Abstract
Objective
Substance use disorders are highly comorbid with and contribute to the increased prevalence of neurocognitive dysfunction observed in HIV infection. Despite their adverse impact on everyday functioning, there are currently no compensatory-based neurorehabilitation interventions validated for use among HIV+ substance users (HIV/SUD). This study examined the effectiveness of Goal Management Training (GMT) alone or GMT as part of a metacognitive training among HIV/SUD individuals with executive dysfunction.
Methods
Ninety HIV/SUD individuals were randomized to a single 15-minute session: 1) GMT (n=30); 2) GMT plus metacognitive training (neurocognitive awareness; GMT+Meta; n=30); or 3) active control (n=30). Following a brief neurocognitive battery and study condition, participants performed a complex laboratory-based function task, Everyday Multitasking Test (Everyday MT), during which metacognition (awareness) was evaluated.
Results
There was an increasing, but nonsignificant tendency for better Everyday MT performances across study conditions (Control≤GMT≤GMT+Meta; ps<0.08). Post-hoc analyses showed that GMT and GMT+Meta groups demonstrated small benefits (d=0.20–0.27) compared to the control arm but did not differ from one another (ds<0.10). When GMT groups were combined, there were significant medium effect-size benefits in Everyday MT performance and metacognitive task appraisals as compared to the control condition. Among participants who underwent GMT, benefits were most prominent in persons with poorer pre-training dual-tasking ability, depression, and methamphetamine use disorders (ds=0.35–1.04).
Conclusions
A brief compensatory strategy has benefits for everyday multitasking and metacognition among HIV+ substance users with executive dysfunction. Future work exploring more intensive trainings, potentially complimentary to other restorative approaches and/or pharmacological treatments, is warranted.
Keywords: Awareness, Executive Functions, Activities of Daily Living, Compensatory Strategies
Introduction
Substance use disorders (SUD) are among the most prevalent comorbidities in HIV disease and are associated with higher frequencies of medical (e.g., cardiovascular disease), psychiatric (e.g., major depressive disorder), and neurocognitive disorders that result in a greatly increased risk of mortality and morbidity than their HIV-only age-matched peers (Lewden, 2010). Both HIV+ and SUD independently, preferentially disrupt frontostriatal circuits (e.g., Langford, Hurford, Hashimoto, Digicaylioglu, & Masliah, 2005; Koob & Volkow, 2010) and together, there have been several mechanisms identified by which substance use may potentiate HIV-associated neural dysfunction (e.g., dopamine transporter dysfunction, increased inflammation/neurotoxicity; Purohit, Rapaka, & Shurtleff, 2011; Carey et al., 2006).
Downstream, HIV-associated neurocognitive disorders (HAND) are indeed disproportionately represented among drug users compared to non-users. Up to 60% of HIV/SUD individuals evidence at least mild-to-moderate levels of impairment in both early and chronic stages of the disease (e.g., Rippeth et al., 2004; Weber, Morgan, et al., 2013), with higher-order executive dysfunction as a predominant characteristic (Heaton et al., 2011; Scott et al., 2007). Not surprisingly, HIV/SUD individuals with such higher-order deficits demonstrate the highest prevalence of related everyday functioning difficulties compared to either singly affected population. For example, Blackstone and colleagues (2013) found that up to 69% of HIV+ methamphetamine users met criteria for global functional dependence (e.g., unemployed, declines on instrumental and/or basic activities of daily living), which was associated with executive functioning impairment. Indeed, both alcohol and drug use appear to be associated with decreased access to and engagement in HIV treatment, as well as increased risk for antiretroviral nonadherence (e.g., Altice et al., 2010; Hinkin et al., 2007; Palepu, Horton, Tibbetts, Meli, & Samet, 2004), all of which have critical implications for HIV disease management (e.g., viral rebound, development of disease-resistant strains). Yet, despite these clear risk factors and the economic burden associated with HIV/SUD (e.g., unemployment, SUD use treatment, poorly controlled HIV disease; Blackstone, Iudicello, et al., 2013; Heaton et al., 2004; Henry, Minassian, & Perry, 2010; Hinkin et al., 2004), no empirically validated techniques exist to treat HAND among SUD individuals.
Goal Management Training (GMT) is one such empirically supported and well-validated neurorehabilitation intervention that has shown efficacy in improving executive-related IADL errors in other clinical populations. GMT aims to improve patients’ organizational and goal-directed behaviors on a global level (Levine et al., 2000). GMT is conceptually based on Duncan’s (1986) theory of behavioral disorganization, which posits that “goal neglect,” or difficulty maintaining intentions, depends on higher-level control over basic cognitive abilities (i.e., executive functions), the symptoms of which were commonly observed among individuals with frontal systems injuries. Robertson and colleagues (1996) subsequently developed a manualized rehabilitation protocol based on Duncan’s theory to address these difficulties clinically. GMT has been validated in several clinical populations (e.g., TBI, stroke), but of relevance to the current study, Alfonso and colleagues (2011) recently examined GMT plus a mindfulness-based meditation in a cohort of polysubstance users using a 7-week protocol. Compared to a treatment as usual group, users who received GMT plus mindfulness meditation showed improved working memory, response inhibition, and decision-making.
Given that engagement with and motivation to apply cognitive strategies is a key component in the neurorehabilitation context and that awareness of illness tracks with both treatment motivation and executive dysfunction (e.g., Prigatano & Wong et al., 1999), we additionally aimed to target metacognitive abilities as part of our study design. Metacognition (i.e., “thinking about thinking”) is comprised of 1) conscious knowledge of cognitive processes (i.e., “metacognitive knowledge”), and 2) ability to monitor and regulate ongoing activities while engaging in a task (i.e., “online awareness”; Toglia & Kirk, 2000). Such abilities are commonly disrupted following injury to the prefrontal systems (i.e., anosgonosia; Kelley et al., 2002; Stuss, 2011), are associated with integrity of executive functions (Fernandez-Duque et al., 2000; Lysaker et al., 2008), and not surprisingly, appear to be disproportionately disrupted in both HIV and SUD compared to their neurologically healthy peers (e.g., Casaletto et al., 2014, 2015; Chiao et al., 2013; Le Berre et al., 2010). Of importance, there is consistent evidence suggesting that limitations in metacognition (i.e., poor awareness of neurocognitive impairments) are significantly associated with poorer motivation for treatment, risk of early attrition, and decreased skills learning (Fleming & Strong, 1999; Trudel, Tryon & Purdum, 1998).
Therefore, given that neither GMT nor metacognitive trainings have been empirically examined in the context of HIV+ substance users, we aimed to explore their independent and combined efficacy as potential neurorehabilitation tools. We examined the effect of GMT only, GMT plus metacognitive feedback training (GMT+Meta), or active control on subsequent everyday multitasking and metacognition among HIV/SUD individuals with executive dysfunction. We hypothesized a stair-step effect, such that HIV/SUD individuals who underwent both GMT and metacognitive feedback training would demonstrate better multitasking and metacognitive awareness as compared to those receiving only the GMT strategy, who in turn would perform better than the active control group. Additionally, we hypothesized that HIV/SUD individuals with the poorest executive functioning abilities prior to training would demonstrate the greatest gains from GMT and GMT+meta trainings.
Methods
Participants
Participants provided written consent for this study, which was approved by the University of California, San Diego Institutional Review Board.
Inclusion criteria
All participants met three major inclusion criteria: 1) HIV infection; 2) lifetime history of a substance use disorder, and 3) demonstrated current executive dysfunction. These inclusion criteria were gathered at participants’ last parent study visit at the University of California, San Diego HIV Neurobehavioral Research Program (<24 months; median 149 days since visit, range 0–719 days).
HIV serostatus was determined via enzyme-linked immunosorbent assay (ELISA) and a confirmatory Western Blot.
In regards to substance use, participants needed to meet Diagnostic and Statistical Manual 4th ed. (DSM-IV) lifetime criteria for any substance abuse or dependence (i.e., any of the following substances alone or in combination: alcohol, cocaine, methamphetamine, cannabis, and/or opioid) as determined by a structured and well-validated clinical interview (18.1% met criteria for current abuse/dependence; Composite International Diagnostic Interview, version 2; World Health Organization, 1997).
Current “executive dysfunction” was determined via impairment (Domain Deficit Score >0.50; Cary et al., 2004) on any of the following neurocognitive domains at participants’ prior parent study visit: Abstract Reasoning/Cognitive Flexibility (i.e., Trail Making Test, Part B; Wisconsin Card Sorting Test-64; Halstead Category Test), Working Memory (i.e., WAIS-III Letter-Number Sequencing; Paced Auditory Serial Addition Test), and/or Verbal Fluency (Letter [F-A-S], Animals, and Action Fluencies). Participants with parent study visits >18 months prior were re-assessed to confirm current executive dysfunction (n=7).
Exclusion criteria
Participants who tested positive for alcohol on a Breathalyzer or evidenced behavior consistent with current, acute intoxication based on examiner evaluation on the day of testing were rescheduled for another day. We also excluded participants with psychosis unrelated to substance use (e.g., schizophrenia), or other neurological conditions that might influence cognitive functioning (e.g., traumatic brain injury with loss of consciousness > 30 min, stroke, seizure disorders).
Final sample
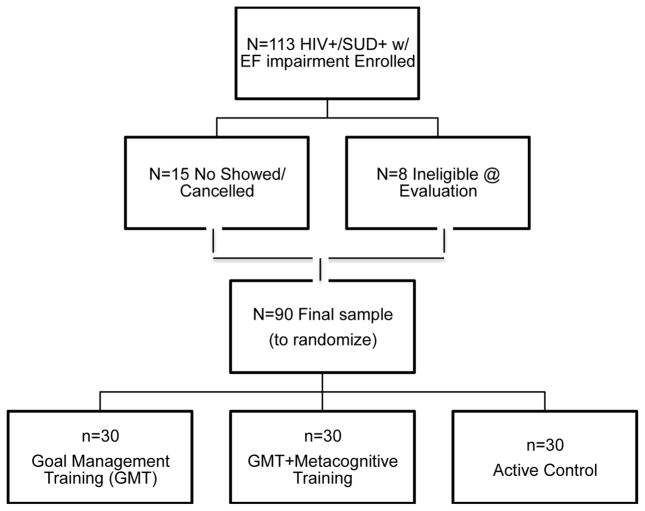
A total of 90 HIV+ participants with substance use disorders (HIV/SUD) and current executive dysfunction completed the study, meeting the enrollment goal of n=30 per study arm (i.e., Goal Management Training (GMT), or GMT+Metacognitive Training (GMT+Meta), or Active Control; see Figure 1 for CONSORT diagram of participant enrollment). On average, participants were about 50-years-old, slightly more than high school educated, majority male, and about half identified as White. In the whole sample, the most common lifetime substances of abuse/dependence were alcohol (72.1%), methamphetamine (58.1%), and cocaine (38.9%). In terms of other psychiatric functioning, a large majority of the sample met DSM-IV-TR criteria for lifetime histories of major depressive disorder (MDD; 70.9%) and participants’ report of current depression symptomology were minimal-to-mild (BDI-II=13.6, SD=11.9). From a neurocognitive standpoint, performances from their last study visit indicated that abstract reasoning/cognitive flexibility was the most commonly impaired area of executive functioning (49.4% impaired), followed by verbal fluency (39.0% impaired), and then working memory (37.5%). Overall, 65.5% of the sample demonstrated global neurocognitive impairment on the gold standard comprehensive battery (see Table 1 for participant clinico-demographic characteristics).
Figure 1.
CONSORT diagram illustrating participant enrollment and randomization procedure.
Note. HIV+/SUD+= HIV+ individuals with histories of substance use disorders; EF=executive functions.
Table 1.
Clinico-demographic characteristics of sample across study arms.
| Active Control (n=30) | Goal Management Training (GMT) (n=30) | GMT + Metacognitive Training (n=30) | p-value | |
|---|---|---|---|---|
| Demographics | ||||
|
| ||||
| Age, y | 47.8 (11.6) | 50.1 (10.1) | 50.6 (9.2) | 0.56 |
| Education, y | 13.1 (2.9) | 13.5 (2.1) | 12.9 (2.8) | 0.69 |
| Sex (% M, n) | 86.7% (26) | 90.0% (27) | 90.0% (27) | 0.89 |
| Race (% White, n) | 56.7% (17) | 53.3% (16) | 56.7% (17) | 0.64 |
|
| ||||
| Substance Use | ||||
|
| ||||
| U-Tox Pos at Eval (%, n) | 36.7% (11) | 26.7% (8) | 20.0% (6) | 0.35 |
| Lifetime Substance Use Disorders (%, n) | ||||
| Alcohol | 75.0% (21) | 62.1% (18) | 79.3% (23) | 0.31 |
| Methamphetamine | 60.7% (17) | 44.8% (13) | 69.0% (20) | 0.17 |
| Cocaine | 46.4% (13) | 34.5% (10) | 32.1% (9) | 0.49 |
| Cannabis | 39.3% (11) | 41.4% (12) | 35.2% (10) | 0.91 |
| Opioid | 14.3% (4) | 6.9% (2) | 17.9% (5) | 0.45 |
| Polysubstance | 63.3% (19) | 60.0% (18) | 70.0% (21) | 0.71 |
| Any Current SUD (%, n) | 18.5% (5) | 14.3% (4) | 21.4% (6) | 0.78 |
| Any Substance Use in Last Year (%, n) | 70.0% (21) | 90.0% (27) | 70.0% (21) | 0.11 |
| Any Substance Last Use, days | 12.6 (27.1) | 20.5 (44.5) | 15.3 (39.7) | 0.80 |
|
| ||||
| HIV Disease | ||||
|
| ||||
| Nadir CD4 | 155 (29, 344.5) | 134 (39.5, 269) | 182 (15.5, 407) | 0.41 |
| Current CD4 | 588.5 (404, 857) | 489 (278.3, 642) | 552 (307, 867) | 0.26 |
| AIDS (%, n) | 66.7% (18) | 64.3% (18) | 55.2% (2) | 0.64 |
| Est. Duration Infection, y | 13.1 (20.8) | 15.5 (8.0) | 11.0 (7.9) | 0.50 |
| VL Detect (plasma; %, n) | 28.6% (6) | 38.9% (7) | 20.8% (5) | 0.44 |
| On ART (%, n) | 85.2% (23) | 92.9% (26) | 82.1% (23) | 0.48 |
| VL Detect on ART (%, n) | 23.5% (4) | 31.3% (5) | 15.8% (3) | 0.56 |
|
| ||||
| Psychiatric and Neurocognitive | ||||
|
| ||||
| BDI-II | 16.3 (11.2) | 10.8 (10.8) | 13.9 (13.4) | 0.27 |
| BDI-II≥17 (%, n) | 50.0% (12) | 24.0% (6) | 30.4% (7) | 0.14 |
| TEA Dual Srch Decr (ss) | 9.7 (4.5) | 10.0 (3.9) | 10.9 (4.2) | 0.52 |
| TEA Dual Srch Decr raw | ||||
| % below median | 46.7% (14) | 53.3% (16) | 50.0% (16) | 0.88 |
|
| ||||
| Comprehensive Neuropsychological (NP) Battery (prior parent study visit) | ||||
|
| ||||
| % Impaired | ||||
| Reason/Flexibility | 50.0% (14) | 48.3% (14) | 50.0% (15) | 0.99 |
| Working Memory | 32.0% (8) | 33.3% (9) | 46.4% (13) | 0.48 |
| Verbal Fluency | 42.3% (11) | 33.3% (9) | 41.4% (12) | 0.76 |
| Global NP | 64.3% (18) | 58.6% (17) | 73.3% (22) | 0.49 |
| Global NP Mean T | 43.3 (7.3) | 43.7 (5.3) | 42.9 (7.0) | 0.89 |
Note. U-Tox Positive at Eval=urine toxicology screen positive at evaluation; SUD=DSM-IV substance use disorder; AIDS=acquired immune deficiency syndrome; ART=antiretroviral therapy; TEA Dual Srch Decr=Test of Everyday Attention Dual Search Decrement.
Study Procedure
Participants were blindly randomized to one of the three study arms via sequential treatment assignment balancing for prognostic factors (i.e., demographics and severity of executive dysfunction; Pocock & Simon, 1975): 1) Goal Management Training (GMT; n=30); 2) Goal Management Training plus Metacognitive Training (GMT+Meta; n=30); or 3) Active Control (n=30). As a result, participants were statistically matched on demographics and severity of executive dysfunction (Table 1).
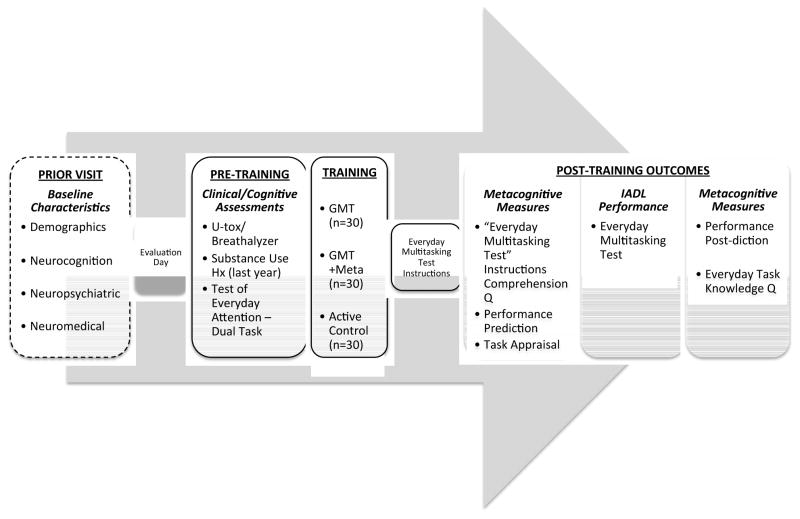
Regardless of condition assignment, all participants received the study assessments and trainings in the following order (approximately 2 hours total): 1) Baseline clinical and cognitive assessment; 2) Study training condition (i.e., GMT only, GMT+Meta, or Active Control conditions); and 3) Outcomes: Everyday Multitasking Test and metacognitive measures. Figure 2 provides an overview of the total study procedure, including the temporal order of the training and assessments.
Figure 2.
Study procedure (~2hr evaluation day) and temporal order of assessments and training.
Note. U-tox = urine drug toxicology screen; GMT = Goal Management Training; Meta = Metacognitive Training; Q = questionnaire; IADL = Instrumental activities of daily living.
Pre-training
Current Substance Use
All participants completed a urine toxicology screen and breathalyzer prior to evaluation. The examiner additionally completed an interval substance use history covering any substances used in the past 12 months.
Test of Everyday Attention – Telephone Search Dual Task
Prior to training, all participants completed the Test of Everyday Attention (TEA) – Telephone Search Dual Task (Robertson, Ward, Ridgeway, Nimmo-Smirth, 1994), in which participants were asked to search a simulated telephone directory for key symbols while simultaneously counting strings of tones presented on an audio recorder. Raw TEA – Dual Task Decrement scores were used in analyses indicating participants’ decrement in performance when completing the dual task compared to the single task (i.e., searching visual key symbols minus searching visual key symbols with auditory counting); lower raw decrement scores indicate better performances. This measure was selected to provide an indicator of participants’ baseline cognitive multitasking capacity (i.e., divided attention). Given that one of the primary outcomes of the study was everyday multitasking performance, we aimed to gather an assessment of participants’ pre-training multitasking ability levels.
Training Conditions
All study conditions were delivered by a Master’s-level doctoral student (K.B.C.). A single-blinded procedure was used in which participants, but not examiner, were blinded to participant condition assignment. Given that the GMT+Meta condition was the longest in duration (~15–20 minutes), we augmented the other conditions (GMT only and active control) to be time equivalent by adding a “non-active” psychoeducational component (described below). In this manner, all three study conditions were time- and attention-matched to approximately 15–20 minutes of examiner interaction/training. Trainings were conducted on Microsoft PowerPoint and presented on a 20” computer monitor prior to completing the Everyday Multitasking Test or metacognitive measures.
Goal Management Training (GMT) only Condition
Thirty HIV/SUD participants were randomized to receive the GMT strategy only. In this condition, participants received a brief (~5 minute) psychoeducation regarding HIV infection, substance use and their effect on the brain, followed by GMT (~10–15 minutes). Of note, the GMT implemented in the current study was an abbreviated version of the training originally described by Levine et al. (2000). In brief, GMT uses goal lists to direct behavior in response to either external or internal demands. GMT is considered an iterative process in which a patient must continually monitor the current situation in order to determine if the current state matches the goal state, and if not, consultation of goal-oriented actions must occur. As such, GMT is divided into five goal monitoring stages: 1) Stop (before beginning a new task), 2) Define (the task goal), 3) List (the steps needed to complete that task), 4) Learn (how you will complete the steps), and 5) Check (ensure behaviors are goal-directed/on-task). Participants were instructed to use the GMT as a strategy to approach the Everyday Multitasking Test (see Table 2 for GMT adaptation for the Everyday Multitasking Test). The general GMT model (i.e., STOP→Define→List→ Learn→ Check, but without details specific to the Everyday MT) was present on a computer monitor throughout test performance.
Table 2.
Application of Goal Management Training to the Everyday Multitasking Test.
| Goal Management Training | Everyday Multitasking Test (MT) Application | |
|---|---|---|
| Stage 1: STOP! | Orient and alert to task | Participants were trained to “Stop and Think” when beginning a new task or engaging in task switch (e.g., cooking to medication management) |
| Stage 2: Define | Goal setting | Participants defined the goals of MT (e.g., to complete as much of the 4 tasks as possible in the 12-minute time limit) |
| Stages 3: List | Outline steps | Participants paraphrased steps of each task into his own words (MT instructions were available as a guide) |
| Stage 4: Learn | Encode/Apply steps | Participants relayed to examiner how s/he would complete each of the steps |
| Stage 5: Check | Checking | Participant trained to cross out each completed step from the MT instructions sheet during task performance as a cue to monitor for errors/omissions |
Goal Management Training plus Metacognitive Training (GMT+Meta)
Thirty HIV/SUD participants were randomized into the Goal Management Training strategy plus Metacognitive Training (GMT+Meta) study arm, which included 5–10 minutes of metacognitive feedback and 10–15 minutes of GMT prior to administration of the Everyday MT and metacognition measures. In the metacognitive feedback component, participants were provided information regarding their specific executive functions impairment(s) as determined by their neurocognitive test performance in the parent study. The training included lay-language definitions of what executive functions are (“starting, stopping, switching, and/or holding things in mind”; Callahan, 2009; Miyake et al., 2000) and how executive dysfunction is known to affect performance in everyday life and on the Everyday MT (e.g., increased likelihood for omission errors; Scott et al., 2011). Importantly, this feedback was framed to illustrate how this information may improve Everyday MT performance (e.g., emphasize that recognition of these difficulties may help participants to reduce these errors during performance). See below for an example of the script for a participant with a deficit in working memory:
“Do you remember the test you took the last time you were here that asked you to add numbers together in your head that were said out loud from an audio-recording? That is a test of ‘holding things in mind.’ You had trouble on that test last time, which tells us that you may have some difficulty holding things in mind. This might mean that you have a hard time getting rid of old information and keeping track of new information in order to complete a new task. You may also find it hard to do mental processing in your head, like solving a problem in your head or maintaining a list in your head.
Tell me about difficulties you’ve noticed “holding things in mind” in your day-to-day life…
The way this might affect your Multitasking Test score is that you may find it hard to keep instructions for each new task in mind. Or, you may find it hard to figure out which step needs to be done next. For example, if you are cooking, it may be hard for you realize that once the pasta is in the water that would be a good time to set the timer.
So, one of the things we’re going to do today is learn a strategy [GMT] that can help with keeping track of what is going on when you are completing a task.”
Active Control Condition
Finally, 30 HIV/SUD participants were randomly assigned to the active control (no treatment) arm. In the control condition, participants were provided the same brief (~5 minutes) HIV and SUD psychoeducation as those in the GMT only condition, and then were subsequently trained to create paper origami structures for 10–15 minutes prior to taking the Everyday MT. This condition aimed to provide a time-equivalent control for the verbal learning, face-to-face interaction, and following directions skills involved in the other trainings.
Post-training Outcomes
Instrumental Activities of Daily Living (IADL) Performance: Everyday Multitasking Test
One of our primary outcomes following completion of a study condition was performance on a validated laboratory-based IADL task, the Everyday Multitasking Test (Everyday MT). The Everyday Multitasking Test was developed in HIV-infected individuals and has been validated in detecting HIV-associated neurocognitive impairment and IADL dependence (i.e., 86% sensitivity to HIV-related IADL dependence; Scott et al., 2011). In the Everyday MT, individuals were required to complete as much of four separate functional tasks as possible within a 12-minute time limit (Scott et al., 2011). The four tasks included: 1) Cooking a Meal; 2) Advanced Finances (e.g., paying bills); 3) Medication Management (e.g., pill dispensing); and 4) Telephone Communication. The task parameters were modified from the Six Elements Test (Shallice & Burgess, 1991) and the first three tasks, including administration and scoring, were adapted from Heaton et al. (2004). Task instruction cue cards were available to participants throughout the test in order to minimize demands on episodic memory and increase ecological validity. Two required task switches were built into the task, one via task instructions (i.e., instructed to complete a phone call to the credit card company before completing finances) and one that was participant-initiated (i.e., when you run out of one medication, call the pharmacy to request a refill), which ensured that participants could not accurately complete any task from beginning to end without switching to another. The primary outcomes of interest examined in the current study were scored via standardized procedures and include: 1) total steps correctly completed; 2) number of errors committed; 3) number of task switches; 4) number of simultaneous task engagements (i.e., engagement in 2 or more tasks at once); and 5) number of overall tasks attempted.
Metacognitive Measures
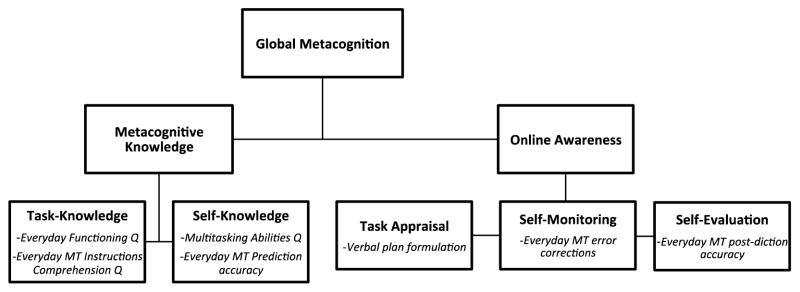
Our second primary outcome of interest was participants’ awareness of their Everyday MT performances (metacognition), which we measured both before and after completion of the Everyday MT test following Toglia and Kirk’s (2000) model (see Figure 2 for temporal order of administration; and Figure 3 illustrates study operationalization of metacognitive constructs):
Figure 3.
Toglia and Kirk’s (2000) conceptual Model of Metacognition (bolded terms) and the corresponding measures (italicized) utilized to operationalize the model in the current study.
Note. Bolded terms indicate theoretical concepts; italicized items represent study measures; Everyday MT = Everyday Multitasking Test; Q = questionnaire.
Metacognitive knowledge includes both task-knowledge and self-knowledge:
Task-knowledge was measured via two questionnaires, Everyday MT Instructions Comprehension questionnaire and the Everyday Functioning questionnaire. Everyday MT Instructions Comprehension questionnaire tapped into knowledge specific to the task itself (i.e., the Everyday MT); while the Everyday Functioning questionnaire ascertained familiarity and prior experience with the four multitasking tasks in their day-to-day lives (i.e., cooking, finances, medication management, and telephone communication).
Self-knowledge was measured by accuracy of participants’ Everyday MT performance predictions and the Multitasking Abilities Questionnaire. Specifically, for Everyday MT performance predictions, participants were asked how many steps of the Everyday MT (out of 60 total possible) they anticipated being able to complete in the 12-minute time limit; participants’ predicted Everyday MT score were then subtracted from their subsequent objective Everyday MT performance score. The Multitasking Abilities Questionnaire was a 15-item measure (rated from 1 (Not Able) to 5 (Highly Able)) that aimed to assess participants’ perceived ability to multitask in everyday life; it demonstrated high face validity (e.g., “I buy groceries while performing another task (e.g., answering the telephone)”) and strong internal consistency (Cronbach’s α=0.92).
Online awareness includes task appraisal, self-monitoring, and self-evaluation around task performance (Toglia & Kirk, 2000):
Task appraisal was measured via verbal formulations of the plan by which the participant anticipated completing the task before completing the Everyday MT. Specifically, participants orally dictated their intended approach for their Everyday MT performance before beginning the task (e.g., “I plan to pour the water into the pot and then start the timer. Then I will call my bank to correct the balance…”). Each oral plan was transcribed and scored for elaboration according to established criteria (e.g., number of executable steps, order and rules for steps; Kliegel, McDaniel, & Einstein, 2000).
Self-monitoring was measured by the number of error corrections the participant demonstrated during Everyday MT performance. That is, as scored by examiner, we calculated the number of times participants recognized an error (e.g., put medication into wrong pill organizer compartment) and corrected it (e.g., moved medication to correct pill organizer compartment) during their Everyday MT performance.
Finally, after completing the Everyday MT, self-evaluation was assessed by the accuracy of participants’ Everyday MT estimated performance after completion (i.e., post-diction). Specifically, after completing the task, participants were asked how many steps of the Everyday MT (out of 60 total possible) they think they completed in the 12-minute time limit (out of a total of 60 possible); participants’ post-dicted Everyday MT scores were then subtracted from their objective Everyday MT performance scores to calculate accuracy.
In order to examine the components of metacognition comparably on the same metric, we created sample-based z-scores (i.e., individual participant score vs. the cohort’s average score) for each metacognitive measure. The measures that comprise each of the metacognitive domains were then averaged together to create the primary metacognitive summary scores (i.e., Metacognitive Knowledge, Online Awareness, Global Metacognition; see Figure 3).
Existing Neuromedical and Psychiatric Data
Data characterizing participants’ basic medical (e.g., HIV disease) and neuropsychiatric (e.g., mood via Beck Depression Inventory-II) backgrounds were drawn from existing data from linked HNRP visits.
Data Analyses
In line with our hypotheses, we conducted a series of omnibus Jonckheere-Terpsta (J-T) tests to assess for monotonic positive trends across the study conditions on metrics of the Everyday Multitasking Test (Everyday MT) performance (e.g., task switches, total errors) and metacognition domains (e.g., self-knowledge, self-evaluation). The J-T test for ordered alternatives tests the null hypothesis that the medians for the study conditions were the same, against the alternative hypothesis that the medians were ordered in magnitude (i.e., Control≤GMT≤GMT+Meta; Siegal, 1988). Follow-up one-sided Wilcoxon tests with false discovery rate adjusted p-values (Benjamini & Hochberg, 1995) examined pairwise study condition differences on the Everyday MT and metacognition. For all nonparametric pairwise comparisons, Cliff’s d was calculated to determine effect sizes, which is an appropriate statistical metric for nonparametric data (Cliff, 1993). Cliff’s d ranges from −1 to +1 and reflects the probability that values for one group are larger than values for another group. The magnitude of the values may be interpreted as follows: d<0.15 “negligible,” d<0.33 “small,” d<0.47 “medium”, otherwise “large” (Romano, 2006).
To determine the moderating effect of pre-training dual-tasking abilities on training condition, we conducted a series of interaction linear regression models with study condition, dual-task performance (TEA-Dual Task raw score), and their interaction (Condition*dual task) predicting Everyday MT performances. To examine directionality of interaction effects, we examined a median split on the TEA-Dual Task raw score (low vs. high) across study condition using post-hoc one-sided t-tests.
Lastly, a series of separate interaction linear regression models were conducted with study condition, clinical covariate of interest, and their interaction (Condition*clinical covariate) on each of the Everyday MT and metacognition outcomes of interest to determine possible individual-level characteristics that may moderate the efficacy of GMT. Models were developed for the following clinical variables of interest: demographics (i.e., age, education, sex, race), sleep, exercise, substance use (i.e., lifetime alcohol, cocaine, methamphetamine, cannabis or opioid use disorders examined separately, and any substance use in the last year), HIV disease (i.e., nadir CD4 count and plasma HIV viral load), mood (i.e., lifetime major depressive disorder and Beck Depression Inventory-II), and everyday functioning (i.e., employment, ADL declines, PAOFI cognitive symptoms). Significant interaction models were followed-up with post-hoc one-sided t-tests to determine directionality of effects.
Results
IADL Performance: Everyday Multitasking Test
Omnibus Jonckheere-Terpstra (J-T) tests indicated trends toward increasing task switches (J*=1.44, p=0.07), simultaneous task engagements (J*=1.41, p=0.08), and tasks attempted (J*=1.4, p=0.08) on the Everyday MT across the study conditions (i.e., monotonic trend Control≤GMT≤GMT+Meta), though these omnibus models did not reach statistical significance at α=0.05.
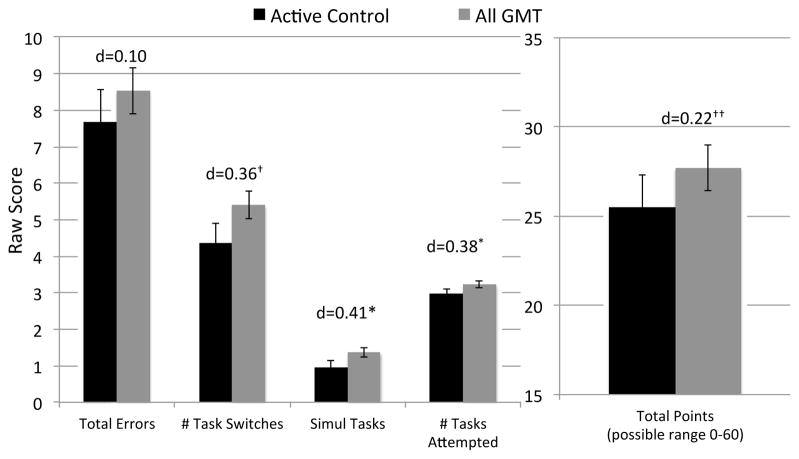
Pairwise one-sided Wilcoxon tests indicated that there were no significant or statistically meaningful (Cliff’s d=−0.14 to −0.01) differences between the GMT and GMT+Meta cohorts on Everyday MT, and the cohorts demonstrated statistical equivalences on the Everyday MT indices (e.g., Everyday MT total score: lower threshold (−5 points) p=0.03 and upper threshold (5 points) p=0.04). Therefore, in order to examine the effect of GMT with greater power, we collapsed these two study conditions (“All GMT”; n=60). One-sided t-tests demonstrated small-to-medium effects, such that HIV/SUD individuals who received the GMT (as part of the Meta training or not) engaged in more simultaneous tasks (p=0.04, d=0.41) and attempted more tasks (p=0.04, d=0.38) than those in the active control condition (Figure 4). There was also a tendency for more task switches (p=0.06, Cohen’s d=0.36) and more total points earned (p=0.16, d=0.22) among HIV/SUD individuals who completed the All GMT compared to controls. The number of total errors on the Everyday MT did not differ between groups (one-sided p=0.79, d=−0.10).
Figure 4.
Performances on the Everyday Multitasking Test comparing HIV/SUD participants who completed the active control (n=30) and those who completed the Goal Management Training (as part of the metacognitive training or not; “All GMT” n=60).
One-sided t-test *p=0.04, †p=0.06, ††p=0.16; Bars=Mean±SE; d=Cohen’s d.
To determine the utility of GMT for Everyday MT as a function of pre-training dual tasking capacity (i.e., cognitive multitasking capacity), we conducted a series of interaction models with study condition (control vs. All GMT), raw dual task performance (TEA-Telephone Search Dual Task), and their interaction (condition*dual task) as predictors of Everyday MT performances (i.e., task switches, simultaneous task engagements, tasks attempted, total points). Significant omnibus multivariable models with significant interaction terms were found for Everyday MT task switches (F(3,86)=2.7, p=0.05), tasks attempted (F(3,86)=3.9, p=0.01), and total points (F(3,86)=3.8, p=0.01). The model predicting simultaneous task engagements approached but did not reach significance (F(3,86)=2.4, p=0.07).
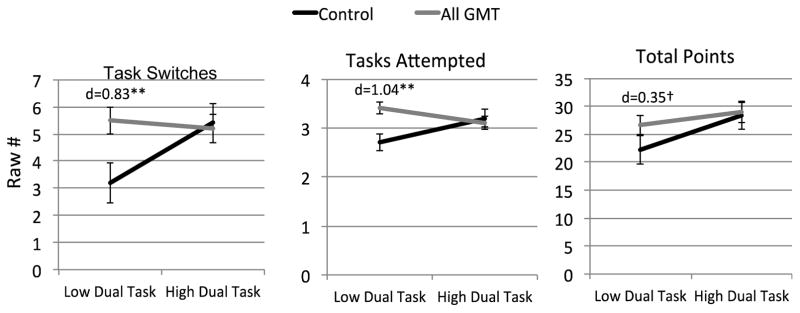
Follow-up analyses using a median split on raw dual task performances (<1.165) found that HIV/SUD individuals with low dual task abilities before the training performed more task switches (one-sided p=0.006, Cohen’s d=0.83), attempted more tasks (one-sided p=0.002, Cohen’s d=1.04), and tended to complete more points (one-sided p=0.08, Cohen’s d=0.35) when in the All GMT condition compared to those in the control condition (Figure 5). In other words, HIV/SUD individuals with poorer dual tasking abilities prior to training benefitted the most from the GMT strategy on the Everyday MT.
Figure 5.
HIV/SUD individuals with poor dual tasking abilities benefitted most from Goal Management Training (“All GMT” n=60 vs. Control n=30) on Everyday Multitasking Test performances.
One-sided t-test **p<0.01, †p=0.08; Bars=Mean±SE; d=Cohen’s d.
Metacognition
Omnibus J-T models were conducted to determine the effect of study condition on metacognitive processes. There was no significant study group effect on Global Metacognition (J*=0.67, p=0.25); however, when examining the two constructs that comprise Global Metacognition (i.e., Metacognitive Knowledge and Online Awareness), Online Awareness showed a significant positive trend across the study conditions (Control≤GMT≤GMT+Meta; J*=1.7, p=0.04), while the Metacognitive Knowledge model was not significant (J*=−1.12, p=0.87). We additionally conducted J-T models to examine which component(s) of Online Awareness (i.e., Task Appraisals, Self-Monitoring, Self-Evaluation) may be driving this study group effect. Only the model for Task Appraisals approached significance with an increasing trend for more elaborate appraisals across study conditions (Control≤GMT≤GMT+Meta; J*=1.4, p=0.08).
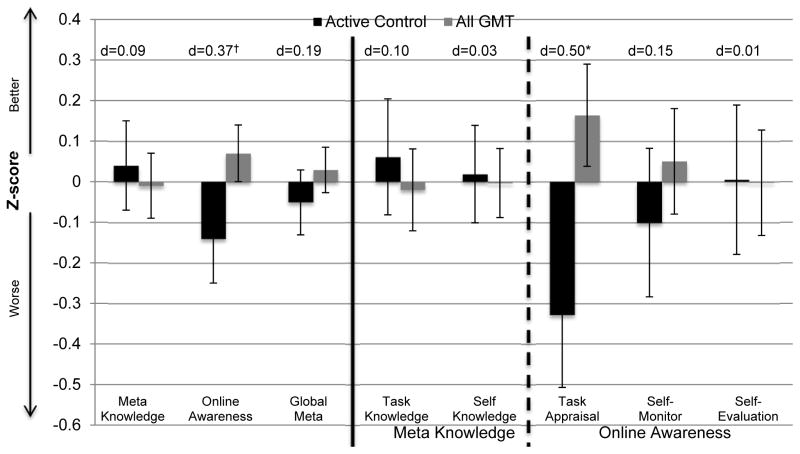
As with the Everyday MT findings, there were no significant or meaningful differences between the GMT and GMT+Meta study groups on metacognitive Online Awareness (z=0.74, Cliff’s d=0.10, p=0.23), and the cohorts demonstrated statistical equivalence (lower threshold (z=−0.5) p<0.001, upper threshold (z=0.5) p=0.02); therefore, we collapsed the latter two groups (“All GMT”) in order to increase power and more closely examine the effect of GMT on metacognitive processes. One-sided t-tests indicated that there was no study group difference on Global Metacognition (p=0.21); however, there was a study group effect that approached significance again for Online Awareness abilities, with those who completed the GMT (as part of Meta training or not) tending to demonstrate better Online Awareness compared to those in the control condition (p=0.052, Cohen’s d=0.37; Figure 6). Importantly, examining the components of Online Awareness, HIV/SUD individuals who completed the All GMT demonstrated significantly more elaborate Task Appraisals (not Self-Monitoring or Self-Evaluation) than controls (p=0.01, Cohen’s d=0.50; Figure 6).
Figure 6.
Goal Management Training (as part of the metacognitive training or not; “All GMT” n=60) demonstrates a small-to-medium effect size benefits for Online Awareness as driven by significantly more elaborate Task Appraisals compared to those who received the control condition (n=30) among HIV/SUD individuals.
†p=0.052; *p=0.01 Bars=Mean, SE, d=Cohen’s d.
Other Moderating Factors
Lastly, we explored the possible roles of other demographic/background, psychiatric and substance use, or clinical factors in moderating the effect of All GMT (control vs. All GMT) via a series of multivariable interaction models. On the Everyday MT, the Beck Depression Inventory-II (BDI-II) demonstrated a significant interaction with study condition (control vs. All GMT) in predicting number of tasks attempted (b (beta)=−0.02, p=0.02) and total points earned (b=−0.26, p=0.02) on the Everyday MT. Specifically, among HIV/SUD individuals with high levels of current depression (BDI-II≥17), those in the All GMT condition attempted more tasks (p=0.047, Cohen’s d=0.93) and tended to complete more points (p=0.051, Cohen’s d=0.82) on the Everyday MT compared to those in the control condition with high symptoms of current depression. Performances between the All GMT and control condition did not differ among participants with low current depressive symptomology (p>0.05; BDI-II<17). Additionally, diagnoses of lifetime methamphetamine use disorders showed a significant interaction with study condition for Everyday MT total points earned (b=2.3, p<0.05). Among HIV/SUD individuals who met criteria for a lifetime methamphetamine use disorder, those who completed the All GMT earned more points on the Everyday MT than methamphetamine users who completed the control condition (p=0.01, Cohen’s d=0.75). Everyday MT performances did not differ between All GMT and control conditions among individuals without lifetime histories of methamphetamine use disorders (p>0.05).
No demographic or background factors, psychiatric or substance use, HIV disease, or daily functioning factors significantly moderated the effect of All GMT study condition for metacognitive online awareness or task appraisals (ps>0.05).
Discussion
HIV+ substance users evidence disproportionate rates of neurocognitive impairment, especially higher-order executive functioning, and related problems in health behaviors and everyday functioning (Altice et al., 2010; Purcell et al., 2001), but to date there are no effective, compensatory-based cognitive neurorehabilitation strategies for use in this vulnerable group. Findings from this study suggest that a brief, single-session, Goal Management Training (GMT) protocol has modest efficacy on naturalistic multitasking performance and related awareness among HIV/SUD. These findings support and extend prior literatures on the effectiveness of GMT in other clinical populations with executive dysfunction, such as traumatic brain injury and frontal lobe stroke (e.g., Levine et al. 2000, 2011; Schweizer et al., 2008; Jackson et al., 2012); additionally, our findings help replicate the positive neurocognitive effect of GMT reported by Alfonso and colleagues (2007) among polysubstance users. Ours also represents the first data supporting a compensatory approach to improve HAND, which complements several recent studies that have demonstrated some efficacy for restorative-based neuroremediation approaches in HIV infection (i.e., computerized brain training programs, such as Posit Science Insight; Vance et al., 2012; Becker et al., 2012; Boivin et al., 2010). Indeed, future work utilizing multimodal rehabilitation approaches including a combination of restorative, compensatory, and pharmacological treatments may be warranted in order to achieve the most optimal results.
Although the mechanisms by which GMT resulted in better Everyday MT performances (i.e., more task switches, simultaneous task engagement, tasks attempted) and metacognitive task appraisals cannot necessarily be teased apart from our study design, there are several conceivable pathways. On the Everyday MT, we may consider multitasking deficits through Shallice and Burgess’ (1991) framework, which posits that following damage to the prefrontal systems there may be an inability to reactivate a previously generated intention after a brief delay (e.g., sub-goal of a multistep task). GMT may interrupt this maladaptive process by instructing participants to verbally self-generate and elaborate the Everyday MT goals, which may have resulted in deeper encoding processes allowing for easier “reactivation” (retrieval) during task performance itself. Another parallel process may be that, by nature, individuals with executive dysfunction are less likely to engage in the reflective, flexible scheduling processes that allow individuals to select appropriate strategies when completing tasks, and ultimately have fewer executive resources to draw from during task performances (Meyer & Kieras, 1997). Therefore, provision of the GMT for these HIV/SUD individuals with executive dysfunction may have reduced the executive cognitive load associated with selection of a strategic approach from the outset. The reduction of strategic cognitive burden afforded by the GMT may have then freed those executive resources to be applied to Everyday MT performances themselves, resulting in greater success on the executive aspects of the test (i.e., task switches, simultaneous task attempts). Supporting this conceptualization, among healthy adults, reduction of load on cognitive control processes is associated with increased activation of task- or process-specific neural circuitry (Kelly & Garavan, 2005; Petersen, van Mier, Fiez, & Raichle, 1998). Subsequently, if the GMT was able to decrease the need for recruitment of cognitive control circuits, a more refined, task-specific network of activation may have resulted in the observed higher number of tasks attempted and overall greater number of steps completed for the Everyday MT.
Indeed, we observed HIV/SUD participants with lower pre-training dual tasking abilities (i.e., those with the most disrupted frontal systems dysfunction) demonstrated disproportionally larger benefits from the GMT-based trainings on Everyday MT (ds=0.35–1.04). This finding suggests that those participants most in need of cognitive support may benefit the most, which further maintains our conceptualization of GMT as an external compensatory aid that helps free control resources to be reallocated to other aspects of task completion. Likely, the GMT helps support several neurocognitive systems; regarding our conceptualizations here, perhaps the GMT reduced the overall cognitive burden of a given task, which may then facilitate the ability to engage in the activation/re-activation processes outlined by Shallice and Burgess (1991) above. Simultaneously, the GMT may also independently promote this sub-goal activation/re-activation process to further promote successful multitasking performance.
Regarding metacognitive task appraisals, HIV/SUD individuals who completed GMT elaborated their conceptualization of and plan for the upcoming task in greater detail than those in the control condition. This ability to identify and delineate task demands relative to one’s own capacity is needed for optimal strategy selection and to define the task expectations that will need to be regulated downstream during performance (Bandura, 1997; Hacker, 1998). A major component of GMT is its provision of an overlaying structure to an otherwise unstructured task by encouraging individuals to define the primary task goals, and then list and learn the steps needed to complete each goal. This process thus promotes the formation of a superordinate hierarchy of goals and steps or, in other words, a plan. Considered in the context of Norman and Shallice’s supervisory attentional model of executive functions (Norman & Shallice, 1980), planning is mediated by the supervisory attentional system (SAS), and is activated when a high-level schema (i.e., goal) are triggered which then passes on the activation to lower-level schemas (i.e., individual action steps). Importantly, the SAS, which is subserved by the prefrontal cortex (Shallice & Burgess, 1991), is the system that is disrupted among individuals with executive dysfunction. Therefore, GMT may have served as an externally fabricated SAS in our HIV/SUD participants with disrupted attentional systems by initiating the activation and development of their planning processes resulting in significantly more elaborate task appraisals. Task appraisals are an important executive skill needed for accurate metacognition (Toglia & Kirk), and their malleability via GMT highlights a potentially fruitful starting point in the development of future executive neurorehabilitation interventions among HIV/SUD individuals.
When developing novel intervention tools, it is critical to ascertain the characteristics of the individual for whom an approach may be especially viable and efficacious. Although there were no individual-level characteristics that moderated the effect of GMT on metacognitive task appraisals, both mood and methamphetamine use influenced how GMT benefitted multitasking abilities. Importantly, these latter two moderating characteristics appeared to impact the benefit of GMT independent of dual tasking abilities. Regarding mood, for HIV/SUD individuals with more severe depressive symptoms providing an aid via GMT may have enhanced perceived self-efficacy of multitasking abilities potentially resulting in greater overall task engagement and ultimately, performance (Bandura, 1997). Similarly, we found that in HIV/SUD participants with lifetime histories of methamphetamine use disorders (n=47), those who received the GMT completed significantly more multitasking points than those in the control condition. Although all study participants met criteria for some lifetime substance use disorder, methamphetamine may have particularly prominent and lasting deleterious effects on the frontostriatal circuits in the context of HIV infection, which are heavily drawn upon when multitasking (Chana et al., 2006; Ellis et al., 2003; Gavrilin, Mathes, & Podell, 2002; Jernigan et al., 2005; Taylor et al., 2007). Indeed, Iudicello and colleagues (2014) demonstrated enduring adverse neurocognitive and functional outcomes following remote methamphetamine dependence particularly among older HIV+ individuals. Therefore, there appears to be significant additional injuries to these neurocognitive systems when multiple risk factors are in play (e.g., HIV infection, methamphetamine use, aging) - with the effects of even remote methamphetamine use being particularly persistent. As a result, HIV+ methamphetamine users may be disproportionately in need of and responsive to such executive neurorehabiltation techniques. Our results indicate a specific pattern of individual characteristics (i.e., HIV+ methamphetamine users with more severe current depression symptomology) that may especially warrant eligibility for GMT implementation. Continued future work developing more individualized neurorehabilitation techniques are needed given that the efficacy of these tools can significantly differ with the constellation of background and comorbid factors of a presenting patient.
Of note, although we hypothesized that HIV/SUD individuals who received the GMT strategy and Metacognitive Training would demonstrate he best outcomes based on prior literature (e.g., Goverover et al., 2007; Ownsworth et al., 2010), this was not necessarily the pattern of results observed. One possibility for the lack of additional benefit of the metacognitive feedback component may have been the brevity of our Metacognitive Training compared to the more intensive 3- to 16-week traditional self-awareness trainings described in previous studies (e.g., Goverover et al., 2007; Ownsworth et al., 2006). By contrast, our single-session training included approximately 10 minutes dedicated to feedback regarding individuals’ executive dysfunction, followed by 10 minutes of training on the executive strategy (GMT). We selected such a brief training both due to the limited scope and resources of this project, but also to develop a potential neurorehabilitation tool that may be applicable in other time-limited settings (e.g., outpatient clinics). It is possible, however, that our brief Metacognitive Training was simply not salient or potent enough to initiate and maintain behavior change (beyond the effects of the GMT) for our HIV/SUD participants. Alternatively, given that we found no statistically significant benefit of adding the metacognitive feedback to GMT, it may have been that providing a concrete executive strategy was the needed “active ingredient” to improve task performances and aspects of metacognition. Indeed, prior studies examining metacognitive trainings have included the selection and application of strategies as part of the global awareness training (Goverover et al., 2007; Ownsworth et al., 2010). Therefore, it is possible that improvements observed in those studies might, in fact, be attributable to the strategy training, and not necessarily the other aspects of the awareness interventions (e.g., feedback). Ours is the first study that has begun to tease apart the most active components in such metacognitive training approaches. We were able to examine the independent effects of strategy application (GMT), however, we were not able to explore the effect of the metacognitive feedback on its own (i.e., a fourth study condition in which participants received the metacognitive feedback alone). Future work is needed to disentangle which aspects of trainings are critical versus those that may not meaningfully contribute to functional outcomes. Improving the economy of treatment approach will help address the limited time demands in the clinic and reduce both patient and provider burden moving forward.
There are several other important methodological limitations to note in our study. First, the current study design had limited statistical power with which it could detect such effects, potentially resulting in Type II error. Development of neurorehabilitation study designs with greater power via larger sample sizes and/or a within-subjects method may reveal a stronger pattern of significant findings. Nonetheless, even with limited sample sizes, our study highlights several medium-sized, significant effects of GMT. Additionally, the primary aim of our brief experimental training was to examine its efficacy on laboratory-based assessments, and did not include indicators of possible generalization or durability of effects. Follow-up evaluations would help determine if the beneficial training effects were robust across time and the extent to which they may have impacted HIV/SUD individuals’ subsequent day-to-day activities. Durability and generalizability are critical concepts when developing neurorehabilitation tools; empirically supported techniques that have a more wide-reaching impact outside of the laboratory are greatly needed and an important future direction in the field (Weber, Blackstone, et al., 2013).
Our brief experimental design demonstrated modest benefits of Goal Management Training (GMT) for both everyday multitasking abilities and metacognitive task appraisals among HIV+ individuals with substance use. Regarding the former, the positive impact of GMT for multitasking was particularly salient among HIV/SUD individuals with poorer multitasking abilities prior to training, as well as those with more severe levels of current depressive symptomology and histories of methamphetamine use disorders. Although the Metacognitive Training did not significantly contribute to multitasking or metacognition beyond the benefits of GMT, further work is needed to better determine if this is indeed a needed or viable neurorehabilitation approach beyond strategy application. Of note, our study was a brief, experimental design that would need to be replicated in future intervention studies and, ultimately, clinical trials before delineating the important active ingredients and best practices for use in clinics. Our study provides initial proof-of-principle of compensatory-based neurorehabilitation interventions with HIV infected substance users (i.e., malleability of behavior) and highlights the potential utility of Goal Management Training even when delivered in a brief, single-session format.
Acknowledgments
This work was supported by the National Institutes of Health (NIDA) F31-DA035708, the Foundation for Rehabilitation Psychology Dissertation Award, and the American Foundation for Psychology Benton-Meier Scholarship. Dr. Scott’s participation was supported by a Department of Veterans Affairs Career Development Award (IK2CX000772).
References
- Alfonso JP, Caracuel A, Delgado-Pastor LC, Verdejo-Garcia A. Combined Goal Management Training and Mindfulness meditation improve executive functions and decision-making performance in abstinent polysubstance abusers. Drug Alcohol Depend. 2011;117(1):78–81. doi: 10.1016/j.drugalcdep.2010.12.025. S0376-8716(11)00049-4 [pii] [DOI] [PubMed] [Google Scholar]
- Altice FL, Kamarulzaman A, Soriano VV, Schechter M, Friedland GH. Treatment of medical, psychiatric, and substance-use comorbidities in people infected with HIV who use drugs. Lancet. 2010;376(9738):367–387. doi: 10.1016/S0140-6736(10)60829-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandura A. Self-efficacy: The exercise of control. New York: W.H. Freeman and Company; 1997. [Google Scholar]
- Becker JT, Dew MA, Aizenstein HJ, Lopez OL, Morrow L, Saxton J, Tárraga L. A pilot study of the effects of internet-based cognitive stimulation on neuropsychological function in HIV disease. Disability and rehabilitation. 2012;34(21):1848–1852. doi: 10.3109/09638288.2012.667188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling for false discovery rate: a practical and powerful approach to multiple testing. Journal of Royal Statistical Society, Series B. 1995;57(1):289–300. [Google Scholar]
- Blackstone K, Iudicello JE, Morgan EE, Weber E, Moore DJ, Franklin DR, Ellis RJ, Grant I, Woods SP the Translational Methamphetamine, Aids Research Center Group. Human immunodeficiency virus infection heightens concurrent risk of functional dependence in persons with long-term methamphetamine use. J Addict Med. 2013;7(4):255–263. doi: 10.1097/ADM.0b013e318293653d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackstone K, Woods SP, Weber E, Grant I, Moore DJ. Memory-based strategies for antiretroviral medication management: an evaluation of clinical predictors, adherence behavior awareness, and effectiveness. AIDS Behav. 2013;17(1):74–85. doi: 10.1007/s10461-012-0308-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boivin MJ, Busman RA, Parikh SM, Bangirana P, Page CF, Opoka RO, Giordani B. A pilot study of the neuropsychological benefits of computerized cognitive rehabilitation in Ugandan children with HIV. Neuropsychology. 2010;24(5):667. doi: 10.1037/a0019312. [DOI] [PubMed] [Google Scholar]
- Callahan CD. The assessment and rehabilitation of executive functions disorders. In: Stonnington BJHH, editor. Rehabilitation of Neuropsychological Disorders: A practical guide for rehabilitation professionals. 2. New York, NY: Taylor & Francis Group, LLC; 2009. [Google Scholar]
- Carey CL, Woods SP, Rippeth JD, Gonzalez R, Heaton RK, Grant I. Additive deleterious effects of methamphetamine dependence and immunosuppression on neuropsychological functioning in HIV infection. AIDS Behav. 2006;10(2):185–190. doi: 10.1007/s10461-005-9056-4. [DOI] [PubMed] [Google Scholar]
- Casaletto KB, Doyle KL, Weber E, Woods SP. Self-predictions of prospective memory in HIV-associated neurocognitive disorders: Evidence of a metamemory deficit. Archives of Clinical Neuropsychology. 2014;29:818–827. doi: 10.1093/arclin/acu061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casaletto KB, Obermeit L, Morgan EE, Weber E, Franklin DR, Grant I, Woods SP the Translational Methamphetamine, Aids Research Center Group. Depression and executive dysfunction contribute to a metamemory deficit among individuals with methamphetamine use disorders. Addict Behav. 2015;40:45–50. doi: 10.1016/j.addbeh.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chana G, Everall IP, Crews L, Langford D, Adame A, Grant I, Cherner M, Lazzareto D, Heaton R, Masliah E. Cognitive deficits and degeneration of interneurons in HIV+ methamphetamine users. Neurology. 2006;67(8):1486–1489. doi: 10.1212/01.wnl.0000240066.02404.e6. 67/8/1486 [pii] [DOI] [PubMed] [Google Scholar]
- Chiao S, Rosen HJ, Nicolas K, Wendelken LA, Alcantar O, Rankin KP, Miller B, Valcour V. Deficits in Self-Awareness Impact the Diagnosis of Asymptomatic Neurocognitive Impairment in HIV. AIDS Res Hum Retroviruses. 2013 doi: 10.1089/AID.2012.0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cliff N. Dominance statistics: Ordinal analyses to answer ordinal questions. Psychological Bulletin. 1993;114(3):494–509. [Google Scholar]
- Ellis RJ, Childers ME, Cherner M, Lazzaretto D, Letendre S, Grant I. Increased human immunodeficiency virus loads in active methamphetamine users are explained by reduced effectiveness of antiretroviral therapy. J Infect Dis. 2003;188(12):1820–1826. doi: 10.1086/379894. JID30762 [pii] [DOI] [PubMed] [Google Scholar]
- Fernandez-Duque D, Baird JA, Posner MI. Executive attention and metacognitive regulation. Conscious Cogn. 2000;9(2 Pt 1):288–307. doi: 10.1006/ccog.2000.0447S1053-8100(00)90447-1. pii. [DOI] [PubMed] [Google Scholar]
- Fleming JM, Lucas SE, Lightbody S. Using occupation to facilitate self-awareness in people who have acquired brain injury: a pilot study. Can J Occup Ther. 2006;73(1):44–55. doi: 10.2182/cjot.05.0005. [DOI] [PubMed] [Google Scholar]
- Gavrilin MA, Mathes LE, Podell M. Methamphetamine enhances cell-associated feline immunodeficiency virus replication in astrocytes. J Neurovirol. 2002;8(3):240–249. doi: 10.1080/13550280290049660. [DOI] [PubMed] [Google Scholar]
- Goverover Y, Johnston MV, Toglia J, Deluca J. Treatment to improve self-awareness in persons with acquired brain injury. Brain Inj. 2007;21(9):913–923. doi: 10.1080/02699050701553205. 781500374 [pii] [DOI] [PubMed] [Google Scholar]
- Hacker DJ. Definitions and empirical foundations. In: Hacker JDDJ, Graesser AC, editors. Metacognition in Educational Theory and Practice. Mahwah, N.J: Lawrence Erlbaum Associates; 1998. pp. 1–23. [Google Scholar]
- Heaton RK, Clifford DB, Franklin DR, Jr, Woods SP, Ake C, Vaida F, Grant I. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75(23):2087–2096. doi: 10.1212/WNL.0b013e318200d727. 75/23/2087 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Marcotte TD, Mindt MR, Sadek J, Moore DJ, Bentley H, McCuthan JA, Reicks C, Grant I. The impact of HIV-associated neuropsychological impairment on everyday functioning. J Int Neuropsychol Soc. 2004;10(3):317–331. doi: 10.1017/S1355617704102130S1355617704102130. pii. [DOI] [PubMed] [Google Scholar]
- Henry BL, Minassian A, Perry W. Effect of methamphetamine dependence on everyday functional ability. Addict Behav. 2010;35(6):593–598. doi: 10.1016/j.addbeh.2010.01.013. S0306-4603(10)00032-8 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkin CH, Hardy DJ, Mason KI, Castellon SA, Durvasula RS, Lam MN, Stefaniak M. Medication adherence in HIV-infected adults: effect of patient age, cognitive status, and substance abuse. AIDS. 2004;18(Suppl 1):S19–25. doi: 10.1097/00002030-200418001-00004. 00002030-200418001-00004 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkin CH, Barclay TR, Castellon SA, Levine AJ, Durvasula RS, Marion SD, Longshore D. Drug use and medication adherence among HIV-1 infected individuals. AIDS and Behavior. 2007;11(2):185–194. doi: 10.1007/s10461-006-9152-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iudicello JE, Morgan EE, Gongvatana A, Letendre SL, Grant I, Woods SP Translational Methamphetamine, Aids Research Center Group. Detrimental impact of remote methamphetamine dependence on neurocognitive and everyday functioning in older but not younger HIV+ adults: evidence for a legacy effect? J Neurovirol. 2014;20(1):85–98. doi: 10.1007/s13365-014-0233-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernigan TL, Gamst AC, Archibald SL, Fennema-Notestine C, Mindt MR, Marcotte TD, Heaton RK, Ellis RJ, Grant I. Effects of methamphetamine dependence and HIV infection on cerebral morphology. Am J Psychiatry. 2005;162(8):1461–1472. doi: 10.1176/appi.ajp.162.8.1461. 162/8/1461 [pii] [DOI] [PubMed] [Google Scholar]
- Kelley WM, Macrae CN, Wyland CL, Caglar S, Inati S, Heatherton TF. Finding the self? An event-related fMRI study. J Cogn Neurosci. 2002;14(5):785–794. doi: 10.1162/08989290260138672. [DOI] [PubMed] [Google Scholar]
- Kelly AM, Garavan H. Human functional neuroimaging of brain changes associated with practice. Cereb Cortex. 2005;15(8):1089–1102. doi: 10.1093/cercor/bhi005. [DOI] [PubMed] [Google Scholar]
- Kliegel M, McDaniel MA, Einstein GO. Plan formation, retention, and execution in prospective memory: a new approach and age-related effects. Mem Cognit. 2000;28(6):1041–1049. doi: 10.3758/bf03209352. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35(1):217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langford D, Hurford R, Hashimoto M, Digicaylioglu M, Masliah E. Signalling crosstalk in FGF2-mediated protection of endothelial cells from HIV-gp120. BMC Neurosci. 2005;6:8. doi: 10.1186/1471-2202-6-8. 1471-2202-6-8 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Berre AP, Pinon K, Vabret F, Pitel AL, Allain P, Eustache F, Beaunieux H. Study of metamemory in patients with chronic alcoholism using a feeling-of-knowing episodic memory task. Alcohol Clin Exp Res. 2010;34(11):1888–1898. doi: 10.1111/j.1530-0277.2010.01277.x. [DOI] [PubMed] [Google Scholar]
- Levine B, Robertson IH, Clare L, Carter G, Hong J, Wilson BA, Duncan J, Stuss DT. Rehabilitation of executive functioning: an experimental-clinical validation of goal management training. J Int Neuropsychol Soc. 2000;6(3):299–312. doi: 10.1017/s1355617700633052. [DOI] [PubMed] [Google Scholar]
- Lewden C. Mortality Working Group of COHERE. Time with CD4 cell count above 500 cells/mm3 allows HIV-infected men, but not women to reach similar mortality rates to those of the general populations: A seven year analysis. Paper presented at the Conference on Retroviruses and Opportunistic Infections; San Francisco, CA. 2010. [Google Scholar]
- Lysaker PH, Warman DM, Dimaggio G, Procacci M, Larocco VA, Clark LK, Dike CA, Nicolo G. Metacognition in schizophrenia: associations with multiple assessments of executive function. J Nerv Ment Dis. 2008;196(5):384–389. doi: 10.1097/NMD.0b013e318171091600005053-200805000-00004. pii. [DOI] [PubMed] [Google Scholar]
- Meyer DE, Kieras DE. A computational theory of executive cognitive processes and multiple-task performance: Part 1. Basic mechanisms. Psychol Rev. 1997;104(1):3–65. doi: 10.1037/0033-295x.104.1.3. [DOI] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex “Frontal Lobe” tasks: a latent variable analysis. Cogn Psychol. 2000;41(1):49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Norman DA, Shallice T. Attention to action. Center for Human Information Processing; 1980. [Google Scholar]
- Ownsworth T, Fleming J, Desbois J, Strong J, Kuipers P. A metacognitive contextual intervention to enhance error awareness and functional outcome following traumatic brain injury: a single-case experimental design. J Int Neuropsychol Soc. 2006;12(1):54–63. doi: 10.1017/S135561770606005X. S135561770606005X [pii] [DOI] [PubMed] [Google Scholar]
- Ownsworth T, Quinn H, Fleming J, Kendall M, Shum D. Error self-regulation following traumatic brain injury: a single case study evaluation of metacognitive skills training and behavioural practice interventions. Neuropsychol Rehabil. 2010;20(1):59–80. doi: 10.1080/09602010902949223. 912451940 [pii] [DOI] [PubMed] [Google Scholar]
- Petersen SE, van Mier H, Fiez JA, Raichle ME. The effects of practice on the functional anatomy of task performance. Proc Natl Acad Sci U S A. 1998;95(3):853–860. doi: 10.1073/pnas.95.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics. 1975;31(1):103–115. [PubMed] [Google Scholar]
- Prigatano GP, Wong JL. Cognitive and affective improvement in brain dysfunctional patients who achieve inpatient rehabilitation goals. Archives of Physical Medicine and Rehabilitation. 1999;80(1):77–84. doi: 10.1016/s0003-9993(99)90311-8. [DOI] [PubMed] [Google Scholar]
- Purcell DW, Parsons JT, Halkitis PN, Mizuno Y, Woods WJ. Substance use and sexual transmission risk behavior of HIV-positive men who have sex with men. J Subst Abuse. 2001;13(1–2):185–200. doi: 10.1016/s0899-3289(01)00072-4. [DOI] [PubMed] [Google Scholar]
- Purohit V, Rapaka R, Shurtleff D. Drugs of abuse, dopamine, and HIV-associated neurocognitive disorders/HIV-associated dementia. Mol Neurobiol. 2011;44(1):102–110. doi: 10.1007/s12035-011-8195-z. [DOI] [PubMed] [Google Scholar]
- Rippeth JD, Heaton RK, Carey CL, Marcotte TD, Moore DJ, Gonzalez R, Wolfson T, Grant I. Methamphetamine dependence increases risk of neuropsychological impairment in HIV infected persons. J Int Neuropsychol Soc. 2004;10(1):1–14. doi: 10.1017/S1355617704101021S1355617704101021. pii. [DOI] [PubMed] [Google Scholar]
- Robertson IH, Ward T, Ridgeway V, Nimmo-Smirth I. The Test of Everyday Attention. Bury St. Emunds: Thames Valley Test Company; 1994. [Google Scholar]
- Robertson IH. Goal Management Training: A clinical manual. Cambridge, UK: PsyConsult; 1996. [Google Scholar]
- Romano J. Appropriate statistics for ordinal level data: Should we really be using t-test and Cohen’s d for evaluating group differences on the NSSE and other surveys?. Paper presented at the Annual meeting of the Florida Association of Institutional Research; Florida. 2006. [Google Scholar]
- Scott JC, Woods SP, Matt GE, Meyer RA, Heaton RK, Atkinson JH, Grant I. Neurocognitive effects of methamphetamine: a critical review and meta-analysis. Neuropsychol Rev. 2007;17(3):275–297. doi: 10.1007/s11065-007-9031-0. [DOI] [PubMed] [Google Scholar]
- Scott JC, Woods SP, Vigil O, Heaton RK, Schweinsburg BC, Ellis RJ, Marcotte TD. A neuropsychological investigation of multitasking in HIV infection: implications for everyday functioning. Neuropsychology. 2011;25(4):511–519. doi: 10.1037/a0022491. 2011-04901-001 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shallice T, Burgess PW. Deficits in strategy application following frontal lobe damage in man. Brain. 1991;114(Pt 2):727–741. doi: 10.1093/brain/114.2.727. [DOI] [PubMed] [Google Scholar]
- Siegal S, Castellan NJ. Nonparametric Statistics for the Behavioral Sciences. 2. McGraw-Hill Humanities/Social Sciences/Languages; 1988. [Google Scholar]
- Stuss DT. Functions of the frontal lobes: relation to executive functions. J Int Neuropsychol Soc. 2011;17(5):759–765. doi: 10.1017/S1355617711000695. S1355617711000695 [pii] [DOI] [PubMed] [Google Scholar]
- Taylor MJ, Schweinsburg BC, Alhassoon OM, Gongvatana A, Brown GG, Young-Casey C, Letendre SL, Grant I. Effects of human immunodeficiency virus and methamphetamine on cerebral metabolites measured with magnetic resonance spectroscopy. J Neurovirol. 2007;13(2):150–159. doi: 10.1080/13550280701194230. 778725301 [pii] [DOI] [PubMed] [Google Scholar]
- Toglia J, Kirk U. Understanding awareness deficits following brain injury. NeuroRehabilitation. 2000;15(1):57–70. [PubMed] [Google Scholar]
- Vance DE, Fazeli PL, Ross LA, Wadley VG, Ball KK. Speed of processing training with middle-age and older adults with HIV: a pilot study. Journal of the Association of Nurses in AIDS Care. 2012;23(6):500–510. doi: 10.1016/j.jana.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber E, Blackstone K, Woods SP. Cognitive Neurorehabilitation of HIV-associated Neurocognitive Disorders: A Qualitative Review and Call to Action. Neuropsychol Rev. 2013;23(1):81–98. doi: 10.1007/s11065-013-9225-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber E, Morgan EE, Iudicello JE, Blackstone K, Grant I, Ellis RJ, Letendre SL, Little S, Morris S, Smith DM, Moore DJ, Woods SP the TMARC Group. Substance use is a risk factor for neurocognitive deficits and neuropsychiatric distress in acute and early HIV infection. J Neurovirol. 2013;19(1):65–74. doi: 10.1007/s13365-012-0141-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Composite International Diagnostic Interview, version 2.1. Geneva, Switzerland: World Health Organization; 1997. [Google Scholar]