Abstract
As the risks of tobacco use become recognized and smoking becomes stigmatized, new smokers may be increasingly driven to smoke by biological or genetic vulnerabilities rather than social desirability. Given that genetic risk for deviant proneness is shared across other psychiatric and addictive disorders, we predicted that as rates of smoking decreased through the latter half of the twentieth century, associations between smoking and psychopathology would increase. Participants (N = 25 412) from a large US study—the National Epidemiologic Survey on Alcohol and Related Conditions, NESARC—were interviewed using the Alcohol Use Disorder and Associated Disabilities Interview Schedule – DSM-IV Version (AUDADIS-IV) and classified into one of five birth cohort decades (1940s to 1980s) and three smoking history (nonsmokers, never-dependent smokers and ever-dependent smokers) groups. We found that the prevalence of smoking decreased across the five birth cohorts, but associations of smoking with drug and AUDs, attention-deficit hyperactivity disorder, bipolar disorder and antisocial personality disorder, each increased monotonically in more recently born cohorts, even after adjusting for concurrent demographic and socioeconomic changes. For drug and AUDs, increases were observed among smokers both with and without a history of nicotine dependence; for other outcomes, increases were entirely driven by nicotine-dependent smokers. Findings suggest that smokers in more recent cohorts have disproportionately high psychiatric vulnerability, and may benefit from greater mental health screenings. Differentiating between casual and dependent smokers may further help prioritize those at greatest risk. Researchers should also be aware of potential variation in psychiatric comorbidity based on cohort of birth when defining groups of smokers, to minimize confounding.
INTRODUCTION
Cigarette smoking has undergone substantial cultural shifts in the Western hemisphere through the last 100 years. The early decades of the twentieth century saw rapidly expanding use, contributed to in part by automation of cigarette manufacturing technology, growing advertising and marketing, free distribution of cigarettes during the World Wars, and increased acceptance of smoking among women.1–3 Subsequently, as tobacco became recognized as a cause of multiple cancers, cardiovascular and other disease, the tide began to shift. Beginning in the early 1960s, a series of prevention measures, including US Surgeon General’s warnings, advertising bans, stringent labeling requirements, and tax policies were introduced. In the latter half of the 20th century, smoking rates declined,4,5 from almost half of the US population in the 1950s to <20% in 2010.6 Comparable shifts in policies and use have occurred in other Western countries.7,8
These shifts raise a larger question of whether smokers today might systematically differ from those who grew up and began smoking in periods when smoking was widespread, and attitudes toward smoking more permissive. Evidence shows that population-level changes in attitudes influence individual behaviors:9,10 for example, individuals who mature in birth cohorts with more restrictive social norms related to drinking and drug use are themselves less likely to use alcohol or cannabis as adolescents and vice versa. Similarly, as adverse consequences of tobacco have become more recognized, smoking rates have fallen. However, as smoking has become increasingly viewed as a medically risky and socially undesirable behavior over the last several decades, and restrictions of smoking have proliferated in many states (for example, smoke-free workplaces and public spaces), initiation of smoking may have become increasingly concentrated within population subgroups who are intrinsically more prone to deviant behaviors.11 Previous research has indicated that individuals who use illicit substances during periods of lower prevalence compared with other periods have higher levels of deviance10 suggesting that a similar mechanism may be possible for smokers over time. If so, then because deviance proneness has genetic underpinnings and is associated with an array of other risky behaviors and externalizing disorders, smokers today may have higher associated rates of associated psychopathology than their counterparts from earlier generations when tobacco use was normative.12–14
Although a relationship between smoking a range of externalizing outcomes and disorders is long established,15–18 few studies have examined whether this relationship itself varies as a function of time. One Canadian study reported increased associations between smoking and depression in the 1990s, as compared with the 50 s and 70 s;19 however, no other psychiatric outcomes were assessed. We previously reported that the relationship between smoking and drug dependence as well as the personality trait of neuroticism increased across cohorts of smokers born in 1920s to 1980s.20 However, these studies were limited by the size and characteristics of the samples, potential lack of differentiation between cohort and age effects, and assessment of few psychiatric disorders.
In the present study, we investigate changes in associations between smoking and psychopathology across five birth cohorts of the twentieth century (1940s to 1980s), using the National Epidemiological Survey of Alcohol and Related Conditions (NESARC),21,22 a large, nationally representative US survey with detailed diagnostic assessments. In order to identify changes in associations, we model six disorders that already have well documented associations with tobacco use: drug-use disorder, alcohol-use disorder (AUD), attention-deficit hyperactivity disorder, bipolar disorder and antisocial personality disorder. We restrict our analysis to cigarette smoking, as not only is the proportion of non-cigarette tobacco users in this sample small,23 but the secular trends motivating our hypotheses may not apply to other forms of tobacco use (for example, cigars, snuff and e-cigarettes). We hypothesize that (1) smokers born in later, more recently born cohorts as compared with earlier birth cohorts will have higher psychiatric comorbidity by age 25, even after adjusting for demographic and socioeconomic changes; and (2) given the greater severity and genetic underpinnings associated with substance dependence relative to substance use,7,24,25 the gradient of change will be larger for smoker groups with, as compared to without, a history of nicotine dependence.
MATERIALS AND METHODS
Sample
The study is based on the participants who completed both Waves 1 and 2 of the National Epidemiological Survey for Alcohol and Related Conditions (NESARC).21,22 The target population was the civilian non-institutionalized population ⩾ 18 years residing in households and group quarters. African-American, Hispanics and adults 18–24 years were oversampled, with data adjusted for oversampling and household- and person-level non-response. Interviews were conducted in 2001–2002 with 43 093 participants by experienced lay interviewers with extensive training and supervision. All procedures, including informed consent, received full ethical review and approval from the US Census Bureau and the US Office of Management and Budget. The Wave 2 interview was conducted ~ 3 years later (2004–2005), with a response rate of 86.7%, reflecting 34 653 completed interviews. Wave 2 weights include a component that adjusts for non-response and demographic factors to ensure that the Wave 2 sample approximated the target population (final composite response across waves was 71%).21 We restricted our analysis to participants who completed both waves, as a broader range of diagnoses related to smoking were assessed in Wave 2.
Assessment
Participants were assessed using a structured diagnostic interview, the AUDADIS-IV.24 At Wave 1, outcomes were assessed in two time frames: past 12 months, and any time before the past 12 months. At Wave 2, the time frames included the past year, and any time before the past year but since the Wave 1 interview. Diagnoses were determined cumulatively across both time periods of both waves.
Classification of independent and dependent variables
Birth cohort
Five consecutive birth cohorts were defined based on the decade of the subject’s birth (1940s through 1980s), which was established based on age at the time of the survey (cohort = survey year − age). We excluded persons born in cohorts before 1940 to reduce potential problems of recall and survivor bias. We only considered comorbidity occurring within the first 25 years of life, in order to equalize the length of exposure across the different cohorts, and to minimize inclusion of later onset illness which often has greater etiological heterogeneity.
Smoking
Participants were divided into three mutually exclusive smoker groups based on smoking history up to age 25: (1) nonsmokers included participants who did not smoke >100 cigarettes in their life; (2) never-dependent smokers included those who smoked ⩾ 100 cigarettes, but never met Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV) criteria for nicotine dependence (ND) and (3) ever-dependent smokers; included participants who met DSM-IV ND criteria. Three hundred and two (<1%) participants who reported not smoking in one wave but were missing data at the other, or were missing data at both waves, were excluded.
To mitigate potential concerns regarding the validity of DSM-IV criteria to adequately assess nicotine dependence,26 we also created an alternative definition of ND based on whether or not participants reported using tobacco shortly after getting up in the morning. ‘Time to first cigarette’ has been found to be one of the most reliable predictors of smoking severity and clinical course,27 and correlates well with biological metabolites (for example, cotinine) of nicotine.26 The main findings of this report were replicated using this alternative classification, as detailed in the online materials.
Psychiatric diagnoses
Diagnoses were based on DSM-IV criteria. We focused on six psychiatric outcomes: drug-use disorders; AUDs; major depression; attention-deficit/hyperactivity disorder (ADHD); bipolar disorder; and antisocial personality disorder.23,28,29 For drug and AUDs, we included both abuse and dependence. For drug-use disorders, the following drug classes were included: hallucinogens, cannabis, cocaine (including crack), inhalants/solvents, heroin and nonmedical sedative, tranquilizer or stimulant use. For antisocial personality disorder, a diagnosis was made if (a) ⩾ 1 required symptom caused distress or social/occupational dysfunction, (b) there was evidence of conduct disorder before age 15 and (3) ⩾ 3 adult antisocial symptoms were present at each wave.8 For ADHD, NESARC required onset of symptoms by age 18,30,31 given concern about excluding later age symptoms.32,33 However, we also retroactively coded the disorder using DSM-IV (onset by age 7) and DSM 5 (by age 12) and the main findings did not change (see Figure 1 caption). Reliability (kappa) for these diagnoses ranges from moderate to high,23,34 and are similar to those of other interviewer and lay-person administered interviews.35 Onset age was also recorded for each diagnosis (except antisocial personality disorder, which required onset of symptoms by age 15).
Figure 1.
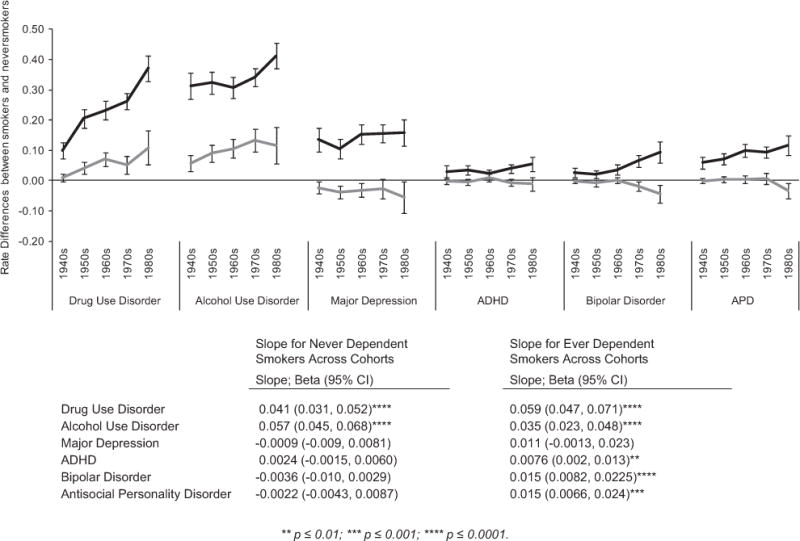
Changes in the association between smoking and psychiatric diagnoses across five birth cohorts. The figure illustrates the difference in the rates of six psychiatric diagnoses between smokers and nonsmokers across five birth cohorts (x-axis). Black lines represent trends among smokers who had a history of nicotine dependence (ND); gray lines represent smokers with no history of ND. The lines do not reflect absolute rates (those are shown in Table 2), but rather, differences in rates of psychiatric outcomes between the corresponding smoking group and the nonsmoker group. Thus, the y-axis value at any point can be interpreted as the ‘excess rate’ of the disorder that is attributed to smoking in that cohort. To formally test whether the differences in outcomes between smokers and nonsmokers were changing, we calculated the slope of each curve (bottom panel of figure; see Materials and Methods for details). A positive coefficient of the slope indicates that the differences in psychiatric outcome between the smoking and never-smoking groups are increasing from the earlier to the later-birth cohorts; negative slope indicates the reverse, and a non-significant slope indicates no change. For example, the 0.041 coefficient for drug-use disorder indicates that there was a 4.1% increase in rates of drug-use disorder per cohort among never-dependent smokers, compared with nonsmokers. Note that a non-significant slope does not indicate that there is no association between smoking and the psychiatric outcome; only that the association does not systematically change across time. The graph and coefficients show that for drug and alcohol-use disorders, attention-deficit/hyperactivity disorder (ADHD)1, bipolar disorder and antisocial personality disorder, risk differences between smokers and never smokers are getting larger as we move from earlier to more recently born cohorts. Furthermore, for the drug and alcohol outcomes, this is true for smokers both with and without a history of nicotine dependence. For ADHD1, bipolar and antisocial personality, however, the risk difference only increases among smokers with nicotine dependence. For major depression, we found no cohort related trends. 1Note, National Epidemiological Survey for Alcohol and Related Conditions (NESARC) required onset of symptoms by age 18 and these criteria have been used in all NESARC publications;30,31 However we also recoded the diagnoses of ADHD requiring onset by 12 (that is, consistent with DSM5; n = 564), or by age 7 (DSM-IV; N = 352). The main findings remain unchanged: in each case, associations between smoking and ADHD increased from earlier- to later-born cohorts, but for nicotine-dependent smokers only (ADHD by age 12: β = 0.006 (95% confidence interval, 0.0021, 0.0099), P = 0.0028; ADHD by age 7: β = 0.0032 (0.0001, 0.0063), P = 0.045). DSM-IV, Diagnostic and Statistical Manual of Mental Disorders, 4th Edition.
The final analytic sample included 25 142 individuals, made up of 15 359 nonsmokers, 5265 never-dependent smokers and 4518 ever-dependent smokers.
Statistical analyses
Analyses were conducted in SAS-callable SUDAAN 11.0.1 (Research Triangle Park, Durham, NC, USA). Nested crosstabs weighting for the complex survey design were used to identify rates of smoking across cohorts and of each diagnosis across smoking and cohort levels. Differences in the relationship between smoking and psychopathology across cohorts were tested by regressing the effects of smoking, cohort and their additive interaction on each psychiatric outcome. A generalized linear model with binomially distributed outcome was used. Interactions were tested on the additive (risk difference) scale in order to identify differences in disease proportions across strata attributable to smoking.11 Because the base rates of smoking themselves vary systematically across cohort, a multiplicative scale could obscure rate differences. Smokers were treated as a 3-level class variable, represented by two mutually exclusive dummy variables D1 and D2 coded as follows: nonsmokers, D1 = 0, D2 = 0 (reference group), never-dependent smokers, D1 = 1, D2 = 0; ever-dependent smokers, D1 = 0, D2 = 1. Models fitted were of the form: diagnosis = β0+β1(cohort)+β2(D1) +β3(D2)+β4(cohort × D1)+β5(cohort × D2)+β6(sex)+β7(sex × cohort) +β8(race)+β9(race × cohort)+ β10(education)+β11(education cohort) cohort)+β12(income)+β13(income × cohort). The β4 and β5 indexed whether the relationship between smoking and cohort varied by cohort, with positive contrast (and slopes shown in Figure 1) estimates reflecting increasing, and negative contrasts, decreasing associations11 between smoking and psychopathology across cohort (null hypothesis, β4 = 0 and β5 = 0). Because smoking varies across demographic strata (sex, race/ethnicity, education and household income),36,37 and we wanted to control for potential changes in these variables across cohorts, we included for each demographic variable not only a stand-alone term (that is, β6,8,10,12) but also an interaction term with cohort (β7,9,11,13). The main findings (namely, β4 and β5 coefficients) did not vary significantly based on whether we controlled or did not control for demographic variables (not shown).
RESULTS
Rates of smoking and nicotine dependence across cohorts
The rates of smoking are shown in Table 1. Overall smoking declined across the five cohorts, but the proportion of smokers with ND increased, from under a third (31%) of smokers born in the 1940s to >70% of those born in the 1980s (linear trend, P<0.0001). These patterns remained significant after adjusting for gender, race, income and education (Table 1, right-most column). There was a small but statistically significant decrease in the average age of onset of smoking and nicotine dependence across the cohorts.
Table 1.
Rates of smoking and nicotine dependence across five birth cohorts
|
Rates
|
Onset age
|
|||||
|---|---|---|---|---|---|---|
|
Any smoker
|
Never-dependent smoker
|
Ever-dependent smoker
|
% Smokers with dependence
|
Smoking
|
Nicotine dependence
|
|
| Individual cohorts | % (95% CI) | % (95% CI) | % (95% CI) | % (95% CI) | Age, years (95% CI) | Age, years (95% CI) |
| 1940s (N = 4,439) | 48.9 (47.04, 50.8) | 33.8 (32.1, 35.6) | 15.09 (13.7, 16.6) | 30.8 (28.3, 33.5) | 16.2 (16.1, 16.4) | 22.6 (22.5, 22.8) |
| 1950s (N = 5,921) | 41.8 (39.9, 43.6) | 25.04 (23.7, 26.4) | 16.7 (15.4, 18.1) | 40.03 (37.6, 42.5) | 16.0 (15.9, 16.4) | 22.5 (22.4, 22.7) |
| 1960s (N = 6,688) | 35.1 (33.5, 36.7) | 18.6 (17.6, 19.8) | 16.5 (15.0, 18.0) | 46.9 (43.9, 49.9) | 15.8 (15.7, 15.9) | 22.4 (22.3, 22.6) |
| 1970s (N = 5,788) | 39.4 (37.4, 41.3) | 13.4 (12.4, 14.4) | 26.0 (24.3, 27.8) | 66.1 (63.6, 68.5) | 15.8 (15.7, 15.9) | 22.5 (22.4, 22.6) |
| 1980s (N = 2,306) | 41.9 (39.0, 44.8) | 12.4 (10.8, 14.2) | 29.5 (27.0, 32.1) | 70.4 (66.7, 73.9) | 15.3 (15.1, 15.4) | 17.9 (17.8, 18.0) |
| Change across cohorts | Beta (95% CI)a | Beta (95% CI)a | Beta (95% CI)a | Beta (95% CI)a | Beta (95% CI)a | Beta (95% CI)a |
| Unadjusted | − 2.7 (−3.2, − 2.2)* | − 5.4 (−5.8, − 5.0)* | 2.9 (2.5, 3.2)* | 10.0 (9.7, 11.0)* | − 0.16 (−0.22, − 0.10)* | − 0.81 (−0.94, − 0.68)* |
| Adjustedb | − 2.8 (−3.3, − 2.2)* | − 5.4 (−5.7, − 5.0)* | 2.3 (1.9, 2.6)* | 9.8 (9.1, 10.6)* | − 0.15 (−0.22, − 0.09)* | − 0.67 (−0.81, − 0.053)* |
Abbreviation: CI, confidence interval.
Betas (β) reflect the linear average percent change of the respective outcome across each cohort; positive βs indicate increasing rates/age as we move from earlier to later-born cohorts; negative βs indicate decreasing rates/age. For example, the unadjusted β − 0.027 in the ‘any smoker’ column indicates that there was on average a 2.7% decrease in smoking rates per cohort, as we move from the earliest (1940s) to latest (1980s) cohorts. Note that the adjusted β is essentially unchanged (−0.028), indicating that these changes are not simply a function of concurrent demographic changes.
Beta values are adjusted for gender, race/ethnicity, income and education (unadjusted values are not adjusted for any variables).
P<0.0001.
Associations between smoking, nicotine dependence and psychopathology across cohorts
Overall frequencies (% of population) of the six psychiatric diagnoses were: drug-use disorder, 11.7%; AUD, 28.6%; major depression, 18.0%; ADHD, 2.7%; bipolar disorder, 3.6%; and antisocial personality, 4.1%; and are similar to those reported in other studies using the full NESARC sample.38
Stratified frequencies of the psychiatric disorders are shown in Table 2, stratified by birth cohort (1940s to 1980s, rows) the three smoking history groups (never smoked, smoked but never had nicotine dependence ‘never dependent’, and smoked with and had nicotine dependence ‘ever dependent’, columns). The difference in frequencies between the smoking and nonsmoking groups, adjusted for sex, race, income and education, are shown in the right-most columns of the table, and can be viewed as the ‘excess’ frequency within the specific cohort that is attributable to smoking.
Table 2.
Rates of psychiatric diagnoses by smoking history across five birth cohorts
| Psychiatric Outcome | Cohort |
Rates, % (95% CI)
|
Risk differencea
% (95% CI)
|
|||
|---|---|---|---|---|---|---|
| Never smoker | Never-dependent smoker | Ever-dependent smoker | Never-dependent smokerb | Ever-dependent smokerb | ||
| Drug-use disorder | 1940s | 1.52 (1.02, 2.28) | 4.81 (3.58, 6.42) | 13.28 (10.14, 17.20) | 0.99 (−0.23, 2.21) | 9.87 (7.13, 12.62) |
| 1950s | 5.45 (4.53, 6.54) | 14.23 (12.03, 16.76) | 29.33 (25.86, 33.05) | 4.12 (2.15, 6.09) | 20.57 (17.50, 23.64) | |
| 1960s | 7.41 (6.43, 8.53) | 18.97 (16.50, 21.72) | 34.82 (30.82, 39.05) | 6.98 (4.69, 9.28) | 23.27 (20.13, 26.42) | |
| 1970s | 6.03 (4.94, 7.35) | 18.97 (15.49, 23.02) | 34.80 (31.39, 38.37) | 5.17 (2.3, 8.05) | 26.31 (23.61, 29.00) | |
| 1980s | 7.36 (5.91, 9.13) | 33.25 (26.29, 41.04) | 51.24 (46.73, 55.74) | 10.84 (5.29, 16.39) | 37.10 (32.83, 41.37) | |
| Alcohol-use disorder | 1940s | 12.51 (10.76, 14.49) | 26.61 (23.72, 29.70) | 51.91 (46.47, 57.30) | 5.68 (2.99, 8.38) | 31.20 (26.84, 35.56) |
| 1950s | 20.24 (18.22, 22.42) | 36.48 (33.20, 39.88) | 58.76 (53.94, 63.42) | 9.07 (6.26, 11.88) | 32.28 (28.71, 35.86) | |
| 1960s | 26.99 (24.55, 29.59) | 42.64 (38.98, 46.37) | 60.72 (56.73, 64.57) | 10.58 (7.52, 13.64) | 30.76 (27.30, 34.22) | |
| 1970s | 27.03 (24.90, 29.28) | 43.99 (40.00, 54.42) | 63.14 (59.91, 66.27) | 13.39 (9.64, 17.13) | 34.19 (31.23, 37.15) | |
| 1980s | 23.67 (21.01, 26.56) | 51.21 (44.43, 57.95) | 69.48 (65.16, 73.48) | 11.61 (5.58, 17.64) | 41.21 (36.92, 45.50) | |
| MDD | 1940s | 10.27 (8.82, 11.94) | 9.97 (8.20, 12.06) | 22.98 (19.07, 27.43) | − 2.34 (−4.37, − 0.31) | 13.46 (9.56, 17.36) |
| 1950s | 14.97 (13.44, 16.64) | 14.08 (11.84, 16.66) | 25.35 (21.85, 29.20) | − 3.93 (−6.03, − 1.83) | 10.38 (7.17, 13.59) | |
| 1960s | 17.37 (15.92, 18.92) | 15.40 (13.27, 17.81) | 33.45 (29.42, 37.74) | − 3.19 (−5.44, − 0.95) | 15.23 (11.91,18.56) | |
| 1970s | 20.32 (18.74, 22.00) | 21.93 (18.28, 26.09) | 35.93 (32.35, 39.68) | − 2.8 (−5.99, 0.39) | 15.61 (12.69, 18.54) | |
| 1980s | 21.86 (18.98, 25.04) | 19.18 (14.45, 25.01) | 36.68 (32.56, 41.02) | − 5.59 (−10.7, − 0.44) | 15.88 (11.51, 20.25) | |
| ADHD | 1940s | 1.39 (0.93, 2.08) | 2.25 (1.33, 3.79) | 3.14 (2.00, 4.89) | − 0.13 (−1.23, 0.97) | 2.77 (0.56, 4.98) |
| 1950s | 1.90 (1.39, 2.60) | 2.21 (1.47, 3.31) | 5.14 (3.66, 7.17) | − 0.45 (−1.34, 0.44) | 3.52 (1.99, 5.05) | |
| 1960s | 1.87 (1.40, 2.49) | 2.15 (1.46, 3.15) | 4.73 (3.32, 6.71) | 0.94 (−0.28, 2.16) | 2.3 (0.92, 3.67) | |
| 1970s | 3.01 (2.32, 3.91) | 3.30 (1.85, 5.82) | 7.42 (5.85, 9.36) | − 0.63 (−1.77, 0.51) | 3.98 (2.53, 5.42) | |
| 1980s | 2.76 (1.95, 3.90) | 3.63 (1.89, 6.89) | 8.72 (6.46, 11.68) | − 1.14 (−3.4, 1.12) | 5.50 (3.09, 7.91) | |
| Bipolar disorder | 1940s | 0.80 (0.47, 1.36) | 1.27 (0.72, 2.24) | 4.00 (2.38, 6.65) | − 0.29 (−0.99, 0.42) | 2.62 (1.08, 4.15) |
| 1950s | 1.34 (0.98, 1.84) | 1.20 (0.73, 1.97) | 3.82 (2.58, 5.60) | − 0.86 (−1.93, 0.21) | 2.07 (0.80, 3.34) | |
| 1960s | 2.27 (1.80, 2.88) | 2.25 (1.52, 3.33) | 7.12 (5.03, 9.98) | − 0.02 (−1.02, 0.97) | 3.56 (1.96, 5.17) | |
| 1970s | 4.69 (3.91, 5.62) | 4.17 (2.64, 6.54) | 10.86 (8.73, 13.44) | − 1.97 (−3.54, − 0.41) | 6.44 (4.59, 8.29) | |
| 1980s | 6.57 (5.24, 8.21) | 5.71 (3.16, 10.13) | 15.61 (12.48, 19.35) | − 4.5 (−7.43, − 1.57) | 9.43 (5.97, 12.89) | |
| Antisocial personality disorder | 1940s | 0.96 (0.54, 1.69) | 2.15 (1.43, 3.21) | 8.17 (5.63, 11.70) | − 0.07 (−0.98, 0.84) | 5.87 (3.80, 7.93) |
| 1950s | 1.23 (0.87, 1.74) | 2.60 (1.84, 3.64) | 9.12 (7.04, 11.75) | 0.27 (−0.74, 1.28) | 7.19 (5.24, 8.96) | |
| 1960s | 1.48 (1.11, 1.97) | 4.92 (3.49, 6.91) | 12.69 (10.06, 15.89) | 0.35 (−0.85, 1.56) | 9.81 (7.72, 11.9) | |
| 1970s | 3.23 (2.58, 4.03) | 7.01 (4.99, 9.77) | 14.54 (12.36, 17.03) | 0.60 (−1.19, 2.40) | 9.34 (7.42, 11.27) | |
| 1980s | 4.03 (2.93, 5.52) | 4.52 (2.42, 8.31) | 14.50 (11.71, 17.82) | − 3.36 (−5.89, −0.83) | 11.60 (8.46, 14.73) | |
Abbreviations: ADHD, attention-deficit/hyperactivity disorder; CI, confidence interval; MDD, major depression.
Adjusted for gender, race/ethnicity, income and education.
Compared with never smokers as a reference group.
To test our main question—is the prevalence of psychopathology among smokers increasing in more recent birth cohorts?—we plotted the excess frequencies for each cohort, and formally tested whether there was a change in these proportions across cohorts (Figure 1). A positive slope indicates that the associations between smoking and psychiatric disorder are increasing across cohorts, and a negative slope, that it is weakening.
Examination of Figure 1 reveals three main patterns. First, smoking was increasingly associated with both drug- and AUDs in a linear manner as we move from earlier to more recently born cohorts. This was true among smokers with (black line) and without (gray line) a history of nicotine dependence. The coefficients (bottom of Figure 1) reflect the slope of the lines, and can be interpreted as the average increase per cohort of the risk of the psychiatric outcome that can be attributed to smoking. Thus, for drug-use disorder, the risk among smokers increased on average 4.1% per decade for never-dependent smokers, and 5.9% for dependent, smokers. For AUD, there was a 5.7% increase for never-dependent and 3.5% for dependent, smokers.
Post hoc analyses to formally test whether the findings in the dependent and never-dependent smoker groups differed from each other confirmed that for drug use, increases in smoking-attributable risks were greater in dependent smokers [β = 0.18 (95% CI: 0.0049, 0.03), P = 0.007], while for AUDs, increases in smoking-attributable risks were greater among non-dependent smokers (β = − 0.017, (−0.033, − 0.0014), P = 0.032). Further exploratory analyses found the patterns to be similar across DSM-IV defined abuse and dependence, and, in the case of drug-use disorder, across cannabis and non-cannabis drug classes (data not shown).
A second pattern was observed for ADHD, bipolar disorder and antisocial personality disorder. Here, the smoking-attributable risk also increased across the five cohorts, but only in nicotine-dependent (black line) smokers. For ADHD, there was a 0.75% average increase per birth cohort, and for bipolar and antisocial personality disorders, a 1.5% average increase. Smokers who did not report ever having nicotine dependence were indistinguishable from nonsmokers in terms of these psychiatric outcomes.
Finally, even though nicotine dependence was associated with major depression, associations did not vary systematically across the five cohorts (coefficients not statistically different from 0).
Similar patterns were observed when the data were examined in non-Hispanic White, non-Hispanic Black and Hispanic subgroups individually (Supplementary E-Table 1); other racial groups could not be examined due to low Ns.
The main findings were also retained when we re-analyzed the results using an alternate classification of nicotine dependence based on smoking shortly after getting up in the morning (see Materials and methods and Supplementary E-Table 2).
Sensitivity analysis
To minimize outlying effects of individual cohorts, we performed a series of sensitivity analyses, removing each cohort, one-by-one, from the analysis and then re-estimating the model. The coefficients, shown in Supplementary E-Table 3, are similar to those shown in Figure 1, and show that the changing associations between smoking and psychopathology did not occur due to undue influence by any single cohort.
DISCUSSION
The relationship between smoking and several psychopathologies is long established.23,28,29 We show here that this relationship itself varies over time. Specifically, smokers in more recently born cohorts had higher drug and alcohol, ADHD, bipolar and antisocial personality disorder comorbidity by the time they reached 25 years of age, and this increased comorbidity persisted after adjusting for concurrent changes in demographic or socioeconomic factors.
The largest increases in smoking-associated psychopathology were observed for drug-use disorders, where more recently born smokers were disproportionately likely to use, abuse and become dependent to one (or more) substances. In each case, having nicotine dependence increased these risks further. For ADHD, bipolar disorder and antisocial personality disorder, changes in the associations between smoking and psychopathology were observed exclusively for nicotine-dependent smokers. Individuals who reported being a smoker but never having nicotine dependence were indistinguishable from never smokers (as illustrated by the overlapping lines in Figure 1). Because nicotine enhances attention and cognition, smokers with more severe externalizing psychopathology may smoke more chronically to control their symptoms, and thus overwhelmingly present as dependent.39 In the case of ADHD, there is also some, albeit mixed, evidence that stimulant treatment may actually reinforce effects of nicotine, leading to increased tobacco consumption and craving.40,41 Increasing stimulant use may contribute to the increased associations between smoking and ADHD.
Ultimately, the relationships between smoking and psychopathology are complex and multi-directional, and our present work does not address causal mechanisms of change in these relationships. We propose that a ‘cohort effect’—that is, varying risk of an outcome by time of birth42—may account for some of the variation observed in the smoking–psychopathology relationship. Within such a framework, the birth cohort can itself be conceptualized as the risk factor, with a person’s birth year reflecting the given exposure to that risk. As we move from birth cohorts with more permissive smoking norms to those with more restrictive norms, fewer smokers emerge, but smoking groups are increasingly composed of persons who smoke despite the growing social pressures not to do so.12–14 Our finding that even as tobacco consumption decreases, the proportion of dependent smokers increases (also reported in other US population-based studies43), is broadly consistent with this model, often referred to as ‘hardening’ of smokers.44 So is our observation that not only did our findings persist after adjusting for demographic changes but that they were largely invariant to whether or not we did so. Indeed, if more recent smokers (1) are more deviance prone and have greater concentrations of behavioral traits (for example, impulsivity and antisociality) or genetic liability to dependence than earlier-born smokers,45 and (2) these liabilities are shared across other externalizing spectrum disorders,46,47 then growing psychiatric comorbidity among more recent smokers logically follows. Only molecular studies can directly test if specific genetic risk factors for nicotine dependence vary across time, and a large recent US-based study of 9000 adults born 1919–1959 hints at just that, finding that heritability of nicotine dependence increases across time,48 though in females only. More genetic studies are needed; meanwhile, a cohort framework allows us to conceptualize changing risks at an aggregate level and identify cohorts at greatest risk.
Occurring in tandem with cohort changes are period and age changes that cannot be formally disaggregated.42 Unlike a cohort effect, a period effect applies to the whole population simultaneously. In the present context, a period effect would have to impact psychiatric profiles of all smokers at a given time point regardless of when they were born. Such a model is inconsistent with our results and with the epidemiologic literature on smoking.49–51 Similarly, because we restricted our analysis framework to outcomes occurring by age 25, age effects do not plausibly account for the directionality of findings. There could, however, be age-related biases. First, recall bias could disproportionately impact earlier-born cohorts. Studies have reported varying degrees of stability for retrospectively collected smoking data.52,53 For example, earlier-born smokers may less accurately recall their smoking history. However, because the key outcome is an interaction—namely, smoking × cohort—smoking and psychopathology would have to be consistently recalled differently from each other to exert a bias. More specifically, individuals with lifetime histories of nicotine dependence would need to be more likely to under-report their psychiatric disorder histories than individuals without nicotine dependence. The possibility for that level of specificity in the recall bias is unlikely, indicating that recall bias alone is not likely to explain our results. Similarly, differential mortality or healthy participant bias could exert disproportionate effects on cohorts by selectively populating the earlier cohorts with healthier smokers due to greater tobacco-related deaths.54 This could be problematic if heavy smokers with psychiatric comorbidity have higher mortality rates than those who do not. We tried to clarify this impact with sensitivity analyses shown in Supplementary E-Table 3, which supported the main findings. However, only a prospectively designed study can comprehensively address these mechanisms.
Other limitations are noted. First, schizophrenia and other psychotic spectrum disorders were not assessed in NESARC. However, even though these may be highly associated with smoking, they are rare. The disorders we tested are more prevalent in the population and thus group differences may have greater clinical relevance. Second, study only documents changes in associations over time. Causality should not be inferred, as unmeasured factors could account for changes in the relationship between smoking and pathology. We also could not explore a number of temporal relationships of interest such as changes in quitting or length of smoking, as these were collinear with cohort. Changes in the onset sequence of the smoking × psychopathology relationship (that is, which comes first) could also not be examined as the proportion of comorbid cases where onset of the psychiatric disorder preceded initiation of smoking was too low (<10% for all disorders, except ADHD, 53%). Finally, though representative of the US, the study findings may not generalize to other countries or cultures with differing social norms and laws. They also may not apply to other forms of smoking (for example, e-cigarettes). Our study also has a number of strengths, including being based on a very large and representative US population sample, and the use of detailed and standardized diagnostic procedures.
CONCLUSIONS
The findings extend previous work20 showing that as smoking becomes less normative, psychiatric vulnerability among smokers increases. Although the current work does not address how this vulnerability is increasing, the increases cannot be accounted for solely by demographic shifts across time. The more recently born smokers thus represent a clinically high-risk cohort, and may benefit from earlier screenings for mental health outcomes, which could in turn help optimize treatment. Differentiating between individuals who smoke only periodically (for example, social smokers and chippers) from those more chronically dependent could be further helpful in identifying patterns of other psychopathology. Yet, smoking cessation guidelines have until recently largely been in the context of physical rather than mental health outcomes,55 and many medical professions have not routinely or systematically assessed smoking history.56,57
There could also be research implications. Overpopulating study groups with younger cohorts of smokers, for example, may necessitate additional controlling or matching to account for the greater expected comorbidity. Furthermore, if the different smoker cohorts vary by underlying genetic architecture, this could impact genetic studies of nicotine dependence as well. Whereas changes in genetic architecture are not tested in the present study, such changes are suggested by other work48 and by evidence for increasing assortative mating among smokers over time.58
Given that much of the research on smoking remains from the perspective of smoking as a risk factor, the present work enmeshes itself within an important and complex question: when a given behavior changes systematically over time (for example, smoking), do correlates or outcomes of that behavior change commensurately as well? Our findings of increased burden of psychopathology among more recent born smokers suggest that this is at least partly so. Further, regardless of what the cause may be of this increasing association of smoking and other psychopathology, smoking is becoming an increasingly important marker of risk. Future studies may thus be well served by incorporating assessments of psychiatric comorbidity into data collection and analysis plans, even when not part of the main study goals, so that temporal changes in smoker profiles can continue to be followed in a more systematic manner.
Supplementary Material
Acknowledgments
The authors express their thanks to Jamie Skipper for assistance with preparation of the manuscript. Dr Talati is funded by K01DA029598 and by Young Investigator NARSAD Grants from the Brain and Behavior Research Foundation. The work of Keyes and Hasin was funded by K01AA021511 and K05AA014223, respectively, and Hasin was also supported by the New York State Psychiatric Institute. None of the funding agencies had any role in study design, in the analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supplementary Information accompanies the paper on the Molecular Psychiatry website (http://www.nature.com/mp)
References
- 1.Brandt AM. The Cigarette Century: Thr Rise, Fall and Demise of the Product that Defined America. Perseus Books Group; New York, NY, USA: 2007. [Google Scholar]
- 2.Lopez AD, Collishaw NE, Piha T. A descriptive model of the cigarette epidemic in developed countries. Tob Control. 1994;3:242–247. [Google Scholar]
- 3.Milmore BK, Conover AG. Tobacco Smoking Patterns in the United States. US Government Printing Office; Washington DC: 1956. (Public Health Monograph No. 45). [Google Scholar]
- 4.Doll R. Tobacco: a medical history. J Urban Health. 1999;76:289–313. doi: 10.1007/BF02345669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murphy M, Di Cesare M. Use of an age-period-cohort model to reveal the impact of cigarette smoking on trends in twentieth-century adult cohort mortality in England and Wales. Popul Stud (Camb) 2012;66:259–277. doi: 10.1080/00324728.2012.678881. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention (CDC) Vital signs: current cigarette smoking among adults aged ≥ 18 years – United States 2005–2010. MMWR Morb Mortal Wkly Rep. 2011;60:1207–1212. [PubMed] [Google Scholar]
- 7.Brown J, West R. Smoking prevalence in England is below 20% for the first time in 80 years. BMJ. 2014;348:g1378. doi: 10.1136/bmj.g1378. [DOI] [PubMed] [Google Scholar]
- 8.Hasin D, Fenton MC, Skodol A, Krueger R, Keyes K, Geier T, et al. Personality disorders and the 3-year course of alcohol, drug, and nicotine use disorders. Arch Gen Psychiatry. 2011;68:1158–1167. doi: 10.1001/archgenpsychiatry.2011.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keyes KM, Schulenberg JE, O’Malley PM, Johnston LD, Bachman JG, Li G, et al. Birth cohort effects on adolescent alcohol use: the influence of social norms from 1976 to 2007. Arch Gen Psychiatry. 2012;69:1304–1313. doi: 10.1001/archgenpsychiatry.2012.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keyes KM, Schulenberg JE, O’Malley PM, Johnston LD, Bachman JG, Li G, et al. The social norms of birth cohorts and adolescent marijuana use in the United States, 1976–2007. Addiction. 2011;106:1790–1800. doi: 10.1111/j.1360-0443.2011.03485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rothman K, Greenland S, Lash TL. Modern Epidemiology. 3rd. Lippincott Williams & Wilkins; Philadelphia: 2008. [Google Scholar]
- 12.Stuber J, Galea S, Link BG. Smoking and the emergence of a stigmatized social status. Soc Sci Med. 2008;67:420–430. doi: 10.1016/j.socscimed.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chassin LPC, Morgan-Lopez A, Sherman SJ. Deviance proneness and adolescent smoking 1980 vs. 2001: has there been a hardening of adolescent smoking? J Appl Dev Psychol. 2007;28:264–276. [Google Scholar]
- 14.Costa ML, Cohen JE, Chaiton MO, Ip D, McDonald P, Ferrence R. “Hardcore” definitions and their application to a population-based sample of smokers. Nicotine Tob Res. 2010;12:860–864. doi: 10.1093/ntr/ntq103. [DOI] [PubMed] [Google Scholar]
- 15.Breslau N, Kilbey M, Andreski P. Nicotine dependence, major depression, and anxiety in young adults. Arch Gen Psychiatry. 1991;48:1069–1074. doi: 10.1001/archpsyc.1991.01810360033005. [DOI] [PubMed] [Google Scholar]
- 16.Oldmeadow C, Wood I, Mengersen K, Visscher PM, Martin NG, Duffy DL. Investigation of the relationship between smoking and appendicitis in Australian twins. Ann Epidemiol. 2008;18:631–636. doi: 10.1016/j.annepidem.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 17.McClave AK, McKnight-Eily LR, Davis SP, Dube SR. Smoking characteristics of adults with selected lifetime mental illnesses: results from the 2007 National Health Interview Survey. Am J Public Health. 2010;100:2464–2472. doi: 10.2105/AJPH.2009.188136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Breslau N, Novak SP, Kessler RC. Psychiatric disorders and stages of smoking. Biol Psychiatry. 2004;55:69–76. doi: 10.1016/s0006-3223(03)00317-2. [DOI] [PubMed] [Google Scholar]
- 19.Murphy JM, Horton NJ, Monson RR, Laird NM, Sobol AM, Leighton AH. Cigarette smoking in relation to depression: historical trends from the Stirling County Study. Am J Psychiatry. 2003;160:1663–1669. doi: 10.1176/appi.ajp.160.9.1663. [DOI] [PubMed] [Google Scholar]
- 20.Talati A, Wickramaratne PJ, Keyes KM, Hasin DS, Levin FR, Weissman MM. Smoking and psychopathology increasingly associated in recent birth cohorts. Drug Alcohol Depend. 2013;133:724–732. doi: 10.1016/j.drugalcdep.2013.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grant BF, Goldstein RB, Chou SP, Huang B, Stinson FS, Dawson DA, et al. Sociodemographic and psychopathologic predictors of first incidence of DSM-IV substance use, mood and anxiety disorders: results from the Wave 2 National Epidemiologic Survey on Alcohol and Related Conditions. Mol Psychiatry. 2009;14:1051–1066. doi: 10.1038/mp.2008.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grant BF, Stinson FS, Dawson DA, Chou SP, Ruan WJ, Pickering RP. Co-occurrence of 12-month alcohol and drug use disorders and personality disorders in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2004;61:361–368. doi: 10.1001/archpsyc.61.4.361. [DOI] [PubMed] [Google Scholar]
- 23.Grant BF, Hasin DS, Chou SP, Stinson FS, Dawson DA. Nicotine dependence and psychiatric disorders in the United States: results from the national epidemiologic survey on alcohol and related conditions. Arch Gen Psychiatry. 2004;61:1107–1115. doi: 10.1001/archpsyc.61.11.1107. [DOI] [PubMed] [Google Scholar]
- 24.Grant BF, Dawson DA, Stinson FS, Chou PS, Kay W, Pickering R. The Alcohol Use Disorder and Associated Disabilities Interview Schedule-IV (AUDADIS-IV): reliability of alcohol consumption, tobacco use, family history of depression and psychiatric diagnostic modules in a general population sample. Drug Alcohol Depend. 2003;71:7–16. doi: 10.1016/s0376-8716(03)00070-x. [DOI] [PubMed] [Google Scholar]
- 25.Fenton MC, Keyes K, Geier T, Greenstein E, Skodol A, Krueger B, et al. Psychiatric comorbidity and the persistence of drug use disorders in the United States. Addiction. 2012;107:599–609. doi: 10.1111/j.1360-0443.2011.03638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baker TB, Breslau N, Covey L, Shiffman S. DSM criteria for tobacco use disorder and tobacco withdrawal: a critique and proposed revisions for DSM-5. Addiction. 2012;107:263–275. doi: 10.1111/j.1360-0443.2011.03657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baker TB, Piper ME, McCarthy DE, Bolt DM, Smith SS, Kim SY, et al. Time to first cigarette in the morning as an index of ability to quit smoking: implications for nicotine dependence. Nicotine Tob Res. 2007;9:S555–S570. doi: 10.1080/14622200701673480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen LS, Saccone NL, Culverhouse RC, Bracci PM, Chen CH, Dueker N, et al. Smoking and genetic risk variation across populations of European, asian, and african american ancestry-a meta-analysis of chromosome 15q25. Genet Epidemiol. 2012;36:340–351. doi: 10.1002/gepi.21627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lasser K, Boyd JW, Woolhandler S, Himmelstein DU, McCormick D, Bor DH. Smoking and mental illness: a population-based prevalence study. JAMA. 2000;284:2606–2610. doi: 10.1001/jama.284.20.2606. [DOI] [PubMed] [Google Scholar]
- 30.Bernardi S, Faraone SV, Cortese S, Kerridge BT, Pallanti S, Wang S, et al. The lifetime impact of attention deficit hyperactivity disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) Psychol Med. 2012;42:875–887. doi: 10.1017/S003329171100153X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Alwis D, Lynskey MT, Reiersen AM, Agrawal A. Attention-deficit/hyperactivity disorder subtypes and substance use and use disorders in NESARC. Addict Behav. 2014;39:1278–1285. doi: 10.1016/j.addbeh.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Faraone SV, Biederman J, Spencer T, Mick E, Murray K, Petty C, et al. Diagnosing adult attention deficit hyperactivity disorder: are late onset and subthreshold diagnoses valid? Am J Psychiatry. 2006;163:1720–1729. doi: 10.1176/ajp.2006.163.10.1720. quiz 859. [DOI] [PubMed] [Google Scholar]
- 33.Kieling C, Kieling RR, Rohde LA, Frick PJ, Moffitt T, Nigg JT, et al. The age at onset of attention deficit hyperactivity disorder. Am J Psychiatry. 2010;167:14–16. doi: 10.1176/appi.ajp.2009.09060796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ruan WJ, Goldstein RB, Chou SP, Smith SM, Saha TD, Pickering RP, et al. The alcohol use disorder and associated disabilities interview schedule-IV (AUDADIS-IV): reliability of new psychiatric diagnostic modules and risk factors in a general population sample. Drug Alcohol Depend. 2008;92:27–36. doi: 10.1016/j.drugalcdep.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goldstein RB, Dawson DA, Chou SP, Ruan WJ, Saha TD, Pickering RP, et al. Antisocial behavioral syndromes and past-year physical health among adults in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. J Clin Psychiatry. 2008;69:368–380. doi: 10.4088/jcp.v69n0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lund M, Lund KE, Kvaavik E. Hardcore smokers in Norway 1996–2009. Nicotine Tob Res. 2011;13:1132–1139. doi: 10.1093/ntr/ntr166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Docherty G, McNeill A. The hardening hypothesis: does it matter? Tob Control. 2012;21:267–268. doi: 10.1136/tobaccocontrol-2011-050382. [DOI] [PubMed] [Google Scholar]
- 38.Hasin DS, Grant BF. The National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) Waves 1 and 2: review and summary of findings. Soc Psychiatry Psychiatr Epidemiol. 2015;50:1609–1640. doi: 10.1007/s00127-015-1088-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sousa NO, Grevet EH, Salgado CA, Silva KL, Victor MM, Karam RG, et al. Smoking and ADHD: an evaluation of self medication and behavioral disinhibition models based on comorbidity and personality patterns. J Psychiatr Res. 2011;45:829–834. doi: 10.1016/j.jpsychires.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 40.Bron TI, Bijlenga D, Kasander MV, Spuijbroek AT, Beekman AT, Kooij JJ. Long-term relationship between methylphenidate and tobacco consumption and nicotine craving in adults with ADHD in a prospective cohort study. Eur Neuropsychopharmacol. 2013;23:542–554. doi: 10.1016/j.euroneuro.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 41.Groenman AP, Oosterlaan J, Rommelse NN, Franke B, Greven CU, Hoekstra PJ, et al. Stimulant treatment for attention-deficit hyperactivity disorder and risk of developing substance use disorder. Br J Psychiatry. 2013;203:112–119. doi: 10.1192/bjp.bp.112.124784. [DOI] [PubMed] [Google Scholar]
- 42.Keyes KM, Utz RL, Robinson W, Li G. What is a cohort effect? Comparison of three statistical methods for modeling cohort effects in obesity prevalence in the United States, 1971–2006. Soc Sci Med. 2010;70:1100–1108. doi: 10.1016/j.socscimed.2009.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Breslau N, Johnson EO, Hiripi E, Kessler R. Nicotine dependence in the United States: prevalence, trends, and smoking persistence. Arch Gen Psychiatry. 2001;58:810–816. doi: 10.1001/archpsyc.58.9.810. [DOI] [PubMed] [Google Scholar]
- 44.Hughes JR. The hardening hypothesis: is the ability to quit decreasing due to increasing nicotine dependence? A review and commentary. Drug Alcohol Depend. 2011;117:111–117. doi: 10.1016/j.drugalcdep.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kendler KS, Thornton LM, Pedersen NL. Tobacco consumption in Swedish twins reared apart and reared together. Arch Gen Psychiatry. 2000;57:886–892. doi: 10.1001/archpsyc.57.9.886. [DOI] [PubMed] [Google Scholar]
- 46.Hicks BM, Schalet BD, Malone SM, Iacono WG, McGue M. Psychometric and genetic architecture of substance use disorder and behavioral disinhibition measures for gene association studies. Behav Genet. 2011;41:459–475. doi: 10.1007/s10519-010-9417-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vrieze SI, McGue M, Miller MB, Hicks BM, Iacono WG. Three mutually informative ways to understand the genetic relationships among behavioral disinhibition, alcohol use, drug use, nicotine use/dependence, and their co-occurrence: twin biometry, GCTA, and genome-wide scoring. Behav Genet. 2013;43:97–107. doi: 10.1007/s10519-013-9584-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Domingue BW, Conley D, Fletcher J, Boardman JD. Cohort effects in the genetic influence on smoking. Behav Genet. 2015 doi: 10.1007/s10519-015-9731-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oesterle S, Hawkins JD, Hill KG. Men’s and women’s pathways to adulthood and associated substance misuse. J Stud Alcohol Drugs. 2011;72:763–773. doi: 10.15288/jsad.2011.72.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Piontek D, Kraus L, Pabst A, Legleye S. An age-period-cohort analysis of cannabis use prevalence and frequency in Germany, 1990–2009. J Epidemiol Community Health. 2011;66:908–913. doi: 10.1136/jech-2011-200180. [DOI] [PubMed] [Google Scholar]
- 51.Weissman MM, Wickramaratne P, Nomura Y, Warner V, Pilowsky D, Verdeli H. Offspring of depressed parents: 20 years later. Am J Psychiatry. 2006;163:1001–1008. doi: 10.1176/ajp.2006.163.6.1001. [DOI] [PubMed] [Google Scholar]
- 52.Hayatbakhsh R, Mamun AA, Williams GM, O’Callaghan MJ, Najman JM. Early childhood predictors of early onset of smoking: a birth prospective study. Addict Behav. 2013;38:2513–2519. doi: 10.1016/j.addbeh.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 53.Twenge JM, Gentile B, DeWall CN, Ma D, Lacefield K, Schurtz DR. Birth cohort increases in psychopathology among young Americans, 1938–2007: a crosstemporal meta-analysis of the MMPI. Clin Psychol Rev. 2010;30:145–154. doi: 10.1016/j.cpr.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 54.Simon GE, VonKorff M, Ustun TB, Gater R, Gureje O, Sartorius N. Is the lifetime risk of depression actually increasing? J Clin Epidemiol. 1995;48:1109–1118. doi: 10.1016/0895-4356(95)00010-2. [DOI] [PubMed] [Google Scholar]
- 55.Hughes JR, Weiss RD. Are differences in guidelines for the treatment of nicotine dependence and non-nicotine dependence justified? Addiction. 2009;104:1951–1957. doi: 10.1111/j.1360-0443.2009.02561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ferketich AK, Khan Y, Wewers ME. Are physicians asking about tobacco use and assisting with cessation? Results from the 2001–2004 national ambulatory medical care survey (NAMCS) Prev Med. 2006;43:472–476. doi: 10.1016/j.ypmed.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 57.Thorndike AN, Stafford RS, Rigotti NA. US physicians’ treatment of smoking in outpatients with psychiatric diagnoses. Nicotine Tob Res. 2001;3:85–91. doi: 10.1080/14622200020032132. [DOI] [PubMed] [Google Scholar]
- 58.Agrawal A, Heath AC, Grant JD, Pergadia ML, Statham DJ, Bucholz KK, et al. Assortative mating for cigarette smoking and for alcohol consumption in female Australian twins and their spouses. Behav Genet. 2006;36:553–566. doi: 10.1007/s10519-006-9081-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


