Abstract
The airway mucosa is responsible for mounting a robust innate immune response (IIR) upon encountering pathogen-associated molecular patterns. The IIR produces protective gene networks that stimulate neighboring epithelia and components of the immune system to trigger adaptive immunity. Little is currently known about how cellular reactive oxygen species (ROS) signaling is produced and cooperates in the IIR. We discuss recent discoveries about 2 nuclear ROS signaling pathways controlling innate immunity. Nuclear ROS oxidize guanine bases to produce mutagenic 8-oxoguanine, a lesion excised by 8-oxoguanine DNA glycosylase1/AP-lyase (OGG1). OGG1 forms a complex with the excised base, inducing its nuclear export. The cytoplasmic OGG1:8-oxoG complex functions as a guanine nucleotide exchange factor, triggering small GTPase signaling and activating phosphorylation of the nuclear factor (NF)κB/RelA transcription factor to induce immediate early gene expression. In parallel, nuclear ROS are detected by ataxia telangiectasia mutated (ATM), a PI3 kinase activated by ROS, triggering its nuclear export. ATM forms a scaffold with ribosomal S6 kinases, inducing RelA phosphorylation and resulting in transcription-coupled synthesis of type I and type III interferons and CC and CXC chemokines. We propose that ATM and OGG1 are endogenous nuclear ROS sensors that transmit nuclear signals that coordinate with outside-in pattern recognition receptor signaling, regulating the IIR.
Key Words: Innate immune response, DNA-damage response, Ataxia telangiectasia mutated, Interferon, 8-Oxoguanine
Role of the Pulmonary Mucosa in Initiating Innate Immunity
The respiratory tract is composed of a surface area that covers over 100 m2 and is highly evolved to facilitate gas exchange and prevent fluid losses. Typically, a normal adult inhales over 11,000 liters of air daily that enter 3 million alveoli for exchanging carbon dioxide with oxygen. The pulmonary tree is lined throughout with a semi-impermeable barrier formed by highly adapted epithelial cells [1]. The airway epithelium is the primary cellular surface that comes in contact with inhaled particulates, pathogens and aeroallergens, and the epithelial cells play a central role in triggering the protective host response.
Upon sensing viral or bacterial pathogens, airway epithelial cells trigger an intracellular host-signaling response known as the innate immune response (IIR). The IIR is initially triggered by germline-encoded pattern recognition receptors (PRRs) normally located on cell membranes in cytosolic and endosomal cellular compartments. These PRRs function to detect cognate pathogen-associated molecular patterns (PAMPs) or damage-associated patterns (DAMPs). PAMPs, which include dsRNA, 5-phosphorylated RNA or CpG-DNA of viral origin and lipopeptides, mannans and flagellins of bacterial origin, indicate the presence of replicating microbial agents [2]. DAMPs include uric acid, Hsp70, or high-mobility group 1 proteins that represent signatures of cellular necrosis/damage. Upon binding their cognate ligands, PRRs trigger an intracellular signaling response that prevents/decreases the spread of a foreign pathogen until the adaptive immune response is mobilized [3].
PRRs in the Pulmonary Host Defense to RNA Virus Infections
The study of the pulmonary host defense to RNA infection has significantly advanced our knowledge of the distinct roles that PRRs play in triggering the IIR and also their interactions (fig. 1). These studies have shown that airway epithelial cells (AECs) play a major modulatory role in the pulmonary IIR to viral infection [4, 5, 6]. In AECs, the 2 major classes of PRRs that sense the presence of RNA virus infection are the cytoplasmic retinoic acid inducible gene (RIG)-I-like RNA helicases (RLHs) and the membrane/endosomal Toll-like receptors (TLRs). The PRRs have been extensively studied and reviewed [7]; only salient aspects will be addressed here.
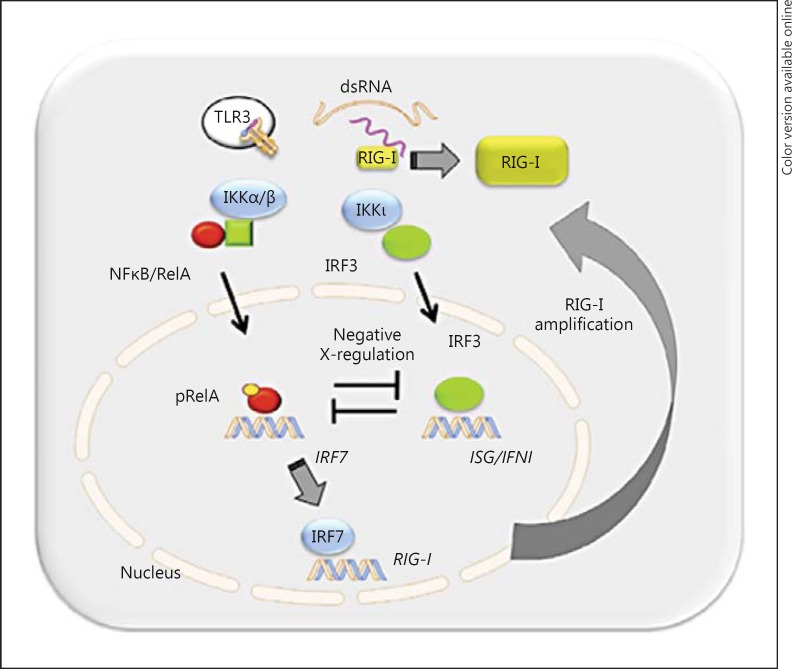
Fig. 1.
Intracellular signaling of the IIR via the IRF3 and RelA effectors. For an idealized AEC, the intracellular RIG-I and endosomal TLR3 PRRs in response to intracellular dsRNA are shown. Downstream of the PRR signaling complexes, the typical IκB kinase (IKKα/β) and atypical IKK (IKKι/TBK1) trigger phosphorylation and nuclear translocation of the NFκB/RelA and IRF3 transcription factors, representing the primary effector arms of the IIR. The 2 pathways are coupled by positive cross-talk via the NFκB-dependent activation of IRF7, involved in amplification of RIG-I-IFN production and negative RelA-IRF3 cross-talk. pRelA = Phospho-RelA.
RIG-I and the melanoma differentiation-associated gene-5 (MDA5) are 2 members of the caspase recruitment domain-rich RIG-I-like receptor helicase family. These are essential PRRs for detecting cytoplasmic viral PAMPs and recognizing ds- and 5-phosphorylated RNA [7]. Although these proteins are highly similar, it is currently thought that RIG-I and MDA5 recognize different types of virus and dsRNAs, with RIG-I responding to most ssRNA viruses and short viral RNAs whereas MDA5 responds to picornavirus RNA and longer dsRNAs [8]. In AECs, RIG-I is the major PRR recognizing ssRNA infections (fig. 1). Activation of RIG-I involves RNA binding that induces energy-dependent conformational changes, followed by its Lys 63-linked ubiquitylation. K63 ubiquitylation is a nondestructive posttranslational modification that promotes protein complex formation with a downstream adaptor known as mitochondrial antiviral signaling (MAVS). MAVS is an integral mitochondrial membrane protein that contains an NH2-terminal CARD domain, which binds cognate CARD domains in activated RIG-I or MDA5. The formation of a RIG-I/MDA5:CARD complex induces MAVS oligomerization into ‘prion-like’ particles, and K63-linked ubiquitylation of the IKKγ, TRAF and RIP adapter proteins, to generate a functional mitochondrial signal generator complex. This complex, in turn, activates both primary effector arms of the IIR, the interferon regulatory factor (IRF)3 and nuclear factor (NF)κB transcription factors, coordinating genes of the antiviral IFN and proinflammatory cytokine genes, respectively (fig. 1).
In AECs, RIG-I is the primary initial PRR sensing the presence of replicating respiratory syncytial virus (RSV) [9]. Using UV cross-linking immunoprecipitation PCR experiments, we found that RIG-I, but not MDA5, binds RSV genomic RNA; siRNA-treated RIG-I-depleted cells show inhibition of early NFκB and IRF3 translocation in response to RSV infection, with decreased type I interferon (IFN) expression and enhanced viral replication [9]. Activation of the RIG-I-IFN pathway produces an amplification loop (fig. 1), an essential component of a robust IIR, the mechanism of which is ROS-dependent and is discussed below. Together, these findings indicate that paramyxovirus-RLH interactions are dynamic, cell type-specific and dependent on viral replication.
TLRsare a family of 12 related membrane-localized PRRs that bind diverse PAMPs including lipopolysaccharide (LPS), lipoteichoic acids, mannans, flagellins, dsRNA and DNA oligonucleotides. TLRs are expressed in distinct compartments of sentinel mucosal cells, the location of which is cell type-specific and dynamic, depending on the state of cellular activation. In resting AECs, TLR9 is cell-surface-associated, TLR3 is endosomal and TLR4 is absent [10]. After viral infection, IFN stimulation upregulates TLR3 expression and redistributes it to the cell surface [9]. TLR3 activation in AECs is coupled to essential phosphorylation of the NFκB/RelA pathway, contributing secondarily to the IIR (fig. 1). In contrast to the intracellular distribution of TLRs in AECs, in dendritic cells (DCs) and alveolar macrophages (AMs), TLR1, TLR2 and TLR4–6 localize at the cell surface, whereas TLR3 and TLR7–9 are contained within the endosomal compartment. TLR signaling is initiated by recruiting specific adapter proteins, including MyD88 and TRIF, to form active signaling complexes. TLR4 binds LPS and induces complex formation with CD14 and MD2. TLR3 is the primary receptor for dsRNA and activates innate signaling by recruiting the cytoplasmic adapter known as TRIF. TLR7-9 bind ssDNA and unmethylated CpGs, respectively. The roles of TLRs are, therefore, compartment- and cell-type-specific.
The NFκB-IRF3 Core Transcription Factor Network
Upon binding to cognate PAMPs, RLH and TLR-type PRRs trigger a coupled intracellular serine kinase-ubiquitin ligase cascade that produces protein complex formation, signal adapter recruitment and kinase activation. Both RLH and TLRs converge on primary transcription effectors of the IIR, known as IRF and NFκB (fig. 1). Activated by coupled phosphorylation-acetylation posttranslational modifications, these effectors form transcriptionally activated complexes that induce the expression of inflammatory cytokines and type I IFN genes.
The IRF family transcription factors primarily control IFN expression. So far, 9 human IRFs have been reported (IRF1–9); of these, IRF1, IRF3 and IRF7 are the key regulators of type I and type III IFN gene expression. These IRF proteins regulate the expression of various genes with antiviral, antiproliferative, apoptotic and immune modulatory functions. Of these, IRF3 is the major early signaling protein constitutively expressed in the cytoplasm (fig. 1). The molecular mechanisms by which RIG-I·MAVS activates IRF3 involves recruitment of TRAF3, a signal adapter that mediates activation of the tank-binding kinase (TBK)1-IKKε/ι, a complex representing the major rate-limiting kinases of IRF3 activation. Phosphorylation of IRF3 on COOH-terminal domain serine residues allows it to homodimerize, associate with p300/CBP and translocate into the nucleus [11]. Phospho-IRF3 activates target genes containing IFN-stimulated response elements, such as IFNβ/IFNα4 and downstream IRF1 and IRF7. The IRF network has been subjected to computational modeling and experimental validation by gene deletion experiments. These studies show that IRF7 upregulation mediated by NFκB stimulates RIG-I amplification, the upregulation of which sustains the antiviral type I IFN response [12] (fig. 1).
NFκB is a family of heterodimeric inducible cytoplasmic complexes tethered in the cytoplasm by association with IκB inhibitors [13]. The well-known ‘canonical’ NFκB pathway is initiated by either activated TNF superfamily receptors or the cytoplasmic PRR RIG-I, which converge on IκB kinase (IKK), the rate-limiting kinase controlling IκBα degradation. By contrast, the ‘noncanonical’ NFκB pathway is activated by RIG-I in an IKKγ-independent manner to converge on the NFκB inducing kinase (NIK)-IKKα complex that initiates NFκB2/p100 processing to liberate both sequestered RelB/p52 and RelA complexes from a cytoplasmic NFκB2/p100 precursor [14]. This latter arm has been termed the ‘cross-talk’ pathway, because it is dependent on noncanonical NIK-IKKα kinases, but liberates the canonical RelA transcriptional activator.
We recently discovered that NFκB activation is a two-step process, mediated by IκBα release from cytoplasmic stores coupled to reactive oxygen species (ROS)-triggered mitogen and stress kinase (MSK)-1 mediated phosphorylation on RelA serine (Ser) 276 [15]; this activating modification is required for RelA acetylation and its stable association with the activated positive transcriptional elongation factor (PTEF-b) complex, Brd4-CDK9 [16, 17, 18]. Activation of the downstream chemokine network is dependent on transcriptional elongation of paused RNA polymerase II, mediated by Brd4-CDK9. The NFκB-CDK9-BRD4 complex mediates a subnetwork of immediate early genes including IL-8 and Groβ, cytokines involved in leukocyte chemotaxis and activation [16, 17, 18]. The essential role of ROS signaling in controlling phospho-NFκB formation in the IIR was a seminal finding that functionally links the intracellular redox signaling and PRR pathways.
Cellular Oxidative Stress Pathways
ROS are ubiquitous components of innate signaling initiated by host-pathogen interactions. Most, if not all, receptor-ligand (e.g. cytokines, chemokines, growth factors and LPS) interactions, cellular metabolic processes, environmental exposures and viral replication elicit the generation of ROS. Cellular sites for ROS generation potentially include the mitochondria, phagosomes and microsomes as well as various enzymes such as COX, lipoxygenase, xanthine oxidase and NADPH oxidases [19, 20, 21, 22]. NADPH oxidases are the most important source of inducible intracellular ROS, generated in response to a variety of stimuli (reviewed extensively [23, 24]).
The primary ROS generated by oxidoreductases such NADPH oxidases (NOX1–3 and NOX5) is the superoxide anion (O2-∙), while others (NOX4 and DOUX2) produce the nonradical oxidant hydrogen peroxide (H2O2[24]). ROS is counterbalanced by intracellular antioxidants (e.g. glutathione and thioredoxin), and by ROS metabolizing antioxidant enzymes (AOEs; e.g. glutathione peroxidases, superoxide dismutase and catalase). AOEs can directly metabolize ROS (superoxide dismutase and catalase) or facilitate their elimination using GSH as a reducing agent. Superoxide dismutases convert the superoxide anion to H2O2 and molecular oxygen, and catalase and glutathione peroxidases convert H2O2 to water and oxygen. Peroxiredoxins are cysteine-dependent peroxidases that catalyze the reduction of H2O2 and alkyl hydroperoxides in the presence of thioredoxin and NADPH (reviewed [19, 20, 21, 22, 24]). In addition to their production through aerobic metabolism, ROS are generated physiologically through PRR signal transduction cascades. Many RNA viruses such as RSV modulate cellular redox via the involvement of NOX/DUOX [23], the depletion of antioxidant enzymes or the induction of antioxidant responses [15, 25, 26]. Ligation of TLRs induces ROS formation, essential for the activation of key transcriptional mediators of the IIR [27, 28]. Similarly, ligation of the TNF receptor induces ROS and oxidative DNA damage [29]. Recently, it was acknowledged that these PRR-generated ROS signals play essential roles in signal transduction by controlling inducible phosphorylation. For example, ROS generation by TNF and RSV is required for increased RelA (Ser 276) phosphorylation and the subsequent induction of a set of phospho-RelA (Ser 276)-dependent and antioxidant-sensitive NFκB-dependent genes [15, 16, 29].
An important question about signal-induced ROS is how ROS signals are detected and transduced into intracellular kinase cascades. We propose and discuss the role of 2 major sensors of oxidative DNA damage, 8-oxoguanine DNA glycosylase1 (OGG1) and ataxia telangiectasia-mutated (ATM), that serve as key transducers linking nuclear ROS to the IIR and inflammation.
Oxidative DNA Damage Response Pathways
More than a hundred products are generated by the interaction of free radicals (primarily ∙OH) with DNA bases and the DNA backbone. Many, if not all, have potential biological consequences. Oxidative damage to the DNA produces oxidized purine and/or pyrimidine bases, apurinic/apyrimidinic sites and DNA single- and double-strand breaks (SSBs and DSBs). Of these, the most abundant oxidation products are 8-oxo-7,8-dihydroguanosine (8-oxoG), 8-oxo-7,8-dihydroadenine and pyrimidine ring saturation products (e.g. uracil glycol, dihydrouracyl, 5-hydroxycytosin and thimidine thymidine glycol). The characterization of these modified purines and pyrimidines has provided useful information on their biological importance [30].
Five oxidized, base-specific DNA glycosylases grouped into 2 mechanistic families have been identified in mammalian cells, and prevent the accumulation of oxidatively modified bases and their biological or mutagenic effects. The first family includes OGG1 and endonuclease III-like protein 1 (NTH1) of the Nth family. OGG1/NTH1 removes base lesions only from duplex DNA and has β-elimination activity, generating an apurinic/apyrimidinic (AP)-site at the resulting SSB. The second family is composed of Nei-like (NEIL1–3) members that preferentially repair oxidative base damage from transcribed genes, suggesting their involvement in DNA repair during replication and transcription. The detailed enzymatic processes in oxidative DNA damage repair have already been extensively reviewed [31].
The Role of OGG1 in Innate Immunity
One of the most abundant DNA base lesions is 8-oxoG, due to the chemical property of guanines having the lowest ionization potential among nucleic acid bases [31, 32]. 8-OxoG is primarily repaired by the OGG1-initiated base excision repair pathway (OGG1-BER; fig. 2a). Accumulation of 8-oxoG in DNA has been linked to accelerated aging and various inflammatory diseases. Intriguingly, accumulation of genomic 8-oxoG in Ogg1−/− mice resulted in a decreased innate and adaptive inflammatory response [33], a finding which suggests that OGG1 may have independent actions in innate inflammation apart from its base excision activity.
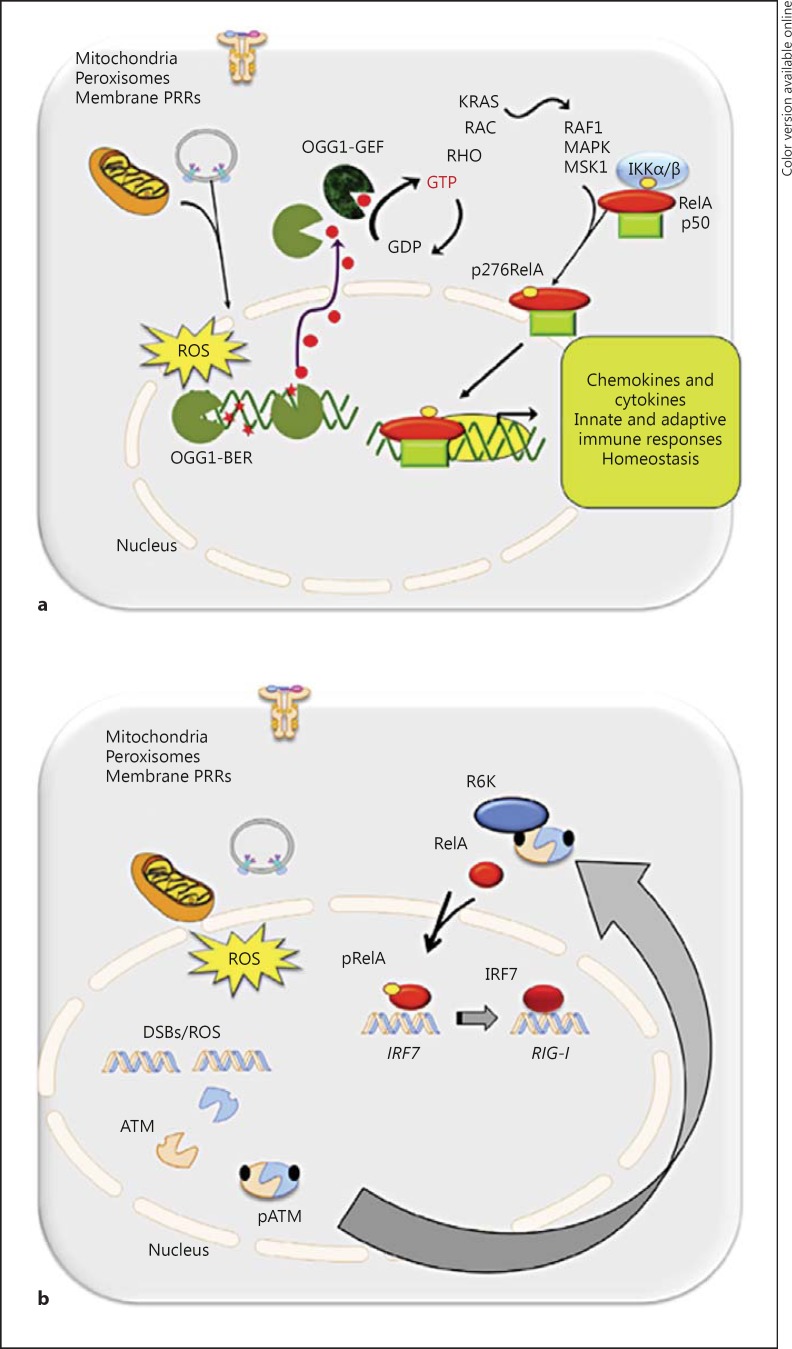
Fig. 2.
The 8-oxoG-OGG1-K-Ras pathway. a ROS generated from mitochondria, peroxisomes or membrane-resident PRRs, resulting in oxidation of guanine residues. Intrahelical 8-oxoG is recognized and excised by OGG1. In the cytoplasm, the free 8-oxoG base is bound by cytoplasmic OGG1, with the complex functioning as a GEF activating K-Ras. Downstream, K-Ras stimulates phosphorylation of NFκB/RelA on Ser 276. b The ATM-RSK pathway. ROS stimulates the autophosphorylation of ATM, either directly or via dsDNA breaks. Phospho-ATM (pATM) is translocated from the nucleus into the cytoplasm, where it forms a scaffold with the RSKs, PKAc and MSK-1, facilitating activation of phospho-Ser 276 RelA (pRelA). Phospho-Ser 276 RelA activates IRF7, the proximal transcription factor of the RIG-I-IFN amplification loop.
Our recent studies showed that OGG1 binds the free 8-oxoG base (product of OGG1-BER) with high affinity, a subnanomolar binding constant [31]. The resulting OGG1:8-oxoG complex interacts with the RAS family GTPases, including Kirsten (K)-RAS, neuroblastoma RAS viral oncogene homolog (N)-RAS and Harvey (H)-RAS, resulting in their activation (fig. 2a). These studies indicated that the cytoplasmic OGG1:8-oxoG complex functions as a guanine nucleotide exchange factor (GEF) [31, 34]. The OGG1:8-oxoG complex activates RAS by exchange of GDP for GTP. Activated RAS-GTPase, in turn, stimulates downstream mitogen-activated protein kinase (MAPK) kinase (MEK1/2) and extracellular signal-regulated kinase (ERK1/2) [10, 31]. Activation of RAS GTPases and their downstream signaling occur upon both the OGG1-BER and 8-oxoG base challenge of cells. Further studies documented that activation of OGG1-BER by oxidative stress increases GTP-bound levels of small GTPases. Intriguingly, in OGG1-deficient cells, oxidative stress failed to induce guanine nucleotide exchange [10, 31, 32, 33, 34, 35, 36]. These observations were unexpected, as many small GTPases are directly redox-sensitive, and ROS have an effect similar to that of GEFs in that they modulate guanine nucleotide binding of GTPase, causing an increase in their GTP-bound form [37].
We further reported that, in addition to the canonical RAS family members, the OGG1:8-oxoG complex physically interacts with guanine nucleotide-free and GDP-bound Ras-related C3 botulinum toxin substrate 1 (RAC1 [35]). OGG1:8-oxoG interaction with RAC1 also results in a rapid increase in the levels of RAC1-GTP, suggesting that OGG1:8-oxoG functions also as a GEF for RAC1 [35]. Downstream from OGG1:8 oxoG-RAC1-GTP, increases in ROS levels were observed via NADPH oxidases (NOX). It is not known whether enhanced ROS result from RAC1-generated signaling or are produced directly by NOX4 [35]. Alternatively, OGG1-BER/RAC1-GTP may be influencing NOX1, NOX2 or NOX3. NOX4 is considered constitutively active; however, a growing number of studies suggest that NOX4 can be acutely activated in response to agonist stimulation [8, 24, 38, 39, 40]. NOX isoform(s) generate H2O2 (either forming H2O2 directly or secondarily to its dismutation) that enters various cellular compartments, including the nucleus, where it serves as a signaling entity and may induce DNA damage by oxidizing bases as well as inducing strand breaks. The biological significance of OGG1-BER-associated ROS generation is not presently known; at first glance, it seems feasible that ROS generated locally could generate cell activation signals and/or inactivate OGG1's glycosylase/AP-lyase activity [41]. To this end, a recent study documented that inactivating OGG1 at cysteine facilitated the recruitment of transacting factors to the promoter of the proinflammatory chemokine CXCL2[42].
Inside-Out Signaling of OGG1 in the IRR
Recent studies have shown that the repair of 8-oxoG via OGG1-BER is a prerequisite for increased expression of proinflammatory chemokines and cytokines as well as for the induction of an IIR in the airways [33, 42]. Downstream of OGG1-BER, K-RAS-GTP, activated phosphatidylinositol-4,5-bisphosphate 3-kinase, mitogen-activated kinases (MEK1,2 and ERK1,2), mitogen-stress related kinase-1 and IκB kinase activate the canonical NFκB pathway, a central mediator of airway mucosal inflammation, which includes RelA phosphorylation at Ser 276 and its nuclear translocation ([33]fig. 2a). Described above, these two events are essential for the full activation of NFκB-dependent inflammation via transcriptional elongation of immediate early proinflammatory gene subnetworks.
When OGG1-expressing and OGG1-deficient mucosal airway epithelia were exposed to oxidative stress, only AECs expressing OGG1 showed activation of K-RAS and increased chemokine/cytokine expression. Interestingly, exposure of the airways to 8-oxoG or the initiation of OGG1-BER (activated by an oxidative burst) increased an identical pattern of chemokine and cytokine expression [33, 42], suggesting that OGG1-BER and formation of OGG1-GEF is an upstream event in proinflammatory gene expression. Accordingly, oxidative challenge failed to induce an IIR in OGG1-deficient airways.
To extend these results, our RNA-sequencing analysis identified 1,592 differentially expressed mRNA transcripts, the expression of which changed by ≥3-fold [33]. The upregulated mRNAs were related to the immune system, macrophage activation, regulation of liquid-surface tension and stimulus-response processes. These biological processes were mediated by chemokines, cytokines, gonadotropin-releasing hormone receptor, integrin and interleukin signaling pathways. These findings point to a new paradigm in which OGG1-BER plays a central role. Specifically, we propose that ROS generate intrahelical 8-oxoG lesions (due to the susceptibility of guanine to oxidation) that are corrected by the OGG1-BER pathway. Through OGG1:8-oxoG's GEF activity, OGG1-BER increases the levels of RAS-GTP, which induces downstream signaling via the NFκB arm of the IIR (fig. 2a).
The Role of ATM in the DSB Response
The presence of DSBs initiates a well-coordinated DNA-damage response (DDR) signaling cascade regulated by sensors, transducers and effector proteins [43]. The net effect of the DDR is to induce cell-cycle arrest. However, if the DNA damage is too extensive for DNA repair, the DDR pathway alternatively induces a proapoptotic signaling program. In mammalian cells, the DDR pathway is coordinated by the members of the phosphoinositide-3-kinase-related protein kinase family consisting of DNA-dependent protein kinase (DNA-PK), ATM and Rad3-related (ATR) kinase and ATM. DNA-PK and ATM are activated in response to DSBs, whereas ATR is activated by replication stress [44]. Once these kinases are activated, they transduce the signal by phosphorylating a wide range of substrates. Genetic alterations or dysfunctional activities of the DDR pathway are linked to a spectrum of human diseases, emphasizing the vital role of the DDR in maintaining cellular viability and function.
ATM is a serine/threonine-specific protein kinase that plays a major role as a cell-cycle checkpoint kinase regulating cell-cycle arrest, DNA repair or apoptosis. Under unstimulated conditions, ATM exists as an inactive dimer (or higher-order multimer) in the nucleus, where its kinase domain is inactivated by its interaction with a Frap ATM Trapp (FAT) domain of an adjacent ATM protein [45]. When DSBs are induced in cells, typically by means of ionizing radiation or chemotherapeutic drugs, a protein complex consisting of meiotic recombination 11, radiation sensitive 50 and Nijmegen breakage syndrome 1 (the MRN complex) is recruited to DNA DSBs [46]. This process induces the hallmark ATM autophosphorylation at Ser 1981, releasing 2 monomeric activated ATM molecules [45]. Activated ATM phosphorylates a network of downstream target proteins, including histone 2A family member X (H2AX), p53, checkpoint kinase 2 homolog (Chk-2), breast cancer 1 (BRCA1, early onset) and p53-binding protein-1 (53BP1) [47].
Although ATM's physiological function is a first line of defense in maintaining genomic integrity, several new functions have recently been attributed to it that could be dependent or independent of extensive double-stranded DNA damage. Outside of its role in cell-cycle control and DNA repair, its role in regulating cellular redox status, mitochondrial function, metabolic control and the activation of innate immune pathway via NFκB or IRF has been a growing area of interest.
ATM-Driven Activation of NFκB
As described above, the NFκB transcription factor exists in the cytoplasm as an inactive form in most cells and its activation, typically either via the canonical or the noncanonical pathway, is induced by engagement of cell surface receptors of the TNF receptor or PRR superfamilies. Unlike these outside-in signaling pathways for activating NFκB transcription factors via the cytosolic IKK complex, genotoxic stress produced by γ-irradiation or topoisomerase-targeted drugs initiates DSB signaling in the nucleus. Upon activation and cytoplasmic export, ATM plays a critical role in NFκB activation (fig. 2b).
This cytosolic ATM response is functionally interdependent with the NFκB signaling pathway. For example, earlier work has shown that activated ATM undergoes nuclear export in a manner dependent on posttranslational modifications of nuclear IKKγ [48, 49]. IKKγ residence in the nucleus is the consequence of its ATM-dependent phosphorylation, coupled to its monoubiquitination at Ser 85 [50]. Upon activation, ATM phosphorylates IKKγ, causing its sumolyation on lysine residues at 277 and 309 and thus promoting export of the ATM-IKKγ complex [48]. Several E3 ligase candidates responsible for IKKγ sumoylation have been identified, including the protein inhibitor of activated STATy (PIASy), p53-induced death domain protein (PIDD)/receptor interacting protein 1 (RIP1) and or poly-ADP-ribose polymerase 1 (PARP1). More work will be required to dissect this important regulatory step in DSB-ATM signaling.
Although IKKγ sumoylation is absolutely essential for ATM-IKKγ nuclear export, this event is not sufficient to facilitate DNA damage-induced NFκB activation [51]. For example, an ATM phosphoacceptor site mutant of IKKγ (S85A) is sumoylated in response to DSB, but is not monoubiquitinated or exported into the cytosol. However, when a monoubiquitin moiety was expressed in frame to the N-terminus of S85A mutant IKKγ, it restored all the downstream events of genotoxic insult-induced IKKγ cytoplasmic translocation in an ATM-dependent manner as well as IKK complex activation and NFκB activation, suggesting an important downstream role of IKKγ monoubiquitination in the nuclear export and subsequent activation of NFκB. The cellular inhibitor of apoptosis 1 (cIAP1) has been recognized as a specific ubiquitin ligase responsible for IKKγ monoubiquitination. Nuclear export of the phospho-Ser 1981 ATM-IKKγ is thought to be CRM1-independent and, instead, may depend on transient association with Ran-GTP in a calcium-dependent pathway.
It is currently thought that cytosolic phospho-Ser 1981 ATM-IKKγ associates with cytosolic ELKS (a protein rich in glutamic acid, leucine, lysine and serine) to regulate IKK activation [50]. DNA damage-induced NFκB activation requires an active IKK complex because the loss of IKKα, IKKβ or IKKγ completely abolishes DSB-induced NFκB activation. To effect IKK activation, the phospho-Ser 1981 ATM-IKKγ complex regulates the activity of TGFβ-activated kinase 1 (TAK1), an IKKβ upstream kinase, the activity of which is essential for NFκB activation by DNA-damaging agents [52]. TAK1 activation requires XIAP-dependent K63-linked polyubiquitination of ELKS, which promotes clustering of activated TAK1 in the complex with TAK1-binding proteins (TABs)-1 or −2, and IKK complex activation [53]. Cytoplasmic ATM can also regulate TAK1 activation by binding to TRAF6 and inducing E3 ligase activity in response to irradiation [54]. TRAF6 K63-linked polyubiquitination leads to TAK1 activation and cIAP1-dependent IKKγ monoubiquitination, resulting in IKK activation [54]. Thus overall, nuclear DSBs are linked to cytoplasmic IKK complex activation via an ATM-dependent IKKγ posttranslational modification and its translocation to the cytoplasm.
ATM Activation by Signal-Induced ROS
In addition to the classic ATM activation pathway, it has recently been reported that ROS can directly induce ATM activation independent of DSBs [46]. Direct oxidation of ATM results in its intermolecular dimerization via disulfide bridges. In the ROS-induced dimer form, one ATM molecule phosphorylates the other, leading to ATM activation. Mutation of the critical Cys residue 2991 involved in the disulfide bridge blocks ATM activation by oxidants. Thus, these findings clearly suggest that ATM may serve as an important nuclear ROS sensor in mammalian cells, regulating the IIR. The fact that ROS can activate ATM and the DDR opens a new frontier for understanding the physiological relevance of this event and provide potential new possibilities for the treatment of ROS-related disease.
In this context, our recent, unbiased, high-throughput siRNA screening studies of 636 members of the human kinome identified ATM as an important regulator of the canonical NFκB pathway triggered by extracellular death domain-containing receptors [55]. In follow-up studies, we observed that ATM is activated by phosphorylation at Ser 1981 and undergoes rapid IKKγ-dependent nuclear export, which controls the NFκB pathway at several distinct points in the cytoplasm [56]. First, cytoplasmic ATM complexes with β-TrCP, an E3-ubiquitin ligase, to promote rapid proteasomal degradation of phospho-IκBα, the rate-limiting step in controlling NFκB entry into the nucleus. Second, cytosolic phospho-ATM binds and activates the catalytic subunit of protein kinase A (PKAc), a ribosomal S6 kinase (RSK) essential for RelA Ser 276 phosphorylation (fig. 2b). In the absence of ATM or in response to a small-molecule inhibitor thereof, TNF-inducible RelA Ser 276 phosphorylation is completely inhibited [56], although inducible Ser 536 phosphorylation is unaffected. Taken together with our previous studies demonstrating that RelA Ser 276 phosphorylation is an oxidative stress- dependent event [29], these data strongly indicate that ATM is a nuclear ROS sensor that couples signal-inducible nuclear ROS to RelA activation via a cytoplasmic scaffolding function. ATM thus favors cell survival and proliferation by increasing NFκB-dependent immediate early and antiapoptotic genes via transcriptional elongation.
Mechanism of ATM in the Virus-Inducible RIG-I-IFN Amplification Loop
As discussed above, the activation of the RIG-I PRR pathway produces a dramatic induction of RIG-I/IFN genes essential for the antiviral response of the IIR (fig. 1). Among the IRF family members, IRF1 and IRF7 are highly inducible isoforms known for their vital role in the IIR. IRF1 mediates its antiviral effect by binding to the virus-inducible enhancer-line element of IFNβ. Additionally, our recent computational model of the integrated IIR predicted and experimentally verified that NFκB-dependent IRF7 expression mediates the induction of the RIG-I gene in response to dsRNA stimulation [12]. This discovery provided a mechanistic link between ROS signaling, NFκB action and antiviral immunity in AECs.
In follow-up studies [57], we have observed that ATM silencing results in enhanced replication of ssRNA viruses, due to decreased expression of type I and type III IFNs and IFN-stimulated genes (fig. 1). Surprisingly, cytoplasmic RSV replication induces activation of the DDR through formation of γH2AX foci and phospho-Ser 1981 ATM. Phospho-Ser 1981 ATM is exported from the nucleus through the shared IKKγ-dependent mechanism. In contrast to the TNF pathway, ssRNA viruses induce RelA phosphorylation via induced mitogen and stress-activated kinase (MSK)-1. Interestingly, ATM-deficient cells show defective RSV-MSK-1 Ser 376 phosphorylation and decreased RelA Ser 276 phosphorylation. The mechanism by which ATM regulates MSK-1 is not currently known.
The above-mentioned study further defined the mechanism for marked RIG-I upregulation characteristic of ssRNA replication. In this study, we observed that RelA inducibly binds to the native IRF7 promoter in an ATM-dependent manner and IRF7 inducibly binds to the endogenous RIG-I gene promoter (fig. 2b). Ectopic IRF7 expression restores RIG-I expression and type I/III IFN expression in ATM-silenced cells. These studies were the first to demonstrate that ssRNA viruses trigger the DDR, a pathway required for MSK1 activation of phospho-Ser 276 RelA formation to induce the IRF7-RIG-I amplification loop necessary for mucosal IFN production [12]. A significant decline in antiviral response in ATM-deficient cells or in the presence of the ATM-specific inhibitor KU-55933 suggests the importance of the ATM-mediated IRF7-RIG-I amplification loop for mucosal IFN production [57].
The Two-Signal Hypothesis
The observations reviewed above show that PAMP signaling stimulates 2 parallel nuclear ROS-sensing pathways mediated via OGG1 (in response to SSBs) and ATM (in response to DSBs/ROS). Intriguingly, both sensors have direct cytosolic actions independent of their roles in the DNA damage response pathway. Upon nuclear export, the activated OGG1:8oxoG and phospho-ATM complexes converge on activation of the phospho-Ser 276 RelA pathway, which controls the rapid expression of CXC chemokines and antiapoptotic proteins to induce tissue inflammation. These observations, taken together with the dramatic decrease in inflammatory responses after the elimination of ROS in TNF and RSV signaling, strongly suggest that 2 signals are required for maximal induction of the mucosal IIR (fig. 3a). One signal, representing a primary trigger of the IIR, is mediated by activated membrane or cytoplasmic-localized PRRs. This pathway controls the initial nuclear translocation of the NFκB and IRF3 transcription factors. Our data show that nuclear translocation is necessary, but is not sufficient for producing a robust IIR. The second signal, representing an activation arm of the IIR, emanates from ROS stress produced in the nucleus. This signal controls post-translational modification of NFκB and IRF3, resulting in the robust activation of the immediate early chemokines controlling inflammation and positive cross-talk with the RIG-I-IFN amplification pathway. We propose that the second ROS signal is an important mechanism for ensuring full activation of the IIR in response to pathogens and preventing a full-blown IIR in response to PAMPs from nonreplicating pathogens or commensal organisms.
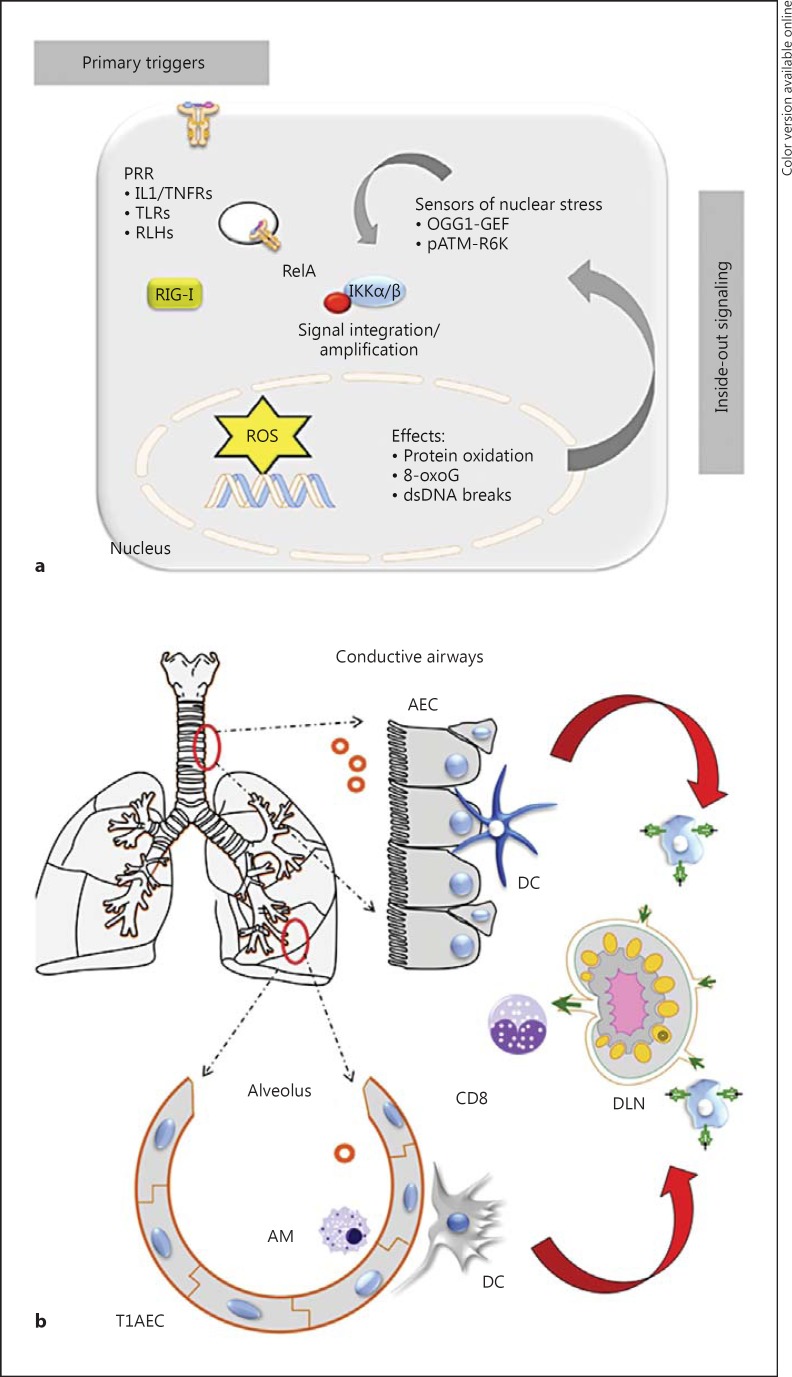
Fig. 3.
Integration of outside-in and inside-out signaling pathways in the IIR. a A schematic illustration of how the formation of nuclear ROS stress is sensed and amplifies the outside-in signaling in the IIR triggered by PRR activation. pATM = Phospho-ATM; TNFRs = TNF receptors. b Topologies of the pulmonary innate response: innate defenses for the conducting airways (top) and the alveolar space (bottom). In each, the AEC plays a major role in the initial triggering of the IIR. DLN = Draining lymph node; T1 = type 1 airway cells.
Implications of the Two-Signal Hypothesis for Pulmonary Innate Immunity
The pulmonary tree is functionally divided into 2 distinct regions, the conductive airways (trachea and bronchi) to move air and the respiratory airways (alveoli) to conduct gas exchange (fig. 3b). These regions are lined by distinct, highly differentiated epithelial cell types [1] that have unique host-defense mechanisms.
The conductive airway cells constitutively produce a protective airway lining fluid, rich in antioxidants, defensins, lysozymes and mucous glycoproteins (mucins), which play an important role in innate immunity [58] by binding viruses or bacteria, preventing their adherence and facilitating their clearance via the mucociliary escalator [59]. Underlying this epithelial monolayer is a dense submucosal network of DCs, with long, finger-like processes that sample luminal antigens [4, 60, 61]. When conductive AECs encounter PAMPs, the consequent IIR signaling pathways result in the expression of CC chemokines (Exodus-1, RANTES, MIP-1α and MIP-1β), CXC chemokines (I-TAC, GRO-α, GRO-β, GRO-χ and IL-8) and the CX3C chemokine Fractalkine [62]. Of these, the CXC chemokine MIP1 and type I/III IFNs induce airway plasmacytoid DCs to express CD-40, CD-80 and CD-86 costimulatory molecules, activating their migration to draining pulmonary lymph nodes. In the draining lymph nodes, activated DCs stimulate cytotoxic T lymphocytes, including natural killer and CD4/8 memory T cells that serve as a second line of protective innate immunity. AECs are also sources of derived thymic stromal lymphoprotein, IL-25 and IL-33; these cytokines activate subepithelial DCs and Th2-type polarization, important in the immunopathogenesis of viral disease [5]. In the absence of the nuclear ROS inside-out signaling pathway, conductive AECs would be uncoupled from DC activation and cytotoxic T lymphocyte activity.
By contrast, the respiratory surface within the alveoli is lined by nonciliated type I and type II epithelial cells highly specialized for gas exchange [1]. Here, the alveolar AECs are associated with AMs (fig. 3b). AMs are phagocytic and play a role in the sequestration of antigens, an activity that suppresses activation of and antigen presentation by DCs under normal conditions. Under resting conditions, AMs closely interact with AECs, resulting in TGFβ-induced expression of integrin αvβ6[4]. Innate activation of AMs through TLR3 produces a loss of integrin αvβ6, detachment from AECs and the acquisition of a phagocytotic phenotype. Activated type II AECs produce surfactants, cytokines and type I/III IFNs, also activating pulmonary DCs and inducing neutrophil recruitment and the survival of AMs (fig. 3b). Type II AECs express TARC, MCP-1 and MDC to a greater degree than the AECs of the conducting airways, suggesting the existence of distinct genetic responses for different types of airway-derived epithelial cell infection [62, 63]. In the absence of the nuclear ROS inside-out signaling pathway, infections of the respiratory surfaces in the lower airways would be expected to have decreased mucosal type I/III expression, CC chemokine expression and AM activation. These different patterns of chemokine mediators produced by AECs in distinct regions of the airways may account for the distinct outcomes of lower and upper respiratory tract infections in humans.
Conclusions/Future Directions
Increased oxidative stress has been implicated in the pathogenesis of a variety of acute and chronic lung diseases such as asthma, COPD and cystic fibrosis [64, 65]. Nuclear ROS sensing in AECs is mediated by a network of specialized proteins triggered by SSBs, DSBs and protein oxidation that serve multiple roles. In addition to roles in the repair of oxidative DNA damage, OGG1 and ATM function in cytosolic amplification of the IIR. The two-signal hypothesis explains how the epithelial cell uses ROS sensing to induce full activation of the IIR in response to appropriate PAMP and DAMP stimuli, and is a unifying mechanism explaining the essential role of ROS in innate pathway activation. By virtue of the regional topologies in pulmonary host-defense, innate ROS-triggered signal transduction pathways in the conductive and respiratory surfaces interface in different manners with innate immunity. Further work will be required to understand how these pathways interact and may be regulated by pathogen interaction, their regional contributions to host defense and whether their modulation could be useful as a therapeutic target for chronic lung disease.
Acknowledgements
This work was supported by National Institutes of Health grants NCATS UL1TR001439 (A.R.B.), NHLBI HHSN268201000037C (A.R.B., I.B.), DMS-1361411/DMS-1361318 and NIAID AI062885 (A.R.B., I.B.), IHII mini-center pilot (A.R.B.) and NIEHS P30 ES006676 (A.R.B., I.B.). We thank Dr. David Konkel for critically editing the manuscript.
References
- 1.Knight DA, Holgate ST. The airway epithelium: structural and functional properties in health and disease. Respirology. 2003;8:432–446. doi: 10.1046/j.1440-1843.2003.00493.x. [DOI] [PubMed] [Google Scholar]
- 2.Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol. 2007;81:1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- 3.Bals R, Hiemstra PS. Innate immunity in the lung: how epithelial cells fight against respiratory pathogens. Eur Respir J. 2004;23:327–333. doi: 10.1183/09031936.03.00098803. [DOI] [PubMed] [Google Scholar]
- 4.Holt PG, Strickland DH, Wikstrom ME, Jahnsen FL. Regulation of immunological homeostasis in the respiratory tract. Nat Rev Immunol. 2008;8:142–152. doi: 10.1038/nri2236. [DOI] [PubMed] [Google Scholar]
- 5.Erle DJ, Sheppard D. The cell biology of asthma. J Cell Biol. 2014;205:621–631. doi: 10.1083/jcb.201401050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lambrecht BN, Hammad H. The airway epithelium in asthma. Nat Med. 2012;18:684–692. doi: 10.1038/nm.2737. [DOI] [PubMed] [Google Scholar]
- 7.Loo Y-M, Gale Jr., M Immune signaling by RIG-I-like receptors. Immunity. 2011;34:680–692. doi: 10.1016/j.immuni.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chiu C, Maddock DA, Zhang Q, Souza KP, Townsend AR, Wan Y. TGF-beta-induced p38 activation is mediated by Rac1-regulated generation of reactive oxygen species in cultured human keratinocytes. Int J Mol Med. 2001;8:251–255. [PubMed] [Google Scholar]
- 9.Liu P, Jamaluddin M, Li K, Garofalo RP, Casola A, Brasier AR. Retinoic acid-inducible gene I mediates early antiviral response and Toll-like receptor 3 expression in respiratory syncytial virus-infected airway epithelial cells. J Virol. 2007;81:1401–1411. doi: 10.1128/JVI.01740-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.German P, Szaniszlo P, Hajas G, Radak Z, Bacsi A, Hazra TK, Hegde ML, Ba X, Boldogh I. Activation of cellular signaling by 8-oxoguanine DNA glycosylase-1-initiated DNA base excision repair. DNA Repair (Amst) 2013;12:856–863. doi: 10.1016/j.dnarep.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hiscott J. Triggering the innate antiviral response through IRF-3 activation. J Biol Chem. 2007;282:15325–15329. doi: 10.1074/jbc.R700002200. [DOI] [PubMed] [Google Scholar]
- 12.Bertolusso R, Tian B, Zhao Y, Vergara LA, Sabree A, Iwanaszko M, Lipniacki T, Brasier A, Kimmel M. Dynamic cross-talk model of the epithelial innate immune response to double-stranded RNA stimulation: coordinated dynamics emerging from cell-level noise. PLoS One. 2014;9:e93396. doi: 10.1371/journal.pone.0093396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brasier AR. The NF-kappaB regulatory network. Cardiovasc Toxicol. 2006;6:111–130. doi: 10.1385/ct:6:2:111. [DOI] [PubMed] [Google Scholar]
- 14.Liu P, Li K, Garofalo RP, Brasier AR. Respiratory syncytial virus induces RelA release from cytoplasmic 100-kDa NF-kappa B2 complexes via a novel retinoic acid-inducible gene-I{middle dot}NF-kappaB-inducing kinase signaling pathway. J Biol Chem. 2008;283:23169–23178. doi: 10.1074/jbc.M802729200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jamaluddin M, Tian B, Boldogh I, Garofalo RP, Brasier AR. Respiratory syncytial virus infection induces a reactive oxygen species-MSK1-phospho-Ser-276 RelA pathway required for cytokine expression. J Virol. 2009;83:10605–10615. doi: 10.1128/JVI.01090-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brasier AR, Tian B, Jamaluddin M, Kalita MK, Garofalo RP, Lu M. RelA Ser 276 phosphorylation-coupled Lys310 acetylation controls transcriptional elongation of inflammatory cytokines in respiratory syncytial virus infection. J Virol. 2011;85:11752–11769. doi: 10.1128/JVI.05360-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tian B, Zhao Y, Kalita M, Edeh CB, Paessler S, Casola A, Teng MN, Garofalo RP, Brasier AR. CDK9-dependent transcriptional elongation in the innate interferon-stimulated gene response to respiratory syncytial virus infection in airway epithelial cells. J Virol. 2013;87:7075–7092. doi: 10.1128/JVI.03399-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nowak DE, Tian B, Jamaluddin M, Boldogh I, Vergara LA, Choudhary S, Brasier AR. RelA Ser 276 phosphorylation is required for activation of a subset of NF-{kappa}B-dependent genes by recruiting cyclin-dependent kinase 9/cyclin T1 complexes. Mol Cell Biol. 2008;28:3623–3638. doi: 10.1128/MCB.01152-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Babior BM. NADPH oxidase: an update. Blood. 1999;93:1464–1476. [PubMed] [Google Scholar]
- 20.Laurindo FR, Araujo TL, Abrahao TB. Nox NADPH oxidases and the endoplasmic reticulum. Antioxid Redox Signal. 2014;20:2755–2775. doi: 10.1089/ars.2013.5605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 22.Holmstrom KM, Finkel T. Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat Rev Mol Cell Biol. 2014;15:411–421. doi: 10.1038/nrm3801. [DOI] [PubMed] [Google Scholar]
- 23.Grandvaux N, Mariani M, Fink K. Lung epithelial NOX/DUOX and respiratory virus infections. Clin Sci (Lond) 2015;128:337–347. doi: 10.1042/CS20140321. [DOI] [PubMed] [Google Scholar]
- 24.Brandes RP, Weissmann N, Schroder K. Nox family NADPH oxidases: molecular mechanisms of activation. Free Radic Biol Med. 2014;76:208–226. doi: 10.1016/j.freeradbiomed.2014.07.046. [DOI] [PubMed] [Google Scholar]
- 25.Hosakote YM, Liu T, Castro SM, Garofalo RP, Casola A. Respiratory syncytial virus induces oxidative stress by modulating antioxidant enzymes. Am J Respir Cell Mol Biol. 2009;41:348–357. doi: 10.1165/rcmb.2008-0330OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Casola A, Burger N, Liu T, Jamaluddin M, Brasier AR, Garofalo RP. Oxidant tone regulates RANTES gene expression in airway epithelial cells infected with respiratory syncytial virus. Role in viral-induced interferon regulatory factor activation. J Biol Chem. 2001;276:19715–19722. doi: 10.1074/jbc.M101526200. [DOI] [PubMed] [Google Scholar]
- 27.Asehnoune K, Strassheim D, Mitra S, Kim JY, Abraham E. Involvement of reactive oxygen species in Toll-like receptor 4-dependent activation of NF-kappa B. J Immunol. 2004;172:2522–2529. doi: 10.4049/jimmunol.172.4.2522. [DOI] [PubMed] [Google Scholar]
- 28.Yang CS, Kim JJ, Lee SJ, Hwang JH, Lee CH, Lee MS, Jo EK. TLR3-triggered reactive oxygen species contribute to inflammatory responses by activating signal transducer and activator of transcription-1. J Immunol. 2013;190:6368–6377. doi: 10.4049/jimmunol.1202574. [DOI] [PubMed] [Google Scholar]
- 29.Jamaluddin M, Wang S, Boldogh I, Tian B, Brasier AR. TNF-alpha-induced NF-kappaB/RelA Ser (276) phosphorylation and enhanceosome formation is mediated by an ROS-dependent PKAc pathway. Cell Signal. 2007;19:1419–1433. doi: 10.1016/j.cellsig.2007.01.020. [DOI] [PubMed] [Google Scholar]
- 30.Wallace SS. Biological consequences of free radical-damaged DNA bases. Free Radic Biol Med. 2002;33:1–14. doi: 10.1016/s0891-5849(02)00827-4. [DOI] [PubMed] [Google Scholar]
- 31.Boldogh I, Hajas G, Aguilera-Aguirre L, Hegde M, Radak Z, Basci A, Sur S, Hazra TK, Mitra S. Activation of Ras signaling by 8-oxoguanine DNA glycosylase bound to its excision product 8-oxoguanine. J Biol Chem. 2012;287:20769–20773. doi: 10.1074/jbc.C112.364620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Radak Z, Boldogh I. 8-Oxo-7,8-dihydroguanine: links to gene expression, aging, and defense against oxidative stress. Free Radic Biol Med. 2010;49:587–596. doi: 10.1016/j.freeradbiomed.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aguilera-Aguirre L, Bacsi A, Radak Z, Hazra TK, Mitra S, Sur S, Brasier AR, Ba X, Boldogh I. Innate inflammation induced by the 8-oxoguanine DNA glycosylase-1-KRAS-NF-kappaB pathway. J Immunol. 2014;193:4643–4653. doi: 10.4049/jimmunol.1401625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lai CC, Boguski M, Broek D, Powers S. Influence of guanine nucleotides on complex formation between Ras and CDC25 proteins. Mol Cell Biol. 1993;13:1345–1352. doi: 10.1128/mcb.13.3.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hajas G, Bacsi A, Aguilera-Aguirre L, Hegde ML, Tapas KH, Sur S, Radak Z, Ba X, Boldogh I. 8-Oxoguanine DNA glycosylase-1 links DNA repair to cellular signaling via the activation of the small GTPase Rac1. Free Radic Biol Med. 2013;61:384–394. doi: 10.1016/j.freeradbiomed.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pandita TK. Unraveling the novel function of the DNA repair enzyme 8-oxoguanine-DNA glycosylase in activating key signaling pathways. Free Radic Biol Med. 2014;73:439–440. doi: 10.1016/j.freeradbiomed.2014.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heo J. Redox control of GTPases: from molecular mechanisms to functional significance in health and disease. Antioxid Redox Signal. 2011;14:689–724. doi: 10.1089/ars.2009.2984. [DOI] [PubMed] [Google Scholar]
- 38.Simone S, Cosola C, Loverre A, Cariello M, Sallustio F, Rascio F, Gesualdo L, Schena FP, Grandaliano G, Pertosa G. BMP-2 induces a profibrotic phenotype in adult renal progenitor cells through Nox4 activation. Am J Physiol Renal Physiol. 2012;303:F23–F34. doi: 10.1152/ajprenal.00328.2011. [DOI] [PubMed] [Google Scholar]
- 39.Ryu J, Lee CW, Shin JA, Park CS, Kim JJ, Park SJ, Han KH. FcgammaRIIa mediates C-reactive protein-induced inflammatory responses of human vascular smooth muscle cells by activating NADPH oxidase 4. Cardiovasc Res. 2007;75:555–565. doi: 10.1016/j.cardiores.2007.04.027. [DOI] [PubMed] [Google Scholar]
- 40.Gianni D, Bohl B, Courtneidge SA, Bokoch GM. The involvement of the tyrosine kinase c-Src in the regulation of reactive oxygen species generation mediated by NADPH oxidase-1. Mol Biol Cell. 2008;19:2984–2994. doi: 10.1091/mbc.E08-02-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morreall J, Limpose K, Sheppard C, Kow YW, Werner E, Doetsch PW. Inactivation of a common OGG1 variant by TNF-alpha in mammalian cells. DNA Repair (Amst) 2015;26:15–22. doi: 10.1016/j.dnarep.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ba X, Aguilera-Aguirre L, Rashid QT, Bacsi A, Radak Z, Sur S, Hosoki K, Hegde ML, Boldogh I. The role of 8-oxoguanine DNA glycosylase-1 in inflammation. Int J Mol Sci. 2014;15:16975–16997. doi: 10.3390/ijms150916975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shiloh Y. ATM and related protein kinases: safeguarding genome integrity. Nat Rev Cancer. 2003;3:155–168. doi: 10.1038/nrc1011. [DOI] [PubMed] [Google Scholar]
- 44.Ciccia A, Elledge SJ. The DNA damage response: making it safe to play with knives. Mol Cell. 2010;40:179–204. doi: 10.1016/j.molcel.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bakkenist CJ, Kastan MB. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature (London) 2003;421:499–506. doi: 10.1038/nature01368. [DOI] [PubMed] [Google Scholar]
- 46.Ditch S, Paull TT. The ATM protein kinase and cellular redox signaling: beyond the DNA damage response. Trends Biochem Sci. 2012;37:15–22. doi: 10.1016/j.tibs.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Matsuoka S, Ballif BA, Smogorzewska A, McDonald ER, Hurov KE, Luo J, Bakalarski CE, Zhao Z, Solimini N, Lerenthal Y, et al. ATM and ATR substrate analysis reveals extensive protein networks responsive to DNA damage. Sci. 2007;316:1160–1166. doi: 10.1126/science.1140321. [DOI] [PubMed] [Google Scholar]
- 48.Huang TT, Wuerzberger-Davis SM, Wu ZH, Miyamoto S. Sequential modification of NEMO/IKKgamma by SUMO-1 and ubiquitin mediates NF-kappaB activation by genotoxic stress. Cell. 2003;115:565–576. doi: 10.1016/s0092-8674(03)00895-x. [DOI] [PubMed] [Google Scholar]
- 49.Tang ED, Wang CY, Xiong Y, Guan KL. A role for NF-kappaB essential modifier/IkappaB kinase-gamma (NEMO/IKKgamma) ubiquitination in the activation of the IkappaB kinase complex by tumor necrosis factor-alpha. J Biol Chem. 2003;278:37297–37305. doi: 10.1074/jbc.M303389200. [DOI] [PubMed] [Google Scholar]
- 50.Wu ZH, Shi Y, Tibbetts RS, Miyamoto S. Molecular linkage between the kinase ATM and NF-{kappa}B signaling in response to genotoxic stimuli. Science (Washington DC) 2006;311:1141–1146. doi: 10.1126/science.1121513. [DOI] [PubMed] [Google Scholar]
- 51.Wu CJ, Conze DB, Li T, Srinivasula SM, Ashwell JD. Sensing of Lys 63-linked polyubiquitination by NEMO is a key event in NF-[kappa]B activation. Nat Cell Biol. 2006;8:398–406. doi: 10.1038/ncb1384. [DOI] [PubMed] [Google Scholar]
- 52.Jin HS, Lee DH, Kim DH, Chung JH, Lee SJ, Lee TH. cIAP1, cIAP2, and XIAP act cooperatively via nonredundant pathways to regulate genotoxic stress-induced nuclear factor-kappaB activation. Cancer Res. 2009;69:1782–1791. doi: 10.1158/0008-5472.CAN-08-2256. [DOI] [PubMed] [Google Scholar]
- 53.Wu ZH, Wong ET, Shi Y, Niu J, Chen Z, Miyamoto S, Tergaonkar V. ATM- and NEMO-dependent ELKS ubiquitination coordinates TAK1-mediated IKK activation in response to genotoxic stress. Mol Cell. 2010;40:75–86. doi: 10.1016/j.molcel.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hinz M, Stilmann M, Arslan SC, Khanna KK, Dittmar G, Scheidereit C. A cytoplasmic ATM-TRAF6-cIAP1 module links nuclear DNA damage signaling to ubiquitin-mediated NF-kappaB activation. Mol Cell. 2010;40:63–74. doi: 10.1016/j.molcel.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 55.Choudhary S, Fang L, Tian B, Wu Z, Rosenblatt K, Brasier AR. High throughput siRNA screening of the human kinome identifies novel kinases controlling the canonical NF-κB activation pathway. J Biol Chem. 2011;286:37187–37195. doi: 10.1074/jbc.M111.224923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fang L, Choudhary S, Zhao Y, Edeh CB, Yang C, Boldogh I, Brasier AR. ATM regulates NF-κB-dependent immediate-early genes via RelA Ser 276 phosphorylation coupled to CDK9 promoter recruitment. Nucleic Acids Res. 2014;42:8416–8432. doi: 10.1093/nar/gku529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fang L, Choudhary S, Tian B, Boldogh I, Yang C, Ivanciuc T, Ma Y, Garofalo RP, Brasier AR. Ataxia telangiectasia mutated kinase mediates NF-kappaB serine 276 phosphorylation and interferon expression via the IRF7-RIG-I amplification loop in paramyxovirus infection. J Virol. 2015;89:2628–2642. doi: 10.1128/JVI.02458-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Message SD, Johnston SL. Host defense function of the airway epithelium in health and disease: clinical background. J Leukoc Biol. 2004;75:5–17. doi: 10.1189/jlb.0703315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Whitsett JA, Alenghat T. Respiratory epithelial cells orchestrate pulmonary innate immunity. Nat Immunol. 2015;16:27–35. doi: 10.1038/ni.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.von Garnier C, Filgueira L, Wikstrom M, Smith M, Thomas JA, Strickland DH, Holt PG, Stumbles PA. Anatomical location determines the distribution and function of dendritic cells and other APCs in the respiratory tract. J Immunol. 2005;175:1609–1618. doi: 10.4049/jimmunol.175.3.1609. [DOI] [PubMed] [Google Scholar]
- 61.Demedts IK, Bracke KR, Maes T, Joos GF, Brusselle GG. Different roles for human lung dendritic cell subsets in pulmonary immune defense mechanisms. Am J Respir Cell Mol Biol. 2006;35:387–393. doi: 10.1165/rcmb.2005-0382OC. [DOI] [PubMed] [Google Scholar]
- 62.Zhang Y, Luxon BA, Casola A, Garofalo RP, Jamaluddin M, Brasier AR. Expression of respiratory syncytial virus-induced chemokine gene networks in lower airway epithelial cells revealed by cDNA microarrays. J Virol. 2001;75:9044–9058. doi: 10.1128/JVI.75.19.9044-9058.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Olzewska B, Casola A, Saito T, Alam R, Crowe S, Mei F, Ogra PL, Garofalo R. Cell-specific expression of RANTES, MCP-1, and MIP-1a by lower airway epithelial cells and eosinophils infected with respiratory syncytial virus. J Virol. 1998;72:4756–4764. doi: 10.1128/jvi.72.6.4756-4764.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Van Eeden SF, Sin DD. Oxidative stress in chronic obstructive pulmonary disease: a lung and systemic process. Can Respir J. 2013;20:27–29. doi: 10.1155/2013/509130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sahiner UM, Birben E, Erzurum S, Sackesen C, Kalayci O. Oxidative stress in asthma. World Allergy Organ J. 2011;4:151–158. doi: 10.1097/WOX.0b013e318232389e. [DOI] [PMC free article] [PubMed] [Google Scholar]