Abstract
Adaptive changes occur in response to repeated exposure to drugs. Although ethanol (EtOH) is known to induce pharmacokinetic tolerance, the effects of EtOH on in vivo, magnetic resonance (MR)-detectable brain measures across repeated exposures have not previously been reported. Of 28 rats weighing 341±22g at baseline, 15 were assigned to the EtOH group and 13 to the control (Ctrl) group. EtOH animals were exposed to 5 cycles of 4-days of EtOH treatment followed by 10 days of recovery. Rats in both groups had structural MR imaging (MRI) scans and whole brain MR spectroscopy (MRS) at baseline, immediately following each binge period, and after each recovery period (total=11 MR scans per rat).
Average blood alcohol levels (BALs) across each of the 5, 4-day binge periods were 298, 300, 301, 312, 318 mg/dL. Cerebrospinal fluid (CSF) volumes of the lateral ventricles and cisterns showed enlargement with each binge EtOH exposure but recovery with each abstinence period. Similarly, changes to MRS metabolites were transient: levels of N-acetyl aspartate (NAA) and total creatine (tCr) decreased, while those of choline-containing compounds (Cho) and glutamate/glutamine (Glx) increased with each binge EtOH exposure cycle, but also recovered during each abstinence period. The directionality of changes in response to EtOH were in expected directions based on previous, single-binge EtOH exposure experiments, but the current results do not provide support for accruing pathology with repeated binge EtOH exposure.
Keywords: repeated withdrawals, alcohol, binge, MRI, MRS, ventricles, glutamate
INTRODUCTION
Repeated withdrawals from cycles of chronic alcohol exposure are hypothesized to contribute to progressive, persisting adaptive changes in the brain that lead to the insidious development and maintenance of alcohol use disorder (AUD). AUD individuals with a history of multiple detoxifications are reported to exhibit more severe withdrawal symptoms (Ballenger and Post, 1978; Booth and Blow, 1993; Brown et al., 1988; Lechtenberg and Worner, 1992) and have a higher risk for relapse (O’Connor et al., 1991) than those without prior detoxification experiences. In some studies, severity of cognitive impairment (based on compromised performance on tasks including the Wechsler Memory Scale (Glenn et al., 1988), the IOWA gambling (Loeber et al., 2009), an emotional Stroop (Duka et al., 2002), incentive conflict (Duka et al., 2011), cognitive flexibility (Trick et al., 2014), and vigilance (Duka et al., 2003)) is associated with the number of prior withdrawal experiences. Patients with multiple detoxifications compared with those experiencing their first detoxification demonstrate significantly lower brain activity (as quantified with brain perfusion of Tc99 m-ECD using single photon emission computed tomography scanning) in temporal lobes and visual cortex (George et al., 1999), greater deficits of frontal gray matter volume (Duka et al., 2011; Trick et al., 2014), and greater connectivity (as determined with functional magnetic resonance imaging, fMRI) in some, but less in other brain regions involved in emotion processing (O’Daly et al., 2012). Together, these results from human studies suggest that recurring rehabilitation attempts and attendant abstinence periods are associated with adaptive changes in the brain that may lead to more severe alcohol dependence.
In human studies, however, factors related to a history of AUD, such as age at initiation, years of heavy drinking, and quantity and frequency of alcohol consumed may confound findings attributed to multiple detoxifications. Animal studies have thus been undertaken to determine whether a history of previous withdrawal episodes from ethanol (EtOH) results in brain adaptations contributing to the development and maintenance of an AUD phenotype. As observed in humans, repeated withdrawals from EtOH in mice are associated with exacerbation of seizure activity (Becker et al., 1997; Becker and Lopez, 2004; Becker et al., 1998; but see Cox et al., 2013). In rats, however, EtOH withdrawal seizures are typically not observed unless stimulated (but see Clemmesen and Hemmingsen, 1984; Cooper et al., 1979; Devaud et al., 2012; audiogenic triggers Ebel et al., 1979; Gonzalez et al., 1989; McCown and Breese, 1990; e.g., with chemoconvulsants Pinel and Van Oot, 1975; electroconvulsants Pinel and Van Oot, 1978; Ruwe et al., 1986; Shen et al., 2012; Ulrichsen et al., 1992). Repeated deprivations from EtOH, even in the absence of overt withdrawal signs (Heyser et al., 1997), are associated with temporary increases in drinking as quantified using both simple consummatory measures and operant procedures (Backstrom et al., 2004; in mice: Becker and Lopez, 2004; Bell et al., 2004; Colombo et al., 2003; Cowen et al., 2003; Cox et al., 2013; Dayas et al., 2004; Fullgrabe et al., 2007; Funk et al., 2004; Heyser et al., 1997; in rats: Holter et al., 2000; Khisti et al., 2006; Lundqvist et al., 1995; Melendez et al., 2006; Oster et al., 2006; Sanchis-Segura et al., 2006; Serra et al., 2003; Spanagel and Holter, 1999; Sparta et al., 2009; but see Stephens et al., 2001; Zghoul et al., 2007).
Studies of behavior in animal models compare repeated withdrawals with single withdrawals or continuous exposure to EtOH. Performance on an avoidance paradigm was worse in female mice subjected to several withdrawals compared with those given continuous EtOH (Freund, 1970). Indeed, repeated withdrawals from EtOH in rats have been associated with deficits in conditioning (Duka et al., 2004; Stephens et al., 2001) and spatial learning (Swartzwelder et al., 2014) and with increased anxiety (Overstreet et al., 2002). Other studies, however, did not observe accruing behavioral deficits associated with repeated withdrawal episodes. Rodents with repeated withdrawal episodes show tolerance to EtOH-induced motor impairment (Kalant et al., 1978; Maier and Pohorecky, 1987; Philibin et al., 2012). Furthermore, prolonged abstinence is associated with recovery: rats tested on days 4–6 of EtOH withdrawal demonstrate impaired performance on the Morris water maze and a novel object recognition task, but do not perform differently from controls when tested on days 11–13 after the final binge period (4 cycles: 4-day binge via oral gavage + 3 day withdrawal) (Zhao et al., 2013). Animals with intermittent (24h/day, 3 days/week) relative to those with continuous (24h/day, 7 days/week) access to EtOH initially (at 24h) perform worse on a delayed nonmatching-to-sample task, but with a protracted abstinence (16–68 days) show comparable performance (George et al., 2012); performance on the Barnes maze is impaired 3–6 days following EtOH (6 days of 3.4g/kg/day), but recovers by day 14 (Kuzmin et al., 2012). Similarly, mice with up to 10 binge drinking episodes (1 period: 4 days drinking in the dark + 3 days withdrawal) did not demonstrate aggravated anxiety-like behaviors (elevated plus maze, open field) or ataxia (rotorod) (Cox et al., 2013).
Whether brain damage accrues with repeated withdrawal episodes is unclear. A single 4-day EtOH binge (via oral gavage) is reported to cause neuronal loss in brain regions including olfactory, entorhinal, piriform, and perirhinal cortices, as quantified by argyrophilia (i.e., silver staining) (e.g., Collins et al., 1996; Collins and Neafsey, 2012). Over a 1-month exposure period, intermittent relative to continuous EtOH exposure (in drinking water) is reported to cause more loss of hippocampal pyramidal neurons as quantified by an optical fractionator (Lundqvist et al., 1995). On the other hand, while fluoro-jade B staining, a marker for neuronal degeneration, is higher in EtOH-exposed animals in entorhinal cortex and hippocampus on the first day after the final binge period (4 cycles: 4-day binge + 3 day withdrawal), by day 14 of abstinence, labeling was not different from controls. Relatedly, dendritic spines on hippocampal and entorhinal neurons initially showed degeneration but demonstrated recovery with extended abstinence from EtOH (Zhao et al., 2013). Indeed, elevated numbers of microglia induced by repeated EtOH exposures (Riikonen et al., 2002) may contribute to brain recovery during abstinence (Zhao et al., 2013).
Together, these findings suggest that repeated withdrawal periods from EtOH, in both humans and animals, influence the morphological and physiological properties of the brain and consequently, behavior. Our in vivo magnetic resonance (MR) studies have consistently shown that a single 4-day EtOH binge is associated with reversible brain abnormality: enlargement of lateral ventricles, reductions in MR Spectroscopy (MRS)-detectable metabolites, N-acetyl-asparate (NAA) and total creatine (tCr), and elevations in choline-containing compounds (Cho) (Zahr et al., 2010; Zahr et al., 2014; Zahr et al., 2013). Together, these reversible events indicate transient cellular injury. However, based on the proposition that progressive, persisting adaptive changes in the brain lead to the development of AUD, the current study tested the hypothesis that repeated binge EtOH exposure and withdrawal periods would be associated with accruing brain pathology as quantified by progressively larger lateral ventricles, reductions in NAA and tCr, and elevations in Cho with later binges.
MATERIALS AND METHODS
Animals
The study group initially included 28 male, wild-type Wistar rats (Charles River Laboratories), singly housed with free access to food and water, with lights on for 12h starting at 6:00. At baseline (i.e., scan 1), rats weighed 340.66±21.93g. Animals used in these experiments were maintained in facilities fully accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care (AAALAC). The Institutional Animal Care and Use Committees (IACUC) at SRI International and Stanford University approved all research protocols in accordance with the guidelines of the IACUC of the National Institute on Drug Abuse, National Institutes of Health, and the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council 1996).
Treatment
After baseline scanning, 15 rats were assigned to the EtOH group. On day 1 of each 4-day binge EtOH exposure (total of 5 treatment cycles), EtOH rats received an initial “loading” dose of 5g/kg 20% EtOH w/v via oral gavage, then a maximum of 3g/kg every 8h (6:00, 14:00, 22:00; on day 1, loading dose at 6:00, extra dose at 8:00) for 4 days. On each of the 4 days, animals were weighed and tail vein blood samples were collected (7:30, 13:00) to determine BALs in plasma assayed for alcohol content based on direct reaction with the enzyme alcohol oxidase (Analox Instruments Ltd., UK).
EtOH was administered according to body weight, BALs, and behavioral intoxication state assessed using a modified Majchrowicz scale (range 0–5: 0-neutrality, 1-sedation, 2-mild ataxia, 3-moderate ataxia, 4-severe ataxia, 5-loss of righting reflex) (Majchrowicz, 1975). Total EtOH administered across each of the 4-day binge periods was as follows: 37.8±3.8, 39.5±2.0, 37.5±2.1, 37.3±2.6, 36.5±1.5 g/kg. Control (Ctrl, n=13) animals received volumes of 5% dextrose equivalent to 3g/kg EtOH at comparable times to the experimental animals.
MR Scanning Procedures and Data Analysis
Schedule
All animals were scanned at baseline (scan 1), after each 4-day binge EtOH exposure period (5 binge scans, within 8h of the last EtOH dose: scans 2, 4, 6, 8, 10), and after each recovery period (5 recovery scans, 10 days following last EtOH dose: scans 3, 5, 7, 9, 11) (Table 1). A total of 14 EtOH and 13 Ctrl rats completed all 11 scans: one EtOH rat died after completing 9 scans (missing binge scan 5 and recovery scan 5). All 28 animals are included in the analyses.
Table 1.
Schedule
| Day | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 | 27 | 28 | 29 | 30 | 31 | 32 | 33 | 34 | 35 | 36 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MR Imaging: | BL | B1 | R1 | B2 | R2 | B3 | ||||||||||||||||||||||||||||||
| scan BALs (mg/dL): | 0 | 289 | 0 | 240 | 0 | 274 | ||||||||||||||||||||||||||||||
| EtOH Administration: | B1 | B2 | B3 | B4 | B1 | B2 | B3 | B4 | B1 | B2 | B3 | B4 | ||||||||||||||||||||||||
| 4-day average dose (g/kg): | 38 | 40 | 38 | |||||||||||||||||||||||||||||||||
| 4-day average BALs (mg/dL): | 297 | 300 | 301 | |||||||||||||||||||||||||||||||||
| 2-Bottle Choice: | X | X | X | X | X | |||||||||||||||||||||||||||||||
| Neurological Exam: | X | X | X | X | X | X | X | X | ||||||||||||||||||||||||||||
| Thiamine Assay: | ||||||||||||||||||||||||||||||||||||
| Liver Extraction: | ||||||||||||||||||||||||||||||||||||
| Euthanasia: |
| Day | 37 | 38 | 39 | 40 | 41 | 42 | 43 | 44 | 45 | 46 | 47 | 48 | 49 | 50 | 51 | 52 | 53 | 54 | 55 | 56 | 57 | 58 | 59 | 60 | 61 | 62 | 63 | 64 | 65 | 66 | 67 | 68 | 69 | 70 | 71 | 72 | 73 | 74 | 75 | 76 | 77 | 78 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MR Imaging: | R3 | B4 | R4 | B5 | R5 | |||||||||||||||||||||||||||||||||||||
| scan BALs (mg/dL): | 0 | 269 | 0 | 284 | 0 | |||||||||||||||||||||||||||||||||||||
| EtOH Administration: | B1 | B2 | B3 | B4 | B1 | B2 | B3 | B4 | ||||||||||||||||||||||||||||||||||
| 4-day average dose (g/kg): | 37 | 37 | ||||||||||||||||||||||||||||||||||||||||
| 4-day average BALs (mg/dL): | 312 | 318 | ||||||||||||||||||||||||||||||||||||||||
| 2-Bottle Choice: | X | X | X | X | X | X | ||||||||||||||||||||||||||||||||||||
| Neurological Exam: | X | X | X | X | X | X | X | X | ||||||||||||||||||||||||||||||||||
| Thiamine Assay: | X | |||||||||||||||||||||||||||||||||||||||||
| Liver Extraction: | X | |||||||||||||||||||||||||||||||||||||||||
| Euthanasia: | X |
BL: baseline, B: binge, R: recovery, BALs: blood alcohol levels
Anesthesia and Monitoring
Animals were held in an MR-invisible structure providing support for a radiofrequency (RF) coil and a nose cone for delivery of isoflurane anesthesia (1.5–2.5%) and oxygen (1.5L/min) (Adalsteinsson et al., 2004). For each rat, blood oxygen saturation, pulse rate, rectal temperature, and respiration were monitored throughout the ~2h MR experiment.
MRI Acquisition
The experiments were conducted on a clinical 3T GE Signa MR scanner equipped with a high-strength insert gradient coil (peak strength=600mT/m, peak slew rate=3200T/m/s, Chronik et al., 2000; Pfefferbaum et al., 2004). A custommade rat brain quadrature head coil (ø=44 mm) was used for both RF excitation and signal reception. A gradient-recalled echo localizer scan was used to position the animals in the scanner and for graphical prescription of the subsequent scans. High resolution, dual-echo, fast spin-echo (FSE) images were acquired in the rat-axial plane, coronal to the magnet system bore (TE1/TE2/TR=12/60/5000ms, field of view (FOV)=64×48mm2, 256×192 matrix, echo train length=8, 26 slices, 0.7mm thick, 0mm separation, in-plane resolution=.25×.25mm2, 2 separate acquisitions each with 2 NEX).
Image Post-processing
Motion-corrected FSE images were computed by aligning the second dual-echo acquisition for each animal with the first using rigid (translation and rotation) image-to-image registration of the early-echo channel. The aligned early- and late- echo images were then averaged. From each motion-corrected early-echo image, a second-order multiplicative intensity bias field was then estimated by entropy minimization (Likar et al., 2001). The same bias field was applied to the corresponding late-echo image to preserve quantities derived from the early-to-late echo ratio such as transverse relaxation time (T2).
A preliminary brain mask was computed for each FSE image pair by 1) thresholding the late-echo image at the 99th percentile, 2) eroding the resulting mask by four pixels, 3) computing connected components, 4) selecting the largest connected component, and 5) dilating the resulting region by seven pixels. The purpose of this coarse, slightly enlarged, approximate brain mask was to exclude the majority of non-brain tissue to facilitate alignment of the image to a whole-head image of a template animal, from which the final brain mask would then be derived by label propagation of the manually-defined template brain mask.
To this end, baseline data from each animal were aligned with the template animal via a sequence of successively refined image transformations: 1) initial alignment based on principal axes of the whole-head late-echo FSE images, 2) rigid alignment of the whole-head late-echo images, 3) affine alignment of the brain-only late-echo template image and the animal late-echo image masked using the preliminary brain mask, and 4) full nonrigid alignment of the brain-only template image to the whole-head late-echo animal image. Steps 2 through 4 all used maximization of normalized cross correlation as the cost function for alignment, which was implemented to exclude non-brain pixels from computation where applicable, rather than set them to zero. This registration sequence resulted from balancing competing needs, such as the observation that brain alignment accuracy improves when brain-only images are co-registered, yet the quality of the initial animal brain masks is too poor for direct use and would interfere with the alignment.
For longitudinal alignment, each follow-up image was aligned with the baseline image of the same animal by co-registration of the whole-head, late-echo FSE images. Because the anatomy of the non-brain tissue is consistent over time in a single animal (as opposed to between different animals), use of the whole-head images for alignment yields better results compared with brain-only images, because it avoids errors that would otherwise arise from longitudinally inconsistent brain masks. All software tools used to perform the processing outlined above were developed in-house and are freely available, in source code, as part of the Computational Morphometry Tool Kit (CMTK; http://nitrc.org/projects/cmtk/).
For ventricular and cistern quantification, a rectangular template encompassing the majority of the lateral ventricles across 7 contiguous slices and a similar region of interest encompassing the posterior cistern, were drawn on the template brain. These templates were reformatted and transformed onto each animal’s native image space and all pixels above a uniform threshold (CSF is much brighter than surrounding gray or white matter) were counted as ventricle or cistern CSF.
MRS Acquisition
FSE images were used to prescribe a voxel (9×7×9mm=567mm 3) fitting within but including as much of the brain as possible while excluding the olfactory bulbs and cerebellum. Single-voxel spectroscopic data were acquired with point-resolved spectroscopy (TE/TR = 133/2283 ms, NEX=128) (Bottomley, 1984) preceded by a 3-pulse chemical shift selective sequence for water suppression. Additionally, data without water suppression from the same voxel were acquired at multiple TEs (TE ranging from 36.6 – 241.4ms, 12.8ms increment, TR=2s) to measure tissue water content for normalization of metabolite signal intensities to the amount of tissue water in the voxel (for details, see Zahr et al., 2009). The 3 singlet resonances (NAA, tC, and Cho) were fitted simultaneously; EtOH and glutamate/glutamine (Glx) resonances were fitted independently, with a Gaussian function within a ±7.95 Hz window using a downhill simplex method (IDL AMOEBA). The integrated area under the fitted Gaussian was used for quantification. The quality of the spectra allowed evaluation of signals of the major proton metabolites: NAA (2.0 ppm), tCr (3.0 ppm), Cho (3.20 ppm), Glx (2.4 ppm), and EtOH (1.2 ppm).
Behavioral Assays: 2-Bottle Choice and Neurological Exam
Animals were removed from their home cages and placed in separate cages for 2-bottle choice sessions (12h) at baseline, 24h after the last EtOH dose of each binge, and on day 4 of each recovery period (Table 1). EtOH (15% w/v) and water reservoirs were standard plastic bottles (300mL) connected to rodent fluid lixit-valves (L-2000 Bedding Resistant Drinking Valve, Systems Engineering Lab Group Inc., Napa, California) via polyethylene tubing (0.5mm OD fiber; 1 mm OD with sheaths) mounted on the wall of a standard rat cage. Bottles were placed on a separate scales (EW-1500i, A&D, San Jose, California) linked to Fusion software (Omnitech Electronics, Inc., Columbus, Ohio) that continuously recorded the weight (in grams) of water or EtOH consumed during the 12h session. Positions of reservoirs were interchanged between sessions. 2-bottle choice data were collected on a total of 15 EtOH and 11 Ctrl rats (i.e., missing data for 2 Ctrl animals because of the limited number of 2-bottle choice stations).
Neurological examination was performed at baseline, on day 4 of each binge, and days 1 and 6 of each recovery period (Table 1). Rats were rated (0=absent, 1=present) for the presence of each of 32 neurological signs (e.g., Becker, 2000; Roberts et al., 1996; Yaksh et al., 1977).
Thiamine Assay
Before euthanasia (conducted within 1 week of the final scan), whole blood (~1.5mL) was collected in EDTA (ethylenediaminetetraacetic acid) tubes and transferred to 15mL conical centrifuge tubes, to which 10ml normal saline was added. The suspension was mixed gently and then centrifuged at 164g at 2 – 8°C for 10min. The supernatant was then discarded with care to prevent disturbing red blood cells (RBCs). This saline wash was repeated 2 more times. After the third wash, 3 – 5mL of saline was left with the RBCs in the tubes. The tubes were wrapped in aluminum foil to protect from light and shipped, cold but not frozen, to Ani Lyitics Inc. (Gaithersburg, MD) for the measurement of thiamine and its phosphate derivatives.
Liver Histopathology
At euthanasia (2.5–3.5% isoflurane, followed by decapitation), left lateral lobe liver specimens from all rats were collected and immersed in 10% buffered formalin solution. After fixation, the specimens were routinely processed for light microscopic examination of hematoxylin and eosin (H&E) stain and Masson’s Trichrome stain and evaluated by the veterinary pathologist (RL) for hepatic pathology on a 0 to 4 scale, where 0=no pathology, 1=minimal (affects <5% of tissue), 2=mild (affects 5–20% of tissue), 3=moderate (affects 20–50% of tissue), and 4=severe (affects > 50% of tissue) (Zahr et al., 2009).
Statistical Analysis
Several approaches were used for longitudinal data analysis. A general linear model (GLM) approach was used in the R statistical package (http://www.r-project.org/) to test the effects of group (i.e., dx = EtOH vs. Ctrl), treatment (i.e., rx = binge or recovery), and observation (i.e., obs = MR scans 1 – 11) on volumes of ventricles or cisterns and levels of metabolites. To correct for multiple comparisons (intercept, dx, rx, obs, rx:obs, rx:dx, obs:dx, rx:dx:obs; i.e., n=8), only p-values ≤ .00625 (α=.05) are reported.
In a further analysis, combining group and treatment resulted in 4 categories: EtOH at binge (EtOH-B), EtOH at recovery (EtOH-R), Ctrl at binge (Ctrl-B), and Ctrl at recovery (Ctrl-R). This approach enabled analysis of intercept and slope differences among the 4 categories to be tested with JMP (http://www.jmp.com/software/) using a repeated-measures analysis of variance (ANOVA) with the same correction for multiple comparisons (4 intercepts and 4 slopes, i.e., n=8, only p-values ≤ .00625 (α=.05) are reported).
Also presented are CSF volumes and MRS metabolites expressed as the percentage change at each scan relative to the previous condition (e.g., percentage change in lateral ventricular volume from baseline to binge 1, from binge 1 to recovery 1, from binge 2 to recovery 1, etc.) for each group (EtOH or Ctrl) separately.
RESULTS
BALs and Weight
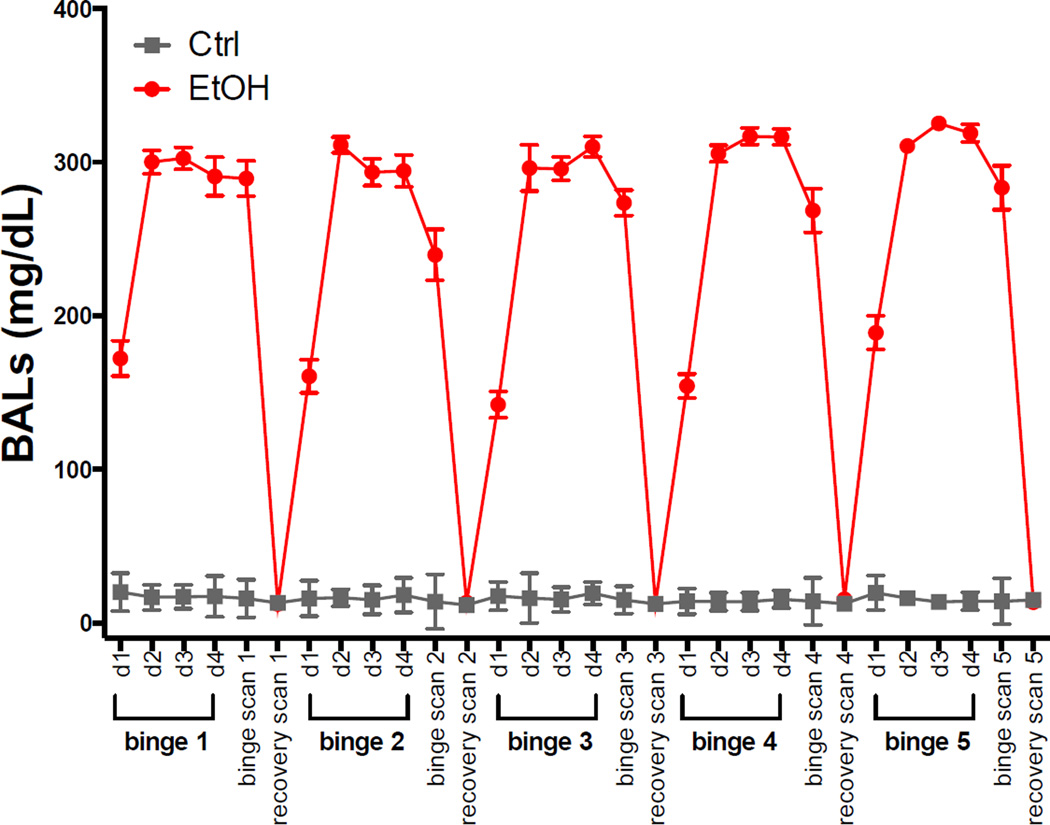
Fig. 1 shows BALs taken on each day of binge treatment (7:30), at each binge scan, and at each recovery scan (immediately following scan). Because on day 1 of each dosing period, the 7:30 BAL measurement was still in the rising phase, average BALs were computed for days 2–4. For each of the 5 binge periods, average BALs were 297.8±41.8, 299.7±35.4, 300.6±37.3, 312.9±20.1, and 318.2±21.8 mg/dL. At each binge scan, average BALs were 289.4±60.2, 239.6±87.5, 273.5±43.9, 268.5±75.3, and 283.5±47.5 mg/dL. Binge scan BALs were slightly lower than averages taken during dosing because there was more variability in the interval between the last EtOH dose and blood collection times for bloods collected after scans than for bloods collected at regular intervals during dosing.
Fig. 1.
Blood Alcohol Levels (BALs) collected on each day of binge EtOH treatment (7:30am) and at each scan. In all figures, the EtOH-exposed group is presented in red circles, the Ctrl group in gray squares.
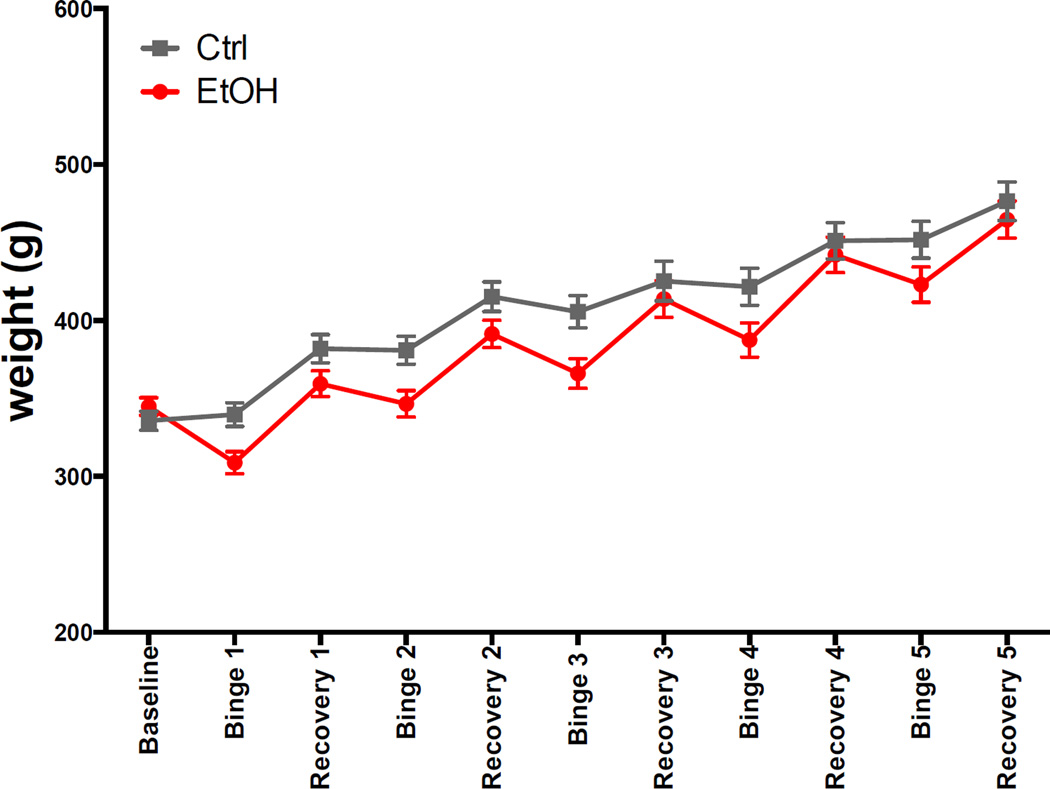
Fig. 2 shows weights for the 2 groups at each of the 11 scans. At the baseline scan, weights of the 2 groups were not significantly different (t(26)=1.1, p=.28). During each binge cycle, EtOH rats lost weight (percentage weight loss at binge scan relative to previous scan: 11, 4, 7, 6, 5 %). During each recovery cycle, EtOH rats gained weight (percentage weight gain at recovery scan relative to previous scan: 16, 13, 13, 13, 10%) so that at each recovery scan weights between the 2 groups were not significantly different. Weights were also not significantly different at the 5th binge scan (t(25)=−1.7, p=.09) despite similar BALs achieved. Over the course of the experiment, both groups of animals demonstrated a similar overall weight gain (Ctrl = 45%, EtOH = 40% increase from baseline weight).
Fig. 2.
Animal weights at baseline and each scan.
Behavior
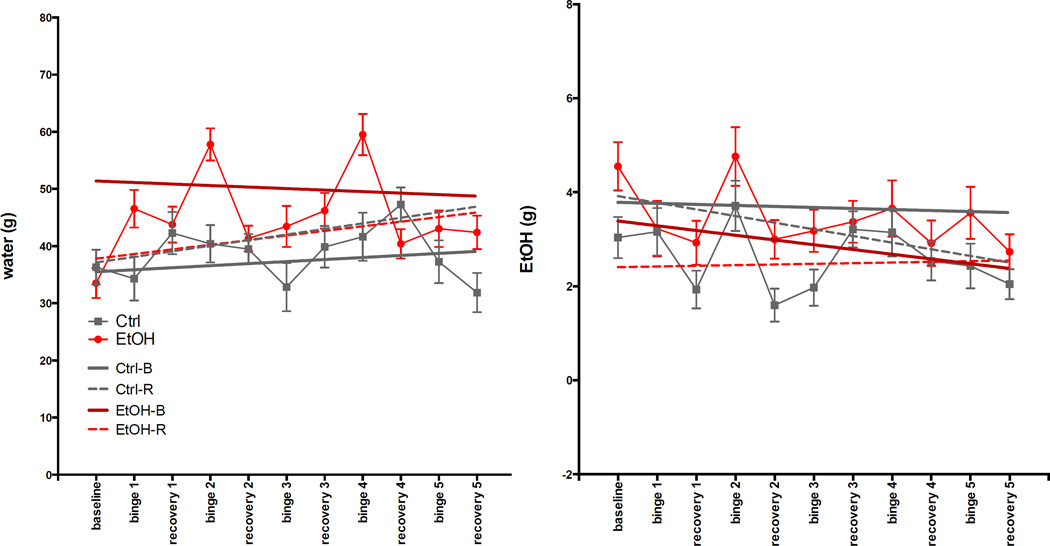
ANOVA showed that EtOH-B (EtOH-treated animals in 2-bottle choice sessions 24h after the last dose of EtOH) drank the most water (higher intercept, t=5.86, p≤.0001). Both EtOH-B and EtOH-R (EtOH-treated animals in 2-bottle choice sessions on day 4 of recovery) rats consumed less EtOH than rats in Ctrl-B and Ctrl-R categories (lower intercept), but only EtOH-R consumption was significantly lower (t= −3.37, p=.0009) (Fig. 3).
Fig. 3.
Results of 2-bottle choice preference for water or 15% EtOH (w/v) assessed for 12h at baseline, 24h after the last EtOH dose of each binge, and on day 4 of each recovery period.
Of 32 neurological variables, 12 were affected by binge EtOH treatment. No signs were evident on day 6 of recovery. The number of EtOH (out of 15) or Ctrl (out of 13) animals showing relevant neurological signs on binge day 4 or recovery day 1 is presented in Table 2. Neurological signs either persisted (e.g., effects on defecation, piloerection, tail tone) or diminished (e.g., startle response, hunched back) with repeated binge EtOH treatment, but none was observed to accumulate.
Table 2.
Behavior at each binge/recovery cycle*
| binge/recovery cycle 1 | binge/recovery cycle 2 | binge/recovery cycle 3 | binge/recovery cycle 4 | binge/recovery cycle 5 | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BL | B1D4 | R1D1 | B2D4 | R2D1 | B3D4 | R3D1 | B4D4 | R4D1 | B5D4 | R5D1 | ||||||||||||
| Con | EtOH | Con | EtOH | Con | EtOH | Con | EtOH | Con | EtOH | Con | EtOH | Con | EtOH | Con | EtOH | Con | EtOH | Con | EtOH | Con | EtOH | |
| Defecation | 0 | 0 | 0 | 15 | 0 | 10 | 0 | 15 | 0 | 10 | 0 | 15 | 0 | 10 | 0 | 15 | 0 | 10 | 0 | 14 | 0 | 9 |
| Respiration | 0 | 0 | 0 | 14 | 0 | 0 | 0 | 15 | 0 | 0 | 0 | 15 | 0 | 0 | 0 | 15 | 0 | 1 | 0 | 14 | 0 | 1 |
| Piloerection | 0 | 0 | 0 | 15 | 0 | 15 | 0 | 15 | 0 | 15 | 0 | 15 | 0 | 15 | 0 | 15 | 0 | 15 | 0 | 14 | 0 | 14 |
| Tail Tone | 0 | 0 | 0 | 14 | 0 | 14 | 0 | 14 | 0 | 14 | 0 | 14 | 0 | 14 | 0 | 13 | 0 | 13 | 0 | 12 | 0 | 12 |
| Wetness around nose/mouth | 0 | 0 | 0 | 4 | 0 | 0 | 0 | 5 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Wetness around eyes | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 |
| Red discharge around eyes | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 2 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Other eye problems | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 9 | 0 | 4 | 0 | 8 | 0 | 4 | 0 | 9 | 0 | 0 | 0 | 7 | 0 | 0 |
| Startle Response | 0 | 0 | 0 | 5 | 0 | 0 | 0 | 4 | 0 | 0 | 0 | 9 | 0 | 0 | 0 | 4 | 0 | 0 | 0 | 5 | 0 | 0 |
| Hunched Back | 0 | 0 | 0 | 6 | 0 | 0 | 0 | 8 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 7 | 0 | 0 | 0 | 6 | 0 | 0 |
| Sits Motionless | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 5 | 0 | 0 | 0 | 6 | 0 | 0 | 0 | 3 | 0 | 0 |
| Tremor | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 0 | 0 | 4 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 |
expressed as # of animals (EtOH n=15, Ctrl n=13, except for binge/recovery cycle 5, EtOH n=14)
BL: baseline, B: binge, R: recovery, D: day
Volumes of Lateral Ventricles and Cisterns
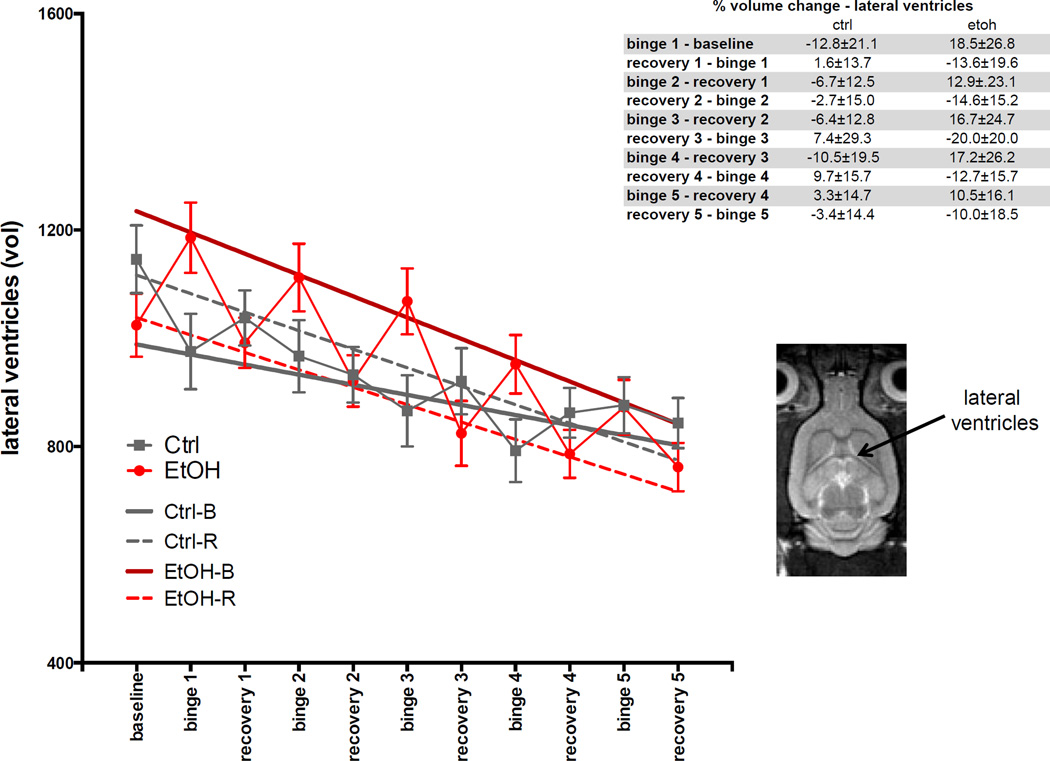
For lateral ventricle volumes (Fig. 4), the GLM showed effects of group (t=3.4, p=.00079) and group-by-treatment (t= −1.6, p=.00161). The ANOVA showed EtOH-B had larger volumes (higher intercept) than the other three categories (t=4.77, p≤.0001). Over the course of the experiment, volume of the lateral ventricles in all animals (both EtOH and Ctrl groups) decreased (t= −6.94, p≤.0001). Percentage change in lateral ventricular volume at each scan relative to the previous scan is presented in Fig. 4, insert.
Fig. 4.
Volumes of lateral ventricles at baseline and each binge and recovery scan. Inset is an axial slice of an FSE image indicating lateral ventricles. In this and all the following figures, the dark red line is the regression for EtOH-exposed rats at binge scans (scans 2, 4, 6, 8, 10), the red dashed line is the regression for EtOH-exposed rats at recovery scans (scans 1, 3, 5, 7, 9, 11), the solid gray line is the regression for Ctrl rats at binge scans (scans 2, 4, 6, 8, 10), the gray dashed line is the regression for Ctrl rats at recovery scans (scans 1, 3, 5, 7, 9, 11). Table insets show the percent change in volume or metabolite levels at each scan relative to the previous scan.
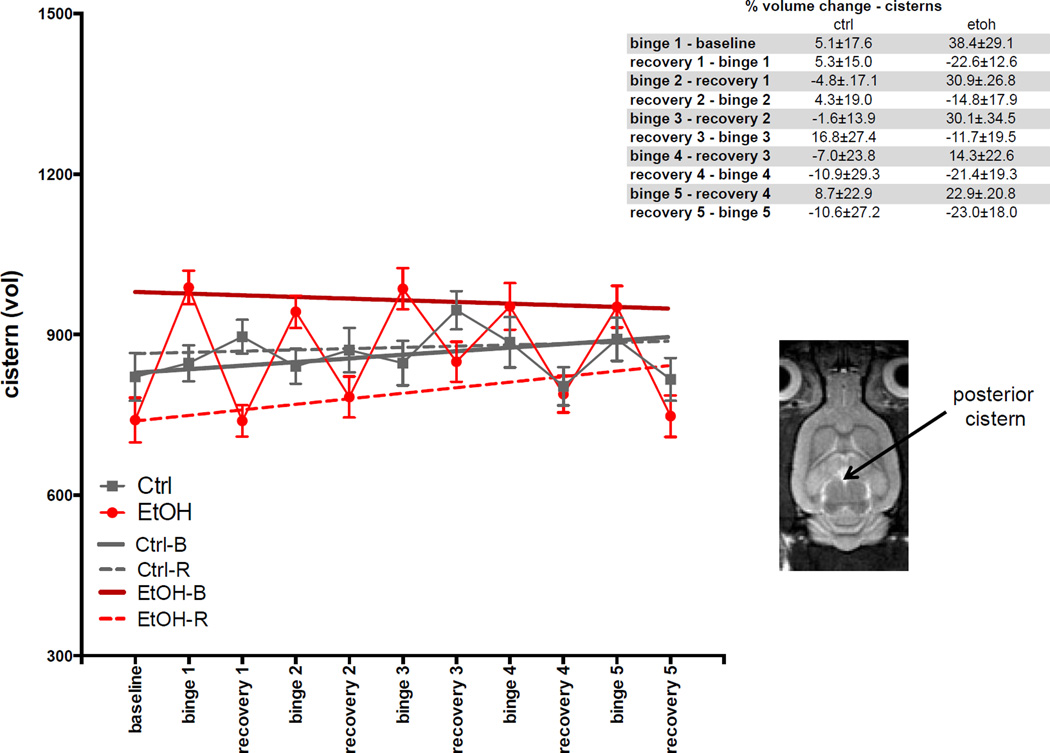
For cistern volume (Fig. 5), the GLM showed effects of group (t=3.1, p=.00254) and group-by-treatment (t= −4.1, p=6.1×10−5). The ANOVA showed EtOH-B had larger volumes (t=6.41, p≤.0001) and EtOH-R had smaller volumes (t=−5.8, p≤.0001) than volumes of Ctrl-B or Ctrl-R. Percentage change in cistern volume at each scan relative to the previous scan is presented in Fig. 5, insert.
Fig. 5.
Volumes of cisterns at baseline and each binge and recovery scan. Inset is an axial slice of an FSE image indicating posterior cistern.
Levels of Metabolites
Percentage change in metabolite levels at each scan relative to the previous scan is presented in the insert for each figure.
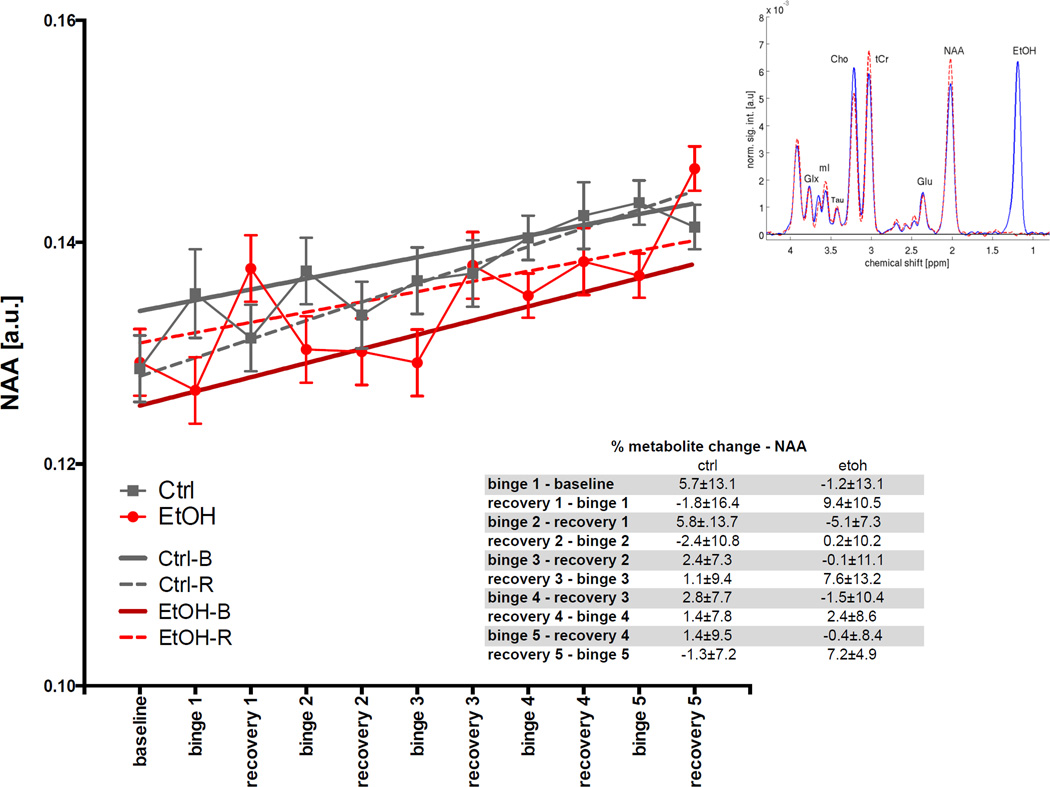
For NAA (Fig. 6), the GLM was not significant for any variable after correction for multiple comparisons. The ANOVA showed EtOH-B had lower NAA levels (t= −3.49, p=.0006) than the other categories. Over the course of the experiment, levels of NAA in all animals (both EtOH and Ctrl groups) increased (t=5.23, p≤.0001).
Fig. 6.
Levels of N-acetyl aspartate (NAA) in arbitrary units (a.u.) at baseline and each binge and recovery scan. Inset shows a typical spectrum acquired during a binge scan with EtOH rats in blue and Ctrl rats in red.
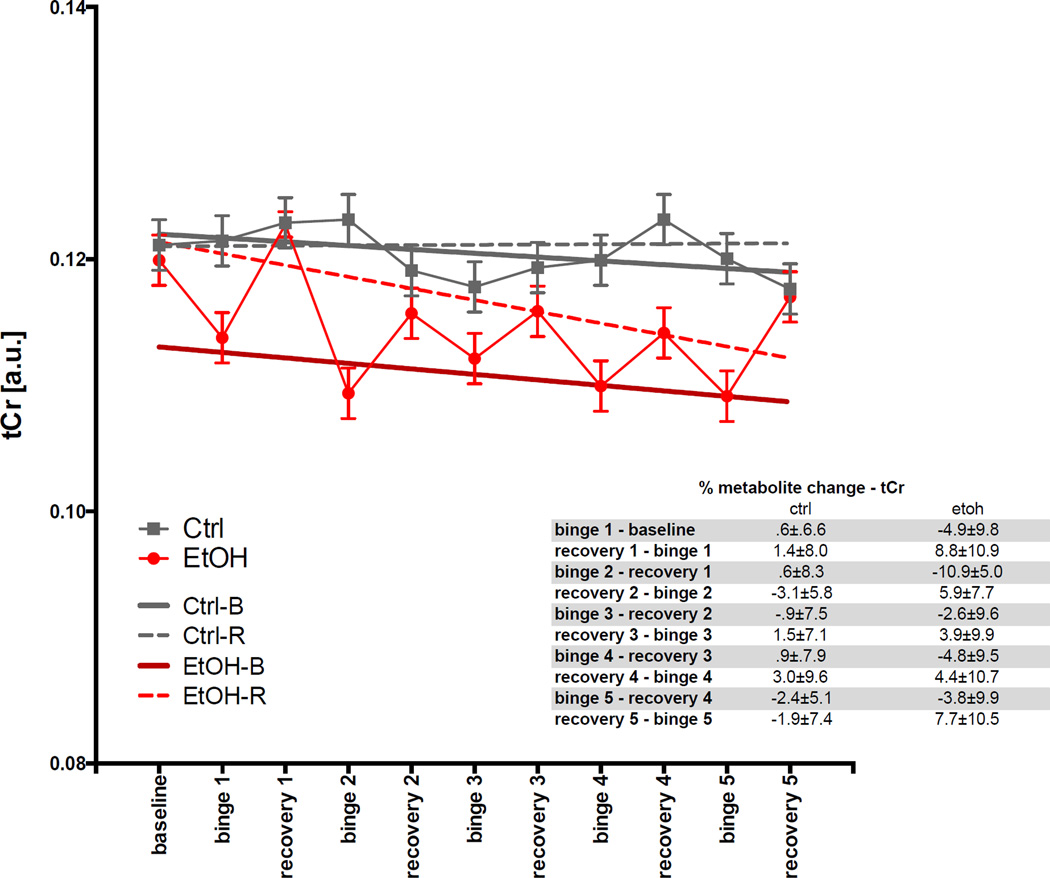
For tCr (Fig. 7), the GLM showed effects of group (t= −3.7, p=.003) and group-by-treatment (t=2.8, p=.0048). The ANOVA showed EtOH-B had a lower tCr levels (t= −8.8, p≤.0001) than the other categories. The apparent decrease in tCr over the course of the experiment (t= −2.7, p=.007) was driven by a significant decline in tCr levels in the EtOH-R group (t= −2.0, p=.046), although this was not significant after correction for multiple comparisons.
Fig. 7.
Levels of total creatine (tCr) in arbitrary units (a.u.) at baseline and each binge and recovery scan.
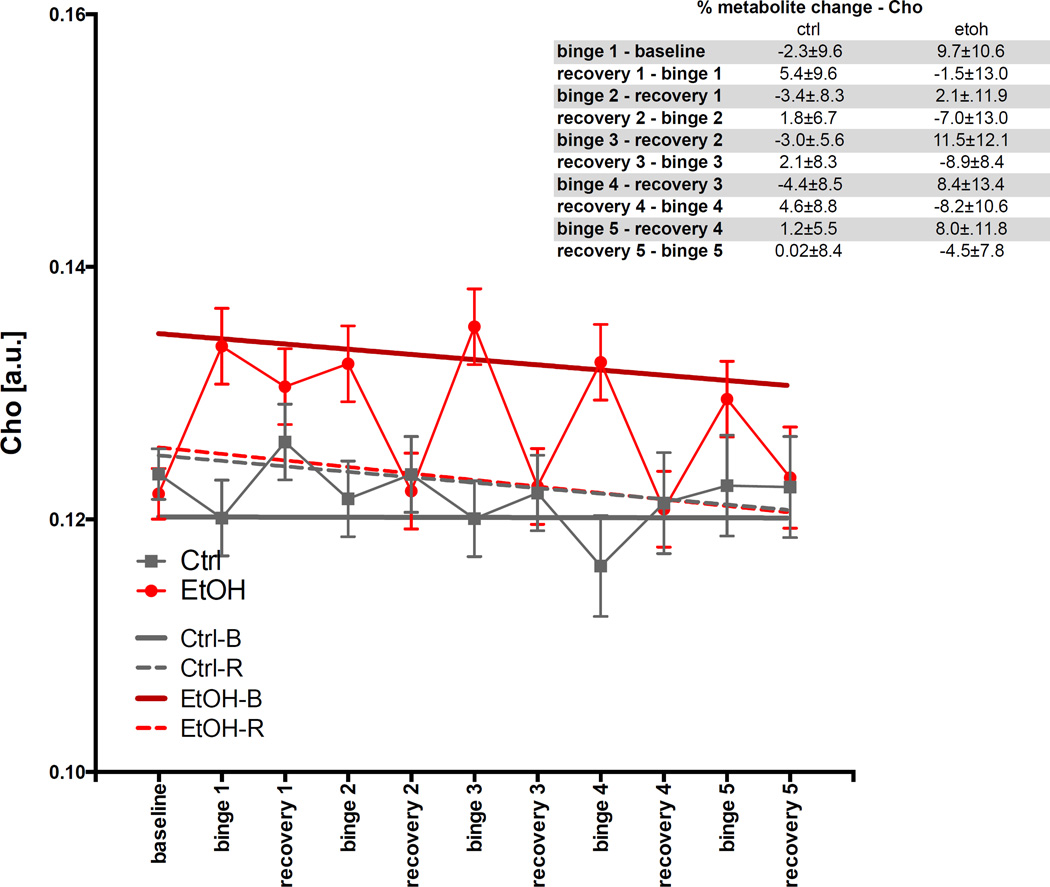
For Cho (Fig. 8), the GLM was significant for group (t=3.7, p=.00022). The ANOVA showed EtOH-B had higher Cho levels (t=6.8, p≤.0001) than the other categories.
Fig. 8.
Levels of choline-containing compounds (Cho) in arbitrary units (a.u.) at baseline and each binge and recovery scan.
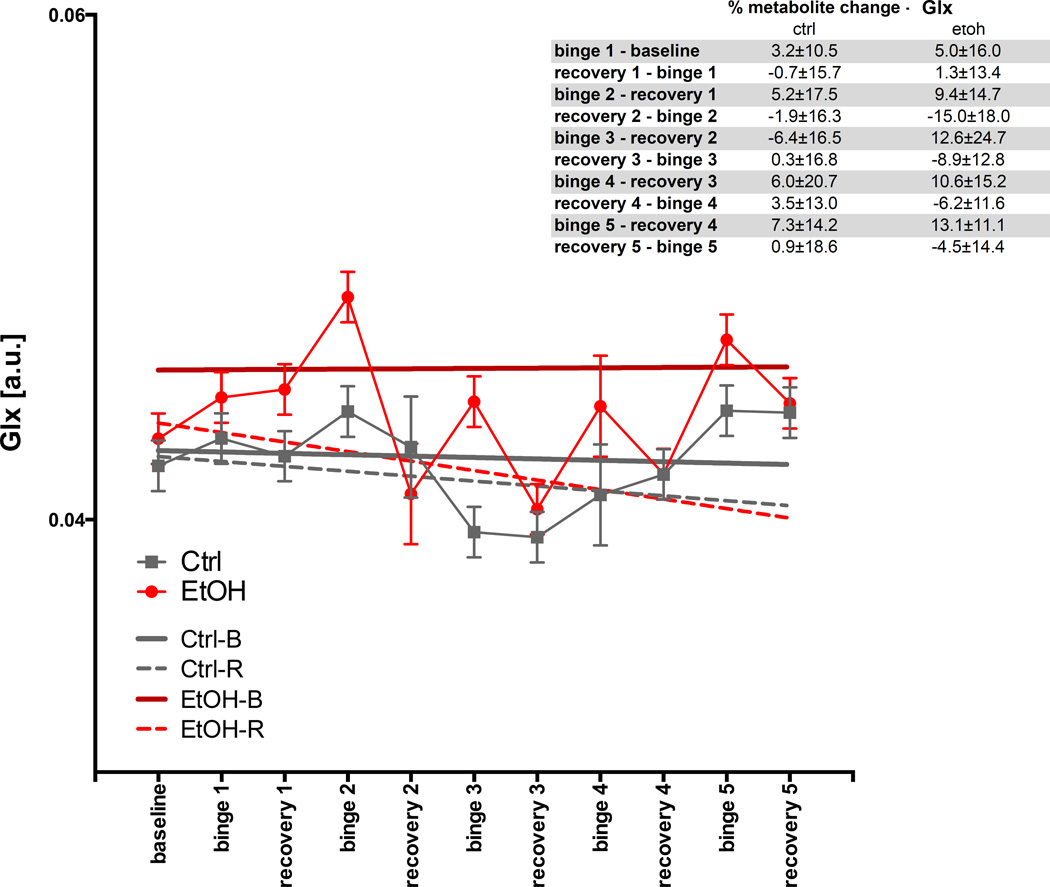
For Glx (Fig. 9), the GLM was not significant. The ANOVA showed EtOH-B had a higher Glx levels (t=5.4, p≤.0001) than the other categories.
Fig. 9.
Levels of glutamate (Glu) in arbitrary units (a.u.) at baseline and each binge and recovery scan.
Liver Histopathology and Thiamine Levels
Postmortem histopathology provided no evidence for alcohol-related hepatic steatosis, hepatitis, or cirrhosis. Both groups scored 0 on a number of hepatic pathology variables (i.e., microvesicular and macrovesicular lipidosis, necrosis, mallory bodies, hepatocytic swelling, hepatic cord atrophy, fibrosis (sinusoidal, portocentric, centrilobular, portal-portal, or central-portal), and regeneration (microscopic or macroscopic)). For scored variables, the highest score given was 2 indicating mild pathology; there were, however, no group differences in inflammation (neutrophilic parenchymal (p=.6), lymphohistiocytic portocentric (p=.6)), or bile duct proliferation (p=.7). There were also no group differences in levels of thiamine (t(25)=−65, p=.52), thiamine monophosphate (t(25)=.35, p=.72), or thiamine diphosphate (t(25)=−.35, p=.73).
DISCUSSION
The current study reports that male Wistar rats exposed to 5 EtOH treatment cycles (4-days of binge EtOH via oral gavage followed by 10 days of abstinence), with each cycle resulting in BALs approaching 300mg/dL, did not show increased voluntary EtOH consumption, unprovoked seizure activity, or persistent brain damage as quantified by in vivo imaging methods. These animals also did not demonstrate liver damage or thiamine deficiency.
Similar BALs were achieved following administration of comparable doses of EtOH over course of experiment (Table 1), indicating an absence of metabolic tolerance (i.e., it was not necessary to increase the dose of EtOH administered to achieve similar BALs) (Cox et al., 2013). That a few behavioral responses to equivalent BALs diminished over time (i.e., startle response, hunched back), however, presents modest evidence for physiological tolerance (Linsenbardt et al., 2011). Similarly, animals lost weight during each binge period and gained weight during each recovery period (Zhao et al., 2013). That weights were not significantly different between EtOH and Ctrl groups at the 5th binge scan despite similar BALs achieved provides evidence for tolerance to the effects of EtOH on weight.
Although a number of studies report increased voluntary EtOH consumption in rats previously exposed to EtOH (Backstrom et al., 2004; Bell et al., 2004; Colombo et al., 2003; Dayas et al., 2004; Fullgrabe et al., 2007; Funk et al., 2004; Heyser et al., 1997; Holter et al., 2000; Oster et al., 2006; Rodd-Henricks et al., 2000; Serra et al., 2003; Spanagel and Holter, 1999), EtOH-exposed rats in the current study did not consume more EtOH when given a 2-bottle choice (Stephens et al., 2001). In fact, EtOH-exposed rats drank less EtOH than Ctrl animals, possibly indicating aversion. One explanation for this discrepancy might be that animals were not assessed at the correct time to observe the transient period of EtOH-preference that might have occurred (Colombo et al., 2003; Heyser et al., 1997). Another likely explanation is that the doses of EtOH administered in the current study, ~9 g/kg/day, are aversive (Froehlich et al., 1988; Linsenbardt et al., 2011; Schulteis et al., 1996; Slawecki and Samson, 1998); to achieve increased voluntary drinking, animals are typically given <6g/kg/day to achieve BALs <200mg/dL (e.g., Bell et al., 2004; Holter et al., 2000; Spanagel and Holter, 1999). Alternatively, access to multiple concentrations of EtOH (e.g., 5%, 10%, 20%) (e.g., Bell et al., 2004; Spanagel and Holter, 1999) or to saccharin-sweetened EtOH (Dayas et al., 2004) might have been necessary to observe increased voluntary EtOH consumption.
Several findings are salient. Gradually increasing body weight (Fig. 2), decreasing size of the lateral ventricles (Fig. 4), and increasing levels of NAA (Fig. 6) over the course of the experiment indicate that the animals were still growing and had not reached their maximum brain size at the start of the experiment, despite the fact that their weight (~340g, >76 days old) indicates they were at least young adults at baseline (Bell et al., 2013). We previously observed that even older rats (~400g, >88 days old) similarly continued to gain weight and show an increase in brain volume over an extended period of observation (up to 700g, >500 days old) (Pfefferbaum et al., 2006a; Sullivan et al., 2006).
Results of in vivo MR imaging are consistent with our previous studies: each binge EtOH exposure cycle was associated with an increase in the volume of the lateral ventricles, a decrease in the levels of NAA and tCr, and an increase in the levels of Cho (Zahr et al., 2010; Zahr et al., 2014; Zahr et al., 2013). As described in great detail, this pattern of changes is consistent with a model of transient fluid redistribution during acute EtOH intoxication and recovery (Zahr et al., 2013). Although we predicted that repeated cycles of binge EtOH exposure would result in persistent and accruing changes in ventricular volumes and metabolite levels, the current results do not support our hypothesis, but are consistent with findings from a similar study in rats using repeated binge EtOH treatments (Zhao et al., 2013). Instead, our findings provide evidence for the remarkable plasticity of the rat brain: even following 5 cycles of EtOH exposure (to BALs consistently approaching 300mg/dL), changes in ventricular volume and metabolite levels were transient. One implication for these results is that perhaps older animals must be used to model AUD: in humans with AUD, older age contributes to greater pathology (Pfefferbaum et al., 2006b; Pfefferbaum et al., 1997; Rosenbloom and Pfefferbaum, 2008). Another consideration is the possibility that longer periods or alternative patterns of EtOH exposure are required to observe persistent pathology (Bell et al., 2013; Braconi et al., 2010; Spanagel, 2000). The only hint of an accumulating change was the decrease in tCr in EtOH exposed animals, indicated by the non-significant decrease in tCr levels in the EtOH group at the recovery scans (Fig. 7). The accruing change in tCr levels in response to repeated binge EtOH treatments might reflect gradually accumulating effects on the brain’s osmotic balance (as tCr has an identified role as an osmolyte, Ross and Bluml, 2001) or energy utilization (as tCr reflects the substrates available for the brain’s high-energy phosphate metabolism, Sartorius et al., 2008).
A novel observation in the current study was an increase in Glx levels following binge EtOH exposure, which we have not previously noted following single bout of binge EtOH treatment. Indeed, by the 5th binge exposure, levels of Glx were elevated to 13% above the previous recovery scan. The Glx signal includes resonances from glutamate and glutamine; given that the concentrations are ~8mM for glutamate and ~3mM for glutamine, however, the majority of the signal is likely from glutamate. We have previously observed that EtOH exposure via vapor chamber for 24 weeks does result in elevations in the MRS-signal derived from glutamate (Zahr et al., 2009), suggesting that prolonged periods of EtOH exposure might be required to affect the MRS-derived glutamate signal. A number of animal studies demonstrate that withdrawal from chronic EtOH is associated with elevated levels of glutamate (Chefer et al., 2011; Dahchour and De Witte, 2003; Hermann et al., 2012; Keller et al., 1983; Rossetti et al., 1999). Furthermore, a single rat was observed to experience unprovoked seizure activity after the 4th binge EtOH exposure: scanning following recovery, i.e., at the 4th recovery scan, revealed that this animal had higher Glx levels than any other animal. None of the other variables (volumes of lateral ventricles or cisterns, levels of NAA, tCr, Cho) uniquely discriminated this animal. The current results, however, do not necessarily support the “glutamate theory” of alcoholism, describing enhanced glutamate-mediated neuronal excitability during EtOH withdrawal and abstinence that might contribute to craving and relapse (Spanagel, 2009), because we did not observe increased voluntary EtOH consumption in any of the EtOH-exposed animals. These findings, however, may indicate a role for glutamate in brain plasticity in response to repeated binge EtOH exposure.
In conclusion, repeated episodes of EtOH intoxication and withdrawal provided little to no support for accruing brain pathology as quantified using in vivo MR imaging and spectroscopy.
Acknowledgments
The research reported herein was supported by grants: AA005965, AA017168, AA013521-INIA. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The authors have no conflicts of interest to declare.
The authors would like to thank Amy Collins, Matthew Serventi, Cheshire Hardcastle, Juan Orduna, Joey Montenegro, Crystal Caldwell, and Priya Asok for all aspects of data collection including animal dosing and acquisition of MRI and behavioral data.
Footnotes
Authors Contribution
NMZ, EVS, and AP designed the experiments and interpreted the data. DM and TR assisted AP with analyzing the data; specifically, TR with structural MRI data and DM with MRS data. RL performed postmortem liver histopathology. NMZ wrote the paper with input from EVS and AP. All author have critically reviewed content and approved the final version for publication.
References
- Adalsteinsson E, Hurd RE, Mayer D, Sailasuta N, Sullivan EV, Pfefferbaum A. In vivo 2D J-resolved magnetic resonance spectroscopy of rat brain with a 3-T clinical human scanner. NeuroImage. 2004;22:381–386. doi: 10.1016/j.neuroimage.2003.12.046. [DOI] [PubMed] [Google Scholar]
- Backstrom P, Bachteler D, Koch S, Hyytia P, Spanagel R. mGluR5 antagonist MPEP reduces ethanol-seeking and relapse behavior. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2004;29:921–928. doi: 10.1038/sj.npp.1300381. [DOI] [PubMed] [Google Scholar]
- Ballenger JC, Post RM. Kindling as a model for alcohol withdrawal syndromes. The British journal of psychiatry : the journal of mental science. 1978;133:1–14. doi: 10.1192/bjp.133.1.1. [DOI] [PubMed] [Google Scholar]
- Becker HC. Animal models of alcohol withdrawal. Alcohol Res Health. 2000;24:105–113. [PMC free article] [PubMed] [Google Scholar]
- Becker HC, Diaz-Granados JL, Hale RL. Exacerbation of ethanol withdrawal seizures in mice with a history of multiple withdrawal experience. Pharmacol Biochem Behav. 1997;57:179–183. doi: 10.1016/s0091-3057(96)00303-6. [DOI] [PubMed] [Google Scholar]
- Becker HC, Lopez MF. Increased ethanol drinking after repeated chronic ethanol exposure and withdrawal experience in C57BL/6 mice. Alcoholism, clinical and experimental research. 2004;28:1829–1838. doi: 10.1097/01.alc.0000149977.95306.3a. [DOI] [PubMed] [Google Scholar]
- Becker HC, Veatch LM, Diaz-Granados JL. Repeated ethanol withdrawal experience selectively alters sensitivity to different chemoconvulsant drugs in mice. Psychopharmacology. 1998;139:145–153. doi: 10.1007/s002130050699. [DOI] [PubMed] [Google Scholar]
- Bell RL, Franklin KM, Hauser SL, Engleman EA. Next Stop Dependence - Binge Drinking on the Road to Alcoholism: Preclinical Findings on its Neurobiology from Rat Animal Models. In: Harris SB, editor. Binge Eating and Binge Drinking. Nova Science Publishers; 2013. [Google Scholar]
- Bell RL, Rodd ZA, Boutwell CL, Hsu CC, Lumeng L, Murphy JM, Li TK, McBride WJ. Effects of long-term episodic access to ethanol on the expression of an alcohol deprivation effect in low alcohol-consuming rats. Alcoholism, clinical and experimental research. 2004;28:1867–1874. doi: 10.1097/01.alc.0000148101.20547.0a. [DOI] [PubMed] [Google Scholar]
- Booth BM, Blow FC. The kindling hypothesis: further evidence from a U.S. national study of alcoholic men. Alcohol and alcoholism. 1993;28:593–598. [PubMed] [Google Scholar]
- Bottomley PA. Selective volume method for preforming localized NMR spectroscopy and NMR chemical shift imaging. 1984 [Google Scholar]
- Braconi S, Sidhpura N, Aujla H, Martin-Fardon R, Weiss F, Ciccocioppo R. Revisiting intragastric ethanol intubation as a dependence induction method for studies of ethanol reward and motivation in rats. Alcoholism, clinical and experimental research. 2010;34:538–544. doi: 10.1111/j.1530-0277.2009.01119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown ME, Anton RF, Malcolm R, Ballenger JC. Alcohol detoxification and withdrawal seizures: clinical support for a kindling hypothesis. Biological psychiatry. 1988;23:507–514. doi: 10.1016/0006-3223(88)90023-6. [DOI] [PubMed] [Google Scholar]
- Chefer V, Meis J, Wang G, Kuzmin A, Bakalkin G, Shippenberg T. Repeated exposure to moderate doses of ethanol augments hippocampal glutamate neurotransmission by increasing release. Addiction biology. 2011;16:229–237. doi: 10.1111/j.1369-1600.2010.00272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chronik B, Alejski A, Rutt BK. Design and fabrication of a three-axis multilayer gradient coil for magnetic resonance microscopy of mice. Magma. 2000;10:131–146. doi: 10.1007/BF02601848. [DOI] [PubMed] [Google Scholar]
- Clemmesen L, Hemmingsen R. Physical dependence on ethanol during multiple intoxication and withdrawal episodes in the rat: evidence of a potentiation. Acta pharmacologica et toxicologica. 1984;55:345–350. doi: 10.1111/j.1600-0773.1984.tb01993.x. [DOI] [PubMed] [Google Scholar]
- Collins MA, Corso TD, Neafsey EJ. Neuronal degeneration in rat cerebrocortical and olfactory regions during subchronic “binge” intoxication with ethanol: possible explanation for olfactory deficits in alcoholics. Alcoholism, clinical and experimental research. 1996;20:284–292. doi: 10.1111/j.1530-0277.1996.tb01641.x. [DOI] [PubMed] [Google Scholar]
- Collins MA, Neafsey EJ. Ethanol and adult CNS neurodamage: oxidative stress, but possibly not excitotoxicity. Front Biosci (Elite Ed) 2012;4:1358–1367. doi: 10.2741/465. [DOI] [PubMed] [Google Scholar]
- Colombo G, Serra S, Brunetti G, Vacca G, Carai MA, Gessa GL. Suppression by baclofen of alcohol deprivation effect in Sardinian alcohol-preferring (sP) rats. Drug and alcohol dependence. 2003;70:105–108. doi: 10.1016/s0376-8716(02)00333-2. [DOI] [PubMed] [Google Scholar]
- Cooper BR, Viik K, Ferris RM, White HL. Antagonism of the enhanced susceptibility to audiogenic seizures during alcohol withdrawal in the rat by gamma-aminobutyric acid (GABA) and “GABA-mimetic” agents. The Journal of pharmacology and experimental therapeutics. 1979;209:396–403. [PubMed] [Google Scholar]
- Cowen MS, Schumann G, Yagi T, Spanagel R. Role of Fyn tyrosine kinase in ethanol consumption by mice. Alcoholism, clinical and experimental research. 2003;27:1213–1219. doi: 10.1097/01.ALC.0000081630.14159.02. [DOI] [PubMed] [Google Scholar]
- Cox BR, Olney JJ, Lowery-Gionta EG, Sprow GM, Rinker JA, Navarro M, Kash TL, Thiele TE. Repeated cycles of binge-like ethanol (EtOH)-drinking in male C57BL/6J mice augments subsequent voluntary EtOH intake but not other dependence-like phenotypes. Alcoholism, clinical and experimental research. 2013;37:1688–1695. doi: 10.1111/acer.12145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahchour A, De Witte P. Excitatory and inhibitory amino acid changes during repeated episodes of ethanol withdrawal: an in vivo microdialysis study. European journal of pharmacology. 2003;459:171–178. doi: 10.1016/s0014-2999(02)02851-0. [DOI] [PubMed] [Google Scholar]
- Dayas CV, Martin-Fardon R, Thorsell A, Weiss F. Chronic footshock, but not a physiological stressor, suppresses the alcohol deprivation effect in dependent rats. Alcohol and alcoholism. 2004;39:190–196. doi: 10.1093/alcalc/agh046. [DOI] [PubMed] [Google Scholar]
- Devaud LL, Walls SA, McCulley WD, 3rd, Rosenwasser AM. Voluntary wheel running attenuates ethanol withdrawal-induced increases in seizure susceptibility in male and female rats. Pharmacol Biochem Behav. 2012;103:18–25. doi: 10.1016/j.pbb.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duka T, Gentry J, Malcolm R, Ripley TL, Borlikova G, Stephens DN, Veatch LM, Becker HC, Crews FT. Consequences of multiple withdrawals from alcohol. Alcoholism, clinical and experimental research. 2004;28:233–246. doi: 10.1097/01.alc.0000113780.41701.81. [DOI] [PubMed] [Google Scholar]
- Duka T, Townshend JM, Collier K, Stephens DN. Kindling of withdrawal: a study of craving and anxiety after multiple detoxifications in alcoholic inpatients. Alcoholism, clinical and experimental research. 2002;26:785–795. [PubMed] [Google Scholar]
- Duka T, Townshend JM, Collier K, Stephens DN. Impairment in cognitive functions after multiple detoxifications in alcoholic inpatients. Alcoholism, clinical and experimental research. 2003;27:1563–1572. doi: 10.1097/01.ALC.0000090142.11260.D7. [DOI] [PubMed] [Google Scholar]
- Duka T, Trick L, Nikolaou K, Gray MA, Kempton MJ, Williams H, Williams SC, Critchley HD, Stephens DN. Unique brain areas associated with abstinence control are damaged in multiply detoxified alcoholics. Biological psychiatry. 2011;70:545–552. doi: 10.1016/j.biopsych.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebel A, Vigran R, Mack G, Durkin T, Mandel P. Cholinergic involvement in ethanol intoxication and withdrawal-induced seizure susceptibility. Psychopharmacology. 1979;61:251–254. doi: 10.1007/BF00432267. [DOI] [PubMed] [Google Scholar]
- Freund G. Science. Vol. 168. New York, NY: 1970. Impairment of shock avoidance learning after long-term alcohol ingestion in mice; pp. 1599–1601. [DOI] [PubMed] [Google Scholar]
- Froehlich JC, Harts J, Lumeng L, Li TK. Differences in response to the aversive properties of ethanol in rats selectively bred for oral ethanol preference. Pharmacol Biochem Behav. 1988;31:215–222. doi: 10.1016/0091-3057(88)90336-x. [DOI] [PubMed] [Google Scholar]
- Fullgrabe MW, Vengeliene V, Spanagel R. Influence of age at drinking onset on the alcohol deprivation effect and stress-induced drinking in female rats. Pharmacol Biochem Behav. 2007;86:320–326. doi: 10.1016/j.pbb.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Funk D, Vohra S, Le AD. Influence of stressors on the rewarding effects of alcohol in Wistar rats: studies with alcohol deprivation and place conditioning. Psychopharmacology. 2004;176:82–87. doi: 10.1007/s00213-004-1859-x. [DOI] [PubMed] [Google Scholar]
- George MS, Teneback CC, Malcolm RJ, Moore J, Stallings LE, Spicer KM, Anton RF, Ballenger JC. Multiple previous alcohol detoxifications are associated with decreased medial temporal and paralimbic function in the postwithdrawal period. Alcoholism, clinical and experimental research. 1999;23:1077–1084. [PubMed] [Google Scholar]
- George O, Sanders C, Freiling J, Grigoryan E, Vu S, Allen CD, Crawford E, Mandyam CD, Koob GF. Recruitment of medial prefrontal cortex neurons during alcohol withdrawal predicts cognitive impairment and excessive alcohol drinking. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:18156–18161. doi: 10.1073/pnas.1116523109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn SW, Parsons OA, Sinha R, Stevens L. The effects of repeated withdrawals from alcohol on the memory of male and female alcoholics. Alcohol and alcoholism. 1988;23:337–342. doi: 10.1093/oxfordjournals.alcalc.a044826. [DOI] [PubMed] [Google Scholar]
- Gonzalez LP, Czachura JF, Brewer KW. Alcohol. Vol. 6. Fayetteville, NY: 1989. Spontaneous versus elicited seizures following ethanol withdrawal: differential time course; pp. 481–487. [DOI] [PubMed] [Google Scholar]
- Hermann D, Weber-Fahr W, Sartorius A, Hoerst M, Frischknecht U, Tunc-Skarka N, Perreau-Lenz S, Hansson AC, Krumm B, Kiefer F, Spanagel R, Mann K, Ende G, Sommer WH. Translational magnetic resonance spectroscopy reveals excessive central glutamate levels during alcohol withdrawal in humans and rats. Biological psychiatry. 2012;71:1015–1021. doi: 10.1016/j.biopsych.2011.07.034. [DOI] [PubMed] [Google Scholar]
- Heyser CJ, Schulteis G, Koob GF. Increased ethanol self-administration after a period of imposed ethanol deprivation in rats trained in a limited access paradigm. Alcoholism, clinical and experimental research. 1997;21:784–791. [PubMed] [Google Scholar]
- Holter SM, Linthorst AC, Reul JM, Spanagel R. Withdrawal symptoms in a long-term model of voluntary alcohol drinking in Wistar rats. Pharmacol Biochem Behav. 2000;66:143–151. doi: 10.1016/s0091-3057(00)00196-9. [DOI] [PubMed] [Google Scholar]
- Kalant H, LeBlanc AE, Gibbins RJ, Wilson A. Accelerated development of tolerance during repeated cycles of ethanol exposure. Psychopharmacology. 1978;60:59–65. doi: 10.1007/BF00429180. [DOI] [PubMed] [Google Scholar]
- Keller E, Cummins JT, von Hungen K. Regional effects of ethanol on glutamate levels, uptake and release in slice and synaptosome preparations from rat brain. Substance and alcohol actions/misuse. 1983;4:383–392. [PubMed] [Google Scholar]
- Khisti RT, Wolstenholme J, Shelton KL, Miles MF. Alcohol. Vol. 40. Fayetteville, NY: 2006. Characterization of the ethanol-deprivation effect in substrains of C57BL/6 mice; pp. 119–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzmin A, Liljequist S, Meis J, Chefer V, Shippenberg T, Bakalkin G. Repeated moderate-dose ethanol bouts impair cognitive function in Wistar rats. Addiction biology. 2012;17:132–140. doi: 10.1111/j.1369-1600.2010.00224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechtenberg R, Worner TM. Seizure incidence enhancement with increasing alcohol intake. Annals of the New York Academy of Sciences. 1992;654:474–476. doi: 10.1111/j.1749-6632.1992.tb26004.x. [DOI] [PubMed] [Google Scholar]
- Likar B, Viergever MA, Pernus F. Retrospective correction of MR intensity inhomogeneity by information minimization. IEEE Trans Med Imaging. 2001;20:1398–1410. doi: 10.1109/42.974934. [DOI] [PubMed] [Google Scholar]
- Linsenbardt DN, Moore EM, Griffin KD, Gigante ED, Boehm SL., 2nd Tolerance to ethanol’s ataxic effects and alterations in ethanol-induced locomotion following repeated binge-like ethanol intake using the DID model. Alcoholism, clinical and experimental research. 2011;35:1246–1255. doi: 10.1111/j.1530-0277.2011.01459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeber S, Duka T, Welzel H, Nakovics H, Heinz A, Flor H, Mann K. Impairment of cognitive abilities and decision making after chronic use of alcohol: the impact of multiple detoxifications. Alcohol and alcoholism. 2009;44:372–381. doi: 10.1093/alcalc/agp030. [DOI] [PubMed] [Google Scholar]
- Lundqvist C, Alling C, Knoth R, Volk B. Alcohol and alcoholism. Vol. 30. Oxford: Oxfordshire; 1995. Intermittent ethanol exposure of adult rats: hippocampal cell loss after one month of treatment; pp. 737–748. [PubMed] [Google Scholar]
- Maier DM, Pohorecky LA. The effect of repeated withdrawal episodes on acquisition and loss of tolerance to ethanol in ethanol-treated rats. Physiology & behavior. 1987;40:411–424. doi: 10.1016/0031-9384(87)90025-4. [DOI] [PubMed] [Google Scholar]
- Majchrowicz E. Induction of physical dependence upon ethanol and the associated behavioral changes in rats. Psychopharmacologia. 1975;43:245–254. doi: 10.1007/BF00429258. [DOI] [PubMed] [Google Scholar]
- McCown TJ, Breese GR. Multiple withdrawals from chronic ethanol “kindles” inferior collicular seizure activity: evidence for kindling of seizures associated with alcoholism. Alcoholism, clinical and experimental research. 1990;14:394–399. doi: 10.1111/j.1530-0277.1990.tb00492.x. [DOI] [PubMed] [Google Scholar]
- Melendez RI, Middaugh LD, Kalivas PW. Development of an alcohol deprivation and escalation effect in C57BL/6J mice. Alcoholism, clinical and experimental research. 2006;30:2017–2025. doi: 10.1111/j.1530-0277.2006.00248.x. [DOI] [PubMed] [Google Scholar]
- O’Connor PG, Gottlieb LD, Kraus ML, Segal SR, Horwitz RI. Social and clinical features as predictors of outcome in outpatient alcohol withdrawal. J Gen Intern Med. 1991;6:312–316. doi: 10.1007/BF02597427. [DOI] [PubMed] [Google Scholar]
- O’Daly OG, Trick L, Scaife J, Marshall J, Ball D, Phillips ML, Williams SS, Stephens DN, Duka T. Withdrawal-associated increases and decreases in functional neural connectivity associated with altered emotional regulation in alcoholism. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2012;37:2267–2276. doi: 10.1038/npp.2012.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oster SM, Toalston JE, Kuc KA, Pommer TJ, Murphy JM, Lumeng L, Bell RL, McBride WJ, Rodd ZA. Alcohol. Vol. 38. Fayetteville, NY: 2006. Effects of multiple alcohol deprivations on operant ethanol self-administration by high-alcohol-drinking replicate rat lines; pp. 155–164. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Breese GR. Accentuated decrease in social interaction in rats subjected to repeated ethanol withdrawals. Alcoholism, clinical and experimental research. 2002;26:1259–1268. doi: 10.1097/01.ALC.0000023983.10615.D7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, Sood R, Mayer D, Bell R, McBride W, Li TK, Sullivan EV. Longitudinal brain magnetic resonance imaging study of the alcohol-preferring rat. Part II: effects of voluntary chronic alcohol consumption. Alcoholism, clinical and experimental research. 2006a;30:1248–1261. doi: 10.1111/j.1530-0277.2006.00146.x. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, Sullivan EV. In vivo structural imaging of the rat brain with a 3-T clinical human scanner. J Magn Reson Imaging. 2004;20:779–785. doi: 10.1002/jmri.20181. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, Sullivan EV. Dysmorphology and microstructural degradation of the corpus callosum: Interaction of age and alcoholism. Neurobiology of aging. 2006b;27:994–1009. doi: 10.1016/j.neurobiolaging.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Mathalon DH, Lim KO. Frontal lobe volume loss observed with magnetic resonance imaging in older chronic alcoholics. Alcoholism: Clinical and Experimental Research. 1997;21:521–529. doi: 10.1111/j.1530-0277.1997.tb03798.x. [DOI] [PubMed] [Google Scholar]
- Philibin SD, Cameron AJ, Schlumbohm JP, Metten P, Crabbe JC. Ethanol withdrawal-induced motor impairment in mice. Psychopharmacology. 2012;220:367–378. doi: 10.1007/s00213-011-2483-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinel JP, Van Oot PH. Generality of the kindling phenomenon: some clinical implications. The Canadian journal of neurological sciences. 1975;2:467–475. doi: 10.1017/s0317167100020618. [DOI] [PubMed] [Google Scholar]
- Pinel JP, Van Oot PH. Increased susceptibility to the epileptic effects of alcohol withdrawal following periodic electroconvulsive shocks. Biological psychiatry. 1978;13:353–368. [PubMed] [Google Scholar]
- Riikonen J, Jaatinen P, Rintala J, Porsti I, Karjala K, Hervonen A. Alcohol and alcoholism. Vol. 37. Oxford: Oxfordshire; 2002. Intermittent ethanol exposure increases the number of cerebellar microglia; pp. 421–426. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Cole M, Koob GF. Intra-amygdala muscimol decreases operant ethanol self-administration in dependent rats. Alcoholism, clinical and experimental research. 1996;20:1289–1298. doi: 10.1111/j.1530-0277.1996.tb01125.x. [DOI] [PubMed] [Google Scholar]
- Rodd-Henricks ZA, McKinzie DL, Murphy JM, McBride WJ, Lumeng L, Li TK. The expression of an alcohol deprivation effect in the high-alcohol-drinking replicate rat lines is dependent on repeated deprivations. Alcoholism, clinical and experimental research. 2000;24:747–753. [PubMed] [Google Scholar]
- Rosenbloom MJ, Pfefferbaum A. Magnetic resonance imaging of the living brain: evidence for brain degeneration among alcoholics and recovery with abstinence. Alcohol Res Health. 2008;31:362–376. [PMC free article] [PubMed] [Google Scholar]
- Ross B, Bluml S. Magnetic resonance spectroscopy of the human brain. The Anatomical record. 2001;265:54–84. doi: 10.1002/ar.1058. [DOI] [PubMed] [Google Scholar]
- Rossetti ZL, Carboni S, Fadda F. Glutamate-induced increase of extracellular glutamate through N-methyl-D-aspartate receptors in ethanol withdrawal. Neuroscience. 1999;93:1135–1140. doi: 10.1016/s0306-4522(99)00250-x. [DOI] [PubMed] [Google Scholar]
- Ruwe WD, Bauce L, Flemons WW, Veale WL, Pittman QJ. Alcohol dependence and withdrawal in the rat An effective means of induction and assessment. J Pharmacol Methods. 1986;15:225–234. doi: 10.1016/0160-5402(86)90052-5. [DOI] [PubMed] [Google Scholar]
- Sanchis-Segura C, Borchardt T, Vengeliene V, Zghoul T, Bachteler D, Gass P, Sprengel R, Spanagel R. Involvement of the AMPA receptor GluR-C subunit in alcohol-seeking behavior and relapse. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:1231–1238. doi: 10.1523/JNEUROSCI.4237-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartorius A, Lugenbiel P, Mahlstedt MM, Ende G, Schloss P, Vollmayr B. Proton magnetic resonance spectroscopic creatine correlates with creatine transporter protein density in rat brain. Journal of neuroscience methods. 2008;172:215–219. doi: 10.1016/j.jneumeth.2008.04.028. [DOI] [PubMed] [Google Scholar]
- Schulteis G, Hyytia P, Heinrichs SC, Koob GF. Effects of chronic ethanol exposure on oral self-administration of ethanol or saccharin by Wistar rats. Alcoholism, clinical and experimental research. 1996;20:164–171. doi: 10.1111/j.1530-0277.1996.tb01060.x. [DOI] [PubMed] [Google Scholar]
- Serra S, Brunetti G, Vacca G, Lobina C, Carai MA, Gessa GL, Colombo G. Alcohol. Vol. 29. Fayetteville, NY: 2003. Stable preference for high ethanol concentrations after ethanol deprivation in Sardinian alcohol-preferring (sP) rats; pp. 101–108. [DOI] [PubMed] [Google Scholar]
- Shen Y, Lindemeyer AK, Gonzalez C, Shao XM, Spigelman I, Olsen RW, Liang J. Dihydromyricetin as a novel anti-alcohol intoxication medication. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:390–401. doi: 10.1523/JNEUROSCI.4639-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slawecki CJ, Samson HH. Alcohol. Vol. 16. Fayetteville, NY: 1998. Exposure to sucrose-quinine solutions does not increase ethanol consumption; pp. 329–335. [DOI] [PubMed] [Google Scholar]
- Spanagel R. Recent animal models of alcoholism. Alcohol Res Health. 2000;24:124–131. [PMC free article] [PubMed] [Google Scholar]
- Spanagel R. Alcoholism: a systems approach from molecular physiology to addictive behavior. Physiological reviews. 2009;89:649–705. doi: 10.1152/physrev.00013.2008. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Holter SM. Long-term alcohol self-administration with repeated alcohol deprivation phases: an animal model of alcoholism? Alcohol and alcoholism. 1999;34:231–243. doi: 10.1093/alcalc/34.2.231. [DOI] [PubMed] [Google Scholar]
- Sparta DR, Ferraro FM, 3rd, Fee JR, Knapp DJ, Breese GR, Thiele TE. The alcohol deprivation effect in C57BL/6J mice is observed using operant self-administration procedures and is modulated by CRF-1 receptor signaling. Alcoholism, clinical and experimental research. 2009;33:31–42. doi: 10.1111/j.1530-0277.2008.00808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens DN, Brown G, Duka T, Ripley TL. Impaired fear conditioning but enhanced seizure sensitivity in rats given repeated experience of withdrawal from alcohol. The European journal of neuroscience. 2001;14:2023–2031. doi: 10.1046/j.0953-816x.2001.01824.x. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Adalsteinsson E, Sood R, Mayer D, Bell R, McBride W, Li TK, Pfefferbaum A. Longitudinal brain magnetic resonance imaging study of the alcohol-preferring rat. Part I: adult brain growth. Alcoholism, clinical and experimental research. 2006;30:1234–1247. doi: 10.1111/j.1530-0277.2006.00145.x. [DOI] [PubMed] [Google Scholar]
- Swartzwelder HS, Hogan A, Risher ML, Swartzwelder RA, Wilson WA, Acheson SK. Alcohol. Vol. 48. Fayetteville, NY: 2014. Effect of sub-chronic intermittent ethanol exposure on spatial learning and ethanol sensitivity in adolescent and adult rats; pp. 353–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trick L, Kempton MJ, Williams SC, Duka T. Impaired fear recognition and attentional set-shifting is associated with brain structural changes in alcoholic patients. Addiction biology. 2014 doi: 10.1111/adb.12175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrichsen J, Clemmesen L, Hemmingsen R. Convulsive behaviour during alcohol dependence: discrimination between the role of intoxication and withdrawal. Psychopharmacology. 1992;107:97–102. doi: 10.1007/BF02244972. [DOI] [PubMed] [Google Scholar]
- Yaksh TL, Kohl RL, Rudy TA. Induction of tolerance and withdrawal in rats receiving morphine in the spinal subarachnoid space. European journal of pharmacology. 1977;42:275–284. doi: 10.1016/0014-2999(77)90294-1. [DOI] [PubMed] [Google Scholar]
- Zahr NM, Mayer D, Rohlfing T, Hasak M, Hsu O, Vinco S, Orduna J, Luong R, Sullivan EV, Pfefferbaum A. Brain Injury and Recovery Following Binge Ethanol: Evidence from In Vivo Magnetic Resonance Spectroscopy. Biological psychiatry. 2010;67:846–854. doi: 10.1016/j.biopsych.2009.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahr NM, Mayer D, Rohlfing T, Hsu O, Vinco S, Orduna J, Luong R, Bell RL, Sullivan EV, Pfefferbaum A. Rat strain differences in brain structure and neurochemistry in response to binge alcohol. Psychopharmacology. 2014;231:429–445. doi: 10.1007/s00213-013-3253-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahr NM, Mayer D, Rohlfing T, Orduna J, Luong R, Sullivan EV, Pfefferbaum A. A mechanism of rapidly reversible cerebral ventricular enlargement independent of tissue atrophy. Neuropsychopharmacology. 2013;38:1121–1129. doi: 10.1038/npp.2013.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahr NM, Mayer D, Vinco S, Orduna J, Luong R, Sullivan EV, Pfefferbaum A. In vivo evidence for alcohol-induced neurochemical changes in rat brain without protracted withdrawal, pronounced thiamine deficiency, or severe liver damage. Neuropsychopharmacology. 2009;34:1427–1442. doi: 10.1038/npp.2008.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zghoul T, Abarca C, Sanchis-Segura C, Albrecht U, Schumann G, Spanagel R. Ethanol self-administration and reinstatement of ethanol-seeking behavior in Per1(Brdm1) mutant mice. Psychopharmacology. 2007;190:13–19. doi: 10.1007/s00213-006-0592-z. [DOI] [PubMed] [Google Scholar]
- Zhao YN, Wang F, Fan YX, Ping GF, Yang JY, Wu CF. Activated microglia are implicated in cognitive deficits, neuronal death, and successful recovery following intermittent ethanol exposure. Behavioural brain research. 2013;236:270–282. doi: 10.1016/j.bbr.2012.08.052. [DOI] [PubMed] [Google Scholar]