Abstract
Chronic kidney disease (CKD) is a global healthcare burden affecting billions of individuals worldwide. The kidney has limited regenerative capacity from chronic insults, and for the most common causes of CKD, no effective treatment exists to prevent progression to end-stage kidney failure. Therefore, novel interventions, such as regenerative cell-based therapies, need to be developed for CKD. Given the risk of allosensitization, autologous transplantation of cells to boost regenerative potential is preferred. Therefore, verification of cell function and vitality in CKD patients is imperative. Two cell types have been most commonly applied in regenerative medicine. Endothelial progenitor cells contribute to neovasculogenesis primarily through paracrine angiogenic activity and partly by differentiation into mature endothelial cells in situ. Mesenchymal stem cells also exert paracrine effects, including pro-angiogenic, anti-inflammatory, and anti-fibrotic activity. However, in CKD, multiple factors may contribute to reduced cell function, including older age, coexisting cardiovascular disease, diabetes, chronic inflammatory states, and uremia, which may limit the effectiveness of an autologous cell-based therapy approach. This review highlights current knowledge on stem and progenitor cell function and vitality, aspects of the uremic milieu that may serve as a barrier to therapy, and novel methods to improve stem cell function for potential transplantation.
Keywords: stem cells, end-stage renal disease, senescence, uremia
INTRODUCTION
Chronic kidney disease (CKD), affecting 8–16% of the population worldwide, is now viewed as part of the rising global non-communicable disease burden1, 2. Given the growing aging population, CKD growth rate is expected to increase exponentially, particularly in the United States and other developed countries. Beyond primary prevention measures, the most common causes of CKD, including diabetes and hypertension, have no effective intervention to stop progression to end-stage kidney failure. The increased morbidity, mortality, and costs of healthcare utilization inherent with initiation of renal replacement therapy thereafter serve as substantial motivation for pursuit of novel therapeutic interventions to prevent or delay progression to end stage renal disease (ESRD)3–8.
Cell-based regenerative therapy is being extensively evaluated as an alternative treatment modality for many with no other treatment options9. Targeting the underlying disease process and boosting the endogenous reparative capacity may reset the clock at least temporarily, allowing structural and functional restoration of the diseased kidney (Figure 1). To achieve this, the chief cell types under investigation are stem and progenitor cells. Endothelial progenitor cells (EPCs), which are derived from the bone-marrow and circulate in the peripheral blood, contribute to neovasculogenesis primarily through the secretion of paracrine angiogenic cytokines, and possibly partly by differentiation into mature endothelial cells in situ. Mesenchymal stem cells (MSCs), isolated from a variety of tissues, are non-embryonic stem cells that possess the ability to differentiate along three main cell lineages. Nevertheless, through paracrine activity on other cell types, MSCs maintain anti-fibrotic, anti-inflammatory, and pro-angiogenic properties, which modulate inflammation, immune activation, and neovasculogenesis. Clinical trials using MSCs have been initiated for acute kidney injury, polycystic kidney disease, and kidney transplantation, but only one trial has been registered for the indication of CKD [Clinicaltrials.gov]10–14. Notably, a recent randomized, multicenter, double-blind, placebo-controlled study applying allogenic MSC for treatment of acute kidney injury in cardiac surgery patients was terminated early due to lack of benefit15, 16.
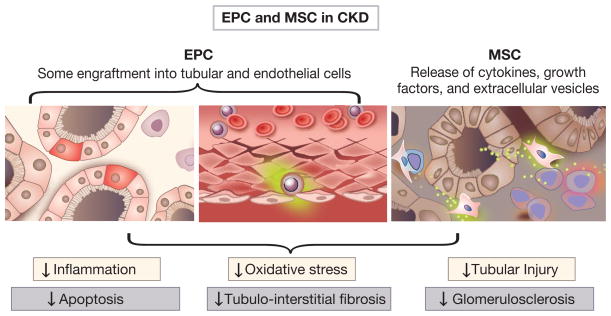
Figure 1.
Endothelial progenitor cell (EPC) and mesenchymal stem cell (MSC) reparative functions in chronic kidney disease. Both cell types possess the capacity to release a variety of cytokines, growth factors, and extracellular vesicles. Cells home to the injured tissue, engraft (although incorporation in kidney structures is often modest), and promote neoangiogenesis. Cumulative effects of the EPC and MSC result in multiple beneficial effects including decreased inflammation, oxidative stress, and apoptosis. Structural effects include minimization of tubular injury, tubulointerstitial fibrosis, and potentially glomerulosclerosis.
Preclinical studies provide ample support for use of EPC and MSC in CKD17–23. In a systematic review and meta-analysis of 71 articles in animal models, Papazova et al20 found that cell-based therapy reduced development and progression of CKD. This was evidenced by decreases in urinary protein and urea, which structurally associated with glomerulosclerosis and interstitial fibrosis. However, direct evidence from glomerulosclerosis and interstitial fibrosis analyses was incomplete. Additionally, Li et al24 found that human MSCs prevented hyperglycemia-induced podocyte apoptosis and injury, and other investigations have shown that in animal models of diabetic nephropathy, MSCs reduced glomerulosclerosis and oxidative stress18, 20, 25, 26. We also previously demonstrated the beneficial effect of EPC and MSC transplantation in a porcine model of renovascular hypertension, wherein intrarenal delivery of cells attenuated renovascular hypertension-induced myocardial injury and decreased renal injury19, 27, 28
Adequate cell functionality and vitality is critical for the success of autotransplantation, Autotransplantation is preferred over allogeneic transplantation, to decrease the risk of allosensitization, particularly in patients that may eventually require kidney transplantation. However, while EPCs and MSCs were identified as the most effective cell types for CKD therapy20, these cells themselves are not impervious to damage and wear. Cell loss and dysfunction may manifest by impaired circulating cell count and pro-angiogenic or anti-inflammatory functionality. Reduced vitality is characterized by premature senescence and increased apoptosis. Cellular senescence is an age-related decline in response to stress and damage originating from exogenous (e.g., disease or oxidative stress) and endogenous (e.g., DNA damage) sources, leading to an irreversible proliferation arrest29–32. Senescence is characterized by cell cycle arrest, telomere shortening, and altered cellular morphology, and is most apparent in disease states and advanced aging, wherein environmental stressors reduce cellular vitality and functionality. Affected cells develop a senescence-associated secretory phenotype, producing excessive inflammatory cytokines, extracellular matrix-modifying proteases, and reactive oxygen species, which impair neighboring cell function and alter tissue structure that may contribute to chronic injury.31, 33–35 Apoptosis, programmed death of damaged cells, aims to minimize necrosis-induced damage, yet nonetheless leads to cellular loss. Notably, CKD may increase cellular propensity for senescence and death. Characterized by a high prevalence of older age, multiple morbidities such as diabetes, hypertension and other cardiovascular diseases, and a uremic milieu, CKD encompasses a poor microenvironment for both harvesting and transplantation of cells, thereby potentially limiting their overall effectiveness in cell-based therapy for CKD.
This review summarizes the relevant evidence regarding EPC and MSC functionality and vitality in the setting of CKD, as well as the associated mechanisms, which may serve as a barrier to therapy. We further provide an overview of promising future strategies to optimize cell function for autotransplantation in cell-based treatments.
Endothelial progenitor cells
Asahara et al36 first isolated endothelial progenitor cells in 1997, and illustrated that these circulating CD34+ cells contributed to angiogenesis. EPCs primarily originate from the bone marrow, and upon expansion in an endothelial-permissive milieu, can adopt an endothelial-like phenotype. They circulate in and patrol the peripheral blood, and home to injured tissues, where they release angiogenic factors and extracellular vesicles that stimulate the local endothelium, promoting angiogenesis and contributing to vascular repair and regeneration37. A small fraction of EPCs may differentiate into mature endothelial cells and incorporate into the damaged endothelium to replace or support existing endothelial cells in forming new blood vessels.
Although the validity of specific EPC surface markers or means to reliably measure EPC levels have been debated, circulating bone marrow-derived cells positive for the surface markers CD34, vascular endothelial growth factor receptor-2 (VEGFR2, or kinase insert domain receptor), and negative for the inflammatory cell marker CD45, are often considered as EPC. Cells with both CD34 and CD133 positivity represent an early (“immature”) circulating EPC population with a potential to develop into mature endothelial cells in-vivo38, 39. The divergent phenotypes of EPC subpopulations applied over the years makes comparison of EPC studies somewhat challenging, given the important prognostic significance associated with these cells for cardiovascular events among patients with cardiovascular disease (CVD)40–42. Nevertheless, low EPC subpopulation numbers or impairment of EPC function consistently associates with the presence of CVD and risk factors43–47.
The primary functional feature of EPC consists of angiogenic potency to induce vascular repair. Angiogenic functionality is demonstrated through secretion of growth factors, as well as their proliferation, migration, and tube formation in in-vitro assays or when injected to murine models. In addition, their ability to home to sites of vascular or tissue injury allowing for both re-endothelization and neovascularization is an important functional feature that can be assessed in-vitro by their migration capacity (in response to VEGF or other chemotactic molecules) and cell-cell or cell-matrix adhesion39, 48. Finally, these cells possess anti-inflammatory capacity, and we have shown their ability to suppress the prevalence of pro-inflammatory macrophages19, 49.
Over the past decade, exogenous administration of EPC has been successfully used in experimental models of CKD. Previous studies have shown that mobilization of EPC contributes to endothelial repair in the kidney immediately after ischemia-reperfusion50, 51. In agreement, we have found in swine renovascular disease that a single intrarenal delivery of autologous EPC improves the renal microvasculature, protecting the stenotic kidney17, 19, 52. Furthermore, intrarenal delivery of autologous EPC in conjunction to renal revascularization restores the hemodynamics, function, and oxygenation of the stenotic swine kidney49, 53, suggesting a therapeutic potential for EPC in preserving the kidney in chronic experimental renovascular disease.
Mesenchymal stem cells
First isolated and characterized by Friedenstein and colleagues in 197454, 55, MSCs have emerged as ideal candidates for cell-based therapies for preservation of the human kidney. Unlike EPCs that are isolated from the bone marrow or peripheral mononuclear cells and cultured for several weeks before transplantation, MSCs can be harvested from a variety of tissues including adipose tissue, bone marrow, skin, and skeletal muscle, and are easily expanded in-vitro within a relatively short period of time56. Minimal criteria for defining MSCs include trilineage differentiation potential (adipocytes, chondrocytes, and osteocytes), plastic-adherence under standard culture conditions, expression of CD29, CD44, CD73, CD90, CD105, and CD166, and no expression of CD45, CD34, CD14 or CD11b, CD79α or CD19 and HLA-DR surface molecules 57.
Importantly, MSCs secrete several growth factors and cytokines that modulate adjacent parenchymal cells, triggering tissue regeneration. Like EPC, MSCs release extracellular vesicles that contain a combination of mRNA and miRNA capable of regulating transcription of genetic information and modulating angiogenesis, inflammation, and other pathways in recipient cells58, 59. Although slightly less potent than EPC, swine adipose tissue-derived MSCs have pro-angiogenic properties, supporting their ability to preserve the renal microvasculature19. Moreover, MSCs possess pronounced immunomodulatory properties that promote tissue repair and decrease inflammation. We have shown that cultured MSCs induce a shift in macrophage phenotype from inflammatory (M1) to reparative (M2), underpinning their anti-inflammatory properties60. Likewise, MSCs inhibit lymphocyte proliferation via the secretion of interleukin (IL)-10 and Fas ligand61. Finally, MSCs lack co-stimulatory molecules such as CD40, CD80, and CD86, which evoke an allogeneic T-cell immune response 62. Therefore, the lower risk of rejection of immunoprivileged MSCs promoted development of off-the-shelf products with the hope for feasible allogeneic administration, although this application remains limited.
The ability of MSCs to preserve renal structure and function has been demonstrated in experimental CKD20, 63, as their administration preserved renal function and attenuated renal injury in several rodent models of diabetic nephropathy 64, partial nephrectomy 65, and chronic allograft nephropathy66. We have shown that intrarenal delivery of adipose tissue-derived MSCs attenuated in swine renovascular disease stenotic kidney injury and dysfunction despite sustained hypertension19, 60. Intrarenal delivery of MSCs in conjunction with renal revascularization also restores renal hemodynamics and function and decreases hypoxia, inflammation, apoptosis, oxidative stress, microvascular loss, and fibrosis 28, 67. Notably, intrarenal delivery of MSCs during reversal of experimental renovascular hypertension subsequently improves cardiac function, uncovering their therapeutic potential to preserve the heart68. Finally, MSCs excellent safety record 65, 69, and their unique immunomodulatory properties and promising results from experimental studies eventually led to their approval by the U.S. Food and Drug Administration for treatment of steroid-resistant graft-versus-host disease, the only stem cell-based drug approval.
EPC and MSC function and vitality in CKD: Human studies
EPC have been thoroughly evaluated in CKD patients, whereas similar studies assessing MSC in this population are lacking (Table). With the exception of one early study70, a large body of evidence shows that EPC number is impaired in patients with CKD45, 47, 48, 71–85. Although different subpopulations have been studied, primarily in patients receiving dialysis, decreased EPC numbers (30–50% lower than in healthy controls) were consistently demonstrated in both CKD and dialysis-dependent states of ESRD40, 86. In addition, EPC function (tube formation, cellular adhesion, and migratory capacity in-vitro) is generally decreased in comparison to healthy controls. Some studies explored the differing effects of renal replacement therapy through dialysis modality (conventional hemodialysis, peritoneal dialysis, nocturnal dialysis) or kidney transplantation on EPC number and function48, 73, 81, 84, 87, 88. Interestingly, augmented efficiency of clearance of uremia appears to restore EPC number and functionality. Fewer studies investigated EPC number and function in patients with CKD45, 71, 73, 74, 77, 80, 84, 89, and reported that even small reductions in glomerular filtration rate (GFR) led to significantly lower EPC numbers compared to healthy controls. In the largest cohort of patients with CKD, Chen et al45 supported earlier findings by Jie et al77, arguing against correlation of the stage of non-dialysis CKD with EPC number. Interestingly, a sharp fall in EPC number was observed at the transition from normal GFR (in healthy controls) to early CKD, providing the impetus to intervene with cell-based therapy even at the early stage of CKD.
Table.
Endothelial progenitor (EPC) and mesenchymal stem cell (MSC) studies in human subjects with chronic kidney disease (CKD)
| Reference | Publication year | Primary aim | CKD-Dialysis Group | Demographics | Age group |
|---|---|---|---|---|---|
| Bahlmann et al.71 | 2003 | Effect of darbepoetin alfa on EPC differentiation, proliferation, and tube formation. | CKD | 8 (5M, 3F) | Adult |
| Bahlmann et al.89 | 2004 | Effect of erythropoietin on EPC mobilization and tube formation. | CKD | 11 (6M, 5F) | Adult |
| Chan et al. (a)48 | 2005 | Effect of uremic clearance by NHD on EPC number and migratory function vs. CHD. | Dialysis-CHD | 12 (8M, 4F) | Adult |
| Chan et al. (b) | 2005 | Effect of uremic clearance by NHD on EPC number and migratory function vs. CHD | Dialysis-NHD | 10 (7M, 3F) | Adult |
| Chen et al. (a)45 | 2013 | Relationship between CKD severity and EPC number. | CKD | 121 (combined:100M, 66F) | Adult |
| Chen et al. (b) | 2013 | Relationship between CKD severity and EPC number. | Dialysis-? | 45 (combined:100M, 66F) | Adult |
| Choi et al.72 | 2004 | Relationship between CHD and EPC number, migratory function, tube formation. | Dialysis-CHD | 44 (44M,0F) | Adult |
| deGroot et al. (a)74 | 2004 | Relationship of uremia and EPC number, differentiation, tube formation, migration. | CKD | 46 (29M, 17F) | Adult |
| deGroot et al (b) | 2004 | Relationship of uremia and EPC number, differentiation, tube formation, migration. | Dialysis-CHD | 6 (5M, 1F) | Adult |
| de Groot et al. (a)73 | 2005 | Relationship between kidney transplant function, and EPC number and differentiation. | Kidney Transplant | 74 (46M, 28F) | Adult |
| de Groot et al. (b) | 2005 | Relationship between kidney transplant function, and EPC number and differentiation. | CKD | 29 (19M, 10F) | Adult |
| Eizawa et al.75 | 2003 | Relationship between CHD and EPC number. | Dialysis CHD | 50 (35M, 15F) | Adult |
| Herbrig et al.70 | 2004 | Relationship between CHD and EPC number and migratory function. | Dialysis-CHD | 20 (14M, 6F) | Adult |
| Herbrig et al.88 | 2006 | Effect of uremic clearance after KTx on EPC number and migratory function. | Dialysis-CHD and PD transition to KTx | 20 (10M, 10F) | Adult |
| Jie et al.77 | 2010 | Relationship between CKD and EPC number and migratory function. | CKD | 49 (22M, 27F) | Adult |
| Jie et al. (a)76 | 2010 | Relationship between CKD, CHD and EPC number and migratory function. | Dialysis Peds | 13 (9M, 3F) | Pediatric |
| Jie et al. (b) | 2010 | Relationship between CKD, CHD and EPC number and migratory function. | CKD Peds | 15 (9M, 6F) | Pediatric |
| Jourde-Chiche et al.78 | 2009 | Relationship between CHD and EPC number. | Dialysis-CHD | 38 (20M, 18F) | Adult |
| Kohagura K. et al.79 | 2008 | Relationship of recombinant human erythropoietin dose in CHD and EPC number. | Dialysis CHD | 35 (18M, 17F) | Adult |
| Krenning et. al. (a)80 | 2009 | Relationship between CKD, CHD and EPC number and proliferation. | CKD | 30 (22M, 8F) | Adult |
| Krenning et. al. (b) | 2009 | Relationship between CKD, CHD and EPC number and proliferation. | Dialysis-CHD and PD | 20 (16M, 4F) | Adult |
| Krieter et. al.81 | 2010 | Effect of differing middle molecule removal in CHD on EPC number. | Dialysis-Low/high flux, HDF | 18 (15M, 3F) | Adult |
| Manfredini et al.165 | 2009 | Effect of exercise in CHD patients on EPC number. | Dialysis-CHD | 30 (20M, 10F) | Adult |
| Maruyama et al.82 | 2008 | Relationship between EPC number in CHD with cardiovascular events and mortality. | Dialysis-CHD | 216 (122M, 94F) | Adult |
| Park et al.164 | 2010 | Effect of green tea consumption in CKD patients on EPC number. | Dialysis-? | 40 (?M,?F) | Adult |
| Schlieper G et al.83 | 2008 | Relationship between cardiovascular disease, CHD and EPC number and migration. | Dialysis CHD | 65 (32M, 33F) | Adult |
| Steiner et al.87 | 2005 | Relationship between PD and EPC number. | Dialysis-PD | 38 (28M, 10F) | Adult |
| Ueno et al. (a)84 | 2010 | Relationship between PD, HD and EPC number. | Dialysis-PD | 67 (45M, 22F) | Adult |
| Ueno et al. (b) | 2010 | Relationship between PD, HD and EPC number. | Dialysis-HD | 142 (104M, 38F) | Adult |
| Ueno et al.46 | 2011 | Relationship between CHD and EPC number. | Dialysis-CHD | 212 (127M, 85F) | Adult |
| Westerweel et al.85 | 2007 | Relationship between CHD and EPC number and tube formation. | Dialysis CHD | 45 (30M, 15F) | Adult |
| Zhao et al.47 | 2014 | Relationship between CHD and EPC proliferation, migratory function, tube formation. | Dialysis-CHD | 15 (9M, 6F) | Adult |
| Reinders et al.91 | 2013 | Relationship between CKD5 and dialysis and MSC differentiation, proliferation, gene expression, cytokine and angiogenic factors, and immune suppression capacity. | Dialysis-PD, HD and CKD5 | 10 (7M, 3F) | Adult |
| Roemeling-van Rhijn et. al.90 | 2012 | Relationship between PD, HD, CKD and MSC differentiation, proliferation, and immunomodulatory function. | Dialysis-PD, HD and CKD | 16 (10M, 6F) | Adult |
| Yamanaka et. al.92 | 2014 | Relationship between dialysis and MSC differentiation, proliferation, function, hypoxic response, angiogenesis activation, gene expression, and senescence. | Dialysis-? | 9 (7M, 2F) | Adult |
To illustrate varying subject enrollment, some references were divided into (a) and (b) groupings.
Abbreviations: CHD: conventional hemodialysis, NHD: nocturnal hemodialysis, PD: peritoneal dialysis, HDF: hemodiafiltration, KTx: Kidney transplantation; ?: unknown data (not provided by authors).
EPC number: generally refers to the circulating number of EPC
Alterations in EPC vitality in CKD, such as the frequency of senescent or apoptotic cells relative to normal or viable cells, have not been fully established. A pattern of increased apoptosis has been identified in dialysis patients, but is yet to be confirmed in subsets of non-dialysis CKD patients81, 85. Overall, the vast body of evidence suggests that EPC number and function are impaired in CKD patients, whereas cellular senescence and apoptosis in this patient population require further studies.
In comparison to EPC, substantially fewer studies have assessed MSC function in human subjects with CKD or ESRD (Table 1). Roemeling-van Rhijn et al90 showed in 16 ESRD patients that adipose-derived MSC proliferative rates were similar to healthy controls, but their vitality was not fully evaluated. Similarly, Reinders et al91 demonstrated that bone marrow-derived MSC from 10 ESRD patients were phenotypically and functionally similar to healthy controls, supporting the feasibility of autologous clinical application. Yamanaka et al92 determined that long-term uremic conditions in 9 ESRD patients led to persistent and systematic downregulation of gene and protein expression of p300/CBP-associated factor, which regulates differentiation, angiogenesis, and gluconeogenesis, as well as poor in-vivo angiogenic potency of adipose-derived MSCs. Given the paucity of human studies of MSC function, much extrapolation on cellular function in CKD is derived from animal models. In bone marrow-derived MSCs harvested from CKD rats, Klinkhammer et al93 found increased senescence, reduced proliferation capacity, accumulation of actin, and a modulated secretion profile. Noh et al94 induced uremia in a CKD mouse model and demonstrated decreased expression of VEGFs its receptor-1, and stromal cell-derived factor-1α, as well as increased cellular senescence, decreased proliferation, and impaired in-vitro tube formation of bone marrow-derived MSC. Taken together, there is conflicting evidence regarding MSC functionality and vitality in CKD states. However, there is a clear need for additional investigations in human subjects to determine whether MSC function and vitality are altered in the noxious milieu that characterizes CKD.
Barriers to therapy: Mechanisms underlying impaired EPC and MSC function and vitality in CKD
Mechanisms by which CKD impairs EPC and MSC function could involve factors common to many pathogenic mechanisms activated by CVD risk factors. In particular in CKD, important roles are ascribed to factors such as inflammation, activation of the renin-angiotensin-aldosterone system, increased oxidative stress, endothelial dysfunction, atherosclerosis, and other CKD-associated conditions (e.g., erythropoietin deficiency, metabolic acidemia, hyperhomocysteinemia, uremic toxins) (Figure 2). Levels of EPCs are believed to be a surrogate marker for vascular function and cumulative CVD risk. A low number of EPCs may reflect a depletion of EPC supply due to either increased demand (e.g., persistent, ongoing endothelial damage), kidney sequestration in the setting of vascular injury95, or impaired EPC mobilization44, 48, 96. EPC supply/demand disparity and endothelial dysfunction, which is common in CKD97, 98, might both contribute to CKD-induced CVD risk99, and vice versa. In the largest study of dialysis patients to date, low circulating EPC was an independent and significant predictor for cardiovascular events and all-cause mortality82. Moreover, emerging evidence suggests that cardiovascular risk factors may in fact elicit a preponderance of EPC capable of contributing to the complications of CVD. For example, EPC bearing osteogenic markers (OCN) have been identified in patients with early CVD, and postulated to contribute to vascular calcifications100–102. Whether a similar population of calcifying EPC might be involved in the increased propensity for calcification observed in patients with CKD is yet to be determined.
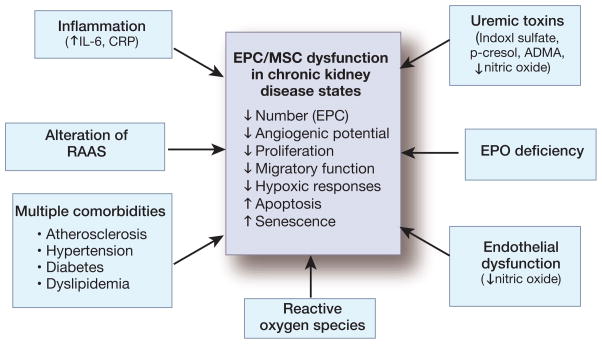
Figure 2.
Causative factors for endothelial progenitor (EPC) and mesenchymal stem cell (MSC) dysfunction in chronic kidney disease (CKD). In the setting of CKD, EPC and MSC become functionally impaired, and EPC demonstrate a reduction in the number of circulating cells. Moreover, the cell vitality might decrease, demonstrated by apoptosis and cellular senescence. A variety of factors shown to contribute to these findings are listed above.
IL-6: interleukin-6, CRP: C-reactive protein, RAAS: renin-angiotensin-aldosterone system, EPO: erythropoietin, ADMA: asymmetric dimethylarginine.
Uremic toxins may also contribute to EPC or MSC depletion and malfunction via endothelial dysfunction observed in CKD. For example, a decrease in bioavailability of nitric oxide (NO), which participates in release of EPC from the bone marrow103, can decrease EPC mobilization in CVD and CKD. Increased reactive oxygen species (ROS) and chronic inflammation secondary to CKD104, 105, dialysis106, or comorbidities107–112 also impair EPC function. Moreover, uremic toxins and aging may exert epigenetic and transcriptional modulation and thereby contribute to pathogenesis in CKD113–117. Finally, co-existing diabetes mellitus in patients with CKD may substantially reduce autologous treatment efficacy using MSC118, 119.
Toxicity of the uremic milieu
Several uremic toxins have been implicated as contributors to endothelial dysfunction, including homocysteine120, advanced glycation end products121, p-cresylsulfate, and indoxyl sulfate122, 123, all of which may indirectly contribute to depletion of EPCs allotted to repair the abnormal endothelium in CKD104. A progressive decline in kidney function leads to progressive accumulation of compounds of differing chemical and physical composition, which are normally efficiently excreted by healthy kidneys. Prominent among the uremic toxins are urea, guanidines, p-cresol, indoles, homocysteine, and advanced glycosylation end (AGE) products. Many of these toxins promote oxidative stress and reduced availability of NO, thereby promoting vascular damage and excess CVD found in patients with CKD124, 125. Urea, a small water-soluble uremic compound, is linked to survival among dialysis-dependent patients 126. Its biochemical effect has not been well demonstrated, but may include increased ROS production127. Guanidines, p-cresols, and indoxylsulfate are among the most frequently studied protein-bound toxins in patients with ESRD. Guanidines are small water-soluble compounds, which are metabolites of L-arginine, the substrate for NO synthesis. Guanidine compounds include creatinine, creatine, asymmetric dimethylarginine (ADMA) and symmetric dimethylarginine (SDMA). Various guanidines are shown to have pro-inflammatory activities by leukocyte proliferation stimulation128, 129. ADMA, often elevated in ESRD, is an inhibitor of NO synthase, marker of endothelial dysfunction, and associated with several adverse vascular outcomes130–133. Although previously considered biologically inactive, recent studies illustrated a role of SDMA in the inflammatory process of CKD, wherein monocytes incubated with SDMA showed increased IL-6 and tumor necrosis factor-α expression134, a rise in active nuclear factor-κB, and inhibition of endothelial NO synthase135. P-cresols are protein-bound, intestinally-derived end-products of tyrosine and phenylalanine catabolism. Their main in-vivo metabolite p-cresylsulfate, exerts pro-inflammatory activity exhibited by increased proportion of leukocytes displaying oxidative burst activity, and has been identified as a predictor of survival in CKD136, 137. Indols, also protein-bound compounds, are produced by intestinal flora as metabolites of tryptophan. Indoxyl sulfate and p-cresol inhibit endothelial proliferation and wound repair contributing to endothelial dysfunction in CKD122. Moreover, indoxyl sulfate induces ROS production by endothelial cells123, and microparticle release, and is associated with vascular disease and mortality in CKD patients104, 123, 136. The amino acid homocysteine associates with CV events in both the CKD and the general population138, with pathogenic mechanisms including up-regulation of multiple genes associated with atherogenesis, increased vascular smooth muscle cell proliferation, C-reactive protein production, endothelial dysfunction, and oxidative damage139. Finally, AGE’s, the products of a non-enzymatic reaction between reducing sugars and amino groups of proteins, lipids, and nucleic acids, accelerate aging of macromolecules and also contribute to endothelial dysfunction140. In the setting of hyperglycemic (diabetes) or oxidative stress (kidney failure) conditions, production and/or accumulation of AGE rise. AGEs stimulate leukocyte activation, inhibit NO synthase, form cross-links between molecules in the basement membrane of the extracellular matrix, and engage the receptor for AGE, resulting in ROS generation and invoking an inflammatory response thereby contributing to the endothelial dysfunction and vascular damage provoked by other uremic toxins140–143. Each of these uremic retention substances, individually or cumulatively, contribute to ongoing endothelial dysfunction through alteration of NO availability and increased ROS production, which may deplete EPC number and impair function of both EPC and MSC. Moreover, direct effects of uremic toxins likely play a major role in their deleterious effect on EPC and MSC.
Effect of uremic toxins on EPCs and MSCs
In-vitro studies have greatly increased our understanding of the effect of uremic toxins on EPC and MSC. de Groot et al74 demonstrated decreased number of circulating EPC in patients with advanced CKD, and their uremic sera inhibited differentiation and migration of healthy EPC. Yet, Jourde-Chiche et al78 found that uremic toxic substances (p-cresylsulfate, indoxylsulfate, indole-3 acetic acid, β-2 microglobulin, and homocysteine) did not induce apoptosis of myeloid EPC from healthy controls, whereas, Wu et al144 determined that indoxyl sulfate inhibited angiogenic function and increased senescence and autophagy of EPC collected from patients with acute kidney injury. Taken together, these data suggest that EPC from patients with kidney disease might be more susceptible to the deleterious effect of uremic toxins. In non-CKD models, homocysteine impairs the homing to injured vasculature, proliferative, migratory, adhesive and in-vitro vasculogenesis capacity of EPC145, 146, and both p-cresol147 and ADMA148, 149 attenuate angiogenic function and proliferation of EPC.
Fewer recent studies assessed the in-vitro effect of uremia or uremic toxins in CKD on MSCs. Kramann et al150, 151 found that uremic serum induced an osteoblast-like phenotype and enhanced MSC proliferation and vascular wall remodeling, but not apoptosis. Lanza et al114 also found a propensity towards osteogenic differentiation of human bone marrow-derived MSC when exposed to either uremic sera or multiple uremic toxins, which likely contributes to bone modification in patients with CKD. Izdiak et al152 showed that p-cresol and indoxyl sulfate decreased human bone marrow-MSC functionality and vitality in vitro, but despite cell membrane damage, apoptosis was not increased. Contrarily, homocysteine-treated MSC manifest increased apoptosis153. Noh et al154 showed that p-cresol induced MSC dysfunction by impairing insulin-induced elevation of hypoxia-inducible factor-1α. These studies demonstrated the potential inhibitory effect of uremia on adequate MSC function in the setting of uremia, which does not necessarily lead to apoptosis. However, more studies are needed to identify specific treatment targets to improve MSC and EPC function.
Strategies to improve EPC and MSC function in CKD
Given that EPC become dysfunctional during the course of a number of prevalent diseases, means for optimization of EPC number and function have been actively sought155. Among the best-documented drugs are 3-hydroxy-3-methylglutaryl-coenzyme-A reductase inhibitors, which increase EPC mobilization and function and reduce apoptosis44, 156–158. Vasa et al44 reported a three-fold increase in circulating progenitor cells after patients with CAD were treated with statins for four weeks, and several randomized trials159 showed a statin-induced increase in EPC number ranging from 25.8% to 223.5%. Similarly, Wu et al144, using a NO-releasing statin, reversed the NO-dependent negative effects of indoxyl sulfate on EPCs in-vitro. Other drugs commonly used in CVD and CKD, such as angiotensin-converting enzyme inhibitors, also lead to mobilization of EPCs, possibly through stimulation of NO activity and a reduction in oxidative stress. Similarly, angiotensin-2 receptor blockers may directly affect EPCs through peroxisome proliferator-activated receptor-γ receptor activity. Figure 3 outlines several drugs shown to affect EPC155, including erythropoietin stimulating agents71, 89, calcium channel blockers160, biguanides (metformin) without or with thiazolidinedione (pioglitazone)161, 162, and dipeptidyl peptidase-4 inhibitors (sitagliptin)163. However, some interventions aimed at improving EPC function in dialysis patients, like green tea consumption164, exercise165, or differing middle molecule removal81, had no discernible effect. Finally, as previously mentioned, optimal clearance of uremic toxins through effective renal replacement therapy, such as kidney transplantation or nocturnal hemodialysis48, 73, is associated with EPC mobilization and improved function, yet is not a uniformly viable option for all ESRD patients.
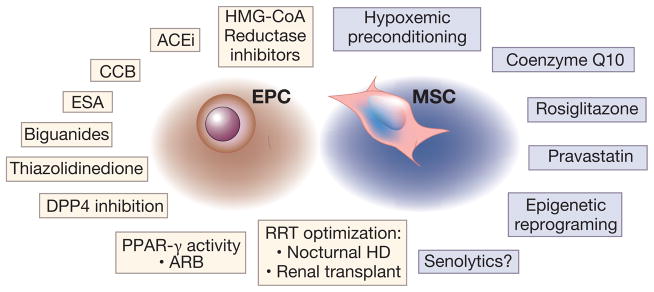
Figure 3.
Preconditioning treatments, pharmacological agents, and other interventions with potential to improve endothelial progenitor (EPC, highlighted in tan boxes) and mesenchymal stem cell (MSC, highlighted in blue boxes) function for stem cell transplantation in chronic kidney disease. Given the EPC and MSC dysfunction in varying disease states, several studies have examined the beneficial effects of drugs and interventions that increase their function and number.
HMG-CoA: 3-hydroxy-3-methylglutaryl-coenzyme-A, ACEi: angiotensin-converting enzyme inhibitors, CCB: calcium channel blockers, ESA: erythropoietin stimulating agent, DPP4: dipeptidyl peptidase-4 inhibitors, PPAR: peroxisome proliferator-activated-receptor, ARB: angiotensin-2 receptor blockers. RRT: renal replacement therapy
Drugs or interventions that may improve function and reduce senescence or apoptosis in MSC include hypoxic and other preconditioning measures166–168, pravastatin169, rosiglitazone170, 171 or coenzyme Q10172 therapies, and epigenetic regulation173, 174. Both preconditioned MSC and EPC have shown improved cell survival, homing to injured sites, and paracrine activities. Finally, given the toxicity of cells acquiring senescence-associated secretory phenotype, their removal with senolytic agents could optimize function and structure of neighboring cells175. To our knowledge, the potential effect of senolytics on MSC or EPC function is yet to be fully explored.
Future directions
Greater investment is needed for better understanding of EPC and MSC function in a diverse group of individuals with varying CKD etiology, race/ethnic, gender, or age groups. For example, African-American hypertensive patients manifest elevated number of circulating pro-inflammatory endothelial cells positive for the inflammatory marker, vascular adhesion-protein-1176. Studies are needed to determine whether comparable phenotypic changes are observed in MSC or EPC of specific population sub-groups. Identification of such harmful cells may facilitate development of targeted treatment modalities to eliminate or improve their function. Furthermore, EPC or MSC function might hypothetically be differently affected in patients with differing CKD etiologies, such as renal-limited IgA nephropathy relative to systemic disease like diabetic nephropathy, despite comparable changes in GFR. This would imply that therapeutic approach cannot be decided based on GFR alone. Additionally, preconditioning protocols may need to be implemented not only on cells in-vitro, but also in-vivo, as a means to optimize the microenvironment in which they will ultimately be implanted.
Several issues in stem cell dysfunction remain unresolved. A documented fall in MSC number and/or function would likely substantiate the motivation to apply their replenishment in CKD. While a decrease in EPC number and function has been well recognized in CKD (Table), the fate of MSC remains to be defined. In as much as MSC derived from different sources (e.g., skin, fat, bone marrow, etc.) are representative of the ubiquitous MSC population177, their availability and functionality might reflect on the endogenous reparative capacity of kidney resident MSC. However, given that MSC are often expanded prior to evaluation, their enumeration is less straightforward than that of circulating EPC. Furthermore, while cell-based therapy has been shown to attenuate tubular and vascular injury, its ability to reverse established glomerulosclerosis remains to be shown. Lastly, some of the paracrine activity of both EPC and MSC is mediated by release of membrane microparticles, which carry genetic and protein cargo derived from their parent cells. Being non-replicating, microparticles are not subject to apoptosis and senescence, and might therefore prove to have a longer lasting impact, as long as local proliferation of delivered vectors is not mandatory for tissue repair. Nonetheless, studies are needed to determine the required residence time of cellular or non-cellular elements, and whether cells derived from subjects with CKD pack harmful (e.g., pro-inflammatory) cargo within the microparticles that they release.
The choice between EPC vs. MSC might be considered based on disease etiology. Kidney diseases associated with predominant microvascular loss would likely benefit from the prominent pro-angiogenic properties of EPC, whereas those characterized by a strong inflammatory or immune components might gain from MSC therapy. Hopefully, future studies will outline specific recommendations for delivery regimens.
CONCLUSION
Regenerative medicine provides new hope for a means to change the trajectory of CKD. In planning for cell-based therapy for CKD, special considerations should be given to patient-related factors that may limit the efficacy of MSC and EPC. Clearly, more studies in humans, particularly of MSC from patients with CKD, are needed to assess the feasibility and efficacy of this approach, as well as the window of opportunity to intervene, prevent, or reverse kidney damage. Advancement in the field is somewhat burdened by expense and expertise of the research laboratories, need for standardization of methodology for cell characterization and cell growth medium, and heterogeneity of the CKD patient population. Nevertheless, identification of means to optimize cell function and/or the microenvironment in which the cells will be transplanted, are of utmost importance as we move into this burgeoning era of treatment.
Acknowledgments
Partly supported by NIH grants DK73608, HL123160, DK102325, DK106427, and DK106427, the Mayo Clinic Center for Regenerative Medicine, and the Satellite Healthcare Foundation.
L.J.H. was supported by the Mayo Clinic Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery.
Footnotes
DISCLOSURE
L.O.L. is on the Advisory Board of Stealth Biopharmaceuticals, Inc.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
LaTonya J. Hickson, Email: Hickson.latonya@mayo.edu.
Alfonso Eirin, Email: EirinMassat.Alfonso@mayo.edu.
Lilach O. Lerman, Email: lerman.lilach@mayo.edu.
References
- 1.Jha V, Garcia-Garcia G, Iseki K, et al. Chronic kidney disease: global dimension and perspectives. Lancet. 2013;382:260–272. doi: 10.1016/S0140-6736(13)60687-X. [DOI] [PubMed] [Google Scholar]
- 2.USRDS USRDS. Annual Data Report. Vol. 2015. Bethesda: 2014. Chapter 1: CKD in the General Population. 2014 Annual Data Report: Epidemiology of Kidney Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. p Chronic Kidney Disease chapter. [Google Scholar]
- 3.Hickson LJ, Cosio FG, El-Zoghby ZM, et al. Survival of patients on the kidney transplant wait list: relationship to cardiac troponin T. Am J Transplant. 2008;8:2352–2359. doi: 10.1111/j.1600-6143.2008.02395.x. [DOI] [PubMed] [Google Scholar]
- 4.Hickson LJ, El-Zoghby ZM, Lorenz EC, et al. Patient survival after kidney transplantation: relationship to pretransplant cardiac troponin T levels. Am J Transplant. 2009;9:1354–1361. doi: 10.1111/j.1600-6143.2009.02636.x. [DOI] [PubMed] [Google Scholar]
- 5.Schoonover KL, Hickson LJ, Norby SM, et al. Risk factors for hospitalization among older, incident haemodialysis patients. Nephrology (Carlton) 2013;18:712–717. doi: 10.1111/nep.12129. [DOI] [PubMed] [Google Scholar]
- 6.USRDS USRDS. Annual Data Report. Vol. 2015. Bethesda: 2014. ESRD in the United States: An Overview of USRDS Annual Data Report Volume 2. 2014 Annual Data Report: Epidemiology of Kidney Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. p ESRD Chapter. [Google Scholar]
- 7.Foley RN, Chen SC, Solid CA, et al. Early mortality in patients starting dialysis appears to go unregistered. Kidney Int. 2014;86:392–398. doi: 10.1038/ki.2014.15. [DOI] [PubMed] [Google Scholar]
- 8.Bradbury BD, Fissell RB, Albert JM, et al. Predictors of early mortality among incident US hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study (DOPPS) Clin J Am Soc Nephrol. 2007;2:89–99. doi: 10.2215/CJN.01170905. [DOI] [PubMed] [Google Scholar]
- 9.Services. UDoHaH. 2020: A New Vision-A Future for Regenerative Medicine. Washington, DC: Dept Health and Human Services; 2006. [Google Scholar]
- 10.Tan J, Wu W, Xu X, et al. Induction therapy with autologous mesenchymal stem cells in living-related kidney transplants: a randomized controlled trial. Jama. 2012;307:1169–1177. doi: 10.1001/jama.2012.316. [DOI] [PubMed] [Google Scholar]
- 11.Westenfelder C, Togel FE. Protective actions of administered mesenchymal stem cells in acute kidney injury: relevance to clinical trials. Kidney Int Suppl (2011) 2011;1:103–106. doi: 10.1038/kisup.2011.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clinicaltrials.gov. Clinicaltrials.gov. Vol. 2015. U.S. National Institues of Health; 2014. Mesenchymal Stem Cells Transplantation in Patients With Chronic Renal Failure Due to Polycystic Kidney Disease. [Google Scholar]
- 13.Clinicaltrials.gov. Autologous Bone Marrow Derived Mesenchymal Stromal Cells (BM-MSCs) in Patients With Chronic Kidney Disease (CKD) Vol. 2015. Clinicaltrials.gov a service of the U.S. National Institutes of Health; 2014. pp We will assess the 18-month safety and potential efficacy of autologous MSCs as a therapy for CKD. A total of 10 patients with CKD IV injection of high doses 12×106/kg of autologous MSCs t, which will be derived from biopsies of their bone marrow. Assessments will be performed at 101, 103, 106, 112 and 118 months after cell injection. Changes in Glomerular Filtration Rate (GFR) were evaluated by scan isotope. [Google Scholar]
- 14.Peng Y, Ke M, Xu L, et al. Donor-derived mesenchymal stem cells combined with low-dose tacrolimus prevent acute rejection after renal transplantation: a clinical pilot study. Transplantation. 2013;95:161–168. doi: 10.1097/TP.0b013e3182754c53. [DOI] [PubMed] [Google Scholar]
- 15.Clinicaltrials.gov. A Study to Evaluate the Safety and Efficacy of AC607 for the Treatment of Kidney Injury in Cardiac Surgery Subjects (ACT-AKI) Vol. 2015. Clinicaltrials.gov a service of the U.S. National Institues of Health; 2014. [Google Scholar]
- 16.2014 KW: ASN Kidney Week 2014 News Release: High impact clinical trials yield results that could lead to improved kidney care. 2014;2015 [Google Scholar]
- 17.Chade AR, Zhu X, Lavi R, et al. Endothelial progenitor cells restore renal function in chronic experimental renovascular disease. Circulation. 2009;119:547–557. doi: 10.1161/CIRCULATIONAHA.108.788653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fang Y, Tian X, Bai S, et al. Autologous transplantation of adipose-derived mesenchymal stem cells ameliorates streptozotocin-induced diabetic nephropathy in rats by inhibiting oxidative stress, pro-inflammatory cytokines and the p38 MAPK signaling pathway. Int J Mol Med. 2012;30:85–92. doi: 10.3892/ijmm.2012.977. [DOI] [PubMed] [Google Scholar]
- 19.Zhu XY, Urbieta-Caceres V, Krier JD, et al. Mesenchymal stem cells and endothelial progenitor cells decrease renal injury in experimental swine renal artery stenosis through different mechanisms. Stem Cells. 2013;31:117–125. doi: 10.1002/stem.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Papazova DA, Oosterhuis NR, Gremmels H, et al. Cell-based therapies for experimental chronic kidney disease: a systematic review and meta-analysis. Dis Model Mech. 2015;8:281–293. doi: 10.1242/dmm.017699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee SR, Lee SH, Moon JY, et al. Repeated administration of bone marrow-derived mesenchymal stem cells improved the protective effects on a remnant kidney model. Ren Fail. 2010;32:840–848. doi: 10.3109/0886022X.2010.494803. [DOI] [PubMed] [Google Scholar]
- 22.van Koppen A, Joles JA, Bongartz LG, et al. Healthy bone marrow cells reduce progression of kidney failure better than CKD bone marrow cells in rats with established chronic kidney disease. Cell Transplant. 2012;21:2299–2312. doi: 10.3727/096368912X636795. [DOI] [PubMed] [Google Scholar]
- 23.van Koppen A, Joles JA, van Balkom BW, et al. Human embryonic mesenchymal stem cell-derived conditioned medium rescues kidney function in rats with established chronic kidney disease. PLoS One. 2012;7:e38746. doi: 10.1371/journal.pone.0038746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li D, Wang N, Zhang L, et al. Mesenchymal stem cells protect podocytes from apoptosis induced by high glucose via secretion of epithelial growth factor. Stem Cell Res Ther. 2013;4:103. doi: 10.1186/scrt314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Castiglione RC, Maron-Gutierrez T, Barbosa CM, et al. Bone marrow-derived mononuclear cells promote improvement in glomerular function in rats with early diabetic nephropathy. Cell Physiol Biochem. 2013;32:699–718. doi: 10.1159/000354473. [DOI] [PubMed] [Google Scholar]
- 26.Ezquer F, Ezquer M, Simon V, et al. Endovenous administration of bone-marrow-derived multipotent mesenchymal stromal cells prevents renal failure in diabetic mice. Biol Blood Marrow Transplant. 2009;15:1354–1365. doi: 10.1016/j.bbmt.2009.07.022. [DOI] [PubMed] [Google Scholar]
- 27.Eirin A, Zhu XY, Ebrahimi B, et al. Intra-renal delivery of mesenchymal stem cells and endothelial progenitor cells attenuates hypertensive cardiomyopathy in experimental renovascular hypertension. Cell Transplant. 2014 doi: 10.3727/096368914X685582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eirin A, Zhu XY, Krier JD, et al. Adipose tissue-derived mesenchymal stem cells improve revascularization outcomes to restore renal function in swine atherosclerotic renal artery stenosis. Stem Cells. 2012;30:1030–1041. doi: 10.1002/stem.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Borodkina A, Shatrova A, Abushik P, et al. Interaction between ROS dependent DNA damage, mitochondria and p38 MAPK underlies senescence of human adult stem cells. Aging. 2014;6:481–495. doi: 10.18632/aging.100673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen J, Goligorsky MS. Premature senescence of endothelial cells: Methusaleh’s dilemma. American journal of physiology Heart and circulatory physiology. 2006;290:H1729–1739. doi: 10.1152/ajpheart.01103.2005. [DOI] [PubMed] [Google Scholar]
- 31.Zhu Y, Armstrong JL, Tchkonia T, et al. Cellular senescence and the senescent secretory phenotype in age-related chronic diseases. Curr Opin Clin Nutr Metab Care. 2014;17:324–328. doi: 10.1097/MCO.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 32.Hoffmann J, Haendeler J, Aicher A, et al. Aging enhances the sensitivity of endothelial cells toward apoptotic stimuli: important role of nitric oxide. Circ Res. 2001;89:709–715. doi: 10.1161/hh2001.097796. [DOI] [PubMed] [Google Scholar]
- 33.Tchkonia T, Morbeck DE, Von Zglinicki T, et al. Fat tissue, aging, and cellular senescence. Aging Cell. 2010;9:667–684. doi: 10.1111/j.1474-9726.2010.00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Passos JF, Nelson G, Wang C, et al. Feedback between p21 and reactive oxygen production is necessary for cell senescence. Mol Syst Biol. 2010;6:347. doi: 10.1038/msb.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tchkonia T, Zhu Y, van Deursen J, et al. Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. J Clin Invest. 2013;123:966–972. doi: 10.1172/JCI64098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 37.Urbich C, Aicher A, Heeschen C, et al. Soluble factors released by endothelial progenitor cells promote migration of endothelial cells and cardiac resident progenitor cells. J Mol Cell Cardiol. 2005;39:733–742. doi: 10.1016/j.yjmcc.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 38.Peichev M, Naiyer AJ, Pereira D, et al. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood. 2000;95:952–958. [PubMed] [Google Scholar]
- 39.Szmitko PE, Fedak PW, Weisel RD, et al. Endothelial progenitor cells: new hope for a broken heart. Circulation. 2003;107:3093–3100. doi: 10.1161/01.CIR.0000074242.66719.4A. [DOI] [PubMed] [Google Scholar]
- 40.Herbrig K, Pistrosch F, Foerster S, et al. Endothelial progenitor cells in chronic renal insufficiency. Kidney Blood Press Res. 2006;29:24–31. doi: 10.1159/000092484. [DOI] [PubMed] [Google Scholar]
- 41.Nowak G, Karrar A, Holmen C, et al. Expression of vascular endothelial growth factor receptor-2 or Tie-2 on peripheral blood cells defines functionally competent cell populations capable of reendothelialization. Circulation. 2004;110:3699–3707. doi: 10.1161/01.CIR.0000143626.16576.51. [DOI] [PubMed] [Google Scholar]
- 42.Werner N, Kosiol S, Schiegl T, et al. Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med. 2005;353:999–1007. doi: 10.1056/NEJMoa043814. [DOI] [PubMed] [Google Scholar]
- 43.Hill JM, Zalos G, Halcox JP, et al. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348:593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- 44.Vasa M, Fichtlscherer S, Aicher A, et al. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res. 2001;89:E1–7. doi: 10.1161/hh1301.093953. [DOI] [PubMed] [Google Scholar]
- 45.Chen YT, Cheng BC, Ko SF, et al. Value and level of circulating endothelial progenitor cells, angiogenesis factors and mononuclear cell apoptosis in patients with chronic kidney disease. Clin Exp Nephrol. 2013;17:83–91. doi: 10.1007/s10157-012-0664-9. [DOI] [PubMed] [Google Scholar]
- 46.Ueno H, Koyama H, Fukumoto S, et al. Advanced glycation end products, carotid atherosclerosis, and circulating endothelial progenitor cells in patients with end-stage renal disease. Metabolism. 2011;60:453–459. doi: 10.1016/j.metabol.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 47.Zhao J, Bolton EM, Randle L, et al. Functional characterization of late outgrowth endothelial progenitor cells in patients with end-stage renal failure. Transpl Int. 2014;27:437–451. doi: 10.1111/tri.12277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chan CT, Li SH, Verma S. Nocturnal hemodialysis is associated with restoration of impaired endothelial progenitor cell biology in end-stage renal disease. Am J Physiol Renal Physiol. 2005;289:F679–684. doi: 10.1152/ajprenal.00127.2005. [DOI] [PubMed] [Google Scholar]
- 49.Eirin A, Zhu XY, Li Z, et al. Endothelial outgrowth cells shift macrophage phenotype and improve kidney viability in swine renal artery stenosis. Arterioscler Thromb Vasc Biol. 2013;33:1006–1013. doi: 10.1161/ATVBAHA.113.301164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kwon O, Miller S, Li N, et al. Bone marrow-derived endothelial progenitor cells and endothelial cells may contribute to endothelial repair in the kidney immediately after ischemia-reperfusion. J Histochem Cytochem. 2010;58:687–694. doi: 10.1369/jhc.2010.956011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Patschan D, Krupincza K, Patschan S, et al. Dynamics of mobilization and homing of endothelial progenitor cells after acute renal ischemia: modulation by ischemic preconditioning. Am J Physiol Renal Physiol. 2006;291:F176–185. doi: 10.1152/ajprenal.00454.2005. [DOI] [PubMed] [Google Scholar]
- 52.Chade AR, Zhu XY, Krier JD, et al. Endothelial progenitor cells homing and renal repair in experimental renovascular disease. Stem Cells. 2010;28:1039–1047. doi: 10.1002/stem.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ebrahimi B, Li Z, Eirin A, et al. Addition of endothelial progenitor cells to renal revascularization restores medullary tubular oxygen consumption in swine renal artery stenosis. Am J Physiol Renal Physiol. 2012;302:F1478–1485. doi: 10.1152/ajprenal.00563.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Friedenstein AJ, Chailakhyan RK, Latsinik NV, et al. Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues. Cloning in vitro and retransplantation in vivo. Transplantation. 1974;17:331–340. doi: 10.1097/00007890-197404000-00001. [DOI] [PubMed] [Google Scholar]
- 55.Friedenstein AJ, Deriglasova UF, Kulagina NN, et al. Precursors for fibroblasts in different populations of hematopoietic cells as detected by the in vitro colony assay method. Exp Hematol. 1974;2:83–92. [PubMed] [Google Scholar]
- 56.Asanuma H, Meldrum DR, Meldrum KK. Therapeutic applications of mesenchymal stem cells to repair kidney injury. J Urol. 2010;184:26–33. doi: 10.1016/j.juro.2010.03.050. [DOI] [PubMed] [Google Scholar]
- 57.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 58.Eirin A, Riester SM, Zhu XY, et al. MicroRNA and mRNA cargo of extracellular vesicles from porcine adipose tissue-derived mesenchymal stem cells. Gene. 2014;551:55–64. doi: 10.1016/j.gene.2014.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Collino F, Bruno S, Incarnato D, et al. AKI Recovery Induced by Mesenchymal Stromal Cell-Derived Extracellular Vesicles Carrying MicroRNAs. J Am Soc Nephrol. 2015;26:2349–2360. doi: 10.1681/ASN.2014070710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Eirin A, Zhang X, Zhu XY, et al. Renal vein cytokine release as an index of renal parenchymal inflammation in chronic experimental renal artery stenosis. Nephrol Dial Transplant. 2014;29:274–282. doi: 10.1093/ndt/gft305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gao K, Chen Y, Wei L, et al. Inhibitory effect of mesenchymal stem cells on lymphocyte proliferation. Cell Biochem Funct. 2008;26:900–907. doi: 10.1002/cbf.1523. [DOI] [PubMed] [Google Scholar]
- 62.Le Blanc K, Ringden O. Immunomodulation by mesenchymal stem cells and clinical experience. J Intern Med. 2007;262:509–525. doi: 10.1111/j.1365-2796.2007.01844.x. [DOI] [PubMed] [Google Scholar]
- 63.Eirin A, Lerman LO. Mesenchymal stem cell treatment for chronic renal failure. Stem Cell Res Ther. 2014;5:83. doi: 10.1186/scrt472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ezquer FE, Ezquer ME, Parrau DB, et al. Systemic administration of multipotent mesenchymal stromal cells reverts hyperglycemia and prevents nephropathy in type 1 diabetic mice. Biol Blood Marrow Transplant. 2008;14:631–640. doi: 10.1016/j.bbmt.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 65.Choi S, Park M, Kim J, et al. The role of mesenchymal stem cells in the functional improvement of chronic renal failure. Stem Cells Dev. 2009;18:521–529. doi: 10.1089/scd.2008.0097. [DOI] [PubMed] [Google Scholar]
- 66.Franquesa M, Herrero E, Torras J, et al. Mesenchymal stem cell therapy prevents interstitial fibrosis and tubular atrophy in a rat kidney allograft model. Stem Cells Dev. 2012;21:3125–3135. doi: 10.1089/scd.2012.0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ebrahimi B, Eirin A, Li Z, et al. Mesenchymal stem cells improve medullary inflammation and fibrosis after revascularization of swine atherosclerotic renal artery stenosis. PLoS One. 2013;8:e67474. doi: 10.1371/journal.pone.0067474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Eirin A, Zhu XY, Ferguson CM, et al. Intra-renal delivery of mesenchymal stem cells attenuates myocardial injury after reversal of hypertension in porcine renovascular disease. Stem Cell Res Ther. 2015;6:7. doi: 10.1186/scrt541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mathiasen AB, Haack-Sorensen M, Kastrup J. Mesenchymal stromal cells for cardiovascular repair: current status and future challenges. Future Cardiol. 2009;5:605–617. doi: 10.2217/fca.09.42. [DOI] [PubMed] [Google Scholar]
- 70.Herbrig K, Pistrosch F, Oelschlaegel U, et al. Increased total number but impaired migratory activity and adhesion of endothelial progenitor cells in patients on longterm hemodialysis. Am J Kidney Dis. 2004;44:840–849. [PubMed] [Google Scholar]
- 71.Bahlmann FH, DeGroot K, Duckert T, et al. Endothelial progenitor cell proliferation and differentiation is regulated by erythropoietin. Kidney Int. 2003;64:1648–1652. doi: 10.1046/j.1523-1755.2003.00279.x. [DOI] [PubMed] [Google Scholar]
- 72.Choi JH, Kim KL, Huh W, et al. Decreased number and impaired angiogenic function of endothelial progenitor cells in patients with chronic renal failure. Arterioscler Thromb Vasc Biol. 2004;24:1246–1252. doi: 10.1161/01.ATV.0000133488.56221.4a. [DOI] [PubMed] [Google Scholar]
- 73.de Groot K, Bahlmann FH, Bahlmann E, et al. Kidney graft function determines endothelial progenitor cell number in renal transplant recipients. Transplantation. 2005;79:941–945. doi: 10.1097/00007890-200504270-00012. [DOI] [PubMed] [Google Scholar]
- 74.de Groot K, Bahlmann FH, Sowa J, et al. Uremia causes endothelial progenitor cell deficiency. Kidney Int. 2004;66:641–646. doi: 10.1111/j.1523-1755.2004.00784.x. [DOI] [PubMed] [Google Scholar]
- 75.Eizawa T, Murakami Y, Matsui K, et al. Circulating endothelial progenitor cells are reduced in hemodialysis patients. Curr Med Res Opin. 2003;19:627–633. doi: 10.1185/030079903125002379. [DOI] [PubMed] [Google Scholar]
- 76.Jie KE, Lilien MR, Goossens MH, et al. Reduced endothelial progenitor cells in children with hemodialysis but not predialysis chronic kidney disease. Pediatrics. 2010;126:e990–993. doi: 10.1542/peds.2009-3346. [DOI] [PubMed] [Google Scholar]
- 77.Jie KE, Zaikova MA, Bergevoet MW, et al. Progenitor cells and vascular function are impaired in patients with chronic kidney disease. Nephrol Dial Transplant. 2010;25:1875–1882. doi: 10.1093/ndt/gfp749. [DOI] [PubMed] [Google Scholar]
- 78.Jourde-Chiche N, Dou L, Sabatier F, et al. Levels of circulating endothelial progenitor cells are related to uremic toxins and vascular injury in hemodialysis patients. J Thromb Haemost. 2009;7:1576–1584. doi: 10.1111/j.1538-7836.2009.03540.x. [DOI] [PubMed] [Google Scholar]
- 79.Kohagura K, Ohya Y, Miyagi S, et al. rHuEPO dose inversely correlated with the number of circulating CD34+ cells in maintenance hemodialysis patients. Nephron Clin Pract. 2008;108:c41–46. doi: 10.1159/000112528. [DOI] [PubMed] [Google Scholar]
- 80.Krenning G, Dankers PY, Drouven JW, et al. Endothelial progenitor cell dysfunction in patients with progressive chronic kidney disease. Am J Physiol Renal Physiol. 2009;296:F1314–1322. doi: 10.1152/ajprenal.90755.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Krieter DH, Fischer R, Merget K, et al. Endothelial progenitor cells in patients on extracorporeal maintenance dialysis therapy. Nephrol Dial Transplant. 2010;25:4023–4031. doi: 10.1093/ndt/gfq552. [DOI] [PubMed] [Google Scholar]
- 82.Maruyama S, Taguchi A, Iwashima S, et al. Low circulating CD34+ cell count is associated with poor prognosis in chronic hemodialysis patients. Kidney Int. 2008;74:1603–1609. doi: 10.1038/ki.2008.495. [DOI] [PubMed] [Google Scholar]
- 83.Schlieper G, Hristov M, Brandenburg V, et al. Predictors of low circulating endothelial progenitor cell numbers in haemodialysis patients. Nephrol Dial Transplant. 2008;23:2611–2618. doi: 10.1093/ndt/gfn103. [DOI] [PubMed] [Google Scholar]
- 84.Ueno H, Koyama H, Fukumoto S, et al. Dialysis modality is independently associated with circulating endothelial progenitor cells in end-stage renal disease patients. Nephrol Dial Transplant. 2010;25:581–586. doi: 10.1093/ndt/gfp358. [DOI] [PubMed] [Google Scholar]
- 85.Westerweel PE, Hoefer IE, Blankestijn PJ, et al. End-stage renal disease causes an imbalance between endothelial and smooth muscle progenitor cells. Am J Physiol Renal Physiol. 2007;292:F1132–1140. doi: 10.1152/ajprenal.00163.2006. [DOI] [PubMed] [Google Scholar]
- 86.Goligorsky MS, Yasuda K, Ratliff B. Dysfunctional endothelial progenitor cells in chronic kidney disease. J Am Soc Nephrol. 2010;21:911–919. doi: 10.1681/ASN.2009111119. [DOI] [PubMed] [Google Scholar]
- 87.Steiner S, Schaller G, Puttinger H, et al. History of cardiovascular disease is associated with endothelial progenitor cells in peritoneal dialysis patients. Am J Kidney Dis. 2005;46:520–528. doi: 10.1053/j.ajkd.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 88.Herbrig K, Gebler K, Oelschlaegel U, et al. Kidney transplantation substantially improves endothelial progenitor cell dysfunction in patients with end-stage renal disease. Am J Transplant. 2006;6:2922–2928. doi: 10.1111/j.1600-6143.2006.01555.x. [DOI] [PubMed] [Google Scholar]
- 89.Bahlmann FH, De Groot K, Spandau JM, et al. Erythropoietin regulates endothelial progenitor cells. Blood. 2004;103:921–926. doi: 10.1182/blood-2003-04-1284. [DOI] [PubMed] [Google Scholar]
- 90.Roemeling-van Rhijn M, Reinders ME, de Klein A, et al. Mesenchymal stem cells derived from adipose tissue are not affected by renal disease. Kidney Int. 2012;82:748–758. doi: 10.1038/ki.2012.187. [DOI] [PubMed] [Google Scholar]
- 91.Reinders ME, Roemeling-van Rhijn M, Khairoun M, et al. Bone marrow-derived mesenchymal stromal cells from patients with end-stage renal disease are suitable for autologous therapy. Cytotherapy. 2013;15:663–672. doi: 10.1016/j.jcyt.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 92.Yamanaka S, Yokote S, Yamada A, et al. Adipose tissue-derived mesenchymal stem cells in long-term dialysis patients display downregulation of PCAF expression and poor angiogenesis activation. PLoS One. 2014;9:e102311. doi: 10.1371/journal.pone.0102311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Klinkhammer BM, Kramann R, Mallau M, et al. Mesenchymal stem cells from rats with chronic kidney disease exhibit premature senescence and loss of regenerative potential. PLoS One. 2014;9:e92115. doi: 10.1371/journal.pone.0092115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Noh H, Yu MR, Kim HJ, et al. Uremia induces functional incompetence of bone marrow-derived stromal cells. Nephrol Dial Transplant. 2012;27:218–225. doi: 10.1093/ndt/gfr267. [DOI] [PubMed] [Google Scholar]
- 95.Eirin A, Gloviczki ML, Tang H, et al. Inflammatory and injury signals released from the post-stenotic human kidney. Eur Heart J. 2013;34:540–548a. doi: 10.1093/eurheartj/ehs197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Heeschen C, Aicher A, Lehmann R, et al. Erythropoietin is a potent physiologic stimulus for endothelial progenitor cell mobilization. Blood. 2003;102:1340–1346. doi: 10.1182/blood-2003-01-0223. [DOI] [PubMed] [Google Scholar]
- 97.Miyazaki H, Matsuoka H, Itabe H, et al. Hemodialysis impairs endothelial function via oxidative stress: effects of vitamin E-coated dialyzer. Circulation. 2000;101:1002–1006. doi: 10.1161/01.cir.101.9.1002. [DOI] [PubMed] [Google Scholar]
- 98.Stam F, van Guldener C, Becker A, et al. Endothelial dysfunction contributes to renal function-associated cardiovascular mortality in a population with mild renal insufficiency: the Hoorn study. J Am Soc Nephrol. 2006;17:537–545. doi: 10.1681/ASN.2005080834. [DOI] [PubMed] [Google Scholar]
- 99.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) Jama. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 100.Flammer AJ, Gossl M, Widmer RJ, et al. Osteocalcin positive CD133+/CD34−/KDR+ progenitor cells as an independent marker for unstable atherosclerosis. Eur Heart J. 2012;33:2963–2969. doi: 10.1093/eurheartj/ehs234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gossl M, Khosla S, Zhang X, et al. Role of circulating osteogenic progenitor cells in calcific aortic stenosis. J Am Coll Cardiol. 2012;60:1945–1953. doi: 10.1016/j.jacc.2012.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gossl M, Modder UI, Gulati R, et al. Coronary endothelial dysfunction in humans is associated with coronary retention of osteogenic endothelial progenitor cells. Eur Heart J. 2010;31:2909–2914. doi: 10.1093/eurheartj/ehq373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chen YH, Lin SJ, Lin FY, et al. High glucose impairs early and late endothelial progenitor cells by modifying nitric oxide-related but not oxidative stress-mediated mechanisms. Diabetes. 2007;56:1559–1568. doi: 10.2337/db06-1103. [DOI] [PubMed] [Google Scholar]
- 104.Brunet P, Gondouin B, Duval-Sabatier A, et al. Does uremia cause vascular dysfunction? Kidney Blood Press Res. 2011;34:284–290. doi: 10.1159/000327131. [DOI] [PubMed] [Google Scholar]
- 105.Popolo A, Autore G, Pinto A, et al. Oxidative stress in patients with cardiovascular disease and chronic renal failure. Free Radic Res. 2013;47:346–356. doi: 10.3109/10715762.2013.779373. [DOI] [PubMed] [Google Scholar]
- 106.Ramirez R, Carracedo J, Merino A, et al. Microinflammation induces endothelial damage in hemodialysis patients: the role of convective transport. Kidney Int. 2007;72:108–113. doi: 10.1038/sj.ki.5002250. [DOI] [PubMed] [Google Scholar]
- 107.Callaghan MJ, Ceradini DJ, Gurtner GC. Hyperglycemia-induced reactive oxygen species and impaired endothelial progenitor cell function. Antioxid Redox Signal. 2005;7:1476–1482. doi: 10.1089/ars.2005.7.1476. [DOI] [PubMed] [Google Scholar]
- 108.Haddad P, Dussault S, Groleau J, et al. Nox2-derived reactive oxygen species contribute to hypercholesterolemia-induced inhibition of neovascularization: effects on endothelial progenitor cells and mature endothelial cells. Atherosclerosis. 2011;217:340–349. doi: 10.1016/j.atherosclerosis.2011.03.038. [DOI] [PubMed] [Google Scholar]
- 109.Tuaillon E, Jaussent I, Morena M, et al. T-Cell Activation and Malnutrition Adversely Impact on Endothelial Progenitor Cell Mobilization in Patients on Extracorporeal Maintenance Dialysis Therapy. Blood Purif. 2015;39:313–322. doi: 10.1159/000381661. [DOI] [PubMed] [Google Scholar]
- 110.Verma I, Syngle A, Krishan P. Endothelial progenitor cell biology in ankylosing spondylitis. Int J Rheum Dis. 2015;18:336–340. doi: 10.1111/1756-185X.12487. [DOI] [PubMed] [Google Scholar]
- 111.Lo Gullo A, Mandraffino G, Bagnato G, et al. Vitamin D Status in Rheumatoid Arthritis: Inflammation, Arterial Stiffness and Circulating Progenitor Cell Number. PLoS One. 2015;10:e0134602. doi: 10.1371/journal.pone.0134602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lo Gullo A, Mandraffino G, Sardo MA, et al. Circulating progenitor cells in rheumatoid arthritis: association with inflammation and oxidative stress. Scand J Rheumatol. 2014;43:184–193. doi: 10.3109/03009742.2013.836564. [DOI] [PubMed] [Google Scholar]
- 113.Kuang W, Xu X, Lin J, et al. Functional and Molecular Changes of MSCs in Aging. Curr Stem Cell Res Ther. 2015;10:384–391. doi: 10.2174/1574888x10666150211162933. [DOI] [PubMed] [Google Scholar]
- 114.Lanza D, Perna AF, Oliva A, et al. Impact of the uremic milieu on the osteogenic potential of mesenchymal stem cells. PLoS One. 2015;10:e0116468. doi: 10.1371/journal.pone.0116468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Perna AF, Luciano MG, Ingrosso D, et al. Hydrogen sulphide-generating pathways in haemodialysis patients: a study on relevant metabolites and transcriptional regulation of genes encoding for key enzymes. Nephrol Dial Transplant. 2009;24:3756–3763. doi: 10.1093/ndt/gfp378. [DOI] [PubMed] [Google Scholar]
- 116.Yamada A, Yokoo T, Yokote S, et al. Comparison of multipotency and molecular profile of MSCs between CKD and healthy rats. Hum Cell. 2014;27:59–67. doi: 10.1007/s13577-013-0082-7. [DOI] [PubMed] [Google Scholar]
- 117.Li Z, Liu C, Xie Z, et al. Epigenetic dysregulation in mesenchymal stem cell aging and spontaneous differentiation. PLoS One. 2011;6:e20526. doi: 10.1371/journal.pone.0020526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kim H, Han JW, Lee JY, et al. Diabetic mesenchymal stem cells are ineffective for improving limb ischemia due to their impaired angiogenic capability. Cell Transplant. 2014 doi: 10.3727/096368914X682792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Saad MI, Abdelkhalek TM, Saleh MM, et al. Insights into the molecular mechanisms of diabetes-induced endothelial dysfunction: focus on oxidative stress and endothelial progenitor cells. Endocrine. 2015 doi: 10.1007/s12020-015-0709-4. [DOI] [PubMed] [Google Scholar]
- 120.Kokame K, Kato H, Miyata T. Homocysteine-respondent genes in vascular endothelial cells identified by differential display analysis. GRP78/BiP and novel genes. J Biol Chem. 1996;271:29659–29665. doi: 10.1074/jbc.271.47.29659. [DOI] [PubMed] [Google Scholar]
- 121.Sengoelge G, Fodinger M, Skoupy S, et al. Endothelial cell adhesion molecule and PMNL response to inflammatory stimuli and AGE-modified fibronectin. Kidney Int. 1998;54:1637–1651. doi: 10.1046/j.1523-1755.1998.00157.x. [DOI] [PubMed] [Google Scholar]
- 122.Dou L, Bertrand E, Cerini C, et al. The uremic solutes p-cresol and indoxyl sulfate inhibit endothelial proliferation and wound repair. Kidney Int. 2004;65:442–451. doi: 10.1111/j.1523-1755.2004.00399.x. [DOI] [PubMed] [Google Scholar]
- 123.Dou L, Jourde-Chiche N, Faure V, et al. The uremic solute indoxyl sulfate induces oxidative stress in endothelial cells. J Thromb Haemost. 2007;5:1302–1308. doi: 10.1111/j.1538-7836.2007.02540.x. [DOI] [PubMed] [Google Scholar]
- 124.Neirynck N, Vanholder R, Schepers E, et al. An update on uremic toxins. Int Urol Nephrol. 2013;45:139–150. doi: 10.1007/s11255-012-0258-1. [DOI] [PubMed] [Google Scholar]
- 125.Vaziri ND. Oxidative stress in uremia: nature, mechanisms, and potential consequences. Semin Nephrol. 2004;24:469–473. doi: 10.1016/j.semnephrol.2004.06.026. [DOI] [PubMed] [Google Scholar]
- 126.Kjellstrand CM, Buoncristiani U, Ting G, et al. Short daily haemodialysis: survival in 415 patients treated for 1006 patient-years. Nephrol Dial Transplant. 2008;23:3283–3289. doi: 10.1093/ndt/gfn210. [DOI] [PubMed] [Google Scholar]
- 127.D’Apolito M, Du X, Zong H, et al. Urea-induced ROS generation causes insulin resistance in mice with chronic renal failure. J Clin Invest. 2010;120:203–213. doi: 10.1172/JCI37672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Glorieux GL, Dhondt AW, Jacobs P, et al. In vitro study of the potential role of guanidines in leukocyte functions related to atherogenesis and infection. Kidney Int. 2004;65:2184–2192. doi: 10.1111/j.1523-1755.2004.00631.x. [DOI] [PubMed] [Google Scholar]
- 129.Schepers E, Glorieux G, Dou L, et al. Guanidino compounds as cause of cardiovascular damage in chronic kidney disease: an in vitro evaluation. Blood Purif. 2010;30:277–287. doi: 10.1159/000320765. [DOI] [PubMed] [Google Scholar]
- 130.Kiechl S, Lee T, Santer P, et al. Asymmetric and symmetric dimethylarginines are of similar predictive value for cardiovascular risk in the general population. Atherosclerosis. 2009;205:261–265. doi: 10.1016/j.atherosclerosis.2008.10.040. [DOI] [PubMed] [Google Scholar]
- 131.Meinitzer A, Seelhorst U, Wellnitz B, et al. Asymmetrical dimethylarginine independently predicts total and cardiovascular mortality in individuals with angiographic coronary artery disease (the Ludwigshafen Risk and Cardiovascular Health study) Clin Chem. 2007;53:273–283. doi: 10.1373/clinchem.2006.076711. [DOI] [PubMed] [Google Scholar]
- 132.Vallance P, Leone A, Calver A, et al. Accumulation of an endogenous inhibitor of nitric oxide synthesis in chronic renal failure. Lancet. 1992;339:572–575. doi: 10.1016/0140-6736(92)90865-z. [DOI] [PubMed] [Google Scholar]
- 133.Zoccali C, Bode-Boger S, Mallamaci F, et al. Plasma concentration of asymmetrical dimethylarginine and mortality in patients with end-stage renal disease: a prospective study. Lancet. 2001;358:2113–2117. doi: 10.1016/s0140-6736(01)07217-8. [DOI] [PubMed] [Google Scholar]
- 134.Schepers E, Barreto DV, Liabeuf S, et al. Symmetric dimethylarginine as a proinflammatory agent in chronic kidney disease. Clinical journal of the American Society of Nephrology: CJASN. 2011;6:2374–2383. doi: 10.2215/CJN.01720211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bode-Boger SM, Scalera F, Kielstein JT, et al. Symmetrical dimethylarginine: a new combined parameter for renal function and extent of coronary artery disease. J Am Soc Nephrol. 2006;17:1128–1134. doi: 10.1681/ASN.2005101119. [DOI] [PubMed] [Google Scholar]
- 136.Liabeuf S, Barreto DV, Barreto FC, et al. Free p-cresylsulphate is a predictor of mortality in patients at different stages of chronic kidney disease. Nephrol Dial Transplant. 2010;25:1183–1191. doi: 10.1093/ndt/gfp592. [DOI] [PubMed] [Google Scholar]
- 137.Schepers E, Meert N, Glorieux G, et al. P-cresylsulphate, the main in vivo metabolite of p-cresol, activates leucocyte free radical production. Nephrol Dial Transplant. 2007;22:592–596. doi: 10.1093/ndt/gfl584. [DOI] [PubMed] [Google Scholar]
- 138.Heinz J, Kropf S, Luley C, et al. Homocysteine as a risk factor for cardiovascular disease in patients treated by dialysis: a meta-analysis. Am J Kidney Dis. 2009;54:478–489. doi: 10.1053/j.ajkd.2009.01.266. [DOI] [PubMed] [Google Scholar]
- 139.Pang X, Liu J, Zhao J, et al. Homocysteine induces the expression of C-reactive protein via NMDAr-ROS-MAPK-NF-kappaB signal pathway in rat vascular smooth muscle cells. Atherosclerosis. 2014;236:73–81. doi: 10.1016/j.atherosclerosis.2014.06.021. [DOI] [PubMed] [Google Scholar]
- 140.Yamagishi S, Maeda S, Matsui T, et al. Role of advanced glycation end products (AGEs) and oxidative stress in vascular complications in diabetes. Biochim Biophys Acta. 2012;1820:663–671. doi: 10.1016/j.bbagen.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 141.Goldin A, Beckman JA, Schmidt AM, et al. Advanced glycation end products: sparking the development of diabetic vascular injury. Circulation. 2006;114:597–605. doi: 10.1161/CIRCULATIONAHA.106.621854. [DOI] [PubMed] [Google Scholar]
- 142.Linden E, Cai W, He JC, et al. Endothelial dysfunction in patients with chronic kidney disease results from advanced glycation end products (AGE)-mediated inhibition of endothelial nitric oxide synthase through RAGE activation. Clinical journal of the American Society of Nephrology: CJASN. 2008;3:691–698. doi: 10.2215/CJN.04291007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Stinghen AE, Massy ZA, Vlassara H, et al. Uremic Toxicity of Advanced Glycation End Products in CKD. J Am Soc Nephrol. 2015 doi: 10.1681/ASN.2014101047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wu VC, Young GH, Huang PH, et al. In acute kidney injury, indoxyl sulfate impairs human endothelial progenitor cells: modulation by statin. Angiogenesis. 2013;16:609–624. doi: 10.1007/s10456-013-9339-8. [DOI] [PubMed] [Google Scholar]
- 145.Chen JZ, Zhu JH, Wang XX, et al. Effects of homocysteine on number and activity of endothelial progenitor cells from peripheral blood. J Mol Cell Cardiol. 2004;36:233–239. doi: 10.1016/j.yjmcc.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 146.Nelson J, Wu Y, Jiang X, et al. Hyperhomocysteinemia suppresses bone marrow CD34+/VEGF receptor 2+ cells and inhibits progenitor cell mobilization and homing to injured vasculature-a role of beta1-integrin in progenitor cell migration and adhesion. Faseb J. 2015 doi: 10.1096/fj.14-267989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ying Y, Yang K, Liu Y, et al. A uremic solute, P-cresol, inhibits the proliferation of endothelial progenitor cells via the p38 pathway. Circ J. 2011;75:2252–2259. doi: 10.1253/circj.cj-11-0046. [DOI] [PubMed] [Google Scholar]
- 148.Fleissner F, Jazbutyte V, Fiedler J, et al. Short communication: asymmetric dimethylarginine impairs angiogenic progenitor cell function in patients with coronary artery disease through a microRNA-21-dependent mechanism. Circ Res. 2010;107:138–143. doi: 10.1161/CIRCRESAHA.110.216770. [DOI] [PubMed] [Google Scholar]
- 149.Thum T, Tsikas D, Stein S, et al. Suppression of endothelial progenitor cells in human coronary artery disease by the endogenous nitric oxide synthase inhibitor asymmetric dimethylarginine. J Am Coll Cardiol. 2005;46:1693–1701. doi: 10.1016/j.jacc.2005.04.066. [DOI] [PubMed] [Google Scholar]
- 150.Kramann R, Couson SK, Neuss S, et al. Exposure to uremic serum induces a procalcific phenotype in human mesenchymal stem cells. Arterioscler Thromb Vasc Biol. 2011;31:e45–54. doi: 10.1161/ATVBAHA.111.228601. [DOI] [PubMed] [Google Scholar]
- 151.Kramann R, Couson SK, Neuss S, et al. Uraemia disrupts the vascular niche in a 3D coculture system of human mesenchymal stem cells and endothelial cells. Nephrol Dial Transplant. 2012;27:2693–2702. doi: 10.1093/ndt/gfr656. [DOI] [PubMed] [Google Scholar]
- 152.Idziak M, Pedzisz P, Burdzinska A, et al. Uremic toxins impair human bone marrow-derived mesenchymal stem cells functionality in vitro. Exp Toxicol Pathol. 2014;66:187–194. doi: 10.1016/j.etp.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 153.Cai B, Li X, Wang Y, et al. Apoptosis of bone marrow mesenchymal stem cells caused by homocysteine via activating JNK signal. PLoS One. 2013;8:e63561. doi: 10.1371/journal.pone.0063561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Noh H, Yu MR, Kim HJ, et al. Uremic toxin p-cresol induces Akt-pathway-selective insulin resistance in bone marrow-derived mesenchymal stem cells. Stem Cells. 2014;32:2443–2453. doi: 10.1002/stem.1738. [DOI] [PubMed] [Google Scholar]
- 155.Lee PS, Poh KK. Endothelial progenitor cells in cardiovascular diseases. World J Stem Cells. 2014;6:355–366. doi: 10.4252/wjsc.v6.i3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Bao XM, Wu CF, Lu GP. Atorvastatin inhibits homocysteine-induced oxidative stress and apoptosis in endothelial progenitor cells involving Nox4 and p38MAPK. Atherosclerosis. 2010;210:114–121. doi: 10.1016/j.atherosclerosis.2009.11.032. [DOI] [PubMed] [Google Scholar]
- 157.Dimmeler S, Aicher A, Vasa M, et al. HMG-CoA reductase inhibitors (statins) increase endothelial progenitor cells via the PI 3-kinase/Akt pathway. J Clin Invest. 2001;108:391–397. doi: 10.1172/JCI13152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Landmesser U, Engberding N, Bahlmann FH, et al. Statin-induced improvement of endothelial progenitor cell mobilization, myocardial neovascularization, left ventricular function, and survival after experimental myocardial infarction requires endothelial nitric oxide synthase. Circulation. 2004;110:1933–1939. doi: 10.1161/01.CIR.0000143232.67642.7A. [DOI] [PubMed] [Google Scholar]
- 159.Hibbert B, Simard T, Ramirez FD, et al. The effect of statins on circulating endothelial progenitor cells in humans: a systematic review. J Cardiovasc Pharmacol. 2013;62:491–496. doi: 10.1097/FJC.0b013e3182a4027f. [DOI] [PubMed] [Google Scholar]
- 160.de Ciuceis C, Pilu A, Rizzoni D, et al. Effect of antihypertensive treatment on circulating endothelial progenitor cells in patients with mild essential hypertension. Blood Press. 2011;20:77–83. doi: 10.3109/08037051.2010.535973. [DOI] [PubMed] [Google Scholar]
- 161.Esposito K, Maiorino MI, Di Palo C, et al. Effects of pioglitazone versus metformin on circulating endothelial microparticles and progenitor cells in patients with newly diagnosed type 2 diabetes--a randomized controlled trial. Diabetes Obes Metab. 2011;13:439–445. doi: 10.1111/j.1463-1326.2011.01367.x. [DOI] [PubMed] [Google Scholar]
- 162.Wang CH, Ting MK, Verma S, et al. Pioglitazone increases the numbers and improves the functional capacity of endothelial progenitor cells in patients with diabetes mellitus. Am Heart J. 2006;152:1051, e1051–1058. doi: 10.1016/j.ahj.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 163.Fadini GP, Boscaro E, Albiero M, et al. The oral dipeptidyl peptidase-4 inhibitor sitagliptin increases circulating endothelial progenitor cells in patients with type 2 diabetes: possible role of stromal-derived factor-1alpha. Diabetes Care. 2010;33:1607–1609. doi: 10.2337/dc10-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Park CS, Kim W, Woo JS, et al. Green tea consumption improves endothelial function but not circulating endothelial progenitor cells in patients with chronic renal failure. Int J Cardiol. 2010;145:261–262. doi: 10.1016/j.ijcard.2009.09.471. [DOI] [PubMed] [Google Scholar]
- 165.Manfredini F, Rigolin GM, Malagoni AM, et al. Exercise training and endothelial progenitor cells in haemodialysis patients. J Int Med Res. 2009;37:534–540. doi: 10.1177/147323000903700229. [DOI] [PubMed] [Google Scholar]
- 166.Wang X, Liu C, Li S, et al. Hypoxia precondition promotes adipose-derived mesenchymal stem cells based repair of diabetic erectile dysfunction via augmenting angiogenesis and neuroprotection. PLoS One. 2015;10:e0118951. doi: 10.1371/journal.pone.0118951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Wei L, Fraser JL, Lu ZY, et al. Transplantation of hypoxia preconditioned bone marrow mesenchymal stem cells enhances angiogenesis and neurogenesis after cerebral ischemia in rats. Neurobiol Dis. 2012;46:635–645. doi: 10.1016/j.nbd.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Yu SP, Wei Z, Wei L. Preconditioning strategy in stem cell transplantation therapy. Transl Stroke Res. 2013;4:76–88. doi: 10.1007/s12975-012-0251-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.van Koppen A, Papazova DA, Oosterhuis NR, et al. Ex vivo exposure of bone marrow from chronic kidney disease donor rats to pravastatin limits renal damage in recipient rats with chronic kidney disease. Stem Cell Res Ther. 2015;6:63. doi: 10.1186/s13287-015-0064-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Benvenuti S, Cellai I, Luciani P, et al. Rosiglitazone stimulates adipogenesis and decreases osteoblastogenesis in human mesenchymal stem cells. J Endocrinol Invest. 2007;30:RC26–30. doi: 10.1007/BF03350807. [DOI] [PubMed] [Google Scholar]
- 171.Contador D, Ezquer F, Espinosa M, et al. Dexamethasone and rosiglitazone are sufficient and necessary for producing functional adipocytes from mesenchymal stem cells. Exp Biol Med (Maywood) 2015 doi: 10.1177/1535370214566565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zhang D, Yan B, Yu S, et al. Coenzyme Q10 inhibits the aging of mesenchymal stem cells induced by D-galactose through Akt/mTOR signaling. Oxid Med Cell Longev. 2015;2015:867293. doi: 10.1155/2015/867293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Fu G, Ren A, Qiu Y, et al. Epigenetic regulation of osteogenic differentiation of mesenchymal stem cells. Curr Stem Cell Res Ther. 2015 doi: 10.2174/1574888x10666150528153313. [DOI] [PubMed] [Google Scholar]
- 174.Teven CM, Liu X, Hu N, et al. Epigenetic regulation of mesenchymal stem cells: a focus on osteogenic and adipogenic differentiation. Stem Cells Int. 2011;2011:201371. doi: 10.4061/2011/201371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Zhu Y, Tchkonia T, Pirtskhalava T, et al. The Achilles’ Heel of Senescent Cells: From Transcriptome to Senolytic Drugs. Aging Cell. 2015 doi: 10.1111/acel.12344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Eirin A, Zhu XY, Woollard JR, et al. Increased circulating inflammatory endothelial cells in blacks with essential hypertension. Hypertension. 2013;62:585–591. doi: 10.1161/HYPERTENSIONAHA.113.01621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006;119:2204–2213. doi: 10.1242/jcs.02932. [DOI] [PubMed] [Google Scholar]