Abstract
Aims
To compare long-term outcomes among participants randomized to buprenorphine or methadone.
Design/Setting/Participants
Follow-up was conducted in 2011–2014 of 1,080 opioid-dependent participants entering 7 opioid treatment programs in the USA between 2006 and 2009 and randomized (within each program) to receive open-label buprenorphine/naloxone or methadone for up to 24 weeks; 795 participants completed in-person interviews (~74% follow-up interview rate) covering on average 4.5 years.
Measurements
Outcomes were indicated by mortality and opioid use. Covariates included demographics, site, cocaine use, and treatment experiences.
Findings
Mortality was not different between the two randomized conditions with 23 (3.6%) of 630 participants randomized to buprenorphine having died, versus 26 (5.8%) of 450 participants randomized to methadone. Opioid use at follow-up was higher among participants randomized to buprenorphine relative to methadone (42.8% vs. 31.7% positive opioid urine specimens, p< .01, effect size (h)=0.23 [0.09, 0.38]; 5.8 days vs. 4.4 days of past 30-day heroin use, p< .05, effect size (d)=0.14 [0.00, 0.28]). Opioid use over the follow-up period by randomization condition was also significant (F(7,39600)=3.16; p < .001) mostly due to less treatment participation among participants randomized to buprenorphine than methadone. Less opioid use was associated with both buprenorphine and methadone treatment (relative to no treatment); no difference was found between the two treatments. Individuals who are white or used cocaine at baseline responded better to methadone than to buprenorphine.
Conclusions
There are few differences in long-term outcomes between buprenorphine and methadone treatment for opioid dependence, and treatment with each medication is associated with a strong reduction in opioid use.
Introduction
Most long-term follow-up studies of individuals with opioid use disorder are based on participants recruited from methadone maintenance treatment, and results have generally shown positive outcomes in reduced opioid use and mortality.1 For many individuals, opioid use disorder is a chronic condition that requires long-term care.2 Both methadone (MET) and buprenorphine (BUP) are effective medications for opioid use disorder,3–7 and are often used as maintenance treatment to stabilize opioid use on a long-term basis.8,9 In the United States, MET is a Schedule II full agonist that can be used in Federal and State approved programs; BUP is a partial agonist that was approved by the U.S. FDA in 2002 and can be used in general health care settings by qualified practitioners. Currently, very limited information is available on the long-term outcomes of participants started on BUP treatment, particularly relative to those receiving MET treatment.
The present study takes advantage of a large multisite prospective U.S. study that randomized participants to BUP (as buprenorphine/naloxone) versus MET and compares their outcomes 2–8 years after randomization. The parent study “Starting Treatment with Agonist Replacement Therapy” (START) was a phase IV, post-marketing study designed to examine the comparative effects of BUP and MET on indices of liver health in opioid-dependent participants.10 The present article reports on these participants’ long-term outcomes in terms of mortality and opioid use. The aims are to: (1) compare mortality by the randomization conditions (BUP vs. MET); (2) compare opioid use status at the follow-up interview and averaged days of opioid use over the 60 month follow-up period by the randomization conditions (BUP vs. MET); (3) estimate treatment participation status at the follow-up interview and treatment retention over the 60 month period by the randomization conditions (BUP vs. MET); and (4) estimate the effects of each type of opioid replacement treatment (i.e., BUP treatment or MET treatment) on level of opioid use over the 60 month period.
METHODS
Study Design
The START study10 randomized 1,269 individuals to BUP (n=740) or MET (n=529) at nine federally licensed opioid treatment programs during 2006–2009. Because of higher dropout in the BUP arm, midway through the trial the randomization scheme was changed from 1:1 to 2:1 to achieve targeted evaluable BUP participants. This change accounts for the higher number randomized to BUP.11
Long-term Follow-up
A follow-up study of all randomized study participants was conducted during 2011–2014, approximately 2 to 8 years (a mean of 4.5 years) post randomization. Two sites (189 participants) were dropped due to logistical difficulties (i.e., one site recruited only 2 participants and the other had difficulty conducting follow-ups). The remaining sites were located in California, Connecticut, Oregon, Pennsylvania, and Washington. Of the 1,080 targeted participants, 89.4% were located with 797 interviewed (73.6% of patients randomized to MET; 73.7% of patients randomized to BUP), 49 deceased, 54 refused to be interviewed, 29 incarcerated, and 36 too mentally dysfunctional to be interviewed or otherwise not interviewed. Among the 797 interviewed, 2 did not provide timeline follow-back data and were excluded from further analysis. There were no differences in the demographic characteristics of participants included (n=795) and omitted (n=285) from analysis.
Participants
Clinical profiles at baseline for the total 1,080 participants and the 795 interviewed participants are provided in Table 1. Demographic information on 795 participants is as follows. Mean age at baseline was 37.4, 34.1% were female, 72.6% white, 11.2% Hispanic, 9.2% African American, and 7.0% other race/ethnicity. The two medication groups were all similar in baseline measures except that more participants in the MET group reported cocaine use (37.2%) than in the BUP group (30.2%).
Table 1.
Clinical profiles at baseline
| Total Sample | Interviewed Sample | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Randomized to Buprenorphine (n=630) | Randomized to Methadone (n=450) | Total (n=1080) | Randomized to Buprenorphine (n=464) | Randomized to Methadone (n=331) | Total (n=795) | |
| Age at baseline (%) | ||||||
| 18–24 | 15.1 | 14.4 | 14.8 | 15.1 | 15.4 | 15.2 |
| 25–34 | 31.3 | 33.6 | 32.2 | 31.7 | 33.5 | 32.5 |
| 35–44 | 22.4 | 22.9 | 22.6 | 22.6 | 20.2 | 21.6 |
| 45–54 | 24.1 | 23.1 | 23.7 | 24.1 | 24.8 | 24.4 |
| 55+ | 7.1 | 6.0 | 6.7 | 6.5 | 6.0 | 6.3 |
| Mean (SD) | 37.7 (11.3) | 37.5 (11.1) | 37.6 (11.2) | 37.4 (11.1) | 37.4 (11.3) | 37.4 (11.2) |
| Gender (%) | ||||||
| Male | 67.8 | 66.9 | 67.4 | 67.2 | 64.1 | 65.9 |
| Female | 32.2 | 33.1 | 32.6 | 32.8 | 35.9 | 34.1 |
| Race/ethnicity (%) | ||||||
| White | 69.4 | 72.7 | 70.7 | 72.0 | 73.4 | 72.6 |
| African American | 8.6 | 9.3 | 8.9 | 8.8 | 9.7 | 9.2 |
| Hispanic | 13.5 | 10.7 | 12.3 | 13.2 | 8.5 | 11.2 |
| Other | 8.6 | 7.3 | 8.1 | 6.0 | 8.4 | 7.0 |
| # of cigarettes smoked per day (%) | ||||||
| 0 | 12.4 | 10.0 | 11.4 | 11.6 | 10.0 | 10.9 |
| <10 | 27.5 | 26.9 | 27.2 | 26.7 | 28.4 | 27.4 |
| 11–20 | 45.7 | 45.6 | 45.7 | 47.2 | 44.4 | 46.0 |
| 21–30 | 12.1 | 11.8 | 11.9 | 12.1 | 10.9 | 11.6 |
| 31+ | 2.4 | 5.8 | 3.8 | 2.4 | 6.3 | 4.0 |
| In past 30 days, self-reported use of…(%) | ||||||
| Alcohol | 28.1 | 31.0 | 29.3 | 28.3 | 34.2 | 30.8 |
| Cocaine | 30.8 | 38.7 | 34.1 ** | 30.2 | 37.2 | 33.1 * |
| Amphetamine | 7.8 | 8.0 | 7.9 | 7.3 | 7.3 | 7.3 |
| Cannabis | 23.0 | 19.6 | 21.6 | 21.6 | 20.8 | 21.3 |
| Drugs by injection | 67.6 | 66.4 | 67.1 | 67.2 | 63.9 | 65.8 |
| SF-36 Physical Component Summary, Mean (SD) | 49.2 (9.1) | 49.0 (9.4) | 49.1 (9.2) | 49.6 (9.0) | 48.9 (9.4) | 49.3 (9.2) |
| SF-36 Mental Component Summary, Mean (SD) | 39.8 (12.3) | 39.2 (13.1) | 39.5 (12.6) | 39.2 (12.5) | 39.4 (13.2) | 39.3 (12.8) |
Chi-square tests for categorical variables and t-tests for continuous variables between buprenorphine and methadone;
p<0.05;
p<0.01
Interview Procedures
Research staff at the clinics where participants were originally recruited conducted face-to-face follow-up interviews. The assessment interview lasted approximately one and a half to two hours. Staff also collected a urine sample for drug testing and a saliva swab for rapid HIV testing. Participants were compensated for their time in accord with local policies. Payment generally consisted of a $50 gift card for the assessment, $10 for a urine sample, and $10 for a saliva sample. All study procedures were approved by the IRB at UCLA and by the local IRB overseeing each study site. A federal Certificate of Confidentiality was obtained to protect against disclosure of sensitive participant information.
Main Measures
Death
Occurrence and date of death before November 2014 (i.e., the most recent date for which data were available) were searched and determined for all 1,080 participants using the National Death Index.
Opioid use
Current use is indicated by (1) self-reported days of opioid use in the past 30 days at the follow-up interview or (2) positive opioid urine test. Opioid use over the follow-up period was measured by self-reported days of opioid use per month from enrollment to the follow-up interview using timeline follow back (TLFB) methodology12 aided by a calendar and other memory prompts.13,14 Opioid use included heroin and prescription opioids (i.e., hydrocodone, oxycodone, other opiates or analgesics).
Treatment participation
TLFB was also used to collect treatment status over time from START enrollment to the follow-up, thus including periods during the original START trial. Types of treatment include (1) BUP, (2) MET, (3) other opioid medication, and (4) treatment with no opioid medication.
Statistical Analysis
Chi-square tests for categorical measures and t-tests for continuous measures were used for group comparisons. Cohen’s effect sizes for comparing proportions (h) and for comparing means (d) with 95% confidence interval of the effect sizes were also computed.15 Proportional hazard (Cox) regression investigated predictors of time to death since randomization.
A growth modeling approach was used to examine opioid use over the 60-month period after randomization. This approach allows for variation in the length of the observation. Patterns of opioid use over time were compared according to the two conditions (BUP and MET) and also in relation to other covariates including demographics, other types of drug use at baseline, and time-varying treatment status over the 60-month period. The 60-month period was chosen to maximize the length of observation, with 39% of BUP participants and 51% of MET participants contributing complete data. Because these longitudinal data exhibit distinct nonlinear time trends, we modeled time trends with piece-wise linear splines,16 which allow the time trend to change its slope at pre-determined time points called knots. We plotted means and box plots of opioid use against time separately for different levels of covariates, particularly MET and BUP, to determine the number of knots. Five knots were evident, at months 1, 5, 9, 26, and 40 following randomization. We selected Antedependence covariance structure available in SAS PROC MIXED, as this model best fit the data with the lowest BIC and AIC values.12
We built and tested models for opioid use with greater complexity by adding covariates. First, we included only the randomization condition (BUP vs. MET) and the randomization by time interaction with time parameterized according to the bent line spline, as described above (Model 1). Next, because participants ending participation in the original trial could enter different types of treatment over time or discontinue it at any time, we tested Model 2, which elaborates on Model 1 by adding treatment status (BUP, MET, and no BUP or MET) at each point as a time-varying covariate. Hereafter, we use the terms BUP or MET groups or conditions to denote the specific randomized medication group or condition, and we use the terms BUP or MET treatment to denote the status or type of treatment that the participant actually received after the baseline assessment. Because the accessibility and availability of BUP and MET treatment varied by study site, in Model 2 we controlled for clinic site at baseline enrollment. The number of sites is small. Therefore, study site was incorporated as a fixed effect. Nevertheless, we also tested study site as a random effect and found it was not significant (in Model 2, as well as in the subsequent models). Further, all fixed site effects modeling results were similar to those produced by the random site effect models. We thus report findings based on the fixed site effect. For Model 3 we added demographics (age, gender, ethnicity) and baseline cocaine use to Model 2. Model 4 also included interactions of ethnicity and baseline cocaine use with BUP or MET treatment. All data analyses were performed in SAS version 9.3 (Cary, NC); longitudinal models were fit using SAS PROC MIXED.17
RESULTS
Results on mortality based on the total target sample (n=1080) are reported first, followed by descriptive summary statistics on opioid use and treatment participation using data collected from the interviewed sample (n=795). The section concludes with the modeling results on opioid use over the follow-up period.
Mortality
There were 23 deaths in the BUP group (n=630, or 3.6%) and 26 deaths in the MET group (n=450, or 5.8%); the difference was not statistically different (X2(1)=2.74; p=.10). The hazard ratio in the Cox regression that included covariates (age, gender, race/ethnicity, cocaine use at the baseline) showed no difference in time to death between the two randomized conditions (X2(1)=2.71; p=.10).
Opioid use: Summary statistics
Current opioid use at the follow-up interview
Opioid use was higher among participants randomized to BUP relative to MET at the follow-up interview (42.8% vs. 31.7% positive opioid urine specimens, p< .01, effect size (h)=0.23 [0.09, 0.38]; 5.8 days vs. 4.4 days of past 30-day heroin use, p< .05, effect size (d)=0.14 [0.00, 0.28]). Overall, 46.8% participants were currently using opioids as indicated by a positive urine test or self-reported past-30-day opioid use with significantly more opioid use among BUP than MET participants (50.9% vs. 41.1%, effect size (h)=0.20 [0.06, 0.34]; Table 2).
Table 2.
Current heroin and opiate use at the follow-up interview
| Randomized to Buprenorphine (n=464) | Randomized to Methadone (n=331) | Total (n=795) | |
|---|---|---|---|
| Heroin use in the past 30 days (%) | |||
| 0 | 63.2 | 69.5 | 65.8 |
| 1–5 | 12.6 | 11.8 | 12.2 |
| 6–20 | 8.9 | 8.1 | 8.6 |
| 21–30 | 15.4 | 10.6 | 13.4 |
| Mean(SD)* | 5.8 (10.5) | 4.4 (9.3) | 5.2 (10.1) |
| Use of other opiates/analgesicsa in the past 30 days (%)* | |||
| 0 | 77.9 | 85.2 | 81.0 |
| 1–5 | 10.6 | 8.5 | 9.7 |
| 6–20 | 5.2 | 2.4 | 4.0 |
| 21–30 | 6.3 | 3.9 | 5.3 |
| Mean(SD)* | 2.7 (7.4) | 1.6 (5.9) | 2.2 (6.9) |
| Positive urine test on heroin or opiate use (%)** | 42.8 | 31.7 | 38.1 |
| Used heroin or opiates as indicated by urine test or self-report (%)** | 50.9 | 41.1 | 46.8 |
Chi-square tests for categorical variables and t-tests for continuous variables;
p<0.05;
p<0.01.
This category was defined to be comparable with the definition of a positive urine test (i.e., opiates 300ng).
Opioid use over the follow-up period
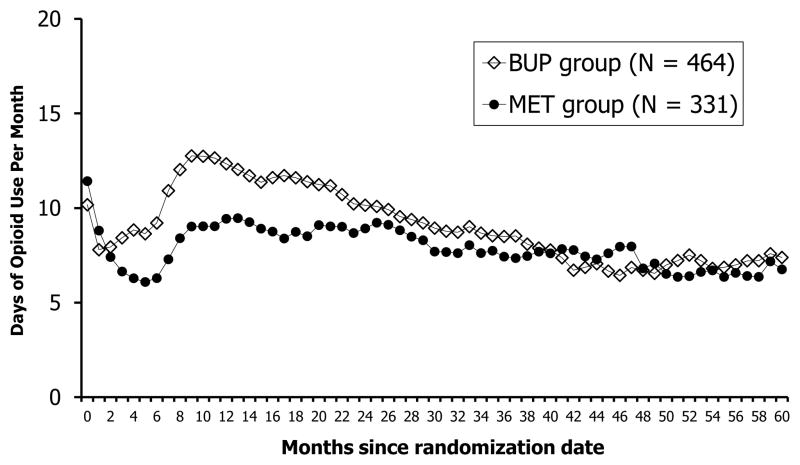
Figure 1 displays the average number of days of opioid use by the two randomized conditions over the follow-up period. Visual inspection of the figure indicates that for both conditions, opioid use drops immediately after entering START, increases somewhat thereafter (approximately 6 months after randomization for both groups), reaches a high point approximately 10 to 12 months post-randomization, and then gradually tapers off; relative to those in BUP, opioid use by individuals in the MET condition dropped more and had lower relapse rates immediately after the trial, although the groups converged in approximately 2 years post-randomization.
Figure 1.
Days of Opioid Use by the Two Randomized Groups (N = 795)
Treatment participation: Summary statistics
Compared to participants randomized to MET, participants randomized to BUP had significantly fewer days in their original treatment (111 vs. 149), spent fewer months in any treatment during the 60 months after randomization (51.7% vs. 62.6% of follow-up months, effect size (h)= −0.22 [−0.36, −0.08]), and fewer were in any treatment at follow-up (53.7% vs. 63.1%, effect size (h)= −0.19 [−0.33, −0.05]) (Table 3). However, being in BUP treatment during the 60-month follow-up period was significantly more common in those randomized to BUP than those randomized to MET (19.8% vs. 6.3% of follow-up months, effect size (h)=0.41 [0.27, 0.56]).
Table 3.
Treatment retention and status
| Randomized to buprenorphine (n=464) | Randomized to methadone (n=331) | Total (n=795) | |
|---|---|---|---|
| During the trial and over the follow-up period | |||
| Days in START over 24 weeks of the trial, mean (SD)** | 111.4 (65.0) | 149.4 (42.0) | 127.3 (59.5) |
| % of months in any treatment during 60 months of follow-up** | 51.7 | 62.6 | 56.2 |
| % of months in methadone treatment** | 28.0 | 53.3 | 38.5 |
| % of months in buprenorphine treatment** | 19.8 | 6.3 | 14.2 |
| % of months in other opioid medication treatmentb | 0.3 | 0.0 | 0.2 |
| % of months in other treatment without opioid medicationsc | 3.6 | 2.9 | 3.3 |
| % of months without any treatment during 60 months of follow-up** | 48.1 | 37.1 | 43.6 |
| Treatment status at follow-up | |||
| Not in any treatment (%)** | 46.3 | 37.0 | 42.4 |
| In methadone treatment (%)** | 37.3 | 48.2 | 41.8 |
| In buprenorphine treatment (%) | 12.3 | 10.0 | 11.3 |
| In other opioid medication treatment (%) | 0.4 | 0 | 0.3 |
| In other treatment without opioid medications (%) | 3.7 | 4.9 | 4.2 |
Chi-square tests for categorical variables and t-tests for continuous variables;
p<0.05;
p<0.01
“Other opioid medication treatment” includes opioid medications other than BUP or MET (e.g., levo-α-acetylmethadol or LAAM).
“Other treatment without opioid medications” includes outpatient, residential, detoxification, or other treatments with no receipt of opioid medications.
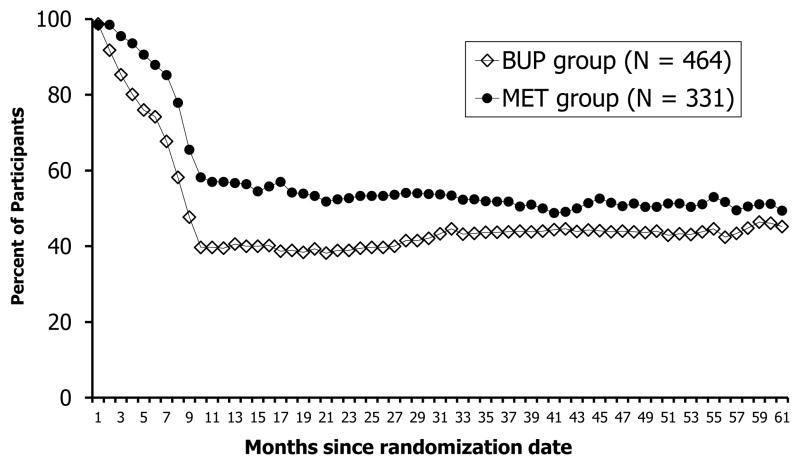
Shown in Figure 2, the percentage of participants in both MET and BUP treatment decreased from months 1 to 10 with fewer BUP participants in treatment compared to those randomized to MET (Chi-square test value at each time point from Month 1 to Month 10 ranged from 16.9 to 33.3; p<.01 for all tests). The proportion in treatment remained relatively stable after Month 10 for both groups.
Figure 2.
Percent of Participants in Treatment† by the Two Randomized Groups (N = 795)
†Treatment is defined as received MET or BUP treatment medication
Modeling opioid use over the follow-up period
Results of four longitudinal models of opioid use over the follow-up period are presented in Table 4. The pattern of results is consistent across models (with various covariates). For parsimony, we describe the results of Model 4 only.
Table 4.
Modeling results predicting days of opioid use over the 60-month follow-up period (N=795)
| Covariatesd | Coefficients
|
|||
|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | |
| Intercept | 11.28** | 21.01** | 21.41** | 20.73** |
| Randomized condition | ||||
| Buprenorphine (vs. Methadone) | −1.09 | −1.10 | −0.89 | −0.81 |
| Male (vs. female) | −0.72 | −0.84 | ||
| White (vs. non-White) | 2.69** | 3.57** | ||
| Age at randomization | −0.08* | −0.08* | ||
| Cocaine use positive at randomization (vs. negative) | 2.44** | 3.00** | ||
| Clinical sites (ref =site 7) | ||||
| Site 1 | −0.84 | −0.83 | −1.16 | |
| Site 2 | −2.36** | −2.40** | −2.41** | |
| Site 3 | −1.23 | −0.99 | −1.56 | |
| Site 4 | −3.57** | −3.55** | −3.64** | |
| Site 5 | −0.24 | 0.01 | −0.14 | |
| Site 6 | −1.28 | −1.08 | −1.15 | |
| Time-varying covariates | ||||
| Methadone treatment (vs. no treatmente) | −8.64** | −8.61** | −6.16** | |
| Buprenorphine treatment (vs. no treatmente) | −6.82** | −6.79** | −7.15** | |
| Interaction of randomized condition and treatment status | ||||
| Buprenorphine group* Methadone treatment | −0.51 | −0.53 | −0.52 | |
| Buprenorphine group* Buprenorphine treatment | −1.78** | −1.78** | −1.76** | |
| Cocaine use* Methadone treatment | −1.44** | |||
| Cocaine use* Buprenorphine treatment | 0.004 | |||
| White* Methadone treatment | −2.69** | |||
| White* Buprenorphine treatment | 0.40 | |||
|
| ||||
| Global F-testf for Intercept and slopesg of spline | ||||
| Intercept and slopes by randomization interactions | F=4.32** | F=2.94** | F=3.09** | F=3.16** |
| Intercept and slopes by gender interactions | F=0.78 | F=0.83 | ||
| Intercept and slopes by ethnicity interactions | F=3.67** | F=3.11** | ||
| Intercept and slopes by age interactions | F=1.95 | F=1.88 | ||
| Intercept and slopes by cocaine use interactions | F=2.08* | F=2.77** | ||
p < 0.05;
p < 0.01
In addition to the covariates summarized in Table 4, other covariates in the models included 6 spline-time indicators (i.e., a slope indicator at month 0–1 and 5 slope change indicators at months 1, 5, 9, 26 and 40), 6 slopes by randomization interactions (i.e., a slope by randomization interaction at month 0–1 and 5 change in slope by randomization interactions at months 1, 5, 9, 26 and 40), 6 slopes by gender interactions (i.e., a slope by gender interaction at month 0–1 and 5 change in slope by gender interactions at months 1, 5, 9, 26 and 40), 6 slopes by ethnicity (i.e., whites vs. non-whites) interactions (i.e., a slope by whites interaction at month 0–1 and 5 change in slope by whites interactions at months 1, 5, 9, 26 and 40), 6 slopes by age interactions (i.e., a slope by age interaction at month 0–1 and 5 change in slope by age interactions at months 1, 5, 9, 26 and 40), and 6 slopes by cocaine-use-status (i.e., positive vs. negative) interactions (i.e., a slope by cocaine-use interaction at month 0–1 and 5 change in slope by cocaine-use interactions at months 1, 5, 9, 26 and 40).
“No treatment” is defined as no BUP or MET treatment, but could include other opioid medication treatment, treatment with no opioid medications, or no treatment.
The degree of freedom for global F-test is F (7, 39600). The 7 indicates the 7 parameters (i.e., 1 intercept and 6 slope and change in slope indicators) simultaneously, and the 39600 indicates the rest of the degrees of freedom, which was computed as the total number of observations-7 (i.e., 39607-7).
Estimated coefficients of slopes and changes in slopes across spline-time indicators were tested simultaneously by Global F-tests.
Association of baseline randomization condition with opioid use
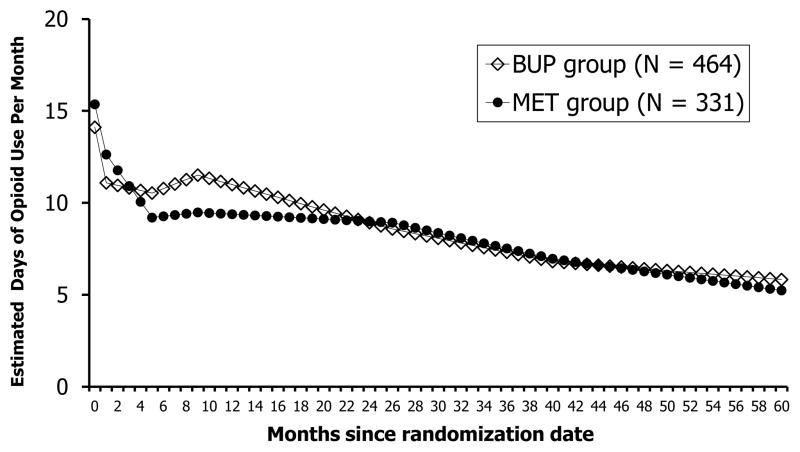
There was no difference by randomization condition in opioid use at baseline however subsequent opioid use patterns were significantly different by randomization condition (F(7,39600)=3.16; p<.01). Adjusting for covariates, the estimated days of opioid use by participants randomized to the MET condition compared to BUP exhibited a greater decrease from month 1 to 6 (as indicated by a steeper drop in slope) and a lower level of use from month 6 onwards, and then the level of use by the two groups gradually merged after about 22 months and for the rest of the observation period (see Figure 3).
Figure 3.
Estimated Days of Opioid Use by the Two Randomized Groups Based on Model 4 (N = 795)
Association of baseline participant characteristics with opioid use
Being white, (compared to non-white), younger age, and cocaine use at baseline were each associated with significantly higher opioid use at baseline. Opioid use over the follow-up period was not related to gender or age but it was related to ethnicity (F (7, 39600)=3.11; p<.01) and cocaine use (F(7, 39600)=2.77; p<.01).
Association of treatment status with opioid use
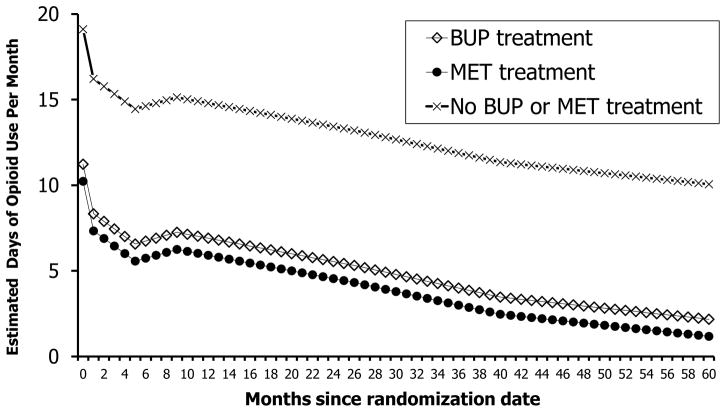
Participation in MET or BUP treatment, relative to no MET or BUP treatment, was associated with reduced opioid use. The estimated reduction on days of opioid use was 8.5 days for MET and 7.8 days for BUP treatment, respectively, with no statistically significant difference between the two types of treatments (F(1,39606)=3.65; p=0.06). Figure 4 presents the estimated days of opioid use over the 60-month period given each type of treatment (i.e., MET treatment, BUP treatment, or neither MET nor BUP treatment).
Figure 4.
Estimated Days of Opioid Use by the Types of Treatment Based on Model 4 (N = 795)††
††The number of participants in each type of treatment varied in each month and is therefore not indicated in the figure; on average over the follow-up period, each month there were about 14.2% of the participants in BUP treatment, 38.5% in MET treatment, and 46.9% in neither BUP nor MET treatment.
Moderation of treatment effects
The effects of MET and BUP treatment on opioid use differed by ethnicity. Compared to being in no treatment, MET treatment was associated with a reduction of opioid use by 9.8 days per month for white participants and by 7.1 days for non-white participants. BUP treatment, compared to being in no treatment, was associated with a reduction of opioid use by 7.6 days for white participants and by 8.0 days for non-white participants. Differences in reduction of days of opioid use between treatments for whites were significant (F (1, 39606)=49.8, p< .01) but not significant among non-whites (F(1, 39606)=2.75, p=.10).
The effects of MET and BUP treatment on opioid use also differed according to whether participants used cocaine. MET treatment was associated with a reduction of opioid use by 9.2 days among cocaine-using participants and by 7.8 days among non-cocaine-using participants. BUP treatment was associated with a reduction of opioid use by 7.8 days among both cocaine-using participants and non-cocaine-using participants. The reduction of opioid use was greater for MET treatment than BUP treatment among cocaine-using participants (reduction of 9.2 vs. 7.8 days; F (1,39606)=8.50, p< .01), while among non-cocaine-using participants the two types of treatment yielded a similar reduction in opioid use (reduction of 7.8 days; F (1,39606)=0.04, p=.84).
Discussion
The ongoing patterns of opioid use in the present study are consistent with many other long-term observations of opioid dependent individuals. Also not surprising is that participation in both MET treatment and BUP treatment was effective at reducing opioid use. There was no difference in the mortality rate according to medication type. Nevertheless, opioid use was higher and treatment participation was consistently lower among BUP participants over the observation period and at the follow-up assessment. These findings are consistent with those reported by prior studies.3,5 We speculate that to improve treatment retention in these two medication conditions, efforts should target factors (at both the patient and contextual level) that contribute to medication discontinuation such as patients’ lack of medication knowledge,18 concurrent use of cocaine or other substances,11,19 inadequate medication dosage,11 co-occurring psychological conditions or stress,19,20 and involuntary medication discontinuation due to strict clinical requirements21 or incarceration.22
Because participants did not always stay in the randomized treatment condition, we included both randomization condition and treatment in the model as separate factors and found similar positive treatment effects of BUP and MET (relative to no treatment) in reduced opioid use. Even after adjusting for covariates (particularly the time-varying treatment status), the difference in opioid use over time between BUP and MET remained significant. Reasons for this finding are not immediately clear. It is important to recognize the overall public health benefits of BUP given that it is more widely available as it can be offered by qualified practitioners in primary care settings as well as in specialty methadone clinics.
In this study, we observed consistently lower treatment participation among participants randomized to BUP than MET. The differential dropout rate between MET and BUP was noted in the original study. That this pattern carried over into the follow-up period is consistent with the differential pharmacology of these two medications. Methadone is a full agonist at the mu opioid receptor and thus more reinforcing and produces a stronger level of physiologic dependence than buprenorphine, which is a partial mu opioid agonist. Since treatment was provided by the study for a limited period, and to remain in treatment participants had to make additional arrangements, the rates of follow-up treatment engagement may not reflect what would occur in routine clinical care. Randomization to MET as opposed to BUP conferred an advantage of having more time in treatment and less drug use over the ensuing 5 years in this study. Nevertheless, participants randomized to BUP were more likely to engage in BUP treatment after completing the study. Given that access routes to BUP treatment, which often occurs in office-based primary care settings, are somewhat different than those for opioid treatment programs, this finding may suggest that a subset of participants randomized to BUP had a sufficiently positive response to the medication such that they sought it out after the study. Nevertheless, opioid users who are unsuccessful with BUP should receive encouragement to enter MET or another effective treatment immediately.
Researchers and practitioners need to better understand which medications work best for which type of drug users. The present study has shown that opioid-dependent users who are white or using cocaine appeared to respond better to MET than BUP in terms of reductions in opioid use. It is also worth noting that cocaine use was associated with negative outcomes among these study participants. Polydrug use, or the concurrent use of multiple substances, has been associated with greater psychopathology;23,24 higher levels of risky health behaviors;25 poorer treatment engagement;26 and worse treatment outcomes.27,28 Thus, treatment for opioid addiction needs to pay special attention to polydrug use, particularly use of cocaine.
The current findings need to be considered within the context of several limitations. The study was based on a randomized medication trial, however many participants dropped from the study and did not remain in their initial randomization condition. Also, the trial lasted for only 6 months and across study sites there was tremendous variability in post-trial treatment availability and local BUP policies. For these reasons, we have included site as a covariate. Finally, the original trial was conducted among community methadone maintenance programs and most participants came to these programs for methadone treatment. Surveillance studies of heroin users indicate that approximately two-thirds have been treated.29 Thus, we believe that our cohort is reasonably representative of opioid-dependent users in the USA. Nevertheless, because the trial recruited from specialty methadone clinics, a setting that is different from general office-based practices, study findings may not be generalizable to those participants typically treated in office-based BUP treatment.
This study, the first to follow opioid dependent individuals randomized to two opioid maintenance treatments prospectively over 5 or more years, is instructive about longer term outcomes and poses a challenge to the field to enhance retention in opioid maintenance treatment. This study shows that many individuals with opioid use disorder cycle in and out of maintenance treatment and confirms that they show better outcomes when retained in maintenance treatment. Efforts are needed, especially in the context of the current opioid epidemic, to improve both BUP and MET treatment retention.
Acknowledgments
The corresponding author, Yih-Ing Hser, has full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Sincere appreciation to our participating networks: the Pacific Northwest Node and Evergreen Treatment Services; the Western States Node and CODA Inc. and Bi-Valley Medical Clinic; the New England Node and Connecticut Counseling Centers and Yale and Hartford Dispensary; the Delaware Valley Node and NET Steps; the Pacific Region Node and Matrix Institute; EMMES Corporation (CCC); the CCTN and NIDA.
Funding source:
Main study funding was provided by the National Institute on Drug Abuse (NIDA) through the Clinical Trials Network (CTN) through a series of grants provided to each participating node:
The Pacific Northwest Node (U10 DA01714)
The Western States Node (U10 DA 015815)
The New England Node (U10 DA13038)
The Delaware Valley Node (U10 DA13043)
The Pacific Region Node (U10 DA13045)
Funding was also provided by NIDA through grant number P30DA016383.
Footnotes
- Walter Ling: Consultant to Reckitt Benckiser Pharmaceuticals.
- Andrew Saxon: Consultant to Reckitt Benckiser Pharmaceuticals, advisory board member for Alkermes, Inc., and receive royalties as an editor for UpToDate.
- George Woody: Consultant to Reckitt Benckiser Pharmaceuticals.
- All other authors report no financial or other possible conflicts of interest.
Trial registration: The START Follow-up Study on ClinicalTrials.gov (NCT01592461).
Contributor Information
Elizabeth Evans, Email: laevans@ucla.edu.
David Huang, Email: yhuang@ucla.edu.
Robert Weiss, Email: robweiss@ucla.edu.
Andrew Saxon, Email: Andrew.Saxon@va.gov.
Kathleen M. Carroll, Email: kathleen.carroll@yale.edu.
George Woody, Email: woody@tresearch.org.
David Liu, Email: dliu@nida.nih.gov.
Paul Wakim, Email: pwakim@nida.nih.gov.
Abigail G. Matthews, Email: amatthews@emmes.com.
Mary Hatch-Maillette, Email: hatchm@u.washington.edu.
Eve Jelstrom, Email: ejelstrom@emmes.com.
Katharina Wiest, Email: KatharinaWiest@codainc.org.
Paul McLaughlin, Email: Paul.McLaughlin@hdisp.org.
Walter Ling, Email: lwalter@ucla.edu.
References
- 1.Hser YI, Evans E, Grella C, Ling W, Anglin D. Long-term course of opioid addiction. Harv Rev Psychiatry. 2015;23:76–89. doi: 10.1097/HRP.0000000000000052. [DOI] [PubMed] [Google Scholar]
- 2.NIH Consensus Statement. Effective medical treatment of opiate addiction. National Consensus Development Panel on Effective Medical Treatment of Opiate Addiction. JAMA. 1998;280:1936–1943. [PubMed] [Google Scholar]
- 3.Bell J, Trinh L, Butler B, Randall D, Rubin G. Comparing retention in treatment and mortality in people after initial entry to methadone and buprenorphine treatment. Addiction. 2009;104:1193–1200. doi: 10.1111/j.1360-0443.2009.02627.x. [DOI] [PubMed] [Google Scholar]
- 4.Connock M, Juarez-Garcia A, Jowett S, et al. Methadone and buprenorphine for the management of opioid dependence: a systematic review and economic evaluation. Health Technol Assess. 2007;11:1–171. iii–iv. doi: 10.3310/hta11090. [DOI] [PubMed] [Google Scholar]
- 5.Mattick RP, Breen C, Kimber J, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2014 Feb 6;2:CD002207. doi: 10.1002/14651858.CD002207.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garcia-Portilla MP, Bobes-Bascaran MT, Bascaran MT, Saiz PA, Bobes J. Long term outcomes of pharmacological treatments for opioid dependence: does methadone still lead the pack? Br J Clin Pharmacol. 2014;77(2):272–284. doi: 10.1111/bcp.12031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Connery HS. Medication-assisted treatment of opioid use disorder: review of the evidence and future directions. Harv Rev Psychiatry. 2015;23(2):63–75. doi: 10.1097/HRP.0000000000000075. [DOI] [PubMed] [Google Scholar]
- 8.Bart G. Maintenance medication for opiate addiction: the foundation of recovery. J Addict Dis. 2012;31:207–225. doi: 10.1080/10550887.2012.694598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kleber HD. Pharmacologic treatments for opioid dependence: detoxification and maintenance options. Dialogues Clin Neurosci. 2007;9(4):455–470. doi: 10.31887/DCNS.2007.9.2/hkleber. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saxon AJ, Ling W, Hillhouse M, et al. Buprenorphine/naloxone and methadone effects on laboratory indices of liver health: a randomized trial. Drug Alcohol Depend. 2013;128:71–76. doi: 10.1016/j.drugalcdep.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hser YI, Saxon AJ, Huang D, et al. Treatment retention among patients randomized to buprenorphine/naloxone compared to methadone in a multi-site trial. Addiction. 2014;109:79–87. doi: 10.1111/add.12333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sobell LC, Sobell MB. Timeline follow-back: a technique for assessing self-reported alcohol consumption. In: Litten RZ, Allen JP, editors. Measuring alcohol consumption. Totowa, NJ: Humana Press; 1992. pp. 41–72. [Google Scholar]
- 13.Hser Y, Anglin MD, Chou CP. Reliability of retrospective self-report by heroin addicts. Psychol Assess. 1992;4:207–213. [Google Scholar]
- 14.Murphy D, Hser Y, Huang D, Brecht L, Herbeck DM. Self-report of longitudinal substance use: A comparison of the UCLA Natural History Interview and the Addiction Severity Index. J Drug Issues. 2010;40:495–516. doi: 10.1177/002204261004000210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cohen J. Statistical power analysis for behavioral sciences. 2. Hillsdale, NJ: Lawrence Earlbaum; 1988. [Google Scholar]
- 16.Weiss RE. Modeling Longitudinal Data. New York: Springer; 2005. [Google Scholar]
- 17.SAS Institute Inc. SAS/STAT(R) 9.3 User’s Guide: The MIXED Procedure. Cary, NC: SAS Institute Inc; 2011. [Google Scholar]
- 18.Teruya C, Schwartz RP, Mitchell SG, Hasson AL, Thomas C, Buoncristiani SH, et al. Patient perspectives on buprenorphine/naloxone: a qualitative study of retention during the starting treatment with agonist replacement therapies (START) study. J Psychoactive Drugs. 2014;46(5):412–26. doi: 10.1080/02791072.2014.921743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haddad MS, Zelenev A, Altice FL. Integrating buprenorphine maintenance therapy into federally qualified health centers: real-world substance abuse treatment outcomes. Drug Alcohol Depend. 2013;131(1–2):127–35. doi: 10.1016/j.drugalcdep.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jaremko KM, Sterling RC, Van Bockstaele EJ. Psychological and physiological stress negatively impacts early engagement and retention of opioid-dependent individuals on methadone maintenance. J Subst Abuse Treat. 2015;48(1):117–127. doi: 10.1016/j.jsat.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bentzley BS, Barth KS, Back SE, Book SW. Discontinuation of buprenorphine maintenance therapy: perspectives and outcomes. J Subst Abuse Treat. 2015;52:48–57. doi: 10.1016/j.jsat.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rich JD, McKenzie M, Larney S, Wong JB, Tran L, Clarke J, et al. Methadone continuation versus forced withdrawal on incarceration in a combined US prison and jail: a randomised, open-label trial. Lancet. 2015;386(9991):350–359. doi: 10.1016/S0140-6736(14)62338-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Booth BM, Leukefeld C, Falck R, Wang J, Carlson R. Correlates of rural methamphetamine and cocaine users: results from a multistate community study. J Stud Alcohol. 2006;67(4):493–501. doi: 10.15288/jsa.2006.67.493. [DOI] [PubMed] [Google Scholar]
- 24.Sumnall HR, Wagstaff GF, Cole JC. Self-reported psychopathology in polydrug users. J Psychopharmacol. 2004;18(1):75–82. doi: 10.1177/0269881104040239. [DOI] [PubMed] [Google Scholar]
- 25.Patterson TL, Semple SJ, Zians JK, Strathdee SA. Methamphetamine-using HIV-positive men who have sex with men: correlates of polydrug use. J Urban Health. 2005;82(1 Suppl 1):i120–126. doi: 10.1093/jurban/jti031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.John D, Kwiatkowski CF, Booth RE. Differences among out-of-treatment drug injectors who use stimulants only, opiates only or both: implications for treatment entry. Drug Alcohol Depend. 2001;64:165–172. doi: 10.1016/s0376-8716(01)00120-x. [DOI] [PubMed] [Google Scholar]
- 27.Bovasso G, Cacciola J. The long-term outcomes of drug use by methadone maintenance patients. J Behav Health Serv Res. 2003;30(3):290–303. doi: 10.1007/BF02287318. [DOI] [PubMed] [Google Scholar]
- 28.Williamson A, Darke S, Ross J, Teesson M. The effect of persistence of cocaine use on 12-month outcomes for the treatment of heroin dependence. Drug Alcohol Depend. 2006;28(81):293–300. doi: 10.1016/j.drugalcdep.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 29.Lipari RN, Hughes A. The NSDUH Report: Trends in Heroin Use in the United States: 2002 to 2013. Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality; Rockville, MD: 2015. Available online at http://www.samhsa.gov/data/sites/default/files/report_1943/ShortReport-1943.pdf. [PubMed] [Google Scholar]