Abstract
Background
Atrial fibrillation (AF) contributes significantly to morbidity and mortality in elderly patients and has been correlated with enhanced age-dependent atrial fibrosis. Reversal of atrial fibrosis has been proposed as therapeutic strategy to suppress AF.
Objective
To test the ability of relaxin to reverse aged-dependent atrial fibrosis and suppress AF.
Methods
Aged F-344 rats (24-months old) were treated with subcutaneous infusion of vehicle or relaxin (0.4 mg/kg/day) for 2-weeks. Rat hearts were excised, perfused on a Langendorff apparatus and stained with voltage and Ca2+ indicator dyes. Optical mapping and programmed electrical stimulation was used to test arrhythmia vulnerability and changes in electrophysiological characteristics. Changes in protein expression and Na+-current density (INa) were measured by tissue immunofluorescence and whole-cell patch clamp technique.
Results
In aged rats, sustained AF was readily induced with a premature pulse (n=7/8) and relaxin treatment suppressed sustained AF by a premature impulse or burst pacing (n=1/6) (p<0.01). Relaxin significantly increased atrial action potential conduction velocity and decreased atrial fibrosis. Relaxin-treatment increased Nav1.5 expression (n=6; 36±10%) and decreased total collagen and collagen I (n=5–6; 55–66±15%) in aged atria (p<0.05) and decreased collagen I&III and TGF-β1 mRNA (p<0.05). Voltage-clamp experiments demonstrated that relaxin-treatment (100nM for 2 days) increased atrial INa by 46±4% (n=12–13/group, p<0.02).
Conclusion
Relaxin suppresses AF through an increase in atrial conduction velocity by decreasing atrial fibrosis and increasing INa. This data provides compelling evidence that relaxin may serve as an effective therapy to manage AF in geriatric patients by reversing fibrosis and modulating cardiac ionic currents.
Keywords: atrial fibrillation, relaxin, fibrosis, sodium channel, aging
Introduction
Atrial fibrillation (AF), a disease associated with high mortality, morbidity, and steep costs, affects tens of millions of people worldwide and is increasing in prevalence.1 A major factor contributing to the increasing prevalence of AF is an aging world population. The prevalence of AF is 0.12%–0.16% in people younger than 49 years, 3.7%–4.2% for those 60–70 years old, and 10%-17% for those 80 years or older.2 The incidence of new onset of AF roughly doubles with each decade of age, independent of the increasing prevalence of known predisposing conditions.3 Age is not a modifiable risk factor and even healthy adults without traditional risk factors for AF are at ever increasing risk of developing AF. In the Framingham Heart Study, AF was associated with a 1.5- to 1.9-fold increase in mortality risk after adjusting for preexisting cardiovascular conditions.4 Therefore, an otherwise healthy adult without modifiable risk factors who develops AF will be at an increased risk of death.
Just like many chronic diseases (hypertension, diabetes, heart failure, etc.), the normal aging process can lead to structural changes of the atrial extracellular matrix (ECM) and enhanced AF vulnerability as a result of the altered myocardial substrate (atrial structural remodeling).5 Fibrosis is a hallmark of arrhythmogenic ECM remodeling creating a barrier to normal cardiac electrical impulse propagation by disrupting inter-myocyte coupling in the atria.6 Increased collagen deposition has been well documented in AF patients compared with control subjects.7 Despite the role of atrial fibrosis in AF, current antiarrhythmic and ablation therapies do nothing to mitigate or alter atrial fibrosis. Furthermore, in the geriatric population, current rate and rhythm control strategies for AF are associated with decreased efficacy and increased morbidity and mortality.
In search for an AF therapy, our group previously showed that the hormone relaxin suppresses AF and reverse atrial fibrosis in the spontaneously hypertensive rat (SHR) model.8 Relaxin treatment suppressed AF by several mechanisms including beneficial effects on atrial structural and electrical remodeling. There was histological evidence of reversal of atrial fibrosis and reduction of atrial myocyte hypertrophy. At the molecular level, relaxin decreased the expression of several pro-fibrotic mediators including TGF-β1, collagen I and III, matrix metalloproteinases 2 and 9 and reduced connexin 43 phosphorylation. As proof of concept for its relevance to human hearts, human cardiomyocytes derived from induced pluripotent stem cells (hiPS-CMs) were exposed to relaxin for 1–2 days to reveal a doubling of the sodium current density, INa, indicative of electrical remodeling.8 The remodeling of the atrial myocardial substrate and its electrophysiology collectively contributed to the suppression of AF by relaxin in the SHR model.
To test the actions of relaxin on age-induced AF, we used Fischer 344/Brown Norway F1 (F-344) rats which are recommended by the National Institute on Aging (NIA) as a model of aged-related changes since this hybrid lives longer and has a more gradual rate of aging than inbred rats.9 Indeed, prior studies by Hayashi et al. demonstrated that 22–24 month-old F 344 rats are readily induced into AF by burst electrical pacing whereas younger F344 rats (2–3 months old) were not inducible.10 Optical mapping of Langendorff-perfused hearts showed a marked increase in conduction time in the atria of old rats and AF was sustained by the propagation of 2–4 independent wave fronts.10 Old atria had significantly higher levels of interstitial fibrosis (15-fold) but cell-cell uncoupling of young atria with heptanol promoted the induction of atrial tachyarrhythmia but not AF.10 Here, we tested the possibility that relaxin may be protective of age-dependent AF and age-related fibrosis which is not secondary to cardiovascular pathology (hypertension, diabetes, heart failure, etc.), but rather the normal aging process. In 24 month-old, otherwise healthy F-344 rats (“aged rats”), we confirmed the prior findings and show that treatment with relaxin suppresses AF by reversing atrial fibrosis and increasing conduction velocity and the sodium current density, INa.
Methods
Detailed descriptions of all methods used are included in the Supplemental Materials section. Studies were performed in accordance with the Guide for the Care and Use of Laboratory Animals and were approved by the IACUC at the University of Pittsburgh.
Animal Study Design
AF inducibility was studied in age-matched (24 months) and sex-matched (male) Fischer 344 rats (F-344 rat colony of the NIA) that were separated into two groups whereby the control group treated with the vehicle (V) and the experimental group was treated with relaxin (RLX; gift from Dr. Mario Bigazzi, Foundation for Research on Relaxin in Cardiovascular and Other Diseases). RLX (400 µg/kg/day) or V were infused by osmotic mini-pumps (ALZET) for 14-days.8 After 14-days, rats were anesthetized, their heart was excised and then perfused on a Langendorff apparatus with physiological Tyrode’s solution.
Optical Mapping, Programmed Stimulation and Inducing AF
Rats were anesthetized with pentobarbital (50 mg/kg), injected with heparin (200 U/kg IV), then the heart was excised and perfused on a Langendorff apparatus with physiological Tyrode’s solution then were stained with bolus injections of a voltage-sensitive dye and a Ca2+ indicator, as previously described.11 Light was collimated, passed through 530 ± 30 nm interference filters, split by a 560 nm dichroic mirror and focused on the atria. Fluorescence from the heart was collected with tandem camera lenses, was split with a 600 nm dichroic mirror to focus images of the atria at short (570–595 nm) and long (610–750 nm) wavelengths on two CMOS cameras.11 Pixel resolution was 150×150 µm2, and the data was recorded and stored in 4–8 second intervals. To reduce motion artifacts, the hearts were placed in a chamber and perfused with blebbistatin (3–5 µM) for 10 min. Local conduction velocity (CV) vectors were calculated for each pixel then were averaged and calculated as mean ± SEM. CV restitution kinetics curves were generated by plotting the mean CV vs. S1–S2 interval in milliseconds. To test AF vulnerability, each heart was paced from the RA using a programmed stimulation protocol consisting of 10 S1 pulses at 250 ms cycle length (CL) followed by a premature S2 pulse with progressively shorter S1–S2 intervals until loss of capture or the initiation of AF occurred. Transient AF was defined as lasting <20 seconds and self-terminated, whereas sustained AF lasted >3 minutes. AF vulnerability was then test by pacing the LA.
F-334 Rat Atrial Myocyte Isolation, Incubation and Immunofluorescence Imaging
Excised rat hearts were perfused for 5 min, treated with collagenase type II (2 mg/ml) for a 20 min at 37°C then the atria were minced in a stopping buffer and myocytes were allowed to settle to form a pellet. The supernatant was removed and myocytes were re-suspended and plated onto laminin-coated coverslips for 2 hours. The plating media was removed and replaced by culture media. Atrial myocytes were incubated with or without 100 nM RLX. After 48 hours, glass cover slips containing rat atrial myocytes were fixed with 2% paraformaldehyde (PFA) followed by incubation with Nav1.5 primary antibody and then a fluorescent secondary antibody conjugated with Alexa488. Images were obtained using an inverted confocal microscope. Once optimal imaging parameters were determined, all subsequent images were obtained using identical conditions. Intensity analysis was performed using NIS Elements imaging analysis software.
RT-PCR Analysis and Immunofluorescence Imaging of Atrial Tissue
RT-PCR analysis was used to measure expression levels of RNAs of interest (collagen I, collagen III, TGF-β), which were normalized to GAPDH. Atrial tissues were fixed in 2% PFA, equilibrated in 30% sucrose, and flash frozen. Frozen sections (7 micron thick) were cut by cryostat and sections incubated with anti-collagen I or anti-Nav1.5 followed by Alexa488-conjugated secondary antibodies and Phalloidin Cy3. Slides were viewed at 10–20× with an inverted confocal microscope. Once optimal imaging parameters were determined, all subsequent images were obtained using the same conditions. Intensity analysis was performed using NIS Elements imaging analysis software. Collagen I to tissue area ratio was calculated as previously described using the area stained with Phalloidin to index tissue area, averaging 20–25 random fields per heart, with 5–7 rats per group.12 Mean intensity analysis was performed using NIS Elements imaging analysis software. Primer sequences are given in “Supplemental Materials”.
Electrophysiological Recordings of F-334 Rat Atrial Myocytes
Rat atrial myocytes were isolated and cultured as stated above. INa was measured using the whole cell configuration of the patch-clamp technique. Currents were measured with an Axopatch 200B amplifier. INa was elicited for a holding potential of −100 mV, and peak INa was recorded during 50 ms voltage-clamp steps from −100 to +35 mV in 5 mV increments proceeding a 200 ms conditioning pulse at −135 mV. INa was normalized to cell capacitance and was reported as peak current density in pA/pF.
Statistics
AF vulnerability between the different groups was compared using Fisher exact test. Parameters recorded under different S1–S2 were compared using ANOVA. For immunofluorescence microscopy, comparisons among the 2 groups were performed using a Student t-test. All results are reported as mean ± SEM. For all tests, a value of P<0.05 was considered to be statistically significant.
For additional details see “supplement materials”.
RESULTS
Aged rats are more susceptible to inducible AF than younger rats
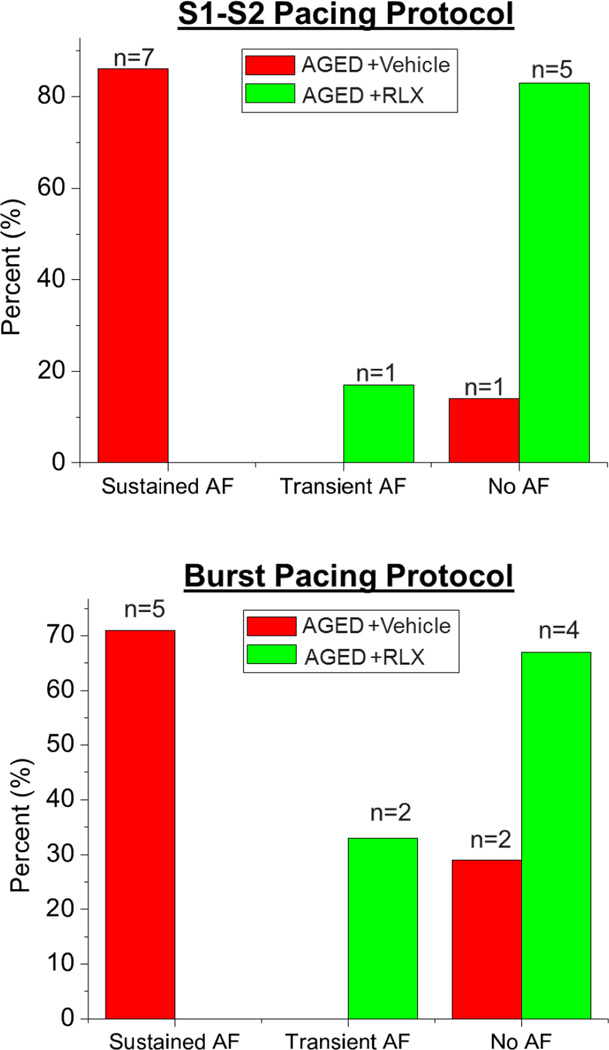
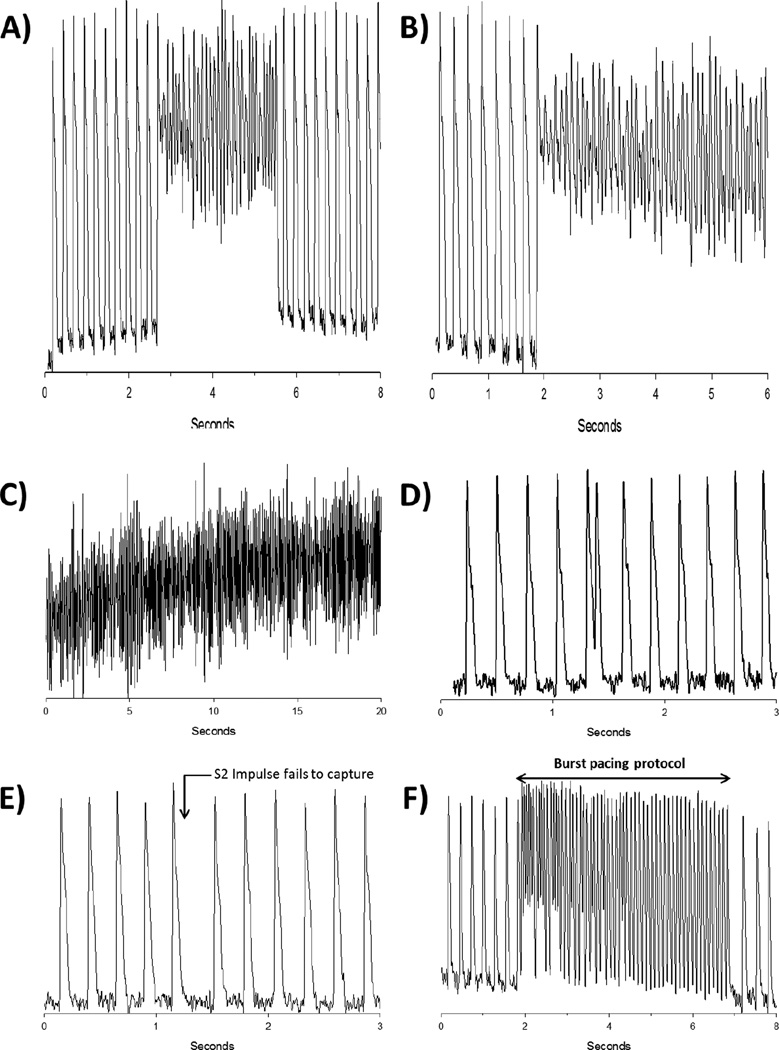
Normotensive Wistar-Kyoto (WKY) rats aged 9–12 months were not vulnerable to AF induced by a premature impulse8 and burst pacing failed to elicit an arrhythmia in young (2–3 months old) F-344 rats.10 Aging has been shown to be an independent risk factor for the development of AF even without concomitant cardiovascular comorbidities;4 hence we wanted to study the effects of Relaxin on AF in a model of age-related AF in 24 month-old F-344) rats that are otherwise ‘normal’ rats.10 Hearts were Langendorff-perfused, mapped optically then programmed stimulation was applied to test AF vulnerability, as previously described.8 AF was induced in 7 of 8 aged rats (Figure 1), which is in contrast to earlier work with adult (9–12 month old) WKY or (2–3 months) F-334 rats where AF could not be induced. After 2- weeks of Relaxin treatment there were no changes in blood pressure in aged rats (see in Supplemental Materials), consistent with findings in SHRs.8 In aged rat hearts, a single premature impulse close to the refractory period (S1–S2= 45 ms) induced a transient arrhythmia (Figure 2A) while an impulse applied at a still shorter interval (S1–S2= 40 ms) produced a sustained AF (Figure 2B and 2C). The use of more provocative arrhythmogenic techniques like burst pacing (10 stimuli, 10-ms apart) consistently induced a sustained AF, which could be terminated with a transient bolus of KCl to restore sinus rhythm.
Figure 1. Inducibility of atrial fibrillation (AF) in aged rats treated with relaxin (RLX) or vehicle.
Aged rats were readily inducible into sustained AF while RLX treated aged rats were considerably more refractory to inducible AF.
Figure 2. Relaxin treatment (RLX) significantly decreases AF inducibility in aged rats.
A: Representative action potential (AP) tracing in an aged rat. A premature pulse (S1–S2 interval 45 ms) elicits a transient episode of AF. B/C: When a shorter S1–S2 interval of 40 ms is delivered, sustained AF is induced. D: Representative AP tracing in an RLX treated aged rat. A premature pulse (S1–S2 interval 45 ms) is longer than the refractory period and captures. E: When an S1–S2 impulse which is shorter than the refractory period is delivered, no capture occurs. F: Burst pacing was unable to elicit AF in RLX treated aged rats.
Relaxin ameliorates inducible AF in aged rats
The most important and consistent finding was that relaxin treatment of aged rats for 2 weeks suppressed AF inducibility (Figure 1, Figure 2 D–F). Of the 6 aged rats treated with relaxin, only 1 rat had an inducible AF which was transient. In contrast, the vehicle treated rats were highly susceptible to inducible AF (n=7/8; P<0.01 versus relaxin treatment). More robust attempts to elicit AF in relaxin treated aged rat hearts, such as varying the location of the pacing electrode and burst pacing (10 stimuli, 10-ms apart) on either the right or left atria, all failed to elicit sustained AF in most of the rats (Figure 1). The refractory periods (between 45–50 ms) were not significantly different between the relaxin treated or vehicle treated aged rats. However, relaxin treated aged rats were able to successfully conduct an S2 impulse of 45 ms without developing transient or sustained AF (Figure 2C), in contrast to untreated aged rats (Figure 2A). Shorter S1–S2 intervals (<45 ms) did not succeed at eliciting a propagated wave and these impulses did not precipitate a sustained AF (Figure 2D) as opposed to untreated aged rats (Figure 2B). In fact, even the most provocative arrhythmogenic burst pacing protocols failed to induce AF in the majority of relaxin treated rats (Figures 1 & 2F).
Relaxin increases conduction velocity and improves restitution in aged rats
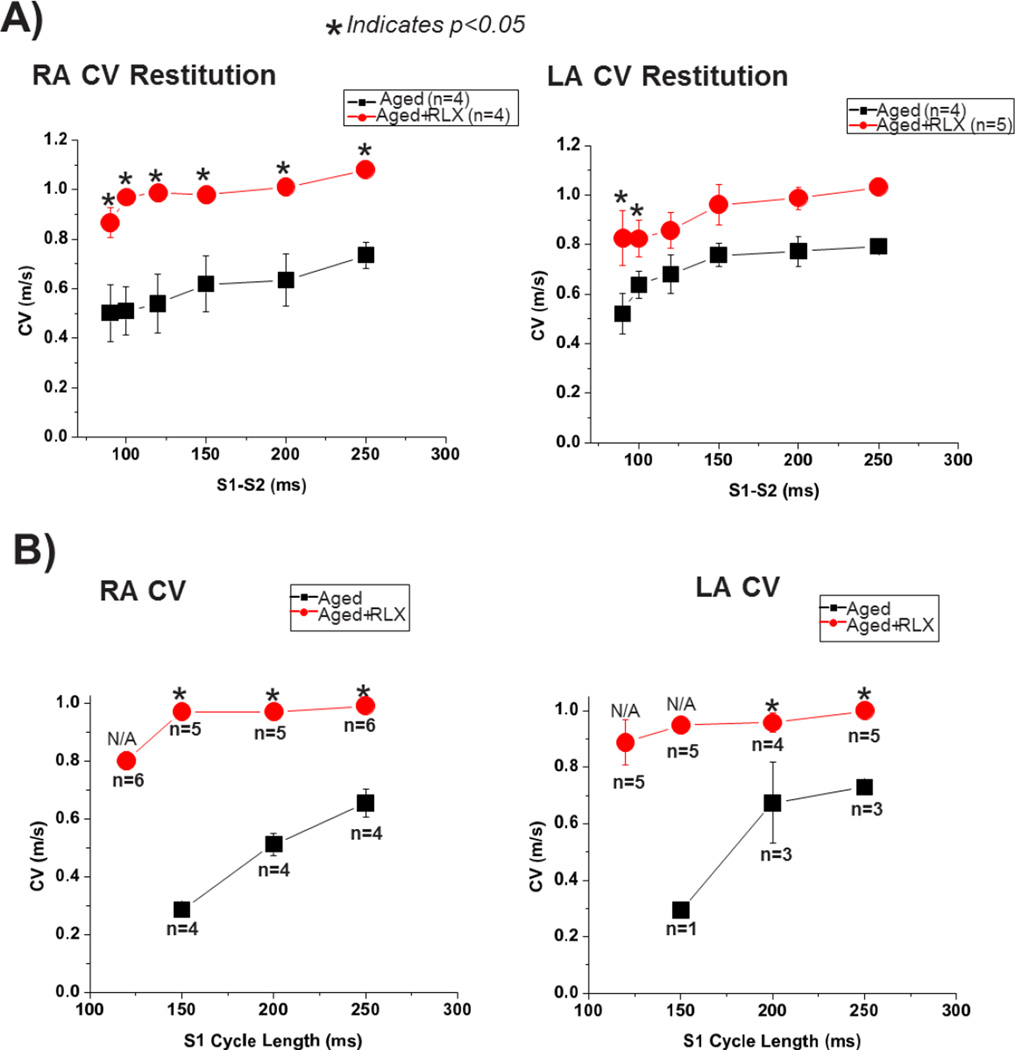
Conduction velocity (CV) and action potential duration (APD) restitution kinetics (RK) were measured from the right and left atria (RA/LA) of aged rats, with or without relaxin administration. Figure 3A shows a marked effect of relaxin on the restitution kinetics of CV in the LA and RA compared with untreated hearts; namely, a large increase in CV particularly for short S1–S2 intervals and a less-steep RK curve. When CV was measured during simple S1 pacing, relaxin treated aged rats had a significantly higher CV regardless of the S1 cycle length (Figure 3B). As the S1 cycle length decreases (heart rate increases) the CV drops precipitously in the untreated aged rat hearts. In fact, at the shortest S1 cycle length tested (125 ms); untreated aged hearts were unable to pace at this highest rate representing a loss of restitution. Conversely, the relaxin treated aged rat hearts were able to pace at every cycle length tested and were able to maintain a consistent CV regardless of S1 cycle length indicating an improvement in CV restitution in the atria. Relaxin treatment did not significantly alter the APD restitution kinetics for LA and RA (data not shown).
Figure 3. Restitution kinetics (RK) of conduction velocity (CV) during S1–S2 (A) and S1 (B) pacing.
RK measured for CV from the left and right atrium (LA and RA), respectively. All values are reported as mean ± SEM.
Relaxin reverses age-related atrial fibrosis
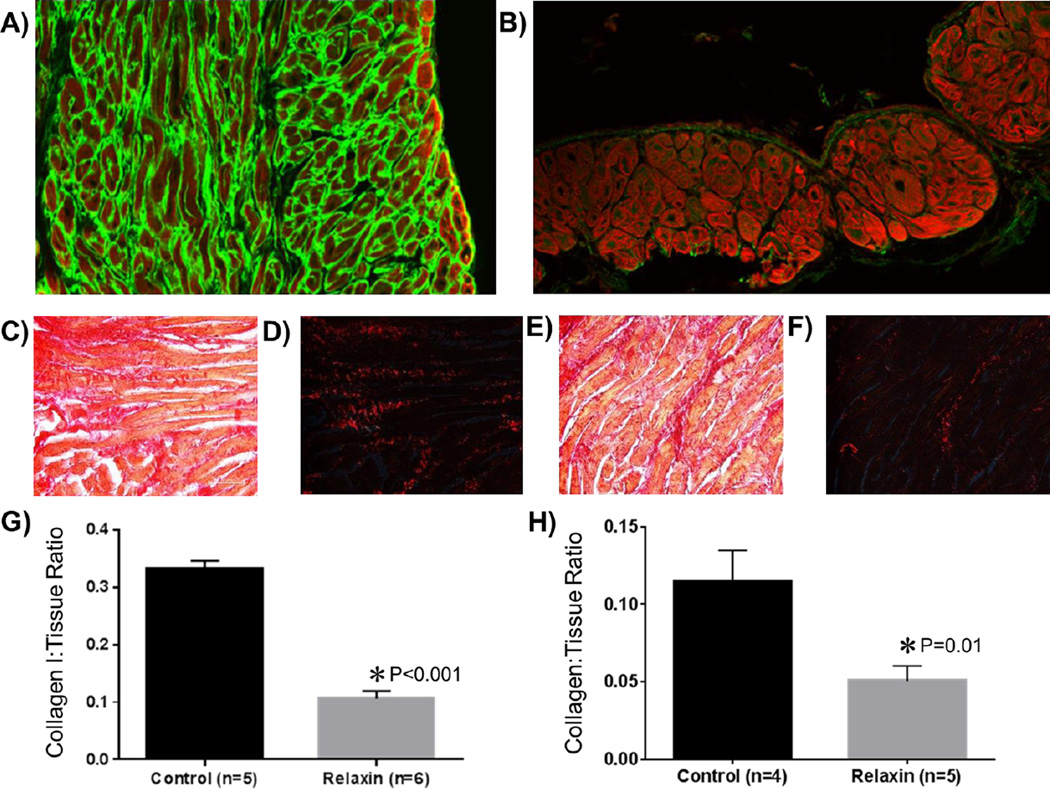
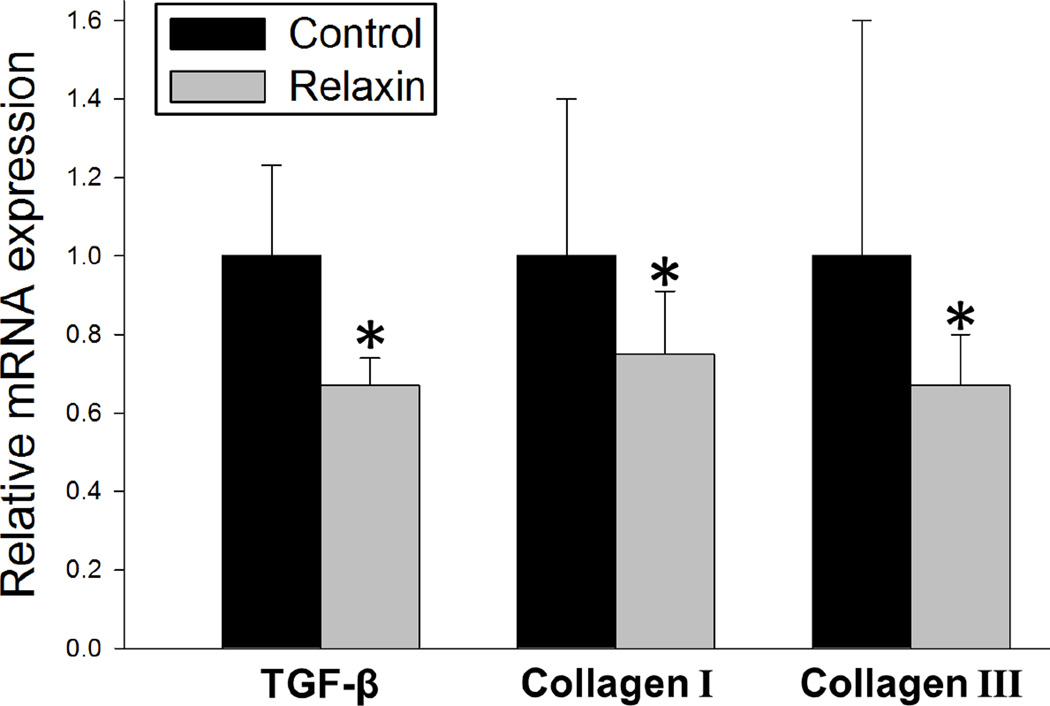
Differences in the amount of atrial fibrosis between the untreated control group and the relaxin treated group are shown in Figure 4A/C/D and B/E/F, respectively. After 2 weeks of treatment, relaxin treated aged rats had an attenuation of fibrosis indicated by a significantly lower atrial collagen to tissue ratio compared with untreated aged rats (P<0.001). Specifically, relaxin decreased the ratio of collagen-I to atrial tissue area by 66% (Figure 4G) and the total collagen-to-tissue ratio by 55% (Figure 4H). Additionally, RT-PCR analysis of atrial tissues demonstrate that relaxin decreases several pro-fibrotic mRNA transcripts for TGF-β, collagen I and collagen III by 25–33% (Figure 5). These findings are consistent with our previous work as well as the work of others.8, 13
Figure 4. Effect of Relaxin on Atrial Fibrosis.
The top panels are atrial immunohistological sections (20×) of aged rat hearts treated with vehicle (A) or relaxin (B). Phalloidin is represented in red and collagen I in green. The middle panels are aged rat atrial sections (40×) stained with picrosirius red. Dark red staining denotes fibrosis. Images C&D are from vehicle treated aged rats imaged under non-polarized (C) and polarized light (D). Images E&F are from relaxin treated aged rats under non-polarized (E) and polarized light (F). G and H quantify the collagen I or collagen to tissue ratio.
Figure 5. Relaxin treatment decreases expression of fibrosis related transcripts.
Relative expression of profibrotic transcripts in relaxin treated aged rats relative to untreated controls isolated from atria tissue. All values are reported as mean ± SEM; *P<0.05.
Relaxin increases the expression of voltage-gated Na+ channels
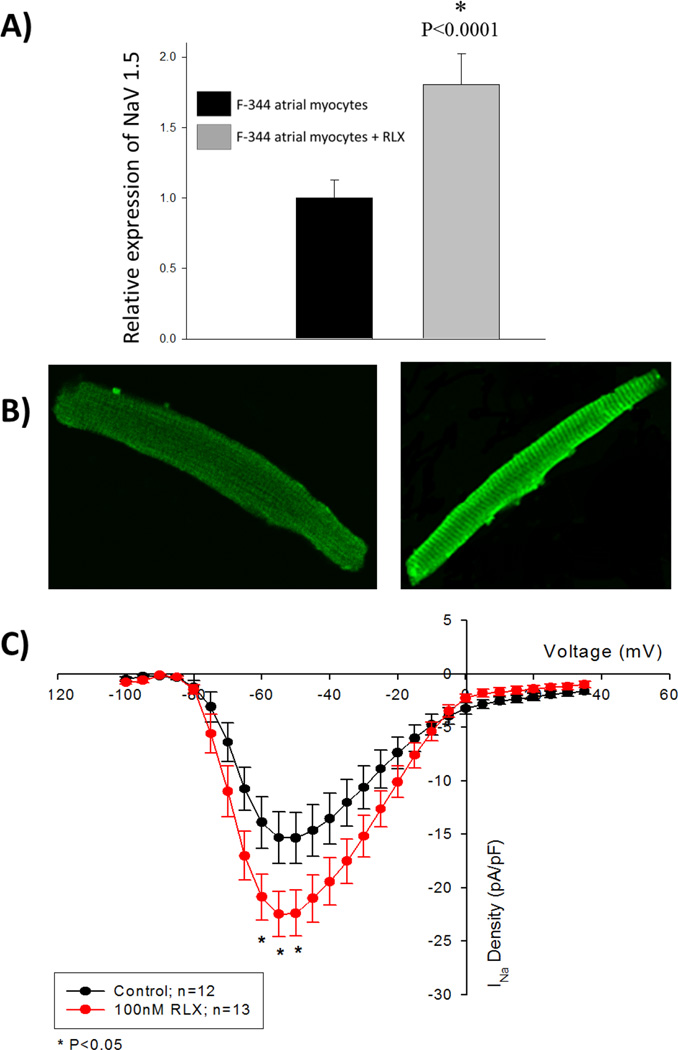
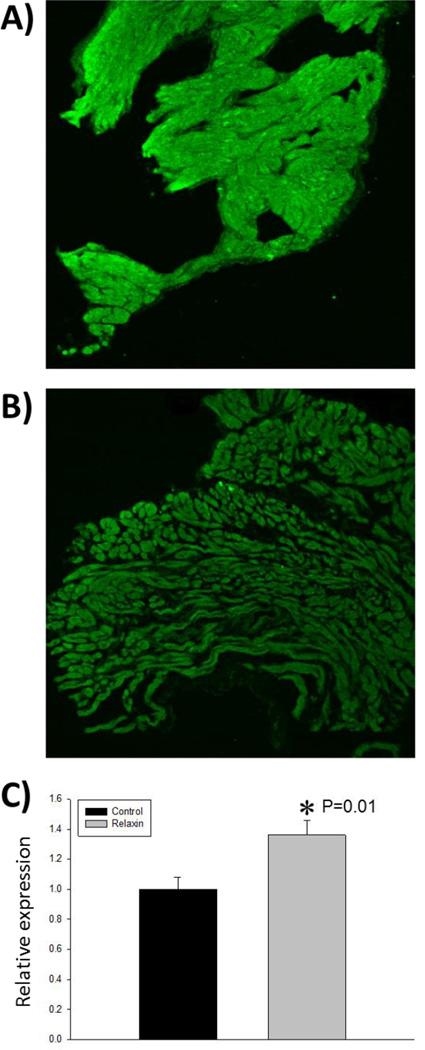
The major electrophysiological change caused by relaxin treatment is a marked increase in CV and the maintenance of those increased CV at decreased cycle lengths (increased heart rate). In addition to reduced atrial fibrosis, an increase in the current density of voltage-gated sodium channels, INa, could help explain these changes in CV. To test the effects of relaxin on INa, we tested the effects of relaxin on INa density in F344 atrial myocytes. F344 rat atrial myocytes were isolated and cultured in the presence or absence of 100 nM relaxin for 48 hours. Immunofluorescence was used to quantify the expression the voltage-gated sodium channel, Nav1.5. Rat atrial myocytes cultured in the presence of relaxin demonstrated an 80% increase in the expression of Nav1.5 (Figure 6A and 6B). To build upon this observation that relaxin upregulates the expression of Nav1.5, freshly isolated F334 atrial myocytes were incubated with or without relaxin (100nM) and the current -to-voltage (I–V) relationship was measured for INa after 48 hours. As shown in Figure 6C, 48 hours of incubation with relaxin significantly increased INa density in atrial myocytes in the voltage range of −60 to −50 mV (P<0.05) compared to untreated cells. At a voltage of −50 mV, exposure to relaxin increased the sodium current density by 46% over the control (−15.33±0.68 pA/pF, controls, n=12; −22.39±0.60 pA/pF, relaxin, n=13; P<0.01). These findings are consistent with the immunocytochemistry data showing that relaxin exposure to atrial myocytes increases the expression of Nav1.5. In our aged rat experiments, immunofluorescence microscopy was performed on LA tissue to determine if relaxin treatment increases the expression of Nav1.5 as was observed in the cell culture experiments. Relaxin treated aged rats had a 36% increased expression of Nav1.5 compared to untreated aged rats (Figure 7).
Figure 6. Relaxin (RLX) treatment of F-344 rat atrial myocytes increases expression of voltage-gated Na+ channels.
A: F-344 rat atrial myocytes were co-incubated with 100 nM RLX for 48 hours. As measured by immunofluorescence, the relative expression of Nav1.5 is increased by RLX. B: The left and right panels (40×) show representative rat atrial myocytes stained with Nav1.5 antibody without and with 100 nM relaxin, respectively. C: Current-to-voltage (I–V) plots for control and RLX-treated rat atrial myocytes demonstrate an upregulation of Na+ current density. All values are reported as mean ± SEM.
Figure 7. Relaxin increases expression of voltage-gated Na+ channels in the atria of aged rats.
A representative image of left atrial tissue from an aged rat treated with vehicle (A) or relaxin (B). As measured by immunofluorescence, the relative expression of Nav1.5 is increased by relaxin (C). All values are reported as mean ± SEM.
DISCUSSION
Previous work has documented the role of aging on cardiac fibrosis, electrical remodeling and increased susceptibility to arrhythmia in the rat heart.14, 15 Aged (F-344) rat hearts exhibit extensive interstitial fibrosis compared to young adult rats and this fibrosis has been shown to facilitate arrhythmias perhaps by promoting generation of early afterdepolarizations and ectopic activity leading to fibrillation.10 Our work builds upon and reaffirms these concepts but more importantly, it also demonstrates that these age-related changes are reversible and modifiable, not inexorable. Previous work has shown that aged rat hearts are prone to atrial and ventricular fibrillation.14–16 These results are distinct from our prior work in SHRs where chronic hypertension was the underlying catalyst for AF susceptibility. Regardless of whether the etiology for AF vulnerability was a modifiable (hypertension) or a non-modifiable risk factor (age), relaxin was able to suppress the development of AF. In both models the mechanism of AF suppression appears to be through an improvement in both atrial electrical and structural remodeling. Specifically, relaxin has been shown to increase slow, pro-arrhythmic CVs by increasing INa in SHR and aged rat hearts. In isolated rat and hiPS-CMs, relaxin increases the INa within 24 hours. In aged rats, we now show that relaxin also has potent anti-fibrotic effects through the suppression of collagen I and III deposition and by decreasing the pro-fibrotic mRNA transcripts of TGF-β, collagen I and collagen III resulting in a significant decrease in the ratio collagen to total tissue in the atria. This reduction in fibrosis also has a beneficial effect on increasing CV. Furthermore Relaxin enhanced the expression of Nav1.5 and its current, INa thereby increasing CV and imparting an important component of the antiarrhythmic effects of relaxin. Until now, it has not previously been demonstrated that aged-associated atrial fibrosis is reversible. Furthermore, our work is exciting because it advances the novel concept that relaxin, a hormone most closely associated with pregnancy, is a potent antiarrhythmic and it’s antiarrhythmic properties are completely unlike all other classes of antiarrhythmic therapies.
In human AF, structural remodeling is characterized by atrial enlargement and tissue fibrosis. Fibrosis promotes AF by interrupting fiber bundle continuity and causing local conduction disturbances.17 Atrial fibrosis appears to be a common endpoint of a wide range of AF-promoting conditions and predicts recurrences.18 Furthermore, AF appears to promote further development of atrial fibrosis, which is an important contributor to therapeutic resistance in patients with long-standing AF.19 To date, no therapies have been developed to effectively reverse atrial fibrosis, however, relaxin may offer promise in addressing this unmet clinical need.
One of the most exciting aspects of relaxin therapy as a potential treatment for AF and underlying atrial fibrosis is that relaxin is already being studied in humans. In the RELAX-AHF trial, over 1100 patients were randomized and 567 received serelaxin therapy.20 Although designed and powered to primarily study relief of dyspnea secondary to acute heart failure, 52% of the patients enrolled had a history of AF and 41% were in AF at the time of enrollment.20 Given that serelaxin has been shown to be safe in clinical trials composed of a high percentage of AF patients and patients at high-risk for the development of AF,20, 21 along with our work demonstrating the ability of relaxin to suppress AF,8 there appears to be a rational basis to study serelaxin in human AF trials. To date, there has been no published subgroup analysis from the RELAX-AHF trial with respect to AF. All currently published data from the RELAX-AHF trial does not extend beyond 180-days post-serelaxin treatment. Therefore, the opportunity does exist for future retrospective analysis of the effects of serelaxin on AF in the RELAX-AHF patient population. Such analysis could be instrumental to better understand the effects of serelaxin on AF and could be useful in generating hypotheses for future randomized clinical trials in AF. A recent subgroup analysis comparing mode of death in patients from the RELAX-AHF trial showed that the 6-month mortality benefit from serelaxin is driven by a reduction in sudden cardiac death and other cardiovascular death (predominately stroke) but not from a reduction in heart failure death.22 Our data indicates that relaxin promotes beneficial cardiac electrical remodeling and suppresses AF. These changes may be occurring in serelaxin treated patients and may help to explain the reduction in sudden cardiac death and death from stroke, respectively.23 Based on the data from clinical trials as well as basic and translational studies, relaxin represents an exciting new and potentially beneficial therapy in AF.
Conclusions
The major findings of our work demonstrate that aged rat hearts have a significant susceptibility to inducible AF triggered by premature atrial impulses and that treatment of aged rats with relaxin for 2-weeks suppressed the development of AF. Relaxin significantly reversed atrial fibrosis, increased atrial CV and improved atrial restitution during fast S1 pacing. The increase in atrial CV and improved restitution is a function of both the upregulation of Nav1.5 resulting in an increase in INa density combined with the reversal of atrial fibrosis. These results suggest that within the aged atria, the pleiotropic hormone relaxin has antifibrotic and antiarrhythmic properties.
Supplementary Material
Clinical Perspectives.
Current management strategies for AF focus on treatment of the underlying rhythm by either ventricular rate control or maintenance of sinus rhythm though ablation or antiarrhythmic therapy. These strategies do not directly address the underlying problem in AF, which is structural and electrical remodeling of the atrium. Furthermore, in the geriatric population, our rate and rhythm control strategies are less efficacious and cause greater morbidity and mortality than in younger AF populations. Our manuscript demonstrates that relaxin suppresses AF in aged rats and does so by directly reversing age-related structural and electrical atrial remodeling which is in contrast to all other currently used treatment modalities for AF. Unlike most preclinical studies which show promising benefit but are totally unproven in humans, relaxin has been extensively studied in human clinical trials and has been shown to be safe and effective in patients. In the RELAX-AHF trial, relaxin was shown to improve 6-month mortality in heart failure patients by decreasing stroke and sudden cardiac death. Our preclinical studies provide a rationale for extending the use of relaxin beyond acute decompensated heart failure to the field of atrial fibrillation. Besides its safety profile, relaxin may represent a superior alternative to our current treatment strategies for geriatric AF patients by directly addressing the underlying atrial remodeling driving AF. Both subgroup analysis of existing clinical trial data and future randomized clinical trials specifically designed for AF end points will be necessary to translate this promising therapy into clinical reality.
Acknowledgements
The study was supported by McGinnis Endowed Chair funds to SGS and grants from the National Institutes of Health (UL1 RR024153 and UL1TR000005 to DS and GS, and the National Heart and Lung Institute HL093074 to GS.
Abbreviations
- AF
atrial fibrillation
- AP
action potential
- CV
conduction velocity
- ECM
extracellular matrix
- F
fluorescence
- hiPS-CMs
human induced pluripotent stem cells derived cardiomyocytes
- LA
left atrium
- PBS
phosphate-buffered saline
- RA
right atrium
- RLX
relaxin
- RK
restitution kinetics
- SHR
spontaneously hypertensive rat
- TGF-β1
Tumor Growth Factor β1
- V
vehicle
- WKY
Wistar-Kyoto
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: none.
REFERENCES
- 1.Lip GY, Brechin CM, Lane DA. The global burden of atrial fibrillation and stroke: A systematic review of the epidemiology of atrial fibrillation in regions outside north america and europe. Chest. 2012;142:1489–1498. doi: 10.1378/chest.11-2888. [DOI] [PubMed] [Google Scholar]
- 2.Zoni-Berisso M, Lercari F, Carazza T, Domenicucci S. Epidemiology of atrial fibrillation: European perspective. Clin Epidemiol. 2014;6:213–220. doi: 10.2147/CLEP.S47385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kannel WB, Wolf PA, Benjamin EJ, Levy D. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: Population-based estimates. Am J Cardiol. 1998;82:2N–9N. doi: 10.1016/s0002-9149(98)00583-9. [DOI] [PubMed] [Google Scholar]
- 4.Benjamin EJ, Wolf PA, D'Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: The framingham heart study. Circulation. 1998;98:946–952. doi: 10.1161/01.cir.98.10.946. [DOI] [PubMed] [Google Scholar]
- 5.Lau YF, Yiu KH, Siu CW, Tse HF. Hypertension and atrial fibrillation: Epidemiology, pathophysiology and therapeutic implications. J Hum Hypertens. 2012;26:563–569. doi: 10.1038/jhh.2011.105. [DOI] [PubMed] [Google Scholar]
- 6.Burstein B, Nattel S. Atrial fibrosis: Mechanisms and clinical relevance in atrial fibrillation. J Am Coll Cardiol. 2008;51:802–809. doi: 10.1016/j.jacc.2007.09.064. [DOI] [PubMed] [Google Scholar]
- 7.Anter E, Callans DJ, Wyse DG. Pharmacological and electrical conversion of atrial fibrillation to sinus rhythm is worth the effort. Circulation. 2009;120:1436–1443. doi: 10.1161/CIRCULATIONAHA.108.824847. [DOI] [PubMed] [Google Scholar]
- 8.Parikh A, Patel D, McTiernan CF, Xiang W, Haney J, Yang L, Lin B, Kaplan AD, Bett GC, Rasmusson RL, Shroff SG, Schwartzman D, Salama G. Relaxin suppresses atrial fibrillation by reversing fibrosis and myocyte hypertrophy and increasing conduction velocity and sodium current in spontaneously hypertensive rat hearts. Circ Res. 2013;113:313–321. doi: 10.1161/CIRCRESAHA.113.301646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boluyt MO, Converso K, Hwang HS, Mikkor A, Russell MW. Echocardiographic assessment of age-associated changes in systolic and diastolic function of the female f344 rat heart. J Appl Physiol (1985) 2004;96:822–828. doi: 10.1152/japplphysiol.01026.2003. [DOI] [PubMed] [Google Scholar]
- 10.Hayashi H, Wang C, Miyauchi Y, Omichi C, Pak HN, Zhou S, Ohara T, Mandel WJ, Lin SF, Fishbein MC, Chen PS, Karagueuzian HS. Aging-related increase to inducible atrial fibrillation in the rat model. Journal of cardiovascular electrophysiology. 2002;13:801–808. doi: 10.1046/j.1540-8167.2002.00801.x. [DOI] [PubMed] [Google Scholar]
- 11.Salama G, Hwang SM. Simultaneous optical mapping of intracellular free calcium and action potentials from langendorff perfused hearts. Curr Protoc Cytom. 2009;Chapter 12(Unit 12):17. doi: 10.1002/0471142956.cy1217s49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li YY, Feng YQ, Kadokami T, McTiernan CF, Draviam R, Watkins SC, Feldman AM. Myocardial extracellular matrix remodeling in transgenic mice overexpressing tumor necrosis factor alpha can be modulated by anti-tumor necrosis factor alpha therapy. Proc Natl Acad Sci U S A. 2000;97:12746–12751. doi: 10.1073/pnas.97.23.12746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lekgabe ED, Kiriazis H, Zhao C, Xu Q, Moore XL, Su Y, Bathgate RA, Du XJ, Samuel CS. Relaxin reverses cardiac and renal fibrosis in spontaneously hypertensive rats. Hypertension. 2005;46:412–418. doi: 10.1161/01.HYP.0000171930.00697.2f. [DOI] [PubMed] [Google Scholar]
- 14.Bapat A, Nguyen TP, Lee JH, Sovari AA, Fishbein MC, Weiss JN, Karagueuzian HS. Enhanced sensitivity of aged fibrotic hearts to angiotensin ii- and hypokalemia-induced early afterdepolarization-mediated ventricular arrhythmias. Am J Physiol Heart Circ Physiol. 2012;302:H2331–H2340. doi: 10.1152/ajpheart.00094.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morita N, Sovari AA, Xie Y, Fishbein MC, Mandel WJ, Garfinkel A, Lin SF, Chen PS, Xie LH, Chen F, Qu Z, Weiss JN, Karagueuzian HS. Increased susceptibility of aged hearts to ventricular fibrillation during oxidative stress. Am J Physiol Heart Circ Physiol. 2009;297:H1594–H1605. doi: 10.1152/ajpheart.00579.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morita N, Lee JH, Bapat A, Fishbein MC, Mandel WJ, Chen PS, Weiss JN, Karagueuzian HS. Glycolytic inhibition causes spontaneous ventricular fibrillation in aged hearts. Am J Physiol Heart Circ Physiol. 2011;301:H180–H191. doi: 10.1152/ajpheart.00128.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burstein B, Comtois P, Michael G, Nishida K, Villeneuve L, Yeh YH, Nattel S. Changes in connexin expression and the atrial fibrillation substrate in congestive heart failure. Circ Res. 2009;105:1213–1222. doi: 10.1161/CIRCRESAHA.108.183400. [DOI] [PubMed] [Google Scholar]
- 18.Oakes RS, Badger TJ, Kholmovski EG, Akoum N, Burgon NS, Fish EN, Blauer JJ, Rao SN, DiBella EV, Segerson NM, Daccarett M, Windfelder J, McGann CJ, Parker D, MacLeod RS, Marrouche NF. Detection and quantification of left atrial structural remodeling with delayed-enhancement magnetic resonance imaging in patients with atrial fibrillation. Circulation. 2009;119:1758–1767. doi: 10.1161/CIRCULATIONAHA.108.811877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Verheule S, Tuyls E, Gharaviri A, Hulsmans S, van Hunnik A, Kuiper M, Serroyen J, Zeemering S, Kuijpers NH, Schotten U. Loss of continuity in the thin epicardial layer because of endomysial fibrosis increases the complexity of atrial fibrillatory conduction. Circ Arrhythm Electrophysiol. 2013;6:202–211. doi: 10.1161/CIRCEP.112.975144. [DOI] [PubMed] [Google Scholar]
- 20.Teerlink JR, Cotter G, Davison BA, Felker GM, Filippatos G, Greenberg BH, Ponikowski P, Unemori E, Voors AA, Adams KF, Jr, Dorobantu MI, Grinfeld LR, Jondeau G, Marmor A, Masip J, Pang PS, Werdan K, Teichman SL, Trapani A, Bush CA, Saini R, Schumacher C, Severin TM, Metra M. Serelaxin, recombinant human relaxin-2, for treatment of acute heart failure (relax-ahf): A randomised, placebo-controlled trial. Lancet. 2013;381:29–39. doi: 10.1016/S0140-6736(12)61855-8. [DOI] [PubMed] [Google Scholar]
- 21.Dschietzig T, Teichman S, Unemori E, Wood S, Boehmer J, Richter C, Baumann G, Stangl K. Intravenous recombinant human relaxin in compensated heart failure: A safety, tolerability, and pharmacodynamic trial. J Card Fail. 2009;15:182–190. doi: 10.1016/j.cardfail.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 22.Felker GM, Teerlink JR, Butler J, Hernandez AF, Miller AB, Cotter G, Davison BA, Filippatos G, Greenberg BH, Ponikowski P, Voors AA, Hua TA, Severin TM, Unemori E, Metra M. Effect of serelaxin on mode of death in acute heart failure: Results from the relax-ahf study. J Am Coll Cardiol. 2014;64:1591–1598. doi: 10.1016/j.jacc.2014.05.071. [DOI] [PubMed] [Google Scholar]
- 23.Henry BL, Schwartzman DS, Salama G. Mode of death prevention by serelaxin. J Am Coll Cardiol. 2015;66:98–99. doi: 10.1016/j.jacc.2014.12.079. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.