Abstract
Aspiration pneumonia is a life-threatening infectious disease often caused by oral anaerobic and periodontal pathogens such as Porphyromonas gingivalis. This organism produces proteolytic enzymes, known as gingipains, which manipulate innate immune responses and promote chronic inflammation. Here, we challenged mice with P. gingivalis W83 and examined the role of gingipains in bronchopneumonia, lung abscess formation, and inflammatory responses. Although gingipains were not required for P. gingivalis colonization and survival in the lungs, they were essential for manifestation of clinical symptoms and infection-related mortality. Pathologies caused by wild-type (WT) P. gingivalis W83, including hemorrhage, necrosis, and neutrophil infiltration, were absent from lungs infected with gingipain-null isogenic strains or WT bacteria preincubated with gingipain-specific inhibitors. Damage to lung tissue correlated with systemic inflammatory responses, as manifested by elevated levels of TNF, IL-6, IL-17, and C-reactive protein. These effects were unequivocally dependent on gingipain activity. Gingipain activity was also implicated in the observed increase in IL-17 in lung tissues. Furthermore, gingipains increased platelet counts in the blood and activated platelets in the lungs. Arginine-specific gingipains made a greater contribution to P. gingivalis-related morbidity and mortality than lysine-specific gingipains. Thus, inhibition of gingipain may be a useful adjunct treatment for P. gingivalis-mediated aspiration pneumonia.
Key Words: Aspiration pneumonia, Porphyromonas gingivalis, Gingipain
Introduction
Extensive clinical and epidemiological data clearly show that periodontitis, a bacterial biofilm-driven chronic inflammatory disease of tooth-supporting tissues, strongly correlates with an increased risk of atherosclerosis, diabetes, adverse events during pregnancy, rheumatoid arthritis, and aspiration pneumonia [1, 2, 3, 4].
Aspiration pneumonia is a life-threatening condition caused by aspiration of oral bacteria during medical procedures, or by aspiration of liquids such as saliva or solid materials such as food. Endogenous anaerobic bacteria residing within the oral cavity are suspected to be the cause of such infections. Indeed, these bacteria are identified in the majority pulmonary infections and can cause lung abscess, necrotizing pneumonia, and empyema [5, 6, 7]. Periodontitis affects up to 30% of the adult population [8,][9]; therefore, it is not surprising that anaerobic periodontal pathogens, such as Porphyromonas gingivalis, Tannerella forsythia, Treponema denticola, and Prevotella intermedia, are often cultured from lung aspirates in around 40% of aspiration pneumonia cases [10, 11, 12, 13, 14, 15].
The best-studied periodontal pathogen implicated in multiple cases of aspiration pneumonia is P. gingivalis. This Gram-negative, oral anaerobic bacterium expresses several well-established virulence factors, including lipopolysaccharides, phosphatases, fimbriae, hemagglutinins, and cysteine proteinases called gingipains [16, 17, 18, 19, 20, 21]. The latter are the most important P. gingivalis virulence factors with respect to pathogenicity and survival in vivo [22, 23, 24]. They are versatile enzymes that are essential for a variety of processes and are encoded by 3 different genes: rgpA, rgpB, and kgp[25]. The 2 arginine-specific gingipains RgpA and RgpB possess practically identical caspase-like catalytic domains and specifically cleave Arg-Xaa peptide bonds. RgpA, however, possesses a large C-terminal extension bearing a hemagglutinin-adhesin domain, which is absent from RgpB. Similar to RgpA, lysine-specific gingipain K, encoded by kgp, harbors a C-terminal hemagglutinin, and its proteolytic activity is limited to Lys-Xaa peptide bonds. Depending on the strain, gingipains are either anchored to the outer membrane of the bacterium via anionic lipopolysaccharide moieties or secreted [26, 27].
The role of gingipains in the development of periodontitis is well characterized and includes degradation of antibacterial peptides (e.g. defensins) [28], inactivation of protease inhibitors [28, 29], exploitation of the complement system (e.g. degradation of different components of the complement cascade) [30, 31], and protection of bacteria from host immune effector cells (e.g. degradation of receptors or mediators required for effector cell activity) [32, 33, 34, 35, 36, 37]. However, although numerous clinical case reports and animal models have shown that P. gingivalis plays an important role in the development of aspiration pneumonia [38, 39, 40], the relevant virulence factors remain unclear.
In line with previous reports, the present study shows that P. gingivalis causes severe experimental pneumonia in mice. On a mechanistic level, we argue that the disease course is critically dependent on gingipain activity. By modulating the immune response of the host, P. gingivalis was capable of producing gingipains, which exacerbated disease manifestation in our experimental model, resulting in severe hemorrhage and extensive tissue damage to the lungs. Surprisingly, however, gingipains were not a prerequisite for colonization and survival of this pathogen in the lungs.
By identifying the specific mechanisms that underlie the development of P. gingivalis-induced aspiration pneumonia, this is the first study to show that gingipains act as important virulence factors during acute, life-threatening cases of pneumonia and not only during chronic diseases such as periodontitis. These findings may have important implications for the development of novel treatment strategies.
Materials and Methods
Bacterial Strains and Culture Conditions
An invasive wild-type (WT) strain of P. gingivalis (W83; American Type Culture Collection, Rockville, Md., USA) and its isogenic single, double, and triple protease-null mutants [Δkgp (ΔKgp), rgpA/rgpB (ΔRgp), and ΔkgpΔrgpAΔrgpB (KRAB)] were used in this study. The general procedure for construction of the Δrgp and Δkgp mutants has been described elsewhere [79]. Homologous recombination of the Δkgp plasmid into the P. gingivalisΔrgp mutant genome resulted in a kgp-rgp-deficient mutant (Δrgp + kgp). The respective phenotypes were confirmed by enzymatic assays and Western blot analysis. All bacteria were grown on blood agar plates (anaerobic blood agar) or in liquid Schaedler broth (BTL, Lodz, Poland) supplemented with hemin (5 μg/ml; Sigma Chemical Co., Steinheim, Germany), L-cysteine (50 μg/ml; Sigma Chemical Co.), menadione (0.5 μg/ml; Sigma Chemical Co.), and 5% sheep blood. The ΔKgp strain was cultured in medium supplemented with tetracycline (1 μg/ml; Sigma Chemical Co.) and the ΔRgp strain was cultured in medium supplemented with erythromycin (5 μg/ml; Sigma Chemical Co.). Bacteria were grown under anaerobic conditions (90% N2, 5% CO2, and 5% H2). To prepare the inoculates, bacteria were grown in an overnight liquid culture and centrifuged. The bacterial pellet was washed 3 times in phosphate-buffered saline (PBS) and then suspended in sterile PBS at a final optical density (λ = 600 nm) of 1 [corresponding to an approximate concentration of 109 colony-forming units (CFU)/ml].
Gingipain Activity
The WT P. gingivalis strain W83 was cultured as described above. Following overnight culture, the bacteria were pelleted by centrifugation (6,000 g, 15 min) and the cells were resuspended in Schaedler medium supplemented with hemin, vitamin K, and L-cysteine (final concentration 109 cells/50 μl). The samples were subsequently divided into 2 equal portions. KYT-1 [carbobenzoxy-Lys-Arg-CO-Lys-N-(CH2)2] and KYT-36 [carbobenzoxy-Glu(NHN[CH3]Ph)-Lys-CO-NHCH2Ph] [both at 1 mM in dimethyl sulfoxide (DMSO)] were added to 1 portion (final concentration 1 μM) and incubated for 15 min at 37°C. KYT-1 and KYT-36 are specific inhibitors of gingipains R and K, respectively. P. gingivalis W83 bacteria treated thus were referred to as W83-KYT. The second portion was supplemented with DMSO at a concentration corresponding to that in the inhibitor-treated bacterial suspension, and the sample was incubated under conditions identical to those described for W83-KYT. To measure the proteolytic activity of gingipains at specific time points (0, 1, 2, 4, 6, 24, and 48 h), 2-µl aliquots of the different strains were added to 98 µl of TNCT buffer (100 mM Tris pH 7.5, 150 mM NaCl, 5 mM CaCl2, and 0.05% Tween 100), supplemented with 10 mM L-cysteine, in a microtiter plate. After a 5-min preincubation, a substrate for gingipain R (BApNA; Sigma-Aldrich, Germany) or gingipain K (N-p-Tos-Gly-Pro-Lys-pNA) was added to the plate. The resulting mixture contained 200 μM substrate, 5% DMSO, and 10 mM L-cysteine. The gingipain-mediated release of p-nitroaniline from the substrates was monitored as an increase in absorbance (405 nm) over time using a microplate reader (SpectraMax Gemini; Molecular Devices).
Animals and Inoculation Procedures
Specific pathogen-free female BALB/c mice aged 8 or 40 weeks were used for the experiments. The mice were purchased from the Mossakowski Medical Research Centre, Polish Academy of Science, Warsaw, Poland. The mice were housed under standard conditions within the animal care facility at Jagiellonian University, Krakow, fed a standard laboratory diet (Labofeed, Morawski, Poland), and allowed water ad libitum. Control and infected mice were housed in separate cages. The outline of the experimental setup is presented in online supplementary figure S1 (for all online suppl. material, see www.karger.com/doi/10.1159/000441724).
The animals were stratified by age and further divided into 4 groups as detailed in online supplementary figure S1. All mice were anesthetized via intraperitoneal injection of ketamine (22 mg/kg, VetaKetam; Vet-Agro, Poland) and xylazine (2 mg/kg, Sedasin; Biowet, Poland) and the eyes were lubricated with ointment (Puralube Vet; Pharmaderm, Melville, N.Y., USA). Following general anesthesia, the mice were laid vertically on their back and bacteria or PBS was delivered intratracheally via a blunt-tipped 22-gauge silicone needle attached to a microliter syringe. Subsequently, the animals were laid at a 45° angle until they fully recovered from anesthesia. The mice were inoculated with 3 × 109 CFU of WT P. gingivalis W83, ΔRgp, ΔKgp, KRAB, or W83-KYT resuspended in a total volume of 50 μl of sterile 1 × PBS (pH 7.4; PAA, Pasching, Austria). Control mice were inoculated with 50 μl of sterile 1 × PBS (sham infected) or not inoculated at all (negative control).
Clinical Evaluation
The mice were evaluated daily for clinical signs and their health status was monitored throughout the experiment. The severity of aspiration pneumonia was quantified by clinical scoring, which was based on mortality, respiratory failure, weight loss, eating disorders, activity loss, ruffled fur, ataxia, and tremor. The results are presented as numbers of animals showing a specific symptom versus the total number of animals per group (n = 15).
Calculation of the CFU
Organs (lungs, liver, spleen, kidneys, and lymph nodes) were removed, weighed, and homogenized in 1 ml of PBS (pH 7.4) using a TissueLyser (Qiagen). Homogenates were then serially diluted (10-fold). In parallel, 50 μl of peripheral blood from each animal was lysed with deionized water. All samples (100 μl each) were plated on blood agar plates as described above and cultured for 7 days in an anaerobic chamber at 37°C. Subsequently, the number of visible colonies was counted and the number of CFU was calculated by multiplying this number by the dilution factor.
Morphological and Histological Analyses
At 24, 48, and 72 h postinoculation with P. gingivalis W83, ΔRgp, ΔKgp, KRAB, or W83-KYT, the animals were bled under deep anesthesia and the lungs were removed surgically. Visible pathological changes, such as edema, necrosis, or hemorrhage, were noted. Subsequently, the lungs were washed in cold PBS (pH 7.5), fixed in 4% formaldehyde (PoCh, Gliwice, Poland) in PBS for 24 h at 4°C, dehydrated in a graded alcohol series, and embedded in paraffin wax. Subsequently, the samples were serially sectioned (6 μm thick) and mounted on silanized glass slides. Prior to staining, the sections were deparaffinized in xylene and rehydrated in a graded alcohol series. The samples were then stained with hematoxylin and eosin (Sigma-Aldrich) according to a standard protocol. The slides were examined under a Nikon Eclipse Ti-S (Tokyo, Japan) microscope and random images were selected for subsequent evaluation.
Immunohistochemistry
The lungs were washed in cold PBS (pH 7.5), fixed in 4% formaldehyde (PoCh) in PBS for 24 h at 4°C, dehydrated in a graded alcohol series, and embedded in paraffin wax. Subsequently, the samples were serially sectioned (6 μm thick) and mounted on silanized glass slides. Prior to staining, the sections were deparaffinized in xylene and rehydrated in a graded alcohol series. Serial sections were stained with monoclonal antibodies against mouse Ly6G (RB6-8C5, FITC conjugated; LifeSpan), human/mouse IL-17A (Santa Cruz Biotechnology), mouse CD31 (LifeSpan), and mouse CD40L (LifeSpan). The specificity of the staining was confirmed using appropriate FITC-conjugated isotype controls or normal rabbit IgG followed by Alexa Fluor 594 goat anti-rabbit IgG. Images were captured under a laser scanning confocal microscope (Olympus FV1000).
Cytokine Measurements
Whole blood was collected into test tubes and allowed to clot at room temperature for 30 min. The clot was removed by centrifugation at 1,000–2,000 g for 10 min in a refrigerated centrifuge. The collected serum was stored at −80°C until further analysis. The concentrations of IL-6, TNF-α, and MCP1 in the sera were measured using commercially available ELISA assays (BD OptEIA™ Set Mouse IL-6, BD OptEIA™ Set Mouse TNF-α, and BD OptEIA™ Set Mouse MCP-1; BD Biosciences, San Diego, Calif., USA) according to the manufacturer's recommendations.
Quantification of IL-17 in Serum and Lung Lysates
Serum was obtained as described in the previous section. To obtain lung tissue homogenates, resected lungs were weighed and homogenized (Tissue Lyser LT; Qiagen, Germany) in PBS, pH 7.4, supplemented with 5 mg/ml hexadecyltrimethylammonium bromide to yield 0.05% (v/v) homogenates. The samples were sonicated for 10 s, freeze-thawed 3 times to release the contents of the neutrophil primary granules, and then sonicated again. The homogenates were centrifuged at 12,000 rpm for 15 min and the supernatants were used immediately. IL-17 levels were measured using a commercial ELISA kit (eBioscience, San Diego, Calif., USA) according to the manufacturer's instructions.
Determination of Myeloperoxidase Activity
Neutrophil influx into the lung tissue was analyzed using myeloperoxidase (MPO) as a marker. Briefly, 10 µl of supernatant from the lung homogenate was mixed with dianisidine reagent (16.7 mg of dianisidine, 90 ml of distilled water, 10 ml of potassium phosphate buffer, and 50 µl of 0.29 M hydrogen peroxide) and incubated for 5 min at room temperature. The reaction was then terminated by adding 50 µl of 2 M H2SO4. The change in absorbance with time was measured at 450 nm and MPO activity was expressed as a percentage of the control value (PBS-treated animals).
Blood Analysis
Briefly, 100 μl of blood was collected into K2-EDTA-coated tubes (Microvette; Sarstedt, Germany) after cardiac puncture. Blood samples were evaluated using an automatic hematology analyzer (Micros 60 Animal Blood Counter; Horiba ABX, UK) with reagents supplied by Horiba ABX. The C-reactive protein (CRP) levels in the serum were measured using a commercially available ELISA (Mouse CRP ELISA Kit; Innovative Research, USA) according to the manufacturer's recommendations.
Statistical Analysis
Significant differences between groups were examined using 2-way repeated-measures ANOVA (multiparameter data) and a 1-way ANOVA with Bonferroni's correction or Dunnett's posttest (multiple comparisons). Kaplan-Meier graphs were analyzed using the log-rank test with Bonferroni's correction (posttest) to correct for multiple comparisons. All statistical analyses (except those shown in fig. 4) were performed using GraphPad Prism software (version 5.0d or 6.0e for Mac; GraphPad). p < 0.05 was considered statistically significant. The significance values shown in figure 4 were calculated using SPSS 20 (IBM, Armonk, N.Y., USA).
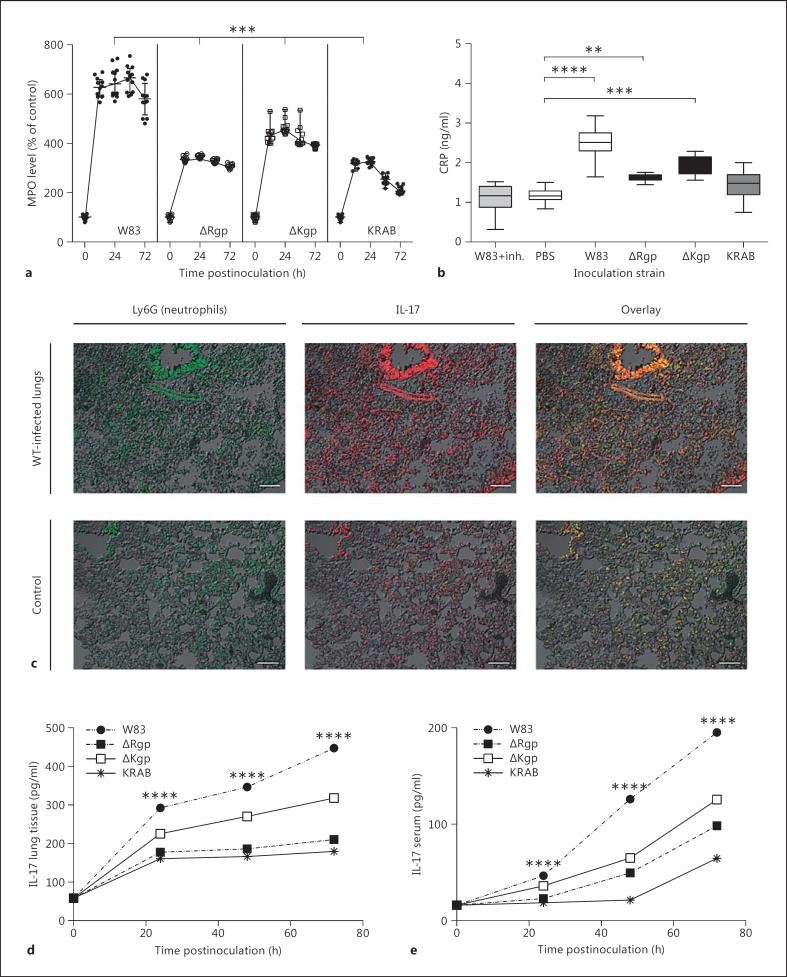
Fig. 4.
Gingipains are important for PMN influx, CRP, IL-17 production in the lung tissue, and elevated levels of IL-17 in the lungs and serum. a, b Animals were intratracheally inoculated with PBS (control) or WT P. gingivalis (W83, ΔKgp, ΔRgp, or KRAB). MPO levels were measured in homogenates of lung tissue at 5 different time points (0, 12, 24, 48, and 72 h postinfection). Each dot represents a single animal. MPO activity is expressed as a percentage of that in the control (mice inoculated with PBS only). Significance was calculated using Student's t test. c Sections of lungs from mice infected with P. gingivalis W83 and from control mice were stained for Ly6G (left) or IL-17 (middle). A merged image is shown on the right. Scale bars = 50 μm. Data are representative of 2 independent experiments with similar results. d, e Animals (n = 38) were intratracheally inoculated with PBS (control) or WT P. gingivalis W83, ΔKgp, ΔRgp, or KRAB. The concentration of IL-17 in lung lysates (d) and in serum (e) was measured at 24 h postinfection. ** p < 0.01, *** p < 0.001, **** p < 0.0001 (2-way ANOVA followed by Dunnett's multiple-comparisons test). Data are expressed as means ± SEM. inh. = Inhibitor.
Results
Experimentally Induced P. gingivalis Pneumonia Is Lethal to Mice
Aspiration pneumonia is prevalent among patients with swallowing impairments or abnormalities [1, 41]. To closely mimic the dynamics of this disease in humans, BALB/c mice (8 and 40 weeks old) were inoculated intratracheally with WT P. gingivalis W83 (doses of 1 × 108, 1 × 109, or 1 × 1010 CFU suspended in 50 μl of sterile PBS). Whereas intratracheal inoculation with 1 × 108 and 1 × 109 CFU of P. gingivalis W83 did not induce pneumonia, inoculation of 1 × 1010 CFU was consistently lethal at 24 h postinfection, independently of age. Based on these results, a dose of 3 × 109 CFU was deemed adequate for all further investigations. The outline of the experimental setup is presented in online supplementary figure S1.
Upon receiving 3 × 109 CFU of bacteria, all 40-week-old animals developed clinical symptoms of pneumonia, with a mortality rate of approximately 20% within 72 h postinfection. Younger animals, i.e. 8 weeks of age, were significantly more susceptible to the bacterial challenge, with mortality reaching 80% within 24 h postinfection (fig. 1a, b). This may be explained, at least in part, by the higher bacterial load relative to the weight of the young animals (16 g) compared to that of their older counterparts (23 g). Based on multiple studies indicating an increased susceptibility of younger animals to infections agents [42, 43, 44, 45], we hypothesize that alterations associated with aging may also account for some of the observed differences in disease penetrance, severity, and mortality.
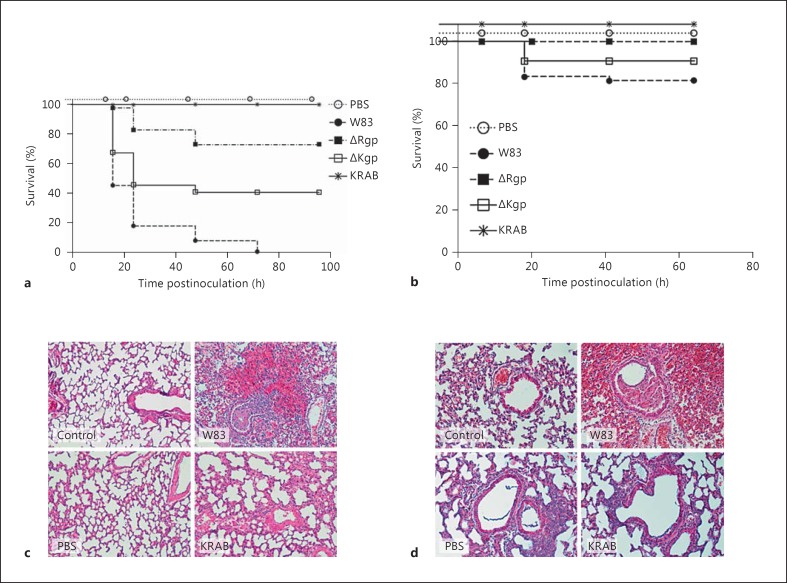
Fig. 1.
Gingipains are essential for the development of lethal P. gingivalis infection. a, b Kaplan-Meier plots showing mortality after a bacterial challenge (log-rank test). Young (8-week-old; a) and old (40-week-old; b) mice were intratracheally inoculated with 3 × 109P. gingivalis W83 (WT), gingipain mutants (ΔKgp, ΔRgp, or KRAB), or an inhibitor-treated bacterial suspension (W83-KYT). Histological evaluation of lung tissue from 8-week-old (c) and 40-week-old (d) mice infected with P. gingivalis. Mice were sham-infected by intratracheal instillation of PBS or intratracheally inoculated with 3 × 109 CFU of P. gingivalis W83 or KRAB. Lungs were surgically removed at 24 h postinfection and formalin-fixed sections were prepared and stained with hematoxylin and eosin. Representative images are shown. Magnification ×200. A section from an untreated animal (control) is also shown. The outline of the experimental setup is presented in online supplementary figure S1.
Although the differences in clinical and morphological findings between the two age groups were minimal, the slower disease course in older mice enabled follow-up examination of immune responses, and of other factors underlying the clinical outcome, over a period of 10 days (compared to the limited 48-hour window of opportunity provided by the younger mice).
To eliminate the above confounding factors during the process of elucidating the mechanisms underlying the pathogenicity of P. gingivalis in the context of pulmonary infection, we focused primarily on disease manifestations in 40-week-old animals. For the interested reader, all data pertaining to the 8-week-old mice are provided in the online supplementary material.
Gingipains Are Pivotal for the Development of Aspiration Pneumonia Caused by P. gingivalis
Apart from proteolytic activity, which is necessary for disruption of the host immune response, gingipains possess several protease-independent activities that are essential for their virulence [27]. To examine the role of gingipains during the development of aspiration pneumonia, animals were intratracheally infected with WT P. gingivalis W83, P. gingivalis deficient in lysine-specific gingipain activity (ΔKgp), or P. gingivalis deficient in arginine-specific (ΔRgp) gingipains. In addition, we examined a knockout strain with no gingipain activity (KRAB). To further clarify the importance of gingipain proteolytic activity during the development of aspiration pneumonia, another group of mice was infected with WT P. gingivalis W83 pretreated with specific inhibitors (KYT-1 and KYT-36) that block the proteolytic activity of gingipains [32]. As shown in online supplementary figure S2, treatment with WT P. gingivalis with KYT-1 and KYT-36 completely abolished cell-associated Kgp activity and reduced Rgp activity by 10-fold. Importantly, in all cases, inhibition was maintained for up to 24 h posttreatment.
A clinical scoring system was used in parallel with histological and morphological examination of lung tissue. The clinical status was assessed daily by visual examination. Respiratory failure, weight loss, eating disorders, activity loss, ruffled fur, ataxia, and tremor were noted. As presented in table 1, animals inoculated with either the ΔRgp strain or the ΔKgp strain developed clinical signs of pneumonia. A more detailed comparison revealed that, compared to animals inoculated with ΔRgp, approximately 25% of the mice inoculated with ΔKgp showed signs of respiratory distress during the experiment. Importantly, pneumonia symptoms were generally very mild in mice infected with the KRAB strain, and no animal developed fatal pneumonia. Reinforcing the notion that aspiration pneumonia caused by P. gingivalis depends on gingipain activity, inoculation of animals with WT P. gingivalis bacteria pretreated with KYT inhibitors (W83-KYT) resulted in an outcome comparable to that observed for KRAB-infected mice.
Table 1.
Development of clinical symptoms in animals intratra-cheally inoculated with PBS (control), P. gingivalis W83 (WT), P. gingivalis gingipain mutants (ΔRgp, ΔKgp, or KRAB), or inhibi-tor-pretreated P. gingivalis W83 (W83-KYT)
| Respiratory | Weight | Loss of | Activity | Ruffled | Ataxia | |
|---|---|---|---|---|---|---|
| Failure | loss | appetite | loss | fur | ||
| PBS | 0/16 | 0/16 | 0/16 | 0/16 | 0/16 | 0/16 |
| WT-W83 | 16/16 | 12/16 | 12/16 | 16/16 | 16/16 | 14/16 |
| ΔKgp | 12/16 | 6/16 | 10/16 | 12/16 | 12/16 | 10/16 |
| ΔRgp | 8/16 | 6/16 | 10/16 | 8/16 | 10/16 | 8/16 |
| KRAB | 2/16 | 6/16 | 0/16 | 2/16 | 0/16 | 0/16 |
| W83-KYT | 2/16 | 6/16 | 0/16 | 2/16 | 0/16 | 0/16 |
Values are presented as numbers of animals showing a specific symptom per group.
The survival rates of young mice infected with the WT strain and either gingipain-deficient or inhibitor-pretreated WT W83 P. gingivalis strains were significant at all time points assessed (p < 0.001; fig. 1a, c). Although the symptoms observed in 40-week-old animals were milder, the overall difference in survival between young and old mice inoculated with WT, ΔRgp, ΔKgp, KRAB, or W83-KYT P. gingivalis was significant at all time points (p < 0.05 at 16, 24, 48, and 72 h; p < 0.001 at 96 h; fig. 1a, b).
Morphological examination of infected lungs and histopathological analysis of lung tissues corroborated the clinical picture. The capacity of the different strains to inflict tissue damage was clearly different and quantifiable upon morphological examination. While 80% of the animals infected with WT W83 developed lung edema, extensive hemorrhage, and necrosis, the lungs of mice inoculated with ΔRgp or ΔKgp showed markedly less damage (table 2). Infection with single gingipain-deficient strains caused lesions in only 50–60% of animals. Inoculation with KRAB or W83-KYT caused sporadic cases of local hemorrhage but no detectable edema or necrosis (table 2).
Table 2.
Edema, necrosis, and hemorrhage in 40-week-old animals intratracheally inoculated with 50 µl of PBS (PBS), P. gingivalis W83 (WT), gingipain mutants (ΔKgp, ΔRgp, or KRAB), or a suspension of P. gingivalis W83 pretreated with inhibitors (W83-KYT)
| Edema | Necrosis | Hemorrhage | |
|---|---|---|---|
| PBS | 0/8 | 0/8 | 0/8 |
| WT-W83 | 7/8 | 4/8 | 8/8 |
| ΔKgp | 6/8 | 4/8 | 5/8 |
| ΔRgp | 3/8 | 2/8 | 4/8 |
| KRAB | 0/8 | 0/8 | 1/8 |
| W83-KYT | 0/8 | 0/8 | 1/8 |
Data were corroborated by histopathological changes in the lungs of infected animals. Values are presented as numbers of animals per group.
Detailed histopathological examination of lung tissue confirmed the primary observations. Infection with WT W83 resulted in severe alveolar and peribronchial hemorrhage. By contrast, only moderate damage was observed in the lungs of animals treated with either KRAB or W83-KYT, and the damage was limited to the 12% of animals that had also developed bronchopneumonia; no marked hemorrhage was detectable in any of these animals. More detailed comparative analyses of tissues from animals infected with WT P. gingivalis versus any of the modified strains were, however, rendered impossible due to the very different histopathological presentations (fig. 1d).
A dose of 3 × 109 CFU WT P. gingivalis administered to 8-week-old animals led to much faster progress of the disease, with 100% mortality within 48 h of the start of the experiment. Evaluation of lung tissues excised from these mice at 24 h postinfection revealed a morphology very similar to that observed in 40-week-old animals. We observed large alveolar and peribronchial hemorrhages in cases of infection with the WT strain (fig. 1c, d), leading to respiratory distress and the subsequent death of the animals. Lungs from control animals (inoculated with sterile PBS) did not show any pathological changes. Taken together, these results suggest that infection with P. gingivalis strains capable of expressing gingipains causes severe pneumonia in mice, whereas inoculation with strains deficient in these proteolytic enzymes or pretreated with inhibitors to abolish their proteolytic activity cause only transient and mild lung inflammation.
Gingipains Are Dispensable for P. gingivalis Colonization and Survival in the Lungs
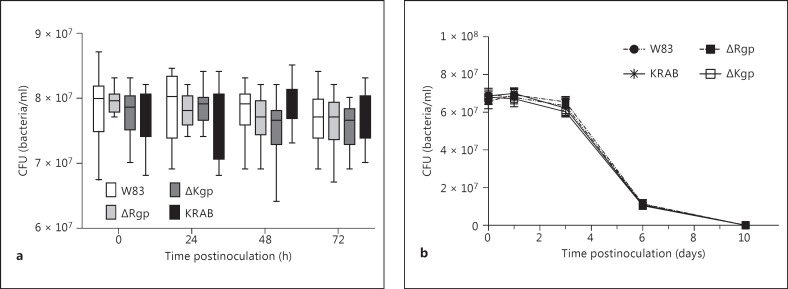
The differences observed in disease outcomes related to gingipain-activity raised the question of whetherthese differences might correlate with the ability of P. gingivalisto colonize and survive in lung tissues. To investigate this further, 40-week-old animals were infected with W83, ΔRgp, ΔKgp, KRAB, or W83-KYT (3 × 109 CFU) bacteria, as previously described. Immediately after inoculation, 4 animals from each group were sacrificed and the bacterial load in the lungs was measured. We found that the initial bacterial load in the lungs was identical for all bacterial strains (median value 6.9 × 107 ± 0.03 × 107 CFU). The bacterial load in the lungs was then assessed every 24 h for 3 days. We found that, irrespectively of the strain, the bacterial load in the lungs remained stable for the first 3 days (fig. 2a; online suppl. fig. S3) before dropping 1 log by day 6; the bacteria were cleared from all surviving animals by day 10 postinoculation (fig. 2b).
Fig. 2.
Gingipains do not affect the colonization and survival of P. gingivalis in infected lungs. a The survival of P. gingivalis was assessed by counting the number of CFU in lung homogenates collected every 24 h for 3 days (n = 24). b Infection was monitored for up to 10 days by examining lung homogenates. For P. gingivalis persistence in the infected lungs of 8-week-old mice, see online supplementary figure S3.
These data provide strong evidence that the gingipain-deficient ΔRgp, ΔKgp, and KRAB and the inhibitor-treated W83-KYT strains were viable, able to colonize the lungs, and able to survive in vivo. Spleen, liver, kidney, lymph node, and peripheral blood samples from sacrificed animals were also examined for the presence of viable bacteria. Viable P. gingivalis was only detected in the peripheral blood of infected animals at a level of 1.97 × 102 ± 0.23 × 102 CFU at 24 h after inoculation.
Taken together, these results suggest that gingipains only play a marginal role in the ability of P. gingivalis to colonize and survive in the lungs.
Gingipain Activity Is Crucial for Systemic Inflammatory Responses during Lung Infection by P. gingivalis
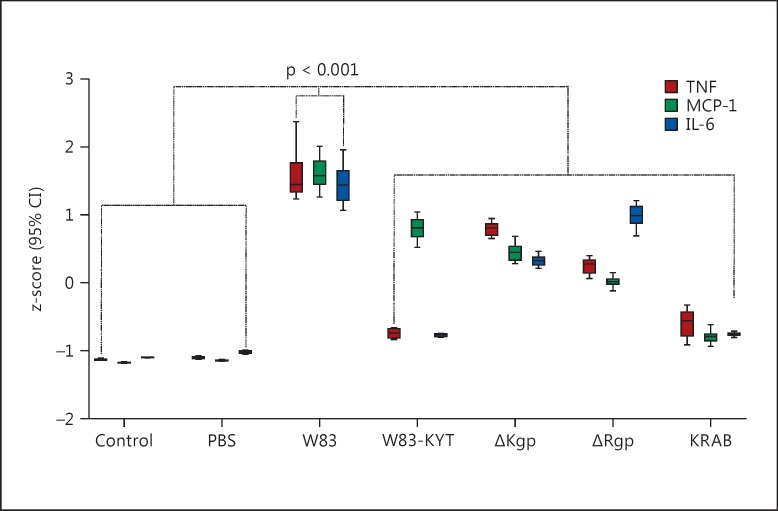
Immune system mediators such as cytokines and chemokines play a crucial role in host defense. Excessive or inappropriate immune responses, however, contribute to tissue damage. Previous studies have demonstrated that intratracheal challenge with P. gingivalis has the potential to elicit strong immune responses [38, 46, 47]. To examine whether gingipains are capable of inducing proinflammatory cytokine responses, we measured the levels of IL-6, TNF-α, and MCP1 in peripheral blood samples at 24 h postinoculation. Animals challenged with WT P. gingivalis showed a sharp increase in TNF-α, IL-6, and MCP1 levels (p < 0.001; fig. 3; online suppl. fig. S4). Infection with ΔRgp and ΔKgp also resulted in increased cytokine production, but to a significantly lesser extent. Interestingly, inoculation of animals with KRAB or W83-KYT did not alter the levels of IL-6 or TNF-α; the levels of these cytokines remained comparable to those detected in PBS-inoculated animals. By contrast, inoculation with W83-KYT led to a significant increase in MCP-1 levels. Similar changes in cytokine levels were observed in 8-week-old animals (online suppl. fig. S5A, B, C, D). These data suggest that, even in their inactive form, Kgp and Rgp can evoke strong inflammatory reactions in the host.
Fig. 3.
Gingipains play a pivotal role in the immune response to P. gingivalis infection. Mice were sham-infected by intratracheal instillation of PBS or infected with 3 × 109 CFU of P. gingivalis W83 (WT), W83-KYT, ΔKgp, ΔRgp, or KRAB. At 24 h postinfection, the concentration of TNF-α, IL-6, and MCP-1 was measured in serum using an ELISA. Dunnett's test (2-tailed) was used subsequent to a 1-way ANOVA (2-tailed) to calculate the significance of the differences between sham-infected mice (PBS) and P. gingivalis-inoculated animals (WT, W83-KYT, ΔKgp, ΔRgp, or KRAB; Dunnett paring to PBS-inoculated mice, 2-way ANOVA, p < 0.001). All values are expressed as standardized values (z-scores), and error bars indicate 95% CI. The raw data of cytokine concentrations in serum are shown in online supplementary figure S4.
We found a statistically significant increase in CRP levels in both young and old animals infected with WT P. gingivalis compared to mice given PBS only (p < 0.0001; online suppl. fig. S6). We also observed a significant upregulation of CRP levels in mice challenged with the ΔKgp and ΔRgp strains compared to the latter, even though they were less pronounced. The CRP levels in mice inoculated with W83-KYT and KRAB remained comparable to those in control mice (fig. 4b).
Gingipain Activity Causes an Influx of Neutrophils into Lung Tissue during Bacterial Infection
As a measure of neutrophil infiltration into lung tissues, MPO activity was determined. The lungs from infected animals showed clear increases in MPO levels, which are indicative of neutrophil infiltration (fig. 4a). The highest MPO concentrations were detected in lung homogenates from animals infected with WT P. gingivalis, whereas those from mice infected with the ΔKgp and ΔRgp strains showed significantly lower MPO activity. Significantly higher levels were observed in ΔKgp-infected mice than in ΔRgp-infected mice (p < 0.05), whereas the MPO levels in lung tissues from KRAB-inoculated mice were not significantly different from those in control animals instilled with PBS. Similar levels of MPO activity were detected in 8-week-old mice infected with P. gingivalis (online suppl. fig. S5E).
Staining of neutrophils confirmed the MPO-based assumption that infection with WT P. gingivalis leads to the accumulation of neutrophils in the target tissues of infected mice (fig. 4b). With respect to neutrophil activity and airway remodeling occurring during chronic respiratory conditions [45], this cell type showed strong expression of IL-17 when infiltrating lungs infected with WT P. gingivalis.
Consistent with the results obtained from immunohistochemical analysis, intratracheal inoculation with either WT P. gingivalis or ΔKgp led to a significant increase in IL-17 expression in lung tissue and peripheral blood. Animals infected with KRAB did not overexpress IL-17, and ΔRgp inoculation induced only a moderate increase in IL-17 production, which was significantly lower than that in WT or ΔKgp-inoculated animals (fig. 4c, d).
P. gingivalis-Induced Pneumonia Affects the Platelet Count and Platelet Activity
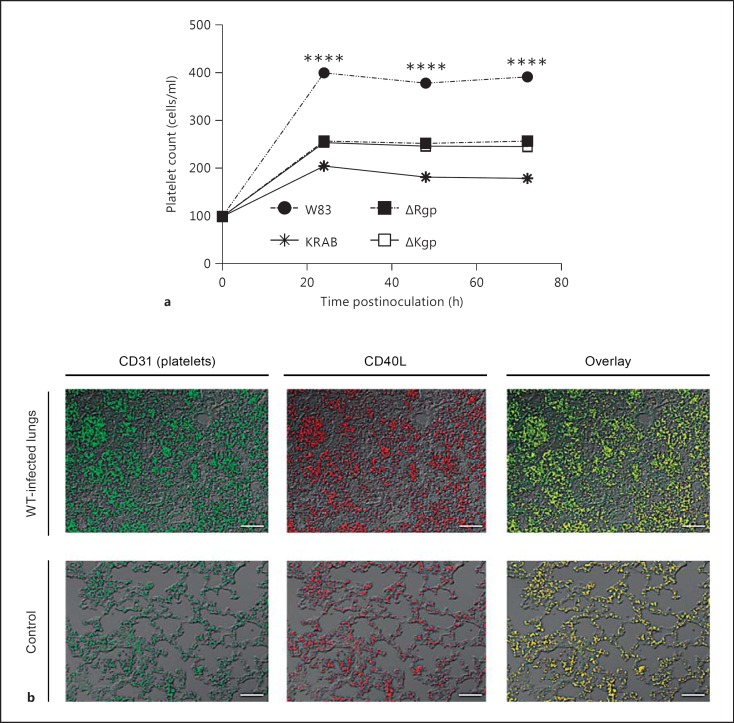
Altered peripheral blood parameters are one of the first symptoms of bacterial infection. In this context, the most visible change occurring subsequent to lung infection with any strain of P. gingivalis was a marked increase in the platelet count. Interestingly, infection with the WT strain resulted in a significantly greater increase in the platelet count than infection with either the KRAB or the W83-KYT strain. Morphological analysis of blood samples from mice inoculated with ΔKgp or ΔRgp also revealed an increase in the platelet count; however, the increase was smaller than that in mice infected with the WT strain. Endotracheal instillation of sterile PBS into the lungs had no effect on the platelet count in the peripheral blood (fig. 5a). This suggests that active gingipains in general, and RgpA and RgpB in particular, play a role in increasing the platelet count. Interestingly, platelets in the lung expressed high levels of CD154 (CD40L; fig. 5b). CD40L is rapidly presented on the platelet surface after stimulation and is subsequently cleaved from the surface to generate soluble fragments that play a key role in the inflammatory response [48]. Also, the translocation of CD40L coincides with the release of platelet-derived growth factor, transforming growth factor-β, platelet factor 4, and thrombospondin from α-granules [49].
Fig. 5.
Gingipains affect the platelet count and strongly activate platelets in lung tissue. a Platelet counts in peripheral blood inoculated with P. gingivalis (W83, KRAB, ΔRgp, or ΔKgp) were performed at 24 h postinfection. b Sections of lung tissue (taken at 24 h postinfection) from mice infected with P. gingivalis W83 and from control mice were stained for CD31 (left) or CD40L (middle). A merged image is shown on the right. Scale bars = 50 μm. **** p < 0.0001. Data are representative of 2 independent experiments with similar results.
Discussion
Aspiration pneumonia is a life-threatening condition caused by aspiration of bacteria into the respiratory system during medical procedures or by accidental aspiration of liquid (e.g. saliva) or solid (e.g. food) material [50, 51]. Here, we evaluated the role of gingipains in the development of lung pathology during P. gingivalis inhalation. A murine model of intratracheal lung inoculation revealed that gingipains play a pivotal role in the development of a severe, often fatal, form of aspiration pneumonia. We confirmed that aspiration of WT P. gingivalis W83 causes severe, fatal pneumonia as previously described [40]; however, we found that inhibition of the proteolytic activity of gingipains, either using specific inhibitors (KYT) or by deleting gingipain-encoding genes, results in almost complete attenuation of the bacterial virulence. In both cases, only mild and transient deterioration of the health status was observed. Moreover, further evaluation revealed that similar numbers of bacteria colonized the lungs of infected animals, regardless of the strain used. This novel observation suggests that gingipains have a minimal impact on the rate of lung infection by P. gingivalis. Other infection models (e.g. a subcutaneous chamber and an abscess model of infection) have shown that gingipains are necessary for P. gingivalis survival in vivo [52, 53]. When all data are taken into account, it is tempting to speculate that the lack of bacterial clearance from the lung in this particular model results from the absence of a gingipain-dependent ‘trigger’ to activate the host immune system. The side effect of immune activation is an exaggerated inflammatory response, leading to bronchopneumonia, lung abscess formation, hemorrhage, and necrosis.
To examine this further, we used a variety of gingipain knockout (ΔRgp, ΔKgp, or KRAB) and WT bacteria pretreated with specific inhibitors (W83-KYT) to analyze the immune response in mice. We found that the severity of clinical symptoms in animals inoculated with WT P. gingivalis correlated with the development of a systemic inflammatory reaction, as indicated by the increased production of CRP and key proinflammatory cytokines. Significantly, no changes in CRP, TNF-α, or IL-6 levels were observed in animals infected with KRAB or W83-KYT. This clearly indicates that the induction of a systemic inflammatory response by P. gingivalis in this lung infection model is solely dependent on the proteolytic activity of gingipains.
The finding that lung infection, morbidity, and mortality was far higher in young animals than in old animals was surprising given that the mortality associated with human aspiration pneumonia is higher in older age groups. However, it is well known that elderly individuals show age-related declines in immune function [54]; thus, the inability to mount a robust response to P. gingivalis in the lungs may explain the survival of older animals at sublethal inoculum levels. Furthermore, aspiration of oral or gastric contents by young and healthy humans is an extremely rare event. The disease is mostly limited to individuals with an incompetent swallowing mechanism, such as stroke survivors, those with Parkinson's disease or severe dementia, and users of antipsychotic drugs, proton pump inhibitors, and angiotensin-converting enzyme inhibitors; all of these factors tend to be associated with older individuals [55].
Several lines of evidence suggest that the proteolytic activity of gingipains alerts and activates the innate immune system. For example, gingipains are involved in the release of anaphylatoxin C5a from the C5 component of complement [56], mediate the release of bradykinin from high-molecular-weight kininogen [57, 58], activate coagulation cascade factors (including factors X and IX and prothrombin) [59, 60, 61, 62], and dysregulate cell signaling via protease-activated receptors (PAR) [63, 64]. In this regard, a previous study showed that IL-6 is induced in fibroblasts by gingipain signaling via PAR-1 [65]. Furthermore, gingipain-mediated stimulation of inflammatory responses (manifested by increased TNF-α and IL-6 levels) in the blood is greatly enhanced by pathogen-associated molecular patterns. This is supported by ex vivo findings showing that gingipains synergistically increase the secretion of proinflammatory cytokines (e.g. from human monocytic cells) by interacting with PAR types 1, 2, and 3 in combination with Toll-like receptors or nucleotide-binding oligomerization domain-containing protein agonists [66]. Also, the severity of edema, which is mediated by the gingipain-dependent release of bradykinin, is increased in the presence of lipopolysaccharides due to transcellular crosstalk between TLR2 and bradykinin receptor-2 [67].
One of the pathogenic outcomes of P. gingivalis-triggered aspiration pneumonia is thrombocytosis. In accordance with findings showing that thrombocytosis is generally associated with inflammatory disease [68], it can be assumed that the observed increase in the platelet count is simply an acute-phase response to inflammation induced by P. gingivalis. However, comparison of the platelet levels in the blood of mice inoculated with KRAB or W83-KYT with those in the blood of mice inoculated with WT W83 suggests that counts are significantly higher in the latter. This difference may be related to the significantly higher levels of IL-6 measured in animals exposed to the WT strain, as this cytokine is known to augment the expression of thrombopoietin, a key factor that stimulates platelet production in the bone marrow [69]. This relatively straightforward process may have a double-edged effect on the development of aspiration pneumonia. Damage to the endothelium of P. gingivalis-infected lungs allows a large number of platelets to interact with collagen and other extracellular matrix proteins. This leads to platelet activation. The large number of activated platelets releases a plethora of molecules, including chemokines and bactericidal peptides, which are involved in modulation of the immune response [70]. In addition, CD154 (CD40L), which is expressed on activated platelets upon interaction with CD40 on endothelial cells, induces these cells to release MCP-1 (CCL-2) [71]. Since platelets can also be activated by nonproteolytic interactions with the RgpA- and Kgp-derived hemagglutinin domain [72], this mechanism may explain why MCP-1 levels increase in mice infected with WT P. gingivalis and W83-KYT but not in mice inoculated with the gingipain-null mutant (KRAB). It is likely that platelet-derived chemokines, IL-1β, histamine, serotonin, and other mediators released from platelets that have extravasated into lung tissues during hemorrhage will exacerbate inflammation and contribute to tissue necrosis, abscess formation, and death in mice infected with WT P. gingivalis. On the other hand, platelets are a rich source of bactericidal peptides [73]; therefore, it is possible that their presence may also limit the growth of P. gingivalis in the lungs.
One interesting aspect of the gingipain-dependent pathology of WT P. gingivalis lung infection is the presence of severe intrapulmonary hemorrhage; this was not observed in animals challenged with gingipain-deficient bacteria (KRAB or W83-KYT). Because no intrapulmonary bleeding was observed in animals intratracheally instilled with PBS, it is clear that the observed symptoms did not result from surgical trauma during inoculation. One may, however, assume that hemorrhage was caused by the effect of gingipains on the respiratory epithelium, the endothelium lining capillary blood vessels, and components of the coagulation cascade. Degradation of tight-junction proteins by gingipains [74], together with enhanced vascular permeability [75], interference with blood clotting due to degradation of fibrinogen by Kgp [76], and protein C consumption by Rgps [77], most likely facilitates blood flow into lung tissues [78]. Also, the increased influx of neutrophils into the lung tissue of animals infected with W83 may exacerbate the local inflammatory response, thereby increasing the tissue damage and, subsequently, bleeding and necrosis.
Taken together, the results of the present study show that proteolytically active gingipains modulate the course of P. gingivalis-associated aspiration pneumonia and aggravate the host immune response; however, gingipains are not required for successful colonization and survival of the bacterium in the lungs. This is the first study to show that P. gingivalis-derived enzymes play an important role not only during chronic disease (e.g. periodontitis) but also during acute, life-threatening pneumonia. This knowledge may provide a new perspective on treatments for P. gingivalis-induced aspiration pneumonia.
Disclosure Statement
The funders had no role in the study design, the data collection and analysis, the decision to publish, or the preparation of this paper. The authors declare that they have no competing interests.
Statement of Ethics
All studies were performed in accordance with European Union regulations for the handling and use of laboratory animals. The protocols were approved by institutional Animal Care and Use Committees (No. 94/2009; Jagiellonian University, Krakow, Poland).
Supplementary Material
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Acknowledgements
We are grateful to Dr. Ravi Jotwani (University of Louisiana School of Dentistry) and Dr. Ky-Anh Nguyen (Sydney University) for assistance with a laser scanning confocal microscope analysis and for critical reading of this paper, respectively.
This work was supported by grants from the National Institutes of Health (NIDCR DE 09761 and DE 022597), the European Community (FP7-HEALTH-F3-2012-306029 ‘TRIGGER’ and Marie Curie ITN RAPID No. 290246), the National Science Center (2012/04/A/NZ1/00051 to J.P., 2014/14/E/NZ6/00162 to P.M.M. and 2011/01/D/NZ6/00269 to K. Pyrc), and the Ministry of Science and Higher Education, Poland (2975/7.PR/13/2014/2 to J.P.). The Faculty of Biochemistry, Biophysics and Biotechnology of Jagiellonian University is a partner of the Leading National Research Center (KNOW) supported by the Ministry of Science and Higher Education.
References
- 1.Terpenning MS, Taylor GW, Lopatin DE, Kerr CK, Dominguez BL, et al. Aspiration pneumonia: dental and oral risk factors in an older veteran population. J Am Geriatr Soc. 2001;49:557–563. doi: 10.1046/j.1532-5415.2001.49113.x. [DOI] [PubMed] [Google Scholar]
- 2.De Pablo P, Chapple ILC, Buckley CD, Dietrich T. Periodontitis in systemic rheumatic diseases. Nat Rev Rheumatol. 2009;5:218–224. doi: 10.1038/nrrheum.2009.28. [DOI] [PubMed] [Google Scholar]
- 3.Friedewald VE, Kornman KS, Beck JD, Genco R, Goldfine A, et al. The American Journal of Cardiology and Journal of Periodontology Editors' Consensus: periodontitis and atherosclerotic cardiovascular disease. Am J Cardiol. 2009;104:59–68. doi: 10.1016/j.amjcard.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 4.Chambrone L, Guglielmetti MR, Pannuti CM, Chambrone LA. Evidence grade associating periodontitis to preterm birth and/or low birth weight. 1. A systematic review of prospective cohort studies. J Clin Periodontol. 2011;38:795–808. doi: 10.1111/j.1600-051X.2011.01755.x. [DOI] [PubMed] [Google Scholar]
- 5.Bartlett JG. How important are anaerobic bacteria in aspiration pneumonia: when should they be treated and what is optimal therapy. Infect Dis Clin North Am. 2013;27:149–155. doi: 10.1016/j.idc.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 6.Bansal M, Khatri M, Taneja V. Potential role of periodontal infection in respiratory diseases - a review. J Med Life. 2013;15:244–248. [PMC free article] [PubMed] [Google Scholar]
- 7.Scannapieco FA, Shay K. Oral health disparities in older adults: oral bacteria, inflammation, and aspiration pneumonia. Dent Clin North Am. 2014;58:771–782. doi: 10.1016/j.cden.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 8.Albandar JM, Rams TE. Global epidemiology of periodontal diseases: an overview. Periodontol. 2000;29:7–10. doi: 10.1034/j.1600-0757.2002.290101.x. [DOI] [PubMed] [Google Scholar]
- 9.Cobb CM, Williams KB, Gerkovitch MM. Is the prevalence of periodontitis in the USA in decline? Periodontol. 2000;50:13–24. doi: 10.1111/j.1600-0757.2008.00284.x. [DOI] [PubMed] [Google Scholar]
- 10.Bartlett JG, Gorbach SL, Finegold SM. The bacteriology of aspiration pneumonia. Am J Med. 1974;56:202–207. doi: 10.1016/0002-9343(74)90598-1. [DOI] [PubMed] [Google Scholar]
- 11.Finegold SM, Strong CA, McTeague M, Marina M. The importance of black-pigmented Gram-negative anaerobes in human infections. FEMS Immunol Med Microbiol. 1993;6:77–82. doi: 10.1111/j.1574-695X.1993.tb00306.x. [DOI] [PubMed] [Google Scholar]
- 12.Zijlstra EE, Swart GR, Godfroy FJ, Degener JE. Pericarditis, pneumonia and brain abscess due to a combined Actinomyces-Actinobacillus actinomycetemcomitans infection. J Infect. 1992;25:83–87. doi: 10.1016/0163-4453(92)93633-2. [DOI] [PubMed] [Google Scholar]
- 13.Morris JF, Sewell DL. Necrotizing pneumonia caused by mixed infection with Actinobacillus actinomycetemcomitans and Actinomyces israelii: case report and review. Clin Infect Dis. 1994;18:450–452. doi: 10.1093/clinids/18.3.450. [DOI] [PubMed] [Google Scholar]
- 14.Scannapieco FA. Role of oral bacteria in respiratory infection. J Periodontol. 1999;70:793–802. doi: 10.1902/jop.1999.70.7.793. [DOI] [PubMed] [Google Scholar]
- 15.Okuda K, Kimizuka R, Abe S, Kato T, Ishihara K. Involvement of periodontopathic anaerobes in aspiration pneumonia. J Periodontol. 2005;76:2154–2160. doi: 10.1902/jop.2005.76.11-S.2154. [DOI] [PubMed] [Google Scholar]
- 16.Ashimoto A, Chen C, Bakker I, Slots J. Polymerase chain reaction detection of 8 putative periodontal pathogens in subgingival plaque of gingivitis and advanced periodontitis lesions. Oral Microbiol Immunol. 1996;11:266–273. doi: 10.1111/j.1399-302x.1996.tb00180.x. [DOI] [PubMed] [Google Scholar]
- 17.Lamont RJ, Jenkinson HF. Life below the gum line: pathogenic mechanisms of Porphyromonas gingivalis. Microbiol Mol Biol Rev. 1998;62:1244–1263. doi: 10.1128/mmbr.62.4.1244-1263.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL. Microbial complexes in subgingival plaque. J Clin Periodontol. 1998;25:134–144. doi: 10.1111/j.1600-051x.1998.tb02419.x. [DOI] [PubMed] [Google Scholar]
- 19.Holt SC, Ebersole JL. Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia: the ‘red complex’, a prototype polybacterial pathogenic consortium in periodontitis. Periodontol. 2000;38:72–122. doi: 10.1111/j.1600-0757.2005.00113.x. [DOI] [PubMed] [Google Scholar]
- 20.Darveau RP. The oral microbial consortium's interaction with the periodontal innate defense system. DNA Cell Biol. 2009;28:389–395. doi: 10.1089/dna.2009.0864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hajishengallis G. Porphyromonas gingivalis-host interactions: open war or intelligent guerilla tactics? Microbes Infect. 2009;11:637–645. doi: 10.1016/j.micinf.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kadowaki T, Takii R, Yamatake K, Kawakubo T, Tsukuba T, et al. A role for gingipains in cellular responses and bacterial survival in Porphyromonas gingivalis-infected cells. Front Biosci. 2007;12:4800–4809. doi: 10.2741/2428. [DOI] [PubMed] [Google Scholar]
- 23.Guo Y, Nguyen K-A, Potempa J. Dichotomy of gingipains action as virulence factors: from cleaving substrates with the precision of a surgeon's knife to a meat chopper-like brutal degradation of proteins. Periodontol 2000. 2010;54:15–44. doi: 10.1111/j.1600-0757.2010.00377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bostanci N, Belibasakis GN. Porphyromonas gingivalis: an invasive and evasive opportunistic oral pathogen. FEMS Microbiol Lett. 2012;333:1–9. doi: 10.1111/j.1574-6968.2012.02579.x. [DOI] [PubMed] [Google Scholar]
- 25.Potempa J, Pavloff N, Travis J. Porphyromonas gingivalis: a proteinase/gene accounting audit. Trends Microbiol. 1995;3:430–434. doi: 10.1016/s0966-842x(00)88996-9. [DOI] [PubMed] [Google Scholar]
- 26.Li N, Collyer CA. Gingipains from Porphyromonas gingivalis - complex domain structures confer diverse functions. Eur J Microbiol Immunol. 2011;1:41–58. doi: 10.1556/EuJMI.1.2011.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Potempa J, Sroka A, Imamura T, Travis J. Gingipains, the major cysteine proteinases and virulence factors of Porphyromonas gingivalis: structure, function and assembly of multidomain protein complexes. Curr Protein Pept Sci. 2003;4:397–407. doi: 10.2174/1389203033487036. [DOI] [PubMed] [Google Scholar]
- 28.Laugisch O, Schacht M, Guentsch A, Kantyka T, Sroka A, et al. Periodontal pathogens affect the level of protease inhibitors in gingival crevicular fluid. Mol Oral Microbiol. 2012;27:45–56. doi: 10.1111/j.2041-1014.2011.00631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Popadiak K, Potempa J, Riesbeck K, Blom AM. Biphasic effect of gingipains from Porphyromonas gingivalis on the human complement system. J Immunol. 2007;178:7242–7250. doi: 10.4049/jimmunol.178.11.7242. [DOI] [PubMed] [Google Scholar]
- 30.Potempa M, Potempa J. Protease-dependent mechanisms of complement evasion by bacterial pathogens. Biol Chem. 2012;393:873–888. doi: 10.1515/hsz-2012-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kadowaki T, Baba A, Abe N, Takii R, Hashimoto M, et al. Suppression of pathogenicity of Porphyromonas gingivalis by newly developed gingipain inhibitors. Mol Pharmacol. 2004;66:1599–1606. doi: 10.1124/mol.104.004366. [DOI] [PubMed] [Google Scholar]
- 32.Baba A, Kadowaki T, Asao T, Yamamoto K. Roles for Arg- and Lys-gingipains in the disruption of cytokine responses and loss of viability of human endothelial cells by Porphyromonas gingivalis infection. Biol Chem. 2002;383:1223–1230. doi: 10.1515/BC.2002.135. [DOI] [PubMed] [Google Scholar]
- 33.Bodet C, Chandad F, Grenier D. Modulation of cytokine production by Porphyromonas gingivalis in a macrophage and epithelial cell co-culture model. Microbes Infect. 2005;7:448–456. doi: 10.1016/j.micinf.2004.11.021. [DOI] [PubMed] [Google Scholar]
- 34.Mezyk-Kopec R, Bzowska M, Potempa J, Bzowska M, Jura N, et al. Inactivation of membrane tumor necrosis factor alpha by gingipains from Porphyromonas gingivalis. Infect Immun. 2005;73:1506–1514. doi: 10.1128/IAI.73.3.1506-1514.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yun PLW, Decarlo AA, Chapple CC, Collyer CA, Hunter N. Binding of Porphyromonas gingivalis gingipains to human CD4(+) T cells preferentially down-regulates surface CD2 and CD4 with little affect on co-stimulatory molecule expression. Microb Pathog. 2005;38:85–96. doi: 10.1016/j.micpath.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 36.Yun LWP, Decarlo AA, Hunter N. Blockade of protease-activated receptors on T cells correlates with altered proteolysis of CD27 by gingipains of Porphyromonas gingivalis. Clin Exp Immunol. 2007;150:217–229. doi: 10.1111/j.1365-2249.2007.03488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hajishengallis G, Wang M, Bagby GJ, Nelson S. Importance of TLR2 in early innate immune response to acute pulmonary infection with Porphyromonas gingivalis in mice. J Immunol. 2008;181:4141–4149. doi: 10.4049/jimmunol.181.6.4141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nelson S, Laughon BE, Summer WR, Eckhaus MA, Bartlett JG, et al. Characterization of the pulmonary inflammatory response to an anaerobic bacterial challenge. Am Rev Respir Dis. 1986;133:212–217. doi: 10.1164/arrd.1986.133.2.212. [DOI] [PubMed] [Google Scholar]
- 39.Nemec A, Pavlica Z, Nemec-Svete A, Eržen D, Milutinović A, et al. Aerosolized clindamycin is superior to aerosolized dexamethasone or clindamycin-dexamethasone combination in the treatment of severe Porphyromonas gingivalis aspiration pneumonia in an experimental murine model. Exp Lung Res. 2012;38:9–18. doi: 10.3109/01902148.2011.632063. [DOI] [PubMed] [Google Scholar]
- 40.Pace CC, McCullough GH. The association between oral microorgansims and aspiration pneumonia in the institutionalized elderly: review and recommendations. Dysphagia. 2010;25:307–322. doi: 10.1007/s00455-010-9298-9. [DOI] [PubMed] [Google Scholar]
- 41.Terpenning MS, Taylor GW, Lopatin DE, Kerr CK, Dominguez BL, Loesche WJ. Aspiration pneumonia: dental and oral risk factors in an older veteran population. J Am Geriatr Soc. 2001;49:557–563. doi: 10.1046/j.1532-5415.2001.49113.x. [DOI] [PubMed] [Google Scholar]
- 42.Kadowaki T, Baba A, Abe N, Takii R, Hashimoto M, et al. Suppression of pathogenicity of Porphyromonas gingivalis by newly developed gingipain inhibitors. Mol Pharmacol. 2004;66:1599–1606. doi: 10.1124/mol.104.004366. [DOI] [PubMed] [Google Scholar]
- 43.Kimizuka R, Kato T, Ishihara K, Okuda K. Mixed infections with Porphyromonas gingivalis and Treponema denticola cause excessive inflammatory responses in a mouse pneumonia model compared with monoinfections. Microbes Infect. 2003;5:1357–1362. doi: 10.1016/j.micinf.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 44.Petelin M, Naruishi K, Shiomi N, Mineshiba J, Arai H, et al. Systemic up-regulation of sTNFR2 and IL-6 in Porphyromonas gingivalis pneumonia in mice. Exp Mol Pathol. 2004;76:76–81. doi: 10.1016/j.yexmp.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 45.Message SD, Johnston SL. Host defense function of the airway epithelium in health and disease: clinical background. J Leukoc Biol. 2004;75:5–17. doi: 10.1189/jlb.0703315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mobarrez F, Sjövik C, Soop A, Hållström L, Frostell C, et al. CD40L expression in plasma of volunteers following LPS administration: a comparison between assay of CD40L on plateletmicrovesicles and soluble CD40L. Platelets. 2014;25:1–5. doi: 10.3109/09537104.2014.932339. [DOI] [PubMed] [Google Scholar]
- 47.Nurden AT. Platelets, inflammation and tissue regeneration. Thromb Haemost. 2011;105((suppl 1)):S13–S33. doi: 10.1160/THS10-11-0720. [DOI] [PubMed] [Google Scholar]
- 48.McGovern Murphy F, Raymond M, Menard PA, Bejar-Ardiles KR, Carignan A, Lesur O. Ventilator associated pneumonia and endotracheal tube repositioning: an underrated risk factor. Am J Infect Control. 2014;42:1328–1330. doi: 10.1016/j.ajic.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 49.Schallom M, Dykeman B, Metheny N, Kirby J, Pierce J. Head-of-bed elevation and early outcomes of gastric reflux, aspiration and pressure ulcers: a feasibility study. Am J Crit Care. 2015;24:57–66. doi: 10.4037/ajcc2015781. [DOI] [PubMed] [Google Scholar]
- 50.Kesavalu L, Holt SC, Ebersole JL. Trypsin-like protease activity of Porphyromonas gingivalis as a potential virulence factor in a murine lesion model. Microb Pathog. 1996;20:1–10. doi: 10.1006/mpat.1996.0001. [DOI] [PubMed] [Google Scholar]
- 51.Genco CA, Odusanya BM, Potempa J, Mikolajczyk-Pawlinska J, Travis J. A peptide domain on gingipain R which confers immunity against Porphyromonas gingivalis infection in mice. Infect Immun. 1998;66:4108–4114. doi: 10.1128/iai.66.9.4108-4114.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Montecino-Rodriguez E, Berent-Maoz B, Dorshkind K. Causes, consequences, and reversal of immune system aging. J Clin Invest. 2013;123:958–965. doi: 10.1172/JCI64096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van der Maarel-Wierink CD, Vanobbergen JN, Bronkhorst EM, Schols JM, de Baat C. Risk factors for aspiration pneumonia in frail older people: a systematic literature review. J Am Med Dir Assoc. 2011;12:344–354. doi: 10.1016/j.jamda.2010.12.099. [DOI] [PubMed] [Google Scholar]
- 54.Discipio RG, Daffern PJ, Kawahara M, Pike R, Travis J, et al. Cleavage of human complement component C5 by cysteine proteinases from Porphyromonas (Bacteroides) gingivalis: prior oxidation of C5 augments proteinase digestion of C5. Immunology. 1996;87:660–667. doi: 10.1046/j.1365-2567.1996.478594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rapala-Kozik M, Bras G, Chruscicka B, Karkowska-Kuleta J, Sroka A, et al. Adsorption of components of the plasma kinin-forming system on the surface of Porphyromonas gingivalis involves gingipains as the major docking platforms. Infect Immun. 2011;79:797–805. doi: 10.1128/IAI.00966-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Imamura T, Pike RN, Potempa J, Travis J. Pathogenesis of periodontitis: a major arginine-specific cysteine proteinase from Porphyromonas gingivalis induces vascular permeability enhancement through activation of the kallikrein/kinin pathway. J Clin Invest. 1994;94:361–367. doi: 10.1172/JCI117330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Imamura T, Potempa J, Tanase S, Travis J. Activation of blood coagulation factor X by arginine-specific cysteine proteinases (gingipain-Rs) from Porphyromonas gingivalis. J Biol Chem. 1997;272:16062–16067. doi: 10.1074/jbc.272.25.16062. [DOI] [PubMed] [Google Scholar]
- 58.Imamura T, Tanase S, Hamamoto T, Potempa J, Travis J. Activation of blood coagulation factor IX by gingipains R, arginine-specific cysteine proteinases from Porphyromonas gingivalis. Biochem J. 2001;353:325–331. doi: 10.1042/0264-6021:3530325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Imamura T, Banbula A, Pereira PJ, Travis J, Potempa J. Activation of human prothrombin by arginine-specific cysteine proteinases (gingipains R) from Porphyromonas gingivalis. J Biol Chem. 2001;276:18984–18991. doi: 10.1074/jbc.M006760200. [DOI] [PubMed] [Google Scholar]
- 60.Imamura T, Potempa J, Pike RN, Moore JN, Barton MH, Travis J. Effect of free and vesicle-bound cysteine proteinases of Porphyromonas gingivalis on plasma clot formation: implications for bleeding tendency at periodontitis sites. Infect Immun. 1995;63:4877–4782. doi: 10.1128/iai.63.12.4877-4882.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lourbakos A, Yuan YP, Jenkins AL, Travis J, Andrade-Gordon P, et al. Activation of protease-activated receptors by gingipains from Porphyromonas gingivalis leads to platelet aggregation: a new trait in microbiology pathogenicity. Blood. 2001;97:3790–3797. doi: 10.1182/blood.v97.12.3790. [DOI] [PubMed] [Google Scholar]
- 62.Lourbakos A, Chinni C, Thompson P, Potempa J, Travis J, et al. Cleavage and activation of proteinase-activated receptor-2 on human neutrophils by gingipain-R from Porphyromonas gingivalis. FEBS Lett. 1998;435:45–48. doi: 10.1016/s0014-5793(98)01036-9. [DOI] [PubMed] [Google Scholar]
- 63.Lourbakos A, Potempa J, Travis J, D'Andrea MR, Andrade-Gordon P, et al. Arginine-specific protease from Porphyromonas gingivalis activates protease-activated receptors on human oral epithelial cells and induces interleukin-6 secretion. Infect Immun. 2001;69:5121–5130. doi: 10.1128/IAI.69.8.5121-5130.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Uehara A, Muramoto K, Imamura T, Nakayama K, Potempa J, Travis J, et al. Arginine-specific gingipains from Porphyromonas gingivalis stimulate production of hepatocyte growth factor (scatter factor) through protease-activated receptors in human gingival fibroblasts in culture. J Immunol. 2005;175:6076–6084. doi: 10.4049/jimmunol.175.9.6076. [DOI] [PubMed] [Google Scholar]
- 65.Monteiro AC, Scovino A, Raposo S, Gaze VM, Cruz C, Svensjö E, et al. Kinin danger signals proteolytically released by gingipain induce fimbriae-specific IFN-gamma- and IL-17-producing T cells in mice infected intramucosally with Porphyromonas gingivalis. J Immunol. 2009;183:3700–3711. doi: 10.4049/jimmunol.0900895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Marwaha N. Thrombocytosis as a predictor of serious bacterial infection. Indian Pediatr. 2010;47:923–924. doi: 10.1007/s13312-010-0154-7. [DOI] [PubMed] [Google Scholar]
- 67.Nagahisa H, Nagata Y, Ohnuki T, Osada M, Nagasawa T, Abe T, et al. Bone marrow stromal cells produce thrombopoietin and stimulate megakaryocyte growth and maturation but suppress proplatelet formation. Blood. 1996;87:1309–1316. [PubMed] [Google Scholar]
- 68.McNicol A, Israels SJ. Mechanisms of oral bacteria-induced platelet activation. Can J Physiol Pharmacol. 2010;88:510–524. doi: 10.1139/y10-029. [DOI] [PubMed] [Google Scholar]
- 69.Gleissner CA, von Hundelshausen P, Ley K. Platelet chemokines in vascular disease. Arterioscler Thromb Vasc Biol. 2008;28:1920–1927. doi: 10.1161/ATVBAHA.108.169417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Naito M, Sakai E, Shi Y, Ideguchi H, Shoji M, et al. Porphyromonas gingivalis-induced platelet aggregation in plasma depends on Hgp44 adhesin but not Rgp proteinase. Mol Microbiol. 2006;59:152–167. doi: 10.1111/j.1365-2958.2005.04942.x. [DOI] [PubMed] [Google Scholar]
- 71.Tang YQ, Yeaman MR, Selsted ME. Antimicrobial peptides from human platelets. Infect Immun. 2002;70:6524–65633. doi: 10.1128/IAI.70.12.6524-6533.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Katz J, Sambandam V, Wu JH, Michalek SM, Balkovetz DF. Characterization of Porphyromonas gingivalis-induced degradation of epithelial cell junctional complexes. Infect Immun. 2000;68:1441–1449. doi: 10.1128/iai.68.3.1441-1449.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Imamura T, Potempa J, Pike RN, Travis J. Dependence of vascular permeability enhancement on cysteine proteinases in vesicles of Porphyromonas gingivalis. Infect Immun. 1995;63:1999–2003. doi: 10.1128/iai.63.5.1999-2003.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Scott CF, Whitaker EJ, Hammond BF, Colman RW. Purification and characterization of a potent 70-kDa thiol lysyl-proteinase (Lys-gingivain) from Porphyromonas gingivalis that cleaves kininogens and fibrinogen. J Biol Chem. 1993;268:7935–7942. [PubMed] [Google Scholar]
- 75.Hosotaki K, Imamura T, Potempa J, Kitamura N, Travis J. Activation of protein C by arginine-specific cysteine proteinases (gingipains-R) from Porphyromonas gingivalis. Biol Chem. 1999;380:75–80. doi: 10.1515/BC.1999.009. [DOI] [PubMed] [Google Scholar]
- 76.Kaminishi H, Cho T, Itoh T, Iwata A, Kawasaki K, Hagihara Y, et al. Vascular permeability enhancing activity of Porphyromonas gingivalis protease in guinea pigs. FEMS Microbiol Lett. 1993;114:109–114. doi: 10.1111/j.1574-6968.1993.tb06559.x. [DOI] [PubMed] [Google Scholar]
- 77.Nguyen KA, Travis J, Potempa J. Does the importance of the C-terminal residues in the maturation of RgpB from Porphyromonas gingivalis reveal a novel mechanism for protein export in a subgroup of gram-negative bacteria? J Bacteriol. 2007;189:833–843. doi: 10.1128/JB.01530-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hosotaki K, Imamura T, Potempa J, Kitamura N, Travis J. Activation of protein C by arginine-specific cysteine proteinases (gingipains-R) from Porphyromonas gingivalis. Biol Chem. 2005;380:75–80. doi: 10.1515/BC.1999.009. [DOI] [PubMed] [Google Scholar]
- 79.Shi Y, Ratnayake B, Okamoto K, Abe N, Yamamoto K, Nakayama K. Genetic analyses of proteolysis, hemoglobin binding, and hemagglutination of Porphyromonas gingivalis. Construction of mutants with a combination of rgpA, rgpB, kgp, and hagA. J Biol Chem. 1999;274:17955–17960. doi: 10.1074/jbc.274.25.17955. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data