Abstract
This report summarizes a 5-year phase 1/2 allogeneic islet transplantation clinical trial conducted at the University of Illinois at Chicago (UIC). Ten patients were enrolled in this single center, open label, and prospective trial in which patients received 1–3 transplants. The first four subjects underwent islet transplantation with the Edmonton immunosuppressive regimen and the remaining six subjects received the UIC immunosuppressive protocol (Edmonton plus etanercept and exenatide). All 10 patients achieved insulin independence after 1–3 transplants. At five years of follow-up, six of the initial 10 patients were free of exogenous insulin. During the follow-up period, 7 of the 10 patients maintained positive C-peptide levels and a composite hypoglycemic (HYPO) score of 0. Most patients maintained HbA1c levels < 6.0% (42.1 mmol/mol) and a significantly improved β-score. In conclusion, this study demonstrated long-term islet graft function without using T-cell depleting induction, with an encouraging outcome that includes 60% of patients remaining insulin independent after five years of initial transplantation.
Keywords: Type 1 diabetes, islet transplantation, immunosuppression, insulin independence
Introduction
Islet transplantation represents a minimally invasive therapy for type 1 diabetic patients [1,2]. Short-term benefits include insulin independence, normal or near-normal hemoglobin A1c (HbA1c) levels, a significant decrease in severe hypoglycemic episodes, and a return of hypoglycemic awareness.
While the insulin independence rate one year post-transplant has more than quadrupled since the Edmonton protocol was introduced [3,4], the long-term therapeutic value remains unclear. Beyond one year post-transplant, insulin independence declines. Only 10% of patients from the original Edmonton Protocol study maintained insulin independence at five years [5]. Since this time, the Collaborative Islet Transplant Registry (CITR) has reported encouraging trends, including an insulin independence rate of 44% at 3 years [3]. Recently, Bellin et al. [6] reported 5-year insulin-independence rates reaching 50% in patients who received FcR nonbinding anti-CD3 antibody alone or T cell depleting antibodies and TNF-α inhibition, indicating the importance of adequate immunosuppression for long-term graft survival. While improvements have been noted in more recent years, the steady functional decline over time remains an unfortunate clinical reality. Proposed responsible factors include alloimmune rejection [7–9], autoimmune recurrence [10,11], immunosuppressive drug toxicity [12–14], and nonimmunologic factors including an inadequate transplanted mass of high-quality islets [15,16].
In order to overcome the multifaceted challenges in islet transplantation, reports of long-term follow-up after islet transplantation in current clinical trials are of importance. Herein, we report the results of a 5-year phase 1/2 clinical trial in 10 patients with type 1 diabetes who underwent islet transplantation at the University of Illinois at Chicago (UIC). The goal of this trial was to test a strategy to achieve insulin independence with a lower initial islet mass, through the use of a tumor necrosis factor (TNF) -α receptor antagonist (etanercept, Enbrel®) and a glucagon-like peptide-1 (GLP-1) analog (exenatide, Byetta®), and without the use of T-cell depleting induction therapy. We have previously reported that the addition of exenatide and etanercept to the Edmonton protocol is associated with a significantly lower number of islets required to achieve insulin independence, during a 15-month follow up [17]. In patients that displayed declining islet graft function, we tested whether additional islet infusions over time could prolong insulin-independence. The current study extends our previous report and provides detailed long-term data on the transplant outcomes of these 10 subjects.
Methods
Islet isolation and transplantation
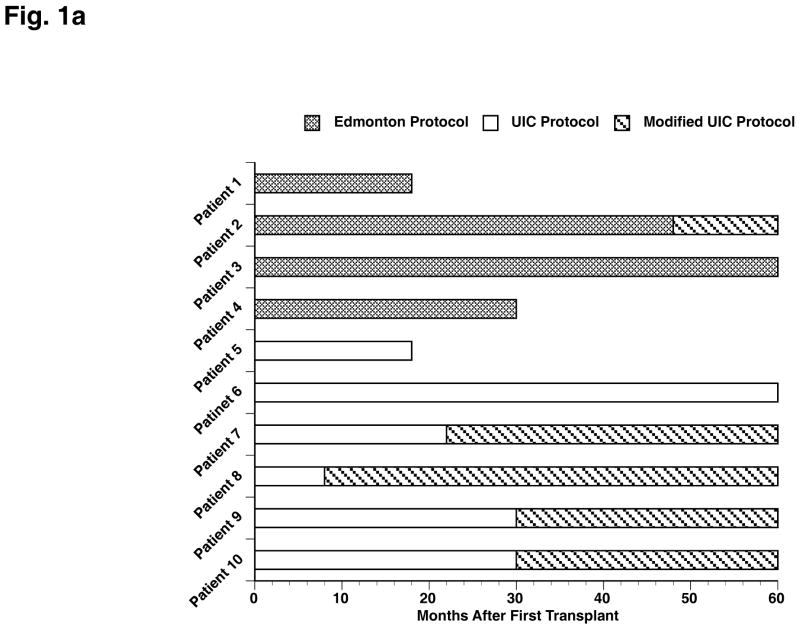
Donor selection and the procedures of islet isolation and transplantation have been previously described [17–20]. Subjects were eligible for further transplant if by the end of the fourth week after the first islet infusion or by the end of the fourth week after the second islet infusion they do not meet insulin-independence criteria [21], or HbA1c increased over 6.5% (47.5 mmol/mol) [22] (three patients required a supplemental islet infusion after the initial study period). Two immunosuppressive protocols were utilized in this clinical trial, as outlined in Figure 1A.
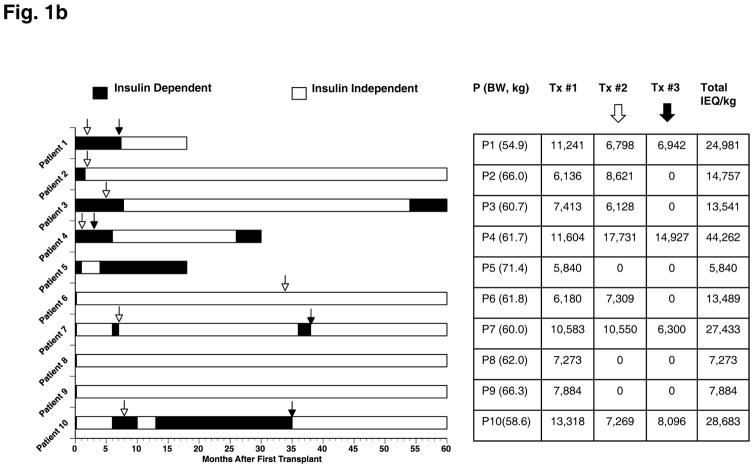
Fig. 1.
(A) Summary of immunosuppressive protocols across transplant recipients and patient status throughout the 60-month follow-up period. (B) Overview of insulin status following islet transplant(s) for each patient. The quantity of islets transplanted is expressed as islet equivalent (IEQ)/kg for each transplant and cumulative, if more than one transplant.
Edmonton Protocol (Patients 1–4): Daclizumab (Zenapax®, Roche Laboratories Inc.), sirolimus (Rapamune®, Wyeth Laboratories), and tacrolimus (Prograf®, Astellas) [4].
UIC Protocol (Patients 5–10): Etanercept and exenatide, in addition to the Edmonton protocol. Patients received etanercept 50 mg intravenously before each islet infusion and 25 mg subcutaneously at 3, 7, and 10 days after each islet infusion. Exenatide 5 μg was administered subcutaneously twice daily for one week within 60 minutes before or after the morning and evening meals. If tolerated well, the dose was increased to 10 μg twice daily for a total of 6 months after each islet infusion. In the case of adverse effects due to immunosuppressive medication, patients were converted to a modified UIC immunosuppression protocol: tacrolimus, mycophenolate mofetil (MMF), and daclizumab. Four of the six patients from the UIC Protocol and one patient from the Edmonton Protocol were converted to the modified protocol after an average follow-up period of 22 months and four years after first transplant, respectively.
Parameters to assess islet graft and renal function
Insulin-independence: the absence of exogenous insulin injection, with fasting glucose levels >140 mg/dL no more than three times per week and two hour post-prandial glucose levels >180 mg/dL no more than four times per week.
C-peptide positive: the presence of measurable levels (>0.1 ng/mL).
HbA1c: values <6.0% (42.1 mmol/mol).
Mixed meal test (MMT): blood glucose concentration <180 mg/dL, 90 minutes after ingesting Boost® 6 mL/kg body weight.
Oral glucose tolerance test (OGTT): blood glucose concentration <140 mg/dL, two hours after ingesting 75g glucose was considered normal. Blood glucose concentration 140–199 mg/dL was considered impaired and >199 mg/dL was considered diabetic.
Intravenous glucose tolerance test (IVGTT): The acute insulin response to glucose (AIRg) was defined as the mean of the first 5-minute insulin values after the glucose injection with the basal values subtracted [23].
Glucagon stimulation test (GST): C-peptide increase > 100%, 6 minutes after 1 mg glucagon intravenous injection.
HYPO score: a composite hypoglycemic score, devised by Ryan et al. [24] was calculated annually. A score of 0 indicated the absence of hypoglycemic events.
β-score: Scoring, described by Ryan et al. [25], ranges from 0 to 8, with 0 indicating the absolute absence of β-cell function.
Renal function: serum creatinine and 24-hrs urine albumin and creatinine levels were measured, and the urine albumin and creatinine ratio (UACR) was calculated. The normal range of UACR is <30 mg/g [26].
Liver Function Tests: Values of 0–1.2 mg/dL for total bilirubin, 40–125 u/L for alkaline phosphate, 10–40 u/L for AST, and 10–50 u/L for ALT.
Antibody measurements: Alloantibodies were assessed by flow cytometry measurement of HLA class I and II panel reactive antibodies (One Lambda Inc, Los Angeles, CA). Anti-islet cell antibodies were assessed by indirect immunofluorescence (normal titers <1:4), and anti-GAD antibodies by radioimmunoassay (normal values 0–70 mGAD-U/mL)
Statistical Analysis
Raw data were presented for each patient. When data were summarized across patients, they were expressed as mean ± SD. Comparisons were performed using Student’s t-test for unpaired data and paired t-tests for paired data. A p-value <0.05 was considered statistically significant.
Results
Islet transplant recipients
The number of islet transplants and the total IEQ/kg for each patient are summarized in Figure 1B. The patients under the UIC protocol required fewer islets to achieve insulin-independence [17]. However, during the 5-year follow up, three patients in the UIC Protocol required supplemental islet infusions to maintain insulin-independence, and the difference between the Edmonton and UIC Protocols in total IEQ/kg transplanted vanished (24385 ± 14209 vs. 16414 ± 9423, p=0.85).
Islet Graft Function
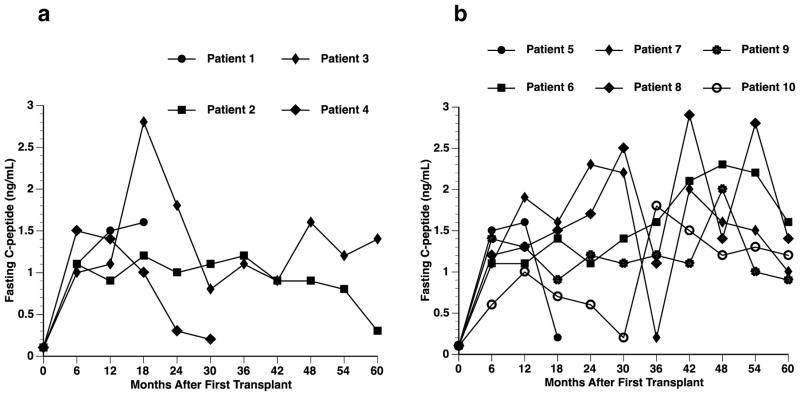
Insulin independence and C-peptide positive status: At least two transplants were conducted for each of the four patients in the Edmonton Protocol to achieve insulin independence (Figure 2). Within the UIC Protocol, all six patients achieved insulin independence after the first transplant and five of the six patients maintained independence at the study end. Patient 6 required one additional transplant and Patients 7 and 10 required two additional transplants to maintain insulin independence. C-peptide concentrations were undetectable in all patients before transplant (<0.1 ng/mL basal and stimulated). However, all patients had detectable C-peptide concentrations (>0.1 ng/mL) at 6 months after first transplant and maintained detectable levels throughout the study (Fig. 2).
-
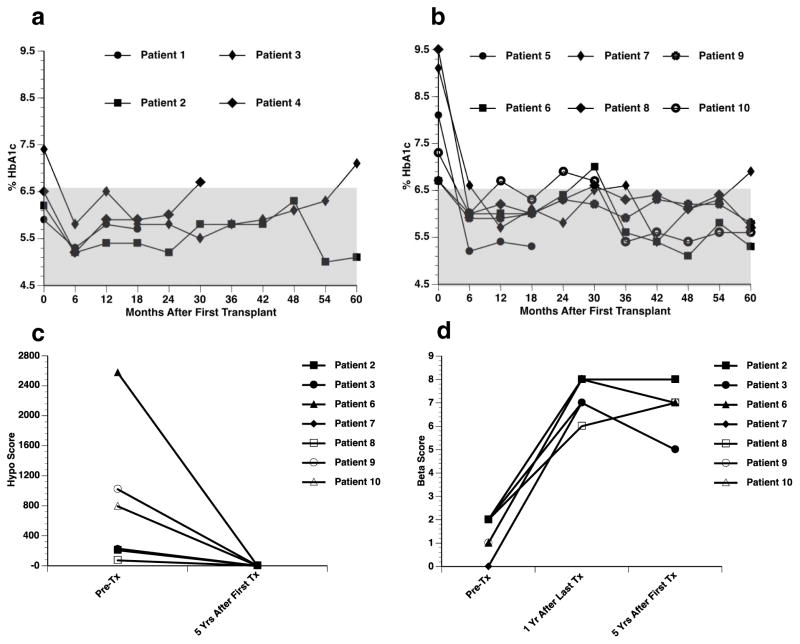
Metabolic assessment: Before transplantation, patients in the Edmonton Protocol presented with significantly lower HbA1c levels than patients in the UIC Protocol (6.5 ± 0.6% vs. 7.9 ± 1.2%, 47.5 ± 7.1 vs. 62.8 ± 13.2 mmol/mol; p=0.045) [17]. After transplantation, HbA1c levels were restored to normal levels (<6.0%) (42.1 mmol/mol) within six months in all but one patient (Fig. 3A, B). Most patients maintained normal levels throughout that period, except for Patients 3 and 7, who presented with elevated HbA1c levels at 5 years.
Metabolic test results are summarized in Table 1. MMT: Before transplant, all patients presented with diabetic response to MMT. 6 out of 7 patients presented with non-diabetic MMT at 1 year. At the study end, Patients 3 and 7 presented with diabetic response, despite expressing lower blood glucose levels than before transplant. OGTT: At 1 year, Patients 2 and 10 demonstrated normal response. At 5 years, Patients 9 and 10 presented with normal response. All other patients presented with either impaired or diabetic responses. IVGTT: Patients 6, 8, and 10 demonstrated a normal AIRg at 1 year after last transplant. Only Patient 10 continued to demonstrate normal IVGTT response at the study end. GST: Before transplant, all subjects demonstrated a diabetic response in the GST. All patients showed an increase in C-peptide at both 1 and 5 years. Patients 7, 8, 9, and 10 demonstrated ≥100% increase at 1 year, and Patients 9 and 10 demonstrated ≥ 100% increase at 5 years. Patients 2 and 3 demonstrated low baseline and stimulated C-peptide levels (<1.0 ng/mL) at 5 years. Patient 7 declined to test OGTT at 1 and 5 years and GST at 5 years, and Patients 7 and 8 declined to test IVGTT at 5 years.
HYPO score and β-score: After five years post-transplant, all patients had a HYPO score of 0 (Fig. 3C). The average score pre-transplant, for the seven patients with 5-year follow-up, was 824.4 (range 71–2668) [17]. The average β-score in this group was 1.3 ± 0.8 before transplantation. The β-score significantly improved at one year after last transplant (7.4 ± 0.8, p<0.001), and remained improved at 5 years (6.7 ± 1.3, p<0.001) (Fig. 3D).
Fig. 2.
Fasting C-peptide levels before and after transplantation. C-peptide levels of transplant recipients over the 5-year follow-up period, according to transplant protocol. (A) Edmonton Protocol (n=4); (B) UIC Protocol (n=6).
Fig. 3.
HbA1c levels before and after transplantation (A, B). HbA1c levels of transplant recipients over the 5-year follow-up period, according to transplant protocol. (A) Edmonton Protocol (n=4); (B) UIC Protocol (n=6). Indirect assessments of islet graft function (C, HYPO Score; D, β-score).
Table 1.
Metabolic test results of the seven patients with 5-year follow-up
| Subjects
|
|||||||
|---|---|---|---|---|---|---|---|
| Test | Patient 2 | Patient 3 | Patient 6 | Patient 7 | Patient 8 | Patient 9 | Patient 10 |
| Mixed Meal Test | |||||||
| Before Tx | |||||||
| Baseline/90 min BG | 103/305 | 78/270 | 198/438 | >590 | 84/255 | 140/382 | 105/369 |
| Baseline/90 min CP | <0.1/<0.1 | <0.1/<0.1 | <0.1/<0.1 | <0.1/ND | <0.1/<0.1 | <0.1/<0.1 | <0.1/<0.1 |
| 1 year after last Tx | |||||||
| Baseline/90 min BG | 95/131 | 109/202 | 90/175 | 98/121 | 101/168 | 93/174 | 87/113 |
| Baseline/90 min CP | 0.9/3 | 1.4/4 | 1.9/5.2 | 1.8/4 | 1.3/5.5 | 1.4/3.4 | 1.2/6 |
| End of study | |||||||
| Baseline/90 min BG | 80/141 | 56/194 | 102/121 | 172/295 | 116/144 | 98/148 | 98/99 |
| Baseline/90 min CP | 0.3/1.5 | 0.2/1.2 | 2.1/3.6 | 1/3 | 1.4/3.3 | 2.0/3.9 | 1.2/3.8 |
| Glucagon Stimulation Test | |||||||
| Before Tx | |||||||
| Baseline/stimulated CP | <0.1/<0.1 | <0.1/<0.1 | <0.1/<0.1 | <0.1/ND | <0.1/<0.1 | <0.1/<0.1 | <0.1/<0.1 |
| 1 year after last Tx | |||||||
| Baseline/stimulated CP | 1.9/3 | 1.8/2.6 | 2.2/3.1 | 1.6/2.3 | 1.5/3.1 | 1.2/2.1 | 2/5.1 |
| End of study | |||||||
| Baseline/stimulated CP | 0.5/0.6 | 0.2/0.4 | 2/2.6 | ND | 1.7/2.4 | 1/2.3 | 1.2/3.3 |
| OGTT | |||||||
| 1 year after last Tx | NR | DM | DM | ND | IM | DM | NR |
| End of study | DM | DM | IM | ND | IM | NR | NR |
| IVGTT (AIRg) | |||||||
| 1 year after last Tx | 33.5 | 27.5 | 59.2 | 8.3 | 45.5 | 21.5 | 50.6 |
| End of study | 0 | 2.3 | 32.2 | ND | ND | 32 | 43.3 |
BG: blood glucose (mg/dL); CP: C-peptide (ng/mL); End of study: 5 years after first islet transplantation; NR: normal; DM: diabetic; IM: impaired; AIRg: acute insulin response to glucose (μU/mL); ND: not done
Adverse Events
Throughout the 5-year follow-up period after transplant, a total of three patients were withdrawn. Patient 1 was withdrawn due to localized breast cancer diagnosis 18 months after first transplant. Patient 4 experienced graft rejection and received whole pancreas transplant; she was removed from the study at 30 months. Patient 5 was withdrawn at 18 months due to the development of diabetic myonecrosis on her neck [27].
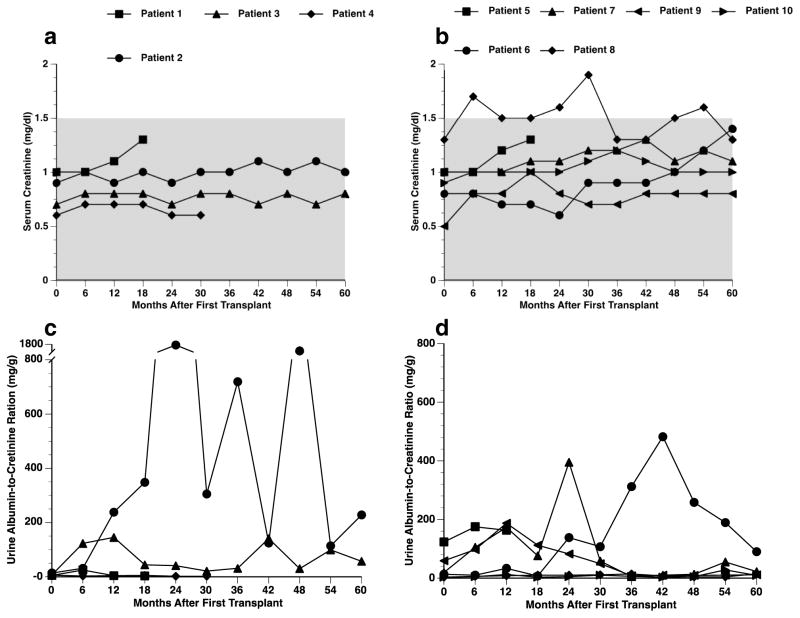
Renal function: Serum creatinine levels remained stable, at values less than 1.5 mg/dL, in all patients throughout their follow up period, except for Patient 8 (Fig. 4A, B). Within the Edmonton Protocol (Fig. 4C), Patient 2 presented with wide-ranging UACR fluctuations (maximum of 1709 mg/g) and Patient 3 presented with UACR values under 100 mg/g during the 5-year period. Within the UIC Protocol (Fig. 4D), four out of six patients (Patients 7–10) presented with relatively stable UACR values and had values < 30 mg/g at the study end. Patient 6 demonstrated an elevated value (482 mg/g) at 30 months, which decreased to 90 mg/g at the end of 5-year follow up.
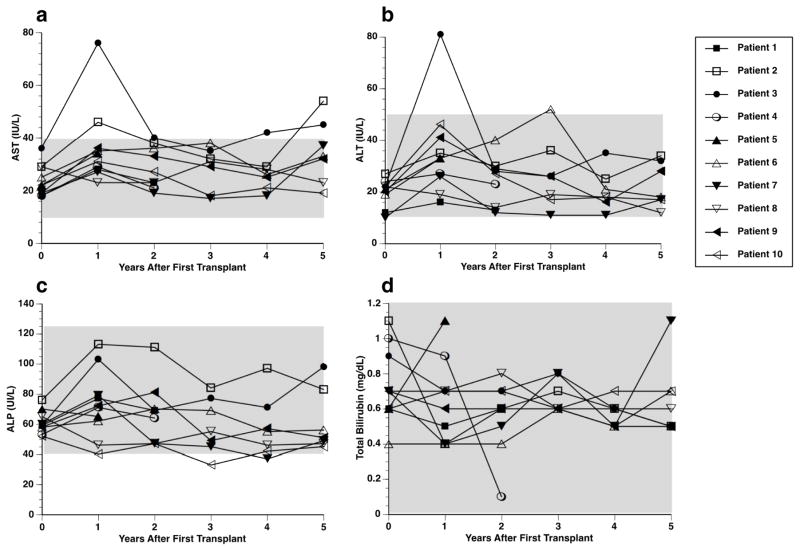
Liver function: Liver function remained stable throughout the 5-year follow up period (Fig. 5). Patient 3 exhibited transient increase in AST and ALT at 1 year, which returned to normal values for the remainder of the study (Fig. 5A, B).
Fig. 4.
Kidney function tests. Serum creatinine (A, B) and UACR levels (C, D) of transplant recipients over the 5-year follow-up period, according to transplant protocol. (A, C) Edmonton Protocol (n=4); (B, D) UIC Protocol (n=6).
Fig. 5.
Liver function test. Aspartate transaminase (AST; A), alanine aminotransferase (ALT; B), Alkaline phosphatase (ALP; C), and total bilirubin (D) levels of transplanted recipients over the 5-year follow-up period. (A, C) Edmonton Protocol (n=4); (B, D) UIC Protocol (n=6).
Antibodies
Prior to transplant, all patients were non-sensitized for both HLA Class I and Class II, except for Patient 8 (PRA 33 for HLA Class I), who after transplant displayed broad sensitization (Table 2). Patient 8 additionally displayed de novo HLA Class II antibodies at year 4 (PRA 52), which disappeared (PRA 0) at year 5. Patients 3 and 4 developed de novo HLA antibodies after five and two years, respectively. At five years, five patients were positive for anti-GAD 65, four of which were positive before transplant, and no patients were positive for anti-ICA 512.
Table 2.
Antibody profiles before and after transplantation
| Subjects
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Test | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | Patient 8 | Patient 9 | Patient 10 |
| %PRA antibodies HLA Class | ||||||||||
| I/Class II | ||||||||||
| Before Tx | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 33/0 | 0/0 | 0/0 |
| 1 yr after first Tx | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 51/0 | 0/0 | 0/0 |
| 2 yrs after first Tx | NA | 0/0 | 0/0 | 16/0 | 0/0 | 0/0 | 0/0 | 45/0 | 0/0 | 0/0 |
| 3 yrs after first Tx | NA | 0/0 | 0/0 | NA | NA | 0/0 | 0/0 | 45/0 | 0/0 | 0/0 |
| 4 yrs after first Tx | NA | 0/0 | 0/0 | NA | NA | 0/0 | 0/0 | 55/52 | 0/0 | 0/0 |
| 5 yrs after first Tx | NA | 0/0 | 0/12 | NA | NA | 0/0 | 0/0 | 54/0 | 0/0 | 0/0 |
| Anti-GAD 65 | ||||||||||
| Before Tx | + | − | − | + | + | − | + | + | + | + |
| 1 yr after first Tx | + | − | − | + | + | − | − | + | − | + |
| 2 yrs after first Tx | NA | − | − | NA | + | − | − | + | − | + |
| 3 yrs after first Tx | NA | − | − | NA | NA | − | + | + | + | + |
| 4 yrs after first Tx | NA | − | − | NA | NA | + | + | + | + | + |
| 5 yrs after first Tx | NA | − | − | NA | NA | + | + | + | + | + |
| Anti-ICA 512 | ||||||||||
| Before Tx | − | − | − | − | + | − | − | − | − | + |
| 1 yr after first Tx | − | − | − | − | + | − | − | − | − | + |
| 2 yrs after first Tx | NA | − | − | − | NA | − | − | − | − | + |
| 3 yrs after first Tx | NA | − | − | NA | NA | − | − | − | − | + |
| 4 yrs after first Tx | NA | − | − | NA | NA | − | − | − | − | + |
| 5 yrs after first Tx | NA | − | − | NA | NA | − | − | − | − | − |
PRA: panel reactive antibodies, expressed by % of positive result; NA: not applicable
Discussion
This study presents the 5-year follow up of ten patients after islet transplantation conducted at UIC. Initially, the UIC protocol enabled the achievement of insulin independence with a significantly lower number of islets [17]. However, this difference vanished with longer follow-up, as most UIC patients required additional islet infusion(s). This suggests a temporary effect of exenatide and etanercept on improved islet function, instead of increased islet mass. Our result is consistent with what has been reported that exenatide can improve transplant outcome during follow-up of 6 [28]and 18 months [29]. The original promise for exenatide and etanercept to decrease the need for multiple donors, which represents a significant limitation of islet transplantation, did not hold long-term in this study. However, re-transplants were effective for maintaining insulin independence in the patients that originally achieved this status with a marginal islet mass. Stable liver function was demonstrated with multiple islet infusions. This is important information for future cell-based therapies for diabetes, and indicates that supplemental infusions are successful with minimal side effects.
Regardless of protocol, all transplanted patients achieved insulin independence within 1 year after initial transplant. Bellin et al. have recently reported promising rates of 50% insulin independence at 5 years in patients that received potent induction immunosuppression regimens of either anti-CD3 monoclonal antibody alone or antithymocyte globulin (ATG) plus TNF-α inhibition, as compared to patients treated only with IL-2RAb [15]. Our study reports comparable results using only TNF-α inhibition. These results are similar to reports of long-term insulin independence in pancreas transplant of 50–60% at 5 years [15].
All patients had detectable C-peptide after transplantation that was maintained for the duration of their follow-up, suggesting islet graft functionality. Such results represent an improvement from previous studies [3]. The patient that resumed low-dose exogenous insulin at 5 years had a detectable C-peptide level of 1.5, indicating partial graft function. Although C-peptide was detected positive in all the patients, the more advanced metabolic tests indicate that glycemic control is less than perfect, with a temporal trend in declining function. The presence of glucose intolerance when challenged by a metabolic test, despite insulin independence, can most likely be explained by the lower than normal islet mass. Even non-diabetic patients that receive autologous islet transplants and display successful glycemic control present with significantly decreased insulin secretory reserve [23]. The glucose intolerance displayed with a majority of the patients reflects a marginal islet mass that functions under daily circumstances, but is insufficient when stressed.
Consistent benefits can be seen in the complete correction of hypoglycemic unawareness and HbA1c levels. Normal HbA1c levels were maintained throughout the study in all but two patients, who displayed slightly raised levels only at the last time point of the study. Islet transplantation has been consistently shown to significantly reduce severe hypoglycemic episodes, as well as the associated fear [30]. All patients displayed significant improvements in the β-score one year after last transplant, and all patients maintained improved β-score at the study end. Only one patient’s β-score declined from one year after last transplant to the study end, which reflected the need for low dose insulin therapy.
The temporal trend of decreasing insulin independence still persists as an unfortunate reality in clinical islet transplantation. Loss of function over time required supplemental infusion(s) in 3 patients. This general trend of decline in islet graft function is largely undefined, and has been attributed to many factors. Immune factors include auto- and allo-immune rejection [10,31] and immunosuppressive drug toxicity [12–14]. Proposed non-immunologic factors include exhaustion and decline of a marginal β cell mass and amyloid-mediated islet apoptosis [32,33]. One patient required 3 transplants to reach insulin independence and then lost islet graft function due to unknown causes after 18 months; this patient subsequently achieved insulin independence successfully after whole pancreas transplant, but had to resume insulin again 4 years later. This case exemplifies the fact that islet transplantation is not successful long-term in all patients, as well as the frustration associated with loss of function over time due to inexplicable reasons, even in whole pancreas transplantation. Another unfortunate reality is the loss of graft function if immunosuppression medication must be stopped, as for the patient that developed breast cancer, or reduced, as for the patient that developed diabetic myonecrosis [27]. Finding a balance between the consequences of too much or too little immunosuppression is not a clinical challenge unique to islet transplantation, but rather represents one of the most difficult clinical problems in all areas of transplantation. Unfortunately, at present, there are no reliable tests available that could help the clinician determine the immunosuppressive level required to prevent rejection without unduly increasing the risk for opportunistic infections.
Drug-related side effects of sirolimus and tacrolimus [34] were comparable to the rates reported by other centers [35]. Proteinuria secondary to sirolimus and reversal after discontinuation has been reported in both renal and islet transplant patients [36–40]. In this study, the most frequent drug-related side effect was proteinuria. Patients who experienced proteinuria were converted to the modified UIC Protocol, replacing sirolimus by MMF. After conversion, all five recipients presented with improved renal function with UACR normalization. The effectiveness of this immunosuppressive regimen was clinically confirmed by the absence of rejection episodes long-term; indeed, the two patients that required only one transplant were converted to MMF at 8 and 30 months after transplant. This study suggests that the combination of tacrolimus, MMF, and extended use Il-2 receptor blockade is overall better tolerated than the combination of tacrolimus and sirolimus, with equal effectiveness in preventing rejection and less nephrotoxic side effects.
A fundamental immunological aspect of any transplant or transfusion procedure is sensitization after exposure to foreign tissue. Sensitization after islet transplant has been previously described in early trials [41] and more recently in larger cohort studies [42]. Antibody profiles were better than expected in this study, as only 2 patients displayed de novo HLA antibodies and one patient sensitized prior to transplant displayed broad sensitization. It was anticipated that sensitized patients would present with poorer transplant outcomes. Inconsistent results were obtained in our limited number of subjects. The patient sensitized before and broadly sensitized after transplant achieved 5-year insulin independence following a single transplant, whereas the patient that developed a relatively low PRA percentage (12%) of Class II antibodies at 5 years had to resume low-dose insulin therapy.
In conclusion, clinical islet transplantation conducted at UIC enabled 60% of the initially transplanted patients to maintain insulin-independence five years after their first transplant. This represents a less drastic decline in insulin independence as compared to past studies and is comparable to whole organ pancreas transplant, albeit at the price of supplemental donor organ use. The difference in outcomes between the two protocols, supporting potential improvement in graft function with the UIC protocol, needs validation in a larger sample. Benefits may be observed through more consistent achievement of long-term insulin independence with the same number of donors. Re-transplants effectively maintain insulin independence, and importantly are well tolerated by the liver, in patients that originally achieve this status with a marginal islet mass using the UIC protocol.
Acknowledgments
Funding/support:
This work was supported by funding from the College of Medicine, University of Illinois at Chicago. J.O. and J. M. were supported by the Islet Cell Resources Center NIH grant (5 U42 RR023245-02), the Christopher Family Foundation, and the Efroymson Foundation. The subjects are evaluated at the UIC Clinical Research Center, which is supported by the University of Illinois at Chicago Center for Clinical and Translational Science (Award Number UL1RR029879) from the National Center for Research Resources. Data acquisition aided by the Collaborative Islet Transplant Registry (CITR), Emmes corporation. The islet program was supported by the Chicago Diabetes Project, and the Washington Square Health Foundation.
Abbreviations
- UIC
University of Illinios at Chicago
- MMF
mycophenolate mofetil
- IEq
islet equivalents
- CITR
Collaborate Islet Transplant Registry
- TNF
tumor necrosis factor
- GLP-1
glucagon-like peptide-1
- MMT
mixed meal test
- OGTT
oral glucose tolerance test
- IVGTT
intravenous glucose tolerance test
- GST
glucagon stimulation test
- UACR
urine albumin and creatinine ratio
Footnotes
Author contributions:
Dr. J.O. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
M.Q. and K.K. attributed to the study design, data collection, and manuscript writing and revision. K.K.D., J.M., B.B., Y.W., J.B., R.C.G, G.K., R.G.R, I.T., attributed to the data collection and manuscript revision. A.H. contributed to data coordination and manuscript revision. M.D. and J.J.M. attributed to data collection and manuscript revision. E.B. and J.O. attributed to study concept, study design, manuscript writing and revision. E.B. and J.O. also revised the manuscript critically for important intellectual content.
Statement of Human and Animal Rights
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008 (5).
Statement of Informed Consent
Informed consent was obtained from all patients for being included in the study.
Conflict of Interest Statement:
Meirigeng Qi, Katie Kinzer, Kirstie K. Danielson, Joan Martellotto, Barbara Barbaro, Yong Wang, James T. Bui, Ron C. Gaba, Grace Knuttinen, Raquel Garcia-Roca, Ivo Tzvetanov, Andrew Heitman, Maureen Davis, James J. McGarrigle, Enrico Benedetti, and Jose Oberholzer declare that they have no conflict of interest.
References
- 1.Ricordi C. Islet transplantation: a brave new world. Diabetes. 2003;52(7):1595–1603. doi: 10.2337/diabetes.52.7.1595. [DOI] [PubMed] [Google Scholar]
- 2.Bertuzzi F, Ricordi C. Beta-cell replacement in immunosuppressed recipients: old and new clinical indications. Acta Diabetol. 2007;44(4):171–176. doi: 10.1007/s00592-007-0020-9. [DOI] [PubMed] [Google Scholar]
- 3.Barton FB, Rickels MR, Alejandro R, Hering BJ, Wease S, Naziruddin B, Oberholzer J, Odorico JS, Garfinkel MR, Levy M, Pattou F, Berney T, Secchi A, Messinger S, Senior PA, Maffi P, Posselt A, Stock PG, Kaufman DB, Luo X, Kandeel F, Cagliero E, Turgeon NA, Witkowski P, Naji A, O'Connell PJ, Greenbaum C, Kudva YC, Brayman KL, Aull MJ, Larsen C, Kay TW, Fernandez LA, Vantyghem MC, Bellin M, Shapiro AM. Improvement in outcomes of clinical islet transplantation: 1999–2010. Diabetes Care. 2012;35(7):1436–1445. doi: 10.2337/dc12-0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343(4):230–238. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 5.Ryan EA, Paty BW, Senior PA, Bigam D, Alfadhli E, Kneteman NM, Lakey JR, Shapiro AM. Five-year follow-up after clinical islet transplantation. Diabetes. 2005;54(7):2060–2069. doi: 10.2337/diabetes.54.7.2060. [DOI] [PubMed] [Google Scholar]
- 6.Bellin MD, Barton FB, Heitman A, Harmon JV, Kandaswamy R, Balamurugan AN, Sutherland DE, Alejandro R, Hering BJ. Potent induction immunotherapy promotes long-term insulin independence after islet transplantation in type 1 diabetes. Am J Transplant. 2012;12(6):1576–1583. doi: 10.1111/j.1600-6143.2011.03977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shapiro AM, Hao EG, Lakey JR, Yakimets WJ, Churchill TA, Mitlianga PG, Papadopoulos GK, Elliott JF, Rajotte RV, Kneteman NM. Novel approaches toward early diagnosis of islet allograft rejection. Transplantation. 2001;71(12):1709–1718. doi: 10.1097/00007890-200106270-00002. [DOI] [PubMed] [Google Scholar]
- 8.Vergani A, Fotino C, D'Addio F, Tezza S, Podetta M, Gatti F, Chin M, Bassi R, Molano RD, Corradi D, Gatti R, Ferrero ME, Secchi A, Grassi F, Ricordi C, Sayegh MH, Maffi P, Pileggi A, Fiorina P. Effect of the purinergic inhibitor oxidized ATP in a model of islet allograft rejection. Diabetes. 2013;62(5):1665–1675. doi: 10.2337/db12-0242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jalili RB, Forouzandeh F, Rezakhanlou AM, Hartwell R, Medina A, Warnock GL, Larijani B, Ghahary A. Local expression of indoleamine 2,3 dioxygenase in syngeneic fibroblasts significantly prolongs survival of an engineered three-dimensional islet allograft. Diabetes. 2010;59(9):2219–2227. doi: 10.2337/db09-1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Monti P, Scirpoli M, Maffi P, Ghidoli N, De Taddeo F, Bertuzzi F, Piemonti L, Falcone M, Secchi A, Bonifacio E. Islet transplantation in patients with autoimmune diabetes induces homeostatic cytokines that expand autoreactive memory T cells. J Clin Invest. 2008;118(5):1806–1814. doi: 10.1172/JCI35197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang G, Wang X, Li Z, Li H, Li X, Zhou Z. Insulin autoantibody could help to screen latent autoimmune diabetes in adults in phenotypic type 2 diabetes mellitus in Chinese. Acta Diabetol. 2012;49(5):327–331. doi: 10.1007/s00592-010-0196-2. [DOI] [PubMed] [Google Scholar]
- 12.Nir T, Melton DA, Dor Y. Recovery from diabetes in mice by beta cell regeneration. J Clin Invest. 2007;117(9):2553–2561. doi: 10.1172/JCI32959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gillard P, Ling Z, Mathieu C, Crenier L, Lannoo M, Maes B, Roep B, Gorus F, Pipeleers D, Keymeulen B. Comparison of sirolimus alone with sirolimus plus tacrolimus in type 1 diabetic recipients of cultured islet cell grafts. Transplantation. 2008;85(2):256–263. doi: 10.1097/TP.0b013e31815e8926. [DOI] [PubMed] [Google Scholar]
- 14.Laugharne M, Cross S, Richards S, Dawson C, Ilchyshyn L, Saleem M, Mathieson P, Smith R. Sirolimus toxicity and vascular endothelial growth factor release from islet and renal cell lines. Transplantation. 2007;83(12):1635–1638. doi: 10.1097/01.tp.0000266555.06635.bf. [DOI] [PubMed] [Google Scholar]
- 15.Bellin MD, Barton FB, Heitman A, Harmon JV, Kandaswamy R, Balamurugan AN, Sutherland DE, Alejandro R, Hering BJ. Potent induction immunotherapy promotes long-term insulin independence after islet transplantation in type 1 diabetes. American Journal of Transplantation. 2012;12(6):1576–1583. doi: 10.1111/j.1600-6143.2011.03977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ngo A, Sutherland DE, Beilman GJ, Bellin MD. Deterioration of glycemic control after corticosteroid administration in islet autotransplant recipients: a cautionary tale. Acta Diabetol. 2014;51(1):141–145. doi: 10.1007/s00592-011-0315-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gangemi A, Salehi P, Hatipoglu B, Martellotto J, Barbaro B, Kuechle JB, Qi M, Wang Y, Pallan P, Owens C, Bui J, West D, Kaplan B, Benedetti E, Oberholzer J. Islet transplantation for brittle type 1 diabetes: the UIC protocol. Am J Transplant. 2008;8(6):1250–1261. doi: 10.1111/j.1600-6143.2008.02234.x. [DOI] [PubMed] [Google Scholar]
- 18.Qi M, Barbaro B, Wang S, Wang Y, Hansen M, Oberholzer J. Human pancreatic islet isolation: Part I: digestion and collection of pancreatic tissue. J Vis Exp. 2009;(27) doi: 10.3791/1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qi M, Barbaro B, Wang S, Wang Y, Hansen M, Oberholzer J. Human pancreatic islet isolation: Part II: purification and culture of human islets. J Vis Exp. 2009;(27) doi: 10.3791/1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soltani SM, O'Brien TD, Loganathan G, Bellin MD, Anazawa T, Tiwari M, Papas KK, Vickers SM, Kumaravel V, Hering BJ, Sutherland DE, Balamurugan AN. Severely fibrotic pancreases from young patients with chronic pancreatitis: evidence for a ductal origin of islet neogenesis. Acta Diabetol. 2013;50(5):807–814. doi: 10.1007/s00592-011-0306-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koh A, Imes S, Kin T, Dinyari P, Malcolm A, Toso C, Shapiro AM, Senior P. Supplemental islet infusions restore insulin independence after graft dysfunction in islet transplant recipients. Transplantation. 2010;89(3):361–365. doi: 10.1097/TP.0b013e3181bcdbe8. [DOI] [PubMed] [Google Scholar]
- 22.Lehmann R, Spinas GA, Moritz W, Weber M. Has time come for new goals in human islet transplantation? Am J Transplant. 2008;8(6):1096–1100. doi: 10.1111/j.1600-6143.2008.02214.x. [DOI] [PubMed] [Google Scholar]
- 23.Teuscher AU, Kendall DM, Smets YF, Leone JP, Sutherland DE, Robertson RP. Successful islet autotransplantation in humans: functional insulin secretory reserve as an estimate of surviving islet cell mass. Diabetes. 1998;47(3):324–330. doi: 10.2337/diabetes.47.3.324. [DOI] [PubMed] [Google Scholar]
- 24.Ryan EA, Shandro T, Green K, Paty BW, Senior PA, Bigam D, Shapiro AM, Vantyghem MC. Assessment of the severity of hypoglycemia and glycemic lability in type 1 diabetic subjects undergoing islet transplantation. Diabetes. 2004;53(4):955–962. doi: 10.2337/diabetes.53.4.955. [DOI] [PubMed] [Google Scholar]
- 25.Ryan EA, Paty BW, Senior PA, Lakey JR, Bigam D, Shapiro AM. Beta-score: an assessment of beta-cell function after islet transplantation. Diabetes Care. 2005;28(2):343–347. doi: 10.2337/diacare.28.2.343. [DOI] [PubMed] [Google Scholar]
- 26.Justesen TI, Petersen JL, Ekbom P, Damm P, Mathiesen ER. Albumin-to-creatinine ratio in random urine samples might replace 24-h urine collections in screening for micro- and macroalbuminuria in pregnant woman with type 1 diabetes. Diabetes Care. 2006;29(4):924–925. doi: 10.2337/diacare.29.04.06.dc06-1555. [DOI] [PubMed] [Google Scholar]
- 27.Salehi P, Stull MA, Martellotto J, Gangemi A, Hatipoglu B, Benedetti E, Oberholzer J. Case report: diabetic myonecrosis of the neck complicated by infection in an islet transplanted patient. J Diabetes Complications. 2009;23(2):140–142. doi: 10.1016/j.jdiacomp.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 28.Froud T, Faradji RN, Pileggi A, Messinger S, Baidal DA, Ponte GM, Cure PE, Monroy K, Mendez A, Selvaggi G, Ricordi C, Alejandro R. The use of exenatide in islet transplant recipients with chronic allograft dysfunction: safety, efficacy, and metabolic effects. Transplantation. 2008;86(1):36–45. doi: 10.1097/TP.0b013e31817c4ab3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Faradji RN, Tharavanij T, Messinger S, Froud T, Pileggi A, Monroy K, Mineo D, Baidal DA, Cure P, Ponte G, Mendez AJ, Selvaggi G, Ricordi C, Alejandro R. Long-term insulin independence and improvement in insulin secretion after supplemental islet infusion under exenatide and etanercept. Transplantation. 2008;86(12):1658–1665. doi: 10.1097/TP.0b013e31818fe448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson JA, Kotovych M, Ryan EA, Shapiro AM. Reduced fear of hypoglycemia in successful islet transplantation. Diabetes Care. 2004;27(2):624–625. doi: 10.2337/diacare.27.2.624. [DOI] [PubMed] [Google Scholar]
- 31.Huurman VA, van der Torren CR, Gillard P, Hilbrands R, van der Meer-Prins EP, Duinkerken G, Gorus FK, Claas FH, Keymeulen B, Roelen DL, Pipeleers DG, Roep BO. Immune responses against islet allografts during tapering of immunosuppression - A pilot study in 5 subjects. Clin Exp Immunol. 2012 doi: 10.1111/j.1365-2249.2011.04605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith RN, Kent SC, Nagle J, Selig M, Iafrate AJ, Najafian N, Hafler DA, Auchincloss H, Orban T, Cagliero E. Pathology of an islet transplant 2 years after transplantation: evidence for a nonimmunological loss. Transplantation. 2008;86(1):54–62. doi: 10.1097/TP.0b013e318173a5da. [DOI] [PubMed] [Google Scholar]
- 33.Westermark GT, Westermark P, Berne C, Korsgren O. Widespread amyloid deposition in transplanted human pancreatic islets. N Engl J Med. 2008;359(9):977–979. doi: 10.1056/NEJMc0802893. [DOI] [PubMed] [Google Scholar]
- 34.Ponte GM, Baidal DA, Romanelli P, Faradji RN, Poggioli R, Cure P, Froud T, Selvaggi G, Pileggi A, Ricordi C, Alejandro R. Resolution of severe atopic dermatitis after tacrolimus withdrawal. Cell Transplant. 2007;16(1):23–30. doi: 10.3727/000000007783464524. [DOI] [PubMed] [Google Scholar]
- 35.Ryan EA, Paty BW, Senior PA, Bigam D, Alfadhli E, Kneteman NM, Lakey JRT, Shapiro AMJ. Five-year follow-up after clinical islet transplantation. Diabetes. 2005;54(7):2060–2069. doi: 10.2337/diabetes.54.7.2060. [DOI] [PubMed] [Google Scholar]
- 36.Bumbea V, Kamar N, Ribes D, Esposito L, Modesto A, Guitard J, Nasou G, Durand D, Rostaing L. Long-term results in renal transplant patients with allograft dysfunction after switching from calcineurin inhibitors to sirolimus. Nephrol Dial Transplant. 2005;20(11):2517–2523. doi: 10.1093/ndt/gfh957. [DOI] [PubMed] [Google Scholar]
- 37.Liew A, Chiang GS, Vathsala A. Factors associated with proteinuria in renal transplant recipients treated with sirolimus. Transpl Int. 2009;22(3):313–322. doi: 10.1111/j.1432-2277.2008.00801.x. [DOI] [PubMed] [Google Scholar]
- 38.Senior PA, Zeman M, Paty BW, Ryan EA, Shapiro AM. Changes in renal function after clinical islet transplantation: four-year observational study. Am J Transplant. 2007;7(1):91–98. doi: 10.1111/j.1600-6143.2006.01573.x. [DOI] [PubMed] [Google Scholar]
- 39.Kaplan B, Schold J, Srinivas T, Womer K, Foley DP, Patton P, Howard R, Meier-Kriesche HU. Effect of sirolimus withdrawal in patients with deteriorating renal function. Am J Transplant. 2004;4(10):1709–1712. doi: 10.1111/j.1600-6143.2004.00569.x. [DOI] [PubMed] [Google Scholar]
- 40.Senior PA, Paty BW, Cockfield SM, Ryan EA, Shapiro AM. Proteinuria developing after clinical islet transplantation resolves with sirolimus withdrawal and increased tacrolimus dosing. Am J Transplant. 2005;5(9):2318–2323. doi: 10.1111/j.1600-6143.2005.01013.x. [DOI] [PubMed] [Google Scholar]
- 41.Olack BJ, Swanson CJ, Flavin KS, Phelan D, Brennan DC, White NH, Lacy PE, Scharp DW, Poindexter N, Mohanakumar T. Sensitization to HLA antigens in islet recipients with failing transplants. Transplant Proc. 1997;29(4):2268–2269. doi: 10.1016/s0041-1345(97)00327-8. [DOI] [PubMed] [Google Scholar]
- 42.Campbell PM, Senior PA, Salam A, Labranche K, Bigam DL, Kneteman NM, Imes S, Halpin A, Ryan EA, Shapiro AM. High risk of sensitization after failed islet transplantation. Am J Transplant. 2007;7(10):2311–2317. doi: 10.1111/j.1600-6143.2007.01923.x. [DOI] [PubMed] [Google Scholar]