Abstract
Dietary-induced obesity (DIO) resulting from high-fat (HF) or high-sugar diets produces a host of deleterious metabolic consequences including adverse bone development. We compared the effects of feeding standard rodent chow (Control), a 30% moderately HF (starch-based/sugar-free) diet, or a combined 30%/40% HF/high-fructose (HF/F) diet for 12 weeks on cancellous/cortical bone development in male Sprague-Dawley rats aged 8 weeks. Both HF feeding regimens reduced the lean/fat mass ratio, elevated circulating leptin, and reduced serum total antioxidant capacity (tAOC) when compared with Controls. Distal femur cancellous bone mineral density (BMD) was 23–34% lower in both HF groups (p<0.001) and was characterized by lower cancellous bone volume (BV/TV, p<0.01), lower trabecular number (Tb.N, p<0.001), and increased trabecular separation versus Controls (p<0.001). Cancellous BMD, BV/TV, and Tb.N were negatively associated with leptin and positively associated with tAOC at the distal femur. Similar cancellous bone deficits were observed at the proximal tibia, along with increased bone marrow adipocyte density (p<0.05), which was negatively associated with BV/TV and Tb.N. HF/F animals also exhibited lower osteoblast surface and reduced circulating osteocalcin (p<0.05). Cortical thickness (p<0.01) and tissue mineral density (p<0.05) were higher in both HF-fed groups versus Controls, while whole bone biomechanical characteristics were not different among groups. These results demonstrate that “westernized” HF diets worsen cancellous, but not cortical, bone parameters in skeletally-immature male rats and that fructose incorporation into HF diets does not exacerbate bone loss. In addition, they suggest that leptin and/or oxidative stress may influence DIO-induced alterations in adolescent bone development.
Keywords: osteoporosis, diet, adipose, adiposity, fat, sugar
1.0 INTRODUCTION
Historically, obesity and body mass index (BMI) have been positively associated with bone mineral density (BMD) in adults, likely a result of higher body mass increasing mechanical strain on the weight bearing portions of the skeleton and/or an altered hormonal milieu resulting from increased adiposity that may positively influence bone acquisition in adulthood.[1] However, the influence of obesity on adolescent bone development requires further clarification because an inverse relationship exists between total body fat mass and total body, lumbar spine, and ultradistal radius areal BMD in children, despite greater lean mass in obese children [2] and because obese adolescents (assessed by BMI) exhibit higher fracture rates than their age-matched normal weight non-obese counterparts,[3] suggesting that obesity may be detrimental to adolescent skeletal health. Consuming a high-fat and/or high-sugar diet is one factor that predisposes children to obesity.[4] Similarly, high-fat [5–10] or high fructose diets [11, 12] lead to dietary-induced obesity (DIO) in skeletally-immature rodents, along with reduced BMD, diminished bone strength, and adverse microarchitectural changes in cancellous bone compartments that persist into adulthood.
Alterations in peripheral [13] and/or central leptin signalling [14] may be factors influencing DIO-induced bone loss, given that rodents fed high-fat [15] or high-fructose diets [16] exhibit chronically elevated circulating leptin and that leptin is negatively associated with BMD in mice with DIO resulting from high-fat feeding.[10] In this regard, leptin is an adipocyte-derived hormone that increases proportionally with adiposity and which influences bone accrual via central [14] and peripheral manners.[17] The central influence of leptin on bone accrual appears to occur indirectly via suppression of CNS serotonin release and subsequent inhibition of serotonergic regulation of bone accrual;[14] although, the role of this central pathway remains controversial with other groups demonstrating hypothalamic leptin administration [18] or leptin gene therapy [17, 19] increase bone formation. In contrast, peripheral leptin is known to directly stimulates bone formation [17] after binding leptin receptors present on osteoblasts and chondrocytes. As evidence, subcutaneous leptin replacement increases longitudinal bone growth, osteoblast number, and mineral apposition rate in ob/ob (leptin deficient) mice.[17]
Increased oxidative stress has also been proposed as one factor underlying adverse skeletal development and the pathogenesis of osteoporosis in a variety of conditions, including diabetes,[20] aging, and sex-steroid deficiency syndromes.[21] As evidence, oxidative stress is associated with increased bone resorption and low BMD in elderly men [22] and postmenopausal women.[23] Furthermore, incubation of human or rodent bone marrow cells with H2O2 (an inducer of oxidative stress) stimulates development of osteoclast-like cells, increases bone resorptive activity,[23] and inhibits bone formation in vitro,[24] effects that are reversed by co-incubation with antioxidants or reactive oxygen species (ROS) inhibitors. In this regard, DIO increases oxidative stress and leptin stimulates ROS generation in endothelial cells,[20] suggesting that increased oxidative stress resulting from ROS generation and/or reduced activity of the antioxidant systems may influence the adverse skeletal development resulting from high-fat or high-fructose diets.
Interestingly, typical “westernized” diets that are high in both fat and fructose are known to produce greater elevations in both leptin and oxidative stress, when compared with high-fat starch-based diets.[25] However, we are unaware of any study examining whether a combined high-fat/high-fructose (HF/F) diet produces greater bone deficits than that of a calorically-matched high-fat (HF) starch-based diet. Our primary purpose was to compare bone development in skeletally-immature rats consuming a moderately HF diet to that of an isocaloric HF/F diet. We hypothesized that the HF/F diet would produce deleterious skeletal adaptations in comparison to the HF (starch-based/sugar-free) diet and that both aforementioned groups would exhibit adverse skeletal development in comparison to animals fed standard low-fat/sugar-free rodent chow. A secondary purpose was to determine whether skeletal outcomes were associated with circulating leptin, systemic oxidative stress, or bone marrow adipocytes (a source of leptin in close proximity to cancellous bone).
2.0 MATERIALS AND METHODS
2.1 Animals
Barrier-raised and specific pathogen-free male Sprague Dawley rats aged 7 weeks were obtained from Harlan Labs (Indianapolis, IN). Upon arrival, rats were examined and remained in quarantine for one week during which they were provided standard rodent chow and water ad libitum. Rats were maintained on a 12:12 hour light-dark cycle. Animals were cared for in accordance with the principles of the Guide to the Care and Use of Experimental Animals and protocols were approved by the University of Florida Institutional Animal Care and Use Committee.
2.2 Experimental Design
We acquired bones from a larger experiment that evaluated cardiovascular and body composition responses to HF and combined HF/F feeding in skeletally-immature rats.[25] Rats were randomized to receive one of three diets: 1) standard rodent chow (Control, n=10; Harlan Teklad Global 18% Protein Rodent Diet, Harlan Laboratories Inc., Madison, WI), 2) a moderately HF (sugar-free) chow with 30% of kcal from fat (n=8; Harlan TD08703), or 3) a moderately HF chow (30% kcal from fat) with 40% kcal from fructose (HF/F, n=8; Harlan TD08702) and water ad libitum (See Supplemental Table 1 for nutrient composition of diets). No glucose was present in either HF diet. Total kcal and macronutrient/micronutrient distributions were matched in the HF and HF/F groups, with the source of carbohydrate (starch vs. fructose) serving as the sole difference between these dietary regimens, which allowed us to distinguish the direct impact of fructose on bone metabolism in the presence of a high-fat diet. All diets exceeded the calcium threshold (2.5 g/kg) necessary to produce normal growth in Sprague-Dawley rats [26] and met the daily phosphorus requirements. Both HF dietary regimens contained 30% kcal from fat, a value that is near the average percentage fat intake of children and adolescents in the United States [27] and within the accepted macronutrient distribution range defined in the 2015–2020 Dietary Guidelines for America recommendations for children and adolescents.[28] Rats were euthanized with an overdose of isoflurane at week 12 and blood was collected via cardiac puncture. The circulatory system was perfused with 20 ml of ice-cold saline and the right and left femurs and tibiae were excised and cleaned of surrounding soft-tissue, weighed, and measured. Blood samples were centrifuged at 4000 rpm for 20 minutes and serum aliquots were separated at stored at −80°C until analysis. Femurs were wrapped in saline-soaked gauze to prevent dehydration and stored at −20°C for microcomputed tomography (μCT) analysis and assessment of bone mechanical properties. Tibiae were cut in half, cross-sectionally, placed in 10% phosphate-buffered formalin for 48h tissue fixation, dehydrated in ethanol, and embedded undecalcified in methyl methacrylate for subsequent sectioning and histologic analysis.
2.3 μCT Analysis of Bone Structure
The left distal femoral metaphysis and diaphysis were scanned by μCT using a Bruker Skyscan 1172 (Kontich, Belgium), as previously described.[29–31] Images were acquired using the following parameters: 80kVP/120μA, 0.5mm aluminium filter, 1k camera resolution, 19.2μm voxel size, 0.5° rotation step, and 180° tomographic rotation. The cancellous regions of interest (ROI) at the distal femoral metaphysis began 1.5mm proximal to the growth plate and encompassed 4mm, including the sponge-like trabecular (cancellous) bone spicules in the medullary cavity and excluding the dense compact (cortical) bone that surrounds the medullary cavity. The cortical ROI at the distal femur began 3mm proximal to the growth plate (in order to completely avoid residual growth plate) and encompassed a total of 2mm, excluding all cancellous bone. The cortical ROI at the femoral diaphysis encompassed a 2mm region beginning at 55% of the femur length in order to avoid the third trochanter. Cross-sectional images were reconstructed using a filtered back-projection algorithm (NRecon, Kontich, Belgium). 2D and 3D morphometric measurements were calculated using CTan software (Bruker Skyscan, Kontich Belgium). Measurements at the distal femur include: cancellous bone volume (as a percentage of bone tissue area, BV/TV %), trabecular thickness (Tb.Th, mm), trabecular number (Tb.N, 1/mm), trabecular separation (Tb.Sp, mm), trabecular pattern factor (Tb.Pf), and structural model index (SMI). Cortical measurements at the distal femur and femoral diaphysis include: total cross-sectional (bone plus medullary) area (Tt.Ar, mm2), cortical bone area (Ct.Ar, mm2), cortical area fraction (Ct.Ar/Tt.Ar, %), and three-dimensional cortical thickness (3D Ct.Th, mm). Additionally, medullary volumetric bone mineral density (vBMD, mg/cm3) and cortical volumetric tissue mineral density (vTMD, mg/cm3) were evaluated in the previously defined distal femur and femoral diaphysis ROIs, respectively. Densities were determined following calibration with hydroxyapatite phantoms.
2.4 Bone Mechanical Characteristics
Subsequent to μCT, the left femora underwent mechanical testing using a previously described method.[32] Briefly, the femora were thawed to room temperature and remained wrapped in salinated gauze except during measurements. The femoral midshaft was subjected to a medial/lateral three-point bending test using a servohydraulic testing machine (MTS 858 Bionix Test System, MTX, Eden Prairie, MN). Before mechanical testing, a preload (10N * 0.1mm/s) was applied on the medial surface of the femur using a steel cross-bar fixture. The bending load was applied at 1.0mm/s until failure. The maximum load, displacement at maximum load, and stiffness were determined from the load deformation curves.
2.5 Cancellous Bone Histology and Bone Marrow Adipogenesis
Cancellous bone parameters were evaluated at the left proximal tibial metaphysis using standard histologic techniques, as described previously.[32] In brief, methyl-methacrylate embedded proximal tibiae were sectioned longitudinally at 4μm thickness, stained with Von Kossa and counterstained with tetrachrome (Polysciences Inc., Warrington, PA, USA) for the assessment of cancellous bone structure. The ROI within the proximal tibial metaphysis began 1mm distal to the growth plate and excluded the primary spongiosa and cancellous bone tissue within 0.25mm of the endocortical surfaces. The following cancellous structural variables were measured with the Osteomeasure System (Osteometrics, Decatur, GA): cancellous bone volume (as a percentage of bone tissue area, BV/TV %), trabecular number (Tb.N, #/mm), trabecular width (Tb.Wi, μm), and trabecular separation (Tb.Sp, μm). Osteoblast (Ob.S/BS) and osteoclast (Oc.S/BS) surfaces were measured as percentages of total cancellous perimeter.
Bone marrow adipogenesis was assessed at the same ROI in which the Von Kossa/tetrachrome stained bone parameters were taken, using a standard laboratory technique.[33] The total number of adipocytes (N.Ad, #) and the adipocyte density [N.Ad/mm2 (total area − bone area)] were evaluated. Adipocytes were identified as unstained round spaces (exclusive of vacuoles) because intracellular fat deposits were removed during histological tissue processing.
2.6 Serum Measurements
Serum measurements were performed in duplicate on a single plate according to manufacturer’s instructions. Osteocalcin (a circulating marker of bone formation) was determined by enzyme immunoassay that has a sensitivity of 50ng/ml and an intra-assay CV <5.0%, and tartrate-resistant acid phosphatase form 5b (TRAP5b, a circulating measure of osteoclast number) was determined by solid phase immunofixed enzyme activity assay that has a sensitivity of 0.1 U/L and an intra-assay CV <5.8% (IDS, Fountain Hills, AZ, USA). Total antioxidant capacity (tAOC) (Abcam, Cambridge, UK) and leptin (Milipore, MA, USA) were measured by ELISA from blood acquired in the fed state at sacrifice.
2.7 Statistical Analysis
One-Way ANOVAs (for normally distributed data) were used to analyze dependent variables and Tukey’s posthoc tests were performed for multiple comparisons among groups when appropriate. The non-parametric Kruskal-Wallis and Mann-Whitney U tests were used when data were not normally distributed. Linear dependence was evaluated between histologically assessed cancellous bone outcomes and adipocyte density and between μCT assessed cancellous bone outcomes and serum measurements with Pearson’s correlations. The Holm-Bonferroni correction was utilized to control type I error that can occur when performing multiple comparisons. Results are reported as means ± standard error (SEM), with threshold for significance defined as p<0.05. Data were analyzed with SPSS v18.0.0 statistical software package (IBM, Chicago, IL, USA).
3.0 RESULTS
3.1 Food/Energy Intake, Body Mass, and Body Composition
Food/energy intake, change in body mass, and body composition data were previously reported in our companion paper.[25] A brief description of these data is included below to provide a proper characterization our model. At baseline, rats exhibited similar whole-body fat and lean mass %, as assessed via time-domain nuclear magnetic resonance (TD-NMR). Food consumption did not differ across groups. In HF and HF/F groups, whole-body fat mass was increased and whole-body lean mass was reduced by week 6 (assessed via TD-NMR). At week 11, whole-body fat mass was 5–7% higher in HF and HF/F groups versus Controls and whole-body lean mass was 6% lower than Controls.[25] No differences in body mass at sacrifice nor delta body mass were present across groups (Table 1). At sacrifice, HF and HF/F groups exhibited a 15% lower lean/fat mass ratio (p<0.001, Table 1) when compared with Controls. No differences in brown adipose tissue or femur/tibia length or mass were present among groups.
Table 1.
Femoral and tibial length and mass from animals fed standard rodent chow (Control), a 30% moderately high-fat (HF) diet, or a combined 30% high-fat/40% high-fructose (HF/F) diet.
| Control | HF | HF/F | |
|---|---|---|---|
| Body mass at sac, g | 406 ± 6 | 409 ± 12 | 428 ± 7 |
| Delta body mass, g | 109 ± 8 | 126 ± 10 | 122 ± 8 |
| Lean/fat ratio | 2.96 ± 0.06 | 2.51 ± 0.08*** | 2.50 ± 0.07*** |
| Femur length, mm | 38.4 ± 0.2 | 38.6 ± 0.4 | 38.7 ± 0.4 |
| Femur mass, g | 1.07 ± 0.02 | 1.09 ± 0.02 | 1.11 ± 0.03 |
| Tibia length, mm | 41.8 ± 0.4 | 42.5 ± 0.6 | 42.8 ± 0.3 |
| Tibia mass, g | 0.86 ± 0.01 | 0.89 ± 0.02 | 0.90 ± 0.02 |
Data are means ± SEM from n = 7–10/group.
p<0.001 vs. Control.
3.2 μCT Analysis of Cancellous Bone Morphometry and Microarchitecture
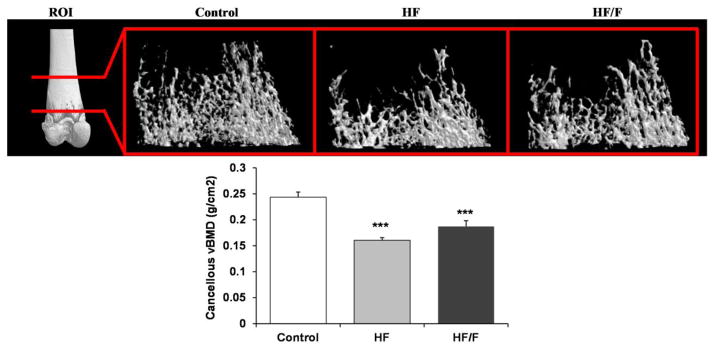
Three-dimensional (3D) μCT analysis of the distal femoral metaphysis indicated that cancellous (medullary) vBMD was 23–34% lower in HF and HF/F animals compared with Controls (p<0.001, Figure 1), with no differences among groups receiving HF diets. This difference was characterized by a 28–41% lower BV/TV (p<0.01), 32–42% lower Tb.N (p<0.001), and a 54–92% higher Tb.Sp (p<0.001) in HF and HF/F animals compared with Controls (Table 2), and by an unexpected 7% increase in Tb.Th in HF animals (p<0.05). Differences in cancellous microarchitectural characteristics were also present among groups, with HF animals exhibiting a higher Tb.Pf (p<0.05) and both HF and HF/F exhibiting a higher SMI (p<0.01) versus Controls.
Figure 1.
Representative microcomputed tomography (μCT) images of cancellous bone at the distal femoral metaphysis region of interest (ROI) from animals fed standard rodent chow (Control), a 30% moderately high-fat (HF) diet, or a combined 30% high-fat/40% high-fructose (HF/F) diet. Animals receiving HF and HF/F diets exhibited reduced cancellous volumetric bone mineral density (vBMD, Lower Panel) and reduced trabecular spicules indicative of cancellous osteopenia (data presented in Table 2). Data are mean ± SEM from n = 7–10/group. ***p<0.001 vs. Control.
Table 2.
Microcomputed tomography (μCT) derived cancellous bone parameters at the distal femoral metaphysis from animals fed standard rodent chow (Control), a 30% moderately high-fat (HF) diet, or a combined 30% high-fat/40% high-fructose (HF/F) diet.
| Control | HF | HF/F | |
|---|---|---|---|
| Distal Femoral Metaphysis | |||
| BV/TV, % | 18.3 ± 1.0 | 10.8 ± 0.5*** | 13.1 ± 1.0** |
| Tb.N, #/mm | 1.5 ± 0.1 | 0.9 ± 0.1*** | 1.0 ± 0.1*** |
| Tb.Th, mm | 0.124 ± 0.002 | 0.133 ± 0.002* | 0.131 ± 0.003 |
| Tb.Sp, mm | 0.61 ± 0.04 | 1.17 ± 0.05*** | 0.94 ± 0.06*** |
| Tb.Pf | 8.0 ± 0.5 | 9.6 ± 0.3* | 9.5 ± 0.5 |
| SMI | 1.94 ± 0.05 | 2.18 ± 0.02** | 2.16 ± 0.06** |
Data are means ± SEM from n = 7–10/group.
p<0.05,
p<0.01,
p<0.001 vs. Control.
BV/TV = cancellous bone volume fraction, Tb.N = trabecular number, Tb.Th = trabecular thickness, Tb.Sp = trabecular separation, Tb.Pf = trabecular pattern factor, SMI = structural model index
3.3 μCT Analysis of Cortical Bone Morphometry and Bone Strength Testing
At the distal femur, 3D Ct.Th was 9% higher in HF and HF/F animals compared with Controls (p<0.01, Table 3). Similarly, HF and HF/F animals exhibited a 6–7% higher Ct.Ar/Tt.Ar versus Controls (p<0.05 for HF and p=0.051 [trend] for HF/F), a change that primary resulted from higher Ct.Ar. At the femoral diaphysis, 3D Ct.Th was 5% higher in HF and HF/F animals compared with Controls (p<0.01, Table 3), with Ct.Ar/Tt.Ar being 4% higher in HF/F animals versus Controls (p<0.05). Cortical bone vTMD was also 2–3% higher in HF and HF/F animals compared with Controls at both the distal femur (p<0.01) and the femoral diaphysis (p<0.05). No other μCT structural differences were present at either skeletal site. No differences in femoral diaphyseal mechanical characteristics were observed among groups (Supplemental Table 2).
Table 3.
Microcomputed tomography (μCT) derived cortical bone parameters at the distal femur and femoral diaphysis from animals fed standard rodent chow (Control), a 30% moderately high-fat (HF) diet, or a combined 30% high-fat/40% high-fructose (HF/F) diet.
| Control | HF | HF/F | |
|---|---|---|---|
| Distal Femur | |||
| 3D Ct.Th, mm | 0.506 ± 0.005 | 0.551 ± 0.007*** | 0.550 ± 0.012** |
| Tt.Ar, mm2 | 15.34 ± 0.29 | 15.05 ± 0.32 | 15.46 ± 0.30 |
| Ct.Ar, mm2 | 6.52 ± 0.07 | 6.87 ± 0.09 | 7.00 ± 0.18* |
| Ct.Ar/Tt.Ar, % | 42.6 ± 0.7 | 45.8 ± 0.8* | 45.3 ± 0.8 |
| vTMD, mg/cm3 | 1.330 ± 0.004 | 1.374 ± 0.007** | 1.364 ± 0.007** |
| Femoral Diaphysis | |||
| 3D Ct.Th, mm | 0.735 ± 0.004 | 0.768 ± 0.009** | 0.772 ± 0.011** |
| Tt.Ar, mm2 | 11.83 ± 0.15 | 12.02 ± 0.39 | 12.01 ± 0.27 |
| Ct.Ar, mm2 | 6.99 ± 0.07 | 7.33 ± 0.21 | 7.40 ± 0.16 |
| Ct.Ar/Tt.Ar, % | 59.6 ± 0.5 | 61.1 ± 0.5 | 61.6 ± 0.4* |
| vTMD, mg/cm3 | 1.432 ± 0.006 | 1.460 ± 0.009* | 1.455 ± 0.006* |
Data are means ± SEM from n = 7–10/group.
p<0.05,
p<0.01,
p<0.001 vs. Control.
3D Ct.Th = three-dimensional cortical thickness, Tt.Ar = total cross-sectional area, Ct.Ar = cortical bone area, Ma.Ar = medullary area, Ct.Ar/Tt.Ar = cortical bone area fraction, vTMD = volumetric tissue mineral density.
3.4 Cancellous Bone Histology and Bone Marrow Adipogenesis
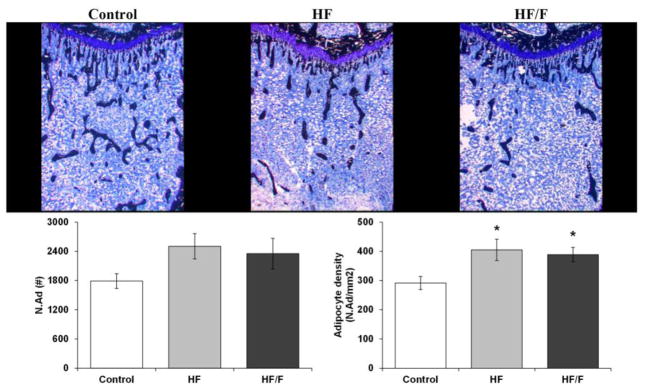
Histological assessment of the proximal tibial metaphysis revealed that cancellous BV/TV was 47–48% lower in HF (p<0.01) and HF/F (p<0.05) animals compared with Controls (Figure 2 and Table 4), an effect primarily resulting from a 45–55% lower Tb.N (p<0.001 for HF and p<0.01 for HF/F) and a >100% higher Tb.Sp in HF animals (p<0.01). Ob.S/BS was 66–72% lower in HF/F animals versus Controls (p<0.05) and HF animals (p=0.071, trend). Oc.S/BS was not different among groups. Bone marrow adipocyte density at the proximal tibia was higher in HF and HF/F animals compared with Controls (Figure 2, p<0.05).
Figure 2.
Histologic images of cancellous bone (stained black) and adipocytes (white) at the proximal tibia from animals fed standard rodent chow (Control), a 30% moderately high-fat (HF) diet, or a combined 30% high-fat/40% high-fructose (HF/F) diet. Animals receiving HF and HF/F diets exhibited increased adipocyte density (lower panel) and reduced cancellous bone volume (data presented in Table 4). Data are mean ± SEM from n = 7–10/group. *p<0.05 vs. Control
Table 4.
Cancellous histological parameters at the proximal tibial metaphysis from animals fed standard rodent chow (Control), a 30% moderately high-fat (HF) diet, or a combined 30% high-fat/40% high-fructose (HF/F) diet.
| Control | HF | HF/F | |
|---|---|---|---|
| BV/TV, % | 10.1 ± 1.3 | 5.2 ± 0.9** | 5.4 ± 0.7* |
| Tb.N, #/mm | 4.2 ± 0.4 | 1.9 ± 0.2*** | 2.3 ± 0.3** |
| Tb.Wi, μm | 29.1 ± 0.8 | 31.8 ± 3.9 | 27.6 ± 1.5 |
| Tb.Sp, μm | 235 ± 28 | 497 ± 74** | 383 ± 30 |
| Oc.S/BS, % | 3.2 ± 0.4 | 4.1 ± 0.9 | 4.2 ± 0.4 |
| Ob.S/BS, % | 6.3 ± 1.0 | 5.2 ± 1.1 | 1.8 ± 0.3* |
Data are mean ± SEM from n = 6–9/group.
p<0.05,
p<0.01,
p<0.001 vs. Control.
BV/TV = % cancellous bone volume, Tb.Wi = trabecular width, Tb.N = trabecular number, Tb.Sp = trabecular separation, Oc.S/BS = osteoclast surface, Ob.S/BS = osteoblast surface
3.5 Serum Measurements
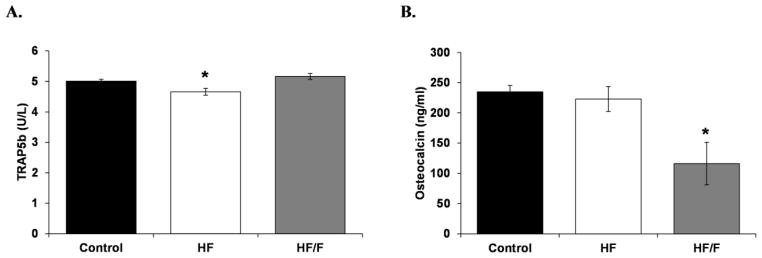
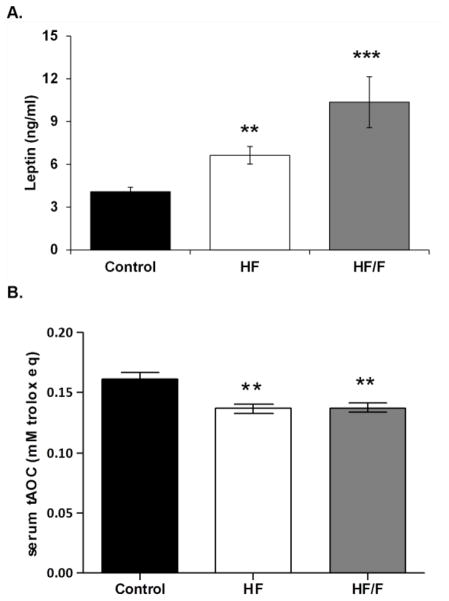
At sacrifice, TRAP5b was lower in HF animals versus Controls (p<0.05) and HF/F animals (p<0.01, Figure 3A), while osteocalcin was lower in HF/F animals compared with Controls and HF animals (p<0.05, Figure 3B). Circulating leptin was 62% and 153% higher in HF and HF/F groups compared with Controls, respectively (p<0.01 for HF and p<0.001 for HF/F, Figure 4A), with a trend indicating leptin was higher in HF/F than HF animals (p=0.074, trend). Additionally, circulating tAOC was reduced in HF and HF/F rats versus respective baseline values (p<0.001 for HF and p<0.01 for HF/F) and values in both HF groups were lower than Controls at sacrifice (p<0.01, Figure 4B).
Figure 3.
Figure 3A–B. Serum measurements of A) TRAP5b (circulating marker of bone resorption) and B) osteocalcin (circulating marker of bone formation) from animals fed standard rodent chow (Control), a 30% moderately high-fat (HF) diet, or a 30% combined high-fat/40% high-fructose (HF/F) diet. Data are mean ± SEM from n = 7–10/group. *p<0.05 vs. Control.
Figure 4.
Figure 4A–B. Serum measurements of A) leptin concentrations and B) total antioxidant capacity (tAOC) from animals fed standard rodent chow (Control), a 30% moderately high-fat (HF) diet, or a combined 30% high-fat/40% high-fructose (HF/F) diet. Data are mean ± SEM from n = 8–10/group. *p<0.05 vs. Control.
3.6 Relationship Among Cancellous Bone Structure, Bone Marrow Adipocytes, and Serum Measurements
Across groups, serum leptin and tAOC were associated with the following μCT-assessed cancellous structural outcomes at the distal femur: vBMD (leptin: r = −0.494, p<0.05; tAOC: r = 0.574, p<0.01), BV/TV (leptin: r = −0.511, p<0.01; tAOC: r = 0.586, p<0.01), and Tb.N (leptin: r = −0.579, p<0.01; tAOC: r = 0.640, p<0.01). Adipocyte density at the proximal tibia was negatively associated with the following histologically-assessed cancellous bone structural outcomes at the same skeletal site: cancellous BV/TV (r = −0.637, p<0.001) and Tb.N (r = −0.697, p<0.001). Leptin was also negatively associated with circulating osteocalcin (r = −0.625, p<0.001).
4.0 DISCUSSION
Moderately HF or HF/F diets are traditionally characterized by elevated fat mass and reduced lean mass.[15] In addition, HF and high-fructose diets produce deleterious skeletal outcomes that may result from changes in leptin signalling, alterations in oxidative stress,[34] increased bone marrow adipogenesis,[9] or from a variety of other consequences of obesity.[35] Interestingly, chronic fructose consumption causes central leptin resistance, independent of dietary fat, predisposing animals to DIO upon exposure to HF diets [16] and also results in detrimental skeletal outcomes.[12] However, the skeletal consequences of combined HF/F feeding regimens have received relatively little attention in the literature. Herein we report that moderately HF and HF/F diets produced similar cancellous bone mineral deficits at the distal femur and proximal tibia in young male rats in comparison to Controls receiving a standard low-fat/sugar-free diet. These deleterious skeletal changes were characterized by reduced cancellous BV/TV and Tb.N and increased Tb.Sp resulting, in-part, from reduced bone formation, as evidenced by lower Ob.S/BS and circulating osteocalcin in HF/F animals. In addition, we observed reduced trabecular connectivity (i.e., increased Tb.Pf) and increased rod-like trabecular geometry (i.e., increased SMI) which suggests that compromised structural integrity [36] persisted in the remaining cancellous network of HF and HF/F animals. In contrast, several cortical bone structural (Ct.Th and Ct.Ar/Tt.Ar) and densitometric characteristics (vTMD) were higher in HF and HF/F animals, indicating that HF feeding produced divergent effects on cancellous and cortical bone compartments.
Long-term consumption of “westernized” diets that are high in both fat and sugar adversely affects bone development. As evidence, Zernicke et al. reported that rodents consuming a combined high-fat/high-sucrose diet for 24 months exhibited reduced cortical thickness and bone strength compared with those consuming a low-fat complex carbohydrate diet.[37] Interestingly, Bass et al. reported that a low-fat/high-glucose diet worsened cancellous bone outcomes in comparison to a low-fat/high-fructose diet in rats,[38] suggesting that the monosaccharides which make up sucrose (i.e., glucose and fructose) may produce differing skeletal responses. Our results expand upon these findings by demonstrating that the addition of fructose to a moderately high-fat diet does not worsen cancellous or cortical bone development in comparison to a moderately high-fat/sugar-free diet. Regardless, fructose consumption produces deleterious skeletal outcomes, as demonstrated by Felice et al. who reported that rats receiving a low-fat diet supplemented with fructose exhibited lower osteocyte number and increased bone resorption markers in comparison to animals on a low-fat/sugar-free diet.[12]
Altered central or peripheral leptin signalling has been proposed as a potential mechanism underlying DIO-induced cancellous bone loss.[10] In this regard, rodents fed HF diets typically develop DIO and experience a temporal increase in circulating leptin.[15] Combined HF/F diets traditionally produce greater elevations in adiposity and leptin when compared with calorically-matched HF (sugar-free) diets.[39] In the current study, we observed no differences in absolute or delta body mass among groups, likely because we examined the effects of a moderately high-fat diet (30% of kcal from fat) that is similar to the average dietary fat consumption of children and adolescents in the United States.[27] In contrast, a variety of other studies have reported weight gain in rodents consuming diets that contain 45–60% of kcal from fat in comparison to low-fat diets.[5, 8, 10, 35] Regardless, HF and HF/F animals exhibited other hallmark characteristics of DIO, a reduced lean/fat mass ratio and increased circulating leptin in comparison to Controls, with leptin being elevated to a greater magnitude in HF/F animals. White adipose tissue is believed to be the primary source of circulating leptin. However, bone marrow adipocytes, which possess characteristics of both white and brown fat,[40] also secrete leptin and are in close proximity to osteoblasts and chondrocytes that express leptin receptors.[41] In our study, both HF groups exhibited higher bone marrow adipocyte density at the proximal tibia in comparison to Controls, suggesting that HF feeding stimulated bone marrow adipogenesis while concomitantly reducing cancellous bone volume in area directly adjacent to adipocyte expansion, similar to the findings of others.[10] One possible explanation for this is that HF diets may increase the adipogenic capacity and/or reduce the osteogenic capacity of bone marrow derived mesenchymal stem cells (MSC), as has been demonstrated in vitro with MSC obtained from fructose supplemented rodents.[12] The higher bone marrow adipocyte density that we observed in concert with elevated circulating leptin indicates that the cancellous bone compartments of HF and HF/F animals were exposed to greater leptin levels than Controls. Interestingly, HF/F animals exhibited the highest circulating leptin concentrations and were the only group that experienced a measureable reduction in bone formation, perhaps suggesting that leptin reduces bone formation after reaching a threshold concentration, as has been reported in other rodent models.[42] We also observed that adipocyte density and circulating leptin were negatively associated with the primary cancellous structural outcomes (vBMD, BV/TV, and Tb.N) across all groups and that leptin was negatively associated with circulating osteocalcin (a marker of bone formation), which supports previous findings indicating that circulating leptin is negatively correlated with cancellous BMD in mice that exhibit DIO resulting from HF feeding.[10]
In spite of the aforementioned observations, we find it unlikely that the cancellous bone deficits in our HF and HF/F animals resulted directly from elevated circulating leptin because Turner et al. have reported that moderate overfeeding, resulting in a leptin concentration similar to our HF animals, did not produce cancellous bone deficits or alter bone turnover in 8-month old female rats.[43] Moreover, overwhelming evidence indicates that peripheral leptin directly stimulates bone formation after binding leptin receptors present in bone [17] and that the low appendicular bone mass phenotype in leptin deficient (ob/ob) and leptin-receptor deficient (db/db) mice [44–46] is reversible (in ob/ob mice) with leptin administration.[19, 47] We believe a more likely explanation may be that the CNS leptin-mediated signalling pathways that indirectly inhibit bone accrual [14] may overtake and oppose the (typically dominant) direct peripheral effects of leptin, only after reaching a threshold concentration, as previously discussed. However, the above discussion remains speculative because a direct causal relationship between DIO-induced changes in leptin and reduced cancellous bone volume has yet to be determined.
Systemic elevations in oxidative stress and/or reduced activity of the antioxidant systems may also influence the pathogenesis of HF feeding-induced cancellous bone loss, similar to that occurring with sex-steroid deficiency [34] and diabetes.[20] For example, Baek et al. reported that circulating 8-Hydroxy-2′-deoxguanosine (a biomarker of systemic oxidative stress) is negatively associated with hip and spine BMD and positively associated with type I collagen C-telopeptide (a marker of bone resorption) in postmenopausal women and that incubation of primary human marrow cells with H2O2 (an inducer of oxidative stress) stimulated formation of osteoclast-like cells and produced concentration-dependent bone resorptive activity in culture, an effect that was inhibited by co-incubation with catalase.[23] In our study, tAOC was lower in HF and HF/F animals versus Controls, indicating reduced systemic antioxidant capacity in these animals and was positively correlated with the primary cancellous structural outcomes across all groups, supporting the contention that antioxidant capacity and/or oxidative stress influence DIO-induced cancellous bone loss. However, no associations were noted among tAOC and Oc.S/BS or circulating Trap5b across groups, despite the lower circulating Trap5b in HF animals. Temporal associations have also been observed between increased oxidative stress and osteoblast apoptosis in C57BL/6 mice [48] and incubation of rabbit [49] or murine bone marrow stromal cells [24] with H2O2 suppresses osteoblastic differentiation and alkaline phosphatase activity (a marker of bone formation), effects that were reversed by a ROS inhibitor; indicating that oxidative stress negatively influences bone formation. In our study, osteocalcin and Ob.S/BS were lower in HF/F animals, although, we did not observe associations between systemic tAOC and the aforementioned bone formation markers.
While HF diets appear detrimental to cancellous bone development, the effects on cortical bone are typically less profound and may be influenced by body mass gains resulting from DIO.[50] For example, Chen et al. reported no effect of high-fat feeding on tibial cortical BMD in young female Sprague Dawley rats fed a high-fat diet intragastrically [9] and others have reported that young male C57BL/6 mice exhibit no change in cortical bone characteristics or bone strength following high-fat feeding regimens lasting 4–24 weeks.[5, 8, 10] In contrast, a combined high-fat/sucrose diet impairs lumbar and femoral neck strength in rodents, likely a result of smaller bone cross-sectional area and/or reduced cortical bone area in comparison to a low-fat/complex carbohydrate diet.[37, 51] Interestingly, we observed that the cortical bone compartments at the distal femur and femoral diaphysis of HF and HF/F animals exhibited increased Ct.Th and Ct.Ar/Tt.Ar and densitometric characteristics, although, these changes did not improve whole bone mechanical characteristics in comparison with Controls. Regardless, our findings indicate that HF feeding produced divergent effects in the cortical and cancellous bone compartments, a concept that has been proposed by others.[10, 52] and that the cortical bone phenotype we observed was independent weight gain, as no differences in absolute or delta body mass were present among groups.
In addition to the discussion above, other factors associated with the dietary regimens that we utilized may have influenced the skeletal outcomes. For example, both HF diets contained lower calcium and phosphorus compared with the Control diet. Although, all diets exceeded the calcium threshold (2.5 g/kg) necessary to produce normal growth in Sprague-Dawley rats, a level beyond which additional calcium does not improve bone structure or bone strength,[26] and met the daily phosphorus requirements. Potential differences in other hormones that are influenced by HF feeding may have also influenced our results. For example, Hawkins et al. reported that female rats exhibited higher serum estradiol after consuming a HF diet,[35] which may explain the increased Ct.Th and Ct.Ar/Tt.Ar in our HF animals; although, we did not measure serum estradiol in our male rats because concentrations are typically at or below the limits of detectability of the most sensitive currently available measurement techniques.[53] Differences also existed in the dietary fat source and composition between the Control (soybean oil) and HF (lard) diets, with both HF diets containing a higher saturated and monounsaturated fat and lower polyunsaturated n-3 (linolenic) and n-6 (linoleic) fatty acids (PUFA), which may affect bone development.[54, 55] However, the above possibilities would likely affect cancellous and cortical bone in a directionally similar manner, which contrasts the divergent cancellous/cortical outcomes that we observed in HF animals.
In summary, moderately HF (starch-based/sugar-free) diets result in adverse cancellous bone development in skeletally-immature male rats, an effect that was not worsened by the replacement of starch with fructose. In addition, both HF and HF/F diets increased circulating leptin and bone marrow adipocyte density and reduced tAOC, changes that were associated with cancellous bone structural outcomes. In contrast, HF and HF/F animals exhibited slightly increased cortical bone structural and densitometric variables and no decrement in whole bone strength, indicating that these diets exerted divergent cancellous/cortical bone outcomes that were independent of body mass changes. In conclusion, the addition of fructose to a calorically-matched HF diet does not exacerbate cancellous bone loss or alter cortical bone development in rodents. Future research delineating the mechanisms underlying HF-induced alterations in bone development remain warranted, with particular focus on determining whether central/peripheral leptin, oxidative stress, sex-steroid hormones, or other hormonal factors influence HF-induced bone loss.
Supplementary Material
HIGHLIGHTS.
A chronic high-fat (sugar-free) diet worsened cancellous, but not cortical, bone development in young male rats.
The addition of fructose to a high-fat diet did not exacerbate cancellous bone loss.
Both diets stimulated bone marrow adipogenesis, increased leptin, and reduced antioxidant capacity, changes associated with adverse cancellous bone development.
The high-fat/high-fructose diet lowered osteoblast number and circulating osteocalcin, a change associated with circulating leptin.
Acknowledgments
FUNDING SOURCE: This work was supported by the National Institutes of Health Grant NIH DK 091710 and by resources provided by the North Florida/South Georgia Veterans Health System, Gainesville, FL. The work reported herein does not represent the views of the US Department of Veterans Affairs or the US Government.
Footnotes
CONFLICT OF INTEREST: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shapses SA, Sukumar D. Bone metabolism in obesity and weight loss. Annu Rev Nutr. 2012;32:287–309. doi: 10.1146/annurev.nutr.012809.104655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dimitri P, Wales JK, Bishop N. Fat and bone in children: differential effects of obesity on bone size and mass according to fracture history. J Bone Miner Res. 2010;25:527–536. doi: 10.1359/jbmr.090823. [DOI] [PubMed] [Google Scholar]
- 3.Dimitri P, Bishop N, Walsh JS, Eastell R. Obesity is a risk factor for fracture in children but is protective against fracture in adults: a paradox. Bone. 2012;50:457–466. doi: 10.1016/j.bone.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 4.Dehghan M, Akhtar-Danesh N, Merchant AT. Childhood obesity, prevalence and prevention. Nutr J. 2005;4:24. doi: 10.1186/1475-2891-4-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patsch JM, Kiefer FW, Varga P, Pail P, Rauner M, Stupphann D, Resch H, Moser D, Zysset PK, Stulnig TM, Pietschmann P. Increased bone resorption and impaired bone microarchitecture in short-term and extended high-fat diet-induced obesity. Metabolism. 2011;60:243–249. doi: 10.1016/j.metabol.2009.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bielohuby M, Matsuura M, Herbach N, Kienzle E, Slawik M, Hoeflich A, Bidlingmaier M. Short-term exposure to low-carbohydrate, high-fat diets induces low bone mineral density and reduces bone formation in rats. J Bone Miner Res. 2010;25:275–284. doi: 10.1359/jbmr.090813. [DOI] [PubMed] [Google Scholar]
- 7.Lac G, Cavalie H, Ebal E, Michaux O. Effects of a high fat diet on bone of growing rats. Correlations between visceral fat, adiponectin and bone mass density. Lipids Health Dis. 2008;7:16. doi: 10.1186/1476-511X-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gautam J, Choudhary D, Khedgikar V, Kushwaha P, Singh RS, Singh D, Tiwari S, Trivedi R. Micro-architectural changes in cancellous bone differ in female and male C57BL/6 mice with high-fat diet-induced low bone mineral density. Br J Nutr. 2014;111:1811–1821. doi: 10.1017/S0007114514000051. [DOI] [PubMed] [Google Scholar]
- 9.Chen JR, Lazarenko OP, Wu X, Tong Y, Blackburn ML, Shankar K, Badger TM, Ronis MJ. Obesity reduces bone density associated with activation of PPARgamma and suppression of Wnt/beta-catenin in rapidly growing male rats. PLoS One. 2010;5:e13704. doi: 10.1371/journal.pone.0013704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fujita Y, Watanabe K, Maki K. Serum leptin levels negatively correlate with trabecular bone mineral density in high-fat diet-induced obesity mice. J Musculoskelet Neuronal Interact. 2012;12:84–94. [PubMed] [Google Scholar]
- 11.Tsanzi E, Light HR, Tou JC. The effect of feeding different sugar-sweetened beverages to growing female Sprague-Dawley rats on bone mass and strength. Bone. 2008;42:960–968. doi: 10.1016/j.bone.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 12.Felice JI, Gangoiti MV, Molinuevo MS, McCarthy AD, Cortizo AM. Effects of a metabolic syndrome induced by a fructose-rich diet on bone metabolism in rats. Metabolism. 2014;63:296–305. doi: 10.1016/j.metabol.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 13.Upadhyay J, Farr OM, Mantzoros CS. The role of leptin in regulating bone metabolism. Metabolism. 2015;64:105–113. doi: 10.1016/j.metabol.2014.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karsenty G, Oury F. The central regulation of bone mass, the first link between bone remodeling and energy metabolism. J Clin Endocrinol Metab. 2010;95:4795–4801. doi: 10.1210/jc.2010-1030. [DOI] [PubMed] [Google Scholar]
- 15.Scarpace PJ, Zhang Y. Leptin resistance: a prediposing factor for diet-induced obesity. Am J Physiol Regul Integr Comp Physiol. 2009;296:R493–500. doi: 10.1152/ajpregu.90669.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shapiro A, Mu W, Roncal C, Cheng KY, Johnson RJ, Scarpace PJ. Fructose-induced leptin resistance exacerbates weight gain in response to subsequent high-fat feeding. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1370–1375. doi: 10.1152/ajpregu.00195.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Turner RT, Kalra SP, Wong CP, Philbrick KA, Lindenmaier LB, Boghossian S, Iwaniec UT. Peripheral leptin regulates bone formation. J Bone Miner Res. 2013;28:22–34. doi: 10.1002/jbmr.1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bartell SM, Rayalam S, Ambati S, Gaddam DR, Hartzell DL, Hamrick M, She JX, Della-Fera MA, Baile CA. Central (ICV) leptin injection increases bone formation, bone mineral density, muscle mass, serum IGF-1, and the expression of osteogenic genes in leptin-deficient ob/ob mice. J Bone Miner Res. 2011;26:1710–1720. doi: 10.1002/jbmr.406. [DOI] [PubMed] [Google Scholar]
- 19.Iwaniec UT, Boghossian S, Lapke PD, Turner RT, Kalra SP. Central leptin gene therapy corrects skeletal abnormalities in leptin-deficient ob/ob mice. Peptides. 2007;28:1012–1019. doi: 10.1016/j.peptides.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamada Y, Fujii H, Fukagawa M. Role of oxidative stress in diabetic bone disorder. Bone. 2009;45(Suppl 1):S35–38. doi: 10.1016/j.bone.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 21.Manolagas SC. From estrogen-centric to aging and oxidative stress: a revised perspective of the pathogenesis of osteoporosis. Endocr Rev. 2010;31:266–300. doi: 10.1210/er.2009-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ostman B, Michaelsson K, Helmersson J, Byberg L, Gedeborg R, Melhus H, Basu S. Oxidative stress and bone mineral density in elderly men: antioxidant activity of alpha-tocopherol. Free Radic Biol Med. 2009;47:668–673. doi: 10.1016/j.freeradbiomed.2009.05.031. [DOI] [PubMed] [Google Scholar]
- 23.Baek KH, Oh KW, Lee WY, Lee SS, Kim MK, Kwon HS, Rhee EJ, Han JH, Song KH, Cha BY, Lee KW, Kang MI. Association of oxidative stress with postmenopausal osteoporosis and the effects of hydrogen peroxide on osteoclast formation in human bone marrow cell cultures. Calcif Tissue Int. 2010;87:226–235. doi: 10.1007/s00223-010-9393-9. [DOI] [PubMed] [Google Scholar]
- 24.Liu AL, Zhang ZM, Zhu BF, Liao ZH, Liu Z. Metallothionein protects bone marrow stromal cells against hydrogen peroxide-induced inhibition of osteoblastic differentiation. Cell Biol Int. 2004;28:905–911. doi: 10.1016/j.cellbi.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 25.Tumer N, Toklu H, Muller-Delp J, Sakarya Y, Oktay S, Matheny M, Kirchenko N, Carter C, Morgan D, Scarpace PJ. High fat plus dietary fructose is not more deleterious than a high fat diet on cardiovascular health. J Hypertension. 2015;33:e303. [Google Scholar]
- 26.Hunt JR, Hunt CD, Zito CA, Idso JP, Johnson LK. Calcium requirements of growing rats based on bone mass, structure, or biomechanical strength are similar. J Nutr. 2008;138:1462–1468. doi: 10.1093/jn/138.8.1462. [DOI] [PubMed] [Google Scholar]
- 27.Ervin RB, Ogden CL. Trends in intake of energy and macronutrients in children and adolescents from 1999–2000 through 2009–2010. NCHS Data Brief. 2013:1–8. [PubMed] [Google Scholar]
- 28.U.S. Department of Health and Human Services and U.S. Department of Agriculture. 2015 – 2020 Dietary Guidelines for Americans. (8) 2015 Dec; Available at http://health.gov/dietaryguidelines/2015/guidelines/
- 29.Beggs LA, Ye F, Ghosh P, Beck DT, Conover CF, Balaez A, Miller JR, Phillips EG, Zheng N, Williams AA, Aguirre JI, Wronski TJ, Bose PK, Borst SE, Yarrow JF. Sclerostin inhibition prevents spinal cord injury-induced cancellous bone loss. J Bone Miner Res. 2015;30:681–689. doi: 10.1002/jbmr.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beck DT, Yarrow JF, Beggs LA, Otzel DM, Ye F, Conover CF, Miller JR, Balaez A, Combs SM, Leeper AM, Williams AA, Lachacz SA, Zheng N, Wronski TJ, Borst SE. Influence of aromatase inhibition on the bone-protective effects of testosterone. J Bone Miner Res. 2014;29:2405–2413. doi: 10.1002/jbmr.2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yarrow JF, Ye F, Balaez A, Mantione JM, Otzel DM, Chen C, Beggs LA, Baligand C, Keener JE, Lim W, Vohra RS, Batra A, Borst SE, Bose PK, Thompson FJ, Vandenborne K. Bone loss in a new rodent model combining spinal cord injury and cast immobilization. J Musculoskelet Neuronal Interact. 2014;14:255–266. [PMC free article] [PubMed] [Google Scholar]
- 32.Yarrow JF, Conover CF, Purandare AV, Bhakta AM, Zheng N, Conrad B, Altman MK, Franz SE, Wronski TJ, Borst SE. Supraphysiological testosterone enanthate administration prevents bone loss and augments bone strength in gonadectomized male and female rats. Am J Physiol Endocrinol Metab. 2008;295:E1213–1222. doi: 10.1152/ajpendo.90640.2008. [DOI] [PubMed] [Google Scholar]
- 33.Aguirre JI, Leal ME, Rivera MF, Vanegas SM, Jorgensen M, Wronski TJ. Effects of basic fibroblast growth factor and a prostaglandin E2 receptor subtype 4 agonist on osteoblastogenesis and adipogenesis in aged ovariectomized rats. J Bone Miner Res. 2007;22:877–888. doi: 10.1359/jbmr.070313. [DOI] [PubMed] [Google Scholar]
- 34.Wauquier F, Leotoing L, Coxam V, Guicheux J, Wittrant Y. Oxidative stress in bone remodelling and disease. Trends Mol Med. 2009;15:468–477. doi: 10.1016/j.molmed.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 35.Hawkins J, Cifuentes M, Pleshko NL, Ambia-Sobhan H, Shapses SA. Energy restriction is associated with lower bone mineral density of the tibia and femur in lean but not obese female rats. J Nutr. 2010;140:31–37. doi: 10.3945/jn.109.111450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mittra E, Rubin C, Qin YX. Interrelationship of trabecular mechanical and microstructural properties in sheep trabecular bone. J Biomech. 2005;38:1229–1237. doi: 10.1016/j.jbiomech.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 37.Zernicke RF, Salem GJ, Barnard RJ, Schramm E. Long-term, high-fat-sucrose diet alters rat femoral neck and vertebral morphology, bone mineral content, and mechanical properties. Bone. 1995;16:25–31. doi: 10.1016/s8756-3282(00)80007-1. [DOI] [PubMed] [Google Scholar]
- 38.Bass EF, Baile CA, Lewis RD, Giraudo SQ. Bone quality and strength are greater in growing male rats fed fructose compared with glucose. Nutr Res. 2013;33:1063–1071. doi: 10.1016/j.nutres.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 39.Shapiro A, Tumer N, Gao Y, Cheng KY, Scarpace PJ. Prevention and reversal of diet-induced leptin resistance with a sugar-free diet despite high fat content. Br J Nutr. 2011;106:390–397. doi: 10.1017/S000711451100033X. [DOI] [PubMed] [Google Scholar]
- 40.Krings A, Rahman S, Huang S, Lu Y, Czernik PJ, Lecka-Czernik B. Bone marrow fat has brown adipose tissue characteristics, which are attenuated with aging and diabetes. Bone. 2012;50:546–552. doi: 10.1016/j.bone.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lecka-Czernik B. Marrow fat metabolism is linked to the systemic energy metabolism. Bone. 2012;50:534–539. doi: 10.1016/j.bone.2011.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martin A, David V, Malaval L, Lafage-Proust MH, Vico L, Thomas T. Opposite effects of leptin on bone metabolism: a dose-dependent balance related to energy intake and insulin-like growth factor-I pathway. Endocrinology. 2007;148:3419–3425. doi: 10.1210/en.2006-1541. [DOI] [PubMed] [Google Scholar]
- 43.Turner RT, Iwaniec UT. Moderate weight gain does not influence bone metabolism in skeletally mature female rats. Bone. 2010;47:631–635. doi: 10.1016/j.bone.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hamrick MW, Pennington C, Newton D, Xie D, Isales C. Leptin deficiency produces contrasting phenotypes in bones of the limb and spine. Bone. 2004;34:376–383. doi: 10.1016/j.bone.2003.11.020. [DOI] [PubMed] [Google Scholar]
- 45.Ealey KN, Fonseca D, Archer MC, Ward WE. Bone abnormalities in adolescent leptin-deficient mice. Regul Pept. 2006;136:9–13. doi: 10.1016/j.regpep.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 46.Williams GA, Callon KE, Watson M, Costa JL, Ding Y, Dickinson M, Wang Y, Naot D, Reid IR, Cornish J. Skeletal phenotype of the leptin receptor-deficient db/db mouse. J Bone Miner Res. 2011;26:1698–1709. doi: 10.1002/jbmr.367. [DOI] [PubMed] [Google Scholar]
- 47.Steppan CM, Crawford DT, Chidsey-Frink KL, Ke H, Swick AG. Leptin is a potent stimulator of bone growth in ob/ob mice. Regul Pept. 2000;92:73–78. doi: 10.1016/s0167-0115(00)00152-x. [DOI] [PubMed] [Google Scholar]
- 48.Manolagas SC, Almeida M. Gone with the Wnts: beta-catenin, T-cell factor, forkhead box O, and oxidative stress in age-dependent diseases of bone, lipid, and glucose metabolism. Mol Endocrinol. 2007;21:2605–2614. doi: 10.1210/me.2007-0259. [DOI] [PubMed] [Google Scholar]
- 49.Bai XC, Lu D, Bai J, Zheng H, Ke ZY, Li XM, Luo SQ. Oxidative stress inhibits osteoblastic differentiation of bone cells by ERK and NF-kappaB. Biochem Biophys Res Commun. 2004;314:197–207. doi: 10.1016/j.bbrc.2003.12.073. [DOI] [PubMed] [Google Scholar]
- 50.Turner RT, Philbrick KA, Wong CP, Olson DA, Branscum AJ, Iwaniec UT. Morbid obesity attenuates the skeletal abnormalities associated with leptin deficiency in mice. J Endocrinol. 2014;223:M1–15. doi: 10.1530/JOE-14-0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lorincz C, Reimer RA, Boyd SK, Zernicke RF. High-fat, sucrose diet impairs geometrical and mechanical properties of cortical bone in mice. Br J Nutr. 2010;103:1302–1308. doi: 10.1017/S0007114509993084. [DOI] [PubMed] [Google Scholar]
- 52.Cao JJ, Gregoire BR, Gao H. High-fat diet decreases cancellous bone mass but has no effect on cortical bone mass in the tibia in mice. Bone. 2009;44:1097–1104. doi: 10.1016/j.bone.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 53.Yarrow JF, Conover CF, Lipinska JA, Santillana CA, Wronski TJ, Borst SE. Methods to quantify sex steroid hormones in bone: applications to the study of androgen ablation and administration. Am J Physiol Endocrinol Metab. 2010;299:E841–847. doi: 10.1152/ajpendo.00384.2010. [DOI] [PubMed] [Google Scholar]
- 54.Lau BY, Cohen DJ, Ward WE, Ma DW. Investigating the role of polyunsaturated fatty acids in bone development using animal models. Molecules. 2013;18:14203–14227. doi: 10.3390/molecules181114203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Corwin RL, Hartman TJ, Maczuga SA, Graubard BI. Dietary saturated fat intake is inversely associated with bone density in humans: analysis of NHANES III. J Nutr. 2006;136:159–165. doi: 10.1093/jn/136.1.159. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.