Abstract
Signal transducer and activator of transcription 3 (STAT3) is constitutively activated in numerous cancer types, including more than 40% of breast cancers. In contrast to tight regulation of STAT3 as a latent transcription factor in normal cells, its signaling in breast cancer oncogenesis is multifaceted. Signaling through the IL6/JAK/STAT3 pathway initiated by the binding of IL6 family of cytokines (i.e., IL-6, IL-11) to their receptors have been implicated in breast cancer development. Receptors with intrinsic kinase activity such as EGFR and VEGFR directly or indirectly induce STAT3 activation in various breast cancer types. Aberrant STAT3 signaling promotes breast tumor progression through deregulation of the expression of downstream target genes which control proliferation (Bcl-2, Bcl-xL, Survivin, Cyclin D1, c-Myc, Mcl-1), angiogenesis (Hif1α, VEGF), and epithelial-mesenchymal transition (Vimentin, TWIST, MMP-9, MMP-7). These multiple modes of STAT3 regulation therefore make it a central linking point for a multitude of signaling processes. Extensive efforts to target STAT3 activation in breast cancer had no remarkable success in the past because the highly-interconnected nature of STAT3 signaling introduces lack of selectivity in pathway identification for STAT3 targeted molecular therapies or because its role in tumorigenesis may not be as critical as it was thought. This review provides a full spectrum of STAT3’s involvement in breast cancer by consolidating the knowledge about its role in breast cancer development at multiple levels: its differential regulation by different receptor signaling pathways, its downstream target genes, and modification of its transcriptional activity by its co-regulatory transcription factors.
Keywords: STAT3, transcription factor, regulation, target genes, breast cancer, tumor growth
The Signal Transducer and Activator of Transcription (STAT) family of transcription factors integrate cytokine and growth factor signaling to transcriptionally regulate a diverse array of cellular processes. STAT3, one of the seven members of the STAT family is constitutively activated in all breast cancer subtypes but it is most often associated with triple negative tumors, which lack the expression of the estrogen receptor (ER) and progesterone receptor (PR), and do not display amplification of HER2/neu receptor (1, 2). Studies to modulate constitutive STAT3 activation by genetic and pharmacological approaches have provided compelling evidence for STAT3’s critical role in cell proliferation, apoptosis, angiogenesis, immune response and metastasis in breast cancer (BC) (3). STAT3 is activated by phosphorylation of its tyrosine and serine residues via signaling from upstream regulators (4, 5). This phosphorylation event induces dimerization between two STAT3 molecules via reciprocal phosphotyrosine-SH2 (Src homology domain 2) interactions. Activated STAT3 dimers then translocate to the nucleus and bind to the consensus promoter sequence of their target genes to initiate transcription, Figure 1 (4–7).
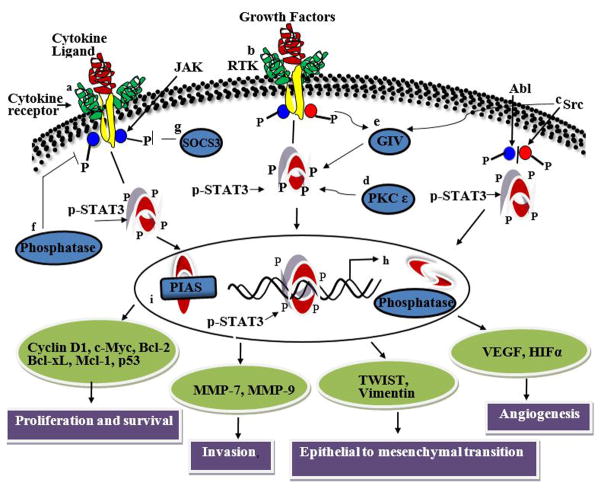
Figure 1.
Schematic representation of STAT3 activation and its pathways. STAT3 is activated by (a) cytokines (e.g., IL6 and non-IL6 family members), (b) growth factors for receptor tyrosine kinases (e.g., EGF, PDGF, VEGF), (c) non receptor tyrosine kinases (e.g., Src), (d) serine kinases (e.g., PKCε), and (e) Gα-interacting vesicle-associated protein (GIV/Girdin)/guanine nucleotide exchange factor (GEF). Binding of extracellular ligands to their cognate cell surface receptors trigger the signaling events that can lead to the phosphorylation of STAT3 molecules. Tyrosine or serine residue phosphorylation activates STAT3, which then forms homodimers and translocates to the nucleus to bind to the consensus sequence of its downstream target genes. The negative regulators of STAT3 (f) protein phosphatases and (g) suppressor of cytokine signaling (SOCS3) block STAT3 activation in the cytoplasm. In the nucleus, (h) nuclear phosphatases can mediate STAT3 dephosphorylation and block phosphorylation. Interactions with proteins such as (i) PIAS also inhibit STAT3 phosphorylation.
This review aims to consolidate a plethora of evidences available from clinical, in vivo and in vitro studies on the role of constitutively activated STAT3 in BC. STAT3 is involved in many cancer types but this review solely focuses on its involvement in BC. We first discuss how STAT3 is activated by i) IL-6 and non-IL-6 family of inflammatory cytokines, and ii) receptor tyrosine kinases, non-receptor tyrosine kinases, serine kinases and G-protein signaling. This is followed by the discussion of the regulation of STAT3’s activity by its negative regulators, which are protein tyrosine phosphatases (PTPs), suppressors of cytokine signaling (SOCS) and protein inhibitor of activated STATs (PIASs). We then discuss how constitutive activation of STAT3 is involved in the regulation of downstream target gene expression in BC. Areas where future research might be highly beneficial are emphasized. Filling these knowledge gaps can improve our understanding and help to design more pertinent studies related to targeting STAT3 for therapeutic interventions.
Activation of STAT3 through secretion of cytokines
IL6 family of cytokines
Chronic low grade inflammation are associated with pathogenesis of breast cancer (8). IL6 family cytokines, which includes IL-6, IL-8, IL-11 and Oncostatin, among others, are key players in this context (9). The tumor microenvironment including bone marrow derived cells, adipocytes, fibroblasts and cancer cells themselves are the source of these proinflammatory cytokines (8). These cytokines are secreted in autocrine and paracrine fashion, and they activate STAT3 in BC cells, Figure 2. BC cell lines exposed to the conditioned medium obtained from cells with high phosphorylated STAT3 (pSTAT3) levels have confirmed the involvement of such autocrine and paracrine signaling (8). These cytokines promote BC development depending on the type of hormone or growth factor receptor present on the surface of the cells (8). In the triple-negative BC subtype, the major mechanism of STAT3 activation is through autocrine and paracrine production of the IL6 family of cytokines (10–12). Functionally the autocrine expressions of IL-6 and IL-8 are critical for anchorage independent growth and resistance to apoptosis (13). The paracrine signaling is the principal mediator of increased STAT3 phosphorylation when ER-α positive breast tumor cells with low basal pSTAT3 levels is co-cultured with mesenchymal stem cells (14). During such paracrine signaling JAK/STAT3 pathway activation is induced by soluble factors secreted from both BC epithelial and associated fibroblast cells with elevated STAT3 phosphorylation (11, 12, 15). Activation of STAT3 under these conditions could be blocked by siRNA for STAT3 gene or neutralizing antibodies targeted against the effector cytokine (16–18).
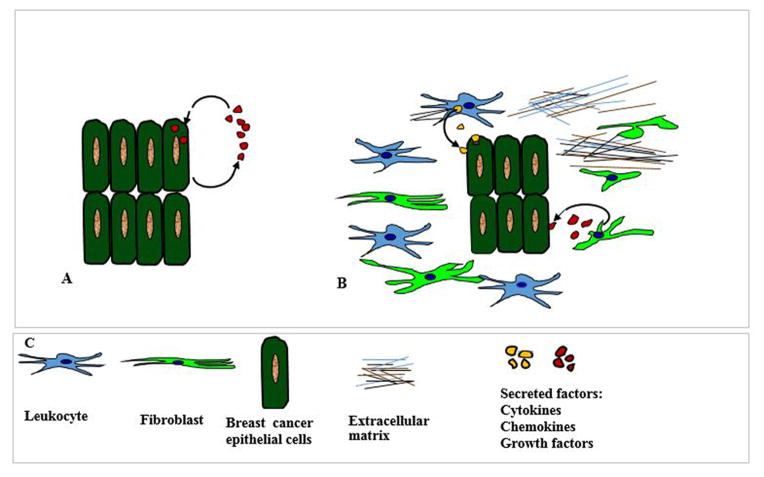
Figure 2.
Major mechanism of STAT3 activation in tumor cells occur by autocrine and paracrine secretion of soluble factors. This figure is a diagrammatic illustration of how autocrine and paracrine signaling activates STAT3 in breast cancer cells. (A) Schematic diagram showing the secretion and uptake of autocrine cytokines, chemokines and growth factors by the same cells. Breast cancer cells themselves secrete soluble factors like cytokines, chemokines and growth factors in the tumor microenvironment. These soluble factors behave as ligands and bind to their receptors present on the surface of the same cell which secreted them. Existence of autocrine loops eliminates the dependence on the availability of external secreted factors for growth and survival. (B) Diagrammatic representation of paracrine mechanism by which secreted factors from the nearby neighboring cells are taken up by breast cancer cells. Paracrine factors are produced by the stroma, fibroblast, leukocyte and infiltrating inflammatory cells. In the paracrine mechanism, cancer cells depend on the availability of secreted factors from the extracellular milieu for their survival. Both autocrine and paracrine signaling can lead to the aggressive growth of cancer cells. (C) Diagrams depict different cell types and components that are typically present in breast tumor microenvironment.
IL-6 regulates cancer stem cell (CSC) self-renewal and promotes breast CSC survival and proliferation when activated by the Notch, Wnt, Hedgehog and TGF-β signaling pathways (8, 19, 20). A clinical study showed that IL-6 is a novel Notch target gene in breast tumor cells and that hyper-activated Notch signaling upregulates IL-6 via the JAK/STAT3 pathway when p53 is mutated (21). High nuclear Notch1 ICD (intracellular domain of the Notch receptor) immunoreactivity, enhanced IL6 mRNA and pSTAT3 levels were observed in a panel of BC tissue samples from ER-α and PR negative patients (21). Other IL6 cytokine family members Oncostatin M (OSM) and IL-11 are functionally associated with cytokine-induced promotion of BC metastasis. IL-11 predominantly functions under hypoxic conditions to regulate remodeling of the extracellular matrix and the epithelial-mesenchymal transition (EMT) (22, 23).
IL6 family of cytokines thus play a role in BC progression in a STAT3 dependent manner. Further studies which profile the array of secreted factors which activate STAT3 in BC pathogenesis could be extremely valuable to develop alternative therapies.
Non-IL6 family of cytokines
Several recent studies have provided evidence that non IL-6 family cytokines IL-10 and IL-32 too have a role in STAT3 activation in BC development. A study conducted to determine the connection between infectious agents and BC development found that viral cmvIL-10 infected cells of human cytomegalovirus (HCMV) positive patients secrete cmvIL-10 to alter the tumor microenvironment in paracrine manner (24). Secreted viral cytokine potentially impacts neighboring cells by activating the IL-10R pathway causing overstimulation of STAT3, enabling tumor cells to grow uncontrollably and resist the induction of apoptosis (24). MDA-MB-231 breast cancer cells cultured in the presence of increasing doses of cmvIL-10 exhibited greater growth, and treatment of cells with either a STAT3 or JAK1 inhibitor blocked the proliferative effects of cmvIL-10, which confirmed that these results were mediated in part by the JAK1/STAT3 signaling cascade (24). Another clinical study which utilized 117 primary breast tumor tissues reported that overexpression of IL-10 conferred tumor growth (25). Since IL-10 is a multifunctional cytokine with both immunosuppressive and anti-angiogenic functions, higher IL-10 expression is associated with reduced number of tumor infiltrating lymphocytes (25). It protects against cytotoxic T lymphocytes by down-regulation of class I and class II major histocompatibility complex (MHC) molecules, thus decreasing the probability of many abnormal peptides produced by cancer cells to be displayed on cell surface to alert the immune system for systematic removal (25). Besides it also promotes resistance to apoptosis (25, 26). IL-10 gene promoter polymorphism were significantly associated with prognostic and predictive factors in a population of Han Chinese BC patients (27).
IL-32β is a pro-inflammatory cytokine that induces secretion of TNF-α and IL-6 by monocytes and macrophages (28). Immunohistochemical (IHC) analysis of primary BC samples showed that the expression of IL-32β positively correlated with tumor size, number of lymph nodes, metastases and tumor stage (29). Under in vitro conditions BC cells expressing exogenous IL-32β exhibited increased migration and invasion, which was associated with enhanced VEGF secretion that activated STAT3 (29). This finding pointed to the involvement of an additional IL-32β-VEGF-STAT3 axis in migration and invasion of BC cells under normoxic and hypoxic conditions (29).
Non-IL6 family of cytokines thus activate STAT3 either directly or indirectly. Therefore, systematic studies which will explore how different classes of cytokines co-regulate the involvement of STAT3 in BC would be very informative.
Activation of STAT3 by receptor tyrosine kinases with intrinsic kinase activity
Various receptor tyrosine kinases (RTKs) with intrinsic tyrosine kinase activity stimulate phosphorylation of STAT3 in BC pathology, including the human epidermal growth factor receptor (EGFR/HER/ErbB) family of receptors and vascular endothelial growth factor receptor (VEGFR) (1, 30).
STAT3 is a central node of the signaling pathways of the HER family of receptors (31). Studies with clinical samples and cell culture models both assert the connection between HER family of receptors and activated STAT3. These studies have established that, when activated simultaneously, HER family receptors and STAT3 form transcriptional complexes and regulate target gene expressions in aggressive cancers (32). These studies have also reported that this cooperation can lead to generation of cancer stem cells (33). Of the four members of the family, expression of its first member EGFR (i.e., HER1/ErbB1) showed a strong association with nuclear STAT3 activation in IHC analysis of primary breast carcinoma samples (32). This assertion was supported with studies on experimental models where interplay between EGFR and STAT3 has been suggested to regulate the expression of TWIST gene, which is responsible for epithelial to mesenchymal transition of BC cells (34).
The second member of the HER family, HER2/Erb2/neu proto-oncogene is amplified in about 25–30% of human BCs and its role may be dependent on the status of hormone receptors ER and PR. A study with both clinical samples and a model BC cell line addressed whether HER2 influenced the activation of STAT3 (33). When 71 ER+ clinical samples were examined, overexpression of HER2 and constitutive phosphorylation of STAT3 in primary BC tissue microarray slides was observed (33). This finding established the involvement of a HER2-ER-STAT3 signaling axis in HER2+ BC subtype (33). Similar findings was observed with model BC cells overexpressing HER2 and ER where HER2-ER-STAT3 activation caused a reduction in epithelial phenotype, increased stem cell like characteristics and created a mesenchymal CD44hi and CD24low microenvironment while expression of Oct-4, Sox-2, and CD44 (which are stem cell markers and indicators of cancer progression to metastasis) were deregulated (33). Similar studies have established that STAT3 is a downstream target of both PR and HER2 (35). Activation of PR by progestin induced nuclear localization of HER2 where it interacts with STAT3 to form a transcriptional complex (35). As a coactivator of STAT3 in the transcriptional complex, HER2 controls proliferation of breast tumors by regulating the expression of cyclin D1 genes (35). HER2 overexpression studies using tumors and model tumor cells found that HER2 activated inflammatory pathways along with other pathways, which synergistically affected IL-6 secretion and STAT3 activation (36). This finding suggested that HER2-IL6-STAT3 autocrine loop is associated with cellular transcriptional changes, anchorage-independent growth and enhanced tumor growth.
VEGF/VEGFR-2 signaling functions as an important survival pathway in BC cells (37). Analysis of a large cohort of primary BC samples (> 1300) showed that VEGF promoted tumor initiating cell self-renewal through VEGFR-2/STAT3 signaling by up-regulating the transcription of Myc, Sox2 and STAT3 (38). High VEGF levels strongly correlated with both STAT3 and Myc expression in these tumor samples (38). Up-regulated expression of Myc and Sox2 contributed to the sustained activation of other transcription factors, which created a feed-forward activation loop driving tumor initiating cell’s self-renewal processes (38). The expression of VEGF and VEGFR-2 may be linked to the adipocytokine Leptin mediated STAT3 activation (39). STAT3 activation in BC may be stemming from Leptin’s role in proliferation and angiogenesis (39–41). Higher levels of leptins correlate with metastasis and lower survival of BC patients (42–45).
When considered together, these findings reveal that EGFR and VEGFR pathways either directly or indirectly crosstalk at different levels in the activation of STAT3, most likely leading to a more aggressive tumor behavior. Further studies in this direction may elucidate the pathologic significance of co-expression of these molecules in patient tumor tissue samples. Such information may help to design therapeutic modalities for synergistic targeting of perturbed pathways to more accurately sensitize tumor cells (46–48).
Activation of STAT3 by non-receptor tyrosine kinases
Src is a non-receptor protein tyrosine kinase which phosphorylates specific tyrosine residues in other proteins including STAT3. Studies have identified important roles for elevated activities of STAT3 and Src in malignant progression of BC (49). A study which enrolled 45 patients with invasive carcinoma showed higher levels of activated STAT3 and Src in tumor tissue than in non-neoplastic ones, and increases in the phosphorylation of STAT3 and Src were correlated (49, 50). Src and JAK family tyrosine kinases cooperate to mediate constitutive STAT3 activation in the absence of EGF induced stimulation in model human BC cell lines (50). IHC detection for c-Src performed on 57 invasive lobular BC lesions showed that increased c-Src activity correlated with activation of STAT3, which is thought to contribute to c-Src-mediated invasion (51). HER2 mediated STAT3 activation may occur through recruitment of non-RTKs like c-Src (52). Several studies have also suggested that there is a cooperation between HER2, Src and STAT3 signaling pathways in BC oncogenesis (51, 53). Existence of hetero-complexes between HER2, STAT3 and Src has been detected by co-immunoprecipitation in SKBR3 cells and in HER2 overexpressing MDA-MB-435 cells (54, 55). Activation of the HER2-Src-STAT3 pathway was found to be critical for HER2 mediated chemoresistance (51, 53–55). These studies have also shown that elevated STAT3 and Src activity correlate with pathologic responses, which suggests that their specific inhibition could offer clinical advantage to BC patients.
Activation by serine kinase PKCε
PKCε is an oncogene whose activation is an initial step for the constitutive STAT3 phosphorylation (56, 57). Its partnership with STAT3 maintains cell invasion in various cancer types, including BC (58, 59). PKCε was shown to physically interact with STAT3 at the S727 residue in human BC cells MCF-7 and MDA-MB-231 (56). This PKCε mediated phosphorylation of STAT3 leads to STAT3-regulated gene expression and maintenance of cell invasion by integration with MAPK cascade (Raf-1, MEK1/2 and Erk1/2) signaling (56). Dependence of STAT3-promoted cell growth on STAT3’s phosphorylation at its S727 residue is also important in tumor-initiating BC cells (60, 61). Further mechanistic studies on the interaction of STAT3 with other PKC family members may provide crucial information on whether other members also participate in cancer related processes via activation of STAT3, and the development of novel drugs which would block STAT3 activation through its interactions with PKCs could therefore be explored.
G-protein signaling and STAT3 activation
Gα-interacting vesicle-associated protein (GIV/Girdin) is a multi-domain signal transducer and a novel guanine nucleotide exchange factor (GEF) that plays a role in cell migration via its ability to scaffold key signaling molecules, i.e., trimeric G-proteins, growth factor receptors, PI3K, Akt, and phosphoinositides, with the actin cytoskeleton (62). GIV protein was expressed positively in 295 of the 820 cases (36%) of BC examined. In cases where GIV was highly expressed, distant metastasis rate was significantly higher than cases with low GIV expression (63). STAT3 activation and GIV expression showed statistically significant correlation at every stage of BC progression implying that GIV is a direct target of STAT3 (62, 64). Transcriptional targets of STAT3, such as RTKs (EGFR), G-protein coupled receptors, and growth factors (VEGF) could further enhance STAT3 signaling through GIV involvement by feed forward regulation (62). Feed forward mechanism here implies that STAT3 up-regulates GIV transcription and that GIV in turn enhances STAT3 activation via its GEF function. The feed forward regulation between GIV and STAT3 could have therapeutic implications for cancer and epithelial tissue regeneration/repair. Further co-expression studies of GIV and STAT3 in patient samples can elucidate whether STAT3-GIV interaction occurs in invading BC.
Role of negative regulators
Protein tyrosine phosphatases (PTPs)
Since phosphorylation and dephosphorylation processes play opposing roles, like kinases, PTPs play a major role in tightly regulating phosphorylated STAT3 levels in cells. TCPTP (PTPN2) potentially serves as a tumor suppressor by decreasing phosphorylation of its substrates, which include RTKs and Src family of kinases (SFKs) (65). A decrease in the level of TCPTP protein was reported in a subset of BC cell lines, and it was absent in a large percentage of triple-negative primary human BCs (65). TCPTP deficiency in human BC cell lines increased SFK and STAT3 signaling and its reconstitution severely impaired cell proliferation and suppressed anchorage-independent growth in vitro and xenograft growth in vivo (65). It has also been shown that down-regulated activity of PTPN9 (non-receptor type 9 PTP; PTPMeg2) is correlated with elevated phosphorylation of STAT3 in BC cells (66). Understanding the molecular mechanisms of regulation of PTPs, dual-specificity phosphatases and low molecular weight phosphatases in both normal and BC tissues would provide a means to increase their levels in triple-negative BC. Increasing PTP levels in combination with the use of kinase inhibitors may constitute an effective therapeutic strategy for aggressive BC phenotypes.
Suppressors of cytokine signaling (SOCS)
Cytokines are important for breast cell function, both as trophic hormones and as mediators of host defense mechanism against BC (5). In BC, loss of SOCS expression is associated with poor clinical outcome (67). SOCS proteins are rapidly induced upon STAT3 activation (5). They suppress the cytokine signals either by direct inhibition of JAKs or by binding to tyrosine phosphorylated receptors thus blocking the binding of other SH2 and PTB domain-containing signaling proteins (5). This negative feedback through SOCS decreases cell sensitivity to cytokines (5), and it appears to be necessary to suppress inflammation and cellular proliferation (67). IHC staining for pSTAT3 antigen and mRNA expression of SOCS-1 and SOCS-3 in 74 surgically dissected patient tumor samples showed no statistical correlation between either SOCS-1 or SOCS-3 mRNA and pSTAT3 expression but reduced expression of SOCS-3 was related to lymph node metastasis thereby indicating that SOCS-3 may be a good predictor for lymph node metastasis (67). A related cross-sectional study was performed in biopsies of 26 patients with BC and 43 patients with benign breast lesions to determine the levels of JAK2, STAT3 and SOCS3 gene expression using RT-qPCR. High levels of STAT3 expression were associated with the early stages of BC development and patients in the control group with obesity showed higher expression of SOCS-3, thus suggesting that SOCS-3 expression can be used as biomarker for risk assessment in obese overweight subjects (68). SOCS-3 was found to be inhibiting the expression of anti-apoptotic gene Survivin in Leptin-mediated activation of the JAK2/STAT3 signaling pathway in MCF-7 BC cells (69). Correlation between Survivin and SOCS-3 expression in Leptin expressing BC patient samples are needed to confirm this finding in a clinical context.
It should also be noted that promoter of SOCS3 gene is often found silenced by methylation in cancers and SOCS3 silencing allows upregulated cell growth (70). Methylation status of SOCS3 promoter has not yet been examined in any patient breast tumor samples although hypermethylation of SOCS3 promoter has been reported for hepatocellular cancer (70, 71).
Protein inhibitor of activated STATs (PIASs)
PIASs are endogenous inhibitors of STAT proteins (72). PIAS3 controls the extent and duration of STAT3 activity in normal cells and prevents its oncogenic function (72, 73). Its expression is post-transcriptionally suppressed in cancer cells, possibly enhancing the oncogenic effects of activated STAT3 (73). MicroRNA miRNA-21, which functionally regulates immune cell recruitment, targets PIAS3 in MCF-7 cells and acts at least in part via its inhibition of PIAS3 expression and oncogenic STAT3 signaling in tumor cells (74). Besides this mechanistic study, not much is known yet about the regulation of STAT3 activity by PIAS in BC. Further research on how the activation of PIAS is suppressed post-transcriptionally either by microRNA or other mechanisms would help to establish whether PIAS can be considered as novel therapeutic targets in BC.
Constitutive activation of STAT3 and regulation of gene expression
STAT3 can regulate gene expression either directly or indirectly through other transcription factors (75). Activated STAT3 translocates to cell nucleus and binds to the interferon-gamma activated sequences of the target promoters to elicit gene transcription (76). A differential pattern of STAT3-dependent target gene expression is observed in various types of BC, which indicates STAT3 as a potential subtype specific regulator of BC malignancy (76, 77). For example, IL-6/JAK2/STAT3 pathway was preferentially active in CD44+CD24− breast cancer cells that have stem cell-like characteristics over CD44−CD24+ cells that resemble more differentiated breast cancer cells and inhibition of several of the STAT3 dependent genes (IL6, PTGIS, HAS1, CXCL3, and PFKFB3) reduced STAT3 activation in these cells (78). STAT3 thus may function as tumor suppressor or may have an opposing effect and promote tumorigenesis depending on cellular context and tumor type.
A recent bioinformatics analysis revealed that basal-like BCs showed a distinct pattern of STAT3-associated gene expression in comparison to luminal A and luminal B type BCs (77). The analysis elucidated that STAT3 signaling may be intricately linked with inflammatory processes in basal BCs but not in luminal A or luminal B subtypes. However, this pattern was not apparent in epithelial BCs (77).
Studies performed in both tumor specimens and BC cell lines identified several STAT3 dependent target genes that are involved in cell proliferation, apoptosis, survival, tumor metastasis/invasion, angiogenesis, and tumor suppression. A study of 45 primary tumor specimens from patients with invasive breast carcinoma showed a correlation between STAT3 activation and Survivin expression (79), which is a member of the inhibitor of apoptosis (IAP) family (79). One of the functions of Survivin (encoded by BIRC5 gene) is to inhibit caspase activation, leading to negative regulation of apoptosis or programmed cell death (79). A direct evidence for the correlation between STAT3 activation and elevated Cyclin D1 protein (encoded by CCND1 gene) expression in primary breast tumors and BC-derived cell lines was reported (80, 81). To control cell cycle progression from G1, D cyclins assemble with the cyclin-dependent kinases 4/6 (CDK4/6), phosphorylate substrates such as retinoblastoma protein (Rb), release E2F transcription factor and promote entry of cells to S-phase (80). Twist-related protein 1 (Twist1) is a basic helix-loop-helix (bHLH) transcription factor encoded by the TWIST gene in humans (34). bHLH transcription factors are implicated in cell lineage determination and differentiation (82). IHC analysis of 130 primary breast carcinoma indicated positive correlations between phosphorylated STAT3 and Twist (34). A similar correlation was observed when EGFR signaling induced binding of nuclear STAT3 to the promoter of TWIST, and EGFR signaling induced EMT via STAT3-mediated TWIST gene expression (34).
Matrix metalloproteinase (MMP) enzymes contribute to cell phenotypic responses by involving in the degradation of extracellular matrix proteins, cleavage of cell surface receptors and release of apoptotic ligands such as FAS (83). Stably transfected MCF-7 cells overexpressing HER2 receptors (MCF-7/HER2 cells) increased mRNA and protein expressions of MMP-7 when promoter activity of STAT3 was increased (83). MMP-7 promoter has three potential STAT3 binding sites and the observed transcriptional up-regulation and protein secretion of MMP-7 was attributed to STAT3 activation (83). This correlation between MMP-7 and STAT3 observed under in vitro conditions still needs to be validated using patient tumor samples. Stable expression of active STAT3 increased matrix metalloproteinase MMP-9 mRNA levels in MCF-7 cells, indicating that it too is regulated by STAT3 (84). STAT3/c-Jun and Fra-1/c-Jun complexes were identified in vivo and activation of MMP-9 promoter was dependent on interactions of Fra-1 and c-Jun with STAT3 (84).
STAT3 is a co-transcriptional activator to a large set of HIF target genes. STAT3 and HIF1α cooperatively activate VEGF and haptoglobin genes during hypoxia in BC cell lines (75). Similar mechanism of STAT3 and NFκB cooperation has been reported in Fascin gene expression that promotes migration of BC cells. STAT3 and NFκB were recruited to the promoter of Fascin and enhanced its expression when MDA-MB-231 cells were treated with IL-6 or OSM (85).
During efficacy testing of hydrazinocurcumin (HC), a synthetic curcumin analogue, inhibition of STAT3 phosphorylation in BC cell lines (MDA-MB-231, MCF-7) decreased the expression of c-Myc, Bcl-xL, Bcl-2, Mcl-1, cyclin D1/D2, VEGF, MMP-2, and MMP-9 among others (86, 87). Another compound benzyl isothiocyanate (BITC), a constituent of edible cruciferous vegetable, inhibited Leptin stimulated migration and invasion of MDA-MB-231 and MCF-7 human BC cells (88). BITC treatment suppressed leptin induced STAT3 phosphorylation and Cyclin D1 transactivation by inhibiting STAT3 recruitment to the Cyclin D1 promoter as detected in chromatin immunoprecipitation (ChIP) analysis (88). STAT3-siRNA induced Fas-mediated apoptosis in vitro and in vivo in BC cells and down-regulation of survival genes Bcl-xL and Survivin were observed (86).
Besides regulating downstream gene expression in its phosphorylated state, STAT3 may be involved in transcriptional regulation in its unphosphorylated form as well. Although data about involvement of unphosphorylated STAT3 in regulating gene expression in BC cells is not available, data obtained for other cells show that unphosphorylated STAT3 participates in transcriptional regulation processes by forming complexes with NFκB (89).
Although a large amount of information is available about STAT3 target gene deregulation in model BC cell lines, gene expression analysis of STAT3 downstream targets in primary tumor samples of patients are still rare. Validation of the exact binding site for STAT3 on promoter of genes that get deregulated in BC can lead to the development of targeted therapy using custom-designed triplex-forming oligonucleotides to selectively interfere with the binding of STAT3 to its target genes.
Future perspectives
Based on the available data and the progress discussed above, ways to improve our understanding of the involvement of STAT3 in BC can be categorized into four major areas: i) Validation of in vitro observations with in vivo studies, ii) identification of the transcription factors that are co-regulators of STAT3, iii) identification of still largely unknown mechanisms of how microRNA mediated regulation of STAT3 contributes to tumor progression and metastasis, and iv) improved profiling of the signaling pathways involved in STAT3 regulation and identification of their crosstalk to establish the cooperation between involved signaling processes.
Most of the studies that investigated the role of STAT3 in BC were pursued using in vitro approaches that utilized model cell lines or their engineered variants. These in vitro studies have established the correlations between activated STAT3 and other proteins, identified the involved signaling pathways and compiled the genes deregulated by STAT3 associated signaling. Unfortunately validation of these in vitro findings in clinical research has been lacking. For example, data about occupancy of target promoters by STAT3 is extremely rare for patient samples. Such data sets can be obtained in ChIP experiments followed by sequencing. This data would provide the information about genes which are direct targets of STAT3. Surprisingly, availability of such data in literature is extremely limited and studies in pursuit of such information could be very useful in better understanding the role of STAT3 in BC.
STAT3 as a transcription factor may target downstream genes in unison with other contextual regulators. Therefore, single candidate approaches for the diagnosis and treatment of BCs with activated STAT3 may not be sufficient for efficient therapy strategies and accurate detection of co-regulatory partners using improved detection and localization methods would be necessary. More emphasis should be placed on the development of a single diagnostic test capable of identifying vast arrays of deregulated proteins, genes, and their modulators simultaneously.
STAT3 is constitutively activated by various cellular mechanisms in BC. It is involved in a large number of phenotypic responses such as cell proliferation, survival, anti-apoptosis, angiogenesis and metastasis. Due to the close relationship and significant overlap between underlying signaling pathways, cells often utilize multiple pathways simultaneously. This allows cells to compensate for blocking a single pathway through pathway substitution, which may lead to the desensitization to blocking of a particular pathway. Therefore, establishing how STAT3 contributes to the regulation of cellular signaling pathways would be extremely beneficial in the target identification for combination therapy. For example, when the inhibition of STAT3 alone is not an efficient treatment, therapy may be adjusted to also inhibit the activity along the substituting pathway by targeting one of the sentinel proteins of that pathway for a much more efficient treatment. For improved clinical outcomes, targeting of the involved signaling nodes can be adjusted to develop optimal personalized therapy strategies.
Because of its involvement in a vast range of cellular processes, past studies have not yet led to a definite STAT3 targeting cancer drug. However, the involvement of STAT3 in breast and other cancers is unquestionably well established. Therefore, continued investigation of its role in cancer is warranted.
Acknowledgments
The research described in this paper was funded by the National Institutes of Health Grant 5R01GM072821 and by the WSU start-up funds to H.R.
Abbreviations used
- BC
breast cancer
- STAT3
Signal Transducer and Activator of Transcription 3
- pSTAT3
phospho-STAT3
- IL
Interleukin
- JAK
Janus Kinases
- EGFR
Epidermal Growth Factor Receptor
- VEGF
Vascular Endothelial Growth Factor
- VEGFR
Vascular Endothelial Growth Factor Receptor
- MMP
Matrix Metalloproteinases
- EMT
epithelial to mesenchymal transition
- IHC
immunohistochemical
- ER
Estrogen Receptor
- PTP
Protein Tyrosine Phosphatases
References
- 1.Levy DE, Darnell JE., Jr Stats: transcriptional control and biological impact. Nature reviews Molecular cell biology. 2002;3(9):651–62. doi: 10.1038/nrm909. [DOI] [PubMed] [Google Scholar]
- 2.Walker SR, Xiang M, Frank DA. Distinct roles of STAT3 and STAT5 in the pathogenesis and targeted therapy of breast cancer. Molecular and cellular endocrinology. 2014;382(1):616–21. doi: 10.1016/j.mce.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yue P, Turkson J. Targeting STAT3 in cancer: how successful are we? Expert opinion on investigational drugs. 2009;18(1):45–56. doi: 10.1517/13543780802565791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klemm JD, Schreiber SL, Crabtree GR. Dimerization as a regulatory mechanism in signal transduction. Annual review of immunology. 1998;16:569–92. doi: 10.1146/annurev.immunol.16.1.569. [DOI] [PubMed] [Google Scholar]
- 5.Rane SG, Reddy EP. Janus kinases: components of multiple signaling pathways. Oncogene. 2000;19(49):5662–79. doi: 10.1038/sj.onc.1203925. [DOI] [PubMed] [Google Scholar]
- 6.Yu H, Jove R. The STATs of cancer--new molecular targets come of age. Nature reviews Cancer. 2004;4(2):97–105. doi: 10.1038/nrc1275. [DOI] [PubMed] [Google Scholar]
- 7.Turkson J, Ryan D, Kim JS, Zhang Y, Chen Z, Haura E, et al. Phosphotyrosyl peptides block Stat3-mediated DNA binding activity, gene regulation, and cell transformation. The Journal of biological chemistry. 2001;276(48):45443–55. doi: 10.1074/jbc.M107527200. [DOI] [PubMed] [Google Scholar]
- 8.Dethlefsen C, Hojfeldt G, Hojman P. The role of intratumoral and systemic IL-6 in breast cancer. Breast cancer research and treatment. 2013;138(3):657–64. doi: 10.1007/s10549-013-2488-z. [DOI] [PubMed] [Google Scholar]
- 9.Bishop JL, Thaper D, Zoubeidi A. The Multifaceted Roles of STAT3 Signaling in the Progression of Prostate Cancer. Cancers. 2014;6(2):829–59. doi: 10.3390/cancers6020829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berishaj M, Gao SP, Ahmed S, Leslie K, Al-Ahmadie H, Gerald WL, et al. Stat3 is tyrosine-phosphorylated through the interleukin-6/glycoprotein 130/Janus kinase pathway in breast cancer. Breast cancer research : BCR. 2007;9(3):R32. doi: 10.1186/bcr1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang Q, Bournazou E, Sansone P, Berishaj M, Gao SP, Daly L, et al. The IL-6/JAK/Stat3 feed-forward loop drives tumorigenesis and metastasis. Neoplasia (New York, NY) 2013;15(7):848–62. doi: 10.1593/neo.13706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lieblein JC, Ball S, Hutzen B, Sasser AK, Lin HJ, Huang TH, et al. STAT3 can be activated through paracrine signaling in breast epithelial cells. BMC cancer. 2008;8:302. doi: 10.1186/1471-2407-8-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hartman ZC, Poage GM, den Hollander P, Tsimelzon A, Hill J, Panupinthu N, et al. Growth of triple-negative breast cancer cells relies upon coordinate autocrine expression of the proinflammatory cytokines IL-6 and IL-8. Cancer research. 2013;73(11):3470–80. doi: 10.1158/0008-5472.CAN-12-4524-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sasser AK, Sullivan NJ, Studebaker AW, Hendey LF, Axel AE, Hall BM. Interleukin-6 is a potent growth factor for ER-alpha-positive human breast cancer. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2007;21(13):3763–70. doi: 10.1096/fj.07-8832com. [DOI] [PubMed] [Google Scholar]
- 15.Schafer ZT, Brugge JS. IL-6 involvement in epithelial cancers. The Journal of clinical investigation. 2007;117(12):3660–3. doi: 10.1172/JCI34237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ling X, Arlinghaus RB. Knockdown of STAT3 expression by RNA interference inhibits the induction of breast tumors in immunocompetent mice. Cancer research. 2005;65(7):2532–6. doi: 10.1158/0008-5472.CAN-04-2425. [DOI] [PubMed] [Google Scholar]
- 17.Zhang X, Zhang J, Wei H, Tian Z. STAT3-decoy oligodeoxynucleotide inhibits the growth of human lung cancer via down-regulating its target genes. Oncology reports. 2007;17(6):1377–82. [PubMed] [Google Scholar]
- 18.Gu J, Li G, Sun T, Su Y, Zhang X, Shen J, et al. Blockage of the STAT3 signaling pathway with a decoy oligonucleotide suppresses growth of human malignant glioma cells. Journal of neuro-oncology. 2008;89(1):9–17. doi: 10.1007/s11060-008-9590-9. [DOI] [PubMed] [Google Scholar]
- 19.Guo S, Liu M, Gonzalez-Perez RR. Role of Notch and its oncogenic signaling crosstalk in breast cancer. Biochimica et biophysica acta. 2011;1815(2):197–213. doi: 10.1016/j.bbcan.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sansone P, Storci G, Tavolari S, Guarnieri T, Giovannini C, Taffurelli M, et al. IL-6 triggers malignant features in mammospheres from human ductal breast carcinoma and normal mammary gland. The Journal of clinical investigation. 2007;117(12):3988–4002. doi: 10.1172/JCI32533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jin S, Mutvei AP, Chivukula IV, Andersson ER, Ramskold D, Sandberg R, et al. Non-canonical Notch signaling activates IL-6/JAK/STAT signaling in breast tumor cells and is controlled by p53 and IKKalpha/IKKbeta. Oncogene. 2013;32(41):4892–902. doi: 10.1038/onc.2012.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu J, Spence MJ, Wallace PM, Forcier K, Hellstrom I, Vestal RE. Oncostatin M-specific receptor mediates inhibition of breast cancer cell growth and down-regulation of the c-myc proto-oncogene. Cell growth & differentiation : the molecular biology journal of the American Association for Cancer Research. 1997;8(6):667–76. [PubMed] [Google Scholar]
- 23.Lim JH. Inhibition of the Interleukin-11-STAT3 Axis Attenuates Hypoxia-Induced Migration and Invasion in MDA-MB-231 Breast Cancer Cells. The Korean journal of physiology & pharmacology : official journal of the Korean Physiological Society and the Korean Society of Pharmacology. 2014;18(5):391–6. doi: 10.4196/kjpp.2014.18.5.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Valle Oseguera CA, Spencer JV. cmvIL-10 stimulates the invasive potential of MDA-MB-231 breast cancer cells. PloS one. 2014;9(2):e88708. doi: 10.1371/journal.pone.0088708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu J, Wang L, Gao W, Li L, Cui X, Yang H, et al. Inhibitory receptor immunoglobulin-like transcript 4 was highly expressed in primary ductal and lobular breast cancer and significantly correlated with IL-10. Diagnostic pathology. 2014;9:85. doi: 10.1186/1746-1596-9-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Llanes-Fernandez L, Alvarez-Goyanes RI, del Arango-Prado MC, Alcocer-Gonzalez JM, Mojarrieta JC, Perez XE, et al. Relationship between IL-10 and tumor markers in breast cancer patients. Breast (Edinburgh, Scotland) 2006;15(4):482–9. doi: 10.1016/j.breast.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 27.Kong F, Liu J, Liu Y, Song B, Wang H, Liu W. Association of interleukin-10 gene polymorphisms with breast cancer in a Chinese population. Journal of experimental & clinical cancer research : CR. 2010;29:72. doi: 10.1186/1756-9966-29-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Player A, Oguamanam T, Okanmelu J, Burrell K, Hollomon M. Preliminary characterization of IL32 in basal-like/triple negative compared to other types of breast cell lines and tissues. BMC research notes. 2014;7:501. doi: 10.1186/1756-0500-7-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park JS, Choi SY, Lee JH, Lee M, Nam ES, Jeong AL, et al. Interleukin-32beta stimulates migration of MDA-MB-231 and MCF-7cells via the VEGF-STAT3 signaling pathway. Cellular oncology (Dordrecht) 2013;36(6):493–503. doi: 10.1007/s13402-013-0154-4. [DOI] [PubMed] [Google Scholar]
- 30.Katz M, Amit I, Yarden Y. Regulation of MAPKs by growth factors and receptor tyrosine kinases. Biochimica et biophysica acta. 2007;1773(8):1161–76. doi: 10.1016/j.bbamcr.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gong C, Zhang Y, Shankaran H, Resat H. Integrated analysis reveals that STAT3 is central to the crosstalk between HER/ErbB receptor signaling pathways in human mammary epithelial cells. Molecular bioSystems. 2015;11(1):146–58. doi: 10.1039/c4mb00471j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berclaz G, Altermatt HJ, Rohrbach V, Siragusa A, Dreher E, Smith PD. EGFR dependent expression of STAT3 (but not STAT1) in breast cancer. International journal of oncology. 2001;19(6):1155–60. doi: 10.3892/ijo.19.6.1155. [DOI] [PubMed] [Google Scholar]
- 33.Chung SS, Giehl N, Wu Y, Vadgama JV. STAT3 activation in HER2-overexpressing breast cancer promotes epithelial-mesenchymal transition and cancer stem cell traits. International journal of oncology. 2014;44(2):403–11. doi: 10.3892/ijo.2013.2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lo HW, Hsu SC, Xia W, Cao X, Shih JY, Wei Y, et al. Epidermal growth factor receptor cooperates with signal transducer and activator of transcription 3 to induce epithelial-mesenchymal transition in cancer cells via up-regulation of TWIST gene expression. Cancer research. 2007;67(19):9066–76. doi: 10.1158/0008-5472.CAN-07-0575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beguelin W, Diaz Flaque MC, Proietti CJ, Cayrol F, Rivas MA, Tkach M, et al. Progesterone receptor induces ErbB-2 nuclear translocation to promote breast cancer growth via a novel transcriptional effect: ErbB-2 function as a coactivator of Stat3. Molecular and cellular biology. 2010;30(23):5456–72. doi: 10.1128/MCB.00012-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hartman ZC, Yang XY, Glass O, Lei G, Osada T, Dave SS, et al. HER2 overexpression elicits a proinflammatory IL-6 autocrine signaling loop that is critical for tumorigenesis. Cancer research. 2011;71(13):4380–91. doi: 10.1158/0008-5472.CAN-11-0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guo S, Colbert LS, Fuller M, Zhang Y, Gonzalez-Perez RR. Vascular endothelial growth factor receptor-2 in breast cancer. Biochimica et biophysica acta. 2010;1806(1):108–21. doi: 10.1016/j.bbcan.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao D, Pan C, Sun J, Gilbert C, Drews-Elger K, Azzam DJ, et al. VEGF drives cancer-initiating stem cells through VEGFR-2/Stat3 signaling to upregulate Myc and Sox2. Oncogene. 2014 doi: 10.1038/onc.2014.257. [DOI] [PubMed] [Google Scholar]
- 39.Gonzalez-Perez RR, Lanier V, Newman G. Leptin’s Pro-Angiogenic Signature in Breast Cancer. Cancers. 2013;5(3):1140–62. doi: 10.3390/cancers5031140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dardeno TA, Chou SH, Moon HS, Chamberland JP, Fiorenza CG, Mantzoros CS. Leptin in human physiology and therapeutics. Frontiers in neuroendocrinology. 2010;31(3):377–93. doi: 10.1016/j.yfrne.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ozbay T, Nahta R. A novel unidirectional cross-talk from the insulin-like growth factor-I receptor to leptin receptor in human breast cancer cells. Molecular cancer research : MCR. 2008;6(6):1052–8. doi: 10.1158/1541-7786.MCR-07-2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hu X, Juneja SC, Maihle NJ, Cleary MP. Leptin--a growth factor in normal and malignant breast cells and for normal mammary gland development. Journal of the National Cancer Institute. 2002;94(22):1704–11. doi: 10.1093/jnci/94.22.1704. [DOI] [PubMed] [Google Scholar]
- 43.Laud K, Gourdou I, Pessemesse L, Peyrat JP, Djiane J. Identification of leptin receptors in human breast cancer: functional activity in the T47-D breast cancer cell line. Molecular and cellular endocrinology. 2002;188(1–2):219–26. doi: 10.1016/s0303-7207(01)00678-5. [DOI] [PubMed] [Google Scholar]
- 44.Tessitore L, Vizio B, Jenkins O, De Stefano I, Ritossa C, Argiles JM, et al. Leptin expression in colorectal and breast cancer patients. International journal of molecular medicine. 2000;5(4):421–6. doi: 10.3892/ijmm.5.4.421. [DOI] [PubMed] [Google Scholar]
- 45.Chang CC, Wu MJ, Yang JY, Camarillo IG, Chang CJ. Leptin-STAT3-G9a Signaling Promotes Obesity-Mediated Breast Cancer Progression. Cancer research. 2015;75(11):2375–86. doi: 10.1158/0008-5472.CAN-14-3076. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 46.Lee MJ, Ye AS, Gardino AK, Heijink AM, Sorger PK, MacBeath G, et al. Sequential application of anticancer drugs enhances cell death by rewiring apoptotic signaling networks. Cell. 2012;149(4):780–94. doi: 10.1016/j.cell.2012.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morton SW, Lee MJ, Deng ZJ, Dreaden EC, Siouve E, Shopsowitz KE, et al. A Nanoparticle-Based Combination Chemotherapy Delivery System for Enhanced Tumor Killing by Dynamic Rewiring of Signaling Pathways. Science Signaling. 2014;7(325) doi: 10.1126/scisignal.2005261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yaffe MB. The Scientific Drunk and the Lamppost: Massive Sequencing Efforts in Cancer Discovery and Treatment. Science Signaling. 2013;6(269) doi: 10.1126/scisignal.2003684. [DOI] [PubMed] [Google Scholar]
- 49.Diaz N, Minton S, Cox C, Bowman T, Gritsko T, Garcia R, et al. Activation of stat3 in primary tumors from high-risk breast cancer patients is associated with elevated levels of activated SRC and survivin expression. Clinical cancer research : an official journal of the American Association for Cancer Research. 2006;12(1):20–8. doi: 10.1158/1078-0432.CCR-04-1749. [DOI] [PubMed] [Google Scholar]
- 50.Garcia R, Bowman TL, Niu G, Yu H, Minton S, Muro-Cacho CA, et al. Constitutive activation of Stat3 by the Src and JAK tyrosine kinases participates in growth regulation of human breast carcinoma cells. Oncogene. 2001;20(20):2499–513. doi: 10.1038/sj.onc.1204349. [DOI] [PubMed] [Google Scholar]
- 51.Zou D, Yoon HS, Anjomshoaa A, Perez D, Fukuzawa R, Guilford P, et al. Increased levels of active c-Src distinguish invasive from in situ lobular lesions. Breast cancer research : BCR. 2009;11(4):R45. doi: 10.1186/bcr2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ren Z, Schaefer TS. ErbB-2 activates Stat3 alpha in a Src- and JAK2-dependent manner. The Journal of biological chemistry. 2002;277(41):38486–93. doi: 10.1074/jbc.M112438200. [DOI] [PubMed] [Google Scholar]
- 53.Hawthorne VS, Huang WC, Neal CL, Tseng LM, Hung MC, Yu D. ErbB2-mediated Src and signal transducer and activator of transcription 3 activation leads to transcriptional up-regulation of p21Cip1 and chemoresistance in breast cancer cells. Molecular cancer research : MCR. 2009;7(4):592–600. doi: 10.1158/1541-7786.MCR-08-0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hosford SR, Miller TW. Clinical potential of novel therapeutic targets in breast cancer: CDK4/6, Src, JAK/STAT, PARP, HDAC, and PI3K/AKT/mTOR pathways. Pharmacogenomics and personalized medicine. 2014;7:203–15. doi: 10.2147/PGPM.S52762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kotha A, Sekharam M, Cilenti L, Siddiquee K, Khaled A, Zervos AS, et al. Resveratrol inhibits Src and Stat3 signaling and induces the apoptosis of malignant cells containing activated Stat3 protein. Molecular cancer therapeutics. 2006;5(3):621–9. doi: 10.1158/1535-7163.MCT-05-0268. [DOI] [PubMed] [Google Scholar]
- 56.Aziz MH, Hafeez BB, Sand JM, Pierce DB, Aziz SW, Dreckschmidt NE, et al. Protein kinase Cvarepsilon mediates Stat3 Ser727 phosphorylation, Stat3-regulated gene expression, and cell invasion in various human cancer cell lines through integration with MAPK cascade (RAF-1, MEK1/2, and ERK1/2) Oncogene. 2010;29(21):3100–9. doi: 10.1038/onc.2010.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kazanietz MG. Eyes wide shut: protein kinase C isozymes are not the only receptors for the phorbol ester tumor promoters. Molecular carcinogenesis. 2000;28(1):5–11. doi: 10.1002/(sici)1098-2744(200005)28:1<5::aid-mc2>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 58.Wu D, Foreman TL, Gregory CW, McJilton MA, Wescott GG, Ford OH, et al. Protein kinase cepsilon has the potential to advance the recurrence of human prostate cancer. Cancer research. 2002;62(8):2423–9. [PubMed] [Google Scholar]
- 59.Wheeler DL, Martin KE, Ness KJ, Li Y, Dreckschmidt NE, Wartman M, et al. Protein kinase C epsilon is an endogenous photosensitizer that enhances ultraviolet radiation-induced cutaneous damage and development of squamous cell carcinomas. Cancer research. 2004;64(21):7756–65. doi: 10.1158/0008-5472.CAN-04-1881. [DOI] [PubMed] [Google Scholar]
- 60.Lee C, Dhillon J, Wang MY, Gao Y, Hu K, Park E, et al. Targeting YB-1 in HER-2 overexpressing breast cancer cells induces apoptosis via the mTOR/STAT3 pathway and suppresses tumor growth in mice. Cancer research. 2008;68(21):8661–6. doi: 10.1158/0008-5472.CAN-08-1082. [DOI] [PubMed] [Google Scholar]
- 61.Zhou J, Wulfkuhle J, Zhang H, Gu P, Yang Y, Deng J, et al. Activation of the PTEN/mTOR/STAT3 pathway in breast cancer stem-like cells is required for viability and maintenance. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(41):16158–63. doi: 10.1073/pnas.0702596104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dunkel Y, Ong A, Notani D, Mittal Y, Lam M, Mi X, et al. STAT3 protein up-regulates Galpha-interacting vesicle-associated protein (GIV)/Girdin expression, and GIV enhances STAT3 activation in a positive feedback loop during wound healing and tumor invasion/metastasis. The Journal of biological chemistry. 2012;287(50):41667–83. doi: 10.1074/jbc.M112.390781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu C, Zhang Y, Xu H, Zhang R, Li H, Lu P, et al. Girdin protein: a new potential distant metastasis predictor of breast cancer. Medical oncology (Northwood, London, England) 2012;29(3):1554–60. doi: 10.1007/s12032-011-0087-6. [DOI] [PubMed] [Google Scholar]
- 64.Jiang P, Enomoto A, Jijiwa M, Kato T, Hasegawa T, Ishida M, et al. An actin-binding protein Girdin regulates the motility of breast cancer cells. Cancer research. 2008;68(5):1310–8. doi: 10.1158/0008-5472.CAN-07-5111. [DOI] [PubMed] [Google Scholar]
- 65.Shields BJ, Wiede F, Gurzov EN, Wee K, Hauser C, Zhu HJ, et al. TCPTP regulates SFK and STAT3 signaling and is lost in triple-negative breast cancers. Molecular and cellular biology. 2013;33(3):557–70. doi: 10.1128/MCB.01016-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Su F, Ren F, Rong Y, Wang Y, Geng Y, Wang Y, et al. Protein tyrosine phosphatase Meg2 dephosphorylates signal transducer and activator of transcription 3 and suppresses tumor growth in breast cancer. Breast cancer research : BCR. 2012;14(2):R38. doi: 10.1186/bcr3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nakagawa T, Iida S, Osanai T, Uetake H, Aruga T, Toriya Y, et al. Decreased expression of SOCS-3 mRNA in breast cancer with lymph node metastasis. Oncology reports. 2008;19(1):33–9. [PubMed] [Google Scholar]
- 68.Santillan-Benitez JG, Mendieta-Zeron H, Gomez-Olivan LM, Ordonez Quiroz A, Torres-Juarez JJ, Gonzalez-Banales JM. JAK2, STAT3 and SOCS3 gene expression in women with and without breast cancer. Gene. 2014;547(1):70–6. doi: 10.1016/j.gene.2014.06.025. [DOI] [PubMed] [Google Scholar]
- 69.Palianopoulou M, Papanikolaou V, Stefanou N, Tsezou A. The activation of leptin-mediated survivin is limited by the inducible suppressor SOCS-3 in MCF-7 cells. Experimental biology and medicine (Maywood, NJ) 2011;236(1):70–6. doi: 10.1258/ebm.2010.010224. [DOI] [PubMed] [Google Scholar]
- 70.Niwa Y, Kanda H, Shikauchi Y, Saiura A, Matsubara K, Kitagawa T, et al. Methylation silencing of SOCS-3 promotes cell growth and migration by enhancing JAK/STAT and FAK signalings in human hepatocellular carcinoma. Oncogene. 2005;24(42):6406–17. doi: 10.1038/sj.onc.1208788. [DOI] [PubMed] [Google Scholar]
- 71.He B, You L, Uematsu K, Zang K, Xu Z, Lee AY, et al. SOCS-3 is frequently silenced by hypermethylation and suppresses cell growth in human lung cancer. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(24):14133–8. doi: 10.1073/pnas.2232790100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schmidt D, Muller S. PIAS/SUMO: new partners in transcriptional regulation. Cellular and molecular life sciences : CMLS. 2003;60(12):2561–74. doi: 10.1007/s00018-003-3129-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Borghouts C, Tittmann H, Delis N, Kirchenbauer M, Brill B, Groner B. The intracellular delivery of a recombinant peptide derived from the acidic domain of PIAS3 inhibits STAT3 transactivation and induces tumor cell death. Molecular cancer research : MCR. 2010;8(4):539–53. doi: 10.1158/1541-7786.MCR-09-0417. [DOI] [PubMed] [Google Scholar]
- 74.Wang Z, Han J, Cui Y, Zhou X, Fan K. miRNA-21 inhibition enhances RANTES and IP-10 release in MCF-7 via PIAS3 and STAT3 signalling and causes increased lymphocyte migration. Biochemical and biophysical research communications. 2013;439(3):384–9. doi: 10.1016/j.bbrc.2013.08.072. [DOI] [PubMed] [Google Scholar]
- 75.Pawlus MR, Wang L, Hu CJ. STAT3 and HIF1alpha cooperatively activate HIF1 target genes in MDA-MB-231 and RCC4 cells. Oncogene. 2014;33(13):1670–9. doi: 10.1038/onc.2013.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Carpenter RL, Lo HW. STAT3 Target Genes Relevant to Human Cancers. Cancers. 2014;6(2):897–925. doi: 10.3390/cancers6020897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tell RW, Horvath CM. Bioinformatic analysis reveals a pattern of STAT3-associated gene expression specific to basal-like breast cancers in human tumors. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(35):12787–92. doi: 10.1073/pnas.1404881111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Marotta LL, Almendro V, Marusyk A, Shipitsin M, Schemme J, Walker SR, et al. The JAK2/STAT3 signaling pathway is required for growth of CD44(+)CD24(−) stem cell-like breast cancer cells in human tumors. The Journal of clinical investigation. 2011;121(7):2723–35. doi: 10.1172/JCI44745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gritsko T, Williams A, Turkson J, Kaneko S, Bowman T, Huang M, et al. Persistent activation of stat3 signaling induces survivin gene expression and confers resistance to apoptosis in human breast cancer cells. Clinical cancer research : an official journal of the American Association for Cancer Research. 2006;12(1):11–9. doi: 10.1158/1078-0432.CCR-04-1752. [DOI] [PubMed] [Google Scholar]
- 80.Leslie K, Lang C, Devgan G, Azare J, Berishaj M, Gerald W, et al. Cyclin D1 is transcriptionally regulated by and required for transformation by activated signal transducer and activator of transcription 3. Cancer research. 2006;66(5):2544–52. doi: 10.1158/0008-5472.CAN-05-2203. [DOI] [PubMed] [Google Scholar]
- 81.Liu LC, Su CH, Wang HC, Chang WS, Tsai CW, Maa MC, et al. Contribution of personalized Cyclin D1 genotype to triple negative breast cancer risk. BioMedicine. 2014;4:3. doi: 10.7603/s40681-014-0003-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, et al. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117(7):927–39. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 83.Yuan G, Qian L, Shi M, Lu F, Li D, Hu M, et al. HER2-dependent MMP-7 expression is mediated by activated STAT3. Cellular signalling. 2008;20(7):1284–91. doi: 10.1016/j.cellsig.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 84.Song Y, Qian L, Song S, Chen L, Zhang Y, Yuan G, et al. Fra-1 and Stat3 synergistically regulate activation of human MMP-9 gene. Molecular immunology. 2008;45(1):137–43. doi: 10.1016/j.molimm.2007.04.031. [DOI] [PubMed] [Google Scholar]
- 85.Snyder M, Huang J, Huang XY, Zhang JJ. A signal transducer and activator of transcription 3.Nuclear Factor kappaB (Stat3.NFkappaB) complex is necessary for the expression of fascin in metastatic breast cancer cells in response to interleukin (IL)-6 and tumor necrosis factor (TNF)-alpha. The Journal of biological chemistry. 2014;289(43):30082–9. doi: 10.1074/jbc.M114.591719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kunigal S, Lakka SS, Sodadasu PK, Estes N, Rao JS. Stat3-siRNA induces Fas-mediated apoptosis in vitro and in vivo in breast cancer. International journal of oncology. 2009;34(5):1209–20. [PMC free article] [PubMed] [Google Scholar]
- 87.Wang X, Zhang Y, Zhang X, Tian W, Feng W, Chen T. The curcumin analogue hydrazinocurcumin exhibits potent suppressive activity on carcinogenicity of breast cancer cells via STAT3 inhibition. International journal of oncology. 2012;40(4):1189–95. doi: 10.3892/ijo.2011.1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kim SH, Nagalingam A, Saxena NK, Singh SV, Sharma D. Benzyl isothiocyanate inhibits oncogenic actions of leptin in human breast cancer cells by suppressing activation of signal transducer and activator of transcription 3. Carcinogenesis. 2011;32(3):359–67. doi: 10.1093/carcin/bgq267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yang J, Liao X, Agarwal MK, Barnes L, Auron PE, Stark GR. Unphosphorylated STAT3 accumulates in response to IL-6 and activates transcription by binding to NFkappaB. Genes & development. 2007;21(11):1396–408. doi: 10.1101/gad.1553707. [DOI] [PMC free article] [PubMed] [Google Scholar]