Abstract
Background
Immune rejection continues to threaten all tissue transplants. Here we sought to determine whether P- and E- selectin mediate T cell recruitment in corneal transplantation and whether their blockade can reduce T cell graft infiltration and improve long-term corneal allograft survival.
Methods
In a murine model of allogeneic corneal transplantation, we used PCR and immunohistochemistry to investigate expression of P- and E-selectin in rejected versus accepted allografts, and lymph node flow cytometry to assess expression of selectin ligands by effector T cells. Using P- and E-selectin neutralizing antibodies we evaluated the effect of blockade on CD4 T cell recruitment, as well as the effect of anti-E selectin on long-term allograft survival.
Results
P- (93.3 fold, p<0.05) and E-selectin (17.1 fold, p<0.005) are upregulated in rejected versus accepted allogeneic transplants. T helper (Th)1 cells from hosts with accepted and rejected grafts express high levels of P-selectin glycoprotein ligand 1 and glycosylated CD43. In vivo blockade of P (0.47±0.03, p<0.05) and E selectin (0.49±0.1, p<0.05) reduced the number of recruited T cells compared to IgG control (0.98±0.1). Anti-E-selectin reduced the number of mature antigen-presenting cells trafficking to lymphoid tissue compared to control (6.96±0.9 vs. 12.67±0.5 p<0.05). Anti-E-selectin treatment delayed graft rejection and increased survival compared to control, although this difference did not reach statistical significance.
Conclusions
In a model of corneal transplantation, P- and E-selectin mediate T cell recruitment to the graft, E-selectin mediates APC trafficking to lymphoid tissue and blockade of E-selectin has a modest effect on improving long-term graft survival.
Introduction
Full-thickness corneal transplantation is an important therapeutic option in the setting of many corneal pathologies, including thinning disorders, certain corneal dystrophies and corneal scars, among others.1,2 With over 40,000 corneal transplants performed each year in the United States alone, it is one of the most commonly performed types of solid-tissue transplantation.3 Grafts placed in uninflamed or ‘low-risk’ host beds enjoy a rate of survival that can exceed 90%, thanks in large part to the cornea's status as an immune-privileged tissue.4-6 However, transplant failure due to immune rejection remains a major threat to all transplants, particularly following transplantation into inflamed or ‘high-risk’ host beds, where survival rates can fall well below 50% despite local immune suppression.7,8
Effector CD4+ T cells, particularly Type 1 T helper (Th1) cells, are the predominant mediators of corneal graft rejection.9-12 These effector T cells must exit the vasculature in order to reach their target tissue. This is accomplished through the leukocyte adhesion cascade, a coordinated series of events involving selectins, integrins and chemokines that results in transendothelial cell migration.15 The first step in this process is mediated by selectins. The selectins are a family of single-chain transmembrane receptors that include platelet (P-), endothelial (E-) and leukocyte (L-) selectin. Of these, P- and E-selectin are expressed by activated vascular endothelial cells.16 The selectins bind to selectin ligands containing the sialyllewisx carbohydrate domain, and although there are a number of known selectin ligands, two of the more well-characterized are P-selectin glycoprotein ligand-1 (PSGL-1) and glycosylated CD43 (glycoCD43). Although PSGL-1 was originally described as a ligand for P-selectin and glycoCD43 was originally associated predominantly with E-selectin binding, it is now recognized that there is considerable overlap in selectin-ligand binding, with PSGL-1 binding all three selectins, and glycoCD43 contributing to P-selectin as well as E-selectin binding.17-20 The binding of ligands on circulating leukocytes by endothelial cell-expressed selectins mediates leukocyte tethering and rolling, a prerequisite for subsequent firm adhesion and migration of effector cells into tissue.21
Due to their crucial role in leukocyte extravasation and migration, selectins are an attractive therapeutic target in the effort to prevent transplant rejection. Selectins have previously been identified in renal allografts,22,23 cardiac allografts24-26 and on vascular endothelium in rejected human corneal allografts.27,28 Both P- and E-selectin have been shown to mediate the recruitment of Th1 cells into inflamed tissues29 and approaches to disrupting selectin/selectinligand binding in models of transplantation have been shown to prevent reperfusion injury and in some cases increase short-term graft survival. However, whether there is any role for selectin blockade in improving long-term transplant survival is currently unknown.30-32 In the present study, we hypothesized that blocking selectin/selectin-ligand interactions in corneal transplantation would prevent T cell homing to the corneal allograft, thereby improving long-term allograft survival.
Materials and Methods
Animals
Male C57BL/6 (donors) and BALB/c (hosts and in vitro experiments) mice 6-8 weeks of age were obtained from Charles River Laboratories (Wilmington, MA). Mice were housed in the Schepens Eye Research Institute animal vivarium and treated according to the guidelines set forth by the Association for Research in Vision and Ophthalmology (ARVO). All animal experiments were reviewed and approved by the Institutional Animal Care and Use Committee.
Corneal transplantation
Allogeneic orthotopic corneal transplantation was performed as described previously.33 In brief, 2 mm diameter donor corneal buttons from C57BL/6 mice were affixed to 1.5 mm diameter BALB/c host beds via 8 interrupted 11-0 nylon sutures. The host eye lids were closed for 3 days via one 8-0 suture in order to promote healing. Seven days following surgery, corneal sutures were removed. Transplantation of a BALB/c donor to BALB/c host served as a syngeneic control. Corneal allograft survival was evaluated by slit-lamp microscopy and graft clarity scored according to an established 0-5+ scale, with scores of 2+ or greater considered rejected.34 To exclude grafts undergoing primary failure, only those grafts with scores of 0+ or 1+ at 10 days following transplantation were used for experimentation and analysis.
Real-Time and RT-PCR
In order to compare allogeneic acceptors and rejectors, mice were evaluated at week 4 for graft clarity. At this time point, allogeneic acceptors, allogeneic rejectors, syngeneic controls and naïve mice were euthanized. Donor buttons plus host corneal beds were collected and stored in Trizol reagent (Invitrogen, Carlsbad, CA) at −80°C. Tissue was homogenized and RNA isolated using the RNeasy micro kit (Qiagen, Valencia, CA) and reverse-transcribed using the Superscript III kit (Invitrogen, Carlsbad, CA). Real-Time PCR was performed using the Taqman Universal PCR Master Mix (Applied Biosystems, Carlsbad, CA) and the following primers: P-selectin Mm01204601_m1, E-selectin Mm01310197_m1 and glyceraldehyde-3-phosphate dehydrogenase Mm99999915_g1 (Applied Biosystems). Comparative threshold (CT) values were measured using a LightCycler 480 II System (Roche, Indianapolis, IN) and target CT values were normalized to the CT value of GAPDH, which served as an endogenous control. Fold change in mRNA level relative to naïve mice was then calculated.
Immunohistochemistry
Corneas from allogeneic acceptors, allogeneic rejectors, syngeneic controls and naïve mice were collected 4 weeks posttransplantation. Corneal epithelium was removed following incubation for 45 minutes in 20 mM EDTA in phosphate buffered saline. Corneas were fixed in acetone for 15 minutes then blocked with 2% BSA and Fc block and stained with FITC-conjugated anti-CD31 (1:100, sc-18916) and either goat anti-P-selectin (1:100, sc-6943) or goat anti-E-selectin (1:100, sc-6939) primary antibodies overnight at 4°C. After washing, corneas were stained for 2 hours at room temperature with Texas Red-conjugated donkey anti-goat secondary antibody (1:400, sc-2783). Corneas were washed in PBS, then mounted using Vectashield mounting medium with DAPI. Microscopic images were taken using a Leica TCS-SP2 confocal microscope (Leica Microsystems, Wetzlar, Germany). All immunohistochemistry antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Flow cytometry
Four weeks following corneal transplantation, the ipsilateral cervical and submandibular lymphoid tissue was collected from allogeneic acceptors and rejectors, syngeneic controls and naïve mice. Single cell suspensions were created by homogenizing lymphoid tissue in 70 μm cell strainers. Cells were stimulated with PMA and Ionomycin in the presence of golgistop for 5 hours at 37° C and 5% CO2, blocked with Fc block then stained with Violet-421 conjugated anti-CD4 antibody (Biolegend, San Diego, CA, USA) and PE-conjugated anti-PSGL-1 (BD Pharmingen, San Jose, CA, USA) or PE-conjugated anti-glycosylated CD43 antibodies (Biolegend, San Diego, CA, USA). Cells were fixed and permeabilized overnight, then stained with FITC-conjugated anti-interferon-gamma antibody. To analyze corneal graft infiltration following in vivo neutralization, corneal grafts and the host bed including the limbus were collected and digested with DNAse and collagenase at 37 °C for 1 hour. Single cell suspensions were stained with PE conjugated anti-CD45 antibody (eBioscience, San Diego, CA, USA) and PE/cy5 conjugated anti-CD4 antibody (eBioscience, San Diego, CA, USA). Antibody staining was read using a BD LSR II flow cytometer and analyzed using Summit v4.3 software (DAKO Corporation).
In vivo neutralization
Commercially available neutralizing antibodies were used for in-vivo blockade. Mice were anesthetized using 2-4% isoflurane and 100% oxygen, and 10 μl of anti-P-selectin (1 mg/ml, clone RB40.34; BD Biosciences 553741), anti-E-selectin (1 mg/ml, clone 10E9.6, BD Biosciences 553748) or rat IgG control (1 mg/ml, R and D Systems 6-001-A) was administered via subconjuctival injection. For injection, the temporal bulbar conjunctiva was grasped with forceps and lifted to expose the potential subjconjunctival space. A Hamilton syringe (10 ul Model 1701, Hamilton Company, Reno, NV, USA) was then used to inject drug or control. Treatment was started at the day of transplantation, and continued on alternating days until flow analysis on day 14. For survival studies, mice were treated on alternating days beginning at the time of transplantation for two weeks, then twice per week thereafter for 6 additional weeks.
Statistical analysis
Data normality was evaluated using Prism 5.0 software. The one-way Anova test with Tukey post test or the Kruskal-Wallis with Dunn's multiple comparison test were used to determine statistical significance (Prism 5.0 software, GraphPad Software, Inc., La Jolla, CA, USA). Graft survival was compared using Kaplan-Meier survival curves, with statistical significance determined using the log-rank test. P ≤ 0.05 was considered statistically significant. Results are presented as the mean ± standard error of the mean (SEM).
Results
Expression of P- and E-selectin is upregulated in allogeneic rejectors
To study the function of P-selectin and E-selectin in mediating Th1 cell migration in corneal transplantation, we first investigated the expression of P- and E-selectin in allogeneic acceptors versus rejectors. Allogeneic corneal transplantation was performed, and at four weeks posttransplantation accepted transplants were distinguished from rejected transplants by a commonly used scale of corneal opacity.34 Grafts plus the host corneal bed were collected for analysis of P- and E-selectin mRNA levels. We found that both P- and E-selectin mRNA levels were significantly upregulated in rejected transplants (P-selectin: 93.3±36.7 fold relative to naïve mice, E-selectin: 17.1±0.05 fold) as compared to both allogeneic acceptors (P-selectin: 17.6±7.4 fold, p<0.05; E-selectin: 7.7±0.03 fold, p<0.005) and syngeneic transplant recipients (P-selectin: 1.3±0.65 fold, p<0.05; E-selectin: 1.4±0.05 fold, p<0.005) (Figures 1A and 1B).
Figure 1. mRNA expression of P-selectin and E-selectin is highest in allogeneic rejectors.
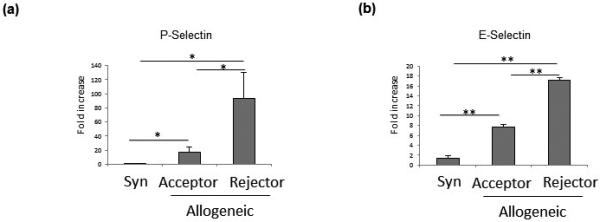
(a) P-selectin and (b) E-selectin messenger RNA (mRNA) transcript levels are significantly upregulated in the corneas (graft and host bed) of allogeneic transplant rejectors as compared to allogeneic transplant acceptors and syngeneic (Syn) graft recipients (n = 4–6 mice/group, *p < 0.05, **p < 0.005; data from one experiment of two are shown).
To further evaluate P- and E-selectin expression in the cornea, immunohistochemistry was performed in order to confirm and localize their expression at the protein level. Grafts plus the host corneal beds from transplanted mice were immunohistochemically stained with DAPI, CD31 (PECAM) and either P- or E-selectin, and confocal micrographs of the limbal vasculature were taken. CD31+ staining was greatest in the allogeneic rejector group, and both P-and E-selectin were exclusively expressed by CD31+ vascular endothelial cells (Figures 2A and B).
Figure 2. P-selectin and E-selectin are expressed by CD31+ endothelial cells.
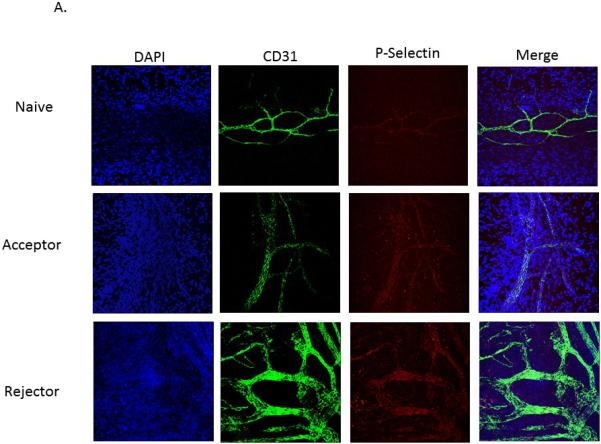
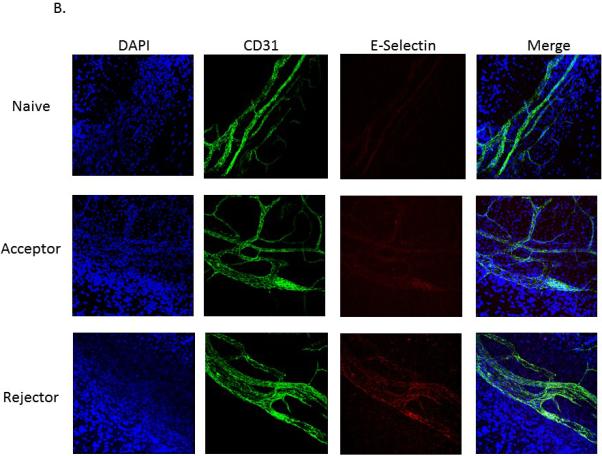
Representative micrographs of the limbal vasculature from whole-mount cornea immunostaining (40×). Corneas from naive, allogeneic transplant acceptors, and allogeneic transplant rejectors were stained with DAPI (blue), anti-CD31 antibody (green), and either (a) anti-P-selectin (red) or (b) anti-E-selectin antibody (red) (n=3 corneas/group, representative micrographs are from one cornea per group).
Selectin ligands are highly expressed by CD4+ T cells
After confirming the presence of P- and E-selectin in corneal transplant recipients, we next sought to determine whether Type 1 T helper (Th1) cells express the selectin ligands PSGL-1 and GlycoCD43. Using flow cytometry, we found no difference in PSGL-1 expression between groups (p>0.05), but PSGL-1 is expressed by nearly 100% of all Th1 cells, regardless of whether those cells are derived from naïve mice (99.9%±0.08), syngeneic transplant recipients (99.9%±0.04), allogeneic transplant acceptors (99.99%±0.006) or rejectors (99.9%±0.04) (Figure 3). Moreover, the level of expression of PSGL-1 by Th1 cells is similar across groups, as indicated by Mean Fluorescence Intensities (naïve mice: 888± 13, syngeneic: 832±29, allogeneic acceptors: 849±45, allogeneic rejectors 816±23) (Figure 3A, p>0.05).
Figure 3. Selectin ligands are highly expressed by Th1 cells from all experimental groups.
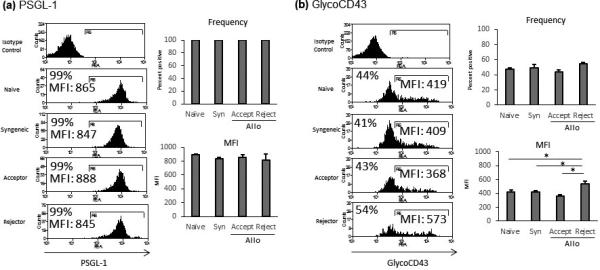
Representative flow cytometry histograms showing the frequency and level of expression (Mean Fluorescent Intensity) of selectin ligands by Th1 cells in the draining LNs of naïve, syngeneic transplant recipients (Syn), allogeneic (Allo) transplant acceptors (Accept), and rejectors (Reject). (a) PSGL-1 is expressed by nearly 100% of Th1 cells, with no significant difference in level of expression between groups. (b) GlycoCD43 is expressed by approximately one half of all Th1 cells, irrespective of experimental group. The level of GlycoCD43 expression (MFI) is significantly upregulated in allogeneic rejectors. (Frequencies and MFI shown are of the representative histogram, n = 5 mice/group, with one animal comprising one flow cytometry sample; *p<0.05; data from one experiment of two are shown).
GlycoCD43 expression by Th1 cells was also evaluated by flow cytometry. The frequency of GlycoCD43 expressing cells was similar across experimental groups, with a trend towards an increase in the frequency of GlycoCD43 expressing cells in the allogeneic rejector group as compared to the allogeneic acceptor group (54% vs. 44%) (Figure 3B). GlycoCD43 expression as measured by MFI was found to be significantly greater in allogeneic rejectors (541 ± 39, p<0.05) as compared to acceptors (361 ± 24), syngeneic recipients (418 ± 17) and naïve mice (421 ± 36) (Figure 3B).
Blockade of P- and E-selectin decreases immune cell infiltration into transplanted corneas
In order to evaluate the functional relevance of selectins in mediating T cell migration in vivo, we treated allogeneic transplant recipients with anti-P or anti-E-selectin neutralizing antibodies. Mice were treated at the time of transplantation and then on alternating days posttransplantation with 10 ul of 1 mg/ml anti-E-selectin, anti-P-selectin or rat IgG via subconjunctival injection. On day 14, the frequencies of cornea-infiltrating CD45+ and CD4+ immune cells were determined by flow cytometry. Compared to treatment with IgG isotype control (19.3±1.4), treatment with both anti-P-selectin (7.8±1.7, p<0.05) and anti-E-selectin (7.8±2.4, p<0.05) reduced the frequency of CD45+ cells (Figure 4, A-B). In addition, treatment with anti-P-selectin (0.47±0.03, p<0.05) and anti-E-selectin (0.49±0.10, p<0.05) also significantly reduced the frequency of corneal CD4+ cells as compared to control treatment (0.98±0.1)(Figure 4, B-C).
Figure 4. In vivo selectin blockade decreases immune cell infiltration of corneal grafts.
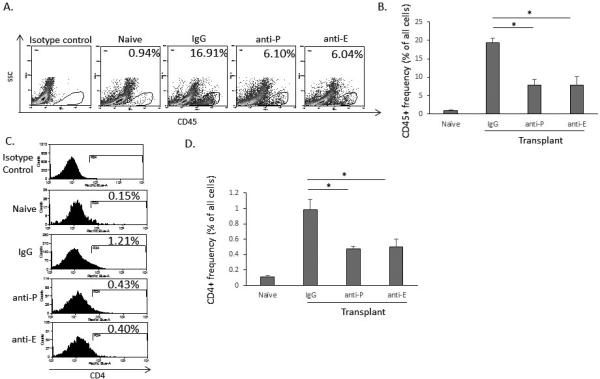
(a) Representative flow cytometry histograms demonstrating that blockade of P- and E-selectin are equally effective in reducing immune cell recruitment to the corneal graft 14 days after allogeneic transplantation. (b) Anti-P (7.8±1.7) and anti-E selectin (7.8±2.4) antibodies significantly reduce the frequency of CD45+ cells compared to IgG control (19.3±1.4). (c) Representative histograms showing CD4+ cells 14 days after selectin treatment and transplantation. (d) Anti-P (0.47±0.03) and anti-E selectin (0.49±0.10) antibodies significantly reduce the frequency of CD4+ cells compared to IgG control (0.98±0.1) (n = 5 mice/group, with one animal comprising one flow cytometry sample; *p<0.05; data from one experiment of two are shown).
Blockade of E-selectin decreases antigen-presenting cell frequency in the draining lymph nodes
We additionally evaluated whether blockade of P- or E-selectin had any effect on mature antigen-presenting cell trafficking by evaluating their frequencies in the draining cervical and submandibular lymph nodes. We found that the CD11c+MHC-IIhi cell frequency was highest in the IgG control group (12.67±0.5) and that treatment with anti-E-selectin (6.96±0.9, p<0.05) but not anti-P-selectin (10.68±0.6) significantly reduced the frequency of these cells as compared to IgG control (Figure 5).
Figure 5. Anti-E-selectin reduces the frequency of mature antigen-presenting cells in the draining lymphoid tissue 14 days after allogeneic transplantation.
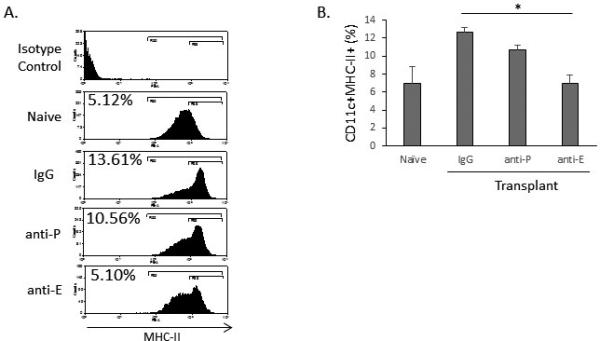
(a) Representative flow cytometry histograms showing MHCII expression by CD11c+ cells. (b) Anti-E-selectin (6.96±0.9) but not anti-P-selectin (10.68±0.6) significantly reduces the number of CD11c+MHCII+ cells compared to IgG control (12.67±0.5). (n = 5 mice/group, with one animal comprising one flow cytometry sample; *p<0.05; data from one experiment of two are shown).
Blockade of E-selectin delays graft rejection
Based on our data demonstrating that treatment with anti-E-selectin antibodies reduced the frequency of both corneal CD4+ T cells and lymph node CD11c+MHC-IIhi cells, we hypothesized that this treatment would also improve long-term corneal allograft survival. Standard allogeneic corneal transplantation was performed and mice were treated with either anti-E-selectin neutralizing antibody or IgG control every other day for 2 weeks then twice per week thereafter (1 mg/ml, 10 ul subconjunctival injections). Beginning at week 3, the anti-E-selectin treatment group exhibited a modestly higher survival rate than IgG control at all time points. From weeks 3 through 5, survival in the anti-E-selectin treatment group was 70% compared to 50% in the IgG control group, and from weeks 5 through 7 survival was 60% and 30%, respectively. Eight weeks posttransplantation, graft survival in the anti-E-selectin treatment group was 40% while the IgG control treatment group was 30% (p>0.05 at all time points, Figure 6).
Figure 6. Blockade of E-selectin improves long-term graft survival.
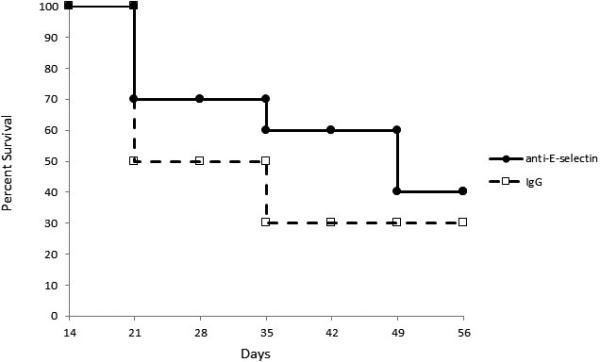
Corneal transplanted mice were treated with anti-E-selectin antibodies beginning at the time of transplantation for 8 weeks. A Kaplan-Meier curve shows graft survival. anti-E-selectin=solid line; IgG control-treated hosts=dashed line (n=10 mice per group).
Discussion
In the present study we show that that P- and E-selectin expression is upregulated in rejected allogeneic corneal transplants, and that effector Th1 cells highly express the selectin ligands PSGL-1 and glycosylated CD43. We provide evidence that in transplant hosts, modulation of selectin/ligand interactions through neutralization of P- or E-selectin reduces the frequency of graft-infiltrating immune cells by 50%, and that blockade of E-selectin also has an inhibitory effect on antigen-presenting cell trafficking, making E-selectin an attractive target for in vivo modulation. Through these mechanisms, neutralization of E-selectin delays graft rejection but yields only a modest improvement in long-term graft survival.
To investigate the role of selectins in corneal transplantation, we first assessed the expression of P- and E-selectin in corneal allografts. We found that both P- and E-selectin are expressed by CD31+ vascular endothelial cells, a pattern of expression consistent with the fact that both P- and E-selectin are upregulated by these cells in response to inflammatory cytokines such as TNF-α and IL-1β,15, 35-36 cytokines which play important roles in mediating corneal inflammation and graft rejection.37 Our data are also supported by previous studies which have identified adhesion molecules, in particular E-selectin, on the vascular endothelium of rejected cornea, cardiac, and pulmonary allografts.24,28,38 In our immunohistochemical analysis, we additionally observed an increase in corneal neovascularization near the graft site, which is consistent with the known contribution of postoperative corneal neovascularization to loss of immune privilege and graft rejection.39,40 Thus, the observed increase in P- and E-selectin expression in allogeneic rejectors may be a result of both increased vascular endothelial cell expression of P- and E-selectin in response to inflammatory cytokines, as well as an increase in corneal neovascularization, resulting in an increase in the total number of selectin-expressing vascular endothelial cells.
We next investigated the expression of selectin ligands by Th1 cells isolated from allogeneic transplant recipients. P- and E-selectin interact with multiple ligands containing the sialyl-lewisx carbohydrate domain, two of the well-characterized of which are PSGL-1 and glycoCD43. Using flow cytometry we evaluated whether Th1 cells express these ligands, and found that PSGL-1 is expressed by nearly 100% of Th1 cells, with no difference in level of expression between Th1 cells isolated from allogeneic acceptors, rejectors and naïve mice. In contrast, glycoCD43 is expressed by approximately 50% of all Th1 cells, and its level of expression is upregulated by Th1 cells isolated from allogeneic rejectors. These results are consistent with a past study examining ligand expression by in vitro generated Th1 cells, in which PSGL-1 and GlycoCD43 were shown to be expressed by 99.5% and 61.5% of Th1 cells, respectively.20 In addition, it was also shown that PSGL-1 MFI is constant across T cell subsets, while GlycoCD43 exhibits significant variation between subsets. These results suggest that PSGL-1 is highly conserved across different T cell subsets and alloimmune environments, while the level of GlycoCD43 expression may be one way in which cells respond and adapt to different immunoinflammatory conditions.
Although glycoCD43 has previously been described as interacting predominantly with E-selectin, glycoCD43 and PSGL-1 can function as ligands for both P- and E-selectin.17-20 Given that P- and E-selectin are significantly upregulated in rejected allogeneic corneal transplants and that effector T cells highly express PSGL-1 and glycoCD43, we next sought to prevent selectin/ligand interactions through neutralization of P- and E-selectin in an in vivo model of corneal transplantation. Treatment with anti-P- and anti-E-selectin antibodies were equally effective in reducing the frequency of T cells recruited to the corneal graft 14 days after transplantation, as each reduced T cell frequency by approximately 50%, indicating their functional relevance in corneal alloimmunity. This finding is supported by past studies showing that inhibiting selectin function can reduce T cell recruitment in models of cutaneous delayed-type hypersensitivity, antigen-induced arthritis and antigen-induced intestinal inflammation.41,42
Interestingly, we found that blocking E-selectin but not P-selectin significantly decreased the number of mature CD11c+ cells in the regional draining lymph nodes of host mice, suggesting that E-selectin may play a role in host sensitization by mediating antigen-presenting cell trafficking from graft to lymphoid tissue. Given that transplantation was performed in low-risk host beds, we would expect the indirect pathway of host sensitization to predominate, and for these APCs to be donor derived.43 Although the exact mechanism by which E-selectin contributes to the process of APC trafficking is unknown, it has been postulated that E-selectin may play a role in APC tethering as well as integrin activation on lymphatic endothelial cells.44 Indeed, lymphatic endothelial cells are capable of expressing E-selectin, particularly under inflammatory conditions,45-48 and past studies have suggested that E-selectin contributes to APC entry from peripheral tissue to the lymphatic vasculature.48-51
Based on our findings demonstrating that E-selectin is functionally relevant in both T cell and APC trafficking, we next evaluated whether treatment with anti-E-selectin could improve long-term corneal allograft survival. We found that treatment with anti-E-selectin delayed graft rejection and improved survival compared to IgG control at all time points, but this difference never reached statistical significance and at week 8, treatment had only a modest effect on graft survival (40% in the anti-E-selectin group vs. 30% in the IgG treatment group). Given that E-selectin neutralization reduces the number of graft-infiltrating CD4+ T cells (the primary mediators of corneal allograft rejection)9-12 and suppresses mature CD11c+ cell trafficking in vivo, it is somewhat surprising that neutralization did not yield a significant improvement in graft survival compared to control. However, this finding may be explained at least in part by the known overlapping function of selectins and the fact that leukocyte adhesion and migration is regulated by multiple additional factors including integrins and chemokines.15 In addition, it may be that local neutralization of adhesion molecules is not sufficient for modulating T cell trafficking, and more systemic administration may be able to improve graft survival by modulating immune cell-vasculature interactions at additional locations other than the allograft, such as in the draining lymphoid tissue. There may also be more effective means of selectin blockade than neutralizing antibodies as other methods for selectin neutralization have proven effective in prior studies, including use of siRNA52,53 and selectin-knockout mice.54,55 The modest increase in graft survival observed here may also be explained by considering the effect of E-selectin neutralization on antigen-presenting cells more closely, and in particular, its effect on tolerogenic dendritic cells.
Tolerogenic dendritic cells are a class of naturally-occurring antigen-presenting cells which suppress alloimmunity in an antigen-specific manner, thus playing an important role in promoting allo-tolerance and transplant survival.56-60 Urzainqui et al. have shown that selectin binding to dendritic cell-expressed PSGL-1 initiates a tolerogenic program in those dendritic cells that includes upregulation of regulatory cytokines and an enhanced ability to generate regulatory T cells. They found that following stimulation, dendritic cell expression of MHC class II, CD40, and CD86 is significantly increased in PSGL-1 (−/−) mice compared to wild-type mice.61 In addition, E-selectin neutralization may also interfere with the migration of host and donor-derived tolerogenic dendritic cells to host lymph nodes. This is supported by the fact that in addition to decreasing the frequency of CD11c+MHCIIhi cells (Figure 5), we found that neutralization of E-selectin also led to a decrease in both total CD11c+ cell and CD11c+MHClo cell frequencies in the draining lymph nodes (data not shown). Thus, while neutralization of E-selectin suppresses CD4+T cell graft infiltration and mature APC trafficking, it may also be promoting alloimmunity by preventing the induction and migration of tolerogenic dendritic cells, leading to the observed modest improvement in long-term graft survival.
In summary, we show that P- and E-selectin mediate T cell recruitment in corneal transplantation and that E-selectin blockade additionally suppresses antigen-presenting cell trafficking. Despite the demonstrated functional relevance of E-selectin in leukocyte infiltration and APC trafficking, neutralization of E-selectin yields only a modest improvement in long-term graft survival, possibly due to the overlapping function of selectins and the large number of factors mediating leukocyte adhesion.
Acknowledgments
This study was supported in part by NIH R01 EY012963 (RD) and NIH K08 EY020575 (PH) and the Eye Bank Association of America/Richard Lindstrom Grant (THD). The authors would like to thank Randy Huang and Don Pottle for expert technical assistance, and Dr. Susanne Eiglmeier (Schepens Eye Research Institute) for helpful discussion and editorial assistance in the preparation of this manuscript.
Abbreviations
- E-selectin
endothelial-selectin
- GlycoCD43
glycosylated CD43
- IL-1β
interleukin-1 beta
- L-selectin
leukocyte-selectin
- P-selectin
platelet-selectin
- PSGL-1
P-selectin glycoprotein ligand-1
- Th1 cell
Type 1 T helper cell
- TNF-α
Tumor necrosis factor alpha
Footnotes
Author contributions:
THD: Performance of the research, data analysis, writing the paper; ADZ: Performance of the research, MO: Performance of the research, data analysis; JH: Performance of the research; JD: Performance of the research; SKC, PH and RD: Research design and writing the paper.
Disclosure:
The authors declare no conflicts of interest.
References
- 1.Dana MR, Qian Y, Hamrah P. Twenty-five-year panorama of corneal immunology: emerging concepts in the immunopathogenesis of microbial keratitis, peripheral ulcerative keratitis, and corneal transplant rejection. Cornea. 2000;19:625. doi: 10.1097/00003226-200009000-00008. PubMed PMID: 11009315. [DOI] [PubMed] [Google Scholar]
- 2.Coster Williams KA. The impact of corneal allograft rejection on the long-term outcome of corneal transplantation. Am J Ophthalmol. 2005;140:1112. doi: 10.1016/j.ajo.2005.07.024. PubMed PMID: 16376660. [DOI] [PubMed] [Google Scholar]
- 3.2013 Eye Banking Statistical Report. Eye Bank Association of America; http://www.restoresight.org/wp-content/uploads/2014/04/2013_Statistical_Report-FINAL.pdf. [Google Scholar]
- 4.Williams KA, Muehlberg SM, Lewis RF, Coster DJ. How successful is corneal transplantation? A report from the Australian Corneal Graft Register. Eye (Lond) 1995;9:219. doi: 10.1038/eye.1995.43. PubMed PMID: 7556721. [DOI] [PubMed] [Google Scholar]
- 5.Patel SP, Dana R. Corneal lymphangiogenesis: implications in immunity. Semin Ophthalmol. 2009;24:1358. doi: 10.1080/08820530902801320. PubMed PMID: 19437348. [DOI] [PubMed] [Google Scholar]
- 6.Niederkorn JY. Corneal transplantation and immune privilege. Int Rev Immunol. 2013;32:57. doi: 10.3109/08830185.2012.737877. doi: 10.3109/08830185.2012.737877. Review. PubMed PMID: 23360158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maguire MG, Stark WJ, Gottsch JD, et al. Risk factors for corneal graft failure and rejection in the collaborative corneal transplantation studies. Collaborative Corneal Transplantation Studies Research Group. Ophthalmology. 1994;101:1536. doi: 10.1016/s0161-6420(94)31138-9. PubMed PMID: 8090456. [DOI] [PubMed] [Google Scholar]
- 8.Kamp MT, et al. Patient-reported symptoms associated with graft reactions in high-risk patients in the collaborative corneal transplantation studies. Collaborative Corneal Transplantation Studies Research Group. Cornea. 1995;14:43. PubMed PMID: 7712736. [PubMed] [Google Scholar]
- 9.Niederkorn JY. Immune mechanisms of corneal allograft rejection. Curr Eye Res. 2007;32:1005. doi: 10.1080/02713680701767884. Pubmed PMID: 18085464. [DOI] [PubMed] [Google Scholar]
- 10.He YG, Ross J, Niederkorn JY. Promotion of murine orthotopic corneal allograft survival by systemic administration of anti-CD4 monoclonal antibody. Invest. Ophthalmol. Vis. Sci. 1991;32:2723. PubMed PMID: 1680112. [PubMed] [Google Scholar]
- 11.Boisgérault F, Liu Y, Anosova N, Ehrlich E, Dana MR, Benichou G. Role of CD4+ and CD8+ T cells in allorecognition: lessons from corneal transplantation. J. Immunol. 2001;167:1891. doi: 10.4049/jimmunol.167.4.1891. PubMed PMID: 11489968. [DOI] [PubMed] [Google Scholar]
- 12.Qian Y, Dana MR. Molecular mechanisms of immunity in corneal allotransplantation and xenotransplantation. Expert Rev Mol Med. 2001;3:1–21. doi: 10.1017/S1462399401003246. PubMed PMID: 14585142. [DOI] [PubMed] [Google Scholar]
- 13.Yamagami S, Kawashima H, Endo H, et al. Cytokine profiles of aqueous humor and graft in orthotopic mouse corneal transplantation. Transplantation. 1998;66:1504. doi: 10.1097/00007890-199812150-00014. PubMed PMID: 9869092. [DOI] [PubMed] [Google Scholar]
- 14.Yamagami S, Hamrah P, Zhang Q, Liu Y, Huq S, Dana MR. Early ocular chemokine gene expression and leukocyte infiltration after high-risk corneal transplantation. Mol Vis. 2005;11:632. PubMed PMID: 16145544. [PubMed] [Google Scholar]
- 15.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678. doi: 10.1038/nri2156. PubMed PMID: 17717539. [DOI] [PubMed] [Google Scholar]
- 16.Impellizzeri D, Cuzzocrea S. Targeting selectins for the treatment of inflammatory diseases. Expert Opin Ther Targets. 2014;18:55. doi: 10.1517/14728222.2013.841140. PubMed PMID: 24074033. [DOI] [PubMed] [Google Scholar]
- 17.Hirata T, Merrill-Skoloff G, Aab M, Yang J, Furie BC, Furie B. P-Selectin glycoprotein ligand 1 (PSGL-1) is a physiological ligand for E-selectin in mediating T helper 1 lymphocyte migration. J Exp Med. 2000;192:1669. doi: 10.1084/jem.192.11.1669. PubMed PMID: 11104809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsumoto M, Atarashi K, Umemoto E, et al. CD43 functions as a ligand for E-selectin on activated T cells. J. Immunol. 2005;175:8042. doi: 10.4049/jimmunol.175.12.8042. PubMed PMID: 11104809. [DOI] [PubMed] [Google Scholar]
- 19.Borges E, Tietz W, Steegmaier M, et al. P-selectin glycoprotein ligand-1 (PSGL-1) on T helper 1 but not on T helper 2 cells binds to P-selectin and supports migration into inflamed skin. J Exp Med. 1997;185:573. doi: 10.1084/jem.185.3.573. PubMed PMID: 9053457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alcaide P, King SL, Dimitroff CJ, Lim YC, Fuhlbrigge RC, Luscinskas FW. The 130-kDa glycoform of CD43 functions as an E-selectin ligand for activated Th1 cells in vitro and in delayed-type hypersensitivity reactions in vivo. J Invest Dermatol. 2007;127:1964. doi: 10.1038/sj.jid.5700805. PubMed PMID: 17392823. [DOI] [PubMed] [Google Scholar]
- 21.Luscinskas FW, Ding H, Lichtman AH. P-selectin and vascular cell adhesion molecule 1 mediate rolling and arrest, respectively, of CD4+ T lymphocytes on tumor necrosis factor alpha-activated vascular endothelium under flow. J Exp Med. 1995;181:1179. doi: 10.1084/jem.181.3.1179. PubMed PMID: 7532680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morgan JD, Lycett A, Horsburgh T, Nicholson ML, Veitch PS, Bell PR. The importance of E-selectin as a marker for renal transplant rejection. Transpl Immunol. 1994;2:326. doi: 10.1016/0966-3274(94)90010-8. PubMed PMID: 7535643. [DOI] [PubMed] [Google Scholar]
- 23.Nagano H, Nadeau KC, Takada M, Kusaka M, Tilney NL. Sequential cellular and molecular kinetics in acutely rejecting renal allografts in rats. Transplantation. 1997;63:1101. doi: 10.1097/00007890-199704270-00009. PubMed PMID: 9133471. [DOI] [PubMed] [Google Scholar]
- 24.Allen MD, McDonald TO, Himes VE, Fishbein DP, Aziz S, Reichenbach DD. E-selectin expression in human cardiac grafts with cellular rejection. Circulation. 1993;88:243–7. PubMed PMID: 7693367. [PubMed] [Google Scholar]
- 25.Briscoe DM, Yeung AC, Schoen FJ, et al. Predictive value of inducible endothelial cell adhesion molecule expression for acute rejection of human cardiac allografts. Transplantation. 1995;59:204. PubMed PMID: 7530872. [PubMed] [Google Scholar]
- 26.Salom RN, Maguire JA, Hancock WW. Endothelial activation and cytokine expression in human acute cardiac allograft rejection. Pathology. 1998;30:24. doi: 10.1080/00313029800169625. PubMed PMID: 9534204. [DOI] [PubMed] [Google Scholar]
- 27.Philipp W, Göttinger W. Leukocyte adhesion molecules in diseased corneas. Invest Ophthalmol Vis Sci. 1993;34:2449. PubMed PMID: 7686892. [PubMed] [Google Scholar]
- 28.Philipp W. Leukocyte adhesion molecules in rejected corneal allografts. Graefes Arch Clin Exp Ophthalmol. 1994;232:87–95. doi: 10.1007/BF00171669. PubMed PMID: 7512518. [DOI] [PubMed] [Google Scholar]
- 29.Austrup F, Vestweber D, Borges E, Löhning M, Bräuer R, Herz U, Renz H, Hallmann R, Scheffold A, Radbruch A, Hamann A. P- and E-selectin mediate recruitment of T-helper-1 but not T-helper-2 cells into inflamed tissues. Nature. 1997;385:81. doi: 10.1038/385081a0. PubMed PMID: 8985251. [DOI] [PubMed] [Google Scholar]
- 30.Takada M, Nadeau KC, Shaw GD, Tilney NL. Early cellular and molecular changes in ischemia/reperfusion injury: inhibition by a selectin antagonist, P-selectin glycoprotein ligand-1. Transplant Proc. 1997;29:1324. doi: 10.1016/s0041-1345(96)00577-5. PubMed PMID: 9123325. [DOI] [PubMed] [Google Scholar]
- 31.Farmer DG, Anselmo D, Da Shen X, et al. Disruption of P-selectin signaling modulates cell trafficking and results in improved outcomes after mouse warm intestinal ischemia and reperfusion injury. Transplantation. 2005;80:828. doi: 10.1097/01.tp.0000174337.53658.b0. PubMed PMID: 16210972. [DOI] [PubMed] [Google Scholar]
- 32.Cai YH, Alvarez A, Alcaide P, et al. Abrogation of functional selectin-ligand expression reduces migration of pathogenic CD8+ T cells into heart. J Immunol. 2006;176:6568. doi: 10.4049/jimmunol.176.11.6568. PubMed PMID: 16709814. [DOI] [PubMed] [Google Scholar]
- 33.Dana MR, Yamada J, Streilein JW. Topical interleukin 1 receptor antagonist promotes corneal transplant survival. Transplantation. 1997;63:1501. doi: 10.1097/00007890-199705270-00022. PubMed PMID: 9175817. [DOI] [PubMed] [Google Scholar]
- 34.Sonoda Y, Streilein JW. Orthotopic corneal transplantation in mice--evidence that the immunogenetic rules of rejection do not apply. Transplantation. 1992;54:694. doi: 10.1097/00007890-199210000-00026. PubMed PMID: 1412761. [DOI] [PubMed] [Google Scholar]
- 35.Bevilacqua MP, Pober JS, Mendrick DL, Cotran RS, Gimbrone MA., Jr Identification of an inducible endothelial-leukocyte adhesion molecule. Proc Natl Acad Sci U S A. 1987;84:9238. doi: 10.1073/pnas.84.24.9238. PubMed PMID: 2827173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weller A, Isenmann S, Vestweber D. Cloning of the mouse endothelial selectins. Expression of both E- and P-selectin is inducible by tumor necrosis factor alpha. J Biol Chem. 1992;267:15176. PubMed PMID: 1378846. [PubMed] [Google Scholar]
- 37.Dana R. Comparison of topical interleukin-1 vs tumor necrosis factor-alpha blockade with corticosteroid therapy on murine corneal inflammation, neovascularization, and transplant survival (an American Ophthalmological Society thesis). Trans Am Ophthalmol Soc. 2007;105:330. PubMed PMID: 18427620. [PMC free article] [PubMed] [Google Scholar]
- 38.Shreeniwas R, Schulman LL, Narasimhan M, McGregor CC, Marboe CC. Adhesion molecules (E-selectin and ICAM-1) in pulmonary allograft rejection. Chest. 1996;110:1143. doi: 10.1378/chest.110.5.1143. PubMed PMID: 8915211. [DOI] [PubMed] [Google Scholar]
- 39.Dana MR, Streilein JW. Loss and restoration of immune privilege in eyes with corneal neovascularization. Invest Ophthalmol Vis Sci. 1996;37:2485. PubMed PMID: 8933765. [PubMed] [Google Scholar]
- 40.Cursiefen C, Cao J, Chen L, et al. Inhibition of hemangiogenesis and lymphangiogenesis after normal-risk corneal transplantation by neutralizing VEGF promotes graft survival. Invest Ophthalmol Vis Sci. 2004;45:2666. doi: 10.1167/iovs.03-1380. PubMed PMID: 15277490. [DOI] [PubMed] [Google Scholar]
- 41.Austrup F, Vestweber D, Borges E, et al. P- and E-selectin mediate recruitment of T-helper-1 but not T-helper-2 cells into inflammed tissues. Nature. 1997;385:81. doi: 10.1038/385081a0. PubMed PMID: 8985251. [DOI] [PubMed] [Google Scholar]
- 42.Haddad W, Cooper CJ, Zhang Z, et al. P-selectin and P-selectin glycoprotein ligand 1 are major determinants for Th1 cell recruitment to nonlymphoid effector sites in the intestinal lamina propria. J Exp Med. 2003;198:369. doi: 10.1084/jem.20020691. PubMed PMID: 12885868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huq S, Liu Y, Benichou G, Dana MR. Relevance of the direct pathway of sensitization in corneal transplantation is dictated by the graft bed microenvironment. J Immunol. 2004;173:4464. doi: 10.4049/jimmunol.173.7.4464. PubMed PMID: 15383577. [DOI] [PubMed] [Google Scholar]
- 44.Johnson LA, Jackson DG. Cell Traffic and the Lymphatic Endothelium. Ann N Y Acad Sci. 2008;1131:119. doi: 10.1196/annals.1413.011. PubMed PMID: 18519965. [DOI] [PubMed] [Google Scholar]
- 45.Sawa Y, Tsuruga E. The expression of E-selectin and chemokines in the cultured human lymphatic endothelium with lipopolysaccharides. J Anat. 2008;212:654. doi: 10.1111/j.1469-7580.2008.00892.x. PubMed PMID: 18410313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brown MN, Fintushel SR, Lee MH, et al. Chemoattractant receptors and lymphocyte egress from extralymphoid tissue: changing requirements during the course of inflammation. J Immunol. 2010;185:4873. doi: 10.4049/jimmunol.1000676. PubMed PMID: 20833836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Teijeira A, Rouzaut A, Melero I. Initial afferent lymphatic vessels controlling outbound leukocyte traffic from skin to lymph nodes. Front Immunol. 2013;4:433. doi: 10.3389/fimmu.2013.00433. PubMed PMID: 24368908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Johnson LA, Clasper S, Holt AP, Lalor PF, Baban D, Jackson DG. An inflammation-induced mechanism for leukocyte transmigration across lymphatic vessel endothelium. J Exp Med. 2006;203:2763. doi: 10.1084/jem.20051759. PubMed PMID: 17116732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Simon SI, Hu Y, Vestweber D, Smith CW. Neutrophil tethering on E-selectin activates beta 2 integrin binding to ICAM-1 through a mitogen-activated protein kinase signal transduction pathway. J Immunol. 2000;164:4348. doi: 10.4049/jimmunol.164.8.4348. PubMed PMID: 10754335. [DOI] [PubMed] [Google Scholar]
- 50.Irjala H, Johansson EL, Grenman R, Alanen K, Salmi M, Jalkanen S. Mannose receptor is a novel ligand for L-selectin and mediates lymphocyte binding to lymphatic endothelium. J Exp Med. 2001;194:1033. doi: 10.1084/jem.194.8.1033. PubMed PMID: 11602634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miteva DO, Rutkowski JM, Dixon JB, Kilarski W, Shields JD, Swartz MA. Transmural flow modulates cell and fluid transport functions of lymphatic endothelium. Circ Res. 2010;106:920. doi: 10.1161/CIRCRESAHA.109.207274. PubMed PMID: 20133901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu ZJ, Tian R, Li Y, et al. Inhibition of tumor angiogenesis and melanoma growth by targeting vascular E-selectin. Ann Surg. 2011;254:450. doi: 10.1097/SLA.0b013e31822a72dc. PubMed PMID: 21795970. [DOI] [PubMed] [Google Scholar]
- 53.Walker T, Saup E, Nolte A, et al. Transfection of short-interfering RNA silences adhesion molecule expression on cardiac microvascular cells. Thorac Cardiovasc Surg. 2011;59:322. doi: 10.1055/s-0030-1271142. PubMed PMID: 21692023. [DOI] [PubMed] [Google Scholar]
- 54.Naka Y, Toda K, Kayano K, Oz MC, Pinsky DJ. Failure to express the P-selectin gene or P-selectin blockade confers early pulmonary protection after lung ischemia or transplantation. Proc Natl Acad Sci U S A. 1997;94:757. doi: 10.1073/pnas.94.2.757. PubMed PMID: 9012858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Carmody IC, Meng L, Shen XD, et al. P-selectin knockout mice have improved outcomes with both warm ischemia and small bowel transplantation. Transplant Proc. 2004;36:263. doi: 10.1016/j.transproceed.2003.12.014. PubMed PMID: 15050128. [DOI] [PubMed] [Google Scholar]
- 56.Coates PT, Thomson AW. Dendritic cells, tolerance induction and transplant outcome. Am J Transplant. 2002;2:299. doi: 10.1034/j.1600-6143.2002.20403.x. PubMed PMID: 12118850. [DOI] [PubMed] [Google Scholar]
- 57.Hattori T, Saban DR, Emami-Naeini P, et al. Donor-derived, tolerogenic dendritic cells suppress immune rejection in the indirect allosensitization-dominant setting of corneal transplantation. J Leukoc Biol. 2012;91:621. doi: 10.1189/jlb.1011500. PubMed PMID: 22291211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sato K, Eizumi K, Fukaya T, et al. Naturally occurring regulatory dendritic cells regulate murine cutaneous chronic graft-versus-host disease. Blood. 2009;113:4780. doi: 10.1182/blood-2008-10-183145. PubMed PMID: 19228924. [DOI] [PubMed] [Google Scholar]
- 59.Stepkowski SM, Phan T, Zhang H, et al. Immature syngeneic dendritic cells potentiate tolerance to pancreatic islet allografts depleted of donor dendritic cells in microgravity culture condition. Transplantation. 2006;82:1756. doi: 10.1097/01.tp.0000250732.30273.9b. PubMed PMID: 17198272. [DOI] [PubMed] [Google Scholar]
- 60.Garrod KR, Chang CK, Liu FC, Brennan TV, Foster RD, Kang SM. Targeted lymphoid homing of dendritic cells is required for prolongation of allograft survival. J Immunol. 2006;177:863. doi: 10.4049/jimmunol.177.2.863. PubMed PMID: 16818740. [DOI] [PubMed] [Google Scholar]
- 61.Urzainqui A, Martínez del Hoyo G, Lamana A, et al. Functional role of P-selectin glycoprotein ligand 1/P-selectin interaction in the generation of tolerogenic dendritic cells. J Immunol. 2007;179:7457. doi: 10.4049/jimmunol.179.11.7457. PubMed PMID: 18025190. [DOI] [PubMed] [Google Scholar]


