: 10.2174/1874609811104030234. [DOI] [PubMed] [Google Scholar]
- 113.Callahan DM, Kent-Braun JA. Effect of old age on human skeletal muscle force-velocity and fatigue properties. J Appl Physiol. 2011;111:1345–52. doi: 10.1152/japplphysiol.00367.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Baudry S, Klass M, Pasquet B, Duchateau J. Age-related fatigability of the ankle dorsiflexor muscles during concentric and eccentric contractions. Eur J Appl Physiol. 2007;100:515–25. doi: 10.1007/s00421-006-0206-9. [DOI] [PubMed] [Google Scholar]
- 115.Dalton BH, Harwood B, Davidson AW, Rice CL. Triceps surae contractile properties and firing rates in the soleus of young and old men. J Appl Physiol. 2009;107:1781–8. doi: 10.1152/japplphysiol.00464.2009. [DOI] [PubMed] [Google Scholar]
- 116.Roos MR, Rice CL, Connelly DM, Vandervoort AA. Quadriceps muscle strength, contractile properties, and motor unit firing rates in young and old men. Muscle Nerve. 1999;22:1094–103. doi: 10.1002/(sici)1097-4598(199908)22:8<1094::aid-mus14>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 117.Power GA, Dalton BH, Rice CL, Vandervoort AA. Reproducibility of velocity-dependent power: before and after lengthening contractions. Appl Physiol Nutr Metab. 2011;36:626–33. doi: 10.1139/h11-068. [DOI] [PubMed] [Google Scholar]
- 118.Hill AV. The heat of shortening and the dynamic constants of muscle. Proc R Soc B. 1938;126:136–95. [Google Scholar]
- 119.Cheng AJ, Rice CL. Fatigue and recovery of power and isometric torque following isotonic knee extensions. J Appl Physiol. 2005;99:1446–52. doi: 10.1152/japplphysiol.00452.2005. [DOI] [PubMed] [Google Scholar]
- 120.Abbott BC, Bigland B, Ritchie JM. The physiological cost of negative work. J Physiol. 1952;117:380–90. doi: 10.1113/jphysiol.1952.sp004755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Vandervoort AA, Kramer JF, Wharram ER. Eccentric knee strength of elderly females. J Gerontol. 1990;45:125–8. doi: 10.1093/geronj/45.4.b125. [DOI] [PubMed] [Google Scholar]
- 122.Phillips SK, Bruce SA, Woledge RC. In mice, the muscle weakness due to age is absent during stretching. J Physiol. 1991;437:63–70. doi: 10.1113/jphysiol.1991.sp018583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Porter MM, Vandervoort AA, Kramer JF. Eccentric peak torque of the plantar and dorsiflexors is maintained in older women. J Gerontol A Biol Sci Med Sci. 1997;52:125–31. doi: 10.1093/gerona/52a.2.b125. [DOI] [PubMed] [Google Scholar]
- 124.Poulin MJ, Vandervoort AA, Paterson DH, Kramer JF, Cunningham DA. Eccentric and concentric torques of knee and elbow extension in young and older men. Can J Sport Sci. 1992;17:3–7. [PubMed] [Google Scholar]
- 125.Klass M, Baudry S, Duchateau J. Aging does not affect voluntary activation of the ankle dorsiflexors during isometric, concentric, and eccentric contractions. J Appl Physiol. 2005;99:31–8. doi: 10.1152/japplphysiol.01426.2004. [DOI] [PubMed] [Google Scholar]
- 126.Phillips SK, Bruce SA, Newton D, Woledge RC. The weakness of old age is not due to failure of muscle activation. J Gerontol. 1992;47:45–9. doi: 10.1093/geronj/47.2.m45. [DOI] [PubMed] [Google Scholar]
- 127.Hasson CJ, Miller RH, Caldwell GE. Contractile and elastic ankle joint muscular properties in young and older adults. PLoS One. 2011;6:15953. doi: 10.1371/journal.pone.0015953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ochala J, Dorer DJ, Frontera WR, Krivickas LS. Single skeletal muscle fiber behavior after a quick stretch in young and older men: a possible explanation of the relative preservation of eccentric force in old age. Pflugers Arch. 2006;452:464–70. doi: 10.1007/s00424-006-0065-6. [DOI] [PubMed] [Google Scholar]
- 129.Faulkner JA, Brooks SV, Zerba E. Muscle atrophy and weakness with aging: contraction-induced injury as an underlying mechanism. J Gerontol A Biol Sci Med Sci. 1995;50:124–9. doi: 10.1093/gerona/50a.special_issue.124. [DOI] [PubMed] [Google Scholar]
- 130.Rader EP, Faulkner JA. Effect of aging on the recovery following contraction-induced injury in muscles of female mice. J Appl Physiol. 2006;101:887–92. doi: 10.1152/japplphysiol.00380.2006. [DOI] [PubMed] [Google Scholar]
- 131.Rader EP, Faulkner JA. Recovery from contraction-induced injury is impaired in weight-bearing muscles of old male mice. J Appl Physiol. 2006;100:656–61. doi: 10.1152/japplphysiol.00663.2005. [DOI] [PubMed] [Google Scholar]
- 132.Buford TW, Macneil RG, Clough LG, Dirain M, Sandesara B, Pahor M, et al. Active muscle regeneration following eccentric contraction-induced injury is similar between healthy young and older adults. J Appl Physiol. 2013 doi: 10.1152/japplphysiol.01350.2012. http://dx.doi.org/10.1152/japplphysiol.01350.2012 [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 133.Edwards RH, Hill DK, Jones DA, Merton PA. Fatigue of long duration in human skeletal muscle after exercise. J Physiol. 1977;272:769–78. doi: 10.1113/jphysiol.1977.sp012072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ingalls CP, Warren GL, Williams JH, Ward CW, Armstrong RB. E-C coupling failure in mouse EDL muscle after in vivo eccentric contractions. J Appl Physiol. 1998;85:58–67. doi: 10.1152/jappl.1998.85.1.58. [DOI] [PubMed] [Google Scholar]
- 135.Payne AM, Delbono O. Neurogenesis of excitation-contraction uncoupling in aging skeletal muscle. Exerc Sport Sci Rev. 2004;32:36–40. doi: 10.1097/00003677-200401000-00008. [DOI] [PubMed] [Google Scholar]
- 136.McGowan CP, Neptune RR, Herzog W. A phenomenological muscle model to assess history dependent effects in human movement. J Biomech. 2013;46:151–7. doi: 10.1016/j.jbiomech.2012.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Abbott BC, Aubert XM. The force exerted by active striated muscle during and after change of length. J Physiol. 1952;117:77–86. [PMC free article] [PubMed] [Google Scholar]
- 138.Rassier DE, Herzog W, Wakeling J, Syme DA. Stretch-induced, steady-state force enhancement in single skeletal muscle fibers exceeds the isometric force at optimum fiber length. J Biomech. 2003;36:1309–16. doi: 10.1016/s0021-9290(03)00155-6. [DOI] [PubMed] [Google Scholar]
- 139.Power GA, Rice CL, Vandervoort AA. Residual force enhancement following eccentric induced muscle damage. J Biomech. 2012;45:1835–41. doi: 10.1016/j.jbiomech.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 140.Herzog W, Leonard TR, Joumaa V, Mehta A. Mysteries of muscle contraction. J Appl Biomech. 2008;24:1–13. doi: 10.1123/jab.24.1.1. [DOI] [PubMed] [Google Scholar]
- 141.de Ruiter CJ, Didden WJ, Jones DA, Haan AD. The force-velocity relationship of human adductor pollicis muscle during stretch and the effects of fatigue. J Physiol. 2000;526:671–81. doi: 10.1111/j.1469-7793.2000.00671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lee HD, Herzog W. Force enhancement following muscle stretch of electrically stimulated and voluntarily activated human adductor pollicis. J Physiol. 2002;545:321–30. doi: 10.1113/jphysiol.2002.018010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Herzog W, Leonard TR. Force enhancement following stretching of skeletal muscle: a new mechanism. J Exp Biol. 2002;205:1275–83. doi: 10.1242/jeb.205.9.1275. [DOI] [PubMed] [Google Scholar]
- 144.Labeit D, Watanabe K, Witt C, Fujita H, Wu Y, Lahmers S, et al. Calcium-dependent molecular spring elements in the giant protein titin. Proc Natl Acad Sci USA. 2003;100:13716–21. doi: 10.1073/pnas.2235652100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Joumaa V, Rassier DE, Leonard TR, Herzog W. The origin of passive force enhancement in skeletal muscle. Am J Physiol Cell Physiol. 2008;294:74–8. doi: 10.1152/ajpcell.00218.2007. [DOI] [PubMed] [Google Scholar]
- 146.DuVall MM, Gifford JL, Amrein M, Herzog W. Altered mechanical properties of titin immunoglobulin domain 27 in the presence of calcium. Eur Biophys J. 2013;42:301–7. doi: 10.1007/s00249-012-0875-8. [DOI] [PubMed] [Google Scholar]
- 147.Edman KA. Residual force enhancement after stretch in striated muscle. A consequence of increased myofilament overlap? J Physiol. 2012;590:1339–45. doi: 10.1113/jphysiol.2011.222729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Power GA, Rice CL, Vandervoort AA. Increased residual force enhancement in older adults is associated with a maintenance of eccentric strength. PLoS One. 2012;7:e48044. doi: 10.1371/journal.pone.0048044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Power GA, Makrakos DP, Rice CL, Vandervoort AA. Enhanced force production in old age is not a far stretch: an investigation of residual force enhancement and muscle architecture. Physiol Rep. 2013;1:e00004. doi: 10.1002/phy2.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Duchateau J, Enoka RM. Neural control of shortening and lengthening contractions: influence of task constraints. J Physiol. 2008;586:5853–64. doi: 10.1113/jphysiol.2008.160747. [DOI] [PMC free article] [PubMed] [Google Scholar]