Abstract
Background
Pancreatic neuroendocrine tumors (PNETs) are rare in nonhuman primates and in humans.
Methods
Twenty-one PNETs from twelve female baboons (Papio spp.) from the Southwest National Primate Research Center were evaluated using histopathology and immunohistochemistry.
Results
Histologically, all tumors were benign and had neuroendocrine packeting. Immunohistochemical staining for synaptophysin and chromogranin was positive in all tumors evaluated (17/17). Insulin was positive in 16/21 tumors. Somatostatin was positive in 9/20 tumors. Multifocal staining for glucagon and pancreatic polypeptide was evident in a minority of tumors (6/20 and 2/17, respectively). Gastrin and vasoactive intestinal peptide were negative in all tumors evaluated. Nine tumors expressed more than one hormone marker.
Conclusions
This is the first detailed pathologic study of pancreatic endocrine tumors in the baboon. The findings suggest that these tumors are generally benign and have similar morphologic and immunohistochemical features as those described in people, including the ability to express multiple hormones.
Keywords: Pancreas, Nonhuman-primate, Neoplasia, Immunohistochemistry, Endocrine, Insulin, Synaptophysin, Chromogranin, Somatostatin, Glucagon
Introduction
Pancreatic neuroendocrine tumors (PNETs) are uncommon tumors in both humans and primates.[2, 7, 14, 20] In humans, they comprise approximately 2% of all pancreatic neoplasms and affect adults between 40 and 60 with no evidence of a sex predilection.[3] Tumors can arise from any of the cells in the pancreatic islets, and tumors within this group include insulinomas, glucagonomas, somatostatinomas, gastrinomas, and VIPomas. Distinct cellular patterns described in humans include solid, acinar, trabecular, and tubular, but these morphologic variants do not appear to differ in biologic behavior. Tumors are typically 1–5 cm in size and size directly correlates with biologic behavior, with larger tumors having a greater risk of malignancy.[3] The three-tiered WHO (2000) classification system for human PNETs consists of: 1. well-differentiated endocrine tumor (subclassifed as 1.1 benign and 1.2 uncertain malignant potential), 2. well-differentiated endocrine carcinoma, and 3. poorly differentiated endocrine carcinoma. [3] The more recent WHO (2010) classification system for PNETs classifies them as low (NET G1), intermediate (NET G2) and high-grade (neuroendocrine carcinoma).[17] Morphologic features do not correlate well with malignancy potential or to tumor type, although amyloid is more likely to be present in an insulinoma, and psammoma bodies are commonly present in somatostatinomas.[1, 3, 20] While activation of oncogenes such as k-ras, P53, and Rb have not been detected in PNETs, molecular and cytogenetic analyses have identified specific chromosomal alterations in PNETs.[20]
Physiologically and anatomically, baboons (Papio spp.) are very similar to humans and share approximately 96% genetic similarity.[23, 24] Baboons have been used for experimental studies affecting the endocrine system, including studies directly affecting the endocrine pancreas.[4, 5, 8, 9, 11, 13, 15] Previous studies have documented the occurrence of pancreatic endocrine tumors in macaques and chimpanzees, [19] owl monkey,[12] colobus monkey,[16] and baboons, [2, 6, 7, 14, 18, 20] with an incidence as high as 4.6% of all baboon tumors in one study,[6] but the pathologic features of pancreatic endocrine neoplasms in Papio spp. have not been reported. The purpose of this study was to characterize the morphologic and immunohistochemical features of pancreatic endocrine tumors in the baboon and to compare them with their human counterparts.
Materials and methods
Baboons were housed in two open-top 6-acre metal and concrete corrals with dirt floors, gang cages with concrete floors, and in individual metal cages if special handling was required (i.e., for medical care). The commercial monkey chows fed over the years were supplemented with an enrichment fare of grains, fruits, and vegetables. The baboons are screened every 6 months for Mycobacterium tuberculosis. All animal care and procedures were approved by the Texas Biomedical Research Institute Animal Care and the Use Committee.
A total of twenty-one tumors were analyzed. The tumors were from twelve female baboons undergoing routine necropsy at the Southwest National Primate Research Center in San Antonio, Texas. The animals ranged in age from 12 to 25 years. Four animals had more than one tumor. All tumors were considered incidental findings.
Tissues were fixed in 10% neutral buffered formalin, processed conventionally, embedded in paraffin, sectioned, and stained with hematoxylin and eosin. Routine immunohistochemistry was performed. Seven antigens were selected based on their specificity for the pancreas and neuroendocrine tumors – insulin, gastrin, glucagon, somatostatin, vasoactive intestinal polypeptide (VIP), synaptophysin, chromogranin, and pancreatic polypeptide (manufacturer information in Table 1). Baboon pancreas was used as a control for insulin, glucagon, somatostatin, synaptophysin, and pancreatic polypeptide. Gastric antrum was used as a control for gastrin and VIP. All seven stains were not performed on every tumor because of the small size of the tumors and resulting limited tissue availability.
Table 1.
Antibodies used for immunohistochemistry
| Primary Antibody | Clone | Dilution | Vendor |
|---|---|---|---|
| Chromogranin A | Rabbit polyclonal |
1:1000 | Dako |
| Gastrin | Rabbit polyclonal |
1:400 | Abcam |
| Glucagon | Rabbit polyclonal |
1:300 | Cell Marque |
| Insulin | Guinea pig | 1:200 | Cell Marque |
| Pancreatic polypeptide |
Goat polyclonal |
1:100 | Abcam |
| Somatostatin | Rabbit polyclonal |
1:1000 | Abcam |
| Synaptophysin | Mouse monoclonal SY38 |
1:500 | Dako |
| Vasoactive intestinal peptide |
Rabbit polyclonal |
1:100 | AbD-Serotec |
Results
Gross Findings
The tumors were small, ranging in size from 0.5mm to 3cm in diameter with most no larger than 2mm in diameter. Only one tumor was noted at necropsy, measuring approximately 3cm in diameter. The rest of the tumors were not evident at necropsy.
Histology and Immunohistochemistry Findings
Histologic and immunohistochemical findings are summarized in Table 2. The majority of tumors (20/21) exhibited a solid pattern. Neuroendocrine packeting was evident in all tumors, and nests of neoplastic cells were separated by delicate fibrovascular trabeculae (Fig. 1). The cells were similar in all tumors and were predominately small and cuboidal to columnar with small to moderate amounts of eosinophilic cytoplasm, round to ovoid nuclei with finely stippled chromatin patterns, and single small nucleoli. There was mild anisokaryosis and anisocytosis, and mitotic figures were either not evident or up to 1 per 10 high powered fields. There was a thin capsule surrounding the tumor in 7/21 (Fig. 2). In 6/21 tumors, there were clusters of cells with eosinophilic cytoplasmic granularity (Fig. 3). These tumors generally had cells with a paler staining cytoplasm in the non-granular areas than tumors without granularity. Amyloid was present within the tumor in 3/21. Amyloid was seen in the non-neoplastic islets of 6/12 baboons, but did not appear to correlate with the presence of amyloid in the PNETs. Diffuse positive insulin staining was seen in 4/21 (Fig. 4), with mild, multifocal insulin staining in 12/21 tumors (Fig. 5). All tumors tested were positive for synaptophysin (Fig. 6) and chromogranin. Only one tumor was diffusely positive for somatostatin; it was the only tumor grossly identified at necropsy and the only neoplasm that was highly vascularized and contained acinar structures (Fig. 7).
| Case # | Tumor # | Signalment | Gross appearance |
Amyloid in tumor |
Islet amyloidosis |
Insulin | Gastrin | Glucagon | Somatostatin | VIP | Synaptophysin | Chromogranin | Pancreatic polypeptide |
Solid | Capsule | Packeting | Very vascular |
Mitotic rate |
Acinar pattern |
Thick stroma Focal (F) or Diffuse (D) |
Granularity | Size on slide |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 23 yr F | 3 cm diameter |
no | no | mild multifocal |
negative | mild multifocal |
positive | negative | positive | positive | negative | − | − | ++ | + | 1 per 10 fields |
+ | F | − | 1.5 × 1cm |
| 2 | NS | no | no | mild multifocal |
negative | negative | mild multifocal |
negative | positive | positive | mild multifocal |
+ | + | ++ | − | 0 per 10 fields |
− | D | − | 5mm | ||
| 2 | 3 | 28 yr F | NS | no | no | mild multifocal |
negative | negative | negative | negative | positive | positive | negative | + | + | + | − | 1 per 10 fields |
− | F | − | 5mm |
| 4 | NS | no | no | mild multifocal |
negative | mild multifocal |
mild multifocal |
negative | positive | positive | negative | + | − | ++ | − | 0 | − | − | + | 2mm | ||
| 5 | NS | no | no | mild multifocal |
negative | mild multifocal |
mild multifocal |
negative | positive | positive | negative | + | + | ++ | − | 0 | − | − | − | 1mm | ||
| 6 | NS | no | no | mild multifocal |
negative | mild multifocal |
mild multifocal |
negative | positive | positive | negative | + | − | ++ | − | 0 | − | − | − | 1 × 0.5mm |
||
| 3 | 7 | 23 yr F | NS | no | no | mild multifocal |
negative | negative | mild multifocal |
negative | positive | positive | mild multifocal |
+ | + | + | − | 1 per 10 fields |
− | F | − | 7 × 5mm |
| 4 | 8 | 26 yr F | NS | no | yes | mild multifocal |
negative | mild multifocal |
mild multifocal |
negative | positive | positive | negative | + | − | + | − | 0 | − | − | + | 2 × 1mm |
| 9 | NS | no | yes | mild multifocal |
negative | negative | negative | negative | positive | positive | negative | + | + | ++ | − | 0 | − | − | − | 1.5 × 1mm |
||
| 5 | 10 | 24 yr F | NS | no | no | negative | negative | negative | negative | negative | positive | positive | negative | + | + | ++ | − | 0 | − | − | − | 2 × 1mm |
| 6 | 11 | 17 yr F | NS | no | no | positive | negative | ND | ND | ND | ND | ND | ND | + | − | ++ | − | 0 | − | − | − | 0.5mm |
| 7 | 12 | 26 yr F | NS | yes | yes | mild multifocal |
negative | negative | negative | negative | positive | positive | negative | + | − | ++ | − | 1 per 10 fields |
− | − | + | 2mm |
| 8 | 13 | 20 yr F | NS | no | no | mild multifocal |
negative | negative | negative | negative | positive | positive | negative | + | − | + | − | 0 | − | − | − | 1mm |
| 9 | 14 | 12 yr F | NS | no | yes | negative | negative | negative | negative | negative | positive | positive | negative | + | + | ++ | − | 0 | − | − | + | 1 × 0.5mm |
| 10 | 15 | 19 yr F | NS | no | yes | negative | negative | negative | negative | negative | positive | positive | negative | + | − | + | − | 0 | − | − | − | 1 × 0.5mm |
| 11 | 16 | 24 yr F | NS | no | yes | positive | negative | negative | negative | negative | positive | positive | negative | + | − | + | − | 0 | − | − | − | 0.75mm |
| 12 | 17 | 26 yr F | NS | yes | yes | negative | negative | negative | negative | negative | positive | ND | negative | + | − | ++ | − | 0 | − | − | − | 1mm |
| 18 | NS | no | yes | positive | negative | negative | negative | ND | ND | ND | ND | + | − | + | − | 0 | − | − | + | 0.5mm | ||
| 19 | NS | yes | yes | positive | negative | mild multifocal |
mild multifocal |
negative | ND | ND | ND | + | − | + | − | 0 | − | − | + | 1 × 0.5mm |
||
| 20 | NS | no | yes | mild multifocal |
negative | negative | mild multifocal |
negative | positive | ND | negative | + | − | ++ | − | 1 | − | − | − | 1mm | ||
| 21 | NS | no | yes | negative | negative | negative | negative | negative | ND | ND | ND | + | − | + | − | 0 | − | − | − | 1 × 0.5mm |
||
| NS = Not seen |
ND = Not done |
ND = Not done |
ND = Not done |
ND = Not done |
ND = Not done |
ND = Not done |
Fig. 1.
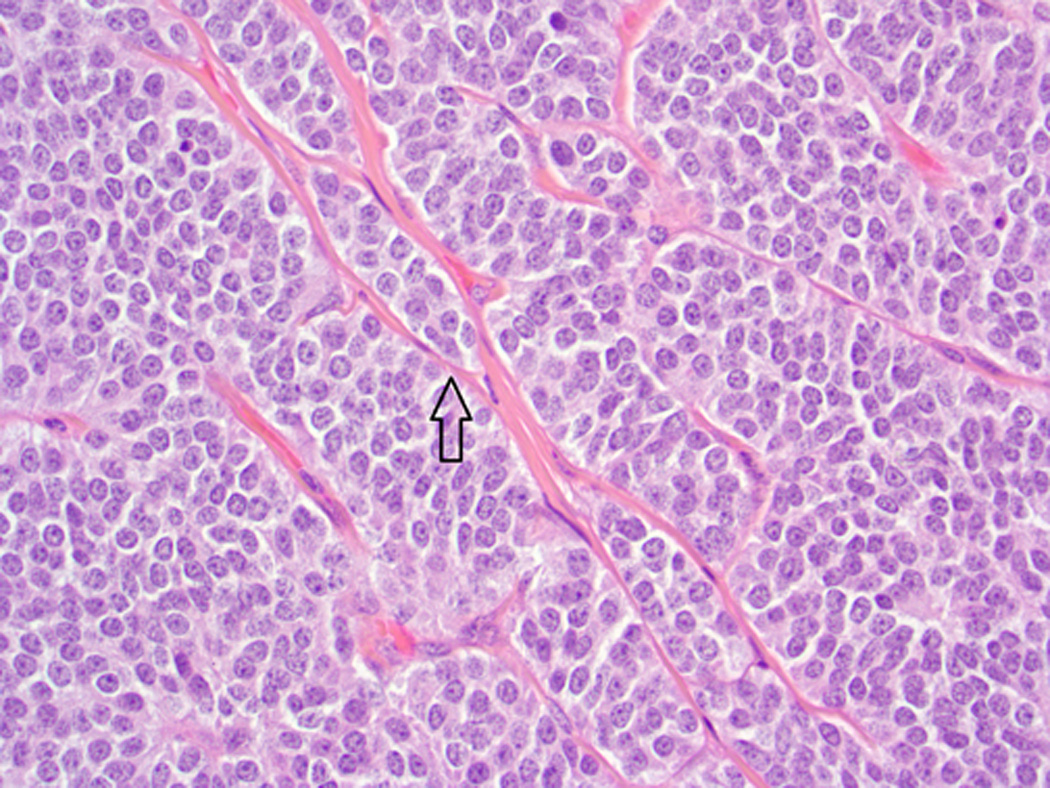
Baboon pancreatic neuroendocrine tumor, Case # 3, Tumor #7. Neuroendocrine packeting is evident, with nests of neoplastic cells separated by delicate fibrovascular stroma (arrow). (H&E, 400×)
Fig. 2.
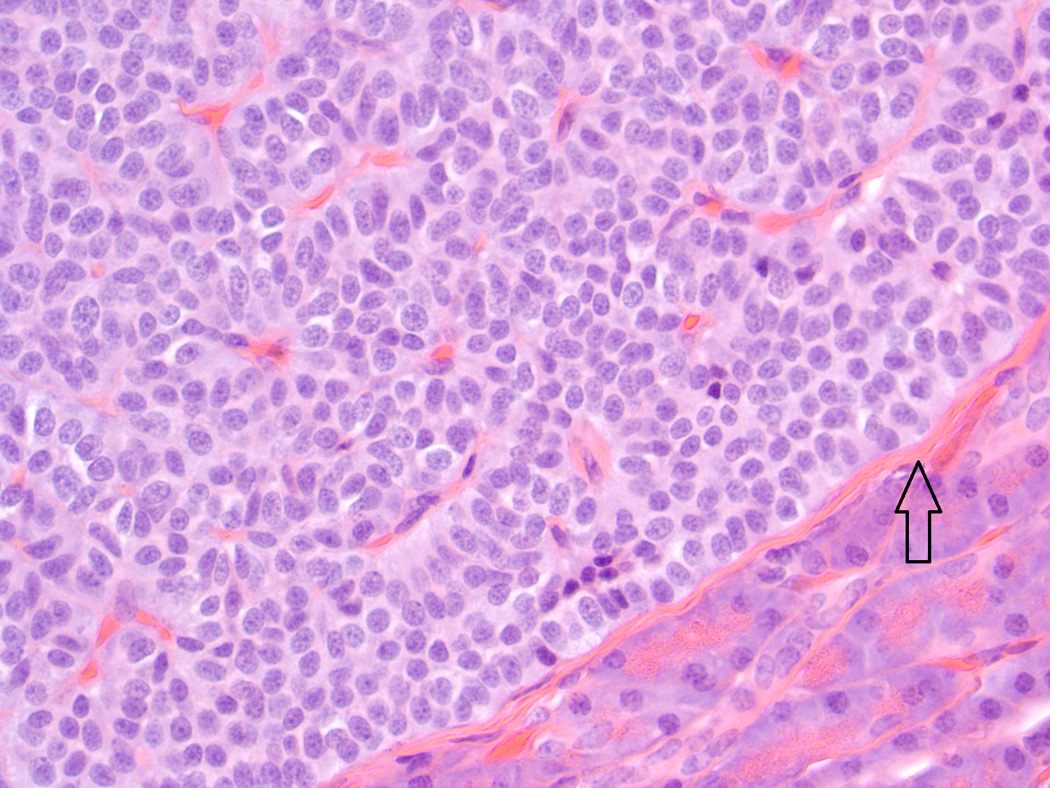
Baboon pancreatic neuroendocrine tumor, Case # 5, Tumor # 10. A thin capsule surrounds the tumor (arrow.) (H&E, 400×)
Fig. 3.
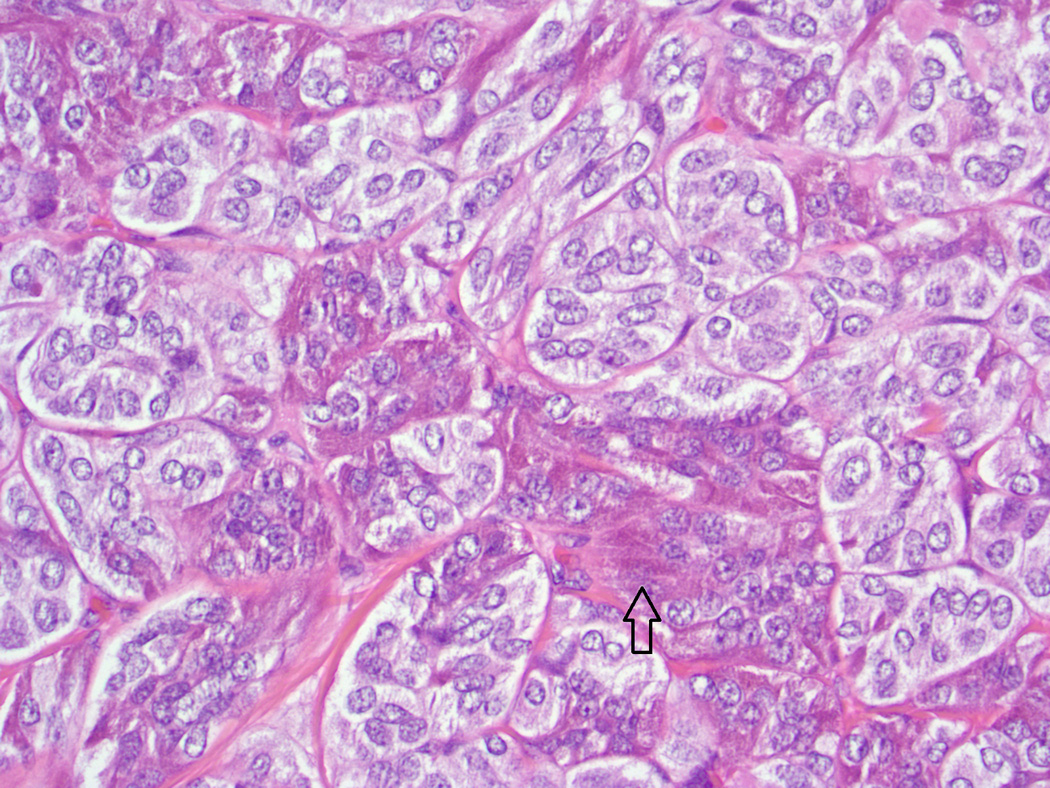
Baboon pancreatic neuroendocrine tumor, Case # 7, Tumor # 12. Note clusters of cells with eosinophilic cytoplasmic granularity (arrow). Neuroendocrine packeting is also visible (H&E, 400×)
Fig. 4.
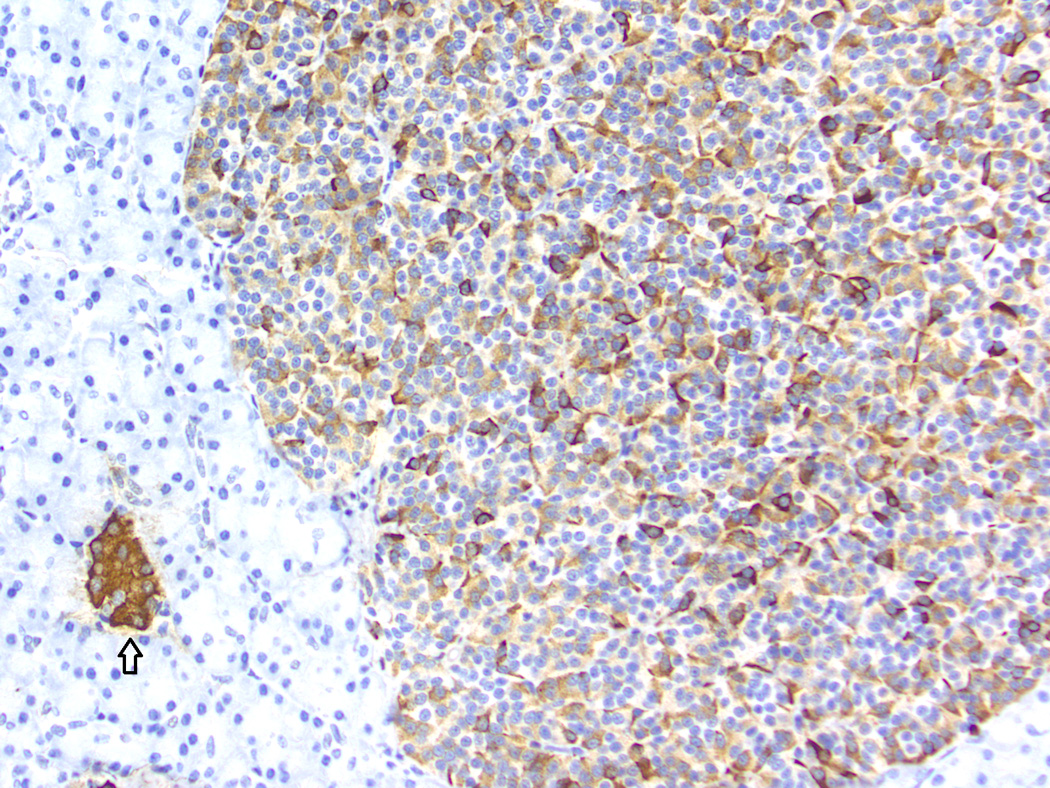
Baboon pancreatic neuroendocrine tumor, Case # 11, Tumor #16. Immunohistochemical staining for insulin. Internal control (islet) indicated by the arrow. The tumor displays a diffusely positive reaction. (200×)
Fig. 5.
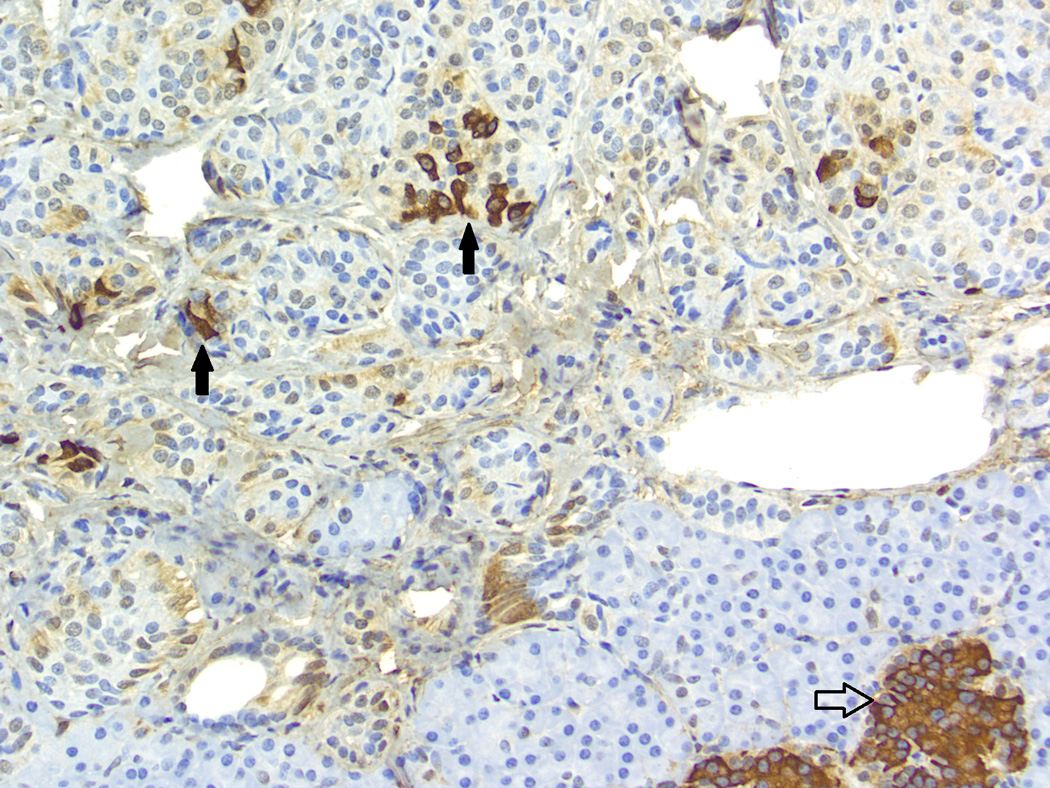
Baboon pancreatic neuroendocrine tumor, Case # 4, Tumor # 8. Immunohistochemical staining for insulin. Mild, multifocal positive reaction (black arrows) was seen in 12/21 tumors. Internal control (islet) indicated by the open arrow. (200×)
Fig. 6.
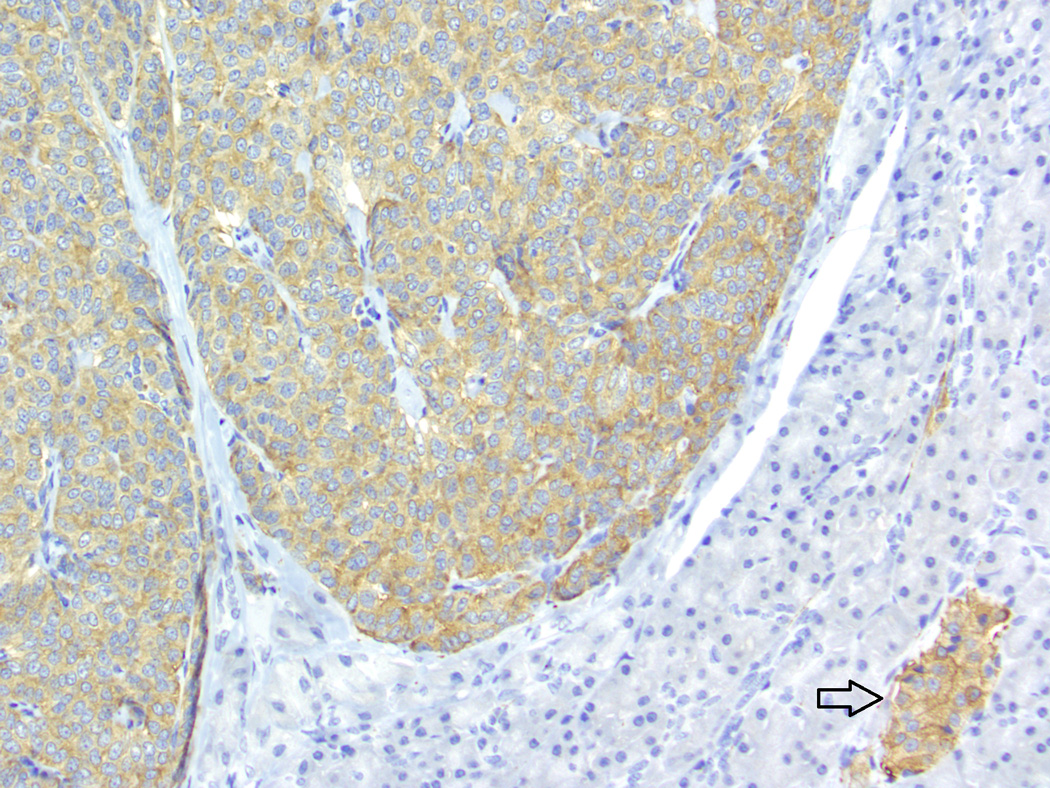
Baboon pancreatic neuroendocrine tumor, Case # 2, Tumor # 3. Immunohistochemical staining for synaptophysin. The tumor displays a diffusely positive reaction. Internal control (islet) indicated by the arrow. (200×)
Fig. 7.
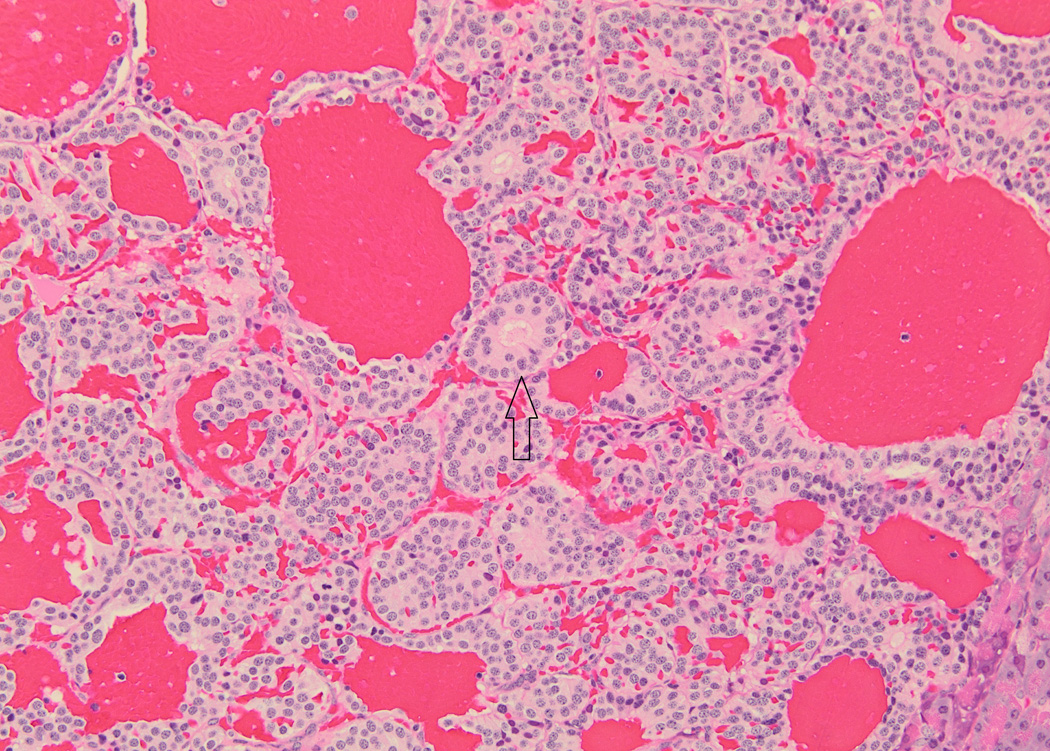
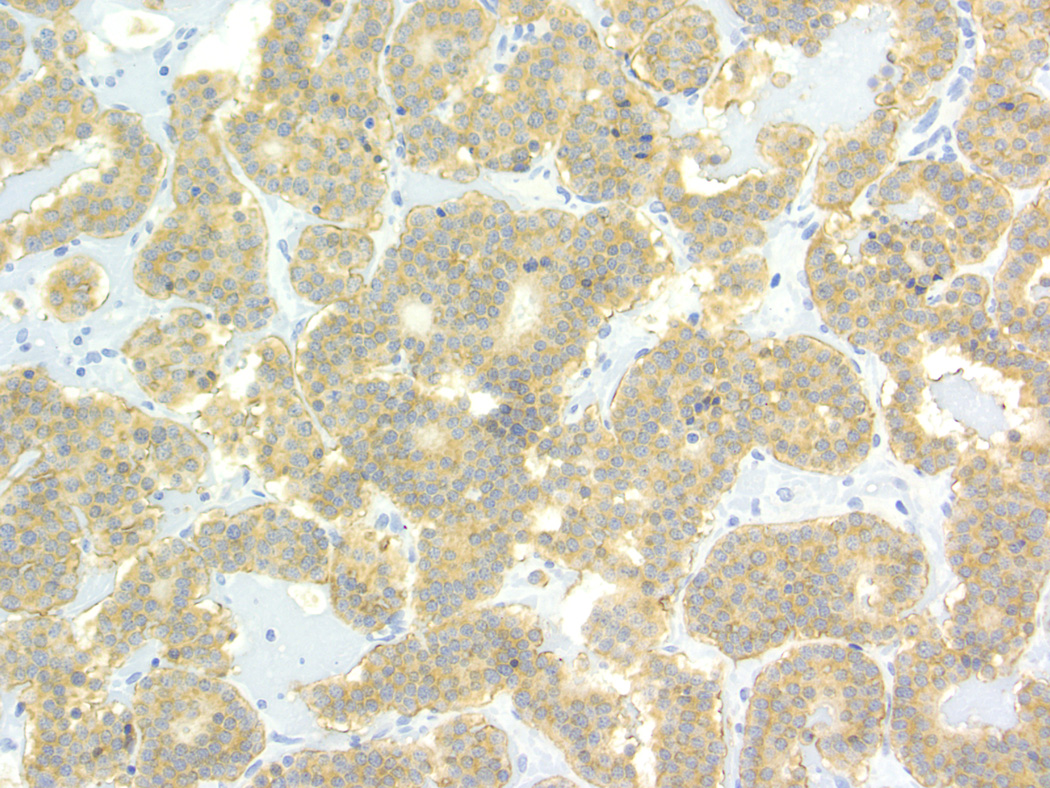
Baboon pancreatic neuroendocrine tumor, Case # 1, Tumor # 1. (A). Tumor is highly vascularized and contains acinar structures (arrow). Cells are columnar with oval nuclei, low mitotic index, and mild anisokaryosis. (200×) (B). Immunohistochemical staining for somatostatin. The tumor displays a diffusely positive reaction. (200×)
Discussion
The cellular features of the tumors in this study are similar to those described in human PNETs. The majority of tumors (20/21) had a solid pattern and showed no angioinvasion or perineural invasion, were less than 2 cm in size, and had less than two mitoses per ten high power fields, all of which are characteristic of WHO (2000) type 1.1 (well differentiated endocrine tumor, benign behavior)[3], or WHO (2010) type NET G1 neuroendocrine tumor (NET) G1.[17] One tumor (Tumor #1) was morphologically distinct, with a larger size (3 cm on gross exam), rich vascular supply, and an acinar pattern, meeting the criteria for a WHO (2000) type 1.2 (well differentiated tumor, uncertain malignant potential), but still WHO (2010) type NET G1 due to the low mitotic rate.[17] The newest WHO classification (2010) is dependent on the mitotic rate, due to the finding that proliferation rate is most significant with regards to malignant potential; the criteria for mitotic rates and KI67 positive cells are as follows: G1: <2/10 mitoses per hpf or <= 2% KI67, G2: 2–20 mitoses per 10 hpf or 3–20% KI67, G3: >20 mitoses per 10 hpf or >20% KI67.[17] The areas of cytoplasmic granularity in a subset of the tumors could represent neuroendocrine granules or an increased number of mitochondria, which has been described in human PNETs as an oncocytic variant.[3] These granules were not further evaluated in this study with cytochemical stains or electron microscopy.
Pancreatic neuroendocrine tumors can express hormones that are normally produced by the pancreas (insulin, glucagon, somatostin, pancreatic polypeptide) or ectopic hormones (gastrin, VIP), and the pattern of staining can greatly differ, with some tumors showing diffuse staining and others only focal staining.[3] Positivity for multiple hormones is common. However, over 90% of PNETs are nonfunctional, secreting no hormones.[22] In this study, insulin was the most common hormone expressed (16/21), and nine tumors expressed more than one hormone. A wide variety of clinical signs are described in people with PNETs, although not all tumors are functional and immunopositivity for a hormone does not imply that the tumor is producing enough hormone to be clinically significant.[1] Clinical evidence of PNET functionality was not evident in the animals in this study, but they received minimal clinical workups. Only two animals had blood work performed, and neither was hypoglycemic.
Whereas the tumors in this paper appeared to be incidental lesions, the knowledge that baboons have these spontaneous lesions is important from a scientific and toxicologic standpoint, especially given recent concerns that currently used glucagon-like peptide-1 based therapies could cause adverse events such as cancer or pancreatitis.[21] Notably, in a large study of baboons at our center, we did not detect neoplasia or pancreatitis in a group of baboons given continuous infusion of exenatide (a GLP-1 agonist) over 14 weeks.[10]
In humans, a subset of PNETs are associated with several genetic conditions that predispose to their development, including multiple endocrine neoplasia type 1 (MEN-1), VHL disease, neurofibromatosis type 1, and tuberous sclerosis complexes 1 and 2. [3] Endocrine lesions in other organs were identified in only two animals in this study (pituitary hyperplasia/adenoma in both); however, the presence of multiple very small PNETs in four animals suggests the possibility of a genetic predisposition to tumor development in these animals. Further evaluation of affected baboons for mutations in the MEN-1 gene and other genes associated with hereditary cancer syndromes is warranted. Human PNETs are typically 1–5 cm in diameter, and those less than 0.5 cm are called “microadenomas” and are seen most commonly in patients with genetic disease.[3] Most PNETs (60–70%) in humans present with metastatic disease, due to the preponderance of nonfunctioning tumors, and often present with mass effects at the head of the pancreas, causing pain, jaundice, nausea, and pancreatitis. However, the tumors in the baboons did not appear to be clinically significant, but additional study into the potential hormonal, clinical pathological, and genetic components of these lesions in the baboon seems warranted.
Acknowledgments
This investigation used resources which were supported by the Southwest National Primate Research Center grant P51 RR013986 from the National Center for Research Resources, National Institutes of Health and which are currently supported by the Office of Research Infrastructure Programs through P51 OD011133. This investigation was conducted in facilities constructed with support from the Office of Research Infrastructure Programs (ORIP) of the National Institutes of Health through Grant Number C06 RR015456 and C06 RR014578. The authors wish to thank Renee Escalona, Tony Perez, and Jesse Martinez for their anatomic pathology support and the clinical support and research staff.
References
- 1.Asa SL. Pancreatic endocrine tumors. Mod Pathol. 2011;24(Suppl 2):S66–S77. doi: 10.1038/modpathol.2010.127. [DOI] [PubMed] [Google Scholar]
- 2.Bommineni YR, Dick EJ, Malapati AR, Owston MA, Hubbard GB. Natural pathology of the Baboon (Papio spp.) J Med Primatol. 2011;40:142–155. doi: 10.1111/j.1600-0684.2010.00463.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Capelli P, Martignoni G, Pedica F, Falconi M, Antonello D, Malpeli G, Scarpa A. Endocrine neoplasms of the pancreas: pathologic and genetic features. Arch Pathol Lab Med. 2009;133:350–364. doi: 10.5858/133.3.350. [DOI] [PubMed] [Google Scholar]
- 4.Chavez AO, Lopez-Alvarenga JC, Tejero ME, Triplitt C, Bastarrachea RA, Sriwijitkamol A, Tantiwong P, Voruganti VS, Musi N, Comuzzie AG, DeFronzo RA, Folli F. Physiological and molecular determinants of insulin action in the baboon. Diabetes. 2008;57:899–908. doi: 10.2337/db07-0790. [DOI] [PubMed] [Google Scholar]
- 5.Chen S, Bastarrachea RA, Roberts BJ, Voruganti VS, Frost PA, Nava-Gonzalez EJ, Arriaga-Cazares HE, Chen J, Huang P, DeFronzo RA, Comuzzie AG, Grayburn PA. Successful β cells islet regeneration in streptozotocin-induced diabetic baboons using ultrasound-targeted microbubble gene therapy with cyclinD2/CDK4/GLP1. Cell Cycle. 2014;13:1145–1151. doi: 10.4161/cc.27997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cianciolo RE, Butler SD, Eggers JS, Dick EJ, Leland MM, de la Garza M, Brasky KM, Cummins LB, Hubbard GB. Spontaneous neoplasia in the baboon (Papio spp.) J Med Primatol. 2007;36:61–79. doi: 10.1111/j.1600-0684.2006.00202.x. [DOI] [PubMed] [Google Scholar]
- 7.Cianciolo RE, Hubbard GB. A review of spontaneous neoplasia in baboons (Papio spp.) J Med Primatol. 2005;34:51–66. doi: 10.1111/j.1600-0684.2005.00092.x. [DOI] [PubMed] [Google Scholar]
- 8.Du Toit DF, Heydenrych J, Smit B, van der Merwe E, Louw G, Zuurmond T, Els D, Weideman A, Du Toit L, Wolfe-Coote S. Islet cell function in long-term surviving primates after segmental pancreatic allotransplantation. J Surg Oncol. 1988;38:63–70. doi: 10.1002/jso.2930380116. [DOI] [PubMed] [Google Scholar]
- 9.Fabbrini E, Higgins PB, Magkos F, Bastarrachea RA, Voruganti VS, Comuzzie AG, Shade RE, Gastaldelli A, Horton JD, Omodei D, Patterson BW, Klein S. Metabolic response to high-carbohydrate and low-carbohydrate meals in a nonhuman primate model. Am J Physiol Endocrinol Metab. 2013;304:E444–E451. doi: 10.1152/ajpendo.00347.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fiorentino TV, Owston M, Abrahamian G, La Rosa S, Marando A, Perego C, Di Cairano ES, Finzi G, Capella C, Sessa F, Casiraghi F, Paez A, Adivi A, Davalli A, Fiorina P, Guardado Mendoza R, Comuzzie AG, Sharp M, DeFronzo RA, Halff G, Dick EJ, Folli F. Chronic continuous exenatide infusion does not cause pancreatic inflammation and ductal hyperplasia in non-human primates. Am J Pathol. 2015;185:139–150. doi: 10.1016/j.ajpath.2014.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goodner CJ, Koerker DJ, Weigle DS, McCulloch DK. Decreased insulin- and glucagon-pulse amplitude accompanying beta-cell deficiency induced by streptozocin in baboons. Diabetes. 1989;38:925–931. doi: 10.2337/diab.38.7.925. [DOI] [PubMed] [Google Scholar]
- 12.Gozalo AS, Zerfas PM, Starost MF, Lambert LE, Elkins WR. Pancreatic endocrine tumour with disseminated pulmonary thromboembolism in an owl monkey (Aotus nancymae) J Comp Pathol. 2013;149:132–136. doi: 10.1016/j.jcpa.2012.11.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guardado-Mendoza R, Davalli AM, Chavez AO, Hubbard GB, Dick EJ, Majluf-Cruz A, Tene-Perez CE, Goldschmidt L, Hart J, Perego C, Comuzzie AG, Tejero ME, Finzi G, Placidi C, La Rosa S, Capella C, Halff G, Gastaldelli A, DeFronzo RA, Folli F. Pancreatic islet amyloidosis, beta-cell apoptosis, and alpha-cell proliferation are determinants of islet remodeling in type-2 diabetic baboons. Proc Natl Acad Sci U S A. 2009;106:13992–13997. doi: 10.1073/pnas.0906471106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guardado-Mendoza R, Dick EJ, Jimenez-Ceja LM, Davalli A, Chavez AO, Folli F, Hubbard GB. Spontaneous pathology of the baboon endocrine system. J Med Primatol. 2009;38:383–389. doi: 10.1111/j.1600-0684.2009.00384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guardado-Mendoza R, Jimenez-Ceja L, Majluf-Cruz A, Kamath S, Fiorentino TV, Casiraghi F, Velazquez AO, DeFronzo RA, Dick E, Davalli A, Folli F. Impact of obesity severity and duration on pancreatic β- and α-cell dynamics in normoglycemic non-human primates. Int J Obes (Lond) 2013;37:1071–1078. doi: 10.1038/ijo.2012.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hobson DJ, Turner PV. Spontaneous pancreatic islet cell tumor in a black and white colobus monkey (Colobus guereza kikuyuensis) J Med Primatol. 2008;37(Suppl 1):11–15. doi: 10.1111/j.1600-0684.2007.00259.x. [DOI] [PubMed] [Google Scholar]
- 17.Klimstra DSAR, Capella C, Hruban RH, Kloppel G, Komminoth P, Solcia E, Rindi G. WHO Classification of Tumours of the Digestive System. Lyon, France: International Agency for Research on Cancer; 2010. [Google Scholar]
- 18.Lowenstine L. Primates: The Road to Self-Sustaining Populations. K (ed) Springer-Verlag; 1985. Neoplasms and proliferative disorders in nonhuman primates; pp. 781–814. [Google Scholar]
- 19.McClure HM, Chandler FW. A survey of pancreatic lesions in nonhuman primates. Vet Pathol Suppl. 1982;7:193–209. [PubMed] [Google Scholar]
- 20.Oberg K, Eriksson B. Endocrine tumours of the pancreas. Best Pract Res Clin Gastroenterol. 2005;19:753–781. doi: 10.1016/j.bpg.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 21.Ryder RE. The potential risks of pancreatitis and pancreatic cancer with GLP-1-based therapies are far outweighed by the proven and potential (cardiovascular) benefits. Diabet Med. 2013;30:1148–1155. doi: 10.1111/dme.12301. [DOI] [PubMed] [Google Scholar]
- 22.Sadaria MR, Hruban RH, Edil BH. Advancements in pancreatic neuroendocrine tumors. Expert Rev Gastroenterol Hepatol. 2013;7:477–490. doi: 10.1586/17474124.2013.811058. [DOI] [PubMed] [Google Scholar]
- 23.Sibley CG, Ahlquist JE. DNA hybridization evidence of hominoid phylogeny: results from an expanded data set. J Mol Evol. 1987;26:99–121. doi: 10.1007/BF02111285. [DOI] [PubMed] [Google Scholar]
- 24.VandeBerg JL, Williams-Blangero S. Advantages and limitations of nonhuman primates as animal models in genetic research on complex diseases. J Med Primatol. 1997;26:113–119. doi: 10.1111/j.1600-0684.1997.tb00042.x. [DOI] [PubMed] [Google Scholar]


