Abstract
Autosomal dominant polycystic kidney disease (ADPKD) is the most common monogenic cause of end-stage renal disease. The molecular pathogenesis of ADPKD is not completely known and there is no approved therapy. To date, there is limited knowledge concerning the molecular consequences of specific disease-causing mutations. Here we show that the ADPKD missense variant TRPP2D511V greatly reduces TRPP2 protein stability, and that TRPP2D511V function can be rescued in vivo by small molecules targeting the TRPP2 degradation pathway. Expression of the TRPP2D511V protein was significantly reduced compared to wildtype TRPP2. Inhibition of lysosomal degradation of TRPP2D511V by the FDA-approved drug chloroquine strongly increased TRPP2 protein levels in vitro. The validation of these results in vivo requires appropriate animal models. However, there are currently no mouse models harboring human PKD2 missense mutations, and screening for chemical rescue of patient mutations in rodent models is time-consuming and expensive. Therefore, we developed a Drosophila melanogaster model expressing the ortholog of TRPP2D511V to test chemical rescue of mutant TRPP2 in vivo. Notably, chloroquine was sufficient to improve the phenotype of flies expressing mutant TRPP2. Thus, this proof-of-concept study highlights the potential of directed therapeutic approaches for ADPKD, and provides a rapid throughput experimental model to screen PKD2 patient mutations and small molecules in vivo.
Keywords: polycystic kidney disease, PKD2, lysosome, chloroquine
Introduction
Autosomal dominant polycystic kidney disease (ADPKD) is the most common fatal monogenic disease in humans and a leading cause of end-stage renal disease. The five-year probability of survival among ADPKD patients with end-stage renal disease is ≤40%.1 There is no approved therapy to cure cystic disease and current treatments include dialysis and transplantation, which are burdensome and costly. Mutations in Polycystic Kidney Disease 2 (PKD2) account for approximately one out of six ADPKD cases, i.e. ~2,000,000 cases worldwide. The PKD2 gene product, Transient Receptor Potential Polycystin-2 (TRPP2), is a non-selective cation channel.2 Several pathogenic missense mutations that impair TRPP2 ion channel function have been reported.3, 4 The confined impact of a single amino acid change makes missense mutations attractive candidates for corrective approaches using small molecules. A successful example of this strategy is the development of a therapeutic intervention for cystic fibrosis (CF), which targets a specific causal mutation rather than disease symptoms.5 Two classes of drugs have been developed for CF: 1) correctors, which target cellular misprocessing of CF Transmembrane conductance Regulator (CFTR), the protein mutated in CF; and 2) potentiators, which restore CFTR ion channel activity at the cell surface.6 The approval of ivacaftor by the U.S. Food and Drug Administration (FDA) for the treatment of patients carrying the rare CFTR variant G551D highlights the feasibility of personalized therapies for inherited channelopathies such as cystic fibrosis and polycystic kidney disease.5 Furthermore, the clinical use of ivacaftor is continuously expanding to larger pools of patients based on common pathogenic mechanisms between G551D and other CFTR missense mutations.7 On the basis of this paradigm, we hypothesized that the analysis of allele-specific pathogenic mechanisms might reveal promising therapeutic targets for ADPKD. Here we show that the ADPKD patient missense mutation TRPP2D511V greatly reduces cellular TRPP2 protein stability and that TRPP2D511V function can be partially rescued in vivo by a FDA-approved drug targeting the default TRPP2 degradation pathway.
Results
A PKD2 missense mutation reduces TRPP2 protein abundance
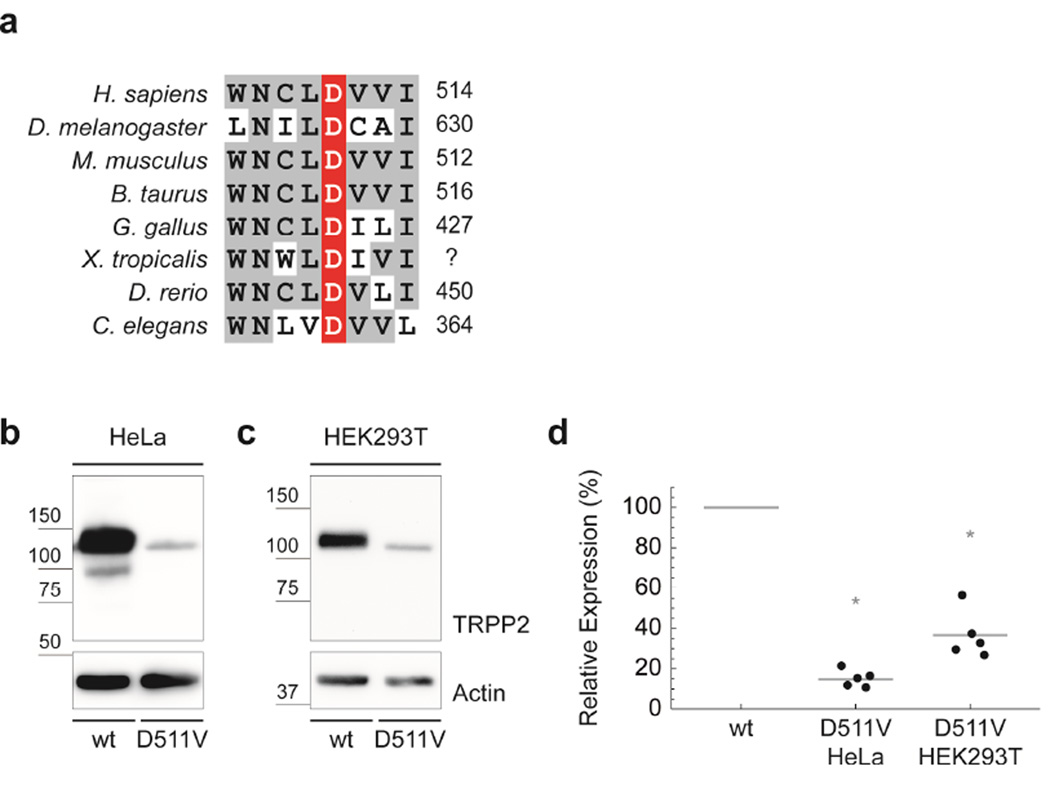
Aspartate 511 of TRPP2 is highly conserved throughout evolution (Fig. 1a). Nonsynonymous substitution of this aspartate with valine has been reported to eliminate TRPP2 ion channel function in vitro.4, 8 We have recently shown that this substition reduces protein abundance and ciliary localization of TRPP2 in D. melanogaster suggesting a pathogenic mechanism involving impaired protein processing in addition to defective channel gating in vivo.8, 9 To test whether protein expression of human TRPP2D511V protein is also reduced and to characterize the molecular impact of the D511V substitution in more detail, we expressed wild-type and mutant protein in different cell lines. TRPP2D511V showed a significant reduction in protein levels by 85% compared to wild-type TRPP2 in HeLa cells 48h after transfection (Fig. 1b, d). This effect was not cell-type-specific, because a similar reduction was observed in HEK293T cells (−63%) (Fig. 1c, d). To ensure that this decrease was not caused by slower TRPP2 protein processing kinetics, we measured TRPP2 levels in HeLa cells 96h after transfection and found a similar reduction of mutant TRPP2 compared to wild-type (mean=−90.6%; n=4; p=0.008). Conceptually, either transcriptional down-regulation or impaired protein processing might account for these significantly reduced TRPP2D511V protein levels. However, PKD2 mRNA transcription or stability was not impaired by the D511V mutation suggesting that TRPP2D511V reduces TRPP2 protein levels by impairing protein processing (Fig. 2a).
FIGURE 1. The ADPKD missense variant TRPP2D511V reduces TRPP2 protein abundance.
a) The human TRPP2D511V mutation is localized in the highly conserved third transmembrane segment (TRPP2506–527) of TRPP2. b) Western blot analysis of wild-type TRPP2 shows two distinct bands.19 Compared to wild-type, TRPP2D511V shows reduced protein levels in HeLa cells. c) This effect is not cell-type-specific, because a similar reduction is observed in HEK293T cells. d) Group data from b and c show a significant reduction of TRPP2D511V protein levels by 85% compared to wild-type TRPP2 in HeLa cells (n=5; p=2·10−6), and by 63% in HEK293T cells (n=5; p=3·10−4).
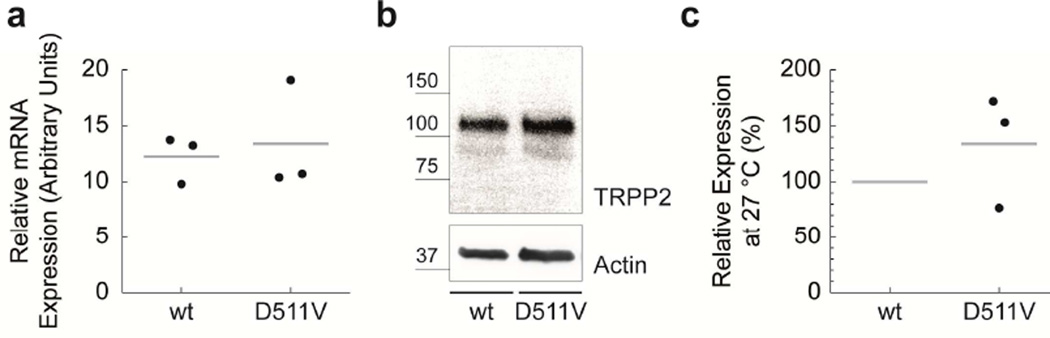
FIGURE 2. TRPP2D511V is temperature-sensitive.
a) mRNA of transiently transfected HeLa cells was isolated. TRPP2 wild-type and TRPP2D511V mRNA abundance is similar as assessed by qPCR. b) Comparison of TRPP2 wild-type and TRPP2D511V expression at 27°C in HeLa cells by Western blot. c) Group data from b.
TRPP2D511V is degraded in lysosomes
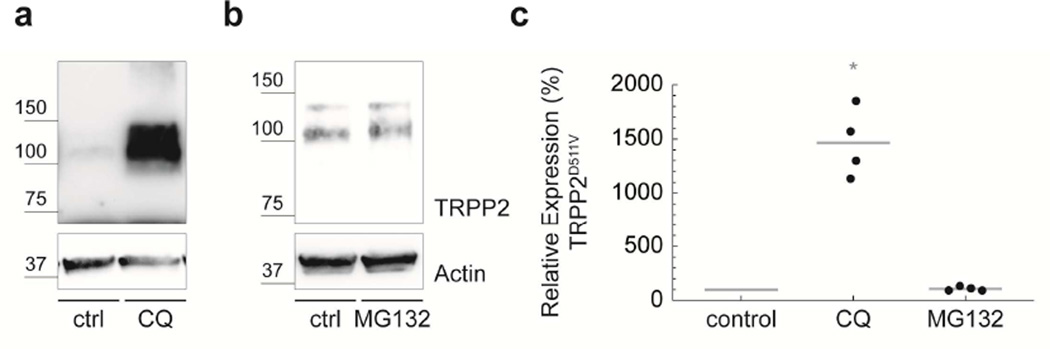
Nascent polypeptides, including TRPP2, transit between a significant number of intermediate folding states before attaining their native state.10 The fidelity of this process can be compromised by amino acid substitutions.10 Cell cultivation at reduced temperatures (25°C–35°C) can rescue the thermodynamic instability of m utant proteins.11 Thermal stress triggers the expression of molecular chaperones promoting protein synthesis, folding and trafficking.11 We found that TRPP2D511V is temperature-sensitive, because incubating cells at 27°C equalized TRPP2 protein levels in wild-type and TRPP2D511V, akin to temperature-sensitive CFTR mutations (Fig. 2b, c). To investigate the mechanism of TRPP2 protein degradation in more detail, we used small molecule inhibitors to target the two major cellular proteolytic pathways: lysosome- and proteasome-mediated degradation. Inhibition of lysosomal function with chloroquine (CQ) significantly increased TRPP2D511V protein levels (Fig. 3). In contrast, the proteasomal inhibitor, MG-132, had no effect on TRPP2D511V, although it induced a significant accumulation of ubiquitin-conjugated proteins (mean=162%; n=6; p=0.002). Taken together, these data suggest that the D511V mutation compromises TRPP2 folding thereby promoting lysosomal degradation.
FIGURE 3. TRPP2D511V is degraded in lysosomes.
a) Western blot analysis shows that TRPP2D511V is more abundant in cells treated with 200 µM chloroquine (CQ) compared with control cells. b) MG-132 (210 µM) does not affect TRPP2D511V protein levels. c) Group data from a and b show that chloroquine significantly increases abundance of TRPP2D511V (mean=1,461%; n=4; p=0.003), whereas MG132 shows no effect (mean=108%; n=4).
Chemical rescue of lysosomal degradation
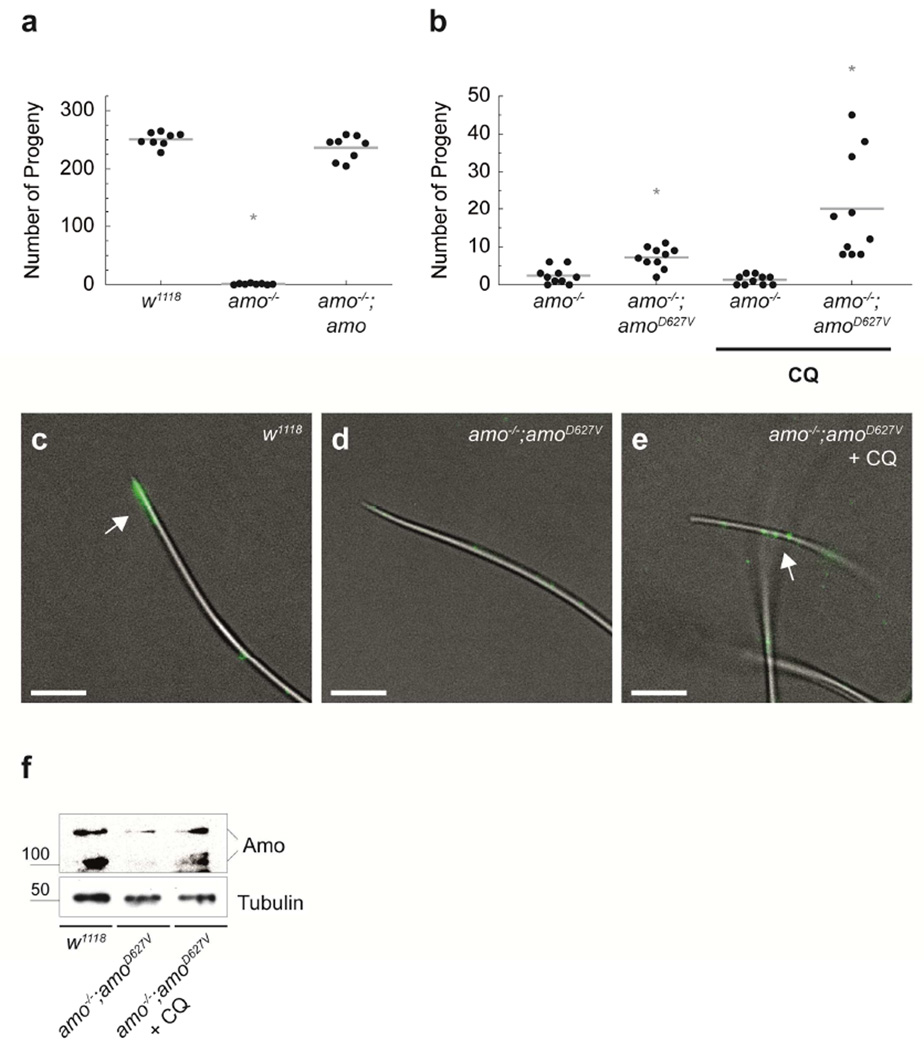
The chemical rescue of TRPP2D511V protein degradation by the FDA-approved drug chloroquine suggested that it might be possible to restore mutant TRPP2 function. Since there is no robust cellular assay of TRPP2 function,12, 13 we turned to a genetically tractable model organism where TRPP2 mutation yields a robust and quantitative phenotype. We have previously shown that ciliary TRPP2 (Amo) is required for male fertility in D. melanogaster.8, 14 In ADPKD patients, sporadic defects in male fertility have been described.15 In contrast to homozygous mutant flies, the underlying mechanism in humans is not understood, as patients are heterozygous for disease causing ADPKD mutations.15–17 Amo-deficient D. melanogaster males produce motile sperm that are transferred to the uterus but do not reach the female sperm storage organs, which is critical for reproductive success. We engineered flies carrying the TRPP2D511V patient mutation to test our hypothesis in vivo. In D. melanogaster, AmoD627V (equivalent to human TRPP2D511V) is a loss of function variant without dominant-negative effects.8 The male infertility phenotype in amo−/− flies can be completely rescued by the expression of transgenic Amo (TRPP2) (Fig. 4a).8, 14 In contrast, AmoD627V failed to restore normal numbers of progeny (Fig. 4a, b), but was able to slightly increase the fertility of amo−/− flies, suggesting that this protein might have some residual function. Notably, feeding of chloroquine (3mM) to male amo−/−;amoD627V flies significantly increased fertility by a factor of three (Fig. 4b). On the molecular level this correlated with an increase of flagellar AmoD627V protein expression in sperm (Fig. 4c–f).
FIGURE 4. Chloroquine rescues mutant D. melanogaster TRPP2 (Amo) function in vivo.
a) Wild-type (w1118) flies have a large number of progeny (mean=251; n=8), while amo mutant males (amo−/−) are infertile (mean number of progeny=2.4; n=8; p=9·10−11). A third chromosomal amo transgene in the amo−/− mutant background can rescue the fertility phenotype (amo−/−;amo) (mean number of progeny=236; n=8). b) Introduction of an amoD627V transgene on the third chromosome fails to rescue male infertility of amo−/− males. Feeding of 3 mM chloroquine to amo−/−;amoD627V males, however, significantly increases fertility compared to untreated flies of the same genotype (mean number of progeny=20; n=10; p=0.007). Lower concentrations of chloroquine (0.2 mM) showed no effect, whereas dietary chloroquine at or above 10 mM caused significant toxicity. Please note that the amoD627V transgene causes a small but significant increase in male fertility compared to amo−/− males suggesting residual function of the amoD627V variant (mean number of progeny: 2.4 versus 7.2, respectively; n=10; p=0.002). In contrast, amo−/− males fed chloroquine have progeny similar to untreated controls (mean number of progeny=1.3; n=10). c)–e) Localization of Amo in mature sperm in wild-type (w1118), amo−/−;amoD627V, and chloroquine-treated amo−/−;amoD627V flies (anti-Amo: green; scale bar 5 µm; n=8). Chloroquine treatment results in an increase of flagellar Amo expression, without enrichment at the tip of the sperm tail. Previous studies have shown that Amo localization in amo−/−;amo flies is similar to wild-type.8 f) Western blot of Amo protein from male flies of the indicated genotypes. Amo was immuno-precipitated from wild-type (w1118), amo−/−;amoD627V, and chloroquine-treated flies (3 mM). Amo expression was reduced in amo−/−;amoD627V flies compared to wild-type (w1118)(−76.1%; standard deviation = 12.8; n=2). Chloroquine-treatment of amo−/−;amoD627V flies increased Amo protein levels (mean=+40.3%; standard deviation=16.7; n=2). Equal numbers of flies were used for all experimental conditions. Tubulin served as a loading control.
Discussion
Loss-of-function mutations in TRPP2 result in autosomal dominant polycystic kidney disease (ADPKD).2 The precise molecular events of how TRPP2 mutations cause renal cyst formation are still unclear. Currently there are no therapies targeting the primary defect of ADPKD. For Polycystin-1-dependent ADPKD, however, proteasome inhibitor therapy has been proposed as an effective therapeutic approach.18 We have previously shown TRPP2 is degraded in lysosomes.19 Here we show that nonsynonymous substitution of aspartate 511 with valine results in lysosomal degradation of TRPP2. Lysosomal inhibition is sufficient to elevate the level of mutant TRPP2 protein and results in a partial rescue of TRPP2 function in vivo.
Our study was inspired by the chemical rescue of specific CFTR missense mutations causing cystic fibrosis. The recent FDA approval of ivacaftor for the treatment of patients carrying the rare CFTR variant G551D highlights the feasibility of personalized therapies for inherited channelopathies such as cystic fibrosis and polycystic kidney disease.5 The efficacy of Ivacaftor suggests that it might be worth exploring similarly targeted corrective therapies for ADPKD.
The ivacaftor success story is remarkable, because human clinical trials were initiated based solely on efficacy studies in cell culture since rodent models harboring pathogenic human mutations weren’t available.7 This was feasible because CFTR channel function in the plasma membrane can be reproducibly measured in heterologous expression systems. Unfortunately, this approach cannot be adapted to the study of small molecule rescue of mutant TRPP2 channels, since there is no robust assay for TRPP2 channel function in heterologous expression systems. While some investigators have reported electrophysiological measurements of TRPP2 channel function, many groups including ourselves have failed to measure TRPP2 whole cell currents in the plasma membrane of cells overexpressing the wild-type channel.4, 13, 20–22 Furthermore, it has not been possible to measure TRPP2 ion channel activity in vertebrate cilia or in D. melanogaster sperm to date.13
To overcome the notorious problem of investigating TRPP2 channel function in heterologous expression systems, we used D. melanogaster as a read-out for TRPP2 channel function in vivo. Cilia are thought to be the critical site of action for TRPP2 channels.16 The ciliary localization and function of TRPP2 is evolutionarily conserved. The function of TRPP2 patient mutations can be studied in a quantitative fashion using the robust fertility defect of TRPP2 mutant flies.8, 14 Our results using chloroquine to rescue male infertility show a small but significant rescue of the male infertility in flies expressing mutant TRPP2. The most likely explanation for the incomplete rescue of chloroquine-treated flies is a gating defect of mutant TRPP2 as has been previously reported.4 It is also possible however, that the smaller relative increase of mutant TRPP2 protein levels in D. melanogaster as compared to cell lines is not sufficient for a complete rescue of the phenotype. Since we cannot measure TRPP2 ion channel activity in vertebrate cilia or in fly sperm,13 we cannot completely exclude the possibility that chloroquine has a salutatory effect on the channel function of mutant TRPP2. However, since this agent increases the expression of TRPP2D511V in cultured cells and AmoD627V in flies, the most parsimonious explanation is that chemical rescue with chloroquine acts primarily by increasing the level of a mutant protein with some residual channel activity. More efficient rescue of mutant TRPP2 function will therefore probably require a combination of corrective drugs such as chloroquine, acting to increase protein abundance, and potentiating drugs similar to ivacaftor,5 which increase ion channel activity.
In summary, our data show that targeted corrective approaches might be feasible in ADPKD. This proof-of-concept study shows that the function of a specific TRPP2 patient missense mutation can be improved in vivo. Non-truncating missense mutations in the PKD2 gene are found in ~20% of PKD2 patients.17 Since other TRPP2 missense mutants are processed similarly,19 we propose that targeting general mechanisms of TRPP2 turnover may have a broader application. The success story of ivacaftor in the treatment of cystic fibrosis demonstrates that an agent which was initially conceived to treat patients with a rare CFTR missense mutation can be used in a broader subset of patient harboring other amino acid substitutions.7 Further development of corrective therapeutic approaches for ADPKD will require high-throughput screening of chemical libraries and characterization of additional patient mutations. To our knowledge, there are currently no mouse models harboring specific human PKD2 mutations and screening for chemical rescue of patient mutations in rodent models is time-consuming and expensive. Alternatively, a two-stage hierarchical screening strategy consisting of in vitro analysis of protein processing followed by in vivo modeling of specific patient mutations in D. melanogaster considerably increases efficiency over testing compounds a priori in mouse models. This platform may help to identify allele-specific pathogenic mechanisms and corrective lead compounds for targeted studies in more complex mouse knock-in models with cystic kidney disease.
Methods
Molecular biology
Full-length human PKD2 (GenBank: U50928) in pcDNA3 (Invitrogen) was kindly provided by Feng Qian (University of Maryland).22 Using this wild-type plasmid TRPP2D511V was generated by site-directed mutagenesis.
Antibodies
Anti-TRPP2 (G-20) antibodies were obtained from Santa Cruz Biotechnology. A monoclonal anti-TRPP2 antibody against amino acid residues 698–799 (anti-TRPP2698–799) was kindly provided by Gerd Walz (Medical Center - University of Freiburg). Clone AC-15 anti-beta-Actin was purchased from Sigma-Aldrich. The monoclonal anti-alpha-Tubulin (AA4.3) antibody developed by C. Walsh was obtained from the Developmental Studies Hybridoma Bank (NICHD). Clone P4D1 anti-ubiquitin antibodies were purchased from Covance. The rabbit anti-Amo antiserum has been described.14 Western blot detection was performed using either an anti-mouse (Dako) or anti-rabbit (GE Healthcare) horseradish peroxidase-coupled secondary antibody. For antigen visualization in immunofluorescence experiments secondary donkey anti-rabbit Alexa TM 488 (Invitrogen) antibodies were used.
Cell culture
HeLa and HEK293T cells were cultivated as adherent monolayers in DMEM media (Lonza) supplemented with 10% heat-inactivated fetal bovine serum (FBS) (Biochrom). Cell lines were maintained in a humidified 10% CO2 incubator at 36.5 °C. Cells were passaged every 3–4 days, using 0.05% Trypsin-EDTA (Gibco). HeLa and HEK293T cells were transfected using Lipofectamine 2000 (Invitrogen) and calcium phosphate, respectively.
Protein isolation
Transiently transfected cells were harvested 24 h after transfection, if not otherwise stated. Samples were lysed in ice-cold IP buffer (1% Triton X-100, 20 mM Tris-HCl at pH 7.5, 50 mM NaCl, 50 mM NaF, 15 mM Na4P2O7, 0.1 mM EDTA at pH 8) supplemented with 2 mM Na3VO4 and Complete Protease Inhibitor Cocktail (Roche). Lysates were first centrifuged at 4 °C for 15 min at 20,000 g and subsequently subjected to ultracentrifugation at 4 °C for 30 min at 100,000 g. Supernatants were denatured 30 min at 42 °C with 2 × Lämmli sample buffer and 100 mM DTT.
SDS-PAGE, Western blot and CCD camera-based ECL detection
Protein samples (~ 20 µg of total protein for cell lysates) were separated by SDS–PAGE using Mini-PROTEAN TGX 4–15% precast gels (Bio-Rad). Proteins were Western blotted semi-dry on Immobilon-P PVDF (Millipore). Membranes were blocked with 5% bovine serum albumin (BSA) (Serva) and probed with antibodies diluted in PBS (Biochrom): anti-TRPP2698–799 1 / 400; anti-beta-Actin 1 / 5000; anti-ubiquitin 1 / 1000; anti-alpha-Tubulin 1 / 80; or anti-Amo 1 / 1000. Immobilized antigens conjugated to horseradish-peroxidase labeled antibodies were detected using SuperSignal West Pico chemiluminescent substrate (Thermo Scientific). A 16-bit ChemoCam (Intas) was used for light-detection; images were taken within the dynamic range of the charge-coupled device (CCD) sensor. None of the depicted bands is saturated.
RNA isolation, reverse transcription polymerase chain reaction (RT-PCR) and real time quantitative PCR (qPCR)
RNA of transiently transfected HeLa cells was isolated (RNeasy Plus Mini Kit, Qiagen) and retrotranscribed to complementary DNA (Quantitect, Qiagen) according to the manufacturers protocols. Subsequently, qPCR (LightCycler 480 SYBR Green I Master, Roche) was performed using the following oligonucleotides: 1) human PKD2, 5'-AGCGGACATAGCTCCAGAAGGAG and 5'-GATGGAATGCTCCATCCGGTC; and 2) human HSPCB, 5'-TCTGGGTATCGGAAAGCAAGCC and 5'-GTGCACTTCCTCAGGCATCTTG. Samples were analyzed on a LightCycler 480 (Roche) and relative quantification was calculated: R = 2−(CP PKD2 − CP HSPCB).23
Temperature assay and small molecule inhibitors
Protein stability at low temperature was assessed after transfected HeLa cells were cultivated for 2 days at 27 °C (humidified 10% CO2 incubator). Drug incubation of HeLa cells was started 24 h after transfection. Small molecule inhibitors were added to DMEM (Lonza) supplemented with 10% heat-inactivated FBS (Biochrom); cells were maintained in a humidified 10% CO2 incubator at 36.5 °C. Drug concentrations and incubation time s: 200 µM chloroquine (CQ; Sigma Aldrich) for 24 h; and 210 µM MG-132 (Enzo Life Sciences) for 6 h. Cells were counted after harvesting using a TC10 automated cell counter (Bio-Rad). Sample volume was normalized for cell count.
Flies, husbandry and drug treatments
All flies were reared according to standard procedures and maintained at 25 °C. Amo mutant flies (amo, amo−/−;amo, and amo−/−;amoD627V) have been described.8, 14 Briefly, transgenic flies expressing wild-type and mutant Amo (D627V) were generated using site-specific recombination with an attP landing site on the third chromosome.8 w1118 flies served as wild-type controls. Chloroquine (0.2 mM or 3 mM) was added to Nutri-Fly Instant White (Genesee Scientific). Using these media, flies were cultured at 25 °C and male offspring was collected for fertility tests, immunofluorescence or protein biochemistry.
Fertility assays
D. melanogaster males of various genotypes were separated upon eclosion and maintained in isolation two days prior to mating. Double pair matings with w1118 virgin females were performed for five days. At that time both parents were removed from the vial. The number of progeny that eclosed from each vial was counted.
Immunofluorescence of D. melanogaster sperm
Dissection and preparation of testis and spermatozoa as well as the anti-Amo antiserum have been described.14 Microscopy images were recorded using a Zeiss Axio Observer microscope (Zeiss).
Protein isolation of D. melanogaster
For each experiment 37 freshly eclosed w1118, amo−/−;amoD627V, and chloroquine-treated (3 mM) amo−/−;amoD627V male flies were collected. Whole flies were lysed and Amo protein precipitated using the Pierce Co-Immunoprecipitation Kit with 10 µl anti-Amo antiserum14 according to the manufacturers protocol. Samples were analyzed by SDS-PAGE and Western blotting as described above. Membranes were probed with anti-Amo antiserum14 diluted 1 / 400 in PBS (Biochrom). Super RX film (Fujifilm) was used for chemiluminescence-detection. Images were developed using a AGFA Curix 40 processor. Amo protein is expressed at rather low levels and was not detectable in lysates. Detection of Amo on Western blots required enrichment of the protein using immunoprecipitation.
Statistical analysis
For data quantitation at least three independent experiments were evaluated. Samples were normalized for beta-Actin Western blot signals. Relative expression was calculated for samples on the same gel. Western blot signal intensities were measured using ImageJ. Student's t-test was performed to assess statistical significance. D. melanogaster fertility tests were evaluated using the Mann-Whitney U test. An asterisk indicates p ≤ 0.05.
Acknowledgments
We would like to thank Feng Qian for providing plasmids through the Baltimore Polycystic Kidney Research and Clinical Core Center (P30 DK090868), Gerd Walz for TRPP2 antibodies, Sebastian Arnold, Anna Köttgen and Wolfgang Kühn for discussions, as well as Simone Diederichsen, Julia Merkel and Susanne Helmstädter for expert technical assistance. Funding was awarded by the Deutsche Forschungsgemeinschaft (DFG KFO 201, CRC 152 and CRC 1140, M.K.), by the Alfried Krupp von Bohlen und Halbach Foundation (M.K.), by the Excellence Initiative of the German Federal and State Governments (GSC-4, Spemann Graduate School), and by the NIH (R01 GM073704; TW).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure
The authors declare that they have no conflict of interest.
Author Contributions
A.H. and C.W. performed the experiments. T.W. made intellectual contributions to experimental design and discussion. A.H. & M.K. designed the study and wrote the manuscript.
References
- 1.Diseases NIoDaDaK, National Institutes of Health, editor. System USRD: USRDS 2012 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. 2012 [Google Scholar]
- 2.Mochizuki T, Wu G, Hayashi T, et al. PKD2, a gene for polycystic kidney disease that encodes an integral membrane protein. Science. 1996;272:1339–1342. doi: 10.1126/science.272.5266.1339. [DOI] [PubMed] [Google Scholar]
- 3.Reynolds DM, Hayashi T, Cai Y, et al. Aberrant splicing in the PKD2 gene as a cause of polycystic kidney disease. Journal of the American Society of Nephrology : JASN. 1999;10:2342–2351. doi: 10.1681/ASN.V10112342. [DOI] [PubMed] [Google Scholar]
- 4.Koulen P, Cai Y, Geng L, et al. Polycystin-2 is an intracellular calcium release channel. Nat Cell Biol. 2002;4:191–197. doi: 10.1038/ncb754. [DOI] [PubMed] [Google Scholar]
- 5.Ramsey BW, Davies J, McElvaney NG, et al. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. The New England journal of medicine. 2011;365:1663–1672. doi: 10.1056/NEJMoa1105185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rowntree RK, Harris A. The phenotypic consequences of CFTR mutations. Annals of human genetics. 2003;67:471–485. doi: 10.1046/j.1469-1809.2003.00028.x. [DOI] [PubMed] [Google Scholar]
- 7.Davis PB, Yasothan U, Kirkpatrick P. Ivacaftor. Nature reviews Drug discovery. 2012;11:349–350. doi: 10.1038/nrd3723. [DOI] [PubMed] [Google Scholar]
- 8.Kottgen M, Hofherr A, Li W, et al. Drosophila sperm swim backwards in the female reproductive tract and are activated via TRPP2 ion channels. PLoS One. 2011;6:e20031. doi: 10.1371/journal.pone.0020031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma R, Li WP, Rundle D, et al. PKD2 functions as an epidermal growth factor-activated plasma membrane channel. Molecular and cellular biology. 2005;25:8285–8298. doi: 10.1128/MCB.25.18.8285-8298.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brodsky JL. Cleaning up: ER-associated degradation to the rescue. Cell. 2012;151:1163–1167. doi: 10.1016/j.cell.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sonna LA, Fujita J, Gaffin SL, et al. Invited review: Effects of heat and cold stress on mammalian gene expression. Journal of applied physiology. 2002;92:1725–1742. doi: 10.1152/japplphysiol.01143.2001. [DOI] [PubMed] [Google Scholar]
- 12.Ma M, Tian X, Igarashi P, et al. Loss of cilia suppresses cyst growth in genetic models of autosomal dominant polycystic kidney disease. Nat Genet. 2013;45:1004–1012. doi: 10.1038/ng.2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeCaen PG, Delling M, Vien TN, et al. Direct recording and molecular identification of the calcium channel of primary cilia. Nature. 2013;504:315–318. doi: 10.1038/nature12832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Watnick TJ, Jin Y, Matunis E, et al. A flagellar polycystin-2 homolog required for male fertility in Drosophila. Curr Biol. 2003;13:2179–2184. doi: 10.1016/j.cub.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 15.Torra R, Sarquella J, Calabia J, et al. Prevalence of cysts in seminal tract and abnormal semen parameters in patients with autosomal dominant polycystic kidney disease. Clinical journal of the American Society of Nephrology : CJASN. 2008;3:790–793. doi: 10.2215/CJN.05311107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Semmo M, Kottgen M, Hofherr A. The TRPP Subfamily and Polycystin-1 Proteins. Handbook of experimental pharmacology. 2014;222:675–711. doi: 10.1007/978-3-642-54215-2_27. [DOI] [PubMed] [Google Scholar]
- 17.Harris PC, Hopp K. The mutation, a key determinant of phenotype in ADPKD. Journal of the American Society of Nephrology : JASN. 2013;24:868–870. doi: 10.1681/ASN.2013040417. [DOI] [PubMed] [Google Scholar]
- 18.Fedeles SV, Tian X, Gallagher AR, et al. A genetic interaction network of five genes for human polycystic kidney and liver diseases defines polycystin-1 as the central determinant of cyst formation. Nat Genet. 2011;43:639–647. doi: 10.1038/ng.860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hofherr A, Wagner C, Fedeles S, et al. N-glycosylation determines the abundance of the transient receptor potential channel TRPP2. The Journal of biological chemistry. 2014 doi: 10.1074/jbc.M114.562264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu Y, Ulbrich MH, Li MH, et al. Structural and molecular basis of the assembly of the TRPP2/PKD1 complex. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:11558–11563. doi: 10.1073/pnas.0903684106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kottgen M, Benzing T, Simmen T, et al. Trafficking of TRPP2 by PACS proteins represents a novel mechanism of ion channel regulation. EMBO J. 2005;24:705–716. doi: 10.1038/sj.emboj.7600566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hanaoka K, Qian F, Boletta A, et al. Co-assembly of polycystin-1 and-2 produces unique cation-permeable currents. Nature. 2000;408:990–994. doi: 10.1038/35050128. [DOI] [PubMed] [Google Scholar]
- 23.Jacob F, Guertler R, Naim S, et al. Careful selection of reference genes is required for reliable performance of RT-qPCR in human normal and cancer cell lines. PLoS One. 2013;8:e59180. doi: 10.1371/journal.pone.0059180. [DOI] [PMC free article] [PubMed] [Google Scholar]