Abstract
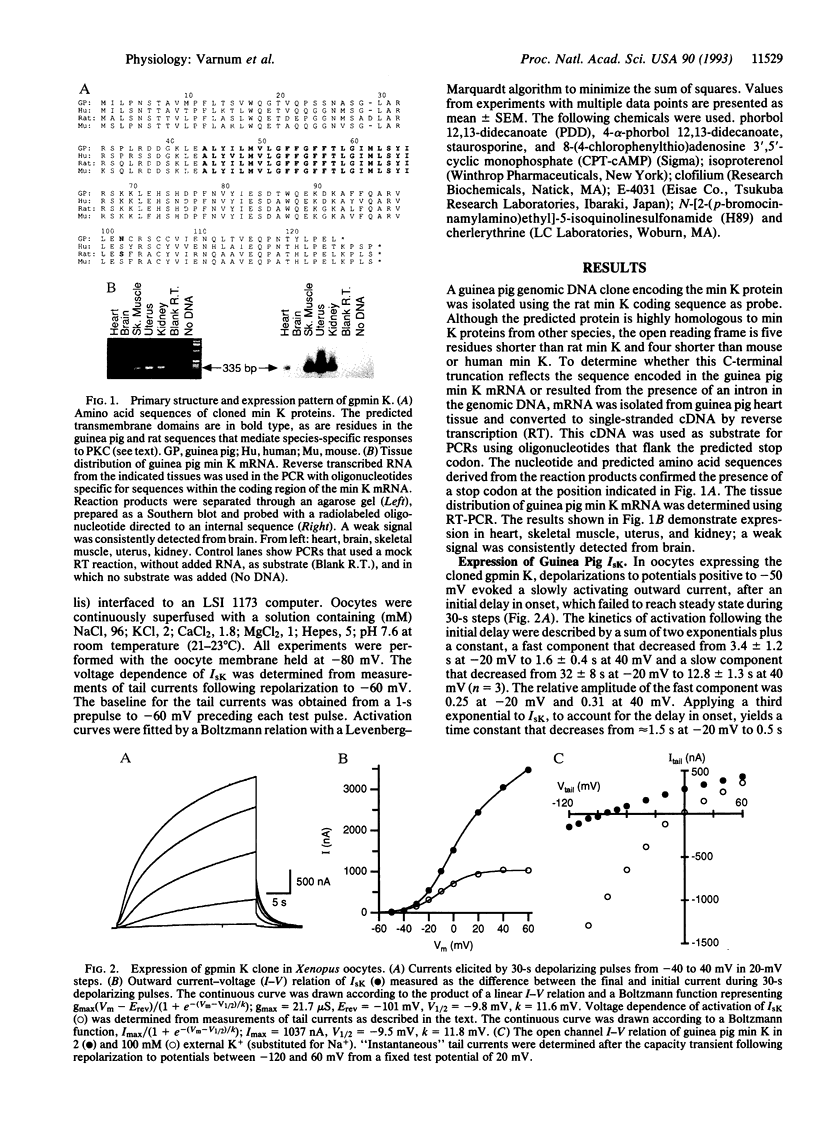
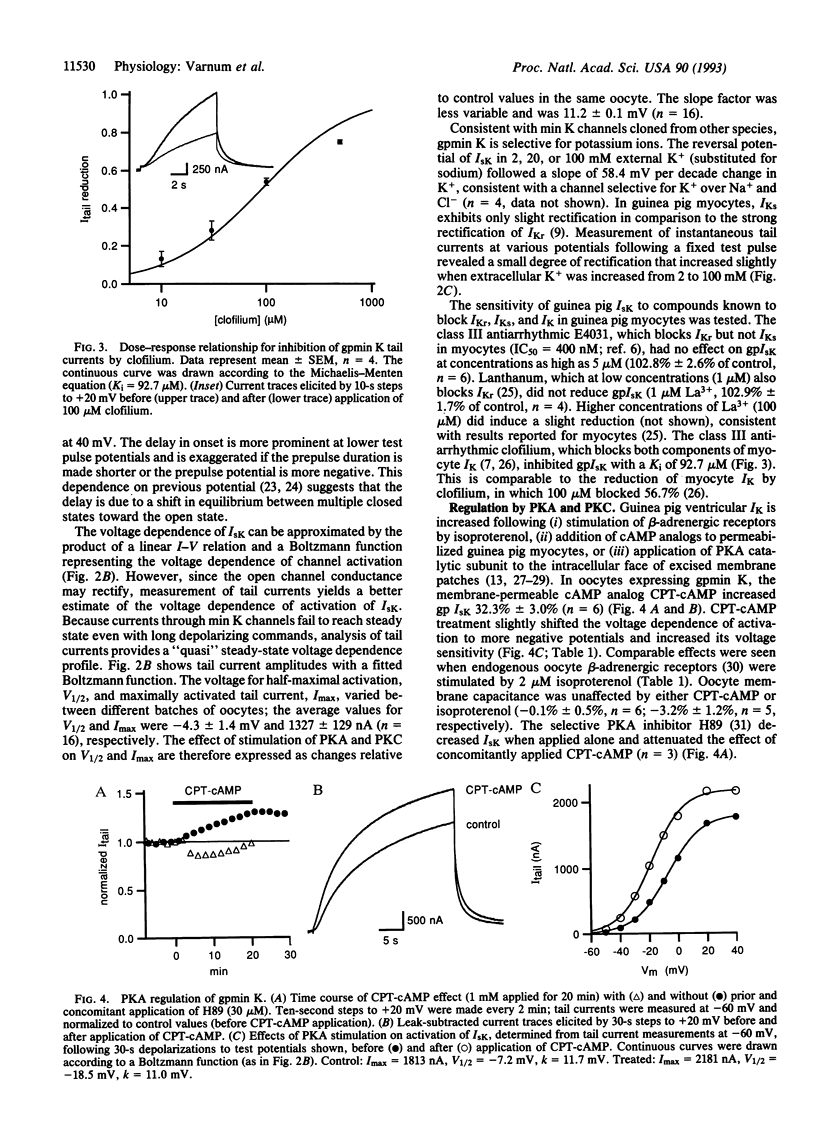
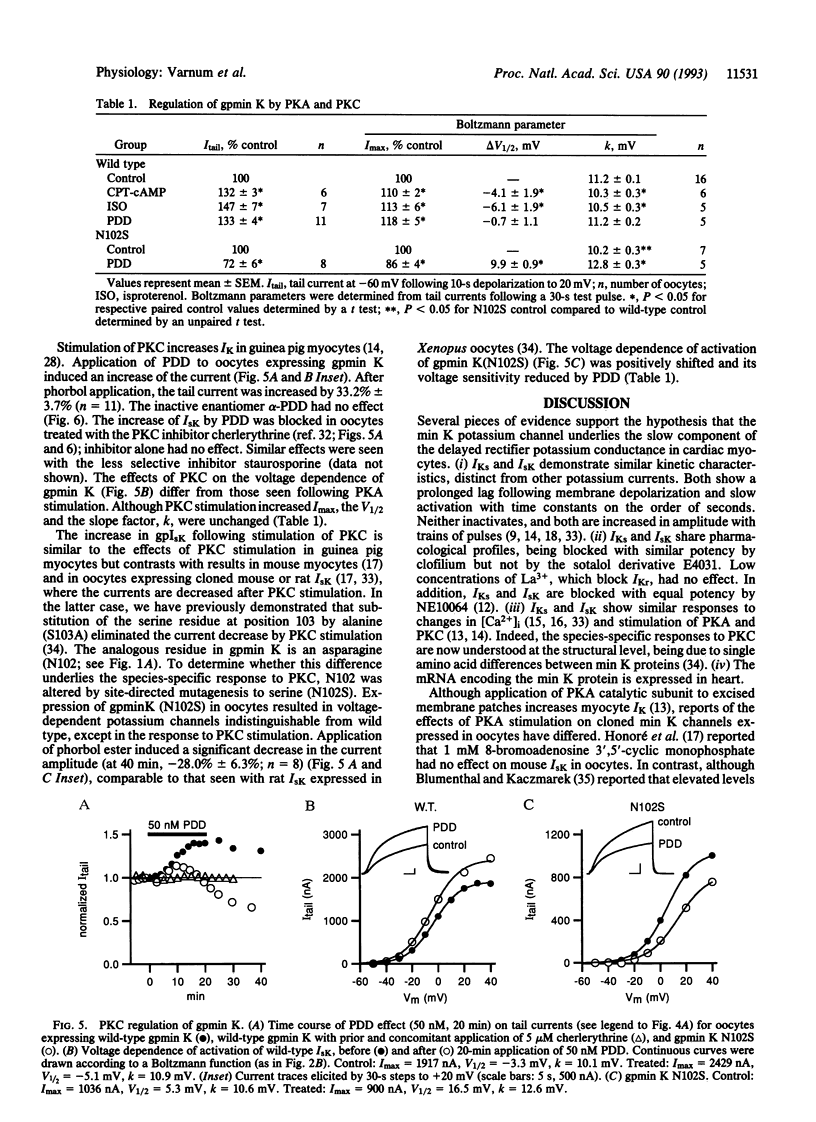
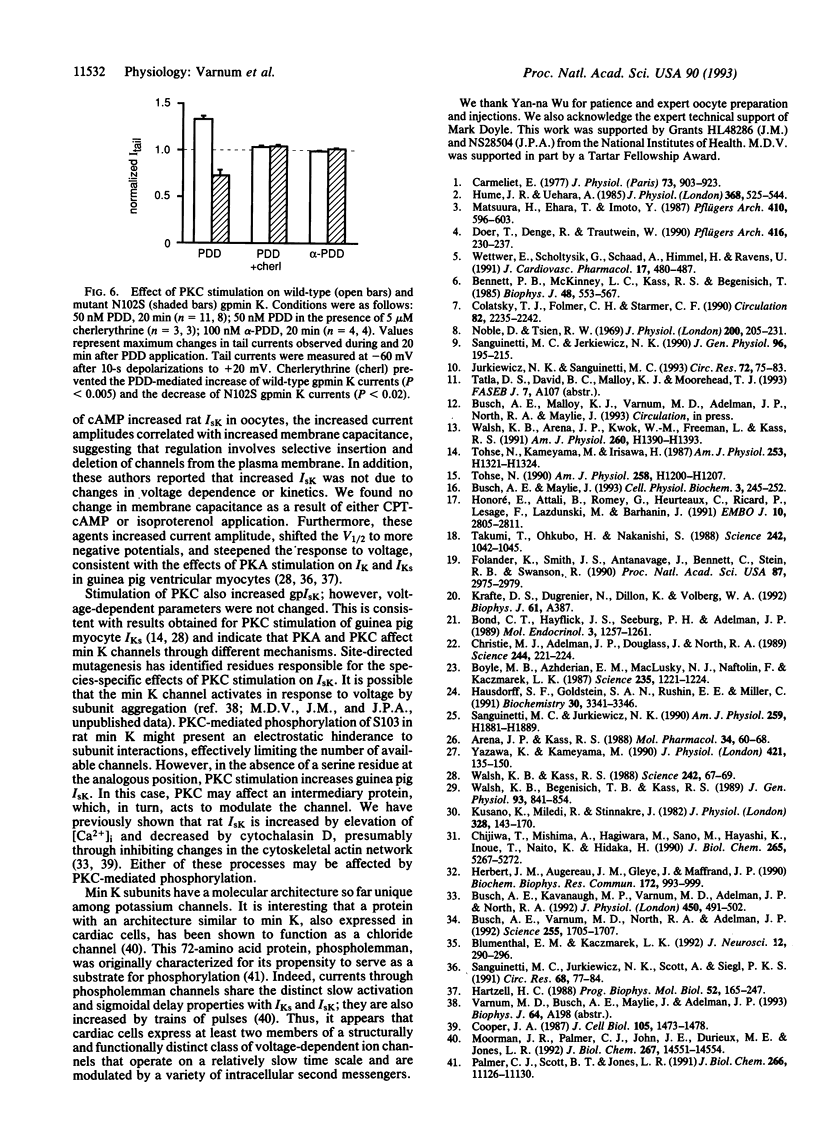
A clone encoding the guinea pig (gp) min K potassium channel was isolated and expressed in Xenopus oocytes. The currents, gpIsK, exhibit many of the electrophysiological and pharmacological properties characteristic of gpIKs, the slow component of the delayed rectifier potassium conductance in guinea pig cardiac myocytes. Depolarizing commands evoke outward potassium currents that activate slowly, with time constants on the order of seconds. The currents are blocked by the class III antiarrhythmic compound clofilium but not by the sotalol derivative E4031 or low concentrations of lanthanum. Like IKs in guinea pig myocytes, gpIsK is modulated by stimulation of protein kinase A and protein kinase C (PKC). In contrast to rat and mouse IsK, which are decreased upon stimulation of PKC, myocyte IK and gpIsK in oocytes are increased after PKC stimulation. Substitution of an asparagine residue at position 102 by serine (N102S), the residue found in the analogous position of the mouse and rat min K proteins, results in decreased gpIsK in response to PKC stimulation. These results support the hypothesis that the min K protein underlies the slow component of the delayed rectifier potassium current in ventricular myocytes and account for the species-specific responses to stimulation of PKC.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett P. B., McKinney L. C., Kass R. S., Begenisich T. Delayed rectification in the calf cardiac Purkinje fiber. Evidence for multiple state kinetics. Biophys J. 1985 Oct;48(4):553–567. doi: 10.1016/S0006-3495(85)83813-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal E. M., Kaczmarek L. K. Modulation by cAMP of a slowly activating potassium channel expressed in Xenopus oocytes. J Neurosci. 1992 Jan;12(1):290–296. doi: 10.1523/JNEUROSCI.12-01-00290.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond C. T., Hayflick J. S., Seeburg P. H., Adelman J. P. The rat gonadotropin-releasing hormone: SH locus: structure and hypothalamic expression. Mol Endocrinol. 1989 Aug;3(8):1257–1262. doi: 10.1210/mend-3-8-1257. [DOI] [PubMed] [Google Scholar]
- Boyle M. B., Azhderian E. M., MacLusky N. J., Naftolin F., Kaczmarek L. K. Xenopus oocytes injected with rat uterine RNA express very slowly activating potassium currents. Science. 1987 Mar 6;235(4793):1221–1224. doi: 10.1126/science.2434999. [DOI] [PubMed] [Google Scholar]
- Busch A. E., Kavanaugh M. P., Varnum M. D., Adelman J. P., North R. A. Regulation by second messengers of the slowly activating, voltage-dependent potassium current expressed in Xenopus oocytes. J Physiol. 1992 May;450:491–502. doi: 10.1113/jphysiol.1992.sp019138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch A. E., Varnum M. D., North R. A., Adelman J. P. An amino acid mutation in a potassium channel that prevents inhibition by protein kinase C. Science. 1992 Mar 27;255(5052):1705–1707. doi: 10.1126/science.1553557. [DOI] [PubMed] [Google Scholar]
- Carmeliet E. Repolarisation and frequency in cardiac cells. J Physiol (Paris) 1977;73(7):903–923. [PubMed] [Google Scholar]
- Chijiwa T., Mishima A., Hagiwara M., Sano M., Hayashi K., Inoue T., Naito K., Toshioka T., Hidaka H. Inhibition of forskolin-induced neurite outgrowth and protein phosphorylation by a newly synthesized selective inhibitor of cyclic AMP-dependent protein kinase, N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide (H-89), of PC12D pheochromocytoma cells. J Biol Chem. 1990 Mar 25;265(9):5267–5272. [PubMed] [Google Scholar]
- Christie M. J., Adelman J. P., Douglass J., North R. A. Expression of a cloned rat brain potassium channel in Xenopus oocytes. Science. 1989 Apr 14;244(4901):221–224. doi: 10.1126/science.2539643. [DOI] [PubMed] [Google Scholar]
- Colatsky T. J., Follmer C. H., Starmer C. F. Channel specificity in antiarrhythmic drug action. Mechanism of potassium channel block and its role in suppressing and aggravating cardiac arrhythmias. Circulation. 1990 Dec;82(6):2235–2242. doi: 10.1161/01.cir.82.6.2235. [DOI] [PubMed] [Google Scholar]
- Cooper J. A. Effects of cytochalasin and phalloidin on actin. J Cell Biol. 1987 Oct;105(4):1473–1478. doi: 10.1083/jcb.105.4.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerr T., Denger R., Doerr A., Trautwein W. Ionic currents contributing to the action potential in single ventricular myocytes of the guinea pig studied with action potential clamp. Pflugers Arch. 1990 May;416(3):230–237. doi: 10.1007/BF00392058. [DOI] [PubMed] [Google Scholar]
- Folander K., Smith J. S., Antanavage J., Bennett C., Stein R. B., Swanson R. Cloning and expression of the delayed-rectifier IsK channel from neonatal rat heart and diethylstilbestrol-primed rat uterus. Proc Natl Acad Sci U S A. 1990 Apr;87(8):2975–2979. doi: 10.1073/pnas.87.8.2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartzell H. C. Regulation of cardiac ion channels by catecholamines, acetylcholine and second messenger systems. Prog Biophys Mol Biol. 1988;52(3):165–247. doi: 10.1016/0079-6107(88)90014-4. [DOI] [PubMed] [Google Scholar]
- Hausdorff S. F., Goldstein S. A., Rushin E. E., Miller C. Functional characterization of a minimal K+ channel expressed from a synthetic gene. Biochemistry. 1991 Apr 2;30(13):3341–3346. doi: 10.1021/bi00227a025. [DOI] [PubMed] [Google Scholar]
- Herbert J. M., Augereau J. M., Gleye J., Maffrand J. P. Chelerythrine is a potent and specific inhibitor of protein kinase C. Biochem Biophys Res Commun. 1990 Nov 15;172(3):993–999. doi: 10.1016/0006-291x(90)91544-3. [DOI] [PubMed] [Google Scholar]
- Honoré E., Attali B., Romey G., Heurteaux C., Ricard P., Lesage F., Lazdunski M., Barhanin J. Cloning, expression, pharmacology and regulation of a delayed rectifier K+ channel in mouse heart. EMBO J. 1991 Oct;10(10):2805–2811. doi: 10.1002/j.1460-2075.1991.tb07829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume J. R., Uehara A. Ionic basis of the different action potential configurations of single guinea-pig atrial and ventricular myocytes. J Physiol. 1985 Nov;368:525–544. doi: 10.1113/jphysiol.1985.sp015874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurkiewicz N. K., Sanguinetti M. C. Rate-dependent prolongation of cardiac action potentials by a methanesulfonanilide class III antiarrhythmic agent. Specific block of rapidly activating delayed rectifier K+ current by dofetilide. Circ Res. 1993 Jan;72(1):75–83. doi: 10.1161/01.res.72.1.75. [DOI] [PubMed] [Google Scholar]
- Kusano K., Miledi R., Stinnakre J. Cholinergic and catecholaminergic receptors in the Xenopus oocyte membrane. J Physiol. 1982 Jul;328:143–170. doi: 10.1113/jphysiol.1982.sp014257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuura H., Ehara T., Imoto Y. An analysis of the delayed outward current in single ventricular cells of the guinea-pig. Pflugers Arch. 1987 Dec;410(6):596–603. doi: 10.1007/BF00581319. [DOI] [PubMed] [Google Scholar]
- Moorman J. R., Palmer C. J., John J. E., 3rd, Durieux M. E., Jones L. R. Phospholemman expression induces a hyperpolarization-activated chloride current in Xenopus oocytes. J Biol Chem. 1992 Jul 25;267(21):14551–14554. [PubMed] [Google Scholar]
- Noble D., Tsien R. W. Outward membrane currents activated in the plateau range of potentials in cardiac Purkinje fibres. J Physiol. 1969 Jan;200(1):205–231. doi: 10.1113/jphysiol.1969.sp008689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer C. J., Scott B. T., Jones L. R. Purification and complete sequence determination of the major plasma membrane substrate for cAMP-dependent protein kinase and protein kinase C in myocardium. J Biol Chem. 1991 Jun 15;266(17):11126–11130. [PubMed] [Google Scholar]
- Sanguinetti M. C., Jurkiewicz N. K. Lanthanum blocks a specific component of IK and screens membrane surface change in cardiac cells. Am J Physiol. 1990 Dec;259(6 Pt 2):H1881–H1889. doi: 10.1152/ajpheart.1990.259.6.H1881. [DOI] [PubMed] [Google Scholar]
- Sanguinetti M. C., Jurkiewicz N. K., Scott A., Siegl P. K. Isoproterenol antagonizes prolongation of refractory period by the class III antiarrhythmic agent E-4031 in guinea pig myocytes. Mechanism of action. Circ Res. 1991 Jan;68(1):77–84. doi: 10.1161/01.res.68.1.77. [DOI] [PubMed] [Google Scholar]
- Sanguinetti M. C., Jurkiewicz N. K. Two components of cardiac delayed rectifier K+ current. Differential sensitivity to block by class III antiarrhythmic agents. J Gen Physiol. 1990 Jul;96(1):195–215. doi: 10.1085/jgp.96.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takumi T., Ohkubo H., Nakanishi S. Cloning of a membrane protein that induces a slow voltage-gated potassium current. Science. 1988 Nov 18;242(4881):1042–1045. doi: 10.1126/science.3194754. [DOI] [PubMed] [Google Scholar]
- Tohse N. Calcium-sensitive delayed rectifier potassium current in guinea pig ventricular cells. Am J Physiol. 1990 Apr;258(4 Pt 2):H1200–H1207. doi: 10.1152/ajpheart.1990.258.4.H1200. [DOI] [PubMed] [Google Scholar]
- Tohse N., Kameyama M., Irisawa H. Intracellular Ca2+ and protein kinase C modulate K+ current in guinea pig heart cells. Am J Physiol. 1987 Nov;253(5 Pt 2):H1321–H1324. doi: 10.1152/ajpheart.1987.253.5.H1321. [DOI] [PubMed] [Google Scholar]
- Walsh K. B., Arena J. P., Kwok W. M., Freeman L., Kass R. S. Delayed-rectifier potassium channel activity in isolated membrane patches of guinea pig ventricular myocytes. Am J Physiol. 1991 Apr;260(4 Pt 2):H1390–H1393. doi: 10.1152/ajpheart.1991.260.4.H1390. [DOI] [PubMed] [Google Scholar]
- Walsh K. B., Begenisich T. B., Kass R. S. Beta-adrenergic modulation of cardiac ion channels. Differential temperature sensitivity of potassium and calcium currents. J Gen Physiol. 1989 May;93(5):841–854. doi: 10.1085/jgp.93.5.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh K. B., Kass R. S. Regulation of a heart potassium channel by protein kinase A and C. Science. 1988 Oct 7;242(4875):67–69. doi: 10.1126/science.2845575. [DOI] [PubMed] [Google Scholar]
- Wettwer E., Scholtysik G., Schaad A., Himmel H., Ravens U. Effects of the new class III antiarrhythmic drug E-4031 on myocardial contractility and electrophysiological parameters. J Cardiovasc Pharmacol. 1991 Mar;17(3):480–487. doi: 10.1097/00005344-199103000-00018. [DOI] [PubMed] [Google Scholar]
- Yazawa K., Kameyama M. Mechanism of receptor-mediated modulation of the delayed outward potassium current in guinea-pig ventricular myocytes. J Physiol. 1990 Feb;421:135–150. doi: 10.1113/jphysiol.1990.sp017937. [DOI] [PMC free article] [PubMed] [Google Scholar]




