Abstract
Aims
To explore the perspectives of structurally vulnerable people who use drugs (PWUD) regarding: (1) the potential integration of harm reduction interventions (e.g., supervised drug consumption services, opioid assisted treatment) into hospitals; and, (2) the implications of these interventions for patient-centered care, hospital outcomes, and drug-related risks and harms.
Design
Semi-structured qualitative interviews.
Setting
Vancouver, Canada.
Participants
30 structurally vulnerable PWUD who had been discharged from hospital against medical advice within the past two years, and hospitalized multiple times over the past five years.
Measurements
Semi-structured interview guide including questions to elicit perspectives on hospital-based harm reduction interventions.
Findings
Participant accounts highlighted that hospital-based harm reduction interventions would promote patient-centered care by: (1) prioritizing hospital care access and risk reduction over the enforcement of abstinence-based drug policies; (2) increasing responsiveness to subjective health needs (e.g., pain and withdrawal symptoms); and, (3) fostering ‘culturally safe’ care.
Conclusions
Hospital-based harm reduction interventions for people who use drugs, such as supervised drug consumption services and opioid assisted treatment, can potentially improve hospital care retention, promote patient-centred care, and reduce adverse health outcomes among people who use drugs.
Keywords: Patient-centered care, drug users, harm reduction, hospitals, qualitative
INTRODUCTION
Patient-centered care (PCC) has gained increased prominence since the publication of reports by the Picker Institute (Europe) and Institute of Medicine (United States) identifying it as a central element of quality health care (1,2). While PCC first emerged in the early 1980s (3), these reports proved instrumental in advancing PCC within medical education and health policy and planning. In many countries, hospitals have since shifted from care models emphasizing ‘compliance’ with physician orders to those seeking to be responsive to patients’ needs, preferences, and values (2,4–7). While there remains no consensus on the definition of PCC, most PCC models include the following dimensions (1,8,9): (i) recognition of bio-psychosocial influences on health; (ii) acknowledgement of subjective health needs and experiences; (iii) shared power and decision-making between patients and health care providers; and, (iv) promotion of patient-provider communication and relationships based on mutual trust. While first advanced as a means to address power relationships between health care provider sand indigenous populations, ‘cultural safety’ has more recently been advanced as a core dimension of PCC (10) and best practice in the care of vulnerable populations (11–13). ‘Cultural safety’ seeks to ensure that care is responsive to power imbalances and institutional policies and practices that produce health inequities on the basis of race, gender identity, sexuality, and socio-economic status, among other characteristics (14).
Previous studies have highlighted the role of PCC in improving patient-provider trust and communication (15–17), satisfaction with care (16,18,19), and health outcomes (20–22). However, there is evidence that not all populations equally receive PCC (23–27). Social-structural forces operating within hospitals often deny the subjective health experiences and agency of ‘structurally vulnerable’ patient populations. These populations occupy marginal positions within social hierarchies due to social-structural inequities (e.g., drug criminalization, racism) and institutional arrangements (e.g., policies and practices), which render them vulnerable to adverse outcomes (28,29). For example, institutionalized racism within hospital settings often constrains patient-centered communication and shared decision-making for racialized populations (e.g., Indigenous or African-American populations) (23,30,31). Here, racialization refers to the processes by which populations are marginalized by differential treatment within social systems (inclusive of hospitals) on the basis of their race or ethnicity (32). Meanwhile, structural forces, such as ‘risk management’ policies, often lead health care professionals to ignore the needs and preferences of certain populations on the grounds that their ‘risky’ behaviors preclude them from sharing in decision-making (33,34). These shortcomings highlight the need to explore how social and structural conditions within hospitals can be altered to reduce disparities in PCC and hospital outcomes.
This need to better align the social-structural contexts of hospital care with the needs of populations affected by health inequities is particularly important in the case of people who use drugs (PWUD). While considerable heterogeneity exists across drug-using populations, those disproportionately impacted by social-structural inequities, such as racialized policing and homelessness, are particularly vulnerable to the transmission of infectious diseases (e.g., HIV, Hepatitis C) (35–37) and other harms. High rates of infectious diseases (38,39) and other drug-related complications (e.g., injection-related infections) (40–42) among structurally vulnerable PWUD lead to frequent hospitalizations (43,44). However, PWUD have among the worst hospital outcomes of any population and are more likely than other populations to be discharged against medical advice (33,34,45–49). This increases their likelihood of hospital readmission, longer hospital stays, and premature mortality (45,49–51). Recent research characterizing hospitals as ‘risk environments’ has demonstrated that social-structural forces in hospitals, such as abstinence-based drug policies and racial discrimination, drive adverse outcomes for PWUD (33,52,53). Moreover, these contextual forces deny the subjective health experiences and agency of structurally vulnerable PWUD (33), and preclude the implementation of PCC models responsive to their needs.
There is mounting evidence that harm reduction interventions, including syringe exchange programs, supervised drug consumption services, and opioid assisted treatment (see descriptions in Table 1), promote access to care (54–57) and strengthen patient-provider relationships (58,59). This highlights the potential of harm reduction interventions to create conditions that facilitate PCC and improve hospital outcomes. One recent qualitative study outlined how the integration of harm reduction services (including supervised drug consumption services) into an HIV care facility improved health access, engagement, and patient-provider relationships (59). Nonetheless, these approaches have not been implemented in hospitals broadly or in a systematic fashion. There remains a need to understand their potential impacts on PCC and hospital outcomes.
TABLE 1.
Harm reduction interventions
| Harm reduction supply distribution |
| Supervised drug consumption services |
|
| Opioid assisted treatment |
|
We undertook this qualitative study to: (i) explore the perspectives of structurally vulnerable PWUD who had been discharged from hospital against medical advice regarding the potential integration of harm reduction services into hospitals, including potential impacts on drug-related risks and care retention; and, (ii) explore the potential of hospital-based harm reduction approaches to facilitate PCC models for structurally vulnerable PWUD.
METHODS
We draw upon qualitative interviews conducted as part of an ethno-epidemiological study exploring social-structural influences on hospital care among structurally vulnerable PWUD (33,52,53), and their perspectives on hospital-based harm reduction services. Consistent with ethno-epidemiological methods (60,61), we deployed qualitative methods alongside an epidemiological research program to examine contextual influences on hospital outcomes to generate insights to inform more targeted interventions. We conducted the qualitative component in connection with two prospective cohort studies in Vancouver, Canada, comprised of more than 2000 structurally vulnerable PWUD: the Vancouver Injection Drug Users Study (HIV-negative) and AIDS Care Cohort to Evaluate Exposure to Survival Services (HIV-positive), which have been described in detail elsewhere (62,63). In brief, cohort participants complete interview-administered questionnaires and provide blood samples for serological analysis and disease monitoring every six months.
From December 2011 to February 2013, we conducted qualitative interviews with cohort participants who reported leaving hospital prior to completing treatment (past six months) during cohort surveys administered since 2010. We focused on this population because they were deemed most likely to identify shortcomings in hospital care and solutions to adverse hospital outcomes. We identified potential participants by querying cohort databases, as well as recruiting individuals reporting discharges against medical advice when completing surveys administered during the study period. We contacted eligible participants by phone or approached them during office visits to invite them to participate. While no one refused to participate, several people did not show up for interview appointments. Ultimately, thirty people were interviewed (see Table 2 for demographic information). Participants were predominantly Aboriginal and vulnerably housed, and nearly all reported multiple hospitalizations over the past five years.
TABLE 2.
Participant characteristics (n=30)
| Age | |
| Mean | 45 years |
| Range | 29–59 years |
| Gender | |
| Male | 16 |
| Female | 13 |
| Transgender | 1 |
| Race | |
| Aboriginal ancestry | 17 |
| Caucasian | 12 |
| African-Canadian | 1 |
| Health Status | |
| HIV-positive | 15 |
| HCV-positive | 22 |
| Housing status (prior to most recent hospitalization) | |
| Single Room Occupancy Hotel | 17 |
| Apartment | 5 |
| Emergency Shelter | 3 |
| Unhoused | 5 |
| Drug Use (thirty days prior to most recent hospitalization) | |
| Crack cocaine | 22 |
| Heroin | 18 |
| Cocaine | 12 |
| Prescription opioids | 7 |
| Reason for most recent hospitalization | |
| Injection-related infections | 8 |
| Pneumonia | 5 |
| Traumatic injury | 4 |
| Other | 13 |
| Number of hospitalizations (past five years) | |
| One | 2 |
| Two to Three | 13 |
| Four or more | 15 |
The lead author conducted all of the interviews at the cohort study research office. Informed consent was obtained prior to commencing interviews. Participants received $20 CAD honoraria after their interviews. Interviews were facilitated using an interview guide including questions on social-structural influences on hospital care and perspectives on hospital-based harm reduction services. Interviews were audio-recorded, averaged 45 minutes, and were transcribed by research assistants.
Interview transcripts were imported into NVivo, a qualitative analysis software program, and analyzed using inductive and deductive methods (64), which are consistent with ethno-epidemiological approaches. Data analysis began at the project mid-point and emerging themes informed subsequent interviews. Inductive and deductive themes were interpreted by drawing on principles of PCC (1,8,9) and Rhodes’ ‘Risk Environment’ framework (65,66). The Risk Environment framework focuses on how the interplay between types of environments (i.e., social, physical, economic, and political) operating across macro, meso, and micro levels of environmental influence produces or reduces adverse health outcomes (65,66). Macro-level influences refer to structural factors, such as legal or policy frameworks, socio-economic conditions, and cultural beliefs (66). Meso-level influences refer to institutional factors, such as organizational policies and practices (66). Micro-level influences refer to immediate or interpersonal influences, such as social relationships or injection settings (66). This framework also focused our attention on how environmental factors could be modified to improve hospital outcomes. We re-coded interview transcripts following the establishment of the final themes to ensure their credibility (67). We obtained feedback on these themes during presentations to local drug user organizations to enhance the rigour of our findings.
Ethical approval was obtained from the Providence Healthcare Research Ethics Board.
RESULTS
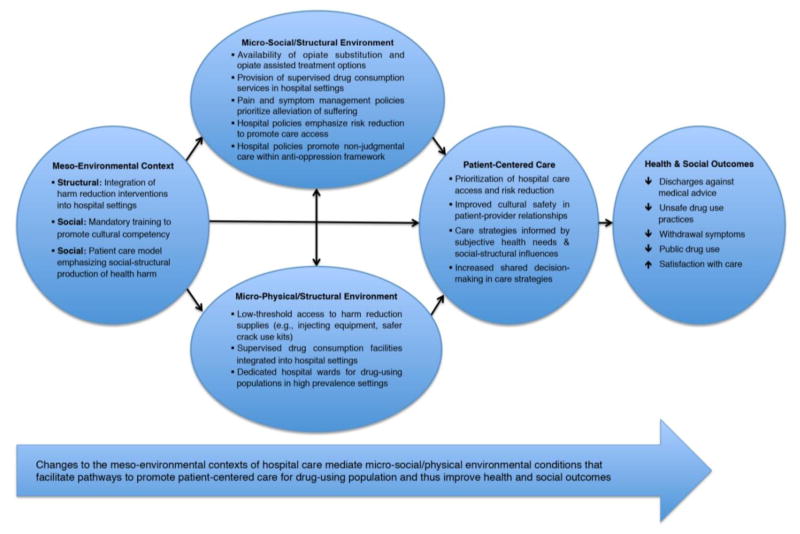
We found that changing the meso-environmental context of hospital care by implementing harm reduction approaches has the potential to mediate micro-social/physical environmental conditions that foster PCC for drug-using populations and improve hospital outcomes. Figure 1 outlines in greater detail the specific environmental changes necessary to align hospital care with the needs, preferences, and values of PWUD. Our findings focus on how these changes would facilitate the adoption of PCC approaches by: (i) prioritizing hospital care retention and risk reduction; (ii) increasing responsiveness to subjective health needs; and, (iii) fostering ‘cultural safety’.
Figure 1.
Conceptual model to promote patient-centered care for PWUD
1. Prioritizing Care Retention & Risk Reduction
1.1. Promoting hospital care retention
Participant accounts suggested that hospital-based harm reduction services would alter the micro-social/physical environmental contexts of hospital care to ensure that continued drug use would not interfere with care access. Participants described how they frequently left hospital before completing treatment to avoid breaching abstinence-based policies, which has been described in detail elsewhere (33). Participants indicated that the expectation that they abstain from drug use while hospitalized was unrealistic due to their drug dependency. Participants emphasized the potential of harm reduction approaches to align environmental conditions in hospital with their needs in ways that would promote care retention. For example, “Mary” described how hospital care retention would be improved if hospitals implemented harm reduction approaches that “listened” to the drug-related needs of PWUD:
If I wouldn’t have been so sick [i.e., experiencing opiate withdrawal], if they would listen about my drug needs, I would stay [in hospital]. Just take it seriously.
Participants singled out hospital-based supervised drug consumption services as having the greatest potential to enable them to complete hospital treatment despite continued drug use. Participants reported that this intervention would allow them to consume drugs in a regulated environment in the hospital as opposed to having to leave hospital or lock themselves in hospital washrooms. “Ellen”, who reported injecting cocaine in locked washrooms during her many hospitalizations, explained:
Just knowing that you’re not having to [consume drugs] in hiding…People aren’t gonna leave the hospital, for one thing, if they feel they need to use and feel safe in the hospital…You don’t have to risk your health care.
1.2. Prioritizing risk reduction over drug abstinence
Whereas current abstinence-based hospital policies produced drug-related risks (e.g., injecting in washrooms, syringe-sharing) for those seeking to avoid “getting caught” using drugs, harm reduction approaches were considered likely to reduce these risks by prioritizing risk reduction over drug abstinence. Participants emphasized that “safer” harm reduction approaches would enable them to enact risk reduction by fostering access to micro-social/physical environmental supports (e.g., medical supervision, injection-related equipment) critical to minimizing HIV and overdose risks. “Allan” described the potential reductions in drug-related risks and harms achievable through hospital-based supervised drug consumption services:
It would save lives. It’d be in a safer environment and it’d be under supervision. It wouldn’t be out on the street…You could watch each other’s back if something should go wrong like an overdose or something.
Additionally, participants emphasized the importance of opioid assisted treatment in reducing the risks (e.g., overdose) associated with opioid dependency under drug criminalization that become particularly pronounced during hospitalizations. For example, given the proliferation of adulterated drugs in the study setting (e.g., heroin mixed with fentanyl), participants explained that opioid assisted treatment meant that they would “know what kind of drugs we’re actually doing.” That is, it would eliminate concerns associated with consuming drugs of unknown purity that are particularly risky when injecting alone (e.g., hospital washrooms) or in situations leading to rushed injections (e.g., avoiding detection by nurses).
2. Responsiveness to Subjective Health Needs
2.1. Acknowledging experiences of pain & withdrawal
Participants considered harm reduction approaches necessary to create micro-environmental conditions in hospitals that acknowledge the subjective health needs and experiences of PWUD. While participants described diverse subjective health needs, they indicated that unmanaged pain and withdrawal symptoms were their most urgent health concerns when hospitalized, and that these framed their experiences in hospitals. Participants reported that “heightened tolerance” to pain medications due to complex co-morbidities (e.g., HIV, traumatic injury) and withdrawal symptoms stemming from the interruption of drug use patterns produced severe suffering when hospitalized. However, participants indicated that hospital staff routinely dismissed these symptoms due to the intersection of abstinence-based policies, anti-drug stigma, and, in the case of those of Aboriginal ancestry, racism. Participants explained that harm reduction approaches had the potential to reorient care by acknowledging these symptoms, among other health care needs, and ensuring that these needs were prioritized over drug abstinence. For example, the following excerpts illustrate how reorienting pain and withdrawal management protocols to alleviate suffering was considered necessary to acknowledge – even symbolically – participants’ subjective health needs and experiences:
You could just show a little more compassion and gentleness. Understand that good people are also addicts. Not everybody is a criminal. Maybe not to deny them proper medications whether it’s an opiate or not just because of their addiction. Give them a chance to heal and get better.
[“Joseph”]
The hospital should ask the person, ‘Do you need anything? Are you using anything?’ Heroin, it’s an addictive drug. If my doctor was giving me pain medication, then I wouldn’t have to use heroin…If you’re a junkie and go in the hospital, they won’t give you anything. How do they expect you to stay?
[“Alexander”]
2.2. Aligning environmental conditions with health needs & decisions
Participants considered the provision of more comprehensive harm reduction supports as being necessary to ensure that hospital care is responsive to and respectful of their needs and experiences. They viewed improvements in pain and withdrawal management as likely to reduce their need to consume drugs. However, participants reported that they would continue to consume drugs due to drug dependency and personal preferences. Harm reduction approaches, such as supervised drug consumption services and opioid assisted treatment, were considered necessary to allow participants’ agency in responding to their needs (e.g., pain and withdrawal symptoms) and in making decisions surrounding drug use. For example, participants indicated that providing opioid assisted treatment in combination with supervised drug consumption services would enable them to manage withdrawal symptoms, in consultation with their physician, stemming from interruptions in drug use patterns. As “Elaine”, who had experienced extreme pain and withdrawal in hospital, explained:
If a person doesn’t have their down [i.e., heroin], they get really sick. I mean, physically sick. You know, instead of being judged by the health care professional, he can just go take a little walk down to this, you know, place [i.e., supervised drug consumption services] and go use [i.e., prescribed opioids] and go back to the bed… They’re able to say, you know, “I am physically sick and I need what I need.”
Importantly, participants articulated that aligning the meso- and micro-social/structural contexts of hospitals with their needs by providing harm reduction supports would ensure that primary focus of care remains their presenting illness. “Otis” explained that this would reduce barriers to hospital care access and retention by partially relieving the stress associated with hospitalization:
I wouldn’t be leaving the hospital. It would make me want to deal with my medical issues. I would stay there ‘cause when I’m up there [in hospital] I want to use drugs. I know a lot of people that go to the hospital that are drug users, and they leave the hospital ‘cause they want to do drugs.
3. Promoting Cultural Safety
3.1. Increased cultural safety in patient-provider relationships
Harm reduction approaches were considered necessary to disrupt stigma operating at the micro (e.g., anti-drug discrimination) and meso (e.g., abstinence-based policies) social-environmental levels and reorient the environmental contexts of hospitals around cultural safety. Participants asserted that harm reduction approaches would reshape the social-environmental contexts of patient-provider interactions by promoting non-judgment and refocusing attention on their ‘personhood’. Participants drew upon their experiences with local harm reduction services when emphasizing that the non-judgment and social inclusion that characterizes these settings could be extended to hospitals through the adoption of similar approaches. “Leslie”, who regularly injected heroin at the local supervised injection facility, explained:
In Insite [i.e., local supervised injection facility], they basically don’t judge. When you go in there, you’re just who you are…You know that nobody’s judging you. They’re keeping an eye out for you just to make sure you’re safe…If [the hospital] was kinda [like] that environment where you feel like you’re not being judged or stereotyped, I think it definitely would be something people [like] myself would be interested in.
Aboriginal participants described how the intersection of anti-drug stigma and racism led to poor treatment in hospitals and contributed to distrust of hospital staff. All participants identified the need to complement harm reduction policies with training, emphasizing social-structural drivers of drug dependence and health inequities. However, Aboriginal participants emphasized the importance of coupling these supports with anti-racist policies and education to extend cultural safety to racialized populations. The following excerpts from interviews with “Beth” and “Natalie”, both Aboriginal women, highlight the intersection of racism and anti-drug stigma, and emphasis Aboriginal participants placed on the need for non-judgmental approaches:
Sometimes, when you’re in a hospital…and you’re an Aboriginal person. You walk in and people just start looking at you and you know there’s a lot of racism in the hospital. […] They mistreat you and they don’t care. […] I think if I was treated equally like the other patients were being treated, like human beings and not mistreated, I would [stay]… treat them for who they are and not just because we’re Aboriginal people and drug addicts.
[“Beth”]
I think they need to learn more about drug addicts and alcoholics and that they need more teaching around addictions instead of judging because of them not having that education…Instead of just being a nurse, maybe be a friend too.
[“Natalie”]
3.2. Creating culturally safe spaces
While emphasizing the importance of cultural safety across the meso- environmental level, participants further pointed to the potential of specialized wards operating under harm reduction and antiracist approaches to create ‘culturally safe spaces’ in hospitals for drug-using populations. They viewed specialized wards as most likely to minimize anti-drug stigma and racial discrimination within the context of patient-provider relationships because the wards would require a greater degree of skill specialization among hospital staff. In turn, participants asserted that hospital staff in specialized wards “[would be] more at ease and know what’s expected if there’s a special area for us”. The following excerpt from an interview with “Roy” illustrates how specialized wards with trained hospital staff were perceived to have potential to minimize stigma and discrimination:
Maybe a separate ward would be better for the addicted… I think a lot of the stigma and misbeliefs about addictions wouldn’t be prevalent because you know that the staff…If they’re there, [it’s] because they really care from the heart…I think the discrimination of addiction would probably, hopefully, be gone.
Participants also acknowledged the potential social-structural barriers (e.g., stigma, anti-drug policies) to integrating harm reduction approaches into general inpatient settings, with one participant noting that these “wouldn’t be very kosher with the other patients”. Participants reported that specialized hospital wards would thus need to be “separated and segregated” in order to be acceptable to other patients and to accommodate those wishing to pursue drug abstinence or drug treatment during hospitalization. Nonetheless, participants indicated that specialized wards for PWUD would be similar to those available to other patient populations. “James” explained:
There should be like a special wing of the hospital that’s just for people [who use drugs] so that their lifestyle doesn’t affect other people that are hospitalized. […] They already have that, where all patients with similar problems are kept in the same wings of the hospital. Like, everybody with spinal problems is kept in the same department together and everybody with cancer are all kept together, so they don’t feel like they’re alone in it, so they have somebody that they can relate to.
DISCUSSION
In summary, our findings highlight the potential of harm reduction approaches to reshape the meso-social/structural contexts of hospital care to foster micro- social/physical environmental conditions that promote PCC for structurally vulnerable PWUD (see Figure 1). Our findings also demonstrate the potential of population-specific PCC to produce improvements in hospital outcomes, including increased satisfaction with care, improved patient-provider relationships, and reductions in discharges against medical advice.
Conceptualizing harm reduction as a necessary condition for PCC for structurally vulnerable PWUD reveals continuities between these approaches. By reducing risk and harm without requiring drug abstinence, harm reduction approaches reflect principles of PCC, such as responsiveness to patient needs, preferences, and values. Conversely, harm reduction approaches are perhaps necessary to orient hospital care to the subjective health needs and experiences of PWUD and foster shared decision-making and mutual trust. However, while efforts to eliminate disparities in PCC prioritize clinical strategies, harm reduction approaches often focus on the need for structural changes (e.g., drug policy reforms) to respond to the needs of PWUD (54,65). Advancing equity in PCC for PWUD would require both clinical and harm reduction approaches to remake the structural-environmental context of hospital care (e.g., reforming drug laws and hospital policies) and facilitate the implementation of population-specific PCC for PWUD. This further suggests that changes to the social-structural contexts of hospital settings can transform them from ‘risk environments’ into ‘safer environments’ for drug-using populations. However, given continued community opposition to harm reduction approaches in much of the world, such changes are unlikely to happen quickly. Advocacy campaigns involving a broad coalition of actors (e.g., researchers, clinicians, drug user organizations) will likely be necessary to foster socio-political conditions leading to the implementation of hospital-based harm reduction services.
Nonetheless, there is an ethical imperative for PCC models to be as responsive to the subjective needs and experiences of drug-using populations as they are to other populations. We found that harm reduction approaches were considered necessary to this end, particularly with regard to pain and withdrawal management. While PWUD have diverse health needs and experiences, there is considerable evidence that their pain and withdrawal symptoms are routinely ignored in hospitals (33,34,52) as the result of anti-drug stigma, racism, and abstinence-based hospital policies (33,34,68). Unmanaged pain and withdrawal symptoms foster severe suffering, and lead to discharges from hospital against medical advice among PWUD seeking to alleviate these symptoms (33). Our findings suggest that enabling PWUD to manage pain and withdrawal in consultation with their attending physician (i.e., opioid assisted treatment) or by injecting on their own (i.e., supervised drug consumption services) may significantly improve hospital care retention and minimize drug-related risks.
Consistent with previous studies on community-based harm reduction interventions (54,55,58,59), our findings demonstrate the potential of hospital-based harm reduction approaches to improve patient-provider relationships by de-stigmatizing drug use, refocusing attention on ‘personhood’, and addressing racialized inequities in patient decision-making. Harm reduction approaches thus appear to be a necessary condition for ‘cultural safety’ in hospital care for PWUD. Importantly, cultural safety extends beyond cultural competence through attention to systemic factors (e.g., power imbalances, institutional policies) that produce and reproduce health inequities among and within populations along classed, gendered, and racialized lines (69). The abovementioned structural changes represent an important step toward fostering conditions that enable cultural safety. However, additional steps, such as improved training and institutional policies, are necessary to further support health professionals in providing more equitable care. Training in harm reduction approaches that emphasizes social-ecological understandings of health inequities will be necessary to promote cultural safety (11,70). The overrepresentation of racialized groups among drug-using populations – and, indeed, Aboriginal persons in our sample – further requires that harm reduction training be combined with antiracist policies and educational programming to extend cultural safety equally across all drug-using populations.
Finally, while integrating harm reduction into PCC models has the potential to reframe hospitals as culturally safe spaces, more targeted approaches, such as specialized wards, might be necessary to ensure cultural safety for PWUD at particularly high-risk of adverse hospital outcomes (e.g., discharges against medical advice). Previous research has suggested that hospital-based harm reduction services may foster conflict between ‘non-drug users’ or ‘good drug users’ and ‘disruptive drug users’ (71). However, our findings suggest that specialized wards for high-risk PWUD might limit these conflicts while enabling hospitals to deploy resources (e.g., trained staff) in a more targeted fashion. Such an approach to hospital care for PWUD is consistent with the emerging view that greater specialization is needed in Addiction Medicine to more fully respond to their needs (72). While specialized wards might not be feasible in all settings, they should be considered in settings where drug epidemics contribute to frequent hospitalizations. However, because racialized groups are often overrepresented among drug-using populations (73,74), these specialized approaches should be implemented only in consultation with these communities and in a manner that does not reproduce segregation but rather addresses larger health inequities.
This study has several limitations. Participants had been discharged against medical advice, and had negative experiences in hospitals. Their views might not be representative of PWUD reporting more favourable hospital treatment. We also undertook this research in a setting with comprehensive harm reduction services. Research undertaken elsewhere should bear in mind that PWUD might have less familiarity with specific interventions. Finally, we did not explore the perspectives of hospital staff regarding hospital-based harm reduction interventions, and additional research exploring their perspectives is needed to more fully account for factors likely to shape the implementation of these approaches.
In conclusion, growing awareness of disparities in hospital outcomes among drug-using populations points to the need for action to better align care with their needs. Hospital-based harm reduction approaches represent a promising avenue for not only fostering population-specific PCC, but also bringing about more comprehensive health improvements among structurally vulnerable PWUD.
References
- 1.Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academies Press; 2001. [PubMed] [Google Scholar]
- 2.Gerteis M, Edgman-Levitan S, Daley J, Delbanco TL. Through the patient’s eyes: understanding and promoting patient-centered care. Jossey-Bass; San Francisco: 1993. [Google Scholar]
- 3.Stewart M. Patient-centered medicine: transforming the clinical method. Radcliffe Publishing; 2003. [Google Scholar]
- 4.NHS. Preventative, people-centred, productive. Department of Health Publications; 2009. 2010–2015: from good to great. [Google Scholar]
- 5.National Health and Hospitals Reform Commission. A healthier future for all Australians—final report. National Health and Hospitals Reform Commission; Canberra, Australia: 2009. [DOI] [PubMed] [Google Scholar]
- 6.The path to a high performance US health system: a 2020 vision and the policies to pave the way: Commonwealth Fund; 2009.
- 7.Ontario Medical Association. Patient-Centred Care. Toronto, Canada: Ontario Medical Association; 2010. [Google Scholar]
- 8.Mead N, Bower P. Patient-centredness: a conceptual framework and review of the empirical literature. Social Science & Medicine. 2000;51(7):1087–110. doi: 10.1016/s0277-9536(00)00098-8. [DOI] [PubMed] [Google Scholar]
- 9.Stewart M. Towards a global definition of patient centred care. BMJ. 2001;322(7284):444–5. doi: 10.1136/bmj.322.7284.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nguyen HT. Patient centred care: cultural safety in indigenous health. Australian Family Physician. 2008;37(12):990. [PubMed] [Google Scholar]
- 11.Pauly B, McCall J, Browne AJ, Parker J, Mollison A. Toward Cultural Safety: Nurse and Patient Perceptions of Illicit Substance Use in a Hospitalized Setting. Advances in Nursing Science. 2015;38(2):121–35. doi: 10.1097/ANS.0000000000000070. [DOI] [PubMed] [Google Scholar]
- 12.Ka’opua L, Diaz TP, Park SH, Bowen T, Patrick K, Tamang S, et al. Colorectal cancer screening at the nexus of HIV, minority statuses, and cultural safety. American Journal of Health Education. 2014;45(1):42–51. doi: 10.1080/19325037.2013.853002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cox LG, Simpson A. Cultural safety, diversity and the servicer user and carer movement in mental health research. Nursing inquiry. doi: 10.1111/nin.12096. In press. [DOI] [PubMed] [Google Scholar]
- 14.Smye V, Browne AJ. ‘Cultural safety’and the analysis of health policy affecting aboriginal people. Nurse Researcher. 2002;9(3):42–56. doi: 10.7748/nr2002.04.9.3.42.c6188. [DOI] [PubMed] [Google Scholar]
- 15.Lee Y-Y, Lin JL. Do patient autonomy preferences matter? Linking patient-centered care to patient–physician relationships and health outcomes. Social Science & Medicine. 2010;71(10):1811–8. doi: 10.1016/j.socscimed.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 16.Fiscella K, Meldrum S, Franks P, Shields CG, Duberstein P, McDaniel SH, et al. Patient trust: is it related to patient-centered behavior of primary care physicians? Medical Care. 2004;42(11):1049–55. doi: 10.1097/00005650-200411000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Ishikawa H, Hashimoto H, Kiuchi T. The evolving concept of ‘patient-centeredness’ in patient–physician communication research. Social Science & Medicine. 2013;96:147–53. doi: 10.1016/j.socscimed.2013.07.026. [DOI] [PubMed] [Google Scholar]
- 18.Mallinger JB, Griggs JJ, Shields CG. Patient-centered care and breast cancer survivors’ satisfaction with information. Patient Education and Counseling. 2005;57(3):342–9. doi: 10.1016/j.pec.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 19.Wolf DM, Lehman L, Quinlin R, Zullo T, Hoffman L. Effect of Patient-Centered Care on Patient Satisfaction and Quality of Care. Journal of Nursing Care Quality. 2008;23(4):316–21. doi: 10.1097/01.NCQ.0000336672.02725.a5. [DOI] [PubMed] [Google Scholar]
- 20.Oates J, Weston WW, Jordan J. The impact of patient-centered care on outcomes. Family Practice. 2000;49:796–804. [PubMed] [Google Scholar]
- 21.Kaplan SH, Greenfield S, Ware JE., Jr Assessing the effects of physician-patient interactions on the outcomes of chronic disease. Medical Care. 1989;27(3):S110–S27. doi: 10.1097/00005650-198903001-00010. [DOI] [PubMed] [Google Scholar]
- 22.Epstein RM, Fiscella K, Lesser CS, Stange KC. Why the nation needs a policy push on patient-centered health care. Health Affairs. 2010;29(8):1489–95. doi: 10.1377/hlthaff.2009.0888. [DOI] [PubMed] [Google Scholar]
- 23.Johnson RL, Roter D, Powe NR, Cooper LA. Patient race/ethnicity and quality of patient-physician communication during medical visits. American Journal of Public Health. 2004;94(12):2084–90. doi: 10.2105/ajph.94.12.2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Radwin LE, Cabral HJ, Woodworth TS. Effects of race and language on patient-centered cancer nursing care and patient outcomes. Journal of Health Care for the Poor and Underserved. 2013;24(2):619–32. doi: 10.1353/hpu.2013.0058. [DOI] [PubMed] [Google Scholar]
- 25.Chapman EN, Kaatz A, Carnes M. Physicians and implicit bias: how doctors may unwittingly perpetuate health care disparities. Journal of General Internal Medicine. 2013;28(11):1504–10. doi: 10.1007/s11606-013-2441-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boulware LE, Cooper LA, Ratner LE, LaVeist TA, Powe NR. Race and trust in the health care system. Public Health Reports. 2003;118(4):358. doi: 10.1016/S0033-3549(04)50262-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shahid S, Finn L, Thompson SC. Barriers to participation of Aboriginal people in cancer care: communication in the hospital setting. Medical Journal of Australia. 2009;190(10):574–9. doi: 10.5694/j.1326-5377.2009.tb02569.x. [DOI] [PubMed] [Google Scholar]
- 28.McNeil R, Kerr T, Anderson S, Maher L, Keewatin C, Milloy MJ, et al. Negotiating structural vulnerability following regulatory changes to a provincial methadone program in Vancouver, Canada: A qualitative study. Social Science & Medicine. 2015;133:168–76. doi: 10.1016/j.socscimed.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quesada J, Hart LK, Bourgois P. Structural vulnerability and health: Latino migrant laborers in the United States. Medical Anthropology. 2011;30(4):339–62. doi: 10.1080/01459740.2011.576725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Benkert R, Peters RM, Clark R, Keves-Foster K. Effects of perceived racism, cultural mistrust and trust in providers on satisfaction with care. Journal of the National Medical Association. 2006;98(9):1532. [PMC free article] [PubMed] [Google Scholar]
- 31.Gordon HS, Street RL, Sharf BF, Kelly PA, Souchek J. Racial differences in trust and lung cancer patients’ perceptions of physician communication. Journal of Clinical Oncology. 2006;24(6):904–9. doi: 10.1200/JCO.2005.03.1955. [DOI] [PubMed] [Google Scholar]
- 32.Bonilla-Silva E. White supremacy and racism in the post-civil rights era. Lynne Rienner Publishers; 2001. [Google Scholar]
- 33.McNeil R, Small W, Wood E, Kerr T. Hospitals as a ‘risk environment’: an ethno-epidemiological study of voluntary and involuntary discharge from hospital against medical advice among people who inject drugs. Social Science & Medicine. 2014;105:59–66. doi: 10.1016/j.socscimed.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Merrill JO, Rhodes LA, Deyo RA, Marlatt GA, Bradley KA. Mutual mistrust in the medical care of drug users: the keys to the “narc” cabinet. Journal of General Internal Medicine. 2002;17(5):327–33. doi: 10.1046/j.1525-1497.2002.10625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Robertson MJ, Clark RA, Charlebois ED, Tulsky J, Long HL, Bangsberg DR, et al. HIV seroprevalence among homeless and marginally housed adults in San Francisco. American Journal of Public Health. 2004;94(7):1207–17. doi: 10.2105/ajph.94.7.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cooper HLF, Des Jarlais DC, Tempalski B, Bossak BH, Ross Z, Friedman SR. Drug-related arrest rates and spatial access to syringe exchange programs in New York City health districts: Combined effects on the risk of injection-related infections among injectors. Health & Place. 2012;18(2):218–28. doi: 10.1016/j.healthplace.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Friedman SR, Cooper HLF, Osborne AH. Structural and social contexts of HIV risk among African Americans. American Journal of Public Health. 2009;99(6):1002. doi: 10.2105/AJPH.2008.140327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aceijas C, Stimson GV, Hickman M, Rhodes T. Global overview of injecting drug use and HIV infection among injecting drug users. AIDS. 2004;18(17):2295–303. doi: 10.1097/00002030-200411190-00010. [DOI] [PubMed] [Google Scholar]
- 39.Mathers BM, Degenhardt L, Phillips B, Wiessing L, Hickman M, Strathdee SA, et al. Global epidemiology of injecting drug use and HIV among people who inject drugs: a systematic review. The Lancet. 2008;372(9651):1733–45. doi: 10.1016/S0140-6736(08)61311-2. [DOI] [PubMed] [Google Scholar]
- 40.Cooper HLF, Brady JE, Ciccarone D, Tempalski B, Gostnell K, Friedman SR. Nationwide increase in the number of hospitalizations for illicit injection drug use-related infective endocarditis. Clinical Infectious Diseases. 2007;45(9):1200–3. doi: 10.1086/522176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lloyd-Smith E, Wood E, Zhang R, Tyndall MW, Montaner JSG, Kerr T. Risk factors for developing a cutaneous injection-related infection among injection drug users: a cohort study. BMC Public Health. 2008;8(1):405. doi: 10.1186/1471-2458-8-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Warner-Smith M, Darke S, Lynskey M, Hall W. Heroin overdose: causes and consequences. Addiction. 2001;96(8):1113–25. doi: 10.1046/j.1360-0443.2001.96811135.x. [DOI] [PubMed] [Google Scholar]
- 43.Fairbairn N, Milloy MJ, Zhang R, Lai C, Grafstein E, Kerr T, et al. Emergency department utilization among a cohort of HIV-positive injecting drug users in a Canadian setting. The Journal of Emergency Medicine. 2012;43(2):236–43. doi: 10.1016/j.jemermed.2011.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kerr T, Wood E, Grafstein E, Ishida T, Shannon K, Lai C, et al. High rates of primary care and emergency department use among injection drug users in Vancouver. Journal of Public Health. 2005;27(1):62–6. doi: 10.1093/pubmed/fdh189. [DOI] [PubMed] [Google Scholar]
- 45.Anis AH, Sun H, Guh DP, Palepu A, Schechter MT, O’Shaughnessy MV. Leaving hospital against medical advice among HIV-positive patients. CMAJ. 2002;167(6):633–7. [PMC free article] [PubMed] [Google Scholar]
- 46.Palepu A, Sun H, Kuyper L, Schechter MT, O’Shaughnessy MV, Anis AH. Predictors of early hospital readmission in HIV-infected patients with pneumonia. Journal of General Internal Medicine. 2003;18(4):242–7. doi: 10.1046/j.1525-1497.2003.20720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yong TY, Fok JS, Hakendorf P, Ben-Tovim D, Thompson CH, Li JY. Characteristics and outcomes of discharges against medical advice among hospitalised patients. Internal Medicine Journal. 2013;43(7):798–802. doi: 10.1111/imj.12109. [DOI] [PubMed] [Google Scholar]
- 48.Ding R, Jung JJ, Kirsch TD, Levy F, McCarthy ML. Uncompleted emergency department care: patients who leave against medical advice. Academic Emergency Medicine. 2007;14(10):870–6. doi: 10.1197/j.aem.2007.06.027. [DOI] [PubMed] [Google Scholar]
- 49.Choi M, Kim H, Qian H, Palepu A. Readmission rates of patients discharged against medical advice: a matched cohort study. PloS One. 2011;6(9):e24459. doi: 10.1371/journal.pone.0024459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Glasgow JM, Vaughn-Sarrazin M, Kaboli PJ. Leaving against medical advice (AMA): risk of 30-day mortality and hospital readmission. Journal of General Internal Medicine. 2010;25(9):926–9. doi: 10.1007/s11606-010-1371-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hwang SW, Li J, Gupta R, Chien V, Martin RE. What happens to patients who leave hospital against medical advice? CMAJ. 2003;168(4):417–20. [PMC free article] [PubMed] [Google Scholar]
- 52.Ti L, Voon P, Dobrer S, Montaner J, Wood E, Kerr T. Denial of pain medication by health care providers predicts in-hospital illicit drug use among individuals who use illicit drugs. Pain Research & Management: The Journal of the Canadian Pain Society. 2015;20(2):84. doi: 10.1155/2015/868746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grewal HK, Ti L, Hayashi K, Dobrer S, Wood E, Kerr T. Drug & Alcohol Review. Illicit drug use in acute care settings. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McNeil R, Small W. ‘Safer environment interventions’: a qualitative synthesis of the experiences and perceptions of people who inject drugs. Social Science & Medicine. 2014;106:151–8. doi: 10.1016/j.socscimed.2014.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Small W, Wood E, Lloyd-Smith E, Tyndall M, Kerr T. Accessing care for injection-related infections through a medically supervised injecting facility: a qualitative study. Drug & Alcohol Dependence. 2008;98(1):159–62. doi: 10.1016/j.drugalcdep.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 56.Tyndall MW, Kerr T, Zhang R, King E, Montaner JG, Wood E. Attendance, drug use patterns, and referrals made from North America’s first supervised injection facility. Drug & Alcohol Dependence. 2006;83(3):193–8. doi: 10.1016/j.drugalcdep.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 57.Oviedo-Joekes E, Marchand K, Lock K, Chettiar J, Marsh DC, Brissette S, et al. A chance to stop and breathe: participants’ experiences in the North American Opiate Medication Initiative clinical trial. Addiction Science & Clinical Practice. 2014;9(1):21. doi: 10.1186/1940-0640-9-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Krüsi A, Small W, Wood E, Kerr T. An integrated supervised injecting program within a care facility for HIV-positive individuals: A qualitative evaluation. AIDS Care. 2009;21(5):638–44. doi: 10.1080/09540120802385645. [DOI] [PubMed] [Google Scholar]
- 59.McNeil R, Dilley LB, Guirguis-Younger M, Hwang SW, Small W. Impact of supervised drug consumption services on access to and engagement with care at a palliative and supportive care facility for people living with HIV/AIDS: a qualitative study. Journal of the International AIDS Society. 2014;17:18855. doi: 10.7448/IAS.17.1.18855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Clatts MC, Welle DL, Goldsamt LA, Lankenau SE. An ethno-epidemiological model for the study of trends in illicit drug use: reflections on the ‘emergence’of crack injection. International Journal of Drug Policy. 2002;13(4):285–95. [Google Scholar]
- 61.Lopez AM, Bourgois P, Wenger LD, Lorvick J, Martinez AN, Kral AH. Interdisciplinary mixed methods research with structurally vulnerable populations: Case studies of injection drug users in San Francisco. International Journal of Drug Policy. 2013;24(2):101–9. doi: 10.1016/j.drugpo.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Strathdee SA, Patrick DM, Currie SL, Cornelisse PGA, Rekart ML, Montaner JSG, et al. Needle exchange is not enough: lessons from the Vancouver injecting drug use study. AIDS. 1997 doi: 10.1097/00002030-199708000-00001. [DOI] [PubMed] [Google Scholar]
- 63.Wood E, Montaner JSG, Yip B, Tyndall MW, Schechter MT, O’Shaughnessy MV, et al. Adherence and plasma HIV RNA responses to highly active antiretroviral therapy among HIV-1 infected injection drug users. CMAJ. 2003;169(7):656–61. [PMC free article] [PubMed] [Google Scholar]
- 64.Bradley EH, Curry LA, Devers KJ. Qualitative data analysis for health services research: developing taxonomy, themes, and theory. Health Services Research. 2007;42(4):1758–72. doi: 10.1111/j.1475-6773.2006.00684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rhodes T. Risk environments and drug harms: a social science for harm reduction approach. International Journal of Drug Policy. 2009;20(3):193–201. doi: 10.1016/j.drugpo.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 66.Rhodes T, Singer M, Bourgois P, Friedman SR, Strathdee SA. The social structural production of HIV risk among injecting drug users. Social Science & Medicine. 2005;61(5):1026–44. doi: 10.1016/j.socscimed.2004.12.024. [DOI] [PubMed] [Google Scholar]
- 67.Creswell JW, Miller DL. Determining validity in qualitative inquiry. Theory Into Practice. 2000;39(3):124–30. [Google Scholar]
- 68.Berg KM, Arnsten JH, Sacajiu G, Karasz A. Providers’ experiences treating chronic pain among opioid-dependent drug users. Journal of General Internal Medicine. 2009;24(4):482–8. doi: 10.1007/s11606-009-0908-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Browne AJ, Smye VL, Varcoe C. The relevance of postcolonial theoretical perspectives to research in Aboriginal health. Canadian Journal of Nursing Research. 2005;37(4):16–37. [PubMed] [Google Scholar]
- 70.McNeil R, Guirguis-Younger M, Dilley LB, Turnbull J, Hwang SW. Learning to account for the social determinants of health affecting homeless persons. Medical Education. 2013;47(5):485–94. doi: 10.1111/medu.12132. [DOI] [PubMed] [Google Scholar]
- 71.Strike C, Guta A, de Prinse K, Switzer S, Chan Carusone S. Living with addiction: The perspectives of drug using and non-using individuals about sharing space in a hospital setting. International Journal of Drug Policy. 2014 doi: 10.1016/j.drugpo.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 72.Wood E, Samet JH, Volkow ND. Physician education in addiction medicine. JAMA. 2013;310(16):1673–4. doi: 10.1001/jama.2013.280377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cooper HLF, Linton S, Kelley ME, Ross Z, Wolfe ME, Chen Y-T, et al. Racialized Risk Environments in a Large Sample of People who Inject Drugs In the United States. International Journal of Drug Policy. doi: 10.1016/j.drugpo.2015.07.015. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Craib KJP, Spittal PM, Wood E, Laliberte N, Hogg RS, Li K, et al. Risk factors for elevated HIV incidence among Aboriginal injection drug users in Vancouver. Canadian Medical Association Journal. 2003;168(1):19–24. [PMC free article] [PubMed] [Google Scholar]
- 75.Strike C, Watson TM, Lavigne P, Hopkins S, Shore R, Young D, et al. Guidelines for better harm reduction: Evaluating implementation of best practice recommendations for needle and syringe programs (NSPs) International Journal of Drug Policy. 2011;22(1):34–40. doi: 10.1016/j.drugpo.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 76.Haydon E, Fischer B. Crack use as a public health problem in Canada: call for an evaluation of’safer crack use kits. Canadian Journal of Public Health. 2005:185–8. doi: 10.1007/BF03403687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wood E, Kerr T, Lloyd-Smith E, Buchner C, Marsh DC, Montaner JSG, et al. Methodology for evaluating Insite: Canada’s first medically supervised safer injection facility for injection drug users. Harm Reduction Journal. 2004;1(1):9. doi: 10.1186/1477-7517-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Oviedo-Joekes E, Nosyk B, Brissette S, Chettiar J, Schneeberger P, Marsh DC, et al. The North American Opiate Medication Initiative (NAOMI): profile of participants in North America’s first trial of heroin-assisted treatment. Journal of Urban Health. 2008;85(6):812–25. doi: 10.1007/s11524-008-9312-9. [DOI] [PMC free article] [PubMed] [Google Scholar]